Abstract
Facial allodynia is a migraine symptom that is generally considered to represent a pivotal point in migraine progression. Treatment before development of facial allodynia tends to be more successful than treatment afterwards. As such, understanding the underlying mechanisms of facial allodynia may lead to a better understanding of the mechanisms underlying migraine. Migraine facial allodynia is modeled by applying inflammatory soup (histamine, bradykinin, serotonin, prostaglandin E2) over the dura. Whether glial and/or immune activation contributes to such pain is unknown. Here we tested if trigeminal nucleus caudalis (Sp5C) glial and/or immune cells are activated following supradural inflammatory soup, and if putative glial/immune inhibitors suppress the consequent facial allodynia. Inflammatory soup was administered via bilateral indwelling supradural catheters in freely moving rats, inducing robust and reliable facial allodynia. Gene expression for microglial/macrophage activation markers, interleukin-1β, and tumor necrosis factor-α increased following inflammatory soup along with robust expression of facial allodynia. This provided the basis for pursuing studies of the behavioral effects of 3 diverse immunomodulatory drugs on facial allodynia. Pretreatment with either of two compounds broadly used as putative glial/immune inhibitors (minocycline, ibudilast) prevented the development of facial allodynia, as did treatment after supradural inflammatory soup but prior to the expression of facial allodynia. Lastly, the toll-like receptor 4 (TLR4) antagonist (+)-naltrexone likewise blocked development of facial allodynia after supradural inflammatory soup. Taken together, these exploratory data support that activated glia and/or immune cells may drive the development of facial allodynia in response to supradural inflammatory soup in unanesthetized male rats.
Keywords: Headache, Dura, Interleukin-1, Tumor necrosis factor alpha, OX42, Migraine
1. Introduction
Allodynia is a lowering of the response threshold to touch/pressure stimuli wherein normally non-noxious stimuli are now perceived as noxious. Facial allodynia is commonly experienced with migraine headache and is poorly understood. Migraine facial allodynia represents a critical point in the progression of the headache in that if the migraine is treated with commonly used medications such as triptans prior to the onset of facial allodynia, the migraine is often halted, but these same medications fail to be effective when administered after allodynia develops (Olesen et al., 2009). Thus an understanding of how facial allodynia initially develops may provide insights to aid the development of more effective therapies.
Rodent models of migraine pain have established that application of ‘‘inflammatory soup” (a standard mixture of histamine, serotonin, bradykinin, and prostaglandin E2) as well as other immunogenic stimuli to the dura induces facial allodynia (Wieseler et al., 2010, 2012). The dura is commonly targeted for the study of migraine due to the innervation by trigeminal nociceptors as well as the abundance of resident immune cells in the associated meninges, including macrophages, dendritic cells, and mast cells (Braun et al., 1993; Zenker et al., 1994; McMenamin, 1999; Rozniecki et al., 1999; Artico and Cavallotti, 2001; Fischer and Reichmann, 2001; Mercier and Hatton, 2004). When activated, the resident immune cells release neuroexcitatory substances that can then act directly on dural nociceptors, leading to activation of pain-responsive neurons in the trigeminal caudal nucleus (Sp5C) (Bernstein and Burstein, 2012). Such inflammation-induced activation of this trigeminal pathway is broadly considered to underlie migraine head pain (Burstein et al., 1998, 2010; Olesen et al., 2009). The original models used to study such pain mechanisms all involved bathing the surgically-exposed dura of anesthetized rats in inflammatory soup. More recently, this approach has been modified so that the potentially confounding effects of anesthesia are eliminated by implanting cannulae and chronically indwelling supradural catheters. In turn, these newer models now allow the study of awake, behaving animals (Wieseler et al., 2010, 2012).
While studies of animal models of migraine pain have targeted neurons and elucidated a number of candidate mediators, there has been little study of whether neuroinflammation within the trigeminal pathway may contribute as well. Historically, studies of the role of glial activation in pain enhancement have focused almost exclusively on spinally-mediated pain. However, attention has more recently shifted to include pain of the head and neck as it has become apparent that mechanisms underlying trigeminal pain can fundamentally differ from those in spinal cord (Bereiter et al., 2000; Sessle, 2000; Chiang et al., 2011). To date, glial/immune contributions to trigeminal pain have been studied only in the context of localized trauma and/or inflammation of trigeminal nerve branches of the face (Shimizu et al., 2009; Lee et al., 2010; Villa et al., 2010; Takahashi et al., 2011).
Thus, the purpose of the present exploratory study is to explore whether glial/immune cell activation may potentially contribute to the initial induction of facial allodynia in an awake, behaving rat model of migraine. Here we show that supradural inflammatory soup increases Sp5C gene expression for markers of glial/immune activation. This result provided the basis for exploratory behavioral studies to define whether treatment with either of 3 diverse glial/immune modulators (minocycline, ibudilast, (+)-naltrexone) could prevent facial allodynia resultant from supradural inflammatory soup. The prevention of facial allodynia corresponds with the ibudilast blockade of glial activation in the Sp5C.
