Abstract
Caloric restriction (CR) has been extensively examined as a preventative strategy against aging and various diseases, but CR effects on cerebral ischemia are largely unknown. We subjected C57BL6/J mice to ad libitum food access (LF) or a diet restricted to 70% of ad libitum food access (RF) for two to four weeks followed by 60 min of transient focal ischemia (tFCI). RF for four weeks protected against subsequent tFCI-induced infarct. RF improved sensorimotor function after stroke in the foot fault and corner tests, as well as performance in the Morris water maze test. In addition, RF preserved ischemic white matter tract integrity assessed by histology and compound action potential. Sirt1 and Sirt3 were both upregulated in RF ischemic brain, but heterozygous deletion of Sirt1 or knockout of Sirt3 did not alter the protection induced by RF against ischemic injury. RF induced significant release of adiponectin, a hormone related to glucose metabolism. Knockout of adiponectin decreased RF-induced protection after tFCI. These data demonstrate the novel finding that white matter, as well as neurons, benefit from CR prior to cerebral ischemic injury, and that adiponectin may contribute to these protective effects.
Keywords: Adiponectin, stroke, sirtuin, cerebral ischemia, caloric restriction
Introduction
Caloric restriction (CR) increases longevity, improves cognitive function, and protects in a variety of disease systems, including ischemia.1–4 The restriction of food intake correlates with a variety of molecular events, including but not limited to changes in mitochondrial function,5,6 global changes in gene expression,7–9 and release of systemic factors, including those that alter metabolic state.10
Stroke pathology is associated with oxidative damage, alterations in metabolic capacity and systemically derived migration of inflammatory factors that impact stroke outcomes and survival. Although several studies have indicated that CR protects against subsequent stroke,11–13 the manner in which this occurs remains unknown. Recent studies have correlated CR with the activation of the sirtuin family, particularly Sirt1 and Sirt3,13–15 as well as the release of hormones from adipocytes classically related to glucose metabolism.10,16 The particular role of these factors has not been well addressed in the context of cerebral ischemia and CR. In addition, although CR has been shown to attenuate age-related decreases in brain white matter integrity,17,18 the extent to which non-neuronal brain cells are impacted by CR after ischemia has not been studied, despite extensive evidence that these cells are susceptible to ischemic stroke.
In the present study, we first sought to better define the histological and functional outcomes associated with CR-induced ischemic neuroprotection, and second, to determine whether sirtuins and/or secreted hormones associated with satiety were associated with and contributed to such ischemic neuroprotection.
Materials and methods
Animals
Wild-type adult male C57BL/6J mice were obtained from the Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). Transgenic mice, including sirt1 heterozygous knockout mice (Sirt1+/−), sirt3 homozygous knockout mice (Sirt3−/−), and adiponectin knockout mice (Adipo−/−) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). Animals were housed in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle. Food and water were available ad libitum, except as stated below. All animal procedures were approved by the Animal Care and Use Committee of Fudan University and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were reported in compliance with the ARRIVE guidelines.19
Animal model of CR
Adult male mice (23–25 g body weight, eight weeks old) were fed either ad libitum (LF) or a restricted diet (70% of the normal diet, RF) before induction of transient focal ischemia (tFCI). Our preliminary observations of daily consumption of diet determined that young adult male mice consumed on average 3.4 g ± 0.2 g diet per day. The mice fed ad libitum were provided with sufficient rodent diet, while the mice on a restricted diet were provided 70% of normal diet (2.38 g ± 0.14 g per mouse) every day for two, three or four weeks before tFCI experiments were conducted. The diet was supplied every 24 h under restricted condition to guarantee the 30% reduction of food intake every day throughout the entire CR period. Physiological parameters, including body weight, serum triglycerides, serum cholesterol, and blood glucose over time following bolus glucose injection (glucose tolerance test (GTT)) were measured after four-week pretreatment with a restricted or ad libitum diet.
Animal model of tFCI
Transient focal cerebral ischemia (tFCI) was induced by middle cerebral artery occlusion (MCAO). Briefly, mice were anesthetized with 1.5% isoflurane in a 30% O2/70% N2O gas mixture, and the carotid arteries were exposed and isolated from branches. The external carotid artery (ECA) was ligated and cut approximately 1.5 mm from the bifurcation and a loose 8–0 silk knot was placed around the origin of the ECA. A 7–0 nylon monofilament coated with a silicone tip (0.21 to 0.23 mm diameter) was inserted into the ECA and advanced 1.8 cm along the internal carotid artery (ICA) to occlude the middle cerebral artery. A suture was placed around the ECA to tighten the nylon monofilament and to prevent bleeding. For the RF group, the suture size was adjusted to account for the reduction in body weight in order to maintain equal blood flow reduction across groups. After 60 min, the filament was withdrawn to establish reperfusion and the wound was sutured. Rectal temperature was maintained at 37.0 ± 0.5℃ during and 2 h after surgery with a temperature-regulated heat lamp. Blood samples were collected from the left femoral artery for blood gas analysis 15 min before ischemia. Cerebral blood flow (CBF) was measured to confirm the induction of ischemia and successful reperfusion. Animals that did not show a CBF reduction of at least 75% on laser-Doppler flowmetry were excluded from further experimentation. Mice in the sham surgery group were similarly anesthetized and operated on but were not subjected to tFCI. Blood glucose and insulin levels were measured after dietary restriction, before ischemic surgery, and within the first 4 h after reperfusion. Weight was monitored beginning 8 days before RF treatment and continuing through 12 days after tFCI (total 49 days). All efforts were made to minimize animal suffering during all of the procedures. All outcome measures were assessed by investigators blinded to experimental group assignment.
Assessment of infarct and atrophy volume
Infarct volume assessment with 2,3,5-triphenyltetrazolium chloride (TTC) staining was performed and quantified as previously described.20 Briefly, brains were removed and sliced into seven coronal sections each 1 mm thick. Sections were immersed in prewarmed 2% TTC (Sigma, Shanghai, China) in saline for 10 min, and then fixed in 4% paraformaldehyde. Animals that developed massive hemorrhage were excluded from further evaluation (about 2% of stroke animals). Microtubule-associated protein 2 (MAP2) immunostaining was used as a determinant of atrophy volume. Briefly, free-floating sections were prepared from the fixed and dehydrated brains and stained with MAP2 antibody (Abcam, Cambridge, UK). Volume of infarct or atrophy was determined with NIH Image J analysis by an observer blinded to experimental group assignment. The volume of infarct/atrophy with correction for edema was calculated as the volume of the contralateral hemisphere minus the non-infarcted volume of the ipsilateral hemisphere from five coronal brain slices (bregma 0.73 to −1.67 mm).
Sensorimotor outcomes
The corner test was used to assess sensorimotor asymmetry following tFCI. Mice were placed in the center of the testing apparatus that was constructed of two plastic boards placed at a 30° degree angle. Upon exploration, mice were able to choose to turn left or right when approaching the corner. Due to some variation among individuals in side preference, mice were screened prior to tFCI to exclude mice with naturally occurring strong asymmetry. Ten trials were performed and the number of left turns per trial was recorded. The foot fault test was used to assess sensorimotor coordination by assessing pad placement during spontaneous locomotion. Mice were placed on an elevated grid surface (30 L × 35 W × 31 H cm) with a grid opening of 2.5 cm2 and videotaped for 1 min for scoring by a blinded experimenter. The total number of steps was counted, and the number of foot-faults (when the animals misplaced a forepaw or hindpaw such that it fell through the grid) was determined within the same minute. The rotarod test was used to assess motor function. Mice were placed on an accelerating rotating rod (4 to 40 r/min over 300 s) and their latency to fall off the rod was recorded as previously reported.21 Mice were trained for three trials per day from three days before the surgery. The average time of the three trials during the last day of training was recorded as the pre-surgery baseline value. After surgery, five trials were performed on each testing day with intervals of 5 min between each trial, and the data for trials #3–5 were used to calculate the mean latency to fall on that day. All tests were carried out by researchers blinded to the experimental conditions of each group.
