Abstract
Acute neurophysiology in the behaving primate typically relies on traditional manufacturing approaches for the instrumentation necessary for recording. For example, our previous approach consisted of distributing single microelectrodes in a fixed plane situated over a circular patch of frontal cortex using conventionally-milled recording grids. With the advent of robust, multisite linear probes, and the introduction of commercially-available, high-resolution rapid prototyping systems, we have been able to improve upon traditional approaches. Here, we report our methodology for producing flexible, MR-informed recording platforms that allow us to precisely target brain structures of interest, including those that would be unreachable using previous methods. We have increased our single-session recording yields by an order of magnitude and recorded neural activity from widely-distributed regions using only a single recording chamber. This approach both speeds data collection, reduces the damage done to neural tissue over the course of a single experiment, and reduces the number of surgical procedures experienced by the animal.
I. INTRODUCTION
Deciphering how behavior arises from the combined activity of millions of neurons relies on our ability to measure neural activity. Early studies used serial recordings of single neurons to correlate firing rates and behavioral variables [1], [2]. While the behavior of individual neurons is still a rich source of insight into behavior [3], [4], it is increasingly clear that the collective dynamics emerging from neural populations can relate to behavior in ways not observable at the level of individual neurons [5]–[8]. Although chronically implanted arrays provide a means to collect multineuronal datasets [9], acute neurophysiology remains a critical tool for studying neural activity in relation to behavior in the head-fixed primate model. Acute recordings allow a broad sampling of the neural environment from day to day, removing sampling bias from analyses. Further, they also enable the study of circuit level activity over the scale of tens of millimeters, and the testing of a many such hypotheses across several experiments within a single subject.
As acute probe technology matures [10], parallel efforts are necessary to optimize how these technologies are used [11]–[13]. Here we provide details of our own efforts to develop a system that allows us to perform recordings from up to eight multisite (e.g. 16-32) commercially-available recording probes. This system is fully customizable such that the number, location, and even individual probe trajectories can be fully tailored to the recording target(s) of interest with precision and repeatability. First, we describe the imaging pipeline we use to develop our anatomical models. Next, we present the basic shuttle and tower designs that can be used in conjunction with traditional planar grids. Third, we demonstrate how we build upon our initial designs to both increase probe density and target distant (30 - 35 mm apart) brain regions within a single recording chamber. Finally, we report neuronal yields from our ongoing experiments.
II. Methods
A. Segmenting MRIs for anatomical models
For each subject, we obtained anatomical images with resolution of 1 × 0.84 × 0.84 mm using a 3T Siemens TIM/Trio MRI scanner with a 2-channel receive-only head coil (Fig. 1a). We manually traced regions of interest in the frontal lobe such as orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (dlPFC), and the striatum (STR) in Slicer3D (slicer.org) [14] using the Paxinos primate atlas [15] as a reference. We used this neuroanatomical model in conjunction with a cranial model (Gray Matter Research, Bozeman, MT) to position custom-fit poly-ether-ether-ketone (PEEK) [16] unilateral acute recording chambers. Chambers were milled using traditional approaches and surgically implanted over the left hemisphere in two subjects (C and V) and the right hemisphere of one subject (T).
Figure 1.
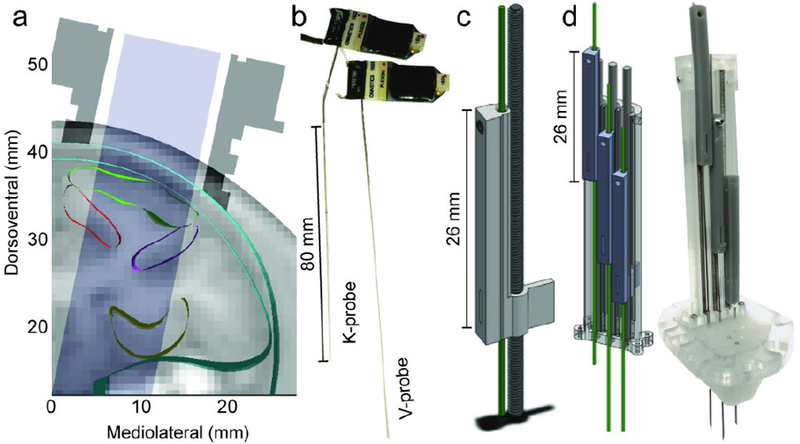
a) Cross-section of anatomical model with MRI slice (subj. C, coronal section 24 mm anterior to the interaural line) with cortical regions outlined. Chamber profile in dark grey; accessible area in light grey. b) Multisite linear probes (left: K-probe, right: V-probe) with headstages and groundwires attached. K-probes have a 20° bend 8 mm above electrode shank, c) Rendering of single shuttle and drive screw. Green shows track for electrode. Scale bar = 26 mm. d) Render (left) and photo (right) of 3 drives assembled into a linear tower and placed on a planar grid (clear). Hypodermic needles serve as guide tubes. Scale bar = 26 mm.
