Abstract
Protein kinase CK2 plays a critical role in cell growth, proliferation, and suppression of cell death. CK2 is overexpressed, especially in the nuclear compartment, in the majority of cancers, including prostate cancer (PCa). CK2-mediated activation of transcription factor nuclear factor kappa B (NF-κB) p65 is a key step in cellular proliferation, resulting in translocation of NF-κB p65 from the cytoplasm to the nucleus. As CK2 expression and activity are also elevated in benign prostatic hyperplasia (BPH), we sought to increase the knowledge of CK2 function in benign and malignant prostate by examination of the relationships between nuclear CK2 and nuclear NF-κB p65 protein expression. The expression level and localization of CK2α and NF-κB p65 proteins in PCa and BPH tissue specimens was determined. Nuclear CK2α and NF-κB p65 protein levels are significantly higher in PCa compared with BPH, and these proteins are positively correlated with each other in both diseases. Nuclear NF-κB p65 levels correlated with Ki-67 or with cytoplasmic NF-κB p65 expression in BPH, but not in PCa. The findings provide information that combined analysis of CK2α and NF-κB p65 expression in prostate specimens relates to the disease status. Increased nuclear NF-κB p65 expression levels in PCa specifically related to nuclear CK2α levels, indicating a possible CK2-dependent relationship in malignancy. In contrast, nuclear NF-κB p65 protein levels related to both Ki-67 and cytoplasmic NF-κB p65 levels exclusively in BPH, suggesting a potential separate impact for NF-κB p65 function in proliferation for benign disease as opposed to malignant disease.
Keywords: BPH, Prostate cancer, CK2, RELA, Nuclear factor kappa B, Ki-67, Biomarker
Introduction
Protein kinase CK2 (previously known as Casein Kinase 2 or II) is a highly conserved and ubiquitous protein Ser/Thr kinase. It is involved in functions related to cell growth, proliferation, and survival, as well as in regulation of cell death. The CK2 holoenzyme is a tetrameric structure with two catalytic subunits (42 kDa α, 38 kDa α′) that are linked through the two regulatory β subunits (28 kDa), with activity toward more than 300 substrates [1, 2]. Consequently, CK2 can affect a diverse range of signaling pathways and influence a variety of cellular functions. CK2 has also been found to play a critical role in many disease conditions, including cancer [3]. It is elevated consistently, predominantly at the protein level, in all the cancer types studied [4, 5]. It is also elevated in normal rapidly proliferating cells. CK2 is localized in the cytoplasmic and nuclear compartments in the cell; however, the nuclear localization of CK2 is significantly enhanced in the cancer phenotype [6–8]. We originally reported that CK2 dysregulation in cancer cells relates to poor prognosis [6, 9, 10]. This observation has since been confirmed in many types of cancer. Specifically in prostate cancer, CK2α elevation and nuclear localization were related to the Gleason score and poor prognosis [11, 12]. Together, these observations suggested the potential of CK2 as a marker of prostate cancer progression and prognosis; however, CK2 expression is also high in benign prostatic hyperplasia (BPH) [11, 12]. At present, there is little information on the functionality of CK2 in BPH compared with malignant prostate disease.
A large number of potential substrates for CK2 have been noted; among them nuclear factor kappa B (NF-κB) p65/RelA and its inhibitor IκBα are well-established substrate proteins [13–15]. Importantly, NF-κB plays a critical role in inflammation and the development of malignant phenotype, and its abnormal activation has been reported in many cancers [16–20]. Typically, NF-κB p65 signal is undetectable to very low in normal prostate tissue and progresses to a predominantly cytosolic signal in BPH, and finally to high level, mainly nuclear signal in PCa which is associated with prognosis [21–25]. Activated nuclear NF-κB p65 (phospho-S276) expression, indicating canonical NF-κB signaling, from early to late stages was correlated with human BPH progression as well [26]. We have observed that NF-κB p65 phosphorylation, especially on S529, can serve as a surrogate for measuring altered CK2 activity in xenograft models of cancer [27, 28]. These observations are germane to those in breast cancer where it was documented that CK2 played a significant role in promoting aberrant activation of NF-κB in breast cancer pathogenesis and survival of breast cancer cells (e.g., [15, 29, 30]).
To our knowledge, there are no published reports relating CK2 and NF-κB protein expression in BPH and PCa human tissue samples. Two studies in cultured breast and prostate cells determined that CK2 phosphorylation and the subsequent degradation of IκB-α causes classical NF-κB (e.g., p50/p65) activation through the EGFR2/HER2 pathway [29, 31]. We examined the coexpression of CK2α and NF-κB p65 proteins in human PCa and BPH specimens to determine how their expression levels in the nuclear compartment relate to one another in the context of benign and malignant proliferative signaling.
