Abstract
Three near-infrared ratiometric fluorescent probes (A–C) based on TBET and FRET near-infrared rhodamine acceptors with different pKa values were designed and synthesized to achieve sensitive ratiometric visualization of pH variations in lysosomes in visible and near-infrared channels. Tetraphenylethene (TPE) was bonded to near-infrared rhodamine dyes through short electrical π -conjugation linkers to prevent an aggregation-caused quenching (ACQ) effect and allow highly efficient energy transfer of up to 98.9% from TPE donors to rhodamine acceptors. Probes A–C respond to pH variation from 7.4 to 3.0 in both buffer solutions and live cells with significant decreases of donor fluorescence and concomitant extraordinary increases of rhodamine acceptor fluorescence because of highly efficient energy transfer. In addition, probe C is capable of determining pH fluctuations in live cells treated with chloroquine. The probes show good photostability, excellent cell membrane permeability, high selectivity to pH, and two well-resolved emission peaks to ensure accurately comparative and quantitative analyses of intracellular pH changes.
1. Introduction
Traditional rhodamine dyes have been widely used to develop fluorescent probes based on a spirocyclic ring-opening switch for detection of a variety of analytes such as cations, ATP, and reactive oxygen, nitrogen and sulfur species. This is due to their excellent photophysical properties such as high absorption coefficient, excellent photostability and high fluorescence quantum yields in the visible region.1–7 To overcome the environmental effects of probe concentration variation and uneven distribution, temperature, solvent polarity, and excitation light fluctuation on a single emission of traditional rhodamine dyes, different fluorophores as donors have been introduced to the rhodamine dyes as acceptors to achieve ratiometric fluorescence imaging with an integrated self-calibration capability of dual well-separated emissions. Naphthalimine, coumarin, dansyl amide, tetraphenyl- ethene (TPE), fluorescein, and BODIPY derivatives have been used as FRET (fluorescence resonance energy transfer) or TBET (through-bond energy transfer) donors to construct fluorescent probes for ratiometric detection of various analytes.4,6 The ratiometric fluorescent probes with large pseudo-Stokes shifts can effectively eliminate the self-absorption properties of traditional rhodamine dyes with very small Stokes shifts of less than 30 nm, and accomplish high resolution fluorescence imaging with low detection limits. However, the fluorescence of these ratiometric sensing platforms is limited below 600 nm due to the distinctive emission of traditional rhodamine dyes.1–7 In order to overcome this short wavelength emission disadvantage, many near-infrared rhodamine dyes have been developed to take advantage of unique near-infrared imaging features such as superior tissue penetration, significantly reduced background fluorescence from biological samples, and low photodamage to tissues and dye photobleaching.8–21 However, ratiometric near-infrared fluorescent probes utilizing rhodamine dyes as acceptors for FRET and TBET have not been well-developed.22 Very recently, we have reported three ratiometric fluorescent probes consisting of TPE and hemicyanine moieties, and demonstrated that probes based on a TBET strategy showed excellent ratiometric responses to pH variations compared with probes based on a π-conjugation modulation strategy.22 However, it is still challenging to construct near-infrared ratiometric fluorescent probes for accurate quantitative and comparative analyses of pH variations because it is not easy to select suitable donors to match the lower fluorescence quantum yields of infrared fluorescent dye acceptors compared with the high fluorescence quantum yields of traditional rhodamine dyes as TBET or FRET acceptors.
In this paper, we develop three ratiometric near-infrared fluorescent probes (A–C) composed of TPE donors with reduced ACQ effects connected to near-infrared rhodamine acceptors to accomplish highly sensitive ratiometric detection of intracellular pH variations utilizing a combined TBET and FRET strategy, see Scheme 1. Probe A bearing a lysosome-targeting morpholine residue has a pKa value of 4.4 which is related to spirolactam ring opening. In order to increase the pKa values of the probes, bulky o-phenylenediamine and a derivative bearing tri(ethylene glycol)methyl ether residues were introduced to rhodamine acceptors to achieve higher pKa values of 4.6 and 4.8 for probes B and C, respectively. The TPE donors of probes A–C maintain aggregation-induced emission (AIE) properties23 with reduced ACQ effects and under excitation at 405 nm show strong fluorescence peaks at 489 nm, 483 nm and 486 nm under neutral and basic conditions, respectively. Probes A, B, and C respond to pH changes from 7.58 to 2.38 in both buffers and living cells ratiometrically exhibiting a decrease in TPE fluorescence under 405 nm excitation, and a concomitant increase of rhodamine fluorescence, and possess well-defined dual emissions and two Stokes shifts with large pseudo-Stokes shifts of 321 nm, 318 nm and 312 nm, respectively. Probes A, B, and C exhibit considerably large signal-to-background fluorescence increases of 365-, 1762- and 131-fold in terms of fluorescence intensity ratios of the TPE donor to the rhodamine acceptor under stimulation of pH decreases from 7.58 to 3.18, respectively. The probes enable accurate double-checked ratiometric fluorescence imaging through ratiometric analysis of visible and near-infrared fluorescence channels, and achieve quantitative analyses of intracellular pH changes. The success of these near-infrared ratiometric sensing platforms based on TPE donors will enable the expansion of a variety of ideal ratiometric fluorescent probes bearing different on–off spirolactam switches for the detection of cations, and reactive oxygen, nitrogen and sulfur species.
Scheme 1.
Chemical structure responses of fluorescent probes to pH changes with p-conjugation alternations.
2. Materials and methods
2.1. Instrumentation
Column chromatographic purification was conducted on silica gel (200–300 mesh) obtained from Sigma-Aldrich while thin-layer chromatography (TLC) analysis was conducted on silica gel plates obtained from Sigma-Aldrich. Intermediates and the probes were characterized using a Varian Unity Inova NMR spectrophotometer at 400 MHz and 100 MHz to record 1H NMR and 13C NMR spectra in CDCl3 or CD3OD solutions, respectively. Either an electrospray ionization mass spectrometer or a fast atom bombardment (FAB) ionization mass spectrometer was used to determine high-resolution mass spectrometer data (HRMS). Absorption spectra were collected by employing a Perkin Elmer Lambda 35 UV/VIS spectrometer while fluorescence spectra were recorded on a Jobin Yvon Fluoromax-4 spectrofluorometer.
2.2. Cell culture and cytotoxicity assay
Standard MTS assay was employed to evaluate the probe cytotoxicity against HeLa cells. HeLa cells were cultured in modified Eagle’s medium (DMEM, Gibco) in the presence of 10% fetal bovine serum (FBS, Fisher Scientific) in an atmosphere of 95% air and 5% CO2 at 37 1C. After the cells were further seeded into a 96-well plate (about 7 × 103 cells per well), and cultured for 24 hours, the cells were put in a fresh culture medium containing probe A, B, or C at concentration levels of 0, 5, 10, 15 to 20 µM, and further incubated for 48 h at 37 1C in a 5% CO2 humidified atmosphere. Cell viability was evaluated by incubating the cells in a fresh culture medium (80 µL) containing 20 µL CellTiter 96® Aqueous for another 2 h. Untreated cells were used as controls. The absorbance of the control cells in the absence of the probe was compared with that of the cells treated with the probe to determine the percentage of cell viability.
