Abstract
The surgical treatment of Fuchs endothelial corneal dystrophy (FECD) has advanced dramatically over the last two decades. Penetrating keratoplasty has been superseded by various iterations of endothelial keratoplasty, and currently, surgical removal of host Descemet membrane without keratoplasty is being investigated. These surgical advances have been accompanied by significant improvement of our understanding of the underlying disease mechanisms, not least the discovery that FECD in western populations is predominantly an intronic trinucleotide repeat expansion disorder in the transcription factor 4 gene that results in RNA toxicity and mis-splicing. Understanding the disease mechanisms augurs well for developing targeted molecular medical therapies, which will require careful clinical investigation through trials to prove their efficacy and safety. As the field advances towards clinical trials, investigators should carefully define the disease state being treated and consider the options for outcome measures relevant to the type of intervention. FECD, and the outcomes of interventions to treat the disease, can be measured in terms of corneal morphology, corneal function and clinical impact. Standardising the approach for defining FECD and careful thought about the outcomes of intervention that are reported will help make the results of future trials for FECD applicable in clinical practice.
Keywords: Fuchs endothelial corneal dystrophy, clinical trials, outcome measures, corneal imaging
TOWARDS CLINICAL TRIALS
Ernst Fuchs described Dystrophia Epithelialis Corneae in 1910 at which time he reported on scarred and vascularised corneas of 13 patients.1 Without the aid of a slit-lamp, the changes he noticed were in the anterior cornea, and he presumed these changes were secondary to dysfunction of the endothelial cell layer. He originally discussed the condition in the 1902 Bowman Lecture, at which time he suggested that the endothelial layer may be dysfunctional and result in corneal oedema.2 Guttae were not described until 1916,3 after the introduction of the slit-lamp in 19114; the presence of guttae by slit-lamp examination remains the diagnostic feature of Fuchs endothelial corneal dystrophy (FECD) to this day.
It took almost a century to make significant advances in the management and understanding of FECD, and these advances have been dramatic over the last two decades now resulting in converging modalities of treatment from surgical and scientific directions.5 6 The likelihood of evaluating potential new treatments for FECD in clinical trials in the near future is real, and thus it is important to be able to define the state of the disease being treated and the outcomes of intervention. This review will briefly discuss the background of surgical and scientific changes in the management of FECD, and consider various methods of evaluating FECD for its classification and the outcomes of intervention.
Surgical advances
Penetrating keratoplasty (PK) was the surgical procedure of choice for FECD for many decades. Based on the outcomes of PK, including limitations in vision from high and irregular astigmatism, and anisometropia, intervention for FECD was reserved for advanced disease when corneas were obviously oedematous and accompanied by impaired vision or even pain.4 7 The risk of spontaneous or traumatic wound dehiscence could also result in loss of the eye because of the large incision, and ocular surface healing and suture-related complications were not uncommon. The evolution of surgical techniques for FECD from large incision PK to small incision endothelial keratoplasty (EK)5 has been a major factor in advancing therapeutic options for FECD. EK has proven successful because of improved uncorrected and best-corrected visual outcomes without detrimentally affecting graft survival,8–10 that is, EK has provided long-term value in terms of surgical outcomes. The absence of large incisions and sutures has also reduced devastating complications. The success and safety of EK has therefore resulted in a lowering of the threshold of intervention11 for FECD compared with when PK was the treatment of choice, akin to how small incision phacoemulsification lowered the threshold for intervening for cataract compared with large incision extracapsular cataract extraction.
Modifying surgical techniques is essentially unregulated, allowing surgeons to pioneer and refine procedures based on their clinical outcomes.12–14 For FECD, EK did not surpass PK because of the results of any carefully designed randomised controlled trial; instead, EK was adopted based on the results of retrospective, uncontrolled clinical series.15 16 Although randomised trials were attempted,17 the field evolved too rapidly for a specific trial to maintain relevance by the time results could be shared. Even though the outcomes of different retrospective series of EK have been convincing for surgeons to adopt the new techniques, the exact state of FECD being treated in each study was typically not well defined, with the inclusion criterion often broadly referred to as ‘Fuchs endothelial corneal dystrophy’ or ‘endothelial keratoplasty candidates’ or similar.18–20 This was not important at the time, but as new comparative studies are undertaken,21 potentially at earlier stages of FECD, there needs to be better definition of what is being treated to reduce heterogeneity, make meaningful interpretation of the results, and to apply the knowledge in clinical practice appropriately. Some EK series have provided more details about FECD severity,10 11 and with some investigators presently evaluating outcomes of newer surgical advances (stripping of the host Descemet membrane without replacement by a graft) often with adjuvant pharmacological therapies, the inclusion criteria have also become more detailed.22 23 Nevertheless, many of the methods used to define the treated state of FECD have limitations (see below) and could be improved.
Improved understanding of FECD disease mechanisms
At the same time that surgical treatments of FECD have advanced, our understanding of the basic pathophysiological mechanisms of the disease has rapidly improved too. The discovery of the major genetic association of FECD in western populations in 2010,24 an intronic CTG-trinucleotide repeat expansion in the transcription factor 4 gene,25 has spurred new research to elucidate the downstream effects, which now appear to be mediated, at least in part, through RNA toxicity and mis-splicing events.26 This has led to the possibility of halting or slowing the progression of the disease by using targeted molecular therapies, such as to block RNA toxicity.27 The recognition of other downstream mechanisms in FECD, including repeat associated non-ATG translation,28 the unfolded protein response29 and oxidative stress,30 may similarly enable novel targeted therapies. The ability to culture human corneal endothelial cells ex vivo31 has already led to promising clinical trials of cultured cell injection therapy, merging advances in basic science with advances in surgical approaches.31 32 In addition, rho-kinase inhibitors have been used as adjuvant agents in culture media of cells for injection therapy,32 as well as topically for promoting endothelial healing after surgical stripping of Descemet membrane without concomitant keratoplasty.23 In contrast to how surgical techniques have evolved, advancing novel non-surgical therapies requires careful scrutiny by ethics, funding and regulatory agencies to demonstrate safety and efficacy in trials. Again, this will require careful definition of the FECD disease state being treated with appropriate measures to determine the outcome of interventions.