Materials and methods
Sample collection
Patients who were on list for the transurethral resection of the prostate (TURP) were recruited for the study. Data were obtained from the records of patients maintained in the Department of Histopathology at the Armed Forces Institute of Pathology, Islamabad, Pakistan. The availability of these data and the protocol followed were approved by the Institutional Ethical Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. All prostate samples were collected from the operation theater of the Armed Forces Institute of Urology (AFIU), Combined Military Hospital (CMH), Rawalpindi, Pakistan. Immediately after the TURP operation, the tissue samples were collected in formalin. The next day, samples were transferred to 10 % buffered formalin for storage until further processing.
Antibodies
The antibodies employed were obtained from Santa Cruz Biotechnology: CK2α (sc-6479; 1:200); Ki-67 (sc-23900; 1:50); NF-κB p65 (sc-109: 1:50).
Immunohistochemistry
Paraffin embedded sections were cut 3- to 4-µm thick. Each section was transferred to positively charged slides with a histogrip coating. Slides were placed in an oven for 1 h at 56 °C. Dewaxing was carried out in absolute xylene for 7–10 min. Slides were rehydrated in descending grades of ethanol (absolute, 80 and 70 % for 2–3 min each). Finally slides were dipped in water for complete rehydration. Antigen retrieval was carried out in 10× EDTA + TRIS antigen retrieval solution at 100 °C for 25 min in a decloaking chamber. Hydrogen peroxidase solution (blocking solution) was added for 5 min followed by washing with PBS (3 times, 5 min each). Slides were incubated with primary antibody for 1 h, then secondary antibody was added for 15 min. Streptavidin-HRP was added for 15 min followed by addition of chromogen DAB for 10 min. Slides were washed with distilled water and counter stained with hematoxylin for 1 min. Once staining was completed, slides were dehydrated in ascending grades of alcohol and xylene and were mounted with cover slips coated with DPX mounting media.
Pathology and scoring
Prostate tissue was collected by TURP and processed for formalin fixation and paraffin embedment. Cancer diagnosis and Gleason scores were assigned based on pathologist analysis of H&E stained tissue sections. After staining for CK2α or NF-κB p65, each slide was scored in a blinded manner by three histopathologists using a method similar to a previously published study [11]. For CK2 nuclear and cytoplasmic staining, the range of scores given was from 0 to 3. For NF-κB p65 staining, the score range for nuclear staining which was from 0 to 4 and for cytoplasmic staining which was from 0 to 3. Ki-67 was quantified by two independent pathologists and one scientist by counting the number of positively stained nuclei out of 30 total in five different randomly selected representative sections within the same tissue specimen. The percentage of positive nuclei was calculated.
Image analysis
Immunostained tissue images were captured using a multihead microscope (Olympus BX41, camera model DP 21) with a cellSens (Olympus) digital imaging system. Whole slides were imaged at ×400 magnification. No adjustments were made to the images.
Statistical analysis
Staining scores were analyzed as continuous measurements. Estimated Pearson’s correlation coefficients were the same as Spearman’s rank order correlation coefficients and therefore were used to test for relationships. Mean differences between PCa and BPH tissue samples were estimated by linear regression analysis. The c-statistic was used as the measure of discrimination of PCa and BPH tissues. DeLongs method was employed to compare c-statistics [32]. Cited p values are not adjusted for the number of comparisons of these two samples of prostate tissues.
Results
Clinical and pathological characteristics of patient samples
A total of 61 patients provided prostate tissue for this study; 30 patients diagnosed with PCa and 31 patients diagnosed with BPH. The mean age of all patients was 69 years (range 28 to 90 years; Table 1). All clinical and pathological grades were assigned by three independent histopathologists. For the PCa tissues, the median Gleason score was 7 and ranged from 5 to 9 (8 tissues had a Gleason score ≤6, and 22 tissues had a Gleason score ≥7). The presence of lymphovascular invasion was detected in 9 PCa tissue (30 %) and perineural invasion was present in 13 PCa patients (43 %). The percentage of malignant disease prostate tissue was estimated to be ≤60 % of the tissue in 11 (37 %) and ≥70 % of the tissue in 19 (63 %) of the patients.
Table 1.