2.3. Probe application in cellular imaging
HeLa cells were seeded into 35 mm ×12 mm glass-bottom culture dishes and incubated for 24 h. Freshly prepared FBS-free medium containing probe A, B or C with concentrations ranging from 5, 10, 15, to 20 µM was used to replace the cell culture medium and the cells were further incubated for 1 h in a 5% CO2 humidified atmosphere. The cells were washed twice with PBS buffer before cellular imaging was conducted. In order to adjust intracellular pH values, the cells were washed with PBS buffer twice before they were incubated with 5 µg mL−1 nigericin in citrate buffers for 30 minutes with pH ranging from 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5 to 7.0 to achieve equilibration between the intracellular and extracellular pH. The cells were incubated for 10 min before they were washed with PBS buffer twice again for imaging. For an experiment under drug stimuli, HeLa cells were cultured in a medium in the presence of different concentrations of chloroquine from 50 µM, 100 µM to 200 µM for 30 min. The cells were washed with PBS buffer twice after the medium was removed. A confocal fluorescence microscope (Olympus IX 81) was employed to collect cellular fluorescence images from 475 to 525 nm for the blue fluorescence of the TPE donor in the blue channel, and those from 650 to 700 nm for the near-infrared fluorescence of the rhodamine acceptor in the red channel under TPE donor excitation at 405 nm. Near-infrared fluorescence images of the rhodamine acceptor from 650 to 700 nm in the green channel were collected under rhodamine acceptor excitation at 559 nm.
2.4. Materials
Unless specifically mentioned, all chemical reagents and solvents were obtained from commercial suppliers and used without further purification. The intermediates were synthesised and characterized, and detailed information is listed in the ESI.†
Synthesis of probe A.
Compounds 5 (61 mg, 0.1 mmol), 6(62 mg, 0.12 mmol), tetrakis (triphenylphosphine)palladium (10 mg, 8 µmol) and Na2CO3 (aq., 0.15 mL, 2 M) were dissolved in a mixed solution of toluene/methanol (10/2 mL), and stirred at 85 1C for 24 h under argon. After the solvents were removed by rotary evaporation, the residue was dissolved in ethyl acetate (20 mL), and washed with water (15 mL). The organic layer was collected, dried over Na2SO4, filtered and concentrated by rotary evaporation. The crude product was purified through flash column chromatography using an eluent of a mixed ethyl acetate and hexane solution (1 : 1, v/v) to yield the product (40 mg, 44%). 1H NMR (400 MHz, CDCl3) δ: 8.04 (d, J = 1.2 Hz, 1H), 7.65–7.62 (m, 3H), 7.54–7.51 (m, 1H), 7.46–7.43 (m, 2H), 7.38 (d, J = 8.4 Hz, 2H), 7.14–7.04 (m, 8H), 6.95 (t, J = 9.2 Hz, 3H), 6.65–6.61 (m, 4H), 6.46–6.44 (m, 2H), 6.32 (dd, J = 8.8 Hz, 2.4 Hz 1H), 3.73 (s, 3H), 3.72 (s, 3H), 3.56 (t, J = 4.4 Hz, 4H), 3.51–3.47 (m, 1H), 3.34–3.27 (m, 1H), 2.94 (s, 6H), 2.63–2.51 (m, 2H), 2.46–2.39 (m, 1H), 2.36–2.26 (m, 4H), 2.20–2.14 (m, 1H), 1.84–1.77 (m, 1H), 1.70–1.65 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 168.5, 158.4, 158.3, 152.8, 151.4, 150.2, 147.2, 147.1, 144.3, 144.1, 141.4, 140.7, 138.9, 138.5, 137.7, 136.5, 132.8, 132.3, 132.2, 131.6, 131.1, 129.0, 128.8, 128.7, 128.0, 126.6, 126.4, 123.9, 123.6, 121.1, 120.6, 114.3, 113.2, 112.7, 109.3, 106.6, 101.3, 99.0, 67.1, 66.3, 56.7, 55.3, 53.7, 40.6, 37.3, 28.5, 22.4. HRMS (ESI): calculated for C60H57N4O5 [MH]+ 913.4329, found 913.4339.
Synthesis of probe B.
Probe B was prepared according to synthesis of probe A by using compounds 10 (61 g, 0.1 mmol) and 6 (62 mg, 0.12 mmol), yielding the product (47 mg, 51%). 1H NMR (400 MHz, CDCl3) δ: 8.18 (d, J = 1.6 Hz, 1H), 7.71 (dd, J = 8.0 Hz, 1.6 Hz, 1H), 7.59 (d, J = 8.4 Hz, 1H), 7.44 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.0 Hz, 1H), 7.11–7.07 (m, 8H), 7.01–6.92 (m, 5H), 6.72 (d, J = 8.0 Hz, 1H), 6.68–6.64 (m, 5H), 6.59–6.56 (m, 1H), 6.48–6.40 (m, 4H), 3.86 (s, 2H), 3.75 (s, 3H), 3.74 (s, 3H), 2.97 (s, 6H), 2.95 (s, 6H), 2.75–2.71 (m, 1H), 2.57–2.53 (m, 1H), 2.15–2.12 (m, 1H), 1.91–1.82 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 166.8, 158.3, 158.2, 153.1, 151.3, 150.8, 150.7, 147.2, 144.3, 144.1, 144.0, 141.4, 140.7, 138.8, 137.6, 136.5, 132.8, 132.2, 132.1, 131.6, 131.4, 129.0, 128.5, 127.9, 126.6, 124.2, 123.3, 121.6, 119.0, 118.7, 118.3, 113.3, 113.2, 111.5, 109.8, 109.2, 101.0, 99.1, 70.4, 55.4, 40.7, 40.6, 29.1, 22.3. HRMS (ESI): calculated for C62H55N4O4 [M + H]+ 919.4223, found 919.4230.
Synthesis of probe C.