B. Initial design components
We previously performed acute recordings using 8 to 16 individually drivable single tungsten microelectrodes lowered bilaterally across two recording chambers [3], [7]. We have since transitioned to multisite linear probes (V/K-probes, Plexon Inc., Dallas, Tx; Fig. 1b) configured with 16 recording sites spaced 100 μm apart. There are two primary challenges to using these probes for high channel count neurophysiology (i.e. 3+ within one brain area). First, whereas metal microelectrodes are solid and can bend to some degree, allowing drives to be angled outwards to increase density [11], multisite probes are hollow, making it critical to prevent damage by inserting them orthogonal to the plane of entry. Second, standard microelectrodes use a single pin connection to route neural signals to an electrode interface board where a headstage connects, allowing for relatively close (1.5 - 2 mm) spacing of electrodes. The multisite probes however interface directly with the headstage, increasing the footprint of a single probe to roughly 11 by 3 mm.
To work within these constraints, we set out to design a modular system that could (1) actuate a probe with a similar precision to our old system (i.e. 1 mm = 3 turns), (2) provide a stable platform for recordings from multiple probes deep within the brain for up to two hours, (3) be modular such that blocks could be reconfigured to cover the breadth of brain available within the craniotomy, and (4) be 3D-printable for rapid and flexible production. To achieve (1), we designed (SolidWorks, Dassault Systèmes, Vélizy-Villacoublay, France) a new shuttle that adds a long vertical arm capable of supporting the length of the thicker hypodermic tubing, which supports the probe shank, at the connector-adjacent end, and secured with a set screw (Fig. 1c). The shuttle interfaces with an 0-80 drive screw that achieves ~330 μm/turn resolution. Tliis drive assembly is slotted into a tower (Fig. 1d) that keeps each drive stabilized via a retaining cap. In the tower shown, we record from 3 probes spaced at 3 mm between probes, at depths of up to 16 - 20 mm (with V probes, > 40 mm with K-probes) from the cortical surface (Fig. 1d, green tracks). The tower assembly is then positioned on a planar grid according to experimental needs (Fig 1d, right, 750 μm resolution shown). Each designed component was printed in-house using the Form 2 stereolithographic (SLA) printer (Form Labs, Cambridge, MA).
C. Increasing probe density
The size of the neural population we can record simultaneously is constrained by the density with which we can arrange the electrodes. For example, the closest possible spacing of 6 electrodes using our initial design is either a linear arrangement within a single tower spanning 15 mm, or split across two towers, covering a 6 × 5.25 mm area. These configurations are inadequate for lowering a large number of probes into a target region.
One strategy to increase density is to decrease the inline spacing between electrodes. We achieve this by angling opposing towers such that their trajectories cross at the target region as shown in Fig. 2a. Here, we offset one tower 1.5 mm in front of the other and tilt its base by 10° medially, resulting in approximately 1.5 mm spacing between 6 electrodes lowered to OFC, effectively halving the recording area.
Figure 2.
