Abstract
Objective
Matching-adjusted indirect comparison (MAIC) can be used to assess the comparative effectiveness of two treatments indirectly using data from randomized placebo-controlled trials. This MAIC assessed the comparative effectiveness of secukinumab (an anti-interleukin-17A) and etanercept (a tumor necrosis factor inhibitor) in a target population of biologic-naïve patients with psoriatic arthritis (PsA).
Methods
Individual patient data pooled from FUTURE 2 (NCT01752634), FUTURE 3 (NCT01989468), and FUTURE 5 (NCT02404350) (secukinumab: 150 mg, n=458 and 300 mg, n=461) were matched to data from the population in the NCT00317499 trial (etanercept 25 mg, n=101) using MAIC methodology, by adjusting for clinical and demographic baseline characteristics. Recalculated outcomes from FUTURE 2, 3, and 5 (150 mg, effective sample size (ESS) post-matching=104; 300 mg, ESS=75; and placebo, ESS=159) were compared with the NCT00317499 trial. Pairwise comparisons using odds ratios (ORs) were performed for the American College of Rheumatology (ACR) 20, 50, and 70 response criteria at week 12 (placebo-adjusted) and week 24 (non-placebo-adjusted).
Results
At week 12, there were no significant differences in ACR responses between secukinumab and etanercept. There was no significant difference between secukinumab 150 mg and etanercept at week 24 with respect to ACR 20 and 50 response rates; however, ACR 70 response rates were higher for secukinumab 150 mg (OR (95% confidence interval (CI)): 4.48 (2.01–9.99), p<0.001). ACR 20, 50, and 70 response rates were higher with secukinumab 300 mg than with etanercept at this time point (OR (95% CI): ACR 20, 3.28 (1.69–6.38), p<0.001; ACR 50, 1.90 (1.04–3.50), p=0.038; and ACR 70, 3.56 (1.51–8.40), p=0.004).
Conclusion
In this MAIC, secukinumab was associated with higher ACR 20 and 50 (secukinumab 300 mg) and 70 (secukinumab 150 mg and 300 mg) response rates at week 24 than etanercept in biologic-naïve patients with active PsA, whereas no significant difference was observed in the short-term at week 12.
Keywords: Comparative effectiveness, etanercept, matching-adjusted indirect comparison, psoriatic arthritis, secukinumab
Introduction
Active psoriatic arthritis (PsA) is typically treated with conventional synthetic biologic disease-modifying anti-rheumatic drugs (csDMARDs) as first systemic treatment (1). Both the European League against Rheumatism (1, 2) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) (3) recommend the use of biologic DMARDs (bDMARDs) when response to csDMARDs is inadequate. The GRAPPA guidelines also recommend biologic therapy, if feasible, in patients with evidence of aggressive disease progression. Currently approved and recommended bDMARDs comprise the tumor necrosis factor inhibitors (TNFis) adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab, and the monoclonal antibodies ustekinumab, which targets interleukin (IL)-12/23, and secukinumab and ixekizumab, which target IL-17A. The targeted synthetic DMARD apremilast, a small-molecule inhibitor that modulates inflammatory cytokine production via the inhibition of phosphodiesterase 4, has also been approved for the treatment of PsA.
Matching-adjusted indirect comparison (MAIC) is a statistical technique that simulates a direct comparison of two therapies (4, 5) by matching individual patient data (IPD) from one trial to published aggregate data from another trial (6, 7). MAIC is one of several methods that can be used to conduct indirect treatment comparisons when patient-level data are not available for all trials (8). When no head-to-head randomized controlled trials (RCTs) have been conducted, such methods may generate the best RCT-based comparative evidence available.
Health technology assessment agencies have acknowledged MAIC as a robust analysis method (9), and the UK’s National Institute for Health and Care Excellence (NICE) has published methodological guidelines on its use (5, 8). MAIC has been used to provide comparative effectiveness evidence in multiple therapeutic areas, including PsA (10–12).
In the present study, secukinumab, a fully human IL-17A inhibitor, with etanercept, a recombinant TNF receptor–immunoglobulin G fusion protein, was compared using pooled IPD from the FUTURE 2 (NCT01752634) (13), FUTURE 3 (NCT01989468) (14), and FUTURE 5 (NCT02404350) (15) trials for secukinumab and aggregate data from the NCT00317499 trial for etanercept (16) in a target population of biologic-naïve patients with PsA.
Methods
MAIC
The MAIC methodology comprises four main steps and has been described in detail in previous studies (5, 11).