Probe C was synthesized similar to the route used for probe A except that compounds 12 (75 mg, 0.1 mmol) and 6 (62 mg, 0.12 mmol) were used to obtain the product (35 mg, 33%). 1H NMR (400 MHz, CDCl3) δ: 8.16 (s, 1H), 7.71 (d, J = 7.6 Hz, 1H), 7.55 (d, J = 8.4 Hz, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.20 (d, J = 8.0 Hz, 1H), 7.12–7.05 (m, 8H), 6.99– 6.91 (m, 5H), 6.68–6.62 (m, 6H), 6.55 (d, J = 6.8 Hz, 1H), 6.45–6.39 (m, 4H), 3.74 (s, 3H), 3.73 (s, 3H), 3.63–3.52 (m, 8H), 3.44–3.42 (m, 2H), 3.29 (s, 3H), 3.18–3.14 (m, 2H), 2.94 (s, 12H), 2.87–2.83 (m, 1H), 2.57–2.49 (m, 1H), 2.07–2.03 (m, 1H), 1.92–1.85 (m, 1H). 13C NMR (100 MHz, CDCl3) δ: 166.9, 158.3, 158.2, 153.3, 151.3, 150.7, 150.3, 147.4, 145.3, 144.3, 144.1, 141.4, 140.7, 138.8, 137.6, 136.4, 132.7, 132.6, 132.1, 131.6, 131.3, 128.9, 127.9, 126.5, 126.3, 124.3, 123.3, 121.6, 118.7, 117.3, 113.3, 113.2, 112.4, 111.4, 109.8, 109.2, 100.7, 99.1, 94.6, 72.1, 70.9, 70.8, 70.5, 70.1, 59.2, 55.4, 43.4, 40.7, 40.6, 29.1, 22.7. HRMS (ESI): calculated for C69H68N4NaO7 [M + Na]+ 1087.4986, found 1087.4977.
2.5. Computational details
Initial structures for probes A, B, and C were obtained using the Chem3D program. These structures were then placed into Avogadro24 and refined with the force field (UFF) calculations for an approximate starting geometry. Files suitable for Gaussian1625 were then generated and the molecules refined using density functional theory (DFT) with a hybrid functional using the TPSS functional,26,27 and with an initial electron basis set at the 6–31g(d,p) level and optimized to convergence. To obtain frequency and excited state information, a split valence and triple zeta basis set for the atoms, namely TZVP, was used.28 Imaginary frequencies were not obtained in any of the frequency calculations. The excited states were assessed on the basis of TD-DFT optimizations29 in a Polarizable Continuum Model (PCM) of water.30 The results were interpreted using GausView for all data and figures. Calculations of bond distances and angles were computed using mercury.31 The results of the calculations are given in detail in the ESI.†
3. Results and discussions
3.1. Probe design and synthetic approach
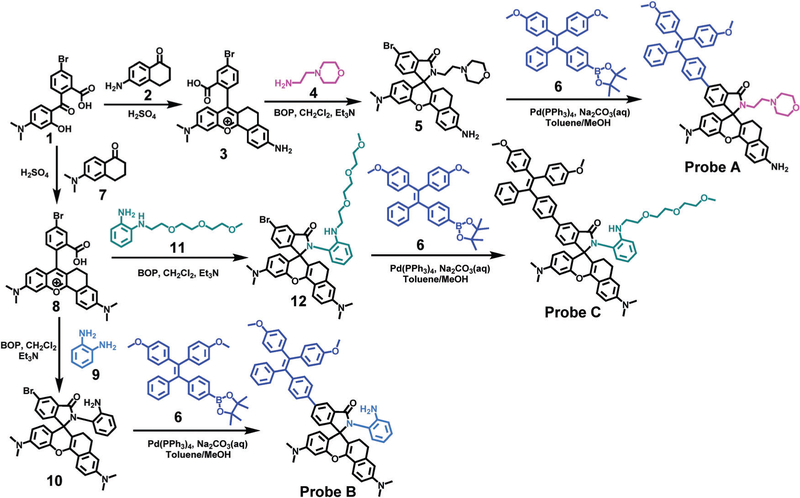
We selected TPE as a donor to take advantage of its unique AIE properties,32–40 and near-infrared rhodamines as acceptors due to their high molar absorptivity, excellent photostability and near-infrared emission.41 Thus, a combined FRET and TBET strategy was utilized to construct ratiometric fluorescent probes for the sensitive ratiometric sensing of lysosomal pH changes. Reaction of 5-bromo-2-(4-(dimethylamino)-2-hydroxybenzoyl)- benzoic acid (1) with 6-amino-1-tetralone (2) and also 6-(dimethyl- amino)-1-tetralone (7) afforded the bromo-functionalized near- infrared rhodamine dyes (3 and 8), respectively, Scheme 2. Fluorescent probe A bearing a lysosome-targeting morpholine residue was prepared by first coupling 4-(2-aminoethyl)morpholine (4) with the carboxylic acid residue of the rhodamine derivative (3) to form a closed spirolactam structure (5), and then further reacting compound 5 with the TPE derivative (6) utilizing a palladium-catalyzed Suzuki coupling reaction. In order to enhance the pKa values of the probes related to spirolactam ring opening, o-phenylenediamine (9) and N1-(2-(2-(2-methoxyethoxy)- ethoxy)ethyl)benzene-1,2-diamine (11) were introduced to the bromo-functionalized rhodamine derivative (8) forming rhodamine derivatives with closed spirolactam structures 10 and 12, respectively. Fluorescent probes B and C were prepared by coupling the TPE derivative (6) with compounds 10 and 12 through the palladium-catalyzed Suzuki coupling reaction, respectively, see Scheme 2.
Scheme 2.
Syntheses of probes A–C.
3.2. Probe AIE properties
We investigated whether TPE donors of probes A, B and C retain aggregation-induced emission properties to prevent ACQ effects (i.e., intermolecular π–π stacking). Increases in water percentages in mixed water and ethanol solutions from 0% to 99% resulted in only moderate absorbance decreases in probes A, B, and C. These absorptions were due to TPE donor absorption in probes containing closed spirolactam ring structures under neutral pH condition (Fig. S17, ESI†). However, increases in water percentage in mixed ethanol and water solutions from 0% and 80% result in significant fluorescence enhancement of TPE donors for probes A, B, and C, indicating that TPE donors possess AIE properties (Fig. S18, ESI†). A picture of probe A with different water percentages in water and ethanol mixed solutions under UV radiation also shows that the fluorescence intensity of probe A increases with water percentage being increased from 0% to 90% in the mixed solutions (Fig. S19, ESI†) presumably due to nanoaggregate formation, which was further confirmed by the results of dynamic light scattering measurements (Fig. S20 and S21, ESI†).32–40
3.3. Probe optical responses to pH variations
We studied probe absorption responses to pH variations in 10 mM citrate buffers containing 30% acetonitrile. Probes A, B and C show the absorption peaks of TPE donors at 314 nm, 325 nm and 325 nm under neutral or basic pH conditions, respectively (Fig. 1–3). As a result, functionalization of rhodamine acceptors with diamine benzene and its derivatives results in absorption red shifts of TPE donors because the benzene rings may partially conjugate with TPE donors. Gradual decreases in the pH from 7.58 to 3.2 result in the appearance and increase of two new absorption peaks, 425 and 585 nm, 445 and 607 nm, and 445 and 608 nm for each of probes A, B, and C, respectively. This is because acidic pH triggers the opening of the spirolactam ring on the rhodamine acceptors in the probes and, presumably significantly enhances π-conjugation of the rhodamine acceptors (Fig. 1). The absorbance of the TPE donor attached to probe A decreases slightly in response to pH decreases from 7.58 to 3.2 (Fig. 1) but those for probes B and C decrease moderately with the corresponding pH changes (Fig. 2 and 3).