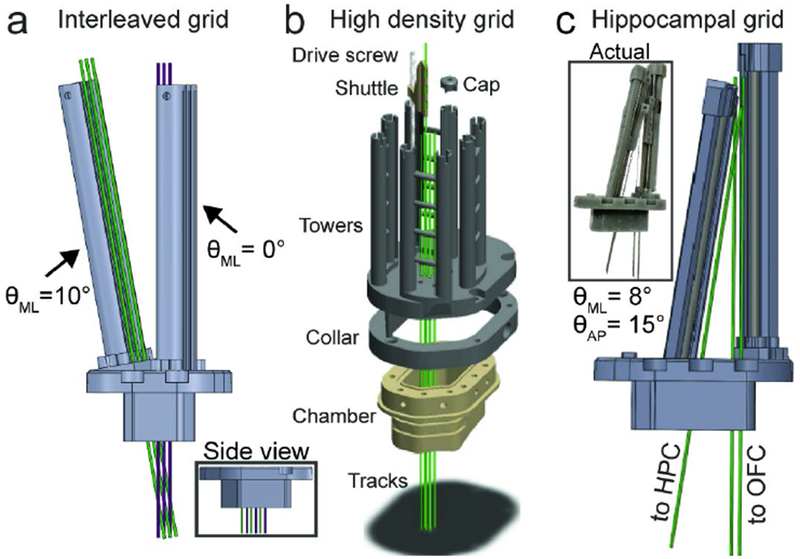
a) Linear dense interleaved grid in coronal profile; inset shows side view. b) High density grid. One shuttle, screw, and cap shown for clarity. Green tracks show electrode trajectories. Grid footprint slots into mating collar that is mounted onto chamber. c) Hippocampal grid render; inset shows photo of assembled system.
A second strategy we are currently piloting is to increase the density per square area of tissue (Fig. 2b). This design will enable us to lower 8 multisite probes within a roughly 2.5 × 2.5 mm area, a comparable footprint to conventional silicon arrays (e.g. Utah array). In order to lower 8 probes in such close proximity, we require a new shuttle design in which the electrode track is offset 12 mm from the microdrive screw. This necessitates extra stabilization, achieved using a non-conductive carbon fiber rod. Due to the extra space required by the elongated shuttles, we developed an adaptor (“collar”) that mates with the recording chamber, accoimnodating the larger footprint required by the 8 electrode towers.
D. Targeting distant brain regions
The custom-designed shuttles provide much greater flexibility with respect to the trajectory with which the probe enters the brain. This is useful for targeting regions around the craniotomy that would be otherwise unreachable with a direct approach. In addition, tailoring individual probe trajectories ensures we can enter cortex perpendicular to the cortical layers, improving our ability to study cortical layer-specific computations [17]. Finally, we can target brain regions separated by relatively large distances (30 mm), as shown in Fig. 2c. In one subject we performed simultaneous recordings from OFC in the frontal lobe and the hippocampus in the temporal lobe from a single chamber, a first in the behaving primate. Other studies have recorded from hippocampus and other prefrontal structures (e.g. lateral PFC), but did so through multiple recording chambers [18]. The pipeline for this approach is straightforward: start and end locations of the desired track are specified via the MRI anatomical model, from which the necessary AP and ML angles are back calculated and translated into a blank recording grid template. The footprint for the tower is laid down around the tracks and either printed as a single piece (Fig. 2a) or modularly (Fig. 2c).
III. Results
Fig. 3 shows an example yield of neurons simultaneously recorded from OFC and the striatum. Here we used six probes in the standard probe/tower configuration (Fig. 1). On average, we recorded approximately 1 - 1.5 well isolated single units per recording site during the first several insertions (mean ± s.e.m. units per 16-channel probe; subj. V: 16 ± 1.6, subj. C: 15 ± 1.6, subj. T: 15 ± 1.8). Although we are still piloting our 8-probe within-region system, using the other systems highlighted in this report, we have yielded 100+ unit counts within the frontal lobe in multiple sessions; we anticipate that lowering 8 32-channel probes into cortex (256 channels) will yield upwards of 200 neurons within a single region based on current data.
Figure 3.
