Abstract
Hypoxic-ischemic encephalopathy (HIE) is a severe neonatal complication responsible for ∼23% of all neonatal deaths. Also, 30–70% of these patients will suffer lifetime disabilities, including learning impairment, epilepsy or cerebral palsy. However, biomarkers for HIE screening, or monitoring disease progression are limited. Herein, we sought to evaluate the clinical usefulness of plasma-type gelsolin (pGSN) and amyloid-beta (Aβ) 40 and 42 as prognostic biomarkers for HIE. pGSN has been previously suggested as a feasible marker in other brain injuries and amyloid-beta 40 and 42 are classically assessed in neurodegenerative diseases. However, to our knowledge, they have not been previously assessed in HIE patients. We have analyzed plasma pGSN and Aβ 40 and 42 levels in 55 newborns (16 controls, 16 mild and 23 moderate-severe HIE) at birth, during 72 h of therapeutic hypothermia, a gold-standard treatment for HIE, and 24 h after hypothermia. Aβ levels were lower in HIE patients, and pGSN levels were progressively reduced in mild and moderate-severe HIE patients. The fact that pGSN reductions could predict the severity of HIE and significantly correlated with the time to undergo hypothermia supports the prognostic value of plasmatic pGSN. Further studies are warranted to investigate the role of pGSN in neonatal HIE.
Keywords: Hypoxic-ischemic encephalopathy, neonate, hypothermia, amyloid-beta, plasma-type-gelsolin
Introduction
Neonatal intensive care has progressed from improving mortality rates to also minimising morbidity rates. In term neonates, the most common perinatal brain injury occurs when oxygen or blood supplies are compromised, resulting in hypoxic-ischemic encephalopathy (HIE).1 HIE is responsible for ∼23% of all neonatal deaths and 30–70% of these patients will suffer lifetime disabilities,2 including learning impairment, epilepsy or cerebral palsy.3 While many efforts are being developed to set new therapeutic modalities for HIE and to increase the knowledge of the pathogenesis of asphyxia-related disorders, it is still difficult to predict which newborn will develop neurological problems.4 Moreover, biomarkers for HIE screening, monitoring disease progression, identifying injured brain regions, and assessing the efficacy of neuroprotective drugs are limited.1 Herein, we sought to evaluate the clinical usefulness of plasma-type gelsolin (pGSN) and amyloid-beta (Aβ) 40 and 42 as prognostic biomarkers for HIE. pGSN is a calcium-dependent actin regulatory protein that has been proposed as a feasible biomarker for subarachnoid haemorrhage-related complications in adults.5 Also, enhanced gelsolin expression might be implicated in neuroprotection after ischemic brain injury.6 Likewise, early reduction sin pGSN levels have been reported in preterm infants, in association with respiratory distress syndrome. Later pSGN reductions are also associated with bronchopulmonary dysplasia or retinopathy of prematurity, suggesting that low pGSN might predict outcomes in preterm infants.7 Taking into account these considerations, it remains possible that pGSN might also be a marker for HIE in neonates. However, to our knowledge, pGSN has not been previously assessed in these patients. Aβ40, Aβ42 and their precursor, Aβ precursor protein (APP), have been widely implicated in neurodegenerative disorders, and specifically in Alzheimer's disease. It has also been reported that gelsolin binds Aβ40 and 42, and may act as a peripheral sink for Aβ, thereby altering the periphery/brain dynamics and by sequestering plasmatic amyloid could reduce or prevent brain amyloidosis.8 Interestingly, the same cognitive skills that are often reduced for relatively well-functioning kids after perinatal asphyxia are similar to the limitations observed in the earliest phases of Alzheimer's disease, including visuospatial or attention deficits.9 However, the physiological function of APP and Aβ in the central nervous system is not well known, while high soluble and insoluble Aβ levels are detected in the brains from Alzheimer's disease patients10 APP-knockout mice show severe behavioural deficits, supporting its relevant physiological role in the central nervous system. Despite these circumstantial observations, to our knowledge, no previous studies have analyzed Aβ levels in HIE neonates.