Descriptive statistics of study population
| BPH (n = 31) |
PCa (n = 30) |
|||||
|---|---|---|---|---|---|---|
| Minimum value | Maximum value | Mean ± SD | Minimum value | Maximum value | Mean ± SD | |
| Age | 55 | 85 | 69.3 ± 9.2 | 28 | 90 | 69.6 ± 12.0 |
| % Ki-67 positive | 2.7 | 6.7 | 3.8 ± 1.3 | 2.0 | 31.3 | 13.6 ± 8.6 |
Nuclear expression of CK2α and NF-κB p65 is higher in PCa than BPH tissues
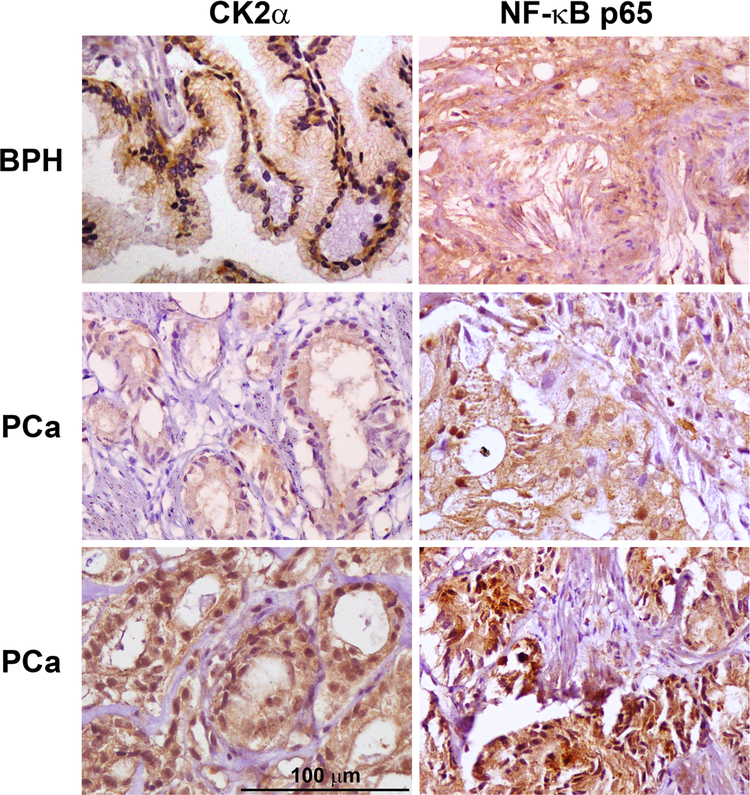
As nuclear localization for both CK2α and for NF-κB p65 protein expression has significance in cancer and in relation to activation, we focused primarily on nuclear expression levels of these two proteins. PCa and BPH tissue sections were stained for the presence of CK2α and NF-κB p65 proteins by immunohistochemical methods. The nuclear and cytoplasmic signals for CK2α and NF-κB p65 were scored in a blinded manner by three histopathologists as described under Materials and methods, and representative staining in BPH and PCa tissues is shown (Fig. 1). The scores for CK2α and NF-κB p65 staining are presented in Table 2. Very low levels of CK2α and NF-κB p65 proteins were detected in normal prostate tissue specimens (data not shown). Both CK2α (mean difference 0.50, 95 % CI 0.29–0.72) and NF-κB p65 (mean difference 0.71, 95 % CI 0.27–1.15) nuclear proteins demonstrated higher expression levels in PCa compared with BPH (Fig. 2a, b). Discrimination of PCa and BPH tissues was not significantly different (p = 0.50) using nuclear CK2α (c-statistic 0.73, 95 % CI 0.61–0.85) or nuclear NF-κB p65 (c-statistic 0.68, 95 % CI 0.56–0.81).
Fig. 1.
Immunohistochemical detection of CK2α and NF-κB p65 proteins in human BPH and malignant prostate tissue. The protein detected is indicated at the top of the images. The type of tissue is indicated on the left side of the images. Scale bar is 100 µm
Table 2.
IHC staining scores for CK2α and NF-κB p65
| Nuclear scores |
Cytoplasmic scores |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | |
| BPH | |||||||||
| CK2α | 17 (54.8 %) | 12 (38.7 %) | 0 | 2 (6.4 %) | N/A | 3 (9.7 %) | 17 (54.8 %) | 11 (35.5 %) | 0 |
| NF-κB p65 | 6 (19.4 %) | 12 (38.75 %) | 13 (41.9 %) | 0 | 0 | 0 | 6 (19.4 %) | 23 (74.2 %) | 2 (6.4 %) |
| PCa | |||||||||
| CK2α | 6 (20.0 %) | 12 (40.0 %) | 8 (13.1 %) | 4 (9.8 %) | N/A | 0 | 12 (40.0 %) | 16 (53.3 %) | 2 (6.7 %) |
| NF-κB p65 | 0 | 13 (43.3 %) | 7 (23.355 %) | 9 (30.0 %) | 1 (3.3 %) | 0 | 4 (13.3 %) | 9 (30.0 %) | 17 (56.7 %) |
The number outside the parenthesis represents the number of patient tissues in that score category. The number in parenthesis is the percent of the patient tissues with that score. BPH n = 31. PCa n = 30
Fig. 2.

