Abstract
Case series
Patient: Male, 66 • Male, 72 • Male, 70
Final Diagnosis: Pancreatic ductal adenocarcinoma
Symptoms: None
Medication: —
Clinical Procedure: —
Specialty: Diagnostics • Laboratory
Objective:
Educational purpose
Background:
Pancreatic ductal adenocarcinoma (PDAC) is a rapidly progressive malignancy that exhibits an extremely poor prognosis, with most cases diagnosed at an advanced stage. To date, few reports have explored the natural history of PDAC, and the period leading up to the detection of PDAC as a tumor with contrast-enhanced computed tomography (CECT) remains unclear. Here, we report 3 PDAC cases diagnosed incidentally by repeating imaging examinations during observation of other organ cancers.
Case Report:
Two patients were undergoing postoperative follow-up for colorectal cancer; owing to the elevation of serum CA19-9 or dilatation of the main pancreatic duct, both cases were finally diagnosed with PDAC. Another patient was administered neoadjuvant chemotherapy for a gastrointestinal stromal tumor; the fluorodeoxyglucose uptake in the pancreas with fluorodeoxyglucose positron emission tomography for the treatment assessment led to the diagnosis of PDAC. All patients underwent frequent CECT for assessment of other diseases, and PDAC became visible with CECT within 3–4 months of the appearance of indirect findings of PDAC.
Conclusions:
The period leading up to the detection of PDAC as a tumor with CECT was approximately 3–4 months. These cases suggest that additional imaging examinations should be performed when the indirect findings of PDAC are noted. This report adds value to the literature by elucidating the natural course of PDAC.
MeSH Keywords: Carcinoma, Pancreatic Ductal; Early Diagnosis; Multidetector Computed Tomography
Background
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies, and it has an increasing prevalence. Globally, pancreatic cancer is the seventh leading cause of cancer-related mortality [1]. Studies have reported that risk factors of PDAC include chronic pancreatitis, intraductal papillary mucinous neoplasm, drinking alcohol, and cigarette smoking [2–5]; however, early diagnosis of PDAC is challenging even for patients with risk factors [6]; to date, few reports have described the natural history of PDAC [7]. While ultrasounds (US), contrast-enhanced computed tomography (CECT), and magnetic resonance imaging (MRI) constitute the diagnostic modality for PDAC, endoscopic ultrasonography (EUS) has established itself as a useful modality for detecting small PDAC [8]. Nevertheless, it remains practically impossible to frequently perform these imaging examinations on all patients who do not have any PDAC risk factors. Most PDAC cases are diagnosed at an advanced stage, and it remains unclear how long it takes a tumor to grow to the point at which it becomes visible by diagnostic imaging. Here, we present 3 PDAC cases diagnosed incidentally with frequent imaging examinations during observation of other organ cancers.
Case Report
At our hospital, 7 patients were diagnosed with PDAC during or after chemotherapy for other malignancies between 2010 and 2018. Here, we present 3 of these cases for which CECT was performed often. All CT scans were performed on 64-channel multidetector CT scanners (Aquilion 64; Toshiba Medical Systems Corporation, Otawara, Japan). All CT images (slice thickness, 5 mm) of the equilibrium phase were obtained with a fixed delay of 180 s after administering the contrast injection.
Case 1
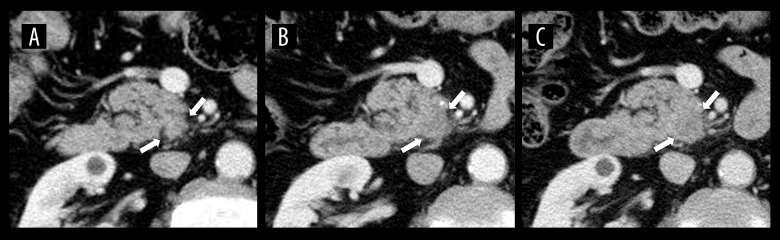
A 66-year-old man was under observation after adjuvant chemotherapy (FOLFOX; 5-fluorouracil, leucovorin, oxaliplatin, intravenously) following surgery for sigmoid colon cancer. As serum carbohydrate antigen (CA19-9) rose to 47 U/mL after 11 months of surgery, colon cancer recurrence was suspected, but colonoscopy revealed no local recurrence of colon cancer. Accordingly, CECT was performed, and it did not reveal recurrence of colon cancer or obvious abnormal findings of the pancreas (Figure 1A). A 3-month follow-up CECT scan retrospectively found a tiny, low-density area in the uncinate process of the pancreas (Figure 1B); however, it could not be confirmed as PDAC at that time. After another 3 months, serum CA19-9 rose to 787 U/mL, and CECT revealed that the low-density area had increased and become recognizable (Figure 1C). Thus, EUS-fine-needle aspiration (EUS-FNA) was performed, and the pathological findings of the specimens indicated well-differentiated adenocarcinoma. Accordingly, pancreaticoduodenectomy was performed, and the pathological diagnosis was invasive ductal adenocarcinoma of the pancreas, T2 N1 M0, stage IIB (Union for International Cancer Control, 8th edition). The patient died due to the PDAC recurrence 25 months postoperatively.