We postulated that pGSN, Aβ40 and Aβ42 could be feasible prognostic biomarkers for HIE. To test this hypothesis, we determined pGSN, Aβ40 and Aβ42 levels in control and HIE neonates at birth, and at 6, 24, 72 h of therapeutic hypothermia, as well as 24 h after hypothermia, and correlated these levels with HIE severity and duration of hypothermia.
Material and methods
Subjects
Fifty-five neonates from the Neonatal Intensive Care Unit (NICU) at Puerta del Mar University Hospital (May 2009–June 2011) were included in the study. A detailed neurological examination was performed at birth (within the first 6 h) and before discharge from the NICU. Subjects were classified according to the severity of their encephalopathy following the Sarnat and Sarnat score,11 which is based on clinical and EEG findings. Mild (stage 1) HIE lasted less than 24 h and included hyperalertness, uninhibited Moro and stretch reflexes, sympathetic effects, and a normal EEG (n = 16). Moderate (stage 2) HIE was characterized by obtundation, hypotonia, strong distal flexion, and multifocal seizures, with the EEG showing a periodic pattern. Severe (stage 3) HIE consisted of stupor, flaccid tone, suppressed brainstem and autonomic functions, and an isopotential EEG or infrequent periodic discharges on EEG. Moderate-severe cases were grouped as previously described12–14 (n = 23). Seven severe HIE patients died. Figure 1(a) summarizes demographic and clinical data. All HIE patients received whole-body cooling to a rectal temperature of 33.5℃ for a period of 72 h, starting before 6 h of life. Hypothermia protocol was performed as previously described.15 Exclusion criteria were intrauterine infection or trauma, central nervous system malformation, chromosomal abnormality and inborn metabolic error. Blood samples were extracted from an umbilical vein catheter before therapeutic hypothermia, after 6, 24 and 72 h of hypothermia, and 24 h after reheating (96 h) for all HIE patients (mild, moderate and severe). Blood samples from term neonates of adequate birth weight obtained at birth (n = 16) served as controls. Samples were centrifuged at 7500 r/min for 7 min and supernatants stored at −80℃.
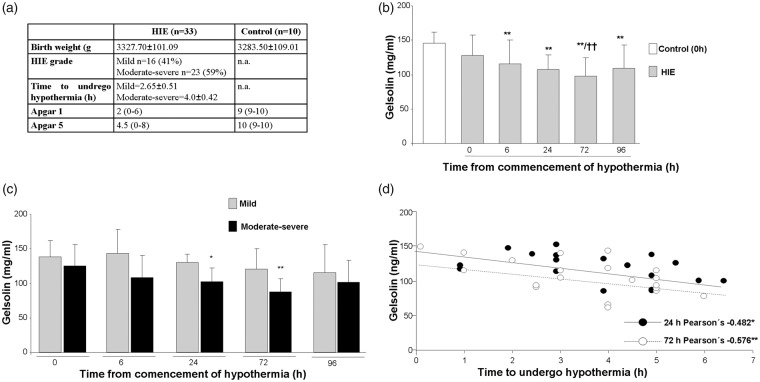
Figure 1.
Data are presented as mean + SD. (a) Patients demographics. (b) pGSN levels were significantly reduced in HIE patients, before the commencement of hypothermia and up to 96 h after the start of hypothermia. Differences were detected by one-way ANOVA followed by Tukey t test [F(5,105) = 7.01, **p < 0.01 vs. Control and HIE 0 h, ††p < 0.01 vs. HIE 0 h] (Control n = 16, HIE 0 h n = 25, HIE 6 h n = 22, HIE 24 h n = 19, HIE 72 h n = 22, HIE 96 h n = 7). (c) We observed an overall reduction of plasma pGSN levels in moderate-severe HIE cases, and this effect was slightly worsened in severe cases when compared with mild HIE patients by Student t test for independent samples, although differences only reached statistical significance at 24 and 72 h; t = 0, p = 0.312 (mild n = 8, moderate-severe n = 17); t = 6, p = 0.052 (mild n = 5, moderate-severe n = 17); t = 24, *p = 0.017 vs. mild (mild n = 4, moderate-severe n = 15); t = 72, **p = 0.004 vs. mild (mild n = 7, moderate-severe n = 15); t = 96, p = 0.645 (mild n = 4, moderate – severe n = 3). (d) Significant correlations were detected between the delay in the start of hypothermia and pGSN levels at 24 and 72 h by Pearson’s correlations (*p = 0.025, **p = 0.007).