Comparison of CK2α and NF-κB p65 nuclear protein expression levels in BPH and PCa patient tissues. Patient tissues were stained for CK2α and NF-κB p65 and scored for expression levels by three independent pathologists. The mean value and standard error were plotted for BPH (n = 31) versus PCa (n = 30) tissues. p values are indicated on the plots. a CK2α nuclear protein expression. b NF-κB p65 nuclear protein expression. c NF-κB p65 nuclear protein expression with means adjusted for differences in CK2α nuclear protein expression
Increased nuclear CK2α expression accounts for the increased nuclear NF-κB p65 levels in PCa
The nuclear protein level of NF-κB p65 was positively correlated with the nuclear protein level of CK2α to the same extent in PCa and BPH tissues (Table 3). Therefore, the higher nuclear CK2α in PCa may help explain the higher nuclear NF-κB p65 expression in the PCa tissue. Using regression analysis to adjust for the difference in nuclear CK2α, the mean difference in nuclear NF-κB p65 expression between PCa and BPH dropped to 0.39 (p = 0.08, Fig. 2c) from the previous mean difference of 0.71 (p = 0.002; Fig. 2b). Furthermore, adding nuclear NF-κB p65 expression did not significantly improve the discrimination of PCa and BPH tissue compared to nuclear CK2α expression alone (c-statistic 0.73, 95 % CI 0.61–0.85 versus 0.76, 95 % CI 0.64–0.88; p = 0.16).
Table 3.
Correlations with nuclear NF-κB p65
| CK2α nuclear | Ki-67 | NF-κB p65 cyto | |
|---|---|---|---|
| BPH | r = 0.43 | r = 0.37 | r = 0.78 |
| p = 0.02 | p = 0.04 | p < 0.001 | |
| PCa | r = 0.44 | r = 0.13 | r = 0.24 |
| p = 0.01 | p = 0.49 | p = 0.19 |
Nuclear NF-κB p65 levels were significantly related to cytoplasmic levels NF-κB p65 in BPH, but not in PCa (Table 3). Nuclear NF-κB p65 expression did not correlate with cytoplasmic CK2α in either BPH (r = 0.01; p = 0.94) or PCa (r = −0.16; p = 0.40) tissue type.
Nuclear NF-κB p65 expression correlates with Ki-67 in BPH but not in PCa
Ki-67 staining, an established marker of proliferation, was markedly higher in PCa compared with BPH tissues (Fig. 3) [33]. The difference in mean percentage of Ki-67 positive cells was 10 % (95 % CI 7–13 %). Proliferation as measured by Ki-67 was related to nuclear NF-κB p65 in BPH, but not in PCa (Table 3). Proliferation was not related to nuclear CK2α in either BPH (r = 0.04; p = 0.84) or PCa (r = 0.16; p = 0.38) tissues.
Fig. 3.
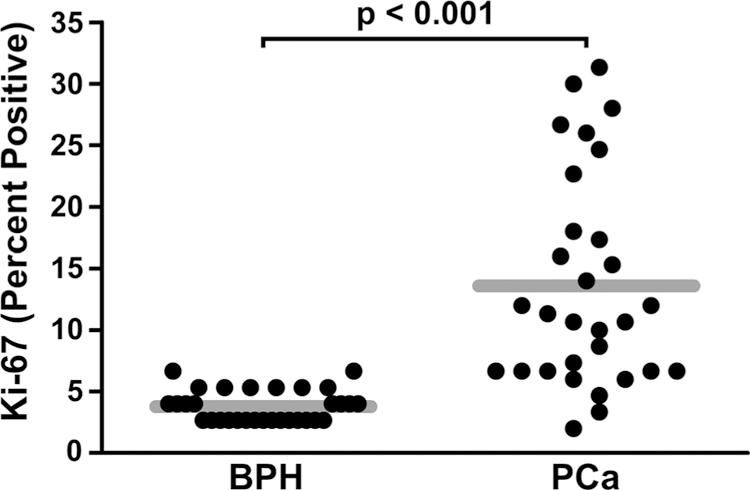
Comparison of Ki-67 percent positive scores in BPH and PCa. The mean values for the percent of Ki-67 positive cells were plotted for BPH (mean = 3.78; standard deviation = 1.29; n = 31) versus PCa (mean = 13.60; standard deviation = 8.59; n = 30) tissues. The gray bar depicts the means
CSNK2A1 and RELA mRNAs do not correlate in PCa
We have thus far demonstrated a correlation between nuclear protein expression levels of CK2α and NF-κB p65 in PCa and BPH. We wished to examine whether mRNA expression data for these genes would also correlate in the prostate. As BPH mRNA expression data were not readily available in public databases, we investigated whether CK2α and NF-κB p65 transcript expression correlated in primary and advanced or metastatic castration-resistant PCa (CRPC) disease states. Using the cBioPortal database, we analyzed coexpression of CSNK2A1 (CK2α) and RELA (NF-κB p65) mRNAs in prostate cancer datasets [34, 35]. No notable correlation of CSNK2A1 and RELA was observed in primary tumors from two datasets (Fig. 4a, b) [36, 37]. In metastatic CRPC tissues, there was some indication of positive correlation between CSNK2A1 and RELA transcripts (Fig. 4c) [38]. In these datasets, from 0 to 2 % and from 0 to 2.7 % of the samples showed amplification, deletion, or mutation of the CSNK2A1 or RELA genes, respectively.