2. Results
2.1. Experiment 1: Supradural inflammatory soup activates Sp5C glia/immune cell gene expression
Experiment 1 tested bilateral supradural saline versus inflammatory soup administered twice, separated by 2 h. Prior to supradural saline versus inflammatory soup, response thresholds to facial stimuli were comparable across groups (Fig. 1A, baseline). Facial allodynia was measured 2 h after the last injection. After testing, rats were transcardially perfused as described and brains were collected and the Sp5C micropunched for gene expression changes.
Fig. 1.
Supradural inflammatory soup induces facial allodynia and increases gene expression for proinflammatory mediators compared to supradural saline, A) facial allodynia, B) complement 3b receptor (CD11b), C) IL-1beta (IL-1), and D) TNF-α (n = 5–6 per group; p < 0.05).
Supradural inflammatory soup was correlated with elevated gene expression for proinflammatory cytokine and/or glial activation markers in Sp5C tissues collected 3 h following induction of robust facial allodynia (2-way repeated measures ANOVA, significant interaction, F (1, 10) = 5.92, p < 0.05; Fig. 1A), as described above. Supradural inflammatory soup significantly increased mRNA for IL-1 (t (9) = 2.491, p < 0.05), TNF-α (t (9) = 2.443, p < 0.05), and CD11b (microglial/macrophage activation marker; t (8) = 2.4.9, p < 0.05) in the Sp5C (Fig. 1B–D). GFAP was analyzed but high variability prevented reliable changes from being detected at this timepoint of tissue collection. Taken together, these data support that at least microglia/macrophages are activated in Sp5C in response to supradural inflammatory soup. This provides the rationale for proceeding with behavioral studies of putative immune/glial inhibitors in the Experiments 2–4, below.
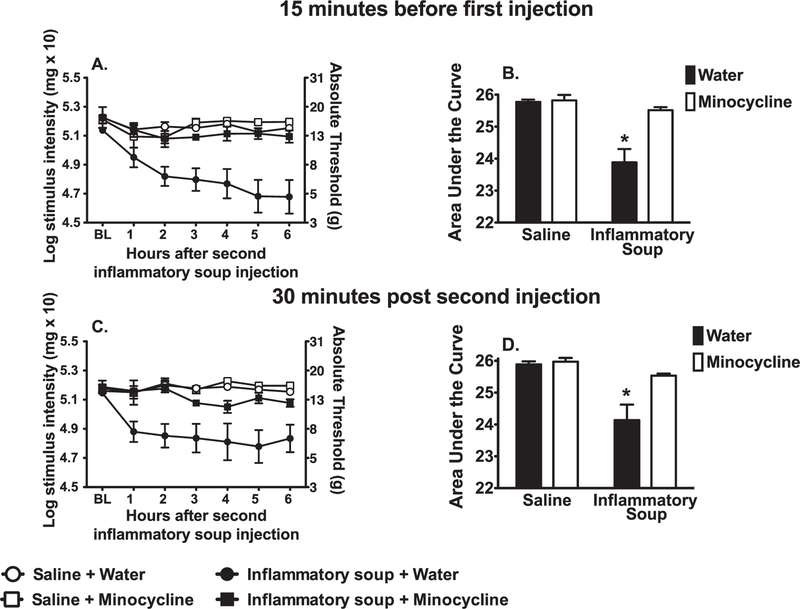
2.2. Experiment 2: Treatment with broadly used, putative glial/immune inhibitors, ibudilast and minocycline, suppresses facial allodynia when administered 15 min prior to or 30 min after inflammatory soup treatment
In Experiment 2, rats were treated with corn oil versus ibudilast (10 mg/kg), or water versus minocycline (20 mg/kg), 15 min prior to supradural saline or inflammatory soup, or 30 min after completion of supradural inflammatory soup and before the development of facial allodynia. Rats were tested every hour after the second inflammatory soup administration through 6 h.
2.2.1. Minocycline
While minocycline does have effects other than on microglia, it is broadly employed in the literature as a microglial inhibitor. Minocycline was used to test its administration would also block the development of facial allodynia. When administered prior to supradural inflammatory soup, minocycline prevented the development of facial allodynia (Fig. 2A). The 2-way ANOVA interaction comparing AUCs among vehicle vs. minocycline and saline vs. inflammatory soup was significant, F (1, 20) = 11.51, p < 0.01 (Fig. 2B). Behavior across groups did not differ statistically prior to experimental manipulations. Pre-treatment robustly prevented facial allodynia. Supradural inflammatory soup robustly induced facial allodynia with water pretreatment (p < 0.05). Facial allodynia did not develop when supradural saline was preceded by minocycline or water (p > 0.05).
Fig. 2.
Minocycline suppresses supradural inflammatory soup induced facial allodynia when administered 15 min prior to the first injection (A, time course of facial allodynia; B, AUC on which statistics were performed) and 30 min after the second injection (C, time course of facial allodynia; D, AUC on which statistics were performed). (n = 6–8 per group; left and right data were averaged; *p < 0.05; AUC, area under the curve).