Fig. 1.
Absorption and fluorescence spectra of 10 µM probe A in 10 mM citrate buffers with the pH range from 7.58 to 2.38 containing 30% acetonitrile under TPE excitation at 405 nm. The ratio between the TPE donor fluorescence and the rhodamine acceptor fluorescence versus pH values for the probe.
Fig. 3.
Absorption and fluorescence spectra of 10 µM probe C in 10 mM citrate buffers with the pH range from 7.58 to 2.38 containing 30% acetonitrile under TPE excitation at 405 nm. The ratio between the TPE donor fluorescence and the rhodamine acceptor fluorescence versus pH values for the probe.
Fig. 2.
Absorption and fluorescence spectra of 10 µM probe B in 10 mM citrate buffers with the pH range from 7.58 to 2.38 containing 30% acetonitrile under TPE excitation at 405 nm. The ratio between the TPE donor fluorescence and the rhodamine acceptor fluorescence versus pH values for the probe.
We also investigated whether probes A–C would show ratio- metric fluorescence responses to pH variations in citrate buffers containing 30% acetonitrile. Under excitation at 405 nm probe A displays only TPE fluorescence at 497 nm with a fluorescence quantum yield of 19.7% at pH 7.58 as the probe rhodamine acceptor preserves its closed spirolactam form at pH levels greater than 7.4. Probes B and C show similar fluorescence responses at pH 7.58 to probe A and display fluorescence from the TPE donors at 490 nm and 485 nm with fluorescence quantum yields of 27.0% and 31.9%, respectively. Probe A exhibits ideal ratiometric fluorescence responses to pH variations as gradual decreases in pH levels from 7.58 to 3.19 result in dramatic decreases of the TPE donor fluorescence, with complete dis- appearance at pH 3.19, and proportional increases of the rhodamine acceptor fluorescence at 630 nm. Probe A exhibits well-defined visible and near-infrared fluorescence peaks, and possesses two Stokes shifts of 183 nm and 45 nm, corresponding to the TPE donor and the rhodamine acceptor, respectively, and shows a big pseudo-Stokes shift of 316 nm (i.e., (rhodamine fluorescence 630 nm)–(TPE absorption 314 nm)). It offers remarkably ratiometric fluorescence responses to pH variations from 7.58 to 3.19, and achieves a significant 365-fold ratio increase in the rhodamine acceptor fluorescence to the TPE donor fluorescence. This presumably occurs through highly efficient energy transfer from the TPE donor to the rhodamine acceptor based on a combined FRET and TBET mechanism, which was confirmed by the fact that the emission spectrum of the TPE donor overlaps significantly with the absorption spectrum of the rhodamine acceptor Fig. S22, ESI,† left). Probe A with a pKa of 4.4 related to spirolactam ring opening has a fluorescence quantum yield of 22.4% with molar absorptivity of 5.8 × 104 cm−1 M−1 at pH 3.2 under TPE excitation at 405 nm. In order to increase the pKa value related to spirolactam ring opening, we introduced o-phenylenediamine and its derivative bearing tri(ethylene glycol)- methyl ether residues to the rhodamine acceptors and formed closed spirolactam ring structures for probes B and C, respectively. Using an o-phenylenediamine derivative bearing tri(ethylene glycol)methyl ether residues is also expected to significantly enhance the hydrophilicity of probe C. Probes B and C respond to pH changes ratiometrically as probe A does (Fig. 2 and 3), but possess higher pKa values of 4.6 and 4.8, respectively. These pKa values were calculated using the Henderson–Hasselbalch equation (Fig. S24–S26, ESI†).17,42–45 The increase in the pKa values for probes B and C may be ascribed to steric hindrance from the bulky o-phenylenediamine and its derivative bearing tri(ethylene glycol)methyl ether residues in the closed spirolactam structures. The highly sensitive ratiometric responses of probes B and C to pH variations also arise from highly efficient energy transfer through a combined FRET and TBET mechanism because their TPE donor emissions also overlap significantly with their rhodamine acceptor absorptions (Fig. S22, ESI,† middle and right). They also provide considerably big signal-to-background fluorescence ratio increases to pH variation from 7.58 to 3.18 as probes B and C show 1762- and 131-fold ratio increases of the TPE donor fluorescence to the rhodamine acceptor fluorescence, respectively. Probe B shows a much more significant signal-to-background fluorescence ratio increase to pH variations because of the almost complete disappearance of the TPE donor fluorescence at pH 3.19 (Fig. 2, right). Probe B also displays dual well-separated fluorescence peaks at 490 nm and 642 nm, and exhibits two Stokes shifts of 165 nm and 35 nm with a large pseudo-Stokes shift of 317 nm. Probe C also shows two well-defined emission peaks at 485 nm and 642 nm, and possesses two Stokes shifts of 160 nm and 34 nm with a pseudo-Stokes shift of 317 nm. Probes B and C possess molar absorptivities of 5.4 × 104 cm−1 M−1 and 4.2 × 104 cm−1 M−1 with fluorescence quantum yields of 25.3% and 27.7% at pH 3.19 under TPE excitation at 405 nm, respectively. Additionally, probes A, B, and C possess fluorescence quantum yields of 23.5%, 26.2% and 28.0% at pH 3.19 under rhodamine acceptor excitation at 555, 570 and 570 nm, respectively. This leads to the conclusion that the high energy transfer efficiencies of probes A, B and C from the TPE donors to the rhodamine acceptors are 95.3%, 96.6% and 98.9% with the combined TBET and FRET mechanism, respectively. The advantage of using TPE as donors is to achieve remarkable ratiometric fluorescence responses of the probes to pH changes.
3.4. Computational analysis
In order to confirm the nature of the electronic transitions, theoretical studies (geometry optimization, frequency and TD-DFT calculations) at the TPSSH46,47/TZVP48 level were conducted for probes A, B and C and their protonated versions, namely, AH+, BH+, and CH+, which feature open spirolactam arrangements. There are some noteworthy changes in the geometries of these structures upon protonation, see Fig. 4. First, there are no significant differences in the torsion twist angles regarding the biphenyl moiety in the center of the structures as they range from —34.01 to —32.91, Fig. 4.
Fig. 4.
Mercury49 drawing of the optimized geometry for probe A (left) and probe AH+ (right). H atoms are omitted for clarity. Grey: carbon; red: oxygen; blue: nitrogen. The labels apply to equivalent atom positions with the other probes which have different groups attached to the rhodamine moiety.
More significant is the increase in the angle labelled C3–C4–O5 which ranges from 134.3–178.2, 141.5–177.5 and 142.1–177.51 for probes X to XH+, (X = A, B, and C) respectively, Fig. 4. This is also accompanied by a shortening of the bond distance between the atoms labelled C3 and C4 in Fig. 4 from approximately 1.52 to 1.49 Å suggesting that a multiple bond exists between these atoms in the protonated versions of the probes. These geometries would suggest that π-conjugation or TBET between TPE and rhodamine is difficult in probes A, B and C but that upon protonation and subsequent spirolactam ring opening, conjugation is possible, and this allows for the transmission of electron density from the TPE donor to the rhodamine acceptor as illustrated above in Fig. 1–3.