Fig. 4.

Comparison of CSNK2A1 and RELA mRNA expression in primary and metastatic castration-resistant PCa patient tissues. All analyses were performed using cBioPortal. a Comparison of RNA Seq data in primary PCa tumor tissues from The Cancer Genome Atlas dataset [37]. n = 333. b Comparison of microarray data in primary PCa tumor tissues from Memorial Sloan Kettering Cancer Center dataset [36]. n = 131. c Comparison of RNA Seq data in metastatic CRPC tissues from Stand Up 2 Cancer dataset [38]. n = 150. RSEM RNA Seq by Expectation–Maximization. RPKM Reads per kilobase million
Discussion
CK2 has long been known to be associated with the normal and malignant proliferative processes of cells. CK2 levels in tumor cells relate to the pathological status of the cancer so that CK2 could serve as a potential prognostic factor [7, 9–11]. However, CK2 is also elevated in benign proliferative conditions such as in the benign prostate growth known as BPH [11]. The comparative functionality of CK2 under conditions of benign versus cancer growth is unclear. Among the large number of its substrates is NF-κB p65 which is also known to be highly involved in normal and cancer cell biology (e.g., [15, 29, 30]). Since we have observed that prostate tumors treated with anti-CK2 therapy demonstrate a large reduction in NF-κB p65, we attempted to determine if there were links in the CK2 and NF-κB pathways in human benign and malignant prostate diseases [27, 39, 40].
In our analysis of PCa and BPH tissues, nuclear staining for CK2α and NF-κB p65 were significantly higher in malignant than in benign tissues and were associated together in both tissues. CK2α transcript levels are increased in prostate adenocarcinoma relative to normal prostate tissue by 1.9–2.2-fold [41, 42]. However, our analysis here and published literature to date support the idea that CK2 protein levels and localization are more informative overall in analysis of CK2 function and contribution to proliferative and malignant signaling. Specifically in this study, there was no nuclear CK2 signal detected in 17 of the 31 BPH tissues, as well as no nuclear NF-κB p65 signal in 6 of the BPH tissues. Interestingly, the difference in means and the significance of increased NF-κB p65 expression in the nuclear compartment of malignant cells, reflecting activated NF-κB, is notably decreased once the contribution of nuclear CK2α expression is removed. Our findings are in line with previous studies focused on PCa which show that nuclear localization and level of CK2 correlates with the pathogenesis and progression of disease [11]. Published literature also indicates the importance of nuclear NF-κB p65 in association with PCa status [43] and pathogenesis and survival of breast cancer [15, 29, 30]. Thus, the present results support the concept that there is an overall increase in nuclear CK2α and NF-κB p65 protein expression in malignant disease. These analyses add the new information that nuclear CK2α and NF-κB p65 protein expression levels correlate in both disease states, but that the enhanced expression of nuclear CK2α contributes to the increased active NF-κB p65 in PCa.
Ki-67 is a nuclear antigen whose expression levels represent the proliferative state of tissue [44–46]. Here we showed that Ki-67 levels are significantly increased in PCa compared with BPH. This finding agrees with previous data available on expression of Ki-67 in malignant conditions [33, 47]. However, our data also demonstrated a significant association of nuclear NF-κB p65 expression with Ki-67 or with cytoplasmic NF-κB p65 in benign disease only. It may be noted that the nature of cell growth in BPH is known to be distinctly different from that in PCa. In BPH, there is a significant involvement of hypertrophic cell growth in addition to hyperplastic growth of epithelial and stromal cells, so that the disease can present itself in multiple forms; in the present work, a distinction on the type of BPH was not made for the various specimens. Multiple factors and pathways, including androgens and estrogens, are implicated in the diverse forms of BPH growth; here, our data support the concept that NF-κB p65 signaling is significantly involved in BPH proliferation [26, 48, 49].
