Abstract
Objectives
Driving pressure (DP) has recently become a promising mediator for the identification of the effects of mechanical ventilation on outcomes in acute respiratory distress syndrome (ARDS). The aim of this study was to systematically and quantitatively update and assess the association between DP and mortality among ventilated patients with ARDS.
Methods
PubMed, the Cochrane Library, ISI Web of Knowledge, and Embase were systematically searched from inception to June 2018. Two investigators conducted the literature search study selection, data extraction, and quality evaluation independently. RevMan 5.3 software was used for all statistical analyses.
Results
A total of seven studies comprising 8010 patients were included in this meta-analysis. Higher DP showed a significant association with higher mortality (pooled risk ratio, 1.10; 95% [CI], 1.05–1.16; I2 =58%). Sensitivity analysis indicated that one study significantly affected the stability of pooled results. One of the subgroups investigated, ARDS severity, could account for the heterogeneity. An exploratory post hoc subgroup analysis and higher DP significantly increased mortality in the mild to severe ARDS subgroup (RR 1.28; 95% [CI], 1.14–1.43; I2 =0), but not in the moderate to severe ARDS subgroup (RR 1.18; 95% [CI], 0.95–1.46; I2 =52%).
Conclusion
Higher DP was significantly associated with an increased risk of death among ventilated patients with ARDS. But it did not seem to predict prognosis to moderate to severe ARDS. Future prospective randomized clinical trials are needed to verify the results of this meta-analysis and address the unresolved questions about optimum cutoff values for DP.
Trial Registration
This trial is registered with PROSPERO (CRD42018102146), on 11 August 2018.
1. Background
Acute respiratory distress syndrome (ARDS) is a common disease that affects up to 20% of mechanically ventilated patients during an intensive care unit (ICU) stay [1]. Despite decades of research, seldom effective therapeutic strategies for treating clinical ARDS have appeared. Current treatments focus on support, as mechanical ventilation is a cornerstone life-saving treatment for ARDS. Lung protective ventilation acts by limiting the iatrogenic injury that is linked to mechanical ventilation [2, 3] and is frequently used ventilation method. It includes several components, the most important of which is lowering tidal volume (VT), limiting plateau (Pplat) to or below 30 cmH2O and higher positive end-expiratory pressures (PEEPs). This combined strategy is indeed the valid ventilator intervention that has been indicated to prominently improve survival to date [4, 5].
To optimize lung protective ventilation, a host of studies have introduced the range of VT to predict body weight (PBW) to normalize VT to lung size [6, 7]. In ARDS, due to the presence of lung disease, there is a commonly significant and nonuniform reduction in the amount of lung available for ventilation among patients [8]. Therefore, a similar tidal volume (VT) dependent on ideal body weight can vary for different degrees of lung stress [9]. In contrast, driving pressure (DP), which is calculated as end-inspiratory plateau pressure (Pplat) minus applied positive end-expiratory pressure (PEEP) and is equivalent to the ratio between the VT and compliance of the respiratory system, can better reflect lung injury compared to VT adjustments based solely on ideal body weight. [10]
A retrospective analysis of several trials in patients with ARDS comparing different PEEP levels at the same VT or different VT levels at the same PEEP, or a combination of both, found that DP was more strongly related to mortality than was Pplat [11]. Similarly, a recent meta-analysis demonstrated an association between DP and mortality [12]. Nevertheless, a few new studies [13, 14] offer additional data that can provide a clearer understanding of the potential value of DP for ARDS. We executed an updated systematic review and meta-analysis to add further documentation that confirms the association of DP with mortality in mechanically ventilated patients with ARDS.
2. Methods
The present meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (PRISMA) [15] (Supplementary Appendix 1). The review protocol was registered at the PROSPERO registry of systematic reviews in August 2018 (registry number: CRD 42018102146).
2.1. Data Sources
We systematically searched four databases, PubMed, the Cochrane Library, ISI Web of Knowledge, and Embase, from inception to June 2018, using a sensitive search strategy (Supplementary Appendix 2). A basic search was performed using the following vocabulary terms (when available), text words, and keywords: (“driving pressure [with related synonyms]”) AND (“acute respiratory distress syndrome [with related synonyms]” OR “ARDS [with related synonyms]”). No language restriction was applied for article selection. Additional studies were identified by reviewing the reference lists of relevant articles.
2.2. Eligibility Criteria
Two reviewers (LEZ and ZC) independently evaluated the resulting studies for their eligibility for inclusion. In cases of disagreement, a consensus was reached by discussion or by consultation with a third reviewer (HJL). Randomized controlled trials (RCTs), controlled studies, cohort studies, secondary analysis studies, and case-control studies were considered eligible if they collected data on mortality in ventilated adult patients with ARDS with DP measurement. The exclusion criteria were as follows: editorials, reviews, abstracts or conference proceedings, expert opinions, animal experiments, unrelated intervention or outcomes, and insufficient information to extract data after contacting the corresponding authors.