Figure 1.
CECT images of Case 1. (A) Axial CECT image obtained with 5-mm slice thickness 6 months before diagnosis of PDAC shows no abnormal findings in the pancreas. (B) Axial CECT image obtained 3 months before diagnosis shows a tiny low-density area in the uncinate process of the pancreas retrospectively. (C) Axial CECT image obtained at time of diagnosis revealed that the low-density area had increased and become recognizable.
Case 2
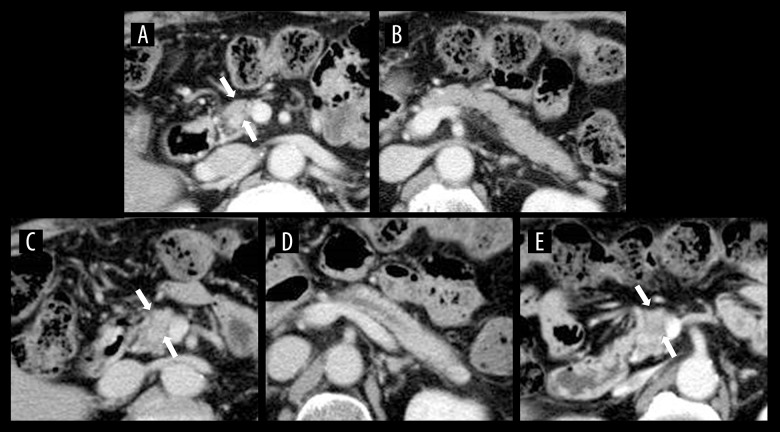
A 72-year-old man was followed up after adjuvant chemotherapy (FOLFOX) after removal of ascending colon cancer. Although CECT performed 18 months postoperatively revealed no tumor in the pancreas (Figure 2A, 2B), the one performed after 4 months revealed the dilatation of the main pancreatic duct, without a visible tumor (Figure 2C, 2D). EUS detected a low echoic mass in the pancreatic body, and we performed EUS-FNA. The pathological findings of the specimens showed moderately-differentiated adenocarcinoma. After 1 month, preoperative CECT revealed a low-density tumor in the pancreatic body (Figure 2E). Pancreaticoduodenectomy was performed, and the pathological diagnosis was invasive ductal adenocarcinoma of the pancreas, T1c N1 M0, stage IIB. The patient was alive without recurrence 15 months postoperatively.
Figure 2.
CECT images of Case 2. (A) Axial CECT image obtained 5 months before diagnosis showed no pancreatic tumors. (B) The main pancreatic duct was not dilatated. (C) Axial CECT image obtained 1 month before diagnosis showed no visible tumor in the pancreas. (D) CECT revealed dilatation of the main pancreatic duct. (E) Axial CECT image obtained preoperatively revealed a low-density mass in the pancreas.