In summary, we have examined the relative expression of CK2α and NF-κB p65 in human BPH and PCa specimens and have observed that the amount of tissue staining for CK2α and NF-κB p65 was much higher in PCa than in BPH. The higher level of nuclear NF-κB p65 in PCa was concordant with the elevated nuclear CK2α. Further, PCa specimens demonstrated higher proliferation than BPH as evidenced by Ki-67 immunostaining of the specimens. Nuclear and cytoplasmic NF-κB and Ki-67 signals correlate in BPH but not in PCa. These various correlations are present at the protein levels and require the ability to discriminate nuclear and cytoplasmic localization of the signals. Thus, the present study adds to our knowledge of CK2 and NF-κB signals, and suggests different modes of their functionality in the biology of benign prostate growth and malignancy.
Acknowledgments
This work was supported by United States Department of Veterans Affairs Merit Review Program Grant numbers I01BX001731 and I01BX003282, and by National Cancer Institute Grant number R01CA150182 (KA). National University of Sciences and Technology provided financial support under the Mega S&T scholarship fund for this work (FQ and SS). Armed Forces Institute of Pathology and Armed Forces Institute of Urology, Rawalpindi, provided technical assistance in the conduct of this work. The results shown here are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Research involving animal and human rights All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Bian Y, Ye M, Wang C, Cheng K, Song C, Dong M, Pan Y, Qin H, Zou H (2013) Global screening of CK2 kinase substrates by an integrated phosphoproteomics workflow. Sci Rep 3:3460 10.1038/srep03460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Núñez de Villavicencio-Díaz T, Mazola Y, Perera Negrín Y, Cruz García Y, Guirola Cruz O, Perea Rodríguez SE (2015) Predicting CK2 beta-dependent substrates using linear patterns. Biochem Biophys Rep 4:20–27. 10.1016/j.bbrep.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerra B, Issinger OG (2008) Protein kinase CK2 in human diseases. Curr Med Chem 15(19):1870–1886. 10.1007/s00018-009-9148-9 [DOI] [PubMed] [Google Scholar]
- 4.Trembley JH, Wang G, Unger G, Slaton J, Ahmed K (2009) Protein kinase CK2 in health and disease: cK2: a key player in cancer biology. Cell Mol Life Sci 66(11–12):1858–1867. 10.1007/s00018-009-9154-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruzzene M, Pinna LA (2010) Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta 3:499–504. 10.1016/j.bbapap.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 6.Faust RA, Niehans G, Gapany M, Hoistad D, Knapp D, Cherwitz D, Davis A, Adams GL, Ahmed K (1999) Subcellular immunolocalization of protein kinase CK2 in normal and carcinoma cells. Int J Biochem Cell Biol 31(9):941–949. 10.1016/S1357-2725(99)00050-3 [DOI] [PubMed] [Google Scholar]
- 7.Giusiano S, Cochet C, Filhol O, Duchemin-Pelletier E, Secq V, Bonnier P, Carcopino X, Boubli L, Birnbaum D, Garcia S, Iovanna J, Charpin C (2011) Protein kinase CK2alpha subunit over-expression correlates with metastatic risk in breast carcinomas: quantitative immunohistochemistry in tissue microarrays. Eur J Cancer 47(5):792–801. 10.1016/j.ejca.2010.11.028 [DOI] [PubMed] [Google Scholar]
- 8.Mandal T, Bhowmik A, Chatterjee A, Chatterjee U, Chatterjee S, Ghosh MK (2014) Reduced phosphorylation of stat3 at Ser-727 mediated by casein kinase 2—protein phosphatase 2A enhances stat3 Tyr-705 induced tumorigenic potential of glioma cells. Cell Signal 26(8):1725–1734. 10.1016/j.cellsig.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Gapany M, Faust RA, Tawfic S, Davis A, Adams GL, Ahmed K (1995) Association of elevated protein kinase CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Mol Med 1(6):659–666 [PMC free article] [PubMed] [Google Scholar]
- 10.Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K (1996) Elevated protein kinase CK2 activity in chromatin of head and neck tumors: association with malignant transformation. Cancer Lett 101(1):31–35. 10.1016/0304-3835(96)04110-9 [DOI] [PubMed] [Google Scholar]
- 11.Laramas M, Pasquier D, Filhol O, Ringeisen F, Descotes JL, Cochet C (2007) Nuclear localization of protein kinase CK2 catalytic subunit (CK2alpha) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer 43(5):928–934. 10.1016/j.ejca.2006.11.021 [DOI] [PubMed] [Google Scholar]