Identification of source data by systematic literature review (SLR)
The SLR conducted to identify evidence is summarized in the Supplementary Methods section.
Studies included
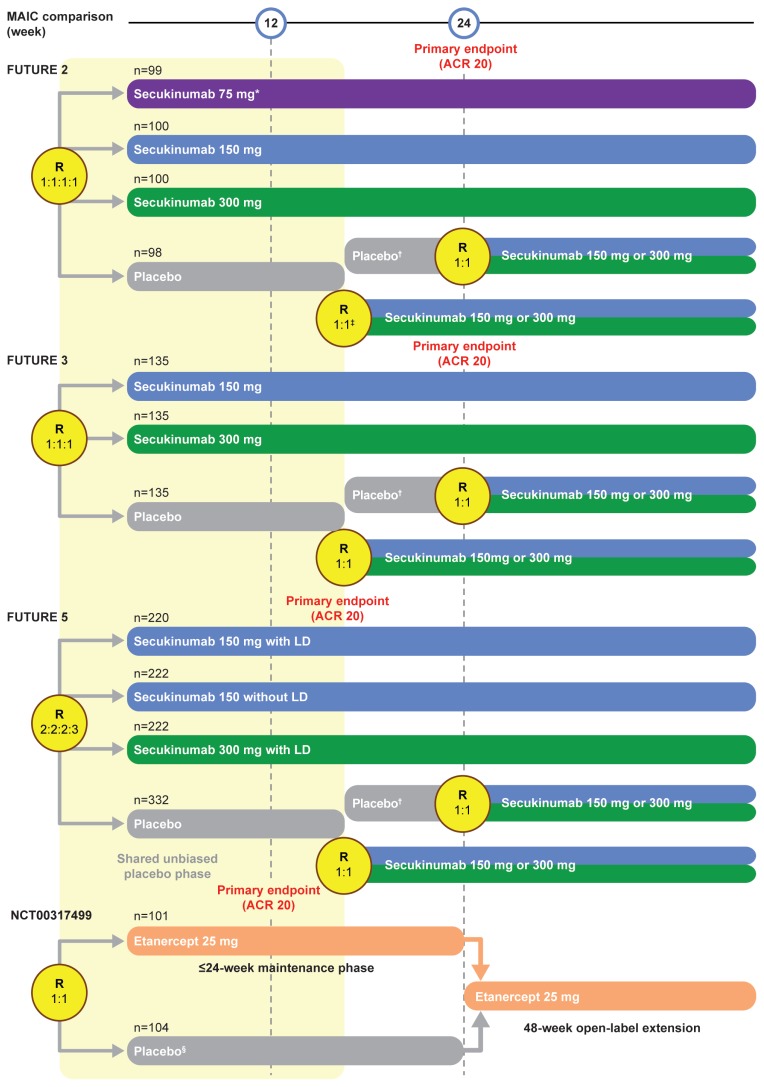
FUTURE 2 (13), FUTURE 3 (14), FUTURE 5 (15), and NCT00317499 (16) were large, randomized, double-blind, placebo-controlled phase 3 studies (Figure 1) with the primary endpoint of proportion of patients achieving a ≥20% improvement in the American College of Rheumatology (ACR) response criteria (ACR 20) (17) at week 24 (FUTURE 2, FUTURE 3, and NCT00317499) or week 16 (FUTURE 5).
Figure 1.
FUTURE 2, 3, and 5 and NCT00317499 trial designs
*Data from the secukinumab 75 mg arm of the FUTURE 2 study were not used in this MAIC.
†Patients who had a ≥20% improvement compared with baseline in TJC and SJC.
‡Patients who had a <20% improvement compared with baseline in TJC and SJC.
§Patients who completed 12 weeks of the study drug in the 24-week placebo-controlled phase were included in the open-label extension.
LD: loading dose; MAIC: matching-adjusted indirect comparison; R: randomization; SJC: swollen joint count; TJC: total joint count
The FUTURE 2, 3, and 5 trials were similar with respect to inclusion/exclusion criteria, follow-up period, and setting, and included the approved 150 mg and 300 mg doses of secukinumab, which allowed us to pool IPD across the three trials, increasing the potential effective sample size (ESS) following matching. Patients in NCT00317499 received etanercept 25 mg twice weekly. This is an approved dosing strategy according to the European Medicines Agency recommendation; however, the US Food and Drug Administration recommends 50 mg once weekly.
Patients who had previously experienced failure of TNFi therapy up to three times were eligible for inclusion in FUTURE 2, 3, and 5, whereas all patients were TNFi-naïve in NCT00317499.