CLASSIFICATION OF FECD
FECD spans a wide range of severity from mild, asymptomatic and inconsequential disease (previously sometimes referred to as cornea guttata4), to severe disease with pain, scarring, vascularisation and loss of vision requiring surgical intervention. It is therefore important to define what state of the disease is being treated in interventional studies. The onset of corneal oedema is gradual,33 34 occurs early in the course of FECD before it can be detected by slit-lamp examination (subclinical oedema),35 and can remain in a chronic subclinical state before becoming manifest. This chronic state of subclinical oedema results in corneal structural changes that can persist, and might affect vision, even after restoring endothelial function by EK.36–39 These changes, notably subepithelial fibrosis,37 40 are more severe after subepithelial bullae develop. To broadly include all categories of FECD in a clinical trial is therefore not appropriate, because intervention is not required in some cases of FECD, and because the outcomes of an intervention are likely to differ based on the extent and chronicity of oedema, and possibly the distribution (area and confluence) of guttae.
Clinical staging
Several methods exist to classify FECD for clinical practice and research purposes. Adamis and colleagues described four clinical stages of FECD that aligned well with clinical decision-making in the era of PK, typically with intervention occurring when eyes reached stage 3, at which time oedema was visible and caused significant pain or impairment in vision.7 As EK has become the standard of care surgical treatment for FECD, the threshold for intervention has decreased enabling patients with milder symptoms to be treated earlier (well before stage 3) and with excellent outcomes.5 The staging proposed by Adamis and colleagues was based on patient symptoms as well as clinical signs; it assumed that stage 1 was asymptomatic (which is not always the case) and did not consider subclinical oedema, and thus is of little assistance to help determine when to proceed to EK. Most patients with FECD now receive EK during stages 1 and 2 of this classification when subclinical oedema may be present and sufficient to warrant intervention.
The severity of FECD is often graded clinically based on the extent and confluence of guttae, and the presence of corneal oedema. Krachmer and colleagues described five grades of disease, with the most advanced stage including corneal oedema.41 This scale implies that corneal oedema can only be present if central confluent guttae occupy an area >5 mm in widest diameter. A modified grading scale added a sixth grade of disease and suggested (somewhat unclearly in the text vs table) that oedema be reported separately to the grade, acknowledging that oedema is sometimes clinically visible with <5 mm of confluent guttae being present.42 Both of these grading scales provide morphological details of FECD, specifically the distribution of guttae, but they do not adequately address corneal endothelial function, or changes in function, by distinguishing between subclinical and clinically detectable oedema.35 Furthermore, these scales are based on subjective clinical assessment, and can result in cornea specialists agreeing less than 50% of the time.34 Photographic grading of guttae distribution is a more objective assessment of the distribution and extent of guttae,43 44 but this method is not simple to standardise within and between clinical practices, and does not help to define the presence or severity of corneal oedema.
Ancillary testing
Clinicians frequently obtain ancillary testing in their evaluation of FECD, especially central corneal thickness (CCT) and endothelial cell density, to help with clinical-decision making in FECD. However, neither test helps to determine the severity of FECD, although changes in CCT can identify clinical progression and response to intervention. Isolated measurements of CCT in FECD are unhelpful because the range of CCT in normal corneas is wide45 and typically overlaps with CCT in FECD with no, subclinical, or clinically definite oedema.35 46 Although corneas with FECD can be much thicker than normal, these corneas usually also have clinically obvious oedema at slit-lamp examination, indicating the need for surgical treatment without CCT measurement. Therefore, proposed cut-off values of CCT to aid in clinical decision-making of when to intervene are of little importance and should not be considered,47 48 as this could erroneously lead to surgical intervention for some patients with naturally thick corneas, or lack of intervention for many patients with subclinical but significant oedema and thickness in an otherwise normal range.35
Similarly, endothelial imaging has limited, if any, role in determining the severity of FECD because of the presence of guttae,49 and should not be used for clinical-decision making.50 When guttae are present, not all endothelial cells are visible for analysis (when guttae are confluent, no cells are visible for analysis), and quantifying cell density requires a specific analysis method that makes assumptions by accounting for the image area occupied by guttae.49 Furthermore, there can be significant regional variation in the distribution of guttae such that cell density measurements of the same cornea can vary widely depending on location and the method of measurement (figure 1). Ultimately, endothelial cell density does not always equate to endothelial function.
Figure 1.