- 12.Yenice S, Davis AT, Goueli SA, Akdas A, Limas C, Ahmed K (1994) Nuclear casein kinase 2 (CK-2) activity in human normal, benign hyperplastic, and cancerous prostate. Prostate 24(1):11–16 [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Westerheide SD, Hanson JL, Baldwin AS (2000) Tumor necrosis factor α-induced phosphorylation of relA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem 275(42):32592–32597 [DOI] [PubMed] [Google Scholar]
- 14.Barroga CF, Stevenson JK, Schwarz EM, Verma IM (1995) Constitutive phosphorylation of I kappa B alpha by casein kinase II. Proc Natl Acad Sci USA 92(17):7637–7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Traish AM, Mercurio F, Sonenshein GE (2001) Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-κB in breast cancer. Cancer Res 61(9):3810–3818 [PubMed] [Google Scholar]
- 16.Dominguez I, Sonenshein GE, Seldin DC (2009) Protein kinase CK2 in health and disease: cK2 and its role in Wnt and NF-kappaB signaling: linking development and cancer. Cell Mol Life Sci 66(11–12):1850–1857. 10.1007/s00018-009-9153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoesel B, Schmid JA (2013) The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 12:86 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C, Rabson AB (2002) Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate 52(3):183–200. 10.1002/pros.10082 [DOI] [PubMed] [Google Scholar]
- 19.Brown M, Cohen J, Arun P, Chen Z, Van Waes C (2008) NF-kappaB in carcinoma therapy and prevention. Exp Opin Ther Targ 12(9):1109–1122. 10.1517/14728222.12.9.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen DP, Li JY, Yadav SS, Tewari AK (2014) Recent insights into NF-kappa B signalling pathways and the link between inflammation and prostate cancer. BJU Int 114(2):168–176. 10.1111/bju.12488 [DOI] [PubMed] [Google Scholar]
- 21.Nunez C, Cansino JR, Bethencourt F, Perez-Utrilla M, Fraile B, Martinez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M (2008) TNF/IL-1/NIK/NF-kappa B transduction pathway: a comparative study in normal and pathological human prostate (benign hyperplasia and carcinoma). Histopathology 53(2):166–176. 10.1111/j.1365-2559.2008.03092.x [DOI] [PubMed] [Google Scholar]
- 22.Guo Z, Xing Z, Cheng X, Fang Z, Jiang C, Su J, Zhou Z, Xu Z, Holmberg A, Nilsson S, Liu Z (2015) Somatostatin derivate (smsDX) attenuates the TAM-stimulated proliferation, migration and invasion of prostate cancer via NF-kappaB regulation. PLoS One 10(5):e0124292 10.1371/journal.pone.0124292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKenzie L, McCall P, Hatziieremia S, Catlow J, Adams C, McArdle P, Seywright M, Tanahill C, Paul A, Underwood M, Mackay S, Plevin R, Edwards J (2012) Nuclear factor kappaB predicts poor outcome in patients with hormone-naive prostate cancer with high nuclear androgen receptor. Hum Pathol 43(9):1491–1500. 10.1016/j.humpath.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 24.McCall P, Bennett L, Ahmad I, Mackenzie LM, Forbes IW, Leung HY, Sansom OJ, Orange C, Seywright M, Underwood MA, Edwards J (2012) NFkappaB signalling is upregulated in a subset of castrate-resistant prostate cancer patients and correlates with disease progression. Br J Cancer 107(9):1554–1563. 10.1038/bjc.2012.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gannon PO, Lessard L, Stevens LM, Forest V, Begin LR, Minner S, Tennstedt P, Schlomm T, Mes-Masson AM, Saad F (2013) Large-scale independent validation of the nuclear factor-kappa B p65 prognostic biomarker in prostate cancer. Eur J Cancer 49(10):2441–2448. 10.1016/j.ejca.2013.02.026 [DOI] [PubMed] [Google Scholar]
- 26.Austin DC, Strand DW, Love HL, Franco OE, Jang A, Grabowska MM, Miller NL, Hameed O, Clark PE, Fowke JH, Matusik RJ, Jin RJ, Hayward SW (2016) NF-kappaB and androgen receptor variant expression correlate with human BPH progression. Prostate 76(5):491–511. 10.1002/pros.23140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trembley JH, Unger GM, Korman VL, Abedin MJ, Nacusi LP, Vogel RI, Slaton JW, Kren BT, Ahmed K (2014) Tenfibgen ligand nanoencapsulation delivers bi-functional anti-CK2 RNAi oligomer to key sites for prostate cancer targeting using human xenograft tumors in mice. PLoS One 10.1371/journal.pone.0109970 [DOI] [PMC free article] [PubMed]