Standard protocol approvals, registrations, and patient consents
Families were informed and written consent was obtained before the inclusion of every patient. The study was approved by Puerta del Mar University Hospital Ethics Committee in accordance with the Declaration of Helsinki.
pGSN levels
Plasma samples were thawed, diluted 1:500 and pGSN levels were quantified by colorimetric ELISA kits in duplicates following the manufacturer's recommendations (Aviscera Bioscience, Santa Clara, ref: SK00384-01). Data were expressed in µg/ml.
Aβ40 and Aβ42 levels
Plasma samples for Aβ40 and Aβ42 levels were diluted 1:2.5 and run in duplicates using colorimetric ELISA kits following the manufacturer's recommendations (Wako, Japan, Aβ40 ref: 294-62501 and Aβ42 ref: 290-62601). Data were expressed as pM.
Statistical analyses
Data are presented as mean + SD. One-way ANOVA test was used to compare Aβ and pGSN across all groups under study followed by Tukey b multiple comparison post-test. Student t test for independent samples was used to compare pGSN levels in mild and moderate-severe HIE cases. Pearson's correlation was used to correlate hypothermia temperature against pGSN levels, and duration of hypothermia against pGSN, Aβ40 and Aβ42 levels.
Results
pGSN levels
pGSN levels were reduced in HIE neonates at all study time points, with a progressive decline between t = 0 and t = 72, when compared to control values. pGN levels in HIE neonates were slightly recovered after the hypothermia concluded at 96 h ([F(5,105) = 7.01, **p < 0.01 vs. control and HIE 0 h, ††p < 0.01 vs. HIE 0 h]) (Figure 1(b)). When HIE cases were plotted separately for mild and moderate-severe, we observed an overall reduction of plasma pGSN levels in moderate-severe cases, although differences only reached statistical significance at 24 and 72 h after onset of hypothermia (Figure 1(c)). The duration of hypothermia (0–72 h) in HIE patients was significantly correlated with pGSN levels (Pearson’s = −0.377**, p < 0.01). We observed a general negative correlation between pGSN levels at different times (0, 6, 24, 72 and 96 h) and the delay in the start of hypothermia (t = 0, Pearson’s = −0.392, p = 0.064; t = 6, Pearsons’s = −0.164, p = 0.517; t = 96, Pearson’s = −660, p = 0.154) although this only reached statistical significance at 24 and 72 h (Pearson’s = −0.526*, p = 0.025; Pearson’s = −0.600, **p = 0.007, respectively) after the start of the hypothermia (Figure 1(d)), supporting the view that pGSN might be a good predictor for central dysfunction in these patients.
Aβ40 and Aβ42 levels
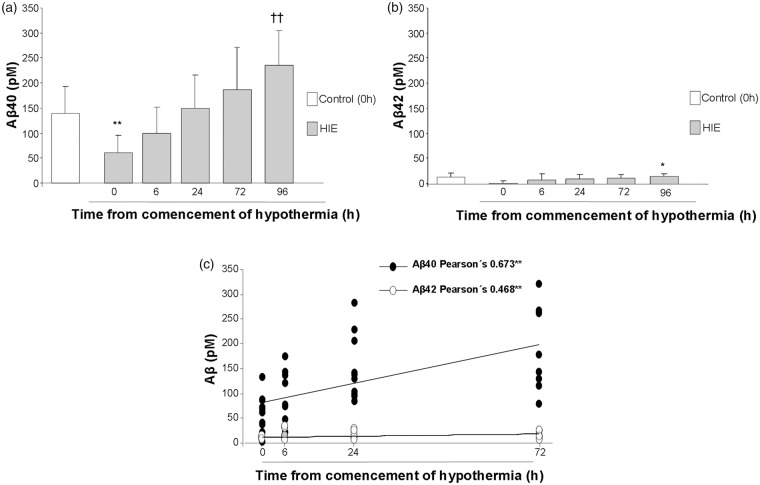
Aβ40 were initially reduced in HIE patients immediately before the commencement of hypothermia and progressively recovered during hypothermia, to end up with higher levels once the hypothermia concluded at 96 h (Figure 2(a)). A similar profile was observed when we compared Aβ42 levels in HIE patients, and the initial reduction in Aβ42 levels was also higher when hypothermia concluded (Figure 2(b)). The duration of hypothermia (0–72 h) in HIE patients was significantly correlated with Aβ40 levels (Pearson’s = 0.673**, **p < 0.01) and Aβ42 levels (Pearson’s = 0.468*, *p = 0.003) (Figure 2(c)).
Figure 2.
(a) A progressive increase of Aβ40 plasma levels was observed after the commencement of hypothermia reaching statistical significance 96 h after commencement of hypothermia, when compared to control patients. Differences were detected by one-way ANOVA followed by Tukey b test [F(5,42) = 7.30, **p < 0.01 vs. Control, HIE 0 h and HIE 6 h, ††p < 0.01 vs. HIE 0 h] (Control n = 7, HIE 0 h n = 11, HIE 6 h n = 8, HIE 24 h n = 10, HIE 72 h n = 8, HIE96 h n = 4). (b) A similar profile was observed when Aβ42 levels were analyzed [F(5,42) = 2.65, *p = 0.036 vs. HIE 0 h] (Control n = 7, HIE 0 h n = 11, HIE 6 h n = 8, HIE 24 h n = 10, HIE 72 h n = 8, HIE96 h n = 4). (c) Significant correlations were detected between time HIE patients spent under hypothermia (0–72 h) and Aβ40 (*p < 0.01) and Aβ42 levels (**p = 0.003).
Discussion
The severity of the complications associated with HIE and the limited available biomarkers make it necessary to explore new prognostic alternatives. Our findings support a feasible role for both pGSN and Aβ40/42 levels as HIE markers. To the best of our knowledge, pGSN has not been previously assessed in HIE newborns. We have detected reductions of pGSN levels in HIE patients, that are more robust in moderate-severe situations, suggesting pGSN prognostic value in these patients. Our results are in line with previous studies showing that pGSN levels are reduced in different animal models and in patients with a wide variety of diseases.16–18 In this sense, early reductions of pGSN plasma levels have been reported in adults with subarachnoid haemorrhage-related vasospasm.5 Similar outcomes have been observed in traumatic brain injury patients, where decreased pGSN levels are reported up to seven days after the injury. Also, decreased pGSN levels are associated with worse prognostic scores in these patients and pGSN has been suggested as a prognostic marker of mortality after traumatic brain injury.19 Likewise, pGSN levels are reduced in newborns with respiratory distress syndrome, who received surfactant therapy or who developed sepsis,7 and in the first month of life in neonates who developed bronchopulmonary dysplasia and retinopathy of prematurity.7 Altogether, these and other studies have led Peddada et al.20 to the hypothesis that pGSN might be a relevant prognostic marker for multiple health complications, qualifying it to be a general health marker. We did not observe any significant improvement of pGSN levels, regardless of the duration of hypothermia, suggesting that hypothermia is not enough to restore pGSN. However, the fact that in our hands pGSN levels, measured at 24 and 72 h after the start of hypothermia, significantly correlate with the delay in starting hypothermia, strengthens the predictive value of plasmatic pGSN in HIE, also suggesting that delaying the start of the treatment reduces the effectiveness of hypothermia to restore pGSN levels. While establishing the accurate levels of gelsolin in human plasma and understanding its variance with age, race, gender and health status is a prerequisite, as previously suggested,20 for pGSN to be used as a biomarker of health, our data supports pGSN as future therapeutic target, in line with previous observations.21 We also observed that Aβ40/42 plasma levels were reduced in HIE patients compared to normal newborns, and a progressive increase of Aβ40/42 plasma levels was detected along with duration of hypothermia. Central-peripheral Aβ balance seems to play a key role in Alzheimer's disease. Plasma concentrations of Aβ40 and 42 have been shown to decrease as the disease progresses and negative correlations between plasma Aβ42 and neocortical amyloid deposition have been shown (for review see Toledo et al.22). Also, a previous study has related encephalopathy and death in infants with abusive head trauma to hypoxic-ischemic injury, accompanied by increased APP levels in the brainstem and the cerebellum.23,24 It is therefore feasible, that reduced plasma Aβ levels in HIE patients might also relate to increased central amyloidosis, while hypothermia counterbalances this effect. However, we cannot unequivocally point towards this possibility. On the other hand, while its physiological role is not well known, Aβ has been implicated in controlling synaptic activity.25 In line with our observations, previous studies have reported reduced Aβ42 levels in the CSF of newborn pigs with perinatal asfixia.9 However, to our knowledge, plasma Aβ levels have not been previously assessed in HIE patients. Brain interstitial fluid Aβ levels have previously been positively correlated with EEG brain activity and with the Glasgow Coma Scale in adults admitted to a neuro-ICU with severe traumatic brain injury,26 supporting that Aβ is secreted by brain neurons in an activity-dependent manner. As expected, Aβ40 and Aβ42 levels were highly correlated (data not shown). However, no significant correlations were observed between Aβ and pGSN levels, suggesting that both biomarkers indicate different aspects of the disease: probably neuronal activity in the case of Aβ and blood–brain barrier integrity in the case of pGSN.
Limitations of our study include the restricted sample size from a single centre, the absence of serial plasma measurements at different time points for the control group (not feasible for ethical reasons), and the lack of long-term follow-up to establish clinical outcomes. Future research should include the Bayley test for infant and toddler development and ultrasound studies. Meanwhile, our present results argue in favour of further study of pGSN and Aβ40/42 as feasible biomarkers for HIE. Following this idea, deep investigations are needed to examine gelsolin role in premature infants and related co-morbidities, as well as its role as a therapeutic agent, as suggested in other pathologies.
Acknowledgements
We thank Dr. Alberto Serrano-Pozo for his help in reviewing the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MG-A: Ministerio de Educación, Cultura y Deporte en el marco del Programa Estatal de Promoción del Talento y su Empleabilidad en I + D + i, Subprograma Estatal de Movilidad, del Plan Estatal de Investigación Científica y Técnica y de Innovación 2013-2016 (PRX16/00246). Programa Estatal de I + D + I orientada a los Retos de la (BFU 2016-75038-R), financed by the Agencia Estatal de Investigación (AEI) and the Fondo Europeo de Desarrollo Regional (FEDER). Proyectos de Excelencia, Consejería de Economía, Innovación, Ciencia y Empleo Junta de Andalucía (P11-CTS-7847). Subvención para la financiación de la investigación y la innovación biomédica y en ciencias de la salud en el marco de la iniciativa territorial integrada 2014-2020 para la provincia de Cádiz. Consejeria de Salud. Junta de Andalucia. Union Europea, financed by the Fondo Europeo de Desarrollo Regional (FEDER) (PI-0008-2017).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Isabel Benavente-Fernandez: study concept, acquisition of data and critical revision. Juan Jose Ramos-Rodriguez and Carmen Infante-Garcia: data acquisition, analysis and interpretation. Gema Jimenez-Gomez, data acquisition and analysis. Alfonso Lechuga-Sancho: critical revision of the manuscript for intellectual content. Simon Lubian-Lopez and Monica Garcia-Alloza: study concept and design, drafting and critical revision of manuscript for intellectual content.