Minocycline was used to further test whether its administration after completion of inflammatory soup administration also blocked development of facial allodynia. The 2-way ANOVA interaction comparing AUCs among vehicle vs. minocycline and saline vs. inflammatory soup was significant, F (1,20) = 6.517, p < 0.02 (Fig. 2D). Behavior across groups did not differ prior to experimental manipulations. Supradural inflammatory soup induced robust facial allodynia when followed by water treatment (p < 0.05). There were no significant differences between groups treated with minocycline or water following supradural saline (p > 0.05) (Fig. 2C).
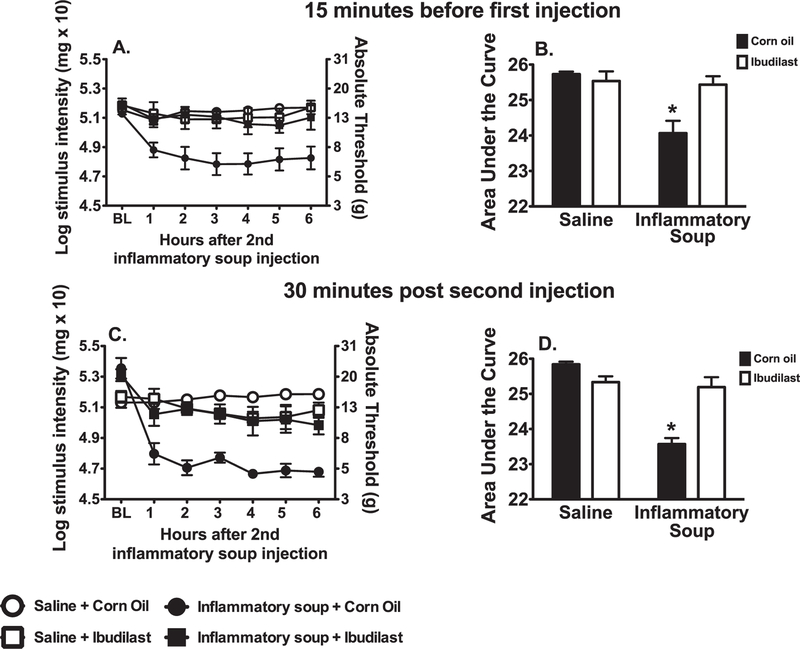
2.2.2. Ibudilast
Ibudilast was also used to test if blocking glial/immune activation would also block the development of facial allodynia. The 2-way ANOVA interaction comparing AUCs among corn oil vs. ibudilast and saline vs. inflammatory soup was significant, F (1, 20) = 9.399, p < 0.01 (Fig. 3B). Experimental groups did not differ behaviorally prior to manipulations. Pre-treatment with ibudilast prevented facial allodynia (Fig. 3A). Supradural inflammatory soup robustly induced facial allodynia when pretreated with corn oil (ibudilast vehicle) (p < 0.05). Behavior was not altered when supradural saline was preceded by ibudilast or corn oil (p > 0.05).
Fig. 3.
Ibudilast suppresses supradural inflammatory soup induced facial allodynia when administered 15 min prior to the first injection (A, time course of facial allodynia; B, AUC on which statistics were performed) and 30 min after the second injection (C, time course of facial allodynia; D, AUC on which statistics were performed). (n = 6–8 per group; left and right data were averaged; *p < 0.05; AUC, area under the curve).
As with the minocycline, ibudilast was further used to test if administration after completion of inflammatory soup administration also prevented development of facial allodynia. Again the 2-way ANOVA interaction comparing AUCs among corn oil vs. ibudilast and saline vs. inflammatory soup was significant, F (1,20) = 31.62, p < 0.0001 (Fig. 3D). Experimental groups did not differ behaviorally prior to manipulations. Treatment with ibudilast following our standard inflammatory soup injection paradigm significantly suppressed facial allodynia (Fig. 3C). Supradural inflammatory soup induced robust facial allodynia when followed by corn oil treatment (p < 0.05). There were no significant differences between groups treated with ibudilast or corn oil (vehicle) following supradural saline (p > 0.05).
2.3. Experiment 3 treatment with the TLR4 antagonist (+)-naltrexone suppress facial allodynia when administered 2 h after inflammatory soup treatment.
In Experiment 3, rats were treated with saline versus (+)-naltrexone (60 mg/kg, s.c.) 2 h after the second inflammatory soup injection. Rats were tested every hour after the second inflammatory soup administration through 6 h.
(+)-Naltrexone was used as a toll-like receptor 4 (TLR4) antagonist (Hutchinson et al., 2008, 2011; Wang et al., 2016), testing whether its administration 2 h after inflammatory soup could prevent facial allodynia. Blockade of TLR4 was chosen for study here given that it is a major activation receptor for microglia (Hutchinson et al., 2011) and has been implicated in diverse models of pain amplification (Hutchinson et al., 2008; Lewis et al., 2012). The 2-way ANOVA interaction comparing AUCs among saline vs. (+)-naltrexone and saline vs. inflammatory soup was significant, F (1, 17) = 6.415, p < 0.05 (Fig. 4B). Experimental groups did not differ behaviorally prior to manipulations. Pre-treatment with (+)-naltrexone prevented facial allodynia (Fig. 4A). Supradural inflammatory soup robustly induced facial allodynia when pretreated with saline ((+)-naltrexone vehicle) (p < 0.05). Behavior was not altered when supradural saline was preceded by (+)-naltrexone or saline (p > 0.05).
Fig. 4.
The non-opioid toll-like receptor 4 antagonist (+)-Naltrexone suppresses supradural inflammatory soup induced facial allodynia when administered 2 h after to the second injection of inflammatory soup (A, time course of facial allodynia; B, AUC on which statistics were performed). (n = 6–8 per group; left and right data were averaged; *p < 0.05; AUC, area under the curve).
3. Discussion
Here we provide converging lines of evidence supportive of an initiating role for glial and/or immune activation in facial allodynia created by supradural inflammatory soup in an awake, behaving rat model of migraine. Supradural inflammatory soup both degranulates meningeal mast cells and acts directly on meningeal nociceptors, leading to decreased periorbital thresholds to mechanical stimuli and sensitization of Sp5C neurons (Bernstein and Burstein, 2012). We show here that it also increases gene expression of proinflammatory mediators and a microglial activation marker. The activating effects of supradural inflammatory soup in Sp5C in association with inflammatory soup-induced facial allodynia has not been previously reported. This served as the basis for exploratory studies of the behavioral effects of 3 diverse immunomodulatory compounds previously implicated has having anti-inflammatory effects on immune and/glial cells. A potential role for glia and/or immune activation in the migraine model was demonstrated using the immunomodulatory drugs minocycline and ibudilast. Each prevented facial allodynia when administered either before or after supradural inflammatory soup. Lastly, facial allodynia in this model was also prevented by (+)-naltrexone, a TLR4 antagonist (Wang et al., 2016).
Supradural inflammatory soup increased gene expression of the key proinflammatory cytokines, IL-1β and TNFα, and the microglial/macrophage activation marker CD11b in Sp5C. These effects of supradural inflammatory soup on glia and/or trafficking immune cells as they relate to periorbital facial allodynia have not previously been shown. What has been previously documented is that these mediators increase in other models of facial pain, including masseter muscle inflammation (Ren and Dubner, 2011), tooth pulp inflammation (Sessle, 2000; Chiang et al., 2011), and subcutaneous (in the lip) administration of immunogenic stimuli (Lee et al., 2010). Here we show an increase in IL-1β, TNFα and CD11b within 3 h of the second injection at the point facial allodynia is robust, supporting that glial/immune activation is associated with perioribital facial allodynia.
A role for activated glia/immune cells in facial allodynia following supradural inflammatory soup is supported by behavioral suppression with systemically administered minocycline, ibudilast, and (+)-naltrexone. These putative glial/immune inhibitors were used to provide converging lines of evidence, as the only known characteristic that these agents share is their ability to inhibit proinflammatory activity demonstrated in both peripheral and central immune cells. Oshinksy et al., showed that minocycline blocked the microglia dependent blood brain barrier breakdown induced by supradural inflammatory soup and the development of chronic facial allodynia in their model of episodic migraine, presented in poster and abstract form (Oshinsky and Maxwell, 2010). Minocycline has not otherwise been tested in such pain models, and ibudilast has not been previously considered in animal models of migraine. Minocycline or ibudilast was administered either prior to application of inflammatory soup, or shortly after the two injections. The prior injection tested if blocking glial/immune activity also blocked the development of facial allodynia. Administration of these immunomodulators after supradural inflammatory soup yet before the detection of facial allodynia was to test if the cascade induced by the supradural inflammatory soup leading to facial allodynia might be halted. Both ibudilast and minocycline robustly blocked facial allodynia independent of when they were given. Likewise, (+)-naltrexone prevented facial allodynia when administered after supradural inflammatory soup as well; administration prior to inflammatory soup was not tested in this initial characterization. Taken together, these data support that neuroinflammatory responses lead to the development of periorbital facial allodynia.
Both minocycline and ibudilast have been used to investigate the mechanisms underlying mechanisms exaggerated pain states. Minocycline is a broad spectrum tetracycline antibiotic commonly used to treat acne and other skin infections (Kim and Suh, 2009). Minocycline has generally been considered a microglial inhibitor, and used to study the role of microglia in pain. Of note, recent evidence reveals effects of minocycline on DRG neurons as well (Mika et al., 2010; Kim et al., 2011). However, minocycline is known to inhibit microglial activation, proinflammatory cytokines, and inducible nitric oxide, and can increase anti-inflammatory cytokine expression (Lee et al., 2003; Wilkins et al., 2004; Ledeboer et al., 2005; Kim and Suh, 2009; O’Connor et al., 2009), and has been shown to have similar effects on macrophages (Zhang et al., 2009; Dutta et al., 2010). Given the preponderance of data on minocycline’s suppressive effects on immune/glial cells, it is reasonable to predict that minocycline is inhibiting the development of facial allodynia by blocking microglial/macrophage activation and the release of proinflammatory signals.
Ibudilast is a phosphodiesterase inhibitor that is used clinically in Japan to treat asthma and post stroke dizziness (Rolan et al., 2009). While a relatively broad-spectrum phosphodiesterase inhibitor, its actions on pain are unique from most other such inhibitors. One of these distinctive actions is its ability to attenuate glial-driven neuropathic pain via unique mechanisms compared to other immune/glial modulators (Mizuno et al., 2004; Ledeboer et al., 2006, 2007; Rolan et al., 2009). Ibudilast blocks macrophage migration inhibitory factor (MIF) (Cho et al., 2010), a proinflammatory cytokine thought to initiate proinflammatory cascade leading to the expression of TNFα and IL1β (Nishihira, 2000). MIF is increased in dorsal spinal cord glia following inflammatory pain, and blocking its activity blocks such pain (Wang et al., 2010). Ibudilast has also been shown to suppress nitric oxide, reactive oxygen species and proinflammatory cytokines, as well as enhance anti-inflammatory cytokines and neurotrophic factors in immune and glial cells (Ledeboer et al., 2007). Ibudilast does not act directly on neurons to suppress neuropathic pain, but rather, the effects of ibudilast are the result of its actions on glia and/or immune cells (Mizuno et al., 2004; Ledeboer et al., 2007; Rolan et al., 2009; Cho et al., 2010).
(+)-Naltrexone is a TLR4 antagonist (Hutchinson et al., 2008, 2011; Wang et al., 2016) that has shown efficacy in suppressing pain enhancement in a number of animal models including chronic constriction injury (Hutchinson et al., 2008), spinal cord injury (Ellis et al., 2014, 2016), spinal nerve ligation (Lewis et al., 2012). Data to date support that it can inhibit macrophage and microglial activation and consequent release of proinflammatory mediators (Hutchinson et al., 2008, 2011; Wang et al., 2016). What the agonist for TLR4 may be in this supradural inflammatory soup model remains to be defined. What is known is that TLR4 is activated in response to a wide array of endogenous danger signals (danger associated molecular patterns; DAMPs). DAMPs (e.g., high mobility group box 1; HMGB1) have been implicated previously in a spreading depression (Takizawa et al., 2016), a procedure often used to model migraine. Whether HMGB1 or other DAMP is causally related to induction of facial allodynia remains to be determined.
Supradural inflammatory soup is commonly used to model the underlying mechanisms of migraine which have most often focused on neuronal activity or meningeal mast cell activity, with facial allodynia (or nociceptor sensitization) being reliably present across supradural inflammatory soup models. We have shown here that supradural inflammatory soup creates a neuroinflammatory state in Sp5C, and that this activation is blocked along with facial allodynia when an immunomodulatory drug is given prior to allodynia onset. Taken together, our data provide initial, exploratory support a role for glia and/or immune cell activation in the development of facial allodynia and that targeting glia/immune therapeutically may lead to better treatment options. Given facial allodynia reflects a critical point in the development of migraine, further characterization of the mechanisms underlying facial allodynia is essential to better treating migraine. Future studies will be needed for a mechanistic understanding of the potent prevention of facial allodynia created by immunomodulatory drugs.
4. Methods and materials
4.1. Animals
Male Sprague-Dawley rats (250–300 gm; Harlan Sprague-Dawley, Indianapolis, IN) were pair-housed with food and water available ad libitum. Room temperature was maintained at 24 ± 1 °C on a 12 h light: 12 h dark cycle. Rats were allowed a minimum of 1 wk to habituate to the colony room prior to initiation of surgery and subsequent testing. Following surgery, animals were singly housed to protect the supradural catheters. For experimental endpoints, n = 6–8 per group for all studies. The University of Colorado Institutional Animal Care and Use Committee approved all protocols.
4.2. Drugs
Inflammatory soup (1 mM bradykinin, serotonin, and histamine, and 0.1 mM prostaglandin E2 in isotonic saline; pH 5.5) (Steen et al., 1995) was made fresh just prior to use from stock solutions. Stock solutions were stored at 4 °C for a maximum of 30 days. Supradural injections (two injections, separated by 2 h) were delivered using an infusion pump at a rate of 6 μl over 2 min 14 s (1 μl/22.3 s; Razel Scientific Instruments, INC., Stamford, CT), as described previously (Wieseler et al., 2010, 2012).
Minocycline and ibudilast were each prepared fresh just prior to use. Minocycline (Sigma/Aldrich) was prepared in water, 20 mg/ml and injected 20 mg/kg intraperitoneally (i.p.). The optimal dose of minocycline was chosen based on pilot studies.
Ibudilast (AV411, Avigen) was prepared in Mazola corn oil, 10 mg/2 ml and injected at 10 mg/kg subcutaneously (s.c.). This ibudilast dose was chosen guided by the literature (Ledeboer et al., 2006, 2007; Hutchinson et al., 2009) and based on pilot studies.
(+)-Naltrexone, a toll-like receptor 4 (TLR4) antagonist (Hutchinson et al., 2008, 2011; Wang et al., 2016) was synthesized by Dr. K.C. Rice (National Institute on Drug Abuse) and prepared fresh in saline. The 60 mg/kg dose was guided by prior publications (Hutchinson et al., 2012; Lewis et al., 2012).
4.3. Preparation and surgery for catheter placement
4.3.1. Catheter preparation
Briefly catheters were prepared as previously described (Wieseler et al., 2010, 2012). polyethylene10 tubing (BD, Franklin Lakes, NJ) was cut in 6–8 cm lengths, filled with sterile saline and heat-sealed at both ends so to exclude air bubbles. Catheters were stored in 75% ethanol for 24 h prior to surgery. Prior to implantation, catheters were transferred to sterile saline and marked with permanent marker at 4 and 6 mm from the end of the catheter that would be threaded under the skull during surgery.
4.3.2. Surgery
Surgery was performed as described previously (Wieseler et al., 2010, 2012), and is described briefly here. Rats were anesthetized under isoflurane anesthesia (1–3%) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). All surgical tools were sterilized before and during the aseptic surgical procedure. The surgical site was shaved and topical Betadine was liberally applied to the skin. A 2 cm incision was made to expose bregma. The skin was retracted, and a handheld electric drill was used to bore two troughs in the skull, bilateral to the midsaggital suture. The catheters were carefully inserted horizontally along the troughs at the point 1 mm caudal to bregma. The catheters were then glued in place at the 4 mm mark with cyanoacrylate (Superglue), catheters clipped and flushed with 5 µl of sterile saline, then heat-sealed. This catheter placement aligns the internal catheter tip so that it lies over the same dural site as previously shown to result in neuronal activation in the trigeminal nucleus caudalis, the result of activation of sensory fibers within the meninges (Wieseler et al., 2010, 2012). The scalp wound was sealed with 9 mm stainless steel wound clips, and powdered Polysporin antibiotic (Pfizer, New York, NY) liberally applied to the surgical site. The rats recovered in a heated recovery box and were individually housed once ambulatory. Catheter placement was verified at the completion of all experiments.
4.4. Facial allodynia
Following 5 days of general habituation to handling and two 5 min habituation sessions to the glove, baseline behavior was assessed, as described previously (Wieseler et al., 2010, 2012). A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) were applied to both the left and right periorbital region. Log stiffness of the hairs is determined by log10 (milligrams × 10). Starting at the smallest filament size used here (log stiffness value, 4.31; 2 grams), a stepwise progression was followed to the maximum filament size used here (log stiffness value, 5.88; 76 grams), or until the rat responded 5 times to a filament. The rat was stimulated 5 times with each filament for 2 s on each side of the face, alternating sides, and separating stimulations by approximately 3 s. Data were analyzed as previously described (Wieseler et al., 2010, 2012).
This range of monofilaments produces a logarithmically graded slope when interpolating a 50% response threshold of stimulus intensity [expressed as log10 (milligrams × 10)]. Assessments were made before (baseline) and at specified times after application of inflammatory soup, as detailed below. The behavioral responses were used to calculate the 50% withdrawal threshold (absolute threshold) by fitting a Gaussian integral psychometric function by using a maximum-likelihood fitting method. This fitting method allows parametric statistical analyses (Harvey, 1986; Treutwein and Strasburger, 1999).
4.5. Tissue collection
In experiments involving tissue collection, rats were overdosed with sodium pentobarbital, and then transcardially perfused with 0.9% saline. For gene expression endpoints, brains were collected 3 h after the second inflammatory soup injection and behavioral verification. Brains were rapidly frozen in dry ice chilled isopentane and stored at −80 °C for the later collection of bilateral Sp5C micropunches. Micropunches were collected using a chilled 18 ga needle from a cyrostat mounted frozen hindbrain.
4.6. Real-time reverse transcription-PCR (RT-PCR)
Micropunches were pooled, left and right for each animal, and processed for gene expression using real-time RT-PCR. Analyzed genes, CD11b (Complement Receptor 3b), interleukin-1 (IL-1) and tumor necrosis factor (TNF)-α, were chosen as they are often indicative of glia/immune activation.
4.6.1. RNA isolation and enrichment
Total RNA was isolated based on the methods of Chomcynski and Sacchi (Chomczynski and Sacchi, 1987), as previously described (Johnston et al., 2004). Samples were DNase treated (DNA-free kit; Ambion, Austin, TX).
4.6.2. cDNA synthesis
Total RNA was reverse transcribed using the SuperScript II First Strand Synthesis system for reverse transciption (RT)-PCR (Invitrogen), per manufacturer’s instructions and as previously described (Johnston et al., 2004). cDNA samples were diluted twofold in DNase free water and stored at −20 °C.
4.6.3. Primer specifications
cDNA sequences for rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH; accession number M17701) were obtained from GenBank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences for rat GAPDH (forward: 5ʹ-GTTTGTGATGGGTGTGAACC-3ʹ; reverse: 5ʹ-TCTTCTGAGTGGCAGTGATG-3ʹ; 162 bp product), CD11b (Complement Receptor 3b) (forward: 5ʹ-CTGGGAGATGTGAATGGAG-3ʹ; reverse: 5ʹ-ACTGATGCTGGCTACTGATG-3ʹ; 114 bp product), TNF α (forward: 5ʹ – CTTCAAGGGACAAGGCTG- 3ʹ; reverse: 5ʹ- GAGGCTGACTTTCTCCTG – 3ʹ; 87 bp product), and IL-1 (forward: 5ʹ – GAAGTCAAGACCAAAGTGG – 3ʹ, reverse: 5ʹ – TGAAGTCAACTATGTCCCG – 3ʹ; 130 bp product) were designed using the Oligo Analysis and Plotting Tool (Qiagen, Valencia, CA; oligos.qiagen.com/ooligos/toolkit.php?) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI. Primer specificity was further verified by melt curve analysis (see below).
4.6.4. Quantitative real-time PCR
PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR kit (Qiagen), as previously described (Johnston et al., 2004). cDNA (1 µl) was added to a reaction master mix (25 µl) containing SYBR Green I, fluorescein (10 nM), and gene-specific primers (500 nM each of forward and reverse primer). Reactions were conducted in triplicate in 200 µl thin wall tubes (Bio-Rad). A melt curve analysis was conducted to confirm uniformity of product formation, primer dimer formation, and amplification of nonspecific products.
4.6.5. Real-time detection and quantitation of PCR product
Formation of PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection system (BioRad). Fluorescence of SYBR Green I was captured 72 °C. Threshold for detection of PCR product fell within the log-linear phase of amplification for each reaction. Relative gene expression was determined using the 2 delta delta ct method, as previously described (Johnston et al., 2004).
4.7. Statistics
Gene expression data were analyzed using the t-test whereas behavioral data were analyzed by repeated two-way ANOVA using GraphPad Prism version 5.00 for Mac OS X (GraphPad Software, San Diego, CA). Left and right behavioral data were averaged for each rat, combined if there were no differences between the two sides, and then analyzed.
Acknowledgements
This work was supported in part by intramural funding from NIH Grant DE021966. The work of the Chemical Biology Research Branch was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA).
Abbreviations:
- AUC
area under the curve
- DAMPs
danger associated molecular patterns
- DRG
dorsal root ganglion
- HMGB1
high mobility group box 1
- IL-1
interleukin-1
- MIF
macrophage migration inhibitory factor
- Sp5C
trigeminal nucleus caudalis
- TLR4
toll-like receptor 4
- TNF
tumor necrosis factor
References
- Artico M, Cavallotti C, 2001. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc. Res. Technol 53, 212–220. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, Hu JW, 2000. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain 88, 221–224. [DOI] [PubMed] [Google Scholar]
- Bernstein C, Burstein R, 2012. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J. Clin. Neurol. Seoul Korea 8, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JS, Kaissling B, Le Hir M, Zenker W, 1993. Cellular components of the immune barrier in the spinal meninges and dorsal root ganglia of the normal rat: immunohistochemical (MHC class II) and electron-microscopic observations. Cell Tissue Res 273, 209–217. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D, 2010. Thalamic sensitization transforms localized pain into widespread allodynia. Ann. Neurol 68, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM, 1998. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol 79, 964–982. [DOI] [PubMed] [Google Scholar]
- Chiang C-Y, Dostrovsky JO, Iwata K, Sessle BJ, 2011. Role of glia in orofacial pain. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 17, 303–320. [DOI] [PubMed] [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ, 2010. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc. Natl. Acad. Sci. U.S.A 107, 11313–11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N, 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A, 2010. Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology 215, 884–893. [DOI] [PubMed] [Google Scholar]
- Ellis A, Grace PM, Wieseler J, Favret J, Springer K, Skarda B, Hutchinson MR, Falci S, Rice KC, Maier SF, Watkins LR, 2016. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain Behav. Immun in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A, Wieseler J, Favret J, Johnson KW, Rice KC, Maier SF, Falci S, Watkins LR, 2014. Systemic administration of propentofylline, ibudilast, and (+)-naltrexone each reverses mechanical allodynia in a novel rat model of central neuropathic pain. J. Pain 15, 407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HG, Reichmann G, 2001. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. Baltim Md 166, 2717–2726. 1950. [DOI] [PubMed] [Google Scholar]
- Harvey LO, 1986. Efficient estimation of sensory thresholds. Behav. Res. Methods Instrum. Comput 18, 623–632. [Google Scholar]
- Hutchinson MR et al. , 2012. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci 32, 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW, 2009. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav. Immun 23, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR, 2011. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol. Rev 63, 772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR, 2008. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur. J. Neurosci 28, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR, 2004. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. Off. J. Soc. Neurosci 24, 7353–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-S, Suh Y-H, 2009. Minocycline and neurodegenerative diseases. Behav. Brain Res 196, 168–179. [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim HI, Kim J, Park M, Song J-H, 2011. Effects of minocycline on Na+ currents in rat dorsal root ganglion neurons. Brain Res 1370, 34–42. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW, 2007. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin. Investig. Drugs 16, 935–950. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, Johnson KW, 2006. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol 2, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR, 2005. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115, 71–83. [DOI] [PubMed] [Google Scholar]
- Lee S, Zhao YQ, Ribeiro-da-Silva A, Zhang J, 2010. Distinctive response of CNS glial cells in oro-facial pain associated with injury, infection and inflammation. Mol. Pain 6, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH, 2003. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma 20, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Loram LC, Hutchinson MR, Li CM, Zhang Y, Maier SF, Huang Y, Rice KC, Watkins LR, 2012. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J. Pain 13, 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, 1999. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J. Comp. Neurol 405, 553–562. [PubMed] [Google Scholar]
- Mercier F, Hatton GI, 2004. Meninges and perivasculature as mediators of CNS plasticity. In: Non-neuronal Cells of the Nervous System: Function and Dysfuction Elsevier. [Google Scholar]
- Mika J, Rojewska E, Makuch W, Przewlocka B, 2010. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience 165, 1420–1428. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A, 2004. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology 46, 404–411. [DOI] [PubMed] [Google Scholar]
- Nishihira J, 2000. Macrophage migration inhibitory factor (MIF): its essential role in the immune system and cell growth. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res 20, 751–762. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R, 2009. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14, 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P, 2009. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol 8, 679–690. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, 2010. Maxwell CR (2010) Glial activation and blood brain barrier permeability following repeated dural stimulation. Proc. Soc. Neurosci 2010 web archive. [Google Scholar]
- Ren K, Dubner R, 2011. The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int. Rev. Neurobiol 97, 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan P, Hutchinson M, Johnson K, 2009. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin. Pharmacother 10, 2897–2904. [DOI] [PubMed] [Google Scholar]
- Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC, 1999. Morphological and functional demonstration of rat dura mater mast cell-neuron interactions in vitro and in vivo. Brain Res 849, 1–15. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, 2000. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol 11, 57–91. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K, 2009. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol. Pain 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen KH, Steen AE, Reeh PW, 1995. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J. Neurosci. Off. J. Soc. Neurosci 15, 3982–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Watanabe M, Suekawa Y, Ito G, Inubushi T, Hirose N, Murasaki K, Hiyama S, Uchida T, Tanne K, 2011. IL-1beta in the trigeminal subnucleus caudalis contributes to extra-territorial allodynia/hyperalgesia following a trigeminal nerve injury. Eur. J. Pain Lond Engl 15 467.e1–14. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Shibata M, Kayama Y, Shimizu T, Toriumi H, Ebine T, Unekawa M, Koh A, Yoshimura A, Suzuki N, 2016. High-mobility group box 1 is an important mediator of microglial activation induced by cortical spreading depression. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H, 1999. Fitting the psychometric function. Percept Psychophys 61, 87–106. [DOI] [PubMed] [Google Scholar]
- Villa G, Ceruti S, Zanardelli M, Magni G, Jasmin L, Ohara PT, Abbracchio MP, 2010. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol. Pain 6, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Shen X, Guo X, Peng Y, Liu Y, Xu S, Yang J, 2010. Spinal macrophage migration inhibitory factor contributes to the pathogenesis of inflammatory hyperalgesia in rats. Pain 148, 275–283. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, Watkins LR, 2016. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol 173, 856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieseler J, Ellis A, Sprunger D, Brown K, McFadden A, Mahoney J, Rezvani N, Maier SF, Watkins LR, 2010. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J. Neurosci. Methods 185, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieseler J, Maier SF, Watkins LR, 2012. An awake behaving rat model for the study of dural inflammation-induced facial allodyna: a rat model relevant to migraine. Methods Mol. Med 851, 99–107. [Google Scholar]
- Wilkins A, Nikodemova M, Compston A, Duncan I, 2004. Minocycline attenuates nitric oxide-mediated neuronal and axonal destruction in vitro. Neuron Glia Biol 1, 297–305. [DOI] [PubMed] [Google Scholar]
- Zenker W, Bankoul S, Braun JS, 1994. Morphological indications for considerable diffuse reabsorption of cerebrospinal fluid in spinal meninges particularly in the areas of meningeal funnels. An electronmicroscopical study including tracing experiments in rats. Anat. Embryol. (Berl) 189, 243–258. [DOI] [PubMed] [Google Scholar]
- Zhang Z-Y, Zhang Z, Fauser U, Schluesener HJ, 2009. Improved outcome of EAN, an animal model of GBS, through amelioration of peripheral and central inflammation by minocycline. J. Cell Mol. Med 13, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]