Selection of baseline characteristics for matching study populations
The choice of baseline characteristics for matching was based on clinical expert advice, targeted literature review, and statistical analyses of effect modifiers, as described previously (18) and in line with the NICE guidelines (5).
Our principal analysis used 11 baseline characteristics for matching, including most of the established prognostic variables used in previous MAICs in PsA as matching parameters (12, 18). The number of patients with active concomitant psoriasis (≥3% body surface area) was used as a matching characteristic; however, Psoriasis Area and Severity Index (PASI) score at baseline could not be used because these data were not published for NCT00317499. PsA disease duration (time since diagnosis), swollen joint count, and C-reactive protein (CRP) levels at baseline were used in matching, but were omitted from the sensitivity analysis, which decreased the matching stringency but provided a larger ESS.
Rates of methotrexate use at baseline were higher for patients in the FUTURE 2, 3, and 5 trials than for those in the NCT00317499 trial, and PsA duration, number of patients with psoriasis >3% of their body surface area, swollen joint count, and CRP levels were all lower, suggesting comparatively less severe disease in these patients (Table 1); this highlights the requirement for MAIC to avoid such potential confounding factors. Owing to these differences between populations, and the matching methodology, the target population for our analysis comprised patients similar to those in the NCT00317499 trial, with characteristics of severe disease, rather than biologic-naïve patients in general.
Table 1.
Baseline characteristics before and after matching in the principal analysis
| NCT00317499 trial | FUTURE 2/3/5 (before matching) | FUTURE 2/3/5 (after matching) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Baseline characteristics | ETN 25 mg BIW (n=101) | PBO (n=104) | SEC 150 mg (n=458) | SEC 300 mg (n=461) | PBO (n=567) | SEC 150 mg (ESS=104) | SEC 300 mg (ESS=75) | PBO (ESS=159) |
| Demographics | ||||||||
| Age (years), mean* | 47.6 | 47.3 | 48.5 (12.3) | 48.6 (12.8) | 49.4 (12.3) | 47.6 (7.9) | 47.6 (6.7) | 47.3 (7.9) |
| Weight (kg), median* | 88.4 | 88.4 | 85.0 | 82.5 | 82.3 | 88.5 | 88.7 | 88.0 |
| Female, n (%) | 43 (42.6) | 57 (54.8) | 231 (50.4) | 235 (51.0) | 308 (54.3) | (42.6)† | (42.6)† | 54.8 |
| p=0.1525 | p=0.1261 | p=1.000 | p=1.000 | |||||
| White, n (%) | 91 (90) | 95 (91) | 397 (86.7) | 410 (88.9) | 501 (88.3) | (90.0)† | (90.0)† | 91.0 |
| p=0.3505 | p=0.7339 | p=1.000 | p=1.000 | |||||
| Disease characteristics | ||||||||
| Methotrexate use at baseline, n (%) | 42 (42) | 43 (41) | 213 (46.5) | 227 (49.2) | 279 (49.2) | (42.0)† | (42.0)† | 41.0 |
| p=0.3686 | p=0.1630 | p=1.000 | p=1.000 | |||||
| Psoriasis (=3% BSA), n (%) | 66 (65.3) | 62 (59.6) | 251 (54.8) | 213 (46.2) | 264 (46.6) | (65.3)† | (65.3)† | 59.6 |
| p=0.0529 | p=0.0005 | p=1.000 | p=1.000 | |||||
| HAQ-DI at baseline, mean* | 1.1 | 1.1 | 1.2 (0.6) | 1.2 (0.6) | 1.2 (0.6) | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.4) |
| p=0.1882 | p=0.1876 | p=1.000 | p=1.000 | |||||
| PsA duration (years) mean (SD)* | 9 | 9.2 | 7.0 (7.8) | 7.3 (8.4) | 6.7 (7.4) | 9.0 (7.3) | 9.0 (5.9) | 9.2 (6.6) |
| SJC, median* | 13 | 12.5 | 9.0 | 8.0 | 8.0 | 13.0 | 13.0 | 13.0 |
| CRP levels, median* | 1.6 | 1.1 | 0.5 | 0.5 | 0.5 | 1.6 | 1.6 | 1.1 |
| TNFi-naïve, n (%) | 101 (100) | 104 (100) | 312 (68.1) | 316 (68.5) | 390 (68.8) | (100.0)† | (100.0)† | 100.0 |
| p<0.0001 | p<0.0001 | p=1.000 | p=1.000 | |||||
| Variables not used for matching | ||||||||
| TJC, median | 18.0 | 17.0 | 16.0 | 15.0 | 16.0 | 19.0 | 17.0 | 18.0 |
| SF-36 PCS, mean | 35.8 | 35.7 | 36.9 (8.0) | 37.7 (8.2) | 36.7 (8.4) | 38.0 (4.5) | 38.9 (4.5) | 38.2 (5.5) |
| SF-36 MCS, mean | 50.9 | 48.4 | 41.8 (10.8) | 44.1 (11.7) | 43.7 (11.2) | 42.6 (6.8) | 43.7 (7.1) | 45.4 (6.4) |
Bold data indicate the post-matching baseline characteristics in each group