There are several close transitions calculated for probes A, B and C which can be represented by broad curves with maxima at 408 (exp. 314), 370 (325) and 380 nm (325 nm) respectively, see Fig. S54, S62 and S70 (ESI†), and are in reasonable agreement with the experimental data. The nature of the transition varies but the main contributors involve π to π* transitions localized on the TPE end of the molecule, see Tables S3, S9 and S15, and corresponding Fig. S55, S63 and S71 (ESI†). Data for experimental and electronic transitions for the probes in the protonated state are summarized in Table 1. It is noteworthy that a good agreement between the calculated and experimentally obtained data was also noted with these compounds as the data in Table 1 illustrate. For probes AH+, BH+ and CH+, two transitions were obtained and are illustrated graphically in Fig. S58, S66 and S74 (ESI†), respectively. In Table 1, the lower energy transition labelled Excited State (ES) 3 for AH+ and BH+ and ES 2 for CH+ mainly originates from π to π* transitions localized on the rhodamine moieties, see Fig. S58, S66 and S74 (ESI†) for the corresponding LCAOs. The higher energy absorption consists of many individual transitions of which the maxima of the curve suggests absorptions at 430 (expt 425), 435 (445) and 435 nm (445 nm) for AH+, BH+ and CH+ respectively, as illustrated in corresponding Fig. S58, S66 and S74 (ESI†). Within these listings, significant contributions based on percentage contribution arise out of the movement from the TPE to the rhodamine end of the probes as signified by transitions 237 → 243 and 242 → 244 for AH+, 238 → 244 and 243 → 245 for BH+ and 278 → 284 and 283 → 285 for CH+ as illustrated in the respective LCAO diagrams, Fig. S59, S67 and S75 (ESI†).
Table 1.
Calculated electronic transitions (nm), experimental data and corresponding oscillator strengths (f) and their percentage contribution to the UV/Vis spectra for the protonated probes
| Probe | Transition | Experiment (nm, eV) | Calculated (nm, eV) | f | % | |
|---|---|---|---|---|---|---|
| AH+ | ES 3 | 240 → 243 | 585, 2.12 | 534, 2.32 | 0.6285 | 9.1 |
| 241 → 243 | 88.9 | |||||
| ES 5 | 237 → 243 | 425, 2.92 | 438, 2.83 | 0.3725 | 37.5 | |
| 238 → 243 | 51.1 | |||||
| 242 → 244 | 7.3 | |||||
| ES 6 | 237 → 243 | 425, 2.92 | 423, 2.93 | 0.3465 | 33.2 | |
| 238 → 243 | 3.4 | |||||
| 242 → 244 | 61.4 | |||||
| BH+ | ES 3 | 241 → 244 | 607, 2.04 | 553, 2.24 | 0.8284 | 3.2 |
| 242 → 244 | 95.0 | |||||
| ES 5 | 238 → 244 | 445, 2.79 | 453, 2.74 | 0.2589 | 5.6 | |
| 239 → 244 | 83.6 | |||||
| 242 → 247 | 2.3 | |||||
| 243 → 245 | 2.8 | |||||
| ES 6 | 237 → 244 | 445, 2.79 | 429, 2.89 | 0.4283 | 13.1 | |
| 243 → 245 | 83.9 | |||||
| CH+ | ES 2 | 281 → 284 | 608, 2.04 | 553, 2.24 | 0.8492 | 5.9 |
| 282 → 284 | 92.2 | |||||
| ES 5 | 277 → 284 | 445, 2.79 | 453, 2.74 | 0.2433 | 2.3 | |
| 278 → 284 | 7.7 | |||||
| 279 → 284 | 81.5 | |||||
| 282 → 287 | 2.1 | |||||
| 283 → 285 | 2.5 | |||||
| ES 6 | 277 → 284 | 445, 2.79 | 430, 2.89 | 0.3205 | 41.4 | |
| 278 → 284 | 5.9 | |||||
| 283 → 285 | 51.3 |
Interestingly, the nature of the transitions can be also gleaned from the different density illustrations for the excited states shown in Fig. 5. The illustrations for probes A, B, and C for the excited states depict the electron density moving from the ends of the molecule and ending up in the middle region as signified by the blue color in the middle. This is only for one specific transition and recall that there were many excited states contributing to the UV-Vis spectra. For the protonated probes, the illustrations in the middle for ES 3, show π to π* transitions localized on the rhodamine end whereas those for ES 6 show movement from the TPE to the rhodamine moieties.
Fig. 5.
Difference density illustrations as isosurfaces of probes for the excited states indicated. Blue/red areas indicate values for different densities of ±1.00e−5 for the middle column and ±5.00e−5 for the outer ones, see the scale on top of the illustration.
3.5. Probe selectivity to pH over metal ions, anions, and amino acids
We investigated the potential interference of metal ions, anions and amino acids with fluorescence responses of the probes to pH at pH 7.6 or 2.4. The presence of 50 µM metal ions such as K+, Mg2+, Ca2+, Al3+, Ag+, Mn2+, Mn2+, Ni2+, Co2+, Cu2+, Zn2+, Fe2+ or Fe3+ shows negligible influence of the probe TPE donor fluorescence and the rhodamine acceptor fluorescence in a buffer with pH 7.6 or 2.4 under excitation at 405, 555 or 570 nm (Fig. S27–S35, ESI†). These results show that probes A–C have excellent selectivity to pH over metal ions. The presence of 50 µM of different anions such as I−, Br−, NO2—, S2—, SO32—, NO3−, SO42—, HCO23— or CO32— does not lead to any significant interference with fluorescence responses of the probe TPE donor and the rhodamine acceptor to pH 7.6 and 2.4 under excitation at 405, 555 or 570 nm (Fig. S27–S35, ESI†). In addition, 50 µM amino acids and biothiols such as DL-alanine, DL-arginine, L-cysteine, DL-leucine, DL-cystine, glycine, DL-proline, DL-methionine, DL-tyrosine, and reduced glutathione display insignificant influences on the fluorescence responses of the probe donor and the acceptor to pH at 7.6 and 2.4 under excitation at 405, 555 or 570 nm (Fig. S27–S35, ESI†). Therefore, probes A–C can provide promising approaches to investigate pH-related biological processes free from interference from the biological environment.
3.6. Probe photostability and reversibility to pH changes
We studied whether probes A–C could respond to pH variations reversibly. The fluorescence of the probe TPE donors and the rhodamine acceptors can well respond to pH changes from 2.4 to 7.6 reversibly under TPE donor excitation at 405 nm or rhodamine acceptor excitation at 555 nm or 570 nm (Fig. S36–S38, ESI†), indicating that probes A–C show reversible responses to pH changes.