Case 3
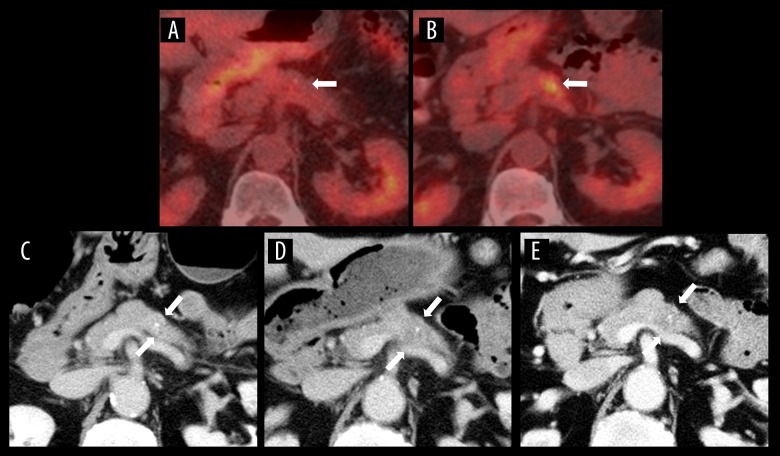
A 70-year-old man with a gastrointestinal stromal tumor (GIST) of the rectum underwent neoadjuvant chemotherapy (imatinib; orally administrated). Four months after treatment initiation, fluorodeoxyglucose positron emission tomography (FDG-PET) revealed mild-enhanced FDG uptake in the pancreatic body, which had not been noted before treatment (Figure 3A, 3B). However, CECT did not show a pancreatic tumor (Figure 3C). After 3 months, CECT revealed a slightly low-density area in the pancreatic body, but the tumor remained unclear (Figure 3D). Accordingly, a laparoscopic low anterior resection was performed for the rectal GIST. Three months postoperatively, a pancreatic tumor became clearly visible on CECT (Figure 3E). The pathological findings of the specimens obtained by EUS-FNA showed moderately-differentiated adenocarcinoma. The patient underwent distal pancreatectomy; the pathological diagnosis was invasive ductal adenocarcinoma of the pancreas, T4 N1 M0, stage III. As a left lung nodule appeared 3 years after the pancreatic surgery, a thoracoscopic left lower lobectomy was performed. Pathological findings indicated adenocarcinoma, and the lung tumor was diagnosed with metastasis of PDAC based on immunostaining results. Although he experienced recurrence of PDAC, he was alive after receiving chemotherapy.
Figure 3.
FDG-PET and CECT images of Case 3. (A) FDG-PET image obtained 10 months before diagnosis showed no abnormal FDG uptake in the pancreas. (B) The FDG-PET image obtained 6 months before diagnosis revealed FDG uptake in the pancreatic body. (C) Axial CECT image obtained at the same time as B showed no visible tumor in the pancreas. (D) Axial CECT image obtained 3 months before diagnosis showed a slightly low-density area in the pancreatic body. (E) Axial CECT image obtained at the time of diagnosis shows the pancreatic tumor has become clearly visible.
Discussion
Despite recent progress in diagnostic imaging, the early detection of PDAC remains challenging, and most PDAC cases are diagnosed at an advanced stage. Although US is recommended for PDAC screening, variations have been reported in the sensitivity and specificity among patients [9]. Reportedly, multi-detector-row CT enhances the sensitivity and specificity of the detection of small PDAC [10], but it remains inadequate, and a dynamic study of CT has been reported to be necessary for the PDAC diagnosis [11]; the diagnostic ability of MRI has been reported to be equivalent to that of CECT [12]. Compared to other diagnostic modalities, EUS is a useful modality for diagnosing PDAC, as it can detect PDAC with markedly higher sensitivity, with a pancreatic tumor detection rate of >90% [13,14]. In addition, EUS can effectively detect small PDAC [8]. However, EUS is not widely used in the general practice, and it is an invasive examination compared with other imaging methods [15]. Although FDG-PET reportedly has superior diagnostic for PDAC, the ability to detect small PDAC varies in the literature, and the assessment of early diagnosis of PDAC remains undetermined [16–18]. Owing to the limitation of diagnostic imaging, the efficacy of liquid biopsy has been reported for the early diagnosis of PDAC, although no established benefit exists at present [19].
In our cases, we did not perform a dynamic study of CT because CECT was performed for assessing other diseases. Although all patients underwent CECT every 3–4 months, confirming the pancreatic tumor at an early stage with nondynamic CECT study was challenging. In a previous report, the volume-doubling time of pancreatic cancer in CT was 132 days on average [7]; similar tumor growth times were assumed in our cases. According to previous reports [20,21], a history of nonpancreatic primary cancers increases the risk of subsequent PDAC, particularly in cases of colorectal cancer. As 2 of our cases had a history of colorectal cancer, we should have been more careful regarding the development of PDAC. Moreover, it has been reported that a history of other cancers is associated with a better prognosis of PDAC, and the intensive surveillance of a prior cancer may contribute to the early detection of PADC [21]. Although we could detect PDAC without distant metastasis by repeating ability CECT, early detection was impossible. In our cases, we should have investigated earlier by combining imaging examinations, including EUS, because we found indirect findings, such as the elevation of the tumor marker, the dilatation of the main pancreatic duct, and the enhanced FDG uptake. In these cases, PDAC became visible as a tumor with CECT after 3–4 months from the appearance of indirect findings, and we should not have missed the timing of additional imaging examinations when the indirect findings of PDAC were noted.
Conclusions
In our cases, the period leading up to the detection of PDAC as a tumor with CECT was considered to be approximately 3–4 months. There are few reports that describe the natural history of PDAC, and no report has assessed the period until the discovery of PDAC by performing frequent CECT. This is a suggestive report following the natural course of PDAC.
Footnotes
Conflict of interest
None.
References:
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: A analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4) Ann Oncol. 2012;23:1880–88. doi: 10.1093/annonc/mdr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381:269–77. doi: 10.1016/j.canlet.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; Aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–58. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: A multicenter study in Japan. Pancreas. 2011;40:364–70. doi: 10.1097/MPA.0b013e31820a5975. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Chen S, Zeng L, et al. New developments in the early diagnosis of pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2017;11:149–56. doi: 10.1080/17474124.2017.1271323. [DOI] [PubMed] [Google Scholar]
- 7.Ahn SJ, Choi SJ, Kim HS. Time to progression of pancreatic cancer: Evaluation with multi-detector computed tomography. J Gastrointest Cancer. 2017;48:164–69. doi: 10.1007/s12029-016-9876-7. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda I, Iwashita T, Doi S, et al. Role of EUS in the early detection of small pancreatic cancer. Dig Endosc. 2011;23:22–25. doi: 10.1111/j.1443-1661.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakaizumi A, Tatsuta M, Uehara H, et al. A prospective trial for early detection of pancreatic cancer by ultrasonographic examination combined with measurement of serum elastase 1. Cancer. 1992;69:936–40. doi: 10.1002/1097-0142(19920215)69:4<936::aid-cncr2820690417>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Bronstein YL, Loyer EM, Kaur H, et al. Detection of small pancreatic tumors with multiphasic helical CT. Am J Roentgenol. 2004;182:619–23. doi: 10.2214/ajr.182.3.1820619. [DOI] [PubMed] [Google Scholar]
- 11.Bakkevold KE, Arnesjø B, Kambestad B. Carcinoma of the pancreas and papilla of Vater: Presenting symptoms, signs, and diagnosis related to stage and tumour site. A prospective multicentre trial in 472 patients. Norwegian Pancreatic Cancer Trial. Scand J Gastroenterol. 1992;27:317–25. doi: 10.3109/00365529209000081. [DOI] [PubMed] [Google Scholar]
- 12.Koelblinger C, Ba-Ssalamah A, Goetzinger P, et al. Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: Prospective evaluation in patients suspected of having pancreatic cancer. Radiology. 2011;259:757–66. doi: 10.1148/radiol.11101189. [DOI] [PubMed] [Google Scholar]
- 13.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–63. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22–29. doi: 10.1055/s-0033-1353603. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen MB, Fristrup C, Holm FS, et al. Prospective evaluation of patient tolerability, satisfaction with patient information, and complications in endoscopic ultrasonography. Endoscopy. 2005;37:146–53. doi: 10.1055/s-2005-861142. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto I, Shirakawa S, Shinzeki M, et al. 18-Fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:712–18. doi: 10.1016/j.cgh.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 17.Strobel K, Heinrich S, Bhure U, et al. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med. 2008;49:1408–13. doi: 10.2967/jnumed.108.051466. [DOI] [PubMed] [Google Scholar]
- 18.Okano K, Kakinoki K, Akamoto S, et al. 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of small pancreatic cancer. World J Gastroenterol. 2011;17:231–35. doi: 10.3748/wjg.v17.i2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Zheng Y, Sun H, et al. K-Ras mutation detection in liquid biopsy and tumor tissue as prognostic biomarker in patients with pancreatic cancer: A systematic review with meta-analysis. Med Oncol. 2016;33:61. doi: 10.1007/s12032-016-0777-1. [DOI] [PubMed] [Google Scholar]
- 20.Amin S, McBride RB, Kline JK, et al. Incidence of subsequent pancreatic adenocarcinoma in patients with a history of nonpancreatic primary cancers. Cancer. 2012;118:1244–51. doi: 10.1002/cncr.26414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Li Y, Su T, et al. The impact of a history of cancer on pancreatic ductal adenocarcinoma survival. United European Gastroenterol J. 2018;6:888–94. doi: 10.1177/2050640618765505. [DOI] [PMC free article] [PubMed] [Google Scholar]