Morris water maze
The Morris water maze test was performed between days 23 and 27 after tFCI, with pre-training prior to tFCI to ensure task compliance. For this test, a 10 cm diameter platform was submerged in a quadrant of a pool that was 110 cm in diameter. The platform was placed 1 cm under the water surface, and the water was made opaque with non-toxic antholeucin. The hidden platform test assessed the ability of the mice to learn the location of the platform without being able to directly see it relative to external spatial cues displayed on the interior of the pool and the surrounding walls. Each mouse was released from each of the three quadrants that did not contain the platform and was allotted 60 s to search for the hidden platform. At the end of each trial, the mouse was placed on the platform or allowed to remain on the platform for 30 s to orient to the prominent spatial cues displayed around the room. Four trials per day for four consecutive days were performed with the location of the platform kept constant. Data are expressed as the time or latency to reach the submerged platform on each day. After the learning trials were completed, the platform was removed, and each mouse was placed in the pool once for 60 s at the same starting location in order to test the memory for the location of the platform. The number of times the mouse crossed the area where the platform used to sit was recorded. All tests were carried out by researchers blinded to group assignment.
Immunohistochemistry
For immunofluorescent staining, brain sections (25µm thick) of interest were washed three times in phosphate-buffered saline (PBS) solution, blocked with 10% goat serum for an hour, followed by 1-h incubation with the primary antibody at 37℃ and overnight at 4℃. After three washes in PBS, sections were incubated with a species-specific secondary antibody conjugated with DyLight 488 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) or DyLight 594 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Primary antibodies included rabbit anti-adiponectin receptor-1 (Abcam, Cambridge, UK), rabbit anti-MAP2 (Abcam, Cambridge, UK), mouse anti-andenomatous polyposis coli (APC, EMD Millipore, Burlington, MA, USA), mouse anti-glial fibrillary acidic protein (GFAP, Abcam, Cambridge, UK), mouse anti-nonphosphorylated neurofilaments (SMI32, Covance, Princeton, NJ, USA) and rat anti-myelin basic protein (MBP, EMD Millipore, Burlington, MA, USA).
Quantification of immunofluorescent images
All immunofluorescent images were captured by a blinded observer. For the calculation of the ratio of SMI32 and MBP, microscopic fields were acquired from striatum, cortex and corpus callosum (CC) in the peri-injury region as indicated (one field per box). The regions of interest in the sham brain and contralateral hemisphere were the same as that in ipsilateral side. Parameters for acquiring the images were identical for each brain slice at the same regions of interests from both hemispheres. The fluorescence intensity was measured using NIH ImageJ analysis by an observer blinded to the experimental group assignment. The ratio of SMI32/MBP in the ipsilateral hemisphere was further normalized by contralateral hemisphere. For the quantification of adiponectin receptor1 (AdipoR1) double labeled cells, microscopic fields were acquired from CC, striatum and cortex in the peri-injury region as indicated (one field per box). The regions of interest in the sham brain were the same as that in ipsilateral side. The number of AdipoR1+/APC+ cells was counted by blinded observer. For the quantification of AdipoR1+/GFAP+ signal, the area of AdipoR1+/GFAP+ was analyzed in both ipsilateral and contralateral hemispheres with identical parameters for acquiring the images. The ratio of AdipoR+/GFAP+ in the ipsilateral hemisphere was normalized by contralateral hemisphere by blinded observer.
Calculation of CC thickness
Callosal thickness was assessed in MBP-stained sections by dividing the CC into 10 equal intervals (117.65 µm/interval). The width of the CC was measured every 117.65 µm from the midline. Experimenters were blinded to the treatment groups.
Western blot
Tissue from CC was harvested at the indicated time points from mice after tFCI for Western blot analysis. Western blot analysis was performed following the standard procedure of the SDS-PAGE method as previously described.22 After blocking, PVDF membranes were incubated at room temperature for an hour and then overnight at 4℃ with the following primary antibodies: rabbit anti-Sirt3 polyclonal antibody (Abcam, Cambridge, UK), rabbit anti-Sirt1 monoclonal antibody (CST, Danvers USA), and rat anti-MBP antibody (Abcam, Cambridge, UK). To detect multiple bands on a single membrane, the membrane was incubated in Restore Plus Western blot stripping buffer (Thermo Fisher Scientific) for 20 min at 37℃ between the various labeling procedures. The blots were semi-quantified using gel densitometry with Quantity One software (Bio-Rad, Hercules, CA, USA). Three independent experiments were performed, with five to nine mice per group for comparison.
Electrophysiology
In order to assess the functionality of white matter tracts in the CC, the compound action potential (CAP) was assessed. Mice were anesthetized with 3% isoflurane, and their brains were rapidly removed. Transverse slices 350 µm thick were cut on a vibrating microtome (VT1000S, Leica Biosystems, Buffalo Grove, IL, USA) and placed in artificial cerebrospinal fluid (aCSF), saturated with 95% O2/5% CO2. The slices were allowed to equilibrate for a half hour at 34℃ followed by 1 h at 26℃ prior to use. The aCSF contained 124 mM NaCl, 2.5 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 1 mM NaH2PO4, 24 mM NaHCO3, 1.3 mM MgSO4, and 10 mM D-glucose. Recordings were performed at 26℃. A concentric bipolar stimulating electrode (25 µm inner pole diameter, FHC, Bowdoin, Maine, USA) was lowered into the CC, at approximately 1.0 mm lateral to midline, and a recording electrode (aCSF-filled glass micropipette; resistance 6–8 MV) was also placed in the CC at a variable distance (0.75 mm and 1.0 mm) from the stimulating electrode. The initial depth of electrodes was 100 µm below the surface of the slice; however, fine adjustments were made in the depths of both stimulating and recording electrodes to optimize the signal amplitude. Evoked callosal CAP was amplified (bandpass = DC to 10 kHz), digitized at 25 kHz, and stored on disk for offline analysis.
ELISA
Blood plasma samples from mice with or without CR pretreatment were collected using heparin as an anticoagulant, and then centrifuged for 20 min at 2000 × g within 30 min of collection. Concentrations of leptin (MOB00, R&D Systems, Minneapolis, MN, USA), adiponectin (MRP300, R&D Systems, Minneapolis, MN, USA), resistin (MBS106432, MyBioSource, San Diego, CA, USA) and insulin (EZRMI-13 K, EMD Millipore, Burlington, MA, USA) were measured with commercial ELISA quantification kits, according to the manufacturer’s instructions.
Statistical analyses
All data are presented as mean ± SD. The statistical differences among means of multiple groups were assessed by one- or two-way ANOVA followed by the Bonferroni post hoc test. The difference in means between two groups was assessed by two-tailed Student t test. Pearson product linear regression analysis was used to correlate the callosal CAP with performance in the spatial memory test (probe test, number of times crossing the location where the removed platform had been formerly placed). A p value of less than 0.05 was deemed statistically significant.
Results
CR exerts ischemic protection and improves functional recovery over an extended time frame
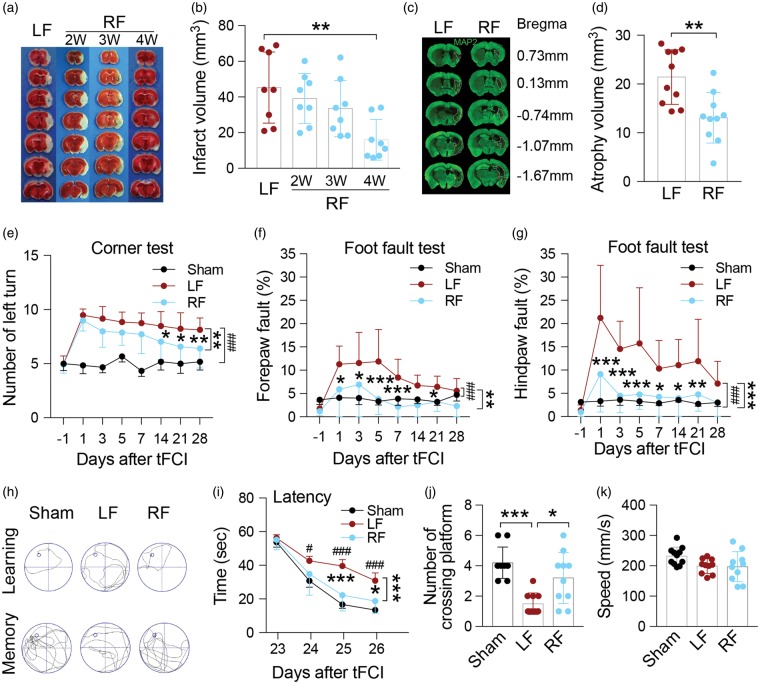
Physiological parameters including blood pH, pO2 and pCO2 were monitored during surgery. There is no significant difference between LF- and RF-fed mice (data not shown). CBF measured by laser Doppler flowmetry was observed over the course of the ischemic episode among groups. No significant difference was detected between two groups (Suppl. Figure 1(d)). After tFCI, brain infarct was observed in mice maintained on the LF diet (Figure 1(a) and (b)). Infarct volume was significantly reduced in mice that underwent four weeks of RF prior to ischemic induction, whereas mice subjected to the RF diet for only two or three weeks displayed minimal differences compared to LF ischemic mice. On this basis, we proceeded with subsequent experiments using mice maintained on RF for four weeks prior to tFCI.
Figure 1.
Food restriction decreases infarct and atrophy volume, and alleviates neurological deficits induced by tFCI. (a) Representative brain images of TTC staining after tFCI of mice maintained on an ad libitum (LF) or restricted diet (RF). (b) Quantification of infarct volume indicating four weeks of RF was required for reduction of infarct volume after tFCI. n = 8 mice/group. **p ≤ 0.01 vs. LF. (c) Representative immunofluorescent images of brain slices stained for MAP2 28 days after tFCI. Dashed lines indicate area of brain atrophy. (d) Quantification of atrophy volume indicating that four weeks of RF-feeding reduces atrophy volume. n = 10 mice/group, **p ≤ 0.01 vs. LF. (e–g) Sensorimotor function and asymmetry as assessed by the corner test (e) and foot fault test (f, g) were improved after tFCI with RF-feeding for four weeks. n = 8 for sham, n = 12 mice/tFCI group. *p ≤ 0.05, **p ≤ 0.01 ***p ≤ 0.001 vs. LF, ###p ≤ 0.001 vs. sham. (h) Representative swim path from each treatment group during the spatial learning (top panel) and memory phase (bottom panel) of the Morris water maze test. (i) Latency in seconds (s) to find the submerged platform assessed during days 23–26 after tFCI shows that learning was improved in mice fed the RF diet. (j) Quantification on day 27 of the number of crossings over the region where the platform used to be indicates that memory was improved in RF-fed mice following tFCI. (k) Swimming speed during the probe test indicating no differences in gross motor function among groups. For i–k: n = 11 mice/sham group, n = 10 mice/ LF group, n = 10 mice/RF group. *p ≤ 0.05, ***p ≤ 0.001 vs. LF group, #p ≤ 0.05, ###p ≤ 0.001 vs. sham. All data are presented as mean ± SD.
Using a variety of acute and long-term functional recovery assays, we next assessed whether four weeks of RF prior to tFCI altered functional outcomes following stroke. No differences in behavioral test performance were observed between LF and RF groups subjected to sham operation (Suppl. Figure 2). Acute analyses of neurological function assayed over the first three days after tFCI indicated that RF consistently improved gross indicators of neurological injury (data not shown). Using more specific assessments of sensorimotor function, RF ischemic animals subjected to the corner test displayed a similar preference for left turns (indicative of disrupted sensorimotor functions) compared to LF ischemic animals until 14 days following ischemia, at which point RF ischemic animals performed significantly better than LF ischemic animals (Figure 1(e)). Paw placement on the grid-walking test was significantly improved in RF ischemic mice compared to LF ischemic mice (Figure 1(f) and (g)), particularly at earlier time points when LF ischemic mice showed the most deficits. These results indicate that RF-treated mice have improved sensorimotor function following ischemic injury.
In addition to acute sensorimotor dysfunction, the tFCI model induced long-term impairment in cognitive function in the Morris water maze test in ischemic mice compared to sham animals up to four weeks following injury (Figure 1(h) to (k)). Four weeks of RF prior to tFCI led to significantly improved performance compared to LF ischemic mice. RF-fed ischemic mice located a hidden platform significantly faster over the four days of learning trials as denoted by a decrease latency to find the platform (Figure 1(h) top panel, and (i)). In addition, RF-fed ischemic mice spent more time crossing over the correct location once the platform was removed on the final day of testing indicative of an improvement in memory function (Figure 1(h) bottom panel, and (j)). No significant differences in swim speed were found among groups (Figure 1(k)), thus deficits in gross motor function cannot account for the observed difference in performance in the Morris water maze test between LF and RF mice. In addition, atrophy volume, measured by MAP2 immunoreactivity 28 days after tFCI, was reduced in RF ischemic mice compared to LF ischemic mice (Figure 1(c) and (d)). Together, these data indicate that four weeks of RF significantly improved acute and long-term histological and functional outcome after ischemic injury compared to the LF diet.
Ischemia-induced white matter injury is reduced in RF mice
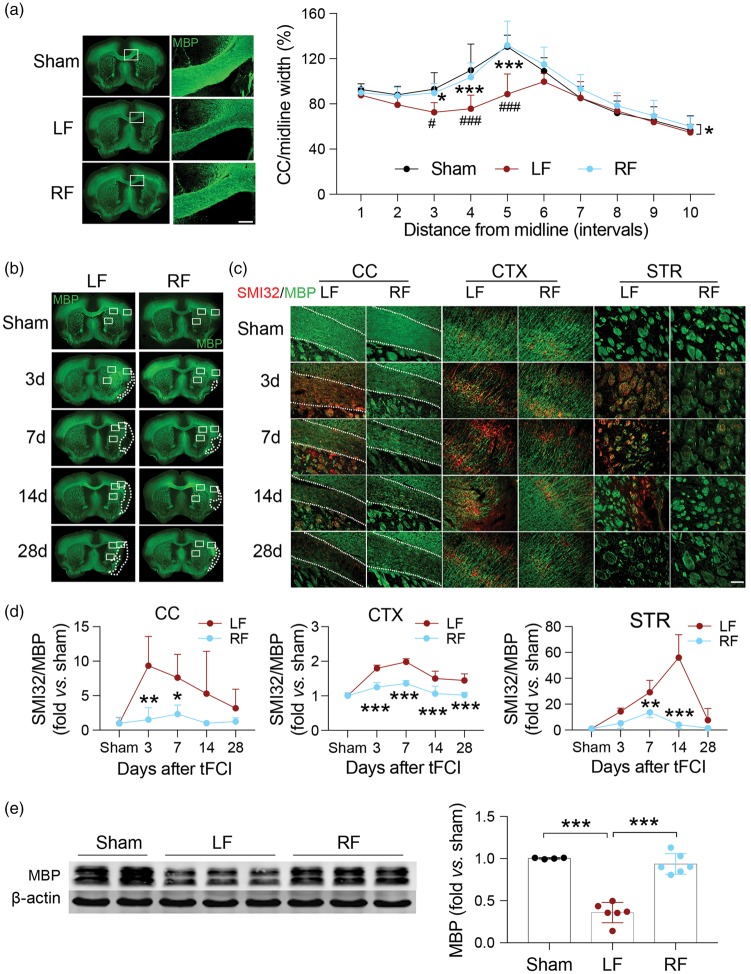
Much of the literature concerning CR in the brain has focused on either functional outcomes or specific populations of neurons.23 However, sensorimotor and learning/memory impairments following ischemic injury have been tightly correlated to extensive damage to white matter tracts throughout the ischemic territory and penumbral regions. Therefore, to examine whether CR could attenuate ischemia-induced white matter damage, we examined immunostaining for MBP, which is distinctly present in mature white matter tracts, and compared it to SMI32 immunostaining, a marker for nonmyelinated neurofilaments. We have previously observed that SMI32 is visible in white matter regions after ischemic brain injury.24 The ratio of the SMI32/MBP in the ipsilateral hemisphere was first compared with that of the contralateral side, and further normalized by the same ratio of sham. In the current study, tFCI led to extensive damage to white matter, as determined by a reduction in MBP immunofluorescent staining and an increase in SMI32 immunostaining in the CC, cortex and striatal fiber bundles (Figure 2(b) to (d)). RF treatment prior to ischemic injury significantly preserved MBP staining in the ischemic brain, and attenuated SMI32 immunoreactivity in the CC and striatum (STR). The differences between groups were more pronounced in the first 14 days following reperfusion. At early time points following ischemia, very little histological damage to white matter tracts was detectable in RF-fed mice, suggesting that RF may function to predominantly protect white matter rather than to spur recovery. In addition, the thickness of the CC between 235.3 µm and 470.6 µm from midline was significantly decreased in LF-fed ischemic mice, whereas no such decrease was observed in RF-fed ischemic mice and sham-treated mice up to 28 days after tFCI (Figure 2(a)). Western blot analysis of tissue MBP levels indicated a substantial loss of MBP in the brains of ischemic LF mice. Maintenance on the RF diet prior to tFCI significantly improved total MBP levels present in the brain 28 days after tFCI (Figure 2(e)). These data suggest that the RF diet preserved the histological structure of white matter tracts in ischemic mice.
Figure 2.
White matter integrity is better preserved in ischemic mice with food restriction. (a) Representative images of MBP immunofluorescent staining of sham (top row) and tFCI mice given LF or RF (middle and bottom rows, respectively) 28 days after ischemic insult. The ischemia-induced reduction in CC thickness is prevented by RF. The white boxes indicate areas that were enlarged in high-power images (right column, scale bar = 200 µm). Quantification of the width of the CC indicates that RF maintained the width of CC 28 days after tFCI. n = 4–7 for each group. *p ≤ 0.05, ***p ≤ 0.001 vs. LF; #p ≤ 0.05, ###p ≤ 0.001 vs. sham. (b) Representative immunofluorescent images of sections from each treatment group stained with MBP. Dashed line depicts brain infarct. (c) Representative high-power images of regions depicted in white rectangles in (b) from each treatment stained with MBP (green) and SMI32 (red). Dashed line depicts the border of the CC. Scale bar = 100 µm. (d) Quantification of the ratio of SMI32/MBP fluorescence intensity in corpus callosum (CC), cortex (CTX) and striatum (STR) of the ipsilateral hemisphere after tFCI indicates that RF-feeding preserves white matter integrity in ischemic mice. n = 5 mice/group at each time point. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. LF. (e) Representative image of Western blot for MBP in corpus callosum of ipsilateral hemisphere (left panel). β-Actin was used as loading control. Semi-quantification of bands relative to sham mice (right panel) shows that RF prevented ischemia-induced loss of MBP. n = 4–6/group. ***p ≤ 0.001. All data are presented as mean ± SD.
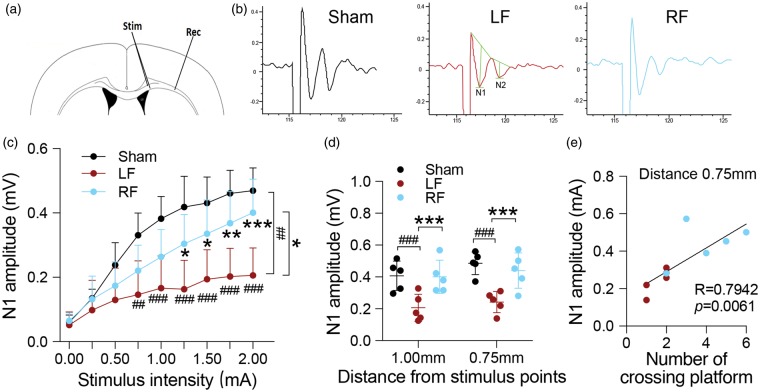
As a measure of functional integrity of white matter, ex vivo slices were used to determine the evoked callosal CAP along white matter tracts. Measurements of CAP amplitude may reflect changes in the functional properties of axonal tracts, as well as in the total number of axons recruited into the CAP field potential.25 The stimulating point (Stim) and recording point (Rec) for the CAP are shown in Figure 3(a). Typically, evoked CAPs yield a biphasic waveform comprising an initial segment (N1, representing fast-conducting myelinated axons) followed by a second segment (N2, representing the slower-conducting unmyelinated axons) in adult mice (Figure 3(b)). With this technique, changes in nerve function along white matter tracts can be ascertained. To determine the conductivity of myelinated axons, amplitude of N1 was compared between LF-and RF-fed mice. tFCI resulted in the significant decrease of N1 amplitude recorded at a distance of 1.0 mm away from stimulating point (Figure 3(c)). The RF ischemic brain displayed more robust sensitivity to the increase in stimulus intensity along myelinated tracts (recorded at 1.0 mm lateral to stimulating point) than the LF ischemic brain (Figure 3(c)). This increased sensitivity reflects recruitment of more intact axonal fibers along the tested tracts, with significantly greater amplitude at the N1 segments compared to ischemic LF brain. Myelinated axons displayed significantly improved nerve conduction in the RF ischemic groups compared to LF ischemic groups when recording and stimulating points were positioned 1.0 mm and 0.75 mm apart (Figure 3(d)), respectively. Pearson correlation analysis was performed to further confirm whether there was any correlation between white matter integrity and cognitive function. The amplitude of N1 was highly correlated with the performance in the spatial memory test (R = 0.7942, p = 0.0061, Figure 3(e)). Taken together, RF prior to ischemia leads to improved white matter functional and structural integrity after tFCI when compared to LF ischemic brain.
Figure 3.
RF preserves functional integrity of white matter tracts after tFCI. (a) Illustration of the position of stimulating and recording electrodes in the CC. (b) Representative traces of evoked CAP in CC (stimulus 2.0 mA, 1.0 mm lateral to the stimulating electrode) at 28 days after tFCI. (c) Recording of the N1 amplitudes as a function of stimulus intensity indicates that RF partially restored sensitivity to the stimulus intensity in the myelinated (N1) tracts. (d) Recording of the N1 amplitudes in response to a 2.0-mA stimulus at different distances (1.0 mm and 0.75 mm) lateral to the stimulating electrode in sham and ischemic LF- and RF-fed mice at 28 days post-tFCI shows that RF restored N1 sensitivity as the distance between the recording and stimulating electrode was increased. No significant difference was detected between LF and RF sham group (data not shown). For c, d *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. LF mice; ##p ≤ 0.01, ###p ≤ 0.001 vs. sham. (e) The amplitude of N1 recorded at 0.75 mm lateral to the stimulating electrode in response to 2.0-mA stimulus was correlated with spatial memory test (number of crossing over the area that formerly contained the platform) at 27 days after tFCI using Pearson linear regression analysis. Data are expressed as mean ± SD. n = 5 mice/group.
Sirt1 and Sirt3 are not required for ischemic neuroprotection induced by restricted feeding
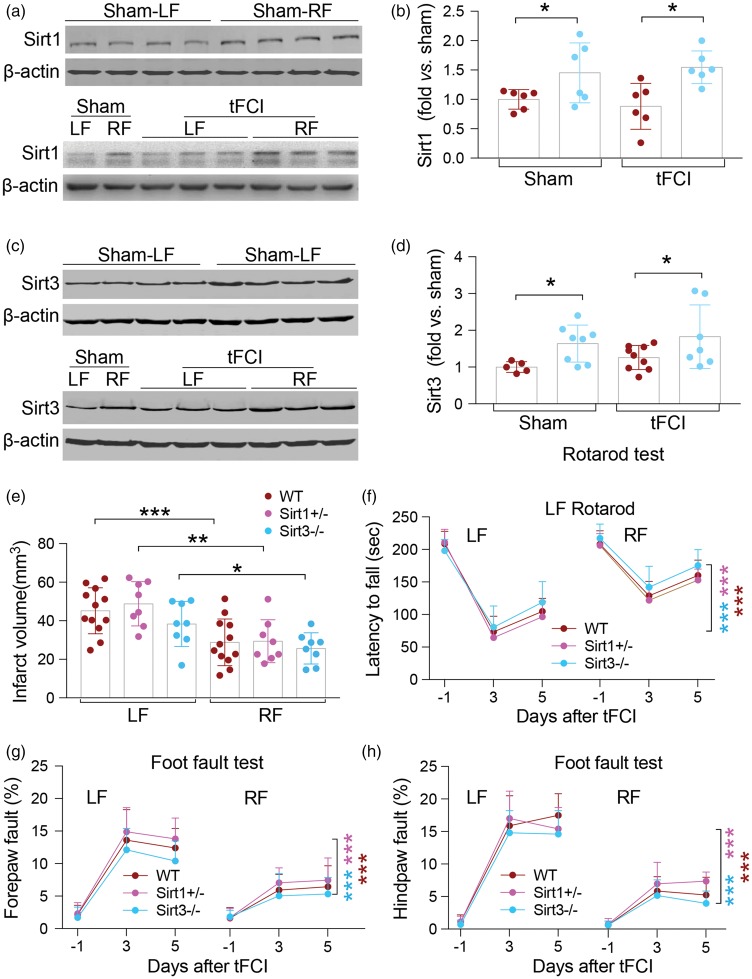
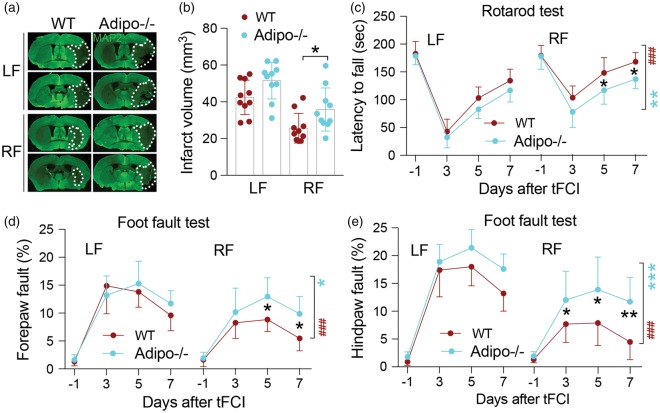
Sirtuins, in particular Sirt1 and Sirt3, have often been associated with ischemic protection26,27 and are thought to mediate the effects of CR in other injury models.28,29 We noted a slight but significant increase in both Sirt1 and Sirt3 protein expression in CC lysates of RF-fed sham-operated mice and RF-fed ischemic mice compared to mice in the LF-fed groups (Figure 4(a) to (d)). Using genetically modified animals, we sought to determine if the presence of Sirt1 or Sirt3 is necessary for RF-induced ischemic protection. To address this question, we used Sirt1+/− mice in order to avoid the severe phenotypic issues with the homozygous knockout (viability), and Sirt3−/− mice, which survive and do not appear to have gross phenotypic changes. Reduction of Sirt1 or Sirt3 did not alter protection induced by RF in terms of infarct volume (Figure 4(e)) or sensorimotor function assessed by rotarod and the foot fault test within five days after tFCI (Figure 4(f) to (h)). These data indicate that neither Sirt1 nor Sirt3 is critical for the protective effects of RF against ischemic injury.
Figure 4.
Sirt1 and Sirt3 are upregulated in RF ischemic mouse brain but are not critical for RF-induced ischemic protection. (a, c) Representative Western blot images of Sirt1 (a) and Sirt3 (c) in sham and ischemic mice fed the LF or RF diet. β-Actin was used as loading control. (b, d) Semi-quantification of bands shows significant upregulation of Sirt1 (b; n = 6 mice per group) and Sirt3 (d; n = 5–9 mice per group) in sham-operated RF-fed and ischemic RF-fed mice. *p ≤ 0.05 between indicated groups. (e–g) Reduction of either Sirt1 (Sirt1+/−) or knockout of Sirt3 (Sirt3−/−) did not alter protection afforded by RF prior to tFCI as assessed by infarct volume (e), performance on the rotarod (f) or the foot fault test (g, h). No significant differences were detected on latency to fall (rotarod test) or forepaw and hindpaw fault rate (foot fault test) between WT and Sirt1+/−or between WT and Sirt3−/− mice within five days after tFCI in LF and RF mice. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. indicated LF group. Brackets indicate comparisons within genotypes between LF and RF treatment (dark red, WT; light red, Sirt1+/−; blue, Sirt3−/−). ***p ≤ 0.001 RF vs. LF in each genotype. Data are expressed as mean ± SD. n = 12 for WT group, and n = 8 for Sirt1+/− and Sirt3−/− mice, respectively.
Restricted feeding induces systemic release of the hormone adiponectin that contributes to protection against tFCI
CR can affect a variety of metabolic factors, including altering glucose metabolism and serum lipid profiles,30 which are likely to impact stroke outcomes.31,32 We screened serum from animals fed LF or RF regimens for levels of relevant factors known to affect injury states. We found no change in serum triglycerides after four weeks of RF, but blood cholesterol levels were significantly decreased in RF mice (Suppl. Figure 1(a) and (b)), consistent with observations from clinical studies.33 In addition, the RF regimen led to a more rapid clearance of glucose in the presence of a glycemic challenge (GTT) (Suppl. Figure 1(c)). Compared with LF mice, blood glucose was significantly decreased in RF mice half an hour after tFCI (Suppl. Figure 1(e)); at later time points (2 and 3 h after tFCI), the level of blood glucose did not differ between groups. Additionally, after tFCI, serum insulin levels were not statistically different between LF and RF groups (Suppl. Figure 1(f)). As expected, the RF groups lost weight compared to LF groups (Suppl. Figure 1(g)). tFCI is well-known to induce spontaneous weight loss in mice within the first week after reperfusion. Indeed, LF mice lost close to 20% of their pre-surgical weight (Suppl. Figure 1(h)). Conversely, RF mice did not appear to lose weight after tFCI, suggesting that the maintenance of body weight following tFCI is associated with better outcome.
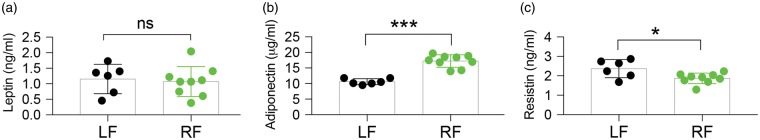
CR causes the release of several hormones from adipose tissue that are involved in satiety, insulin sensitivity, and glucose metabolism. These hormones, which include leptin, adiponectin, and resistin, can also exert varied effects on cellular metabolism and inflammatory states, and increased adiponectin serum levels have been associated with improved functional outcomes, including after ischemia.4,34,35 Following four weeks of RF, wild-type mice did not display altered blood levels of leptin, as assayed by ELISA (Figure 5(a)). However, serum levels of adiponectin were significantly increased (Figure 5(b)), and resistin serum levels were significantly decreased (Figure 5(c)), albeit to a lesser extent.
Figure 5.
RF alters the levels of adiponectin and resistin in blood circulation. (a–c) Assessment of plasma protein levels of leptin (a), adiponectin (b), and resistin (c) by ELISA after four-week treatment with LF or RF indicates that RF-feeding altered circulating levels of adiponectin and resistin. Data are mean ± SD. n = 6 for LF and n = 9 for RF group. *p ≤ 0.05, ***p ≤ 0.001, vs. LF; ns = no significant difference.
To determine if adiponectin contributes to the cerebral ischemic protection induced by RF, we maintained adiponectin knockout mice (Adipo−/−) or wild-type mice for four weeks on the RF regimen, and then induced tFCI. Compared with LF-fed wild-type mice, knockout of adiponectin showed comparable infarct volume in LF-fed mice seven days after tFCI, suggesting its critical role in neuroprotection against ischemic brain injury. However, knockout of adiponectin led to significantly increased infarct volume in RF-fed mice at seven days after tFCI compared to wild-type mice fed the RF diet (Figure 6(a) and b)). In LF-fed ischemic mice, knockout of adiponectin did not alter sensorimotor performance; however, the beneficial effects of the RF diet prior to tFCI were significantly attenuated in the Adipo−/− mice on the rotarod and grid walking (foot fault) tests (Figure 6(c) to (e)). The differences between LF and RF in WT and adiponectin knockout mice were further compared. RF significantly improved sensorimotor outcomes when compared to LF within genotypes (Figure 6(c) to (e), brackets)). However, knockout of adiponectin partially blocked the neuroprotective effect afforded by RF pretreatment when compared to WT RF groups, as observed by sensorimotor performances in the foot fault and rotarod tests within seven days after tFCI (Figure 6(c) to (e)). These data suggest that adiponectin contributes at least in part to RF-induced neuroprotection against ischemic insult and promotion of functional recovery.
Figure 6.
Adiponectin contributes to RF-induced neuroprotection against tFCI. (a) Representative immunofluorescent images of MAP2 staining seven days after tFCI in wild-type (WT) and adiponectin knockout (Adipo−/−) mice. Dashed line depicts infarct region. (b) Measurement of infarct volume in WT and Adipo−/− mice indicates that knockout of adiponectin exacerbates ischemic injury in RF-fed mice. No significant difference was detected between WT and Adipo−/− in LF-fed mice. n = 10/group, *p ≤ 0.05 between groups as indicated. (c–e) Sensorimotor deficits induced by tFCI were significantly alleviated in both wild type and Adipo−/− mice pretreated with RF within seven days after ischemic insult. RF-induced improvements in sensorimotor function as assessed in the rotarod (c) and grid walking test (d, e) were partly abolished by knockout of adiponectin. *p ≤ 0.05, **p ≤ 0.01 between groups as indicated. Brackets indicate comparisons within genotypes between LF and RF treatment (red, WT; blue, Adipo−/−). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 Adipo−/− RF vs. Adipo−/− LF; ###p ≤ 0.001 WT RF vs. WT LF. Data are mean ± SD. n = 10 mice per group.
As mentioned above, sensorimotor function can be highly impacted by the integrity of white matter fiber bundles. Although the receptor for adiponectin, AdipoR1, has been identified in brain, localization to white matter cell types has not been reported. We found that AdipoR1 is expressed on GFAP and APC positive cells (astrocytes and oligodendrocytes, respectively) in both LF- and RF-fed mice (Figure 7(c) and Suppl. Figure 3(a)). AdipoR1 expression significantly increased on astrocytes in CC and STR seven days after cerebral ischemia in LF mice (Suppl. Figure 3(a) and (e)), but this increase was significantly attenuated in RF ischemic brain. However, no significant difference in AdipoR1 expression was detected between LF- and RF-fed in the cortex (Suppl. Fig 3(a) to (d)). To further examine the expression of AdipoR1 in white matter, the number of APC (a mature oligodendrocyte marker) and AdipoR1 double-labeled cells was calculated (Figure 7(f)). RF significantly elevated the number of APC+/AdipoR1+ cells in the STR of sham mice, compared to LF sham. The ischemic insult appeared to slightly decrease APC+/AdipoR1+ immunostaining in the CC and STR of LF groups, but significant downregulation was only detected in the cortex (Figure 7(c), Suppl. Figure 3(a) and (e)). RF preserved expression of AdipoR1 in mature oligodendrocytes in the peri-infarct area (Figure 7(c) and (f) and Suppl. Figure 3(a) and (c)). Indeed, RF ischemic brain consistently contained significantly more APC+/AdipoR1+ cells compared to LF ischemic brain in the CC, STR (Figure 7(c)) and cortex (Suppl. Figure 3(c)).
Figure 7.
Adiponectin contributes to the preservation of white matter after tFCI. (a) Representative images of SMI32 and MBP double staining in corpus callosum (CC) and striatum (STR) in wild-type and adiponectin knockout (Adipo−/−) mice, subjected to either LF or RF, then sacrificed following seven days after tFCI. Scale bar = 50 µm. (b) Knockdown of adiponectin abolishes the RF protection of myelination in CC and STR as assessed by quantification of SMI32/MBP ratio. **p < 0.01; n = 10 per group. (c) Representative images of double-labeling of adiponectin receptor 1 (AdipoR1, red) with mature oligodendrocyte (APC, green) markers in CC and STR of LF and RF mice at seven days after tFCI. White boxes indicate areas in high-power images in (e). Scale bar = 25 µm. (d) Representative image of MBP immunofluorescence (green) at seven days after tFCI. The white boxes depict the peri-infarct area in CC and striatum (STR), whereas high-power images were taken in (c) Scale bar = 1 mm. (e) Enlarged areas in CC and STR depicting co-labeling with APC (green), AdipoR1 (red), and DAPI (blue). Arrow: APC+/AdipoR1+ cells. Scale bar = 25 µm. (f) Quantification of APC+/AdipoR1+ cells indicates that RF-fed increases the number of APC+/AdipoR1+ cells in STR in sham-treated mice, and in the CC and STR of ischemic-mice. n = 5 mice per group. Data are represented as mean ± SD, *p ≤ 0.05, **p ≤ 0.01 between groups as indicated; ns means no significant difference.
In order to determine whether adiponectin is necessary for the preservation of white matter resulting from RF, we evaluated histological visualization of SMI32 for damaged axons and MBP for intact myelinated fibers seven days after reperfusion in Adipo−/− mice. tFCI induced expression of SMI32 in the CC and STR was decreased by RF-feeding in wild-type mice (Figure 7(b)). Adipo−/− mice no longer benefitted from RF-induced protection in the CC or STR following tFCI (Figure 7(a) and (b)), in contrast to wild-type mice. Together, these data indicate that adiponectin-mediated effects in white matter cells play a critical role in improving histological and functional recovery in ischemic RF-treated mice.
Discussion
CR protects various neuronal populations in numerous disease models1–4 and improves age-related deterioration of white matter integrity.17,18 Our study extends these findings with the novel discovery that CR protects white matter as well as neurons against ischemic injury at both the histological and functional levels. Furthermore, we present data to demonstrate that, although the upregulation in Sirt1 and Sirt3 protein expression is correlated with CR, neither of these two molecules appears to be necessary for the protective effects of CR against cerebral ischemic injury. Instead, the release of adiponectin, which is also associated with CR, contributes to the protection induced by a month-long restricted diet prior to cerebral ischemia.
To date, many studies have indicated that CR paradigms exert robust protection against a variety of diseases, including ischemic injury. Missing from the literature is whether CR preserves white matter as well as neurons against ischemic stress. White matter damage is a major component of cerebral ischemic injury,36 and sensorimotor and cognitive functional recovery is associated with attenuation of white matter damage. Therefore, preserving white matter integrity after stroke is likely to lead to better clinical outcomes in stroke patients. Our study examined the histological and functional integrity of white matter tracts under ad libitum and restricted food diets in mice subjected to transient ischemic injury. We found that extended RF (four weeks) prior to stroke significantly improved white matter tract integrity after tFCI, compared to LF ischemic animals. Using correlational analyses, the improved functional integrity of white matter tracts of the CC, which feed into the hippocampus, was tightly linked to improved memory function assessed by the Morris water maze task. Indeed, the Morris water maze task is most often associated in the literature with hippocampal function in memory consolidation, in part due to the involvement of spatial cues and orientation, which necessitate hippocampal place cells for intact spatial maps. Memory, however, is a more complex phenomenon that requires not just hippocampal function, but also higher order cognitive processing. The CC provides interhemispheric functional connectivity that is becoming more appreciated to be associated with higher order processes, including memory. Studies in humans have noted CC disturbances correlated to cognitive or memory impairment in agenesis,37,38 AD,39 and multiple sclerosis.40 Animal studies have also found correlation between white matter changes and spatial memory impairment.41 Because memory storage/retrieval is not a simple process, involvement of interhemispherical connectivity is likely. Thus, we have started to include correlational analyses between histological and functional perturbations of white matter and behavioral outcomes. These novel findings extend the beneficial effects of CR to white matter and link this protection to improved behavioral function. In addition, we identified the presence of AdipoR1 on white matter cells, including astrocytes and oligodendrocytes. This novel finding, along with the protection of white matter tracks linked to RF treatment, suggests that, in addition to neurons being a target of adiponectin, RF-released adiponectin may also affect white matter.
Much of the focus concerning potential mechanisms underlying CR has been on glucose sensitivity, mitochondria, or more recently, the revascularization process.4,42,43 Sirtuins have previously been demonstrated to be upregulated following CR and have been implicated in ischemic protection in several non-brain injury models (e.g. kidney hypoxia44 and myocardial ischemia45). Sirt1, a nuclear sirtuin has been extensively investigated in neurons and neural stem and progenitor cells, regulating neuronal survival,46 synaptic plasticity,47 and differentiation.48 Sirt3 is localized in mitochondria, preserving energy metabolism and mitochondrial integrity under oxidative stress.49 We compared the expression of Sirt1 and Sirt3 after CR from the infarct region of brain from LF and RF mice. Consistent with our present study, others have demonstrated that CR stimulated the expression and activity of Sirt1 and Sirt3 in brain.50 In a recent study of tFCI in rats, Sirt1 knockdown by siRNA was reported to reverse the protective effects of CR after ischemia.51,52 Although we found that Sirt1 and Sirt3 were significantly upregulated in RF animals even after tFCI, surprisingly, when these genes were decreased by genetic deletion, we did not observe any noticeable effect on protection induced by RF. Differences between our study and the previous report could relate to the methods used to delete Sirt1; the previous study used intraventricular injection of siRNA, which has limited diffusion in the brain parenchyma, and assessed animals at an early time point (24 h). We used genetic heterozygotes (Sirt1) or full knockdown (Sirt3) mice to more robustly decrease Sirt1 or Sirt3 expression and extended the endpoint to seven days. In our study, the upregulation and deletions were not specific for white matter; since global deletions may lead to different effects in different cell types, global targeting may counteract effects that may have been observed in different models. Thus, although Sirt1 and Sirt3 expression are associated with RF, on the basis of the present study, neither Sirt1 nor Sirt3 appear to be required for RF-induced cerebral ischemic protection.
In assessing the metabolic capacity of ischemic and sham animals, we found that RF leads to a more rapid clearance of glucose in the glucose challenge test (Suppl. Figure 2). As we delved more into potential contributors to the observed protective effects of RF, an increase in circulating levels of adiponectin may explain, at least in part, the observed glucose sensitivity in non-ischemic RF mice. How adiponectin contributes to protection against ischemia in brain is unknown. One potential mechanism may involve the slight reduction in serum glucose levels observed 30 min after tFCI. Clinical observations have pointed to an association between post-ischemic hyperglycemia and worsened clinical outcome53; however, clinical trials that attempted to correct hyperglycemia in stroke patients report only moderate effects on clinical outcome.32 An alternative mechanism may be a local effect on adiponectin in the brain. Adiponectin exerts its effect by binding to its receptors (adiponectin receptor 1 and 2) in targeted cells.54 The presence of adiponectin receptors has previously been reported in the brain,55 and AdipoR1 is more pronounced than AdipoR2 in mouse cortical neurons.56 We now extend those findings to include localization of AdipoR1 to white matter cells.55 Although we did not assess brain content or direct function of adiponectin on glial cells following RF or stroke, these receptors could trigger a variety of signaling pathways.55 Lastly, in addition to its role as a factor impacting glucose sensitivity, adiponectin may modulate the role of circulating immune cells. Immune cell infiltration into the stroke brain is a relatively new concept. However, recent literature suggests strongly that infiltrating immune cells drastically impact the outcomes of stroke.57 Further studies in ischemic Adipo−/− mice (with or without RF) examining the role of immune cell infiltration and the potential consequences are merited.
In addition to increased release of adiponectin, we also found a slight but significant decrease in resistin serum levels. Resistin can interfere with insulin sensitivity58; thus, the reduction of resistin could theoretically contribute to the protection induced by RF in collaboration with adiponectin. The role of resistin in recovery or protection from cerebral ischemia may provide interesting insights into the interplay between adipocyte-secreted factors.
Although CR has been supported in the literature over the last 30 years to increase longevity and confer protection to a variety of systems, its functions and mechanisms in the stroke brain are still under investigation. Our findings further extend the benefits of CR to white matter. In addition, we have linked adiponectin as a significant contributor to CR induced protection against ischemic protection.
Supplemental Material
Supplemental material for Preconditioning with partial caloric restriction confers long-term protection against grey and white matter injury after transient focal ischemia by Jia Zhang, Wenting Zhang, Xuguang Gao, Yongfang Zhao, Di Chen, Na Xu, Hongjian Pu, R Anne Stetler and Yanqin Gao in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Chinese Key R&D Plan of the State Ministry of Science and Technology 2017YFC1308403 (to Y.G), and Chinese Natural Science Foundation grants 81571285 (to Y.G), and 81529002, 81471332, and 81771419 (to W.Z). The authors are indebted to Dr. Amanda Smith for editing the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
JZ, WZ, XG, YZ and DC performed the experiments. NX and HP analyzed the data. RAS and WZ wrote the manuscript. YQ and RAS designed the study and edited the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell 2015; 161: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stranahan AM, Mattson MP. Metabolic reserve as a determinant of cognitive aging. J Alzhimers Dis 2012; 30(Suppl 2): S5–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lucia C, Gambino G, Petraglia L, et al. Long-term caloric restriction improves cardiac function, remodeling, adrenergic responsiveness, and sympathetic innervation in a model of postischemic heart failure. Circ Heart Fail 2018; 11: e004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondo M, Shibata R, Miura R, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem 2009; 284: 1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanza IR, Zabielski P, Klaus KA, et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab 2012; 16: 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin AL, Coman D, Jiang L, et al. Caloric restriction impedes age-related decline of mitochondrial function and neuronal activity. J Cereb Blood Flow Metab 2014; 34: 1440–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schleit J, Johnson SC, Bennett CF, et al. Molecular mechanisms underlying genotype-dependent responses to dietary restriction. Aging Cell 2013; 12: 1050–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon HF, Shepherd HM, Reed TT, et al. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging 2006; 27: 1020–1034. [DOI] [PubMed] [Google Scholar]
- 9.Radak Z, Koltai E, Taylor AW, et al. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic Biol Med 2013; 58: 87–97. [DOI] [PubMed] [Google Scholar]
- 10.Zhu M, Miura J, Lu LX, et al. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol 2004; 39: 1049–1059. [DOI] [PubMed] [Google Scholar]
- 11.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem 2005; 16: 129–137. [DOI] [PubMed] [Google Scholar]
- 12.Bruce-Keller AJ, Umberger G, McFall R, et al. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol 1999; 45: 8–15. [PubMed] [Google Scholar]
- 13.Stetler RA, Leak RK, Gan Y, et al. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol 2014; 114: 58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haigis MC, Guarente LP. Mammalian sirtuins – emerging roles in physiology, aging, and calorie restriction. Genes Dev 2006; 20: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 15.Guarente L. Mitochondria – a nexus for aging, calorie restriction, and sirtuins? Cell 2008; 132: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinmura K, Tamaki K, Saito K, et al. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 2007; 116: 2809–2817. [DOI] [PubMed] [Google Scholar]
- 17.Bendlin BB, Canu E, Willette A, et al. Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol Aging 2011; 32: 2319.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, Bakshi V, Lin AL. Early shifts of brain metabolism by caloric restriction preserve white matter integrity and long-term memory in aging mice. Front Aging Neurosci 2015; 7: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilkenny C, Altman DG. Improving bioscience research reporting: ARRIVE-ing at a solution. Lab Anim 2010; 44: 377–378. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Gan Y, Sun BL, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol 2013; 74: 458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 2015; 272: 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Shi Y, Zhang L, et al. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis 2014; 68: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira JN, Mari RB, Stabille SR, et al. Benefits of caloric restriction in the myenteric neuronal plasticity in aging rats. An Acad Bras Cienc 2014; 86: 1471–1481. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Wang H, Zhang H, et al. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Exp Neurol 2015; 272: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G, Shi Y, Jiang X, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A 2015; 112: 2853–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koronowski KB, Khoury N, Saul I, et al. Neuronal SIRT1 (Silent information regulator 2 homologue 1) regulates glycolysis and mediates resveratrol-induced ischemic tolerance. Stroke 2017; 48: 3117–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury N, Koronowski KB, Young JI, et al. The NAD(+)-dependent family of sirtuins in cerebral ischemia and preconditioning. Antioxid Redox Signal 2018; 28: 691–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouchiroud L, Houtkooper RH, Auwerx J. NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol 2013; 48: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guarente L. Calorie restriction and sirtuins revisited. Genes Dev 2013; 27: 2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron KM, Miwa S, Walker C, et al. Male mice retain a metabolic memory of improved glucose tolerance induced during adult onset, short-term dietary restriction. Longev Healthspan 2012; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile NT, Seftchick MW, Huynh T, et al. Decreased mortality by normalizing blood glucose after acute ischemic stroke. Acad Emerg Med 2006; 13: 174–180. [DOI] [PubMed] [Google Scholar]
- 32.Kruyt ND, Biessels GJ, Devries JH, et al. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 2010; 6: 145–155. [DOI] [PubMed] [Google Scholar]
- 33.Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis 2006; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation 2008; 117: 216–223. [DOI] [PubMed] [Google Scholar]
- 35.Efstathiou SP, Tsioulos DI, Tsiakou AG, et al. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke 2005; 36: 1915–1919. [DOI] [PubMed] [Google Scholar]
- 36.Shindo A, Liang AC, Maki T, et al. Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab 2016; 36: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinkley LB, Marco EJ, Findlay AM, et al. The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One 2012; 7: e39804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul LK, Erickson RL, Hartman JA, et al. Learning and memory in individuals with agenesis of the corpus callosum. Neuropsychologia 2016; 86: 183–192. [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Liu S, Hilal S, et al. Inter-hemispheric functional dysconnectivity mediates the association of corpus callosum degeneration with memory impairment in AD and amnestic MCI. Sci Rep 2016; 6: 32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lafosse JM, Mitchell SM, Corboy JR, et al. The nature of verbal memory impairment in multiple sclerosis: a list-learning and meta-analytic study. J Int Neuropshchol Soc 2013; 19: 995–1008. [DOI] [PubMed] [Google Scholar]
- 41.Chahboune H, Ment LR, Stewart WB, et al. Hypoxic injury during neonatal development in murine brain: correlation between in vivo DTI findings and behavioral assessment. Cereb Cortex 2009; 19: 2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 2005; 6: 298–305. [DOI] [PubMed] [Google Scholar]
- 43.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev 2009; 59: 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kume S, Uzu T, Horiike K, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 2010; 120: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto T, Tamaki K, Shirakawa K, et al. Cardiac Sirt1 mediates the cardioprotective effect of caloric restriction by suppressing local complement system activation after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 2016; 310: H1003–H1014. [DOI] [PubMed] [Google Scholar]
- 46.Castro V, Skowronska M, Lombardi J, et al. Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J Cereb Blood Flow Metab 2018; 38: 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H, Kim S, Choi JE, et al. Decreased neuron number and synaptic plasticity in SIRT3-knockout mice with poor remote memory. Neurochem Res Epub ahead of print 26 October 2017. DOI: 10.1007/s11064-017-2417-3. [DOI] [PubMed] [Google Scholar]
- 48.Jablonska B, Gierdalski M, Chew LJ, et al. Sirt1 regulates glial progenitor proliferation and regeneration in white matter after neonatal brain injury. Nat Commun 2016; 7: 13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Pinzon MA, Stetler RA, Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab 2012; 32: 1362–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris KC, Lin HW, Thompson JW, et al. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab 2011; 31: 1003–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol 2008; 295: H2348–H2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ran M, Li Z, Yang L, et al. Calorie restriction attenuates cerebral ischemic injury via increasing SIRT1 synthesis in the rat. Brain Res 2015; 1610: 61–68. [DOI] [PubMed] [Google Scholar]
- 53.Bevers MB, Vaishnav NH, Pham L, et al. Hyperglycemia is associated with more severe cytotoxic injury after stroke. J Cereb Blood Flow Metab 2017; 37: 2577–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banks WA, Kovac A, Morofuji Y. Neurovascular unit crosstalk: pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J Cereb Blood Flow Metab 2018; 38: 1104–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thundyil J, Pavlovski D, Sobey CG, et al. Adiponectin receptor signalling in the brain. Br J Pharmacol 2012; 165: 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thundyil J, Tang SC, Okun E, et al. Evidence that adiponectin receptor 1 activation exacerbates ischemic neuronal death. Exp Transl Stroke Med 2010; 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.An C, Shi Y, Li P, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol 2014; 115: 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature 2001; 409: 307–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Preconditioning with partial caloric restriction confers long-term protection against grey and white matter injury after transient focal ischemia by Jia Zhang, Wenting Zhang, Xuguang Gao, Yongfang Zhao, Di Chen, Na Xu, Hongjian Pu, R Anne Stetler and Yanqin Gao in Journal of Cerebral Blood Flow & Metabolism