We investigated the probe photostability under continuous probe excitation with 5 min intervals and through the assessment of the fluorescence intensity of the TPE donors or the rhodamine acceptors for every 10 min. Probes A, B, and C exhibit good photostability with TPE fluorescence decreases by 2.4%, 3.7%, and 2.8% in pH 7.6 citrate buffers, rhodamine fluorescence decreases by 4.3%, 4.3% and 2.2% in pH 2.5 citrate buffer under 1 h TPE excitation at 405 nm, and with rhodamine fluorescence decreases by 2.0%, 3.5% and 3.5% under 1 h rhodamine excitation at 555 nm for probe A, and at 570 nm for probes B and C, respectively (Fig. S39, ESI†). The TPE fluorescence intensities of probes A, B, and C decrease by 7.5%, 9.5% and 5.5% in pH 7.6 citrate buffers under 3 h TPE excitation at 405 nm while the rhodamine fluorescence intensities of probes A, B, and C decrease by 10%, 13% and 8.6% in pH 2.4 citrate buffer under 3 h TPE excitation at 405 nm, and by 7.4%, 11% and 7.4% at pH 2.4 under 3 h rhodamine excitation at 555 nm for probe A, and at 570 nm for probes B and C, respectively (Fig. S39, ESI†). The results indicate that the probes possess good photostability.
3.7. Cytotoxicity of the fluorescent probes
We investigated the effect of the probes on the viability of HeLa cells with various probe concentrations ranging from 5 µM to 20 µM by using MTS assay to assess the cytotoxicity of the probes. No significant toxicity was observed with 20 µM probes A–C since the cell viability is greater than 82% at 20 µM probe concentration, indicating that the probes have a potential application in intracellular imaging of live cells because of their low cytotoxicity and good biocompatibility (Fig. S40, ESI†).
3.8. Probe applications in cellular imaging
We investigated whether the probes could penetrate and stain the cells for potential cellular imaging applications. Three color channels were employed for these experiments: blue fluorescence channel from 475 to 525 nm under TPE donor excitation at 405 nm, and two near-infrared fluorescence channels from 650 to 700 nm (pseudo-colored as red and green for clarity) under TPE donor excitation at 405 nm and under rhodamine acceptor excitation at 559 nm, respectively. 15 µM of the probe A–C level shows both strong cellular visible fluorescence intensities of the TPE donors in the first column and intense cellular near- infrared fluorescence of the rhodamine acceptors in the second column under TPE donor excitation at 405 nm (Fig. S41–S43, ESI†). In addition, they also display strong cellular near-infrared fluorescence intensities of the rhodamine acceptors in the third column at the 15 µM concentration level under rhodamine excitation at 559 nm (Fig. S41–S43, ESI†). Increases of the probe concentrations significantly enhance the fluorescence signals of both TPE donors in the blue channel and rhodamine acceptors in the red or green channel under TPE donor excitation at 405 nm or under rhodamine acceptor excitation at 559 nm. Probe C shows stronger donor and acceptor fluorescence intensities than probes A and B at the same probe concentration and pH levels because probe C possesses a higher pKa value of 4.8 related to the spirolactam ring opening than probes A and B. These results demonstrate that the probes possess excellent cell membrane permeability. In order to prove the probes’ potential targeting lysosomes in live cells, we conducted colocalization correlation analysis by incubating HeLa cells with a near-infrared lysotracker42 and probes A, B and C, respectively (Fig. S44, ESI†). The Pearson’s colocalization coefficients between the donor excitation blue channel and the near-infrared Lysotracker channel for probes A, B and C are 0.917, 0.896 and 0.934 respectively, indicating that our probes stay with the near- infrared Lysotracker together in the lysosomes in live cells.
With these cellular imaging data in hand, we further investigated whether probes A, B and C could be applied in quantification of intracellular pH values. HeLa cells were incubated in a series of buffers with different pH values of 3.5, 4.0, 4.5, 5.5, 6.0, 6.5 and 7.0 in the presence of 5 µM nigericin (K+/H+ ionophore) to equilibrate the intracellular pH with external media.50 Probe A responds to intracellular pH variations from 7.0 to 3.5 ratiometrically with gradual decreases in blue intracellular TPE donor emission in the first row, and gradual concomitant increases in near-infrared intracellular rhodamine acceptor emission (using red channel) in the second row under TPE donor excitation at 405 nm. These observations match well with the fluorescence spectral results in buffer solutions (Fig. S45, ESI†). In addition, the near-infrared intracellular emission of the rhodamine acceptor (using green channel) in the third row also gradually increases under rhodamine acceptor excitation at 559 nm when pH decreases from 7.0 to 3.5, which is also consistent with the fluorescence spectral results in buffer solutions (Fig. S24, ESI†). The overlapped images in the first and second rows show dramatic color changes from deep blue, purple, pink to deep red when pH changes from 7.0 to 3.5, demonstrating that probe A can respond to pH changes in live cells ratiometrically (Fig. S45, ESI†). Probe A shows dual Stokes shifts of 177 nm and 48 nm with a big Stokes shift of 321 nm, and possesses dual well-separated emissions under dual excitations (Fig. 1 and Fig. S24, ESI†). The overlapped images of the blue and green channels also show substantial color changes from deep blue, cyan, to deep green in the fifth row in response to intracellular pH variations from 7.0 to 3.5 (Fig. S45, ESI†). Moreover, the overlapped images of the two near-infrared channels in the second and third rows under TPE donor and rhodamine acceptor excitations also show significant color changes from light yellow to deep yellow in response to intracellular pH changes from 7.0 to 3.5 (Fig. S45, ESI†). These results convincingly demonstrate that probe A can enable the accurately double-checked fluorescence ratiometric sensing of intracellular pH changes through imaging colocalization of visible and near-infrared fluorescence channels. In addition, ratiometric images (red channel/blue channel) show significant color changes from strong brownish white to weakly brownish white upon intracellular pH changes from 3.5 to 6.0 (Fig. S46, ESI†). Probe B possesses a higher pKa value than probe A since the weak near-infrared fluorescence of probe B can be observed at pH 6.0 in the third row under rhodamine excitation (Fig. S47, ESI†). Probe C also exhibits similar ratiometric fluorescence responses to pH variations in living cells, but possesses a higher pKa value related to the opening of the rhodamine acceptor spirolactam ring than probe B because the near-infrared fluorescence of probe C can be observed at pH 6.0 under TPE donor excitation (the second row), and at pH 6.5 (the third row) under rhodamine excitation (Fig. 6). The higher pKa values of probes B and C are due to significant steric hindrance in their spirolactam ring structures with bulky o-phenylenediamine and its derivative residues (Scheme 1). The statistical analysis of the confocal imaging data in Fig. 8 also demonstrates that probes A, B and C show remarkable ratiometric fluorescence responses to intra- cellular pH variations from 7.0 to 3.5 with considerable fluorescence decreases of TPE donors, and concomitant increases of rhodamine acceptors (Fig. 7, Fig. S48, S49 and S51, ESI†), which is consistent with ratiometric fluorescence responses to pH variations in buffers (Fig. 1–3).