- 28.Unger GM, Kren BT, Korman VL, Kimbrough TG, Vogel RI, Ondrey FG, Trembley JH, Ahmed K (2014) Mechanism and efficacy of sub-50-nm tenfibgen nanocapsules for cancer cell-directed delivery of anti-CK2 RNAi to primary and metastatic squamous cell carcinoma. Mol Cancer Ther 13(8):2018–2029. 10.1158/1535-7163.MCT-14-0166-7163.MCT-14-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE (2002) Protein kinase CK2 promotes aberrant activation of nuclear factor-{kappa}B, transformed phenotype, and survival of breast cancer cells. Cancer Res 62(22):6770–6778 [PubMed] [Google Scholar]
- 30.Eddy SF, Guo S, Demicco EG, Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE (2005) Inducible I{kappa}B kinase/I{kappa}B kinase varepsilon expression is induced by CK2 and promotes aberrant nuclear factor-{kappa}B activation in breast cancer cells. Cancer Res 65(24):11375–11383. 10.1158/0008-5472.can-05-1602 [DOI] [PubMed] [Google Scholar]
- 31.Le Page C, Koumakpayi IH, Lessard L, Saad F, Mes-Masson AM (2005) Independent role of phosphoinositol-3-kinase (PI3 K) and casein kinase II (CK-2) in EGFR and Her-2-mediated constitutive NF-kappaB activation in prostate cancer cells. Prostate 65(4):306–315. 10.1002/pros.20291 [DOI] [PubMed] [Google Scholar]
- 32.DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845 [PubMed] [Google Scholar]
- 33.Fisher G, Yang ZH, Kudahetti S, Moller H, Scardino P, Cuzick J, Berney DM (2013) Prognostic value of Ki-67 for prostate cancer death in a conservatively managed cohort. Br J Cancer 108(2):271–277. 10.1038/bjc.2012.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cbioportal. Sci Signal 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed]
- 35.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) The cbio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2(5):401–404. 10.1158/2159-8290.cd-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cancer Genome Atlas Research Network (2015) The Molecular taxonomy of primary prostate cancer. Cell 163(4):1011–1025. 10.1016/j.cell.2015.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM (2015) Integrative clinical genomics of advanced prostate cancer. Cell 161(5):1215–1228. 10.1016/j.cell.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trembley JH, Unger GM, Gomez OC, Abedin J, Korman VL, Vogel RI, Niehans G, Kren BT, Ahmed K (2014) Tenfibgen-DMAT nanocapsule delivers CK2 inhibitor DMAT to prostate cancer xenograft tumors causing inhibition of cell proliferation. Mol Cell Pharmacol 6(2):15–25 [PMC free article] [PubMed] [Google Scholar]
- 40.Trembley JH, Unger GM, Tobolt DK, Korman VL, Wang G, Ahmad KA, Slaton JW, Kren BT, Ahmed K (2011) Systemic administration of antisense oligonucleotides simultaneously targeting CK2alpha and alpha’ subunits reduces orthotopic xenograft prostate tumors in mice. Mol Cell Biochem 356(1–2):21–35. 10.1007/s11010-011-0943-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortega CE, Seidner Y, Dominguez I (2014) Mining CK2 in cancer. PLoS One 9(12):e115609 10.1371/journal.pone.0115609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo W, Yu W-D, Ma Y, Chernov M, Trump DL, Johnson CS (2013) Inhibition of protein kinase CK2 reduces Cyp24a1 expression and enhances 1,25-dihydroxyvitamin D3 antitumor activity in human prostate cancer cells. Cancer Res 73(7):2289–2297. 10.1158/0008-5472.can-12-4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F (2003) NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int 91(4):417–420 [DOI] [PubMed] [Google Scholar]
- 44.Endl E, Gerdes J (2000) The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res 257(2):231–237. 10.1006/excr.2000.4888 [DOI] [PubMed] [Google Scholar]
- 45.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133(4):1710–1715 [PubMed] [Google Scholar]
- 46.Landberg G, Roos G (1993) Proliferating cell nuclear antigen and Ki-67 antigen expression in human haematopoietic cells during growth stimulation and differentiation. Cell Prolif 26(5):427–437 [DOI] [PubMed] [Google Scholar]
- 47.Sulik M, Maruszak K, Puchalska J, Misiukiewicz-Poć M (2011) Expression of Ki-67 as a proliferation marker in prostate cancer. Pol Ann Med 18(1):12–19 [Google Scholar]
- 48.Nicholson TM, Ricke WA (2011) Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation 82(4–5):184–199. 10.1016/j.diff.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin-Tsai O, Clark PE, Miller NL, Fowke JH, Hameed O, Hayward SW, Strand DW (2014) Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate 74(6):669–679. 10.1002/pros.22785 [DOI] [PMC free article] [PubMed] [Google Scholar]