References
- 1.Douglas-Escobar M, Weiss MD. Biomarkers of hypoxic-ischemic encephalopathy in newborns. Front Neurol 2012; 3: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Eur J Pediatr 2013; 1: 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aly H, Hamed Z, Mohsen L, et al. Serum amyloid A protein and hypoxic ischemic encephalopathy in the newborn. J Perinatol 2011; 31: 263–268. [DOI] [PubMed] [Google Scholar]
- 5.Chou SH, Lee PS, Konigsberg RG, et al. Plasma-type gelsolin is decreased in human blood and cerebrospinal fluid after subarachnoid hemorrhage. Stroke 2011; 42: 3624–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yildirim F, Gertz K, Kronenberg G, et al. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol 2008; 210: 531–542. [DOI] [PubMed] [Google Scholar]
- 7.Kose M, Elmas T, Gokahmetoglu S, et al. Predictive value of gelsolin for the outcomes of preterm neonates: a pilot study. Pediatr Int 2014; 56: 856–859. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka Y, Saito M, LaFrancois J, et al. Novel therapeutic approach for the treatment of Alzheimer's disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci 2003; 23: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benterud T, Pankratov L, Solberg R, et al. Perinatal asphyxia may influence the level of beta-amyloid (1–42) in cerebrospinal fluid: an experimental study on newborn pigs. PLoS One 2015; 10: e0140966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 2011; 1: a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976; 33: 696–705. [DOI] [PubMed] [Google Scholar]
- 12.Ancora G, Testa C, Grandi S, et al. Prognostic value of brain proton MR spectroscopy and diffusion tensor imaging in newborns with hypoxic-ischemic encephalopathy treated by brain cooling. Neuroradiology 2013; 55: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 13.Horn AR, Swingler GH, Myer L, et al. Defining hypoxic ischemic encephalopathy in newborn infants: benchmarking in a South African population. J Perinat Med 2013; 41: 211–217. [DOI] [PubMed] [Google Scholar]
- 14.Umran RM, Al-Tahir M, Jagdish D, et al. Insulin-like growth factor-1 levels in term newborns with hypoxic-ischemic encephalopathy. Am J Perinatol 2016; 33: 640–645. [DOI] [PubMed] [Google Scholar]
- 15.Blanco D, Garcia-Alix A, Valverde E, et al. [Neuroprotection with hypothermia in the newborn with hypoxic-ischaemic encephalopathy. Standard guidelines for its clinical application]. An Pediatr 2011; 75: 341 e1–20. [DOI] [PubMed] [Google Scholar]
- 16.Guntert A, Campbell J, Saleem M, et al. Plasma gelsolin is decreased and correlates with rate of decline in Alzheimer's disease. J Alzheimers Dis 2010; 21: 585–596. [DOI] [PubMed] [Google Scholar]
- 17.Le HT, Hirko AC, Thinschmidt JS, et al. The protective effects of plasma gelsolin on stroke outcome in rats. Exp Transl Stroke Med 2011; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Liu J, Liang S, et al. Plasma gelsolin protects HIV-1 gp120-induced neuronal injury via voltage-gated K+ channel Kv2.1. Mol Cell Neurosci 2013; 57: 73–82. [PMC free article] [PubMed] [Google Scholar]
- 19.Xu JF, Liu WG, Dong XQ, et al. Change in plasma gelsolin level after traumatic brain injury. J Trauma Acute Care Surg 2012; 72: 491–496. [DOI] [PubMed] [Google Scholar]
- 20.Peddada N, Sagar A, Ashish, ret al. Plasma gelsolin: a general prognostic marker of health. Med Hypotheses 2012; 78: 203–210. [DOI] [PubMed]
- 21.Chou SH, Lo EH, Ning M. Plasma-type gelsolin in subarachnoid hemorrhage: novel biomarker today, therapeutic target tomorrow? Crit Care 2014; 18: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements – a desired but elusive Alzheimer's disease biomarker. Alzheimers Res Ther 2013; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matschke J, Buttner A, Bergmann M, et al. Erratum to: encephalopathy and death in infants with abusive head trauma is due to hypoxic-ischemic injury following local brain trauma to vital brainstem centers. Int J Legal Med 2015; 129: 115–116. [DOI] [PubMed] [Google Scholar]
- 24.Matschke J, Buttner A, Bergmann M, et al. Encephalopathy and death in infants with abusive head trauma is due to hypoxic-ischemic injury following local brain trauma to vital brainstem centers. Int J Legal Med 2015; 129: 105–114. [DOI] [PubMed] [Google Scholar]
- 25.Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol 2006; 575(Pt 1): 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brody DL, Magnoni S, Schwetye KE, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 2008; 321: 1221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]