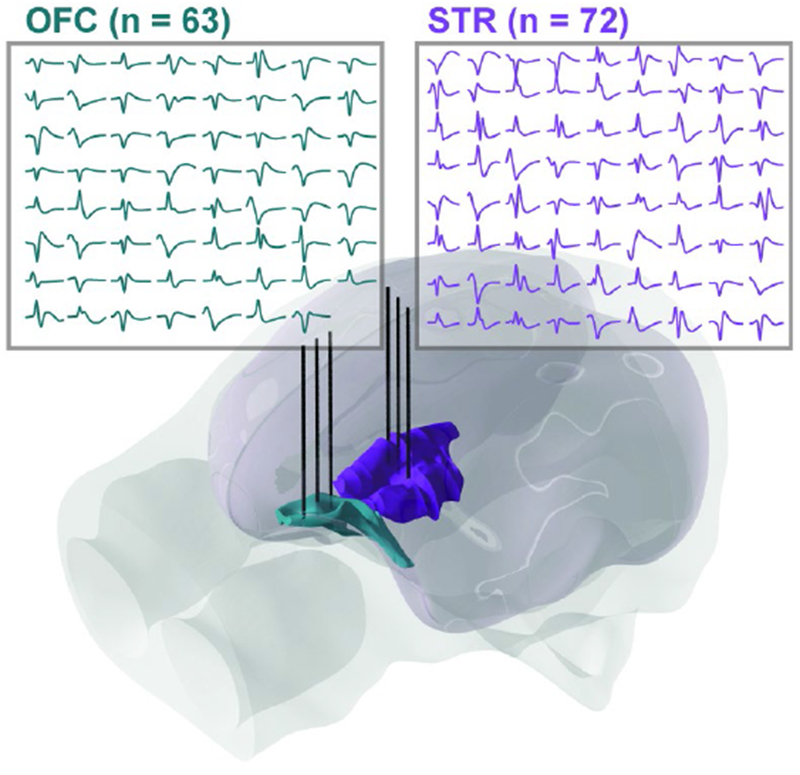
Average waveforms for well-isolated single units from six probes lowered to OFC (cyan) and striatum (purple; subj. V).
IV. Discussion
We outlined a general approach to recording increasingly large ensembles of neurons in the behaving non-human primate. starting with anatomical scans, we model brain regions of interest in order to localize a form fitting chamber. Once implanted, we have the option to use generic planar grids combined with modular multi-probe shuttle and tower groups. To increase density of recording, we can interleave probes along a single axis, or cluster several probes in close proximity by increasing the effective footprint of our recording chamber. To record from distant brain regions simultaneously, we use our models to generate a number of different trajectories that can be easily printed using commercially-available 3D printing techniques.
There are several advantages to our approach over those reported by others [10] and options developed by commercial suppliers of multisite linear probes (Plexon, NeuroNexus, etc.). First, that we know of, our approach is the only one that enables high density, high channel count (>100 channel) electrophysiology in deep structures of the primate brain. Orbitofrontal cortex sits at the base of the frontal lobe superior to the eye orbits, approximately 15 mm below the cortical surface, as does much of the striatum; hippocampus in primates is located about twice as deep at 30 mm. Thus, as investigators begin to hypothesize about the network properties of large populations of neurons in these deep regions, it is critical that they be able to collect the data. Unlike surface recordings, these regions cannot be accessed through high channel count chronic arrays.
The next advantage to our approach is the decreased insertion to yield ratio. To collect data from 100 neurons with our approach requires 5-6 probe insertions, while yielding the same with conventional microelectrodes would optimistically require 30-50 insertions (assuming 2-3 units per electrode). Not only do we benefit from collecting the data simultaneously, allowing for a more diverse set of analyses [7], the damage done to the neural tissue is drastically reduced. This increases the amount of data that can be collected from a single subject thereby reducing the number of subjects needed. Finally, the flexibility our approach affords allows us to implant and maintain only a single unilateral recording chamber. Our previous work [3]–[7] relied on bilateral recording chambers to increase yields (16-20 neurons maximum); by switching to a single implant, we reduce the maintenance requirements of the sterile environment inside the chamber(s), and reduce the likelihood that either chamber gets infected, necessitating a removal of both.
Rapid prototyping technology has enabled a quiet revolution in neuroscience [11]–[13], [19]–[21]. Combined with medical imaging and computer aided design, much of the guesswork that previously dictated the precision of neurophysiological experimentation has been eliminated. While recording technology continues to advance [10], it is only natural that new pipelines are developed. Our flexible approach has allowed us to reliably improve single neuron yields compared to our previous work. This ability allows us to test new hypotheses about both population-level dynamics of prefrontal cortex [6], [7], and how PFC interacts with far-reaching regions as part of the larger network, all while reducing damage to neural tissue caused by repeated microelectrode insertions.
V. Conclusions
We presented here one methodological approach to using multiple commercially-available linear multisite recording probes for acute primate neurophysiology. our approach combines MR imaging, computer aided design, and rapid prototyping techniques to enable precisely-targeted high channel count distributed and concentrated neural recording.
Footnotes
Research supported by R01-MH117763.