Fig. 6.
Cellular fluorescence images of 15 µM probe C incubated with HeLa cells in 10 mM citrate buffers with the pH range from 3.5 to 7.0 in the presence of 5 µg mL−1 nigericin. The blue channel in the first row was collected from 475 to 525 nm, and two NIR channels (pseudo-colored as red and green for clarity) in the second and third rows were collected from 650 to 700 nm under excitation of the TPE donor and the rhodamine acceptor at 405 nm and 559 nm, respectively. A confocal fluorescence microscope was employed to obtain fluorescence images at 60× magnification with scale bars of 50 µM.
Fig. 8.
Cellular fluorescence images of 20 µM probe C incubated with HeLa cells in 10 mM citrate buffers with pH 7.4 in the absence and in the presence of 100 and 200 µM chloroquine. The blue channel in the first column was obtained from 475 to 525 nm, and two NIR channels (pseudo-colored as red and green for clarity) in the second and third columns were got from 650 to 700 nm under excitation of the TPE donor and the rhodamine acceptor at 405 nm and 559 nm, respectively. A confocal fluorescence microscope was used to acquire the images at 60× magnification with scale bars of 50 µM.
Fig. 7.
Cellular fluorescence intensities of TPE donors and rhodamine acceptors in probes A (left), B (middle) and C (right) in 10 mM citrate buffers with the pH range from 3.5 to 7.0 having 5 µg mL−1 nigericin under TPE excitation at 405 nm. Statistical analysis of the confocal imaging data in Fig. 6 and Fig. S45, S47 (ESI†) generates the donor and acceptor fluorescence intensities of probes A–C in HeLa live cells.
We further applied probe C in monitoring the pH fluctuations in live cells under a drug stimulus. We treated HeLa cells with 100 and 200 µM chloroquine since chloroquine as a lysosomotropic agent can increase the lysosomal pH by inhibiting autophagy and protein degradation (Fig. 8 and Fig. S50, ESI†).51–54 Compared with control cells without chloroquine treatment, the cellular blue fluorescence intensity of the TPE donor in the first column slightly increases, and the cellular near-infrared fluorescence intensities of the rhodamine acceptor in the second and third columns decrease after the cells were treated with 100 and 200 µM chloroquine, indicating that the drug stimulus results in an increase in lysosomal pH. The fluorescence intensity of the overlapped fluorescence images in the sixth column also becomes weaker after chloroquine treatment of the cells. The overlapped images in the fourth column change colors from pink to blue with slightly pink under the chloroquine stimulus while the overlapped images in the fifth column also undergo significant color changes from ice blue to azure blue. In addition, the ratiometric images (red channel/blue channel) show significant color changes from reddish yellow to reddish blue before and after the chloroquine stimulus (Fig. S50, ESI†). These results have convincingly demonstrated that the probe shows excellent performance with high contrast in visualizing intracellular pH and its fluctuations.
4. Conclusion
In summary, we have designed and synthesized three ratiometric fluorescent probes consisting of TPE donors and near-infrared acceptors in order to achieve sensitive, quantitative and comparative analyses of intracellular pH variations. Probes A–C with different pKa values have good photostability, excellent cell membrane permeability, biocompatibility, high selectivity, sensitivity, and remarkable ratiometric fluorescence responses to pH changes in both solutions and living cells. They are capable of visualizing intracellular minor pH fluctuations under a drug stimulus. The probes allow for the development of various ratiometric fluorescent probes for quantitative and comparative reliable analyses of cations, and reactive nitrogen, oxygen and sulfur species by conjugating various biosensing groups into the near-infrared rhodamine acceptors.
Supplementary Material
Acknowledgements
The research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R15GM114751 (to Haiying Liu) and the China Scholarship Council (to Jianbo Wang). We would like to thank Professor Ashutosh Tiwari for insightful discussion.
Footnotes
Electronic supplementary information (ESI) available.
Conflicts of interest
There are no conflicts to declare.
References
- 1.Kaur B, Kaur N and Kumar S, Coord. Chem. Rev, 2018, 358, 13–69. [Google Scholar]
- 2.Sivaraman G, Iniya M, Anand T, Kotla NG, Sunnapu O, Singaravadivel S, Gulyani A and Chellappa D, Coord. Chem. Rev, 2018, 357, 50–104. [Google Scholar]
- 3.Gupta A and Kumar N, RSC Adv, 2016, 6, 106413–106434. [Google Scholar]
- 4.Zhang RQ, Yan FY, Huang YC, Kong DP, Ye QH, Xu JX and Chen L, RSC Adv, 2016, 6, 50732–50760. [Google Scholar]
- 5.Zhu H, Fan JL, Wang BH and Peng XJ, Chem. Soc. Rev, 2015, 44, 4337–4366. [DOI] [PubMed] [Google Scholar]
- 6.Yuan L, Lin WY, Zheng KB and Zhu SS, Acc. Chem. Res, 2013, 46, 1462–1473. [DOI] [PubMed] [Google Scholar]
- 7.Beija M, Afonso CAM and Martinho JMG, Chem. Soc. Rev, 2009, 38, 2410–2433. [DOI] [PubMed] [Google Scholar]
- 8.Zheng QS and Lavis LD, Curr. Opin. Chem. Biol, 2017, 39, 32–38. [DOI] [PubMed] [Google Scholar]
- 9.Ikeno T, Nagano T and Hanaoka K, Chem. – Asian J, 2017, 12, 1435–1446. [DOI] [PubMed] [Google Scholar]
- 10.Umezawa K, Citterio D and Suzuki K, Anal. Sci, 2014, 30, 327–349. [DOI] [PubMed] [Google Scholar]
- 11.Yuan L, Lin WY, Zheng KB, He LW and Huang WM, Chem. Soc. Rev, 2013, 42, 622–661. [DOI] [PubMed] [Google Scholar]
- 12.Mao ZQ, Jiang H, Song XJ, Hu W and Liu ZH, Anal. Chem, 2017, 89, 9620–9624. [DOI] [PubMed] [Google Scholar]