Endothelial photograph of a cornea with Fuchs endothelial corneal dystrophy with irregularly distributed guttae. Measuring endothelial cell density in the presence of guttae is inaccurate and unhelpful, because guttae obscure cells that may be overlying them and regional variations in guttae can lead to vastly different endothelial cell densities of the same cornea.49
Revised classification
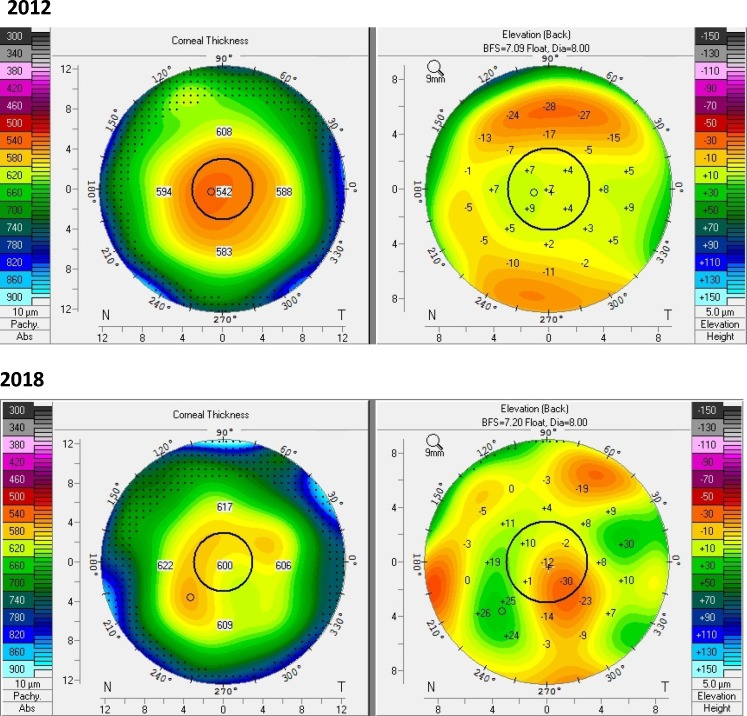
A revised and simplified classification of FECD incorporating Scheimpflug tomography (table 1) may be more objective and more clinically relevant as an indirect functional assessment of the corneal endothelium, especially in the current era of EK and with newer treatments on the horizon.35 The diagnosis of FECD (presence of guttae) and the presence of clinically definite oedema are made by slit-lamp examination. Slit-lamp examination should rule out other corneal pathologies that might interfere with tomographic analysis. Tomographic analysis is performed when oedema is not clinically visible to assess for three features in the pachymetry map and posterior float patterns: irregular isopachs, displacement of the thinnest point of the cornea, and posterior surface depression towards the anterior chamber (figure 2). The classification is independent of CCT, easily obtained in most cornea clinical practices, and of high practical relevance to general ophthalmologists who may be the first to evaluate symptomatic patients with FECD or when needing to determine the surgical approach in the setting of a cataract. The presence of two or three tomographic features of subclinical oedema has been associated with a several fold-increased risk of disease progression over a median of 4 years compared with when one or no tomographic features are present (Patel SV et al; Prognosis of eyes with FECD based on Scheimpflug tomography as part of a revised classification; ARVO E-Abstract #2223, 2019) (figure 3). Further analyses are needed to quantify the tomographic patterns, disease progression and sensitivity in assessing response to intervention. Nevertheless, this classification method may be the most relevant to date for clinical practice and clinical research.
Table 1.
Revised classification of corneas with Fuchs endothelial corneal dystrophy (FECD) (reproduced from Sun et al with permission from Elsevier35)
| Classification*† | Required findings | |
| Slit-lamp examination | Scheimpflug tomography‡ | |
| FECD with clinically definite oedema | Guttae present and typically confluent§; clinically visible corneal oedema¶ present | Not required; classification made by slit-lamp examination alone If obtained, tomography would typically show all three features of corneal oedema,** possibly with focal anterior elevation associated with any area of focal posterior depression |
| FECD with subclinical oedema | Guttae present and typically confluent,§ without clinically definite oedema | Required Shows the features of corneal oedema**; frequently all three features are present, and typically at least two features are present |
| FECD without oedema | Guttae present and could be non-confluent or confluent§ without clinically definite oedema | Required Shows no features consistent with corneal oedema, that is, appears consistent with a normal cornea |
| No FECD | No guttae | Not required |
*Slit-lamp examination is required first to diagnose FECD by the presence of guttae and to determine if clinically definite edema is present. Tomography is only required for FECD without clinically definite edema.
†This classification is independent of central corneal thickness, traditional morphologic grading, and patients’ visual dysfunction; however, the afore-mentioned characteristics may be considered as adjunctive information.
‡Assessment of the pachymetry and posterior corneal elevation maps (Pentacam HR; Oculus, Lynnwood, Washington, USA), typically found in the ‘4-Maps Refractive’ display.
§Authors recommend that confluence be confirmed by specular reflection at slit-lamp examination; visible cells between guttae by this method indicates non-confluent guttae in that region of examination.
¶Clinically definite oedema is oedema that is obviously visible by slit-lamp examination based on thickening of the stroma (with a visible change in corneal contour of the anterior or posterior surface), Descemet or deep stromal folds, microcystic epithelial oedema or bedewing, or subepithelial bullae. The specific finding should be documented to support this classification.
**Specific features of tomographic corneal oedema are (1) loss of parallel isopachs, (2) displacement of the thinnest point of the cornea and (3) presence of focal posterior corneal depression.
Figure 2.
Pachymetry (left) and posterior float (right) maps, derived from Scheimpflug tomography, of the same eye with Fuchs endothelial corneal dystrophy before and after Descemet membrane endothelial keratoplasty (DMEK). Subclinical oedema can be detected by the presence of irregular isopachs, displacement of the thinnest point of the cornea, and posterior float depression (towards the anterior chamber).35 After DMEK, these changes have resolved resulting in normal pachymetry and posterior float maps.
Figure 3.
Pachymetry (left) and posterior float (right) maps, derived from Scheimpflug tomography, of the same eye with Fuchs endothelial corneal dystrophy 6 years apart. In 2012, there was subtle nasal displacement of the thinnest point of the cornea with mildly irregular isopachs suggesting oedema was present inferotemporal to the centre of the cornea; there was no posterior float depression. In 2018, the thinnest point of the cornea was obviously displaced with profoundly irregular isopachs; obvious posterior float depression was present inferotemporal to the centre of the cornea, indicating progression of the disease.
Genetic classification of FECD will also be important for future clinical trials, either as an inclusion criterion, depending on the type of intervention, or for understanding the response to an intervention. Although approximately 75% of FECD patients in western populations have a trinucleotide repeat expansion disorder,25 the genetic basis of the other 25% of FECD patients remains poorly characterised at this time.6 In addition, the clinical disease state at the time of intervention can be variable even for the same underlying genetic abnormality, and thus clinical classification of FECD is critical.
OUTCOME MEASURES
Future trials of interventions for FECD will require meaningful outcome measures, and the measures used will vary according to the nature of the specific intervention, for example, whether a graft is performed or not, whether central Descemet membrane and guttae are removed or not and so on. Regardless of the intervention, outcomes should include measures of corneal morphology, corneal function, and most importantly, clinical impact (table 2). There should also be demonstration of a sustained effect over time to help assess long-term value of any specific intervention.
Table 2.
Recommended measurements of Fuchs endothelial corneal dystrophy for clinical trials. Before intervention, some parameters help classify the disease state, and some are necessary to compare to post-intervention measures. Not all parameters may be necessary to measure, depending on the type of intervention.
| Before intervention (Classifying the disease state) |
After intervention (Outcome measures) |
|
| Anatomy (endothelial morphology) |
Guttae distribution* Peripheral ECD |
Central ECD† or Guttae distribution* Peripheral ECD |
| Physiology (endothelial function) |
Central corneal thickness‡ Tomography maps§ Corneal backscatter¶ |
Central corneal thickness‡ Tomography maps§ Corneal backscatter¶ |
| Clinical impact | Best-corrected visual acuity Visual disability (PRO)** Other domains of vision†† |
Best-corrected visual acuity Visual disability (PRO)** Other domains of vision†† |
*Guttae distribution can include objective grading of the confluency and area of guttae from endothelial images (ideally if retaining host Descemet membrane) or subjective clinical grading (reasonable if host Descemet membrane will be removed). It is unknown if guttae distribution improves with any interventions that will retain host Descemet membrane at this time.
†Central ECD can be measured post-intervention if host endothelium has been removed (whether replaced with donor endothelium or not), but has limited role if guttae are present (pre-intervention or post-intervention).
‡Central corneal thickness does not classify the disease state, but changes after intervention are important for assessing corneal function.
§Pachymetry and posterior float maps derived from Scheimpflug tomography.35
¶Corneal backscatter can be derived from Scheimpflug tomography39 69 and measurements should be standardised.71 The role of backscatter for disease classification is uncertain at present, but changes post-intervention can be indicative of changes in corneal function.
**The Visual Function and Corneal Health Status instrument has been validated for FECD.77
††Consider low-contract visual acuity and disability glare (straylight).
ECD, endothelial cell density; PRO, patient-reported outcome.
Corneal morphology
Endothelial cell density is a frequent outcome measure of corneal endothelial studies,51–53 often being considered as a surrogate for corneal endothelial function. Although lower endothelial cell density is usually associated with worse endothelial cell function, this is not always the case. Nevertheless, central endothelial cell density should continue to be measured, though results should be interpreted cautiously if guttae are still present post-intervention (ie, if Descemet membrane has not been surgically removed), as these will prevent accurate analyses (figure 1).49 It is unknown if any treatments will result in the resolution of guttae (without excising host Descemet membrane) at this time; endothelial imaging might therefore be helpful to assess the confluency and area occupied by guttae before and after intervention. The latter will require sampling multiple regions of cornea because of regional variation of guttae49; retroillumination photography may have a role too.43 44
Peripheral endothelial cell density analysis54 should also be considered depending on the type of intervention, but especially when migration of cells is expected from the periphery to the centre of the cornea.22 55 Peripheral endothelial cell density can be measured accurately preoperatively because these regions are usually devoid of guttae. Sustained health of the peripheral corneal endothelium in FECD is important, as poor peripheral endothelial cell reserve is associated with an increased risk of graft failure56–58 (it is unknown if this can be extrapolated to failure of non-graft interventions at this time). Changes in endothelial cell morphology, specifically a decrease in the percentage of hexagonal cells and an increase in the coefficient of variation of cell size, have provided limited information in long-term studies of corneal transplantation.53 Such changes have been associated with hypoxia in the setting of contact lens wear,59 and metabolic changes in the setting of diabetes,60 61 but it is unclear if they might be useful parameters to measure in studies of endothelial cell rejuvenation. All studies measuring corneal endothelial cell density and morphology require careful calibration of the specular or confocal microscope, and a consistent method of cell analysis that measures an adequate number of cells.62–64
Corneal function
Ultimately, all interventions for FECD should improve corneal endothelial function. The simplest indirect measurement of corneal endothelial function is CCT. A decrease in CCT after an intervention indicates improved endothelial function, assuming tissue has not been added or removed as part of the intervention. Increasing CCT after reaching steady-state after an intervention can be an excellent indicator of declining endothelial function with time,65 irrespective of the intervention. CCT needs to be measured in a consistent manner, using the same instrument and ideally at the same location of the cornea; precision and accuracy vary between instruments.66 Changes in pachymetry map and posterior float patterns derived from tomography might also provide useful information related to endothelial function.35 These could indicate changes in the paracentral, and not just central, cornea that may be relevant depending on the type of intervention. Normalisation of these maps is definitely seen after Descemet membrane endothelial keratoplasty (figure 2),35 and progression of FECD over the longer term can also be found (figure 3). Whether or not small changes in thickness that reflect subtle changes in endothelial function can be reliably detected with this method is uncertain at this time. In addition, detecting changes after Descemet stripping endothelial keratoplasty might be confounded by the irregular posterior surface shape67 at steady-state.
True endothelial function studies involve measuring the percentage of recovery per hour of corneal thickness after induced swelling of the cornea.68 69 These studies take hours to complete and will not be convenient for trials involving large numbers of subjects. Corneal backscatter derived from Scheimpflug tomography is associated with true corneal endothelial function,69 although not highly predictive by itself, in part because the backscatter signal from corneal oedema is mixed with the backscatter signal from more permanent corneal structural changes.37 40 Determining backscatter accurately and prospectively requires standardisation measurements to account for variations in the light source intensity and camera sensitivity of the instrument.70 71 Nevertheless, changes in backscatter before and after an intervention can be indicative of changes in corneal function, and is easily measured with Scheimpflug tomography. Combining tomographic backscatter with information from tomographic pachymetry map and posterior float patterns should be investigated further as a composite indirect measure of endothelial function.
Clinical impact
Ideally, vision would be the primary outcome of all interventional trials for FECD because most patients will be seeking improved vision, and most treatments will be indicated to treat impaired vision (rather than pain). Measuring visual outcomes in the PK era was not easy because of variability in refraction caused by high and irregular astigmatism, and the need to account for the presence of any remaining sutures at the primary endpoint. EK advanced in part because surgeons pursued better vision outcomes, and although best-corrected visual acuity (BCVA) should be easier to measure after EK compared with after PK, few EK studies have measured visual acuity in a standardised and rigorous manner typically expected in research studies.72 Standardised measurement of high-contrast BCVA should be one vision outcome of any new treatment for FECD; other facets of vision could be measured too, such as low-contrast visual acuity or disability glare.10 40 73 74
Perhaps the most important assessment of visual function after any intervention for any disease is that reported by the patient. Similar to measuring patient-reported outcomes after cataract surgery,75 76 interventions for FECD should also measure the impact on visual disability and quality of life, as these improvements might not always be reflected by changes in visual acuity. The Visual Function and Corneal Health Status (V-FUCHS) instrument has been validated for assessing visual disability across a range of severity of FECD.77 The instrument determines Rasch-based scores for two dimensions, visual acuity and glare, based on 15 self-administered or interviewer-administered questions. Patient-reported outcome measures related to vision should factor the status of the fellow-eye and any comorbidities affecting vision. Formal testing of V-FUCHS before and after intervention has yet to be undertaken.
Graft survival
Historically, the classic outcome measure for corneal transplant surgery has been graft survival, which is determined by the converse, graft failure.9 53 78 However, graft survival, or failure, is not ideal as an isolated outcome measure because patients receiving an intervention, and clinicians administering an intervention, are usually seeking better vision (rather than longevity of a graft that does not confer improvement in vision).79 Ideally, an intervention for a disease would confer improved function that is sustained over a long period of time. Furthermore, the definition of graft survival has varied between large studies, although sometimes to vaguely incorporate an accompanying detriment in vision.53 78 80–82 While graft survival should certainly continue to be an important outcome of interventions that involve a graft, it should not be the sole outcome measure and will not be relevant to interventions that do not involve a graft.
Recommendations
Several methods exist for assessing corneas with FECD. Tomographic pachymetry and posterior float maps provide a simple and relevant classification for clinical practice and research purposes (table 1). Genetic classification of FECD might prove to be important for understanding the response to certain interventions too. The disease state can also be characterised in terms of corneal morphology, corneal function and clinical impact, enabling the same or similar outcomes to be measured after intervention to determine efficacy (table 2). Although clinical impact outcomes are most important for patient care, corneal parameters should be measured to show the specific effect of interventions on the cornea; this is important when clinical outcomes might be confounded by concomitant treatments, such as concurrent cataract surgery. A standardised approach by investigators for defining the disease state being treated and careful thought about the outcomes reported will help to make the results of future trials for FECD applicable in clinical practice.
Footnotes
Presented at: Presented in part at the 21st Bowman Club Annual Meeting, London, UK, 29 March 2019.
Funding: Supported by Mayo Foundation.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fuchs E. Dystrophia epithelialis corneae. Graefes Arch Klin Exp Ophthalmol 1910:76478–508. [Google Scholar]
- 2.Fuchs E. On keratitis, being the Bowman lecture. Trans Ophthalmol Soc U K 1902:2215–34. [Google Scholar]
- 3.Koeppe L. Klinische Beobachtungen MIT Der Nernstspaltlampe und dem Hornhautmikroskop. Graefes Arch Klin Exp Ophthalmol 1916:91363–79. [Google Scholar]
- 4.Wilson SE, Bourne WM. Fuchs?? dystrophy. Cornea 1988;7:2???18–18. 10.1097/00003226-198801000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Patel SV. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Experimental Eye Research 2012;95:40–7. 10.1016/j.exer.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wacker K, Baratz K, Fautsch M, et al. . Medical and semi-surgical treatments for Fuchs endothelial corneal dystrophy. Klin Monatsbl Augenheilkd 2018;235:709–13. 10.1055/a-0577-7953 [DOI] [PubMed] [Google Scholar]
- 7.Adamis AP, Filatov V, Tripathi BJ, et al. . Fuchs' endothelial dystrophy of the cornea. Survey of Ophthalmology 1993;38:149–68. 10.1016/0039-6257(93)90099-S [DOI] [PubMed] [Google Scholar]
- 8.Price MO, Calhoun P, Kollman C, et al. . Descemet stripping endothelial keratoplasty: ten-year endothelial cell loss compared with penetrating keratoplasty. Ophthalmology 2016;123:1421–7. 10.1016/j.ophtha.2016.03.011 [DOI] [PubMed] [Google Scholar]
- 9.Rosenwasser GO, Szczotka-Flynn LB, Ayala AR, et al. . Effect of cornea preservation time on success of Descemet stripping automated endothelial keratoplasty. JAMA Ophthalmology 2017;135:1401–9. 10.1001/jamaophthalmol.2017.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wacker K, Baratz KH, Maguire LJ, et al. . Descemet stripping endothelial keratoplasty for Fuchs' endothelial corneal dystrophy: five-year results of a prospective study. Ophthalmology 2016;123:154–60. 10.1016/j.ophtha.2015.09.023 [DOI] [PubMed] [Google Scholar]
- 11.Lass JH, Szczotka-Flynn LB, Ayala AR, et al. . Cornea preservation time study: methods and potential impact on the cornea donor pool in the United States. Cornea 2015;34:601–8. 10.1097/ICO.0000000000000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melles GRJ, Eggink FAGJ, Lander F, et al. . A surgical technique for posterior lamellar keratoplasty. Cornea 1998;17:618–26. 10.1097/00003226-199811000-00010 [DOI] [PubMed] [Google Scholar]
- 13.Melles GRJ, Wijdh RHJ, Nieuwendaal CP. A technique to excise the Descemet membrane from a recipient cornea (descemetorhexis). Cornea 2004;23:286–8. 10.1097/00003226-200404000-00011 [DOI] [PubMed] [Google Scholar]
- 14.Melles GR. Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea 2006;25:879–81. [DOI] [PubMed] [Google Scholar]
- 15.Price FW, Price MO. Descemet's stripping with endothelial keratoplasty in 200 eyes: early challenges and techniques to enhance donor adherence. J Cataract Refract Surg 2006;32:411–8. [DOI] [PubMed] [Google Scholar]
- 16.Chen ES, Terry MA, Shamie N, et al. . Descemet-stripping automated endothelial keratoplasty: six-month results in a prospective study of 100 eyes. Cornea 2008;27:514–20. 10.1097/ICO.0b013e3181611c50 [DOI] [PubMed] [Google Scholar]
- 17.Patel SV, McLaren JW, Hodge DO, et al. . Scattered light and visual function in a randomized trial of deep lamellar endothelial keratoplasty and penetrating keratoplasty. American Journal of Ophthalmology 2008;145:97–105. 10.1016/j.ajo.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 18.JY L, Terry MA, Goshe J, et al. . Three-year visual acuity outcomes after Descemet's stripping automated endothelial keratoplasty. Ophthalmology 2012;119:1126–9. [DOI] [PubMed] [Google Scholar]
- 19.Price MO, Giebel AW, Fairchild KM, et al. . Descemet's membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009;116:2361–8. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Calvo-de-Mora M, Quilendrino R, Ham L, et al. . Clinical outcome of 500 consecutive cases undergoing Descemet's membrane endothelial keratoplasty. Ophthalmology 2015;122:464–70. 10.1016/j.ophtha.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain W, Lin CC, Austin A, et al. . Descemet endothelial thickness comparison trial: a randomized trial comparing ultrathin Descemet stripping automated endothelial keratoplasty with Descemet membrane endothelial keratoplasty. Ophthalmology 2019;126:19–26. [DOI] [PubMed] [Google Scholar]
- 22.Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea 2016;35:1267–73. 10.1097/ICO.0000000000000915 [DOI] [PubMed] [Google Scholar]
- 23.Moloney G, Petsoglou C, Ball M, et al. . Descemetorhexis without grafting for Fuchs endothelial Dystrophy—Supplementation with topical Ripasudil. Cornea 2017;36:642–8. 10.1097/ICO.0000000000001209 [DOI] [PubMed] [Google Scholar]
- 24.Baratz KH, Tosakulwong N, Ryu E, et al. . E2-2 protein and Fuchs's corneal dystrophy. N Engl J Med 2010;363:1016–24. 10.1056/NEJMoa1007064 [DOI] [PubMed] [Google Scholar]
- 25.Wieben ED, Aleff RA, Tosakulwong N, et al. . A common trinucleotide repeat expansion within the transcription factor 4 (Tcf4, E2-2) gene predicts Fuchs corneal dystrophy. PLoS ONE 2012;7:e49083 10.1371/journal.pone.0049083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieben ED, Aleff RA, Tang X, et al. . Trinucleotide repeat expansion in the transcription factor 4 (TCF4) gene leads to widespread mRNA splicing changes in Fuchs' endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 2017;58:343–52. 10.1167/iovs.16-20900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarouchlioti C, Sanchez-Pintado B, Hafford Tear NJ, et al. . Antisense therapy for a common corneal dystrophy ameliorates Tcf4 repeat Expansion-Mediated toxicity. The American Journal of Human Genetics 2018;102:528–39. 10.1016/j.ajhg.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soragni E, Petrosyan L, Rinkoski TA, et al. . Repeat-associated non-ATG (Ran) translation in Fuchs' endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2018;59:1888–96. 10.1167/iovs.17-23265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engler C, Kelliher C, Spitze AR, et al. . Unfolded protein response in Fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? American Journal of Ophthalmology 2010;149:194–202. 10.1016/j.ajo.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurkunas UV. Fuchs endothelial corneal dystrophy through the prism of oxidative stress. Cornea 2018;37(Suppl 1):S50–S54. 10.1097/ICO.0000000000001775 [DOI] [PubMed] [Google Scholar]
- 31.Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest. Ophthalmol. Vis. Sci. 2004;45:1743–51. 10.1167/iovs.03-0814 [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita S, Koizumi N, Ueno M, et al. . Injection of cultured cells with a rock inhibitor for bullous keratopathy. N Engl J Med 2018;378:995–1003. 10.1056/NEJMoa1712770 [DOI] [PubMed] [Google Scholar]
- 33.Kopplin LJ, Przepyszny K, Schmotzer B, et al. . Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Archives of Ophthalmology 2012;130:433–9. 10.1001/archopthalmol.2011.1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Repp DJ, Hodge DO, Baratz KH, et al. . Fuchs' endothelial corneal dystrophy. subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology 2013;120:687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun SY, Wacker K, Baratz KH, et al. . Determining subclinical edema in Fuchs endothelial corneal dystrophy: revised classification using scheimpflug tomography for preoperative assessment. Ophthalmology 2019;126:195–204. 10.1016/j.ophtha.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 36.Hecker LA, McLaren JW, Bachman LA. Anterior keratocyte depletion in Fuchs endothelial dystrophy. Arch Ophthalmol 2011;129:555–61. 10.1001/archophthalmol.2010.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SV, McLaren JW. In vivo confocal microscopy of Fuchs endothelial dystrophy before and after endothelial keratoplasty. JAMA Ophthalmol 2013;131:611–8. 10.1001/jamaophthalmol.2013.799 [DOI] [PubMed] [Google Scholar]
- 38.Amin SR, Baratz KH, McLaren JW, et al. . Corneal abnormalities early in the course of Fuchs' endothelial dystrophy. Ophthalmology 2014;121:2325–33. 10.1016/j.ophtha.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wacker K, McLaren JW, Amin SR, et al. . Corneal high-order aberrations and Backscatter in Fuchs' endothelial corneal dystrophy. Ophthalmology 2015;122:1645–52. 10.1016/j.ophtha.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baratz KH, McLaren JW, Maguire LJ, et al. . Corneal haze determined by confocal microscopy 2 years after Descemet stripping with endothelial keratoplasty for Fuchs corneal dystrophy. Arch Ophthalmol 2012;130:868–74. 10.1001/archophthalmol.2012.73 [DOI] [PubMed] [Google Scholar]
- 41.Krachmer JH, Purcell JJ, Young CW, et al. . Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol 1978;96:2036–9. [DOI] [PubMed] [Google Scholar]
- 42.Louttit MD, Kopplin LJ, Igo RP, et al. . A multicenter study to map genes for Fuchs endothelial corneal dystrophy: baseline characteristics and heritability. Cornea 2012;31:26–35. 10.1097/ICO.0b013e31821c9b8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eghrari AO, Mumtaz AA, Garrett B, et al. . Automated Retroillumination photography analysis for objective assessment of Fuchs corneal dystrophy. Cornea 2017;36:44–7. 10.1097/ICO.0000000000001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottsch JD, Sundin OH, Rencs EV, et al. . Analysis and documentation of progression of Fuchs corneal dystrophy with retroillumination photography. Cornea 2006;25:485–9. 10.1097/01.ico.0000178726.11693.14 [DOI] [PubMed] [Google Scholar]
- 45.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol 2000;44:367–408. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed KA, McLaren JW, Baratz KH, et al. . Host and graft thickness after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. American Journal of Ophthalmology 2010;150:490–7. 10.1016/j.ajo.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 47.Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs' corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology 2005;112:441–6. [DOI] [PubMed] [Google Scholar]
- 48.AAo O. Cataract in the adult eye preferred practice pattern. San Francisco: American Academy of Ophthalmology, 2016. [Google Scholar]
- 49.McLaren JW, Bachman LA, Kane KM, et al. . Objective assessment of the corneal endothelium in Fuchs' endothelial dystrophy. Invest. Ophthalmol. Vis. Sci. 2014;55:1184–90. 10.1167/iovs.13-13041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun SY, Wacker K, Baratz KH, et al. . Re: Sun et al.: Determining subclinical edema in Fuchs endothelial corneal dystrophy: revised classification using Scheimpflug tomography for preoperative assessment (Ophthalmology 2019;2:195-204). Ophthalmology 2019;126:e22 10.1016/j.ophtha.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 51.Lass JH, Gal RL, Dontchev M, et al. . Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. specular microscopy ancillary study results. Ophthalmology 2008;115:627–32. 10.1016/j.ophtha.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lass JH, Benetz BA, Verdier DD, et al. . Corneal endothelial cell loss 3 years after successful Descemet stripping automated endothelial keratoplasty in the cornea preservation time study. JAMA Ophthalmology 2017;135:1394–400. 10.1001/jamaophthalmol.2017.4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel SV, Diehl NN, Hodge DO. Donor risk factors for graft failure in a 20-year study of penetrating keratoplasty. Arch Ophthalmol 2010;128:418–25. 10.1001/archophthalmol.2010.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka H, Okumura N, Koizumi N, et al. . Panoramic view of human corneal endothelial cell layer observed by a prototype slit-scanning wide-field contact specular microscope. Br J Ophthalmol 2017;101:655–9. 10.1136/bjophthalmol-2016-308893 [DOI] [PubMed] [Google Scholar]
- 55.Moloney G, Chan U-T, Hamilton A, et al. . Descemetorhexis for Fuchs’ dystrophy. Canadian Journal of Ophthalmology 2015;50:68–72. 10.1016/j.jcjo.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 56.Sugar A, Tanner JP, Dontchev M, et al. . Recipient risk factors for graft failure in the cornea Donor Study. Ophthalmology 2009;116:1023–8. 10.1016/j.ophtha.2008.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terry MA, Aldave AJ, Szczotka-Flynn LB, et al. . Donor, recipient, and operative factors associated with graft success in the cornea preservation time study. Ophthalmology 2018;125:1700–9. 10.1016/j.ophtha.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lass JH, Benetz BA, Patel SV, et al. . Donor, recipient, and operative factors associated with increased endothelial cell loss in the cornea preservation time study. JAMA Ophthalmol 2018. doi: 10.1001/jamaophthalmol.2018.5669. [Epub ahead of print: 26 Oct 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourne WM. The effect of long-term contact lens wear on the cells of the cornea. Clao J 2001;27:235–30. [PubMed] [Google Scholar]
- 60.Larsson L-I, Bourne WM, Pach JM. Structure and function of the corneal endothelium in diabetes mellitus type I and type II. Arch Ophthalmol 1996;114:9–14. 10.1001/archopht.1996.01100130007001 [DOI] [PubMed] [Google Scholar]
- 61.Lass JH, Riddlesworth TD, Gal RL, et al. . The effect of donor diabetes history on graft failure and endothelial cell density 10 years after penetrating keratoplasty. Ophthalmology 2015;122:448–56. 10.1016/j.ophtha.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008;27:1–16. 10.1097/ICO.0b013e31815892da [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel SV, McLaren JW, Bachman LA, et al. . Comparison of flex-center, center, and corner methods of corneal endothelial cell analysis. Cornea 2010;29:1042–7. 10.1097/ICO.0b013e3181cc7a60 [DOI] [PubMed] [Google Scholar]
- 64.Benetz BA, Gal RL, Ruedy KJ, et al. . Specular microscopy ancillary study methods for donor endothelial cell density determination of cornea Donor Study images. Current Eye Research 2006;31:319–27. 10.1080/02713680500536738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. American Journal of Ophthalmology 2005;139:311–9. 10.1016/j.ajo.2004.09.045 [DOI] [PubMed] [Google Scholar]
- 66.McLaren JW, Nau CB, Erie JC, et al. . Corneal thickness measurement by confocal microscopy, ultrasound, and scanning slit methods. American Journal of Ophthalmology 2004;137:1011–20. 10.1016/j.ajo.2004.01.049 [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi T, Ohnuma K, Tomida D, et al. . The contribution of the posterior surface to the corneal aberrations in eyes after keratoplasty. Invest. Ophthalmol. Vis. Sci. 2011;52:6222–9. 10.1167/iovs.11-7647 [DOI] [PubMed] [Google Scholar]
- 68.Mandell RB, Polse KA, Brand RJ, et al. . Corneal hydration control in Fuchs' dystrophy. Invest Ophthalmol Vis Sci 1989;30:845–52. [PubMed] [Google Scholar]
- 69.Wacker K, McLaren JW, Kane KM, et al. . Corneal hydration control in Fuchs' endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2016;57:5060–5. 10.1167/iovs.16-20205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McLaren JW, Bourne WM, Patel SV. Standardization of corneal haze measurement in confocal microscopy. Invest. Ophthalmol. Vis. Sci. 2010;51:5610–6. 10.1167/iovs.10-5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McLaren JW, Wacker K, Kane KM, et al. . Measuring corneal haze by using scheimpflug photography and confocal microscopy. Invest. Ophthalmol. Vis. Sci. 2016;57:227–35. 10.1167/iovs.15-17657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wacker K, Bourne WM, Patel SV. Effect of graft thickness on visual acuity after Descemet stripping endothelial keratoplasty: a systematic review and meta-analysis. Am J Ophthalmol 2016:16318–28. [DOI] [PubMed] [Google Scholar]
- 73.van der Meulen IJE, Patel SV, Lapid-Gortzak R. Quality of vision in patients with Fuchs endothelial dystrophy and after Descemet stripping endothelial keratoplasty. Arch Ophthalmol 2011;129:1537–42. 10.1001/archophthalmol.2011.247 [DOI] [PubMed] [Google Scholar]
- 74.Van Den Berg TJTP, Van Rijn L.J. (René), Michael R, et al. . Straylight effects with aging and lens extraction. American Journal of Ophthalmology 2007;144:358–63. 10.1016/j.ajo.2007.05.037 [DOI] [PubMed] [Google Scholar]
- 75.Lundström M, Pesudovs K. Catquest-9SF patient outcomes questionnaire: Nine-item short-form Rasch-scaled revision of the Catquest questionnaire. J Cataract Refract Surg 2009;35:504–13. [DOI] [PubMed] [Google Scholar]
- 76.Lundström M, Roos P, Jensen S, et al. . Catquest questionnaire for use in cataract surgery care: description, validity, and reliability. Journal of Cataract & Refractive Surgery 1997;23:1226–36. 10.1016/S0886-3350(97)80321-5 [DOI] [PubMed] [Google Scholar]
- 77.Wacker K, Baratz KH, Bourne WM, et al. . Patient-Reported Visual Disability in Fuchs’ Endothelial Corneal Dystrophy Measured by the Visual Function and Corneal Health Status Instrument. Ophthalmology 2018;125:1854–61. 10.1016/j.ophtha.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 78.gal RL, Dontchev M, Beck RW, et al. . The effect of donor age on corneal transplantation outcome results of the cornea Donor Study. Ophthalmology 2008;115:620–6. 10.1016/j.ophtha.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel SV. Graft survival after penetrating keratoplasty. Am J Ophthalmol 2011;151:397–8. 10.1016/j.ajo.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams KA, Roder D, Esterman A, et al. . Factors predictive of corneal graft survival. Report from the Australian corneal graft registry. Ophthalmology 1992;99:403–14. [DOI] [PubMed] [Google Scholar]
- 81.Thompson RW, Price MO, Bowers PJ, et al. . Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003;110:1396–402. 10.1016/S0161-6420(03)00463-9 [DOI] [PubMed] [Google Scholar]
- 82.Anshu A, Lim LS, Htoon HM, et al. . Postoperative risk factors influencing corneal graft survival in the Singapore corneal Transplant Study. American Journal of Ophthalmology 2011;151:442–8. 10.1016/j.ajo.2010.09.002 [DOI] [PubMed] [Google Scholar]