References
- [1].Fuster JM, “Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory,” J. Neurophysiol, vol. 36, no. 1, pp. 61–78, January 1973. [DOI] [PubMed] [Google Scholar]
- [2].Niki H and Watanabe M, “Prefrontal and cingulate unit activity during timing behavior in the monkey,” Brain Res, vol. 171, no. 2, pp. 213–224, August 1979. [DOI] [PubMed] [Google Scholar]
- [3].Kennerley SW, Behrens TEJ, and Wallis JD, “Double dissociation of value computations in orbitofrontal and anterior cingulate neurons,” Nat. Neurosci, vol. 14, no. 12, pp. 1581–1589, December 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hosokawa T, Kennerley SW, Sloan J, and Wallis JD, “Singleneuron mechanisms underlying cost-benefit analysis in frontal cortex,” J. Neurosci, vol. 33, no. 44, pp. 17385–17397, October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mante V, Sussillo D, Shenoy KV, and Newsome WT, “Context-dependent computation by recurrent dynamics in prefrontal cortex,” Nature, vol. 503, no. 7474, pp. 78–84, November 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lara AH and Wallis JD, “Executive control processes underlying multi-item working memory,” Nat. Neurosci, vol. 17, no. 6, pp. 876–883, April 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rich EL and Wallis JD, “Decoding subjective decisions from orbitofrontal cortex,” Nat. Neurosci, vol. 19, no. 7, pp. 973–980, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rich EL and Wallis JD, “Spatiotemporal dynamics of information encoding revealed in orbitofrontal high-gamma,” Nat. Commun, vol. 8, no. 1, p. 1139, October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ganguly K, Dimitrov DF, Wallis JD, and Cannena JM, “Reversible large-scale modification of cortical networks during neuroprosthetic control,” Nat. Neurosci, vol. 14, no. 5, pp. 662–667, May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jun JJ et al. , “Fully integrated silicon probes for high-density recording of neural activity,” Nature, vol. 551, p. 232, November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Patel SR, Ghose K, and Eskandar EN, “An open source 3-d printed modular micro-drive system for acute neurophysiology,” PLoS One, vol. 9, no. 4, p. e94262, April 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baden T, Chagas AM, Gage G, Marzullo T, Prieto-Godino LL, and Euler T, “Open Labware: 3-D Printing Your Own Lab Equipment,” PLoS Biol, vol. 13, no. 3, p. e1002086, March 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen X, Possel JK, Wacongne C, van Ham AF, Klink PC, and Roelfsema PR, “3D printing and modelling of customized implants and surgical guides for non-human primates,” J. Neurosci. Methods, vol. 286, pp. 38–55, July 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fedorov A et al. , “3D Slicer as an image computing platform for the Quantitative Imaging Network,” Magn. Reson. Imaging, vol. 30, no. 9, pp. 1323–1341, November 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Paxinos G, Huang X-F, and Toga AW, “The Rhesus Monkey Brain in Stereotaxic Coordinates,” 2000.
- [16].Mulliken GH et al. , “Custom-fit radiolucent cranial implants for neurophysiological recording and stimulation,” J. Neurosci. Methods, vol. 241, pp. 146–154, February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bastos AM, Loonis R, Kornblith S, Lundqvist M, and Miller EK, “Laminar recordings in frontal cortex suggest distinct layers for maintenance and control of working memory,” Proc. Natl. Acad. Sci. U. S. A, p. 201710323, January 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brincat SL and Miller EK, “Frequency-specific hippocampal-prefrontal interactions during associative learning,” Nat. Neurosci, vol. 18, no. 4, pp. 576–581, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kloosterman F et al. , “Micro-drive array for chronic in vivo recording: drive fabrication,” J. Vis. Exp, no. 26, April 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Headley DB, DeLucca MV, Haufler D, and Paré D, “Incorporating 3D-printing technology in the design of head-caps and electrode drives for recording neurons in multiple brain regions,” J. Neurophysiol, vol. 113, no. 7, pp. 2721–2732, April 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Freedman DS, Schroeder JB, Telian GI, Zhang Z, Sunil S, and Ritt JT, “OptoZIF Drive: a 3D printed implant and assembly tool package for neural recording and optical stimulation in freely moving mice,” J. Neural Eng, vol. 13, no. 6, p. 066013, December 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