- 13.Grimm JB, Muthusamy AK, Liang YJ, Brown TA, Lemon C, Patel R, Lu RW, Macklin JJ, Keller PJ, Ji N and Lavis LD, Nat. Methods, 2017, 14, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm JB, Brown TA, Tkachuk AN and Lavis LD, ACS Cent. Sci, 2017, 3, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang HX, Liu J, Liu CL, Yu PC, Sun MJ, Yan XH, Guo JP and Guo W, Biomaterials, 2017, 133, 60–69. [DOI] [PubMed] [Google Scholar]
- 16.Chen YC, Zhang WJ, Cai YJ, Kwok RTK, Hu YB, Lam JWY, Gu XG, He ZK, Zhao Z, Zheng XY, Chen B, Gui C and Tang BZ, Chem. Sci, 2017, 8, 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang MX, Adhikari R, Bi JH, Mazi W, Dorh N, Wang JB, Conner N, Ainsley J, KarabenchevaChristova TG, Luo FT, Tiwari A and Liu HY, J. Mater. Chem. B, 2017, 5, 9579–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu GL, Zhang PP, Liu WM, Wang MQ, Zhang HY, Wu JS, Zhang LP and Wang PF, Anal. Chem, 2017, 89, 1922–1929. [DOI] [PubMed] [Google Scholar]
- 19.Ren TB, Xu W, Zhang W, Zhang XX, Wang ZY, Xiang Z, Yuan L and Zhang XB, J. Am. Chem. Soc, 2018, 140, 7716–7722. [DOI] [PubMed] [Google Scholar]
- 20.Ren TB, Xu W, Jin FP, Cheng D, Zhang LL, Yuan L and Zhang XB, Anal. Chem, 2017, 89, 11427–11434. [DOI] [PubMed] [Google Scholar]
- 21.Chen TH, Zhang SW, Jaishi M, Adhikari R, Bi JH, Fang MX, Xia S, Luck RL, Pati R, Lee HM, Luo FT, Tiwari A and Liu HY, ACS Appl. Bio Mater, 2018, 1, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JB, Xia S, Bi JH, Fang MX, Mazi WF, Zhang YB, Conner N, Luo FT, Lu HP and Liu HY, Bioconjugate Chem, 2018, 29, 1406–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Chi Z, Li H, Zhang X, Li X, Liu S, Zhang Y and Xu J, J. Phys. Chem. C, 2011, 115, 17574–17581. [DOI] [PubMed] [Google Scholar]
- 24.Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E and Hutchison GR, J. Cheminf, 2012, 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM,Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O¨, Foresman JB, Ortiz JV, Cioslowski J and Fox DJ, Gaussian 16, Gaussian, Inc., Wallingford CT, 2016. [Google Scholar]
- 26.Staroverov VN, Scuseria GE, Tao JM and Perdew JP, J. Chem. Phys, 2003, 119, 12129–12137. [Google Scholar]
- 27.Tao JM, Perdew JP, Staroverov VN and Scuseria GE, Phys. Rev. Lett, 2003, 91, 146401–146404. [DOI] [PubMed] [Google Scholar]
- 28.Schafer A, Huber C and Ahlrichs R, J. Chem. Phys, 1994, 100, 5829–5835. [Google Scholar]
- 29.Casida ME, Jamorski C, Casida KC and Salahub DR, J. Chem. Phys, 1998, 108, 4439–4449. [Google Scholar]
- 30.Cances E, Mennucci B and Tomasi J, J. Chem. Phys, 1997, 107, 3032–3041. [Google Scholar]
- 31.Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J and Wood PA, J. Appl. Crystallogr, 2008, 41, 466–470. [Google Scholar]
- 32.Mei J, Hong YN, Lam JWY, Qin AJ, Tang YH and Tang BZ, Adv. Mater, 2014, 26, 5429–5479. [DOI] [PubMed] [Google Scholar]
- 33.Qian J and Tang BZ, Chem, 2017, 3, 56–91. [Google Scholar]
- 34.Hu R, Leung NLC and Tang BZ, Chem. Soc. Rev, 2014, 43, 4494–4562. [DOI] [PubMed] [Google Scholar]
- 35.Ding D, Li K, Liu B and Tang BZ, Acc. Chem. Res, 2013, 46, 2441–2453. [DOI] [PubMed] [Google Scholar]
- 36.Kwok RTK, Leung CWT, Lam JWY and Tang BZ, Chem. Soc. Rev, 2015, 44, 4228–4238. [DOI] [PubMed] [Google Scholar]
- 37.Feng GX and Liu B, Small, 2016, 12, 6528–6535. [DOI] [PubMed] [Google Scholar]
- 38.Hu F and Liu B, Org. Biomol. Chem, 2016, 14, 9931–9944. [DOI] [PubMed] [Google Scholar]
- 39.Liang J, Tang B and Liu B, Chem. Soc. Rev, 2015, 44, 2798–2811. [DOI] [PubMed] [Google Scholar]
- 40.Liu HY, Fang MX, Xia SH, Bi JH, Wigstrom TP, Valenzano L, Wang JB, Mazi W, Tanasova M and Luo F-T, Chem. Commun, 2018, 54, 7625–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong YJ, Zhang XB, Mao GJ, Su L,Meng HM, Tan WH, Feng SL and Zhang GS, Chem. Sci, 2016, 7, 2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang SW, Chen TH, Lee HM, Bi JH, Ghosh A, Fang MX, Qian ZC, Xie F, Ainsley J, Christov C, Luo FT, Zhao F and Liu HY, ACS Sens, 2017, 2, 924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JT,Yang M, Li C, Dorh N, Xie F, Luo FT, Tiwari A and Liu HY, J. Mater. Chem. B, 2015, 3, 2173–2184. [DOI] [PubMed] [Google Scholar]
- 44.Vegesna GK, Janjanam J, Bi JH, Luo FT, Zhang JT, Olds C, Tiwari A and Liu HY, J. Mater. Chem. B, 2014, 2, 4500–4508. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JT, Yang M, Mazi WF, Adhikari K, Fang MX, Xie F, Valenzano L, Tiwari A, Luo FT and Liu HY, ACS Sens, 2016, 1, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao J, Perdew JP, Staroverov VN and Scuseria GE, Phys. Rev. Lett, 2003, 91, 146401. [DOI] [PubMed] [Google Scholar]
- 47.Staroverov VN, Scuseria GE, Tao J and Perdew JP, J. Chem. Phys, 2003, 119, 12129–12137. [Google Scholar]
- 48.Scha¨fer A, Huber C and Ahlrichs R, J. Chem. Phys, 1994, 100, 5829–5835. [Google Scholar]
- 49.Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J and Wood PA, J. Appl. Crystallogr, 2008, 41, 466–470. [Google Scholar]
- 50.Wei YF, Cheng D, Ren TB, Li YH, Zeng ZB and Yuan L, Anal. Chem, 2016, 88, 1842–1849. [DOI] [PubMed] [Google Scholar]
- 51.Dong BL, Song XZ, Wang C, Kong XQ, Tang YH and Lin WY, Anal. Chem, 2016, 88, 4085–4091. [DOI] [PubMed] [Google Scholar]
- 52.Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB and Stitt AW, Curr. Eye Res, 2004, 28, 277–284. [DOI] [PubMed] [Google Scholar]
- 53.Wu LL, Li XL, Huang CS and Jia NQ, Anal. Chem, 2016, 88, 8332–8338. [DOI] [PubMed] [Google Scholar]
- 54.Zhu H, Fan JL, Xu QL, Li HL, Wang JY, Gao P and Peng XJ, Chem. Commun, 2012, 48, 11766–11768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.