2.3. Data Extraction
Two reviewers (GLL and XXW) independently extracted study characteristics and data from each eligible study, including the authors, year of study, country of origin, study design, study settings, relevant population, sample size, mean age, the optimal cutoff values, outcome assessment, and follow-up period. The diagnosis of ARDS met the Berlin criteria [16] or was based on the American-European consensus definition criteria [17]. Our primary outcome was hospital mortality; if not available, we used mortality at the latest reported time point. If a meta-analysis noted that unpublished data were provided by the primary authors, we extracted those data from forest plots of the meta-analysis [12] and reviewed original articles to confirm whether the trials met our inclusion criteria. When those data were our outcomes of interest, we pooled them with the data from primary trials. Additionally, a study by Villar et al. [18] contained two data sets (derivation cohort and validation cohort). The authors have shared the corresponding data, which can be downloaded from http://links.lww.com/CCM/C436.
2.4. Quality Assessment
The Newcastle Ottawa Scale for cohort studies was used to assess the reporting quality of the included component studies [19]. This scale comprises eight items evaluating the quality of observational cohort studies in terms of selection, comparability, and outcome. Observational cohort studies receiving seven or more stars were considered to be of high quality. The assessment was performed independently by two reviewers (XXW and ZC). Disagreements were resolved by consensus.
2.5. Statistical Analyses
RevMan 5.3 software from the Cochrane Collaboration was utilized for the meta-analysis. Relative risk (RR) was used as the common measure of association across studies. To this end, the hazard ratios (HRs) were directly considered the RRs. In addition, an adjusted RR or HR was selected as the effect size from eligible studies based on multivariate analysis. RR was reported to estimate the association between DP and mortality among ventilated patients with ARDS. RR and the associated 95% CI were pooled using fixed-effect (Mantel-Haenszel method) or random-effect models (DerSimonian and Laird method) [20]. RRs greater than 1 indicated a beneficial effect of the exposure for mortality.
Heterogeneity in a meta-analysis indicates the degree of variability in the results across studies and was appraised using the Q test, p value, and I2 index, which included thresholds for low (I2 < 50%), moderate (50% < I2 <75%), and high (I2 >75%) heterogeneity [21].
In addition, to investigate the potential sources of heterogeneity in the eligible studies, sensitivity analyses were performed in RevMan with sequential exclusion of each study to explore the heterogeneity observed. Sensitivity analysis was also investigated by removing trials with characteristics different from the others.
To evaluate whether the association between higher DP and mortality among ventilated patients with ARDS was modified by clinical characteristics, several subgroups were examined based on ARDS severity (mild to severe ARDS vs. moderate to severe ARDS), sample size (>500 vs.≤500), and cutoff value (>15 vs.≤15). On account of a trial by Chiu et al. [13] employing ECOM as the main cotreatment and a study by Raymondos et al. [14] missing important parameter (cutoff value of DP), we will exclude the above two trials to perform an exploratory post hoc subgroup analysis based on ARDS severity (mild to severe ARDS vs. moderate to severe ARDS). Analysis was performed to assess whether the difference between the subgroups was statistically significant. We used χ2 to test for subgroup differences—that is, whether the observed differences in the subgroups are compatible with chance alone. A low P value (or a large χ2 statistic relative to its degree of freedom) provides evidence of heterogeneity beyond chance.
Funnel plots were used to screen for potential publication bias. We calculated κ statistics to assess the agreement between the two investigators for assessment of methodological quality.
3. Results
3.1. Identification of Studies
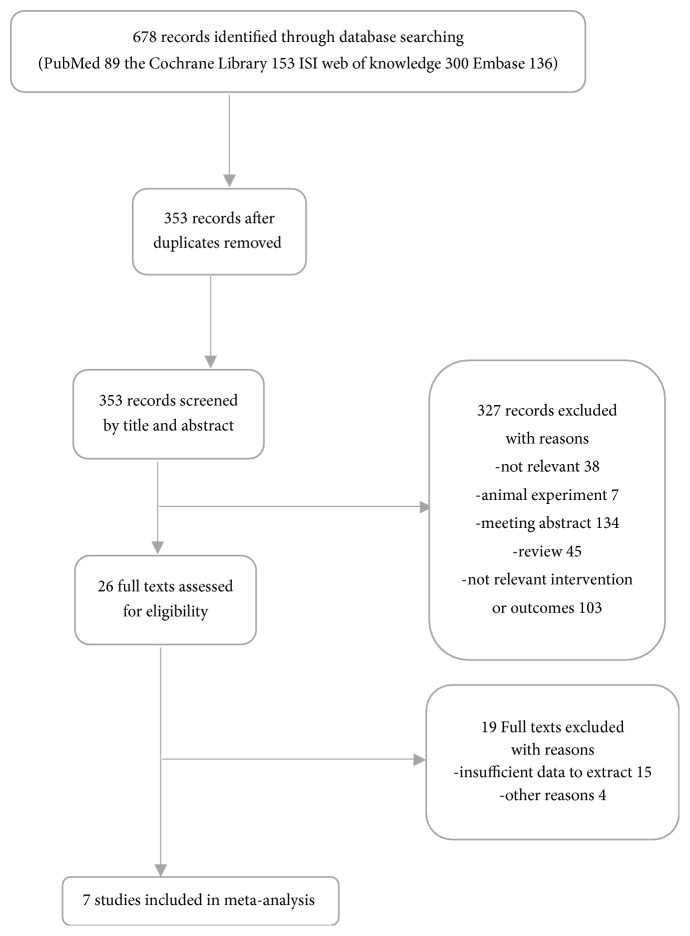
The flow chart of the study selection procedure is shown in Figure 1. The initial search identified 89 citations from PubMed, 153 from the Cochrane Library, 300 from ISI Web of Knowledge, and 136 from Embase. After removing 325 duplicates, the titles and abstracts of the remaining 353 papers were screened. After 326 records were eliminated by inspection of the titles and abstracts, 26 articles were subsequently scrutinized by a reading of the full text. As a result, 7 studies [11, 13, 14, 18, 22–24] fulfilled our eligibility criteria and were included in the final meta-analysis.
Figure 1.
Flow diagram of literature search and selection process of the studies.
3.2. Characteristics of the Included Studies
The characteristics of the eligible studies are presented in Table 1. Two secondary analyses of previous RCTs [11, 22], one secondary analysis of previous cohorts [18], one retrospective observational study (ROS) [13], and three prospective observational studies (POS) [14, 23, 24] were included, all [11, 13, 14, 18, 22–24] of which were published between 2015 and 2017. With respect to clinical setting, all seven studies [11, 13, 14, 18, 22–24] were conducted in an intensive care unit (ICU). The sample sizes varied across the studies, ranging from 150 to 3562, and the mean age of the patients was between 50.3 and 62.8 years. In terms of population, four studies [11, 14, 23, 24] focused on mild to severe ARDS patients, and three studies [13, 18, 22] focused on moderate to severe ARDS patients. With regard to criteria for selecting the DP thresholds, each study provided optimum cutoff points. The cutoff values of DP for the prediction varied across the studies, ranging from 13 to 21 cmH2O, with the exception of the study by Raymondos et al. [14], in which the cutoff point was not reported.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Study | Study design |
Country | Setting | Population | Sample Size |
Male (%) |
Age (years) |
Mortality (%) |
Cut-off value (cmH20) |
Follow-up (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| Amato et al. 2015 |
ROS | Brazil | ICU | Mild to Severe ARDS | 3562 | NR | NR | 34.7 | 15 | Mortality at day 60 |
| Chiu et al. 2017 | ROS | Taiwan | ICU | Severe ARDS with ECMO | 158 | 68.4 | 50.3 | 55.1 | 21 | ICU mortality |
| Chiumello et al. 2016 | POS | Italy | ICU | Mild to Severe ARDS | 150 | 68 | 62 | 32 | 15 | ICU mortality |
| Guerin et al. 2016 | POS | France | ICU | Moderate to severe ARDS | 787 | 68.9 | 59 | 32.3 | 13 | Mortality at day 90 |
| Laffey et al . 2016 |
POS | Canada | ICU | Mild to severe ARDS | 2377 | 61.9 | 60.5 | 40.1 | 14 | Hospital mortality |
| Raymondos et al. 2017 | POS | Germany | ICU | Mild to Severe ARDS | 198 | 71.7 | 59/62.8 | 57.5 | NR | Hospital mortality |
| Villar et al. 2017a | ROS | Spain | ICU | Moderate to Severe ARDS | 478 | 69.9 | 54 | 42.2 | 19 | Hospital mortality |
| Villar et al. 2017b | ROS | Spain | ICU | Moderate to Severe ARDS | 300 | 67 | 57 | 42.3 | 19 | Hospital mortality |
ICU=intensive care unit, ARDS=acute respiratory distress syndrome, NR=not reported, ROS= retrospective observational study, ECMO=extracorporeal membrane oxygenation, POS= prospective observational study.
3.3. Results of the Quality Assessment
The interrater reliability for the assessment of quality items was 0.73 (P<0.0001). Overall, the methodological quality was moderate. Details of the methodological assessment are shown in Table 2. Table 2 displays the quality assessment using the Newcastle Ottawa Scale for observational studies. The results showed that three studies scored 9 points, and the remaining studies scored 8 points. That is, the included studies showed a low risk of bias.
Table 2.
Quality Assessment with Newcastle Ottawa Scale for cohort study.
| Study | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| REC | SNC | AE | AOI | Design and Analysis | Assessment | Enough Follow-up |
Adequate Follow-up |
Score | |
| Amato et al. 2015 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Chiu et al. 2017 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Chiumello et al. 2016 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Laffey et al. 2016 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Raymondos et al. 2017 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Villar et al. 2017 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | |
| Guerin et al. 2016 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
REC=Representative of Exposed Cohort, SNC=Selection of Nonexposed Cohort, AE=Ascertainment of Exposed, AOI=Absence of Outcome of Interest, star (☆) was allocated to a particular item when it was adequately reported and addressed. The item “comparability” could be allocated with a maximum of two stars. Dashes indicate this item was not adequately reported or addressed.
3.4. Data Synthesis
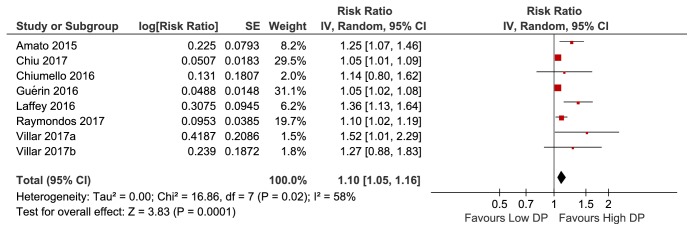
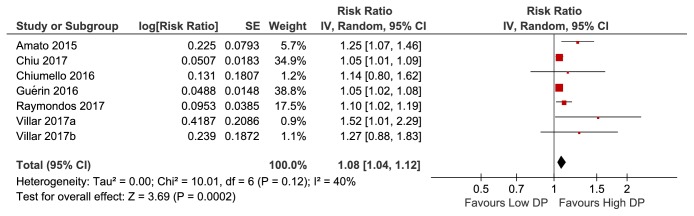
In the meta-analysis of eight studies involving 8010 patients, higher DP was significantly associated with increased mortality among mechanically ventilated ARDS patients (pooled risk ratio, 1.10; 95% [CI], 1.05–1.16; I2 =58%) (Figure 2). Considering the remarkable heterogeneity across studies observed, a sensitivity analysis was performed to explore the heterogeneity. After omitting one study by Laffey et al. [24], the heterogeneity of the pooled RR (1.08; 95% [CI], 1.04–1.12; I2 =40%) showed a relative decrease from moderate to low heterogeneity (Figure 3), with the I2 index decreasing from 58% to 40%. Given a study by Chiu et al. [13] with ECMO as the main cointervention, a sensitivity analysis in which the trials by Chiu et al. were excluded showed a pooled RR of 1.16 (95% [CI], 1.07–1.26; I2 =63%) (Supplementary Figure S1). On account of a study by Raymondos et al. missing important parameter (cutoff value of DP), a sensitivity analysis in which the trials by Raymondos et al. [14] were excluded showed a pooled RR of 1.11 (95% [CI], 1.05–1.18; I2 =63%) (Supplementary Figure S2).
Figure 2.
Forest plots for pooled risk ratio of high DP versus low DP from eligible studies.
Figure 3.
Sensitivity analysis by excluding study by Laffey et al.
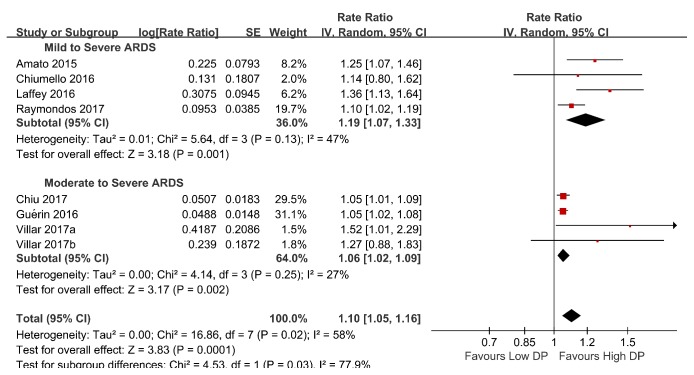
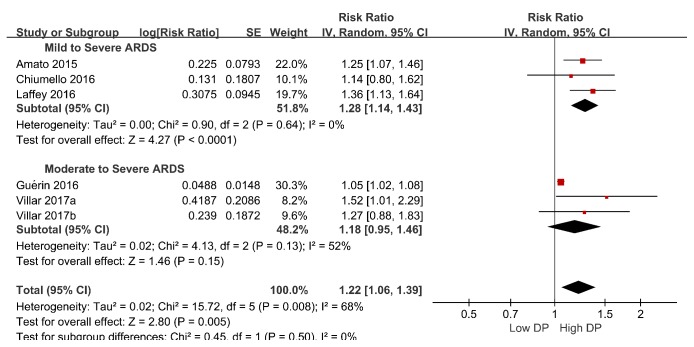
Additionally, subgroup analyses were performed based on ARDS severity, sample size, and cutoff values. One of the subgroups investigated, ARDS severity, could account for the heterogeneity. In the mild to severe ARDS subgroup, the pooled RR was 1.19 (95% CI, 1.07–1.33; I2 =47%). In the moderate to severe ARDS subgroup, the pooled RR was 1.06 (95% CI, 1.02–1.09; I2 =20%) (Figure 4). The relative risk was higher in the mild to severe ARDS subgroup than the moderate to severe ARDS subgroup (χ2= 4.53, P = 0.03). The results of all subgroup analyses are presented in Table 3. An exploratory post hoc subgroup analysis based on ARDS severity was performed. In the mild to severe ARDS subgroup, the pooled RR was 1.28 (95% CI, 1.14–1.43; I2 =0). In the moderate to severe ARDS subgroup, the pooled RR was 1.18 (95% CI, 0.95–1.46; I2 =52%) (Figure 5). Inspection of the corresponding funnel plot revealed no evidence of significant publication bias (Figure 6).
Figure 4.
Subgroup analysis-ARDS severity for the predictive value of elevated DP for mortality in ARDS with mechanical ventilation.
Table 3.
Results of subgroup analysis based on different standards.
| K | N | RR [95% CI] | P | Study heterogeneity | P (between-groupcomparison) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Chi2 | df | I2 (%) | P | ||||||
| ARDS Severity | 0.03 | ||||||||
| Mild to Severe ARDS | 4 | 6287 | 1.19 [1.07, 1.33] | 0.001 | 5.64 | 3 | 47 | 0.13 | |
| Moderate to Severe ARDS | 4 | 1723 | 1.06 [1.02, 1.09] | 0.002 | 4.14 | 3 | 27 | 0.25 | |
| Sample size | 0.28 | ||||||||
| Sample Size≤500 | 5 | 1284 | 1.08 [1.02, 1.14] | 0.005 | 5.09 | 4 | 21 | 0.28 | |
| 500<Sample Size | 3 | 6726 | 1.19 [1.00, 1.42] | 0.05 | 11.76 | 2 | 83 | 0.003 | |
| Cut-off value | 0.97 | ||||||||
| DP≤15cmH2O | 4 | 6876 | 1.18 [1.02, 1.37] | 0.03 | 11.91 | 3 | 75 | 0.008 | |
| 15cmH2O<DP | 3 | 936 | 1.18 [0.95, 1.46] | 0.14 | 4.06 | 2 | 51 | 0.13 | |
Figure 5.
An exploratory post hoc subgroup analysis based on ARDS severity.
Figure 6.
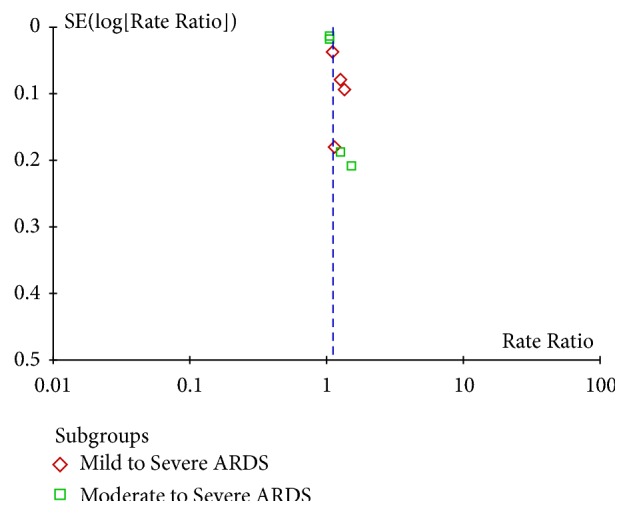
Funnel plot of publication bias.
4. Discussion
The present systematic review and meta-analysis investigated the significant association of DP with mortality among ventilated patients with ARDS. Accordingly, the pooled risk ratio is 1.10 (95% CI, 1.05–1.16), indicating that higher DP is a bedside-available parameter for the prediction of mortality in ventilated patients with ARDS that may help identify patients who are at increased risk of death. An exploratory post hoc subgroup analysis indicated it seems to have no prognostic effect on moderate to severe ARDS.
A previous meta-analysis reported a similar topic [12]. The differences between the present meta-analysis and the previous one are as follows. First, our meta-analysis included two additional trials providing sufficient information for analysis that were not included in previous meta-analyses. As the latest and most comprehensively updated meta-analysis, the present study further reinforces the results of previous meta-analyses. Second, we registered the protocol of this study on PROSPERO. A registered protocol may increase the transparency and quality of meta-analysis.
Our meta-analysis confirmed that the higher DP significantly increased mortality among ventilated patients with ARDS, in accord with result of published meta-analyses [12]. Significant heterogeneity was observed in the present study. We conducted a sensitivity analysis with serial exclusion of individual studies. After omitting one study by Laffey et al. [24], it could account for the heterogeneity. A possible explanation is that the trial did not primitively report the relative risk for higher DP and mortality in entire ARDS populations. We extract the effect size from a published meta-analysis [12], which may affect the accuracy. To investigate other possible reasons for study heterogeneity, we performed subgroup analysis. A subgroup analysis based on ARDS severity indicated DP was consistently associated with increased mortality with low heterogeneity in both subgroups. However, analysis of subgroup differences found the relative risk was significantly higher in the mild to severe ARDS subgroup than the moderate to severe ARDS subgroup (P = 0.03). Given two trials by Chiu et al. [13] and Raymondos et al. [14] including their own limitations, we removed the above two trials to perform an exploratory post hoc subgroup analysis based on ARDS severity. The result showed that higher DP is still related to increased mortality in the mild to severe ARDS subgroup, but not in the moderate to severe ARDS subgroup. Higher DP appears to have no prognostic effect on moderate to severe ARDS. Future prospective randomized clinical trials are needed to verify the results of this meta-analysis.
Likewise, the importance of DP in determining the effects of ventilator settings has been subsequently confirmed by a recent epidemiological study involving more than 2000 patients with ARDS in 50 countries [25]. Higher survival was detected in patients with DP≤14 cmH2O at the onset of the syndrome. Furthermore, in a recent secondary analysis study, [26] DP showed an independent association with mortality [adjusted OR, 1.04 (95% CI, 1.01 - 1.07)] among mechanically ventilated patients without ARDS. DP may be the most useful ventilator variable for stratifying patients' disease severity and the risk of ventilator-induced lung injury (VILI) among mechanically ventilated patients, whether or not the patients suffer from ARDS.
To date, several ventilator variables, such as PEEP and VT, have been determined and monitored for their relative effects on survival among ventilated patients with ARDS. In a previous systematic review and meta-analysis, [27, 28] PEEP or VT was not related to increased mortality in ARDS patients receiving lung protective ventilation. Nevertheless, the current study confirms the results of prior individual studies indicating the association of higher DP with higher mortality. Gattinoni et al. [29] recently obtained deep insight into the importance of the primary components of lung-protective mechanical ventilation. The authors calculated the mechanical power applied to respiratory system based on the assumption that the greater the power is, the greater the likelihood of lung injury becomes. The main variables associated with the ventilator, including VT, respiratory rate, flow rate, PEEP, DP, and patient compliance and airway resistance, all exert an influence on lung injury. The strongest effects are due to VT and DP, with the weakest effect derived from the application of PEEP. Hence, all these parameters must be considered when predicting outcomes. The diverse etiologies and varying severities of ARDS imply that prediction of the risk of death is impossible to determine by a single parameter. Apparently, all ARDS patients should be ventilated with a lung-protective Vt, PEEP, Pplat, and DP.
Currently, no study has prospectively evaluated whether systematic interventions titrated to DP reduction may provide a relevant clinical benefit. Thus, a well-performed RCT that evaluates outcomes from VT adjustments based on DP compared to VT adjustments based solely on ideal body weight must be performed to ascertain the benefit of using DP to set volume. Moreover, a safety and feasibility trial is also needed to understand how a protocol targeting DP can be developed and implemented by bedside clinicians (e.g., adjustment of tidal volume or other ventilatory variables, lung recruitment with PEEP) and whether there are any adverse effects of such a strategy compared to convention mechanical ventilation strategies.
Several limitations of this study should be discussed. First, marked heterogeneity existed across the included studies in terms of ARDS severity, sample size, and optimal cutoff values. Although we performed sensitivity and subgroup analyses to explore the sources of potential heterogeneity between studies, the heterogeneity of each parameter was not entirely reduced. Additional high-quality studies with well-designed may be required. Second, the cutoff points of DP varied, ranging from 13 to 21 cmH2O, and we could not determine ideal cutoff values for DP because we did not have the raw data to construct ROC curves. To confirm whether one or more DP thresholds exist, further explorations in larger, prespecified groups of patients are required. Third, one study by Amato et al. [11] is an analysis of several prior trials, and another study by Guerin et al. [22] is an analysis of two prior trials. An individual patient data (IPD) meta-analysis should be appropriately performed to account for the random effects of the studies whose data involved 2-3 trials. However, we do not have access to the data underlying the original studies. Finally, we could not determine whether the data rooted in each trial, namely, the numerical value of the DP, are contaminated by several confounding factors that strongly affect results (e.g., spontaneous effort, chest wall stiffness, and position). [30] Simultaneously, virtually none of the trials ensured that DP was recorded under passive conditions because plateau pressure can be displayed by most ventilators even when the patient is actively breathing. Besides, DP cannot be recorded when there is reverse triggering occurring during inspiration [31, 32]. Again, chest wall stiffness and, in some cases, position affect those values as well. DP will change in the same patient with variation of disease and ventilation settings. Therefore, the predictive accuracy of DP needs to be further studied to evaluate.
5. Conclusion
Higher DP was significantly associated with an increased risk of death among ventilated patients with ARDS. Yet it did not appear to predict prognosis to moderate to severe ARDS. Future prospective randomized clinical trials are needed to verify the results of this meta-analysis and address unresolved questions about the optimum cutoff values of DP.
Abbreviations
- ARDS:
Acute respiratory distress syndrome
- ICU:
Intensive care unit
- VT:
Tidal volume
- Pplat:
Plateau
- PBW:
Predicted body weight
- DP:
Driving pressure
- PEEP:
Positive end-expiratory pressures
- PRISMA:
Preferred reporting items for systematic reviews and meta-analyses statement
- RCTs:
Randomized controlled trials
- RR:
Relative risk
- ROS:
Retrospective observational study
- ECMO:
Extracorporeal membrane oxygenation
- POS:
Prospective observational study
- VILI:
Ventilator-induced lung injury
- IPD:
Individual patient data.
Contributor Information
Haijin Lv, Email: haijinlv@163.com.
Liuer Zuo, Email: 13627479179@163.com.
Data Availability
All data generated or analyzed during this study are included in this published article. The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Study concept and design were made by Zhen Chen, Genglong Liu, Xuxia Wei, Haijin Lv, and Liuer Zuo. Acquisition of data was performed by Haijin Lv, Zhen Chen, and Xuxia Wei. Analysis and interpretation of data were made by Xuxia Wei, Haijin Lv, and Zhen Chen. Haijin Lv, Qiang Tai, Donghua Zheng, Wenfeng Xie, and Xuxia Wei drafted the manuscript. Critical revision of the manuscript for important intellectual content was performed by Zhen Chen, Li Chen, Ganping Wang, Jia-Qi Sun, and Genglong Liu. Liuer Zuo, Siqi Wang, Na Liu, and Xuxia Wei conducted the statistical analysis. Administrative, technical, and material support was by Liuer Zuo and Zhen Chen. Study supervision was done by Haijin Lv and Genglong Liu. All authors have read and approved the manuscript for publication. Zhen Chen, Xuxia Wei, Genglong Liu, and Qiang Tai have equally contributed.
Supplementary Materials
Supplementary Appendix 1. PRISMA Checklist.
Supplementary Appendix 2. Search strategy used for the literature review.
Supplementary Figure S1. Sensitivity analysis by excluding study by Chiu et al.
Supplementary Figure S2. Sensitivity analysis by excluding study by Raymondos et al.
References
- 1.Villar J., Martín-Rodríguez C., Domínguez-Berrot A. M., et al. A quantile analysis of plateau and driving pressures. Critical Care Medicine. 2017;45(5):843–850. doi: 10.1097/CCM.0000000000002330. [DOI] [PubMed] [Google Scholar]
- 2.Liu G., Lv H., An Y., Wei X., Yi X., Yi H. Tracking of transplanted human umbilical cord-derived mesenchymal stem cells labeled with fluorescent probe in a mouse model of acute lung injury. International Journal of Molecular Medicine. 2018;41(5):2527–2534. doi: 10.3892/ijmm.2018.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laffey J. G., Bellani G., Pham T., et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Medicine. 2016;42(12):1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 4.Villar J., Blanco J., Kacmarek R. M. Current incidence and outcome of the acute respiratory distress syndrome. Current Opinion in Critical Care. 2016;22(1):1–6. doi: 10.1097/MCC.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 5.Cortes-Puentes G. A., Cortes-Puentes L. A., Adams A. B., Anderson C. P., Marini J. J., Dries D. J. Experimental intra-abdominal hypertension influences airway pressure limits for lung protective mechanical ventilation. Journal of Trauma and Acute Care Surgery. 2013;74(6):1468–1473. doi: 10.1097/TA.0b013e31829243a7. [DOI] [PubMed] [Google Scholar]
- 6.Mekontso Dessap A., Boissier F., Charron C., et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Medicine. 2016;42(5):862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 7.Terragni P. P., Rosboch G., Tealdi A., et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2007;175(2):160–166. doi: 10.1164/rccm.200607-915oc. [DOI] [PubMed] [Google Scholar]
- 8.Gattinoni L., Marini J. J., Pesenti A., Quintel M., Mancebo J., Brochard L. The "baby lung" became an adult. Intensive Care Medicine. 2016;42(5):663–673. doi: 10.1007/s00134-015-4200-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q., Li W., Zeng Q., Zhong N., Chen R. Lung stress and strain during mechanical ventilation in animals with and without pulmonary acute respiratory distress syndrome. Journal of Surgical Research. 2013;181(2):300–307. doi: 10.1016/j.jss.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Grieco D. L., Chen L., Dres M., Brochard L. Should we use driving pressure to set tidal volume? Current Opinion in Critical Care. 2017;23(1):38–44. doi: 10.1097/MCC.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 11.Amato M. B. P., Meade M. O., Slutsky A. S., et al. Driving pressure and survival in the acute respiratory distress syndrome. The New England Journal of Medicine. 2014;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 12.Aoyama H., Pettenuzzo T., Aoyama K., Pinto R., Englesakis M., Fan E. Association of driving pressure with mortality among ventilated patients with acute respiratory distress syndrome. Critical Care Medicine. 2018;46(2):300–306. doi: 10.1097/CCM.0000000000002838. [DOI] [PubMed] [Google Scholar]
- 13.Chiu L., Hu H., Hung C., et al. Dynamic driving pressure associated mortality in acute respiratory distress syndrome with extracorporeal membrane oxygenation. Annals of Intensive Care. 2017;7(1):p. 12. doi: 10.1186/s13613-017-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raymondos K., Dirks T., Quintel M., Molitoris U., Ahrens J., Dieck T. Outcome of acute respiratory distress syndrome in university and non-university hospitals in germany. Critical Care. 2017;21(1):p. 122. doi: 10.1186/s13054-017-1687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Ranieri V. M., Rubenfeld G. D., Thompson B. T., et al. Acute respiratory distress syndrome: the Berlin definition. The Journal of the American Medical Association. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Bernard G. R., Artigas A., Brigham K. L., et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine. 1994;149(3):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 18.Villar J., Martín-Rodríguez C., Domínguez-Berrot A. M., et al. A quantile analysis of plateau and driving pressures. Critical Care Medicine. 2017;45(5):843–850. doi: 10.1097/CCM.0000000000002330. [DOI] [PubMed] [Google Scholar]
- 19.Cook D. A., Reed D. A. Appraising the quality of medical education research methods. Academic Medicine: Journal of the Association of American Medical Colleges. 2015;90(8):1067–1076. doi: 10.1097/ACM.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 20.Thompson S. G., Sharp S. J. Explaining heterogeneity in meta-analysis: A comparison of methods. Statistics in Medicine. 1999;18(20):2693–2708. doi: 10.1002/(SICI)1097-0258(19991030)18:20<2693::AID-SIM235>3.0.CO;2-V. doi: 10.1002/(SICI)1097-0258(19991030)18:20<2693::AID-SIM235>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Lijmer J. G., Bossuyt P. M. M., Heisterkamp S. H. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Statistics in Medicine. 2002;21(11):1525–1537. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 22.Guérin C., Papazian L., Reignier J., Ayzac L., Loundou A., Forel J.-M. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilationin two randomized controlled trials. Critical Care. 2016;20(1):p. 384. doi: 10.1186/s13054-016-1556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiumello D., Carlesso E., Brioni M., Cressoni M. Airway driving pressure and lung stress in ARDS patients. Critical Care. 2016;20(1):p. 276. doi: 10.1186/s13054-016-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laffey J. G., Bellani G., Pham T., et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Medicine. 2016;42(12):1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 25.Bellani G., Laffey J. G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. Journal of the American Medical Association. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 26.Fuller B. M., Page D., Stephens R. J., et al. Pulmonary mechanics and mortality in mechanically ventilated patients without acute respiratory distress syndrome: a cohort study. Shock. 2018;49(3):311–316. doi: 10.1097/SHK.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walkey A. J., Del Sorbo L., Hodgson C. L., et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome. a systematic review and meta-analysis. Annals of the American Thoracic Society. 2017;14(Supplement_4):S297–S303. doi: 10.1513/AnnalsATS.201704-338OT. [DOI] [PubMed] [Google Scholar]
- 28.Walkey A. J., Goligher E. C., Del Sorbo L., et al. Low Tidal volume versus non–volume-limited strategies for patients with acute respiratory distress syndrome. a systematic review and meta-analysis. Annals of the American Thoracic Society. 2017;14(Supplement_4):S271–S279. doi: 10.1513/AnnalsATS.201704-337OT. [DOI] [PubMed] [Google Scholar]
- 29.Gattinoni L., Tonetti T., Cressoni M., et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Medicine. 2016;42(10):1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 30.Bugedo G., Retamal J., Bruhn A. Driving pressure: A marker of severity, a safety limit, or a goal for mechanical ventilation? Critical Care. 2017;21(1):p. 199. doi: 10.1186/s13054-017-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murias G., de Haro C., Blanch L. Does this ventilated patient have asynchronies? Recognizing reverse triggering and entrainment at the bedside. Intensive Care Medicine. 2016;42(6):1058–1061. doi: 10.1007/s00134-015-4177-3. [DOI] [PubMed] [Google Scholar]
- 32.Akoumianaki E., Lyazidi A., Rey N., et al. Mechanical ventilation-induced reverse-triggered breaths. CHEST. 2013;143(4):927–938. doi: 10.1378/chest.12-1817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix 1. PRISMA Checklist.
Supplementary Appendix 2. Search strategy used for the literature review.
Supplementary Figure S1. Sensitivity analysis by excluding study by Chiu et al.
Supplementary Figure S2. Sensitivity analysis by excluding study by Raymondos et al.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The data used to support the findings of this study are available from the corresponding author upon request.