p values are for secukinumab compared with etanercept
For continuous baseline characteristics in the NCT00317499 study, either only the median or the mean without SD was reported. Therefore, in both cases, it was not possible to perform a statistical test
Integer population (n) values were not available because pooled SEC ESS values were calculated using the equation
BIW: twice weekly; BSA: body surface area; CRP: C-reactive protein; ESS: effective sample size; ETN: etanercept; HAQ-DI: Health Assessment Questionnaire Disability Index; MCS: mental component summary; PBO: placebo; PCS: physical component summary; PsA: psoriatic arthritis; SD: standard deviation; SEC: secukinumab; SF-36: 36-Item Short-Form Health Survey; SJC: swollen joint count; TJC: tender joint count; TNFi: tumor necrosis factor inhibitor
Matching and adjusting IPD to published aggregate data
Pooled FUTURE 2, 3, and 5 IPD were weighted to match selected patient baseline characteristics using published aggregate data from the etanercept arm of NCT00317499 (16). Placebo arms were also matched. This methodology was based on Signorovitch et al. (19), subsequent publications (4, 10, 20), and NICE guidelines (5).
The regression results were used to weigh patients in FUTURE 2, 3, and 5 so that each patient’s weight corresponded to their relative propensity for enrolling in FUTURE 2, 3, or 5 versus NCT00317499; following this, the weighted mean baseline characteristics of the pooled FUTURE 2, 3, and 5 populations matched those reported for NCT00317499, and the sample size of the pooled FUTURE 2, 3, and 5 data set was reduced to the ESS.
Comparing outcomes using recalculated patient data
After matching, the weights were used to recalculate outcomes for FUTURE 2, 3, and 5. These were aggregated and used to estimate the comparative effectiveness of secukinumab and etanercept using published aggregate data from NCT00317499.
Analyses
Missing data reporting
All published outcomes included in this MAIC were from the intention-to-treat population. All missing ACR response data were derived using non-responder imputation. For 36-Item Short-Form Health Survey (SF-36) summary scores, missing values were imputed using the last observation carried forward (LOCF) method in the NCT00317499 trial; LOCF was also applied to analyze SF-36 data in FUTURE 2, 3, and 5 to ensure consistency in the definition of these outcome variables.
Outcomes
American College of Rheumatology 20, 50, and 70 response rates and SF-36 scores were selected for comparison, as recommended for PsA clinical trial data sets in the Outcomes Measures in Rheumatology (21, 22) and GRAPPA (23) guidelines. Outcomes were compared at weeks 12 and 24.
All trials measured health-related quality of life (HRQoL) at week 24 using the SF-36 and reported the physical component summary (PCS) and the mental component summary (MCS).
Placebo-adjusted and non-placebo-adjusted outcome comparisons
Our MAIC analyses were conducted using the Bucher method (7). Placebo-adjusted comparisons were possible at week 12, but not at week 24; placebo arms were no longer comparable at this time point because patients could receive active treatment from week 16 in the FUTURE trials. Therefore, after week 12, outcomes from the etanercept arm of NCT00317499 were compared with outcomes from the adjusted and recalculated pooled secukinumab 150 mg and 300 mg arms of FUTURE 2, 3, and 5.
Pairwise comparisons
American College of Rheumatology outcomes are reported as odds ratios (ORs) with 95% confidence intervals (CIs) and p values. A standard p value threshold of ≤0.05 was used; furthermore, p values between 0.1 and 0.001 were considered as “increasing evidence” and p≤0.001 as “strong evidence” against the null hypothesis (Supplementary Methods section).
Psoriasis Area and Severity Index outcomes were not reported in the present study because baseline characteristic data for the NCT00317499 psoriasis subgroup were not available, and therefore matching for this subgroup could not be performed appropriately.
SF-36 MCS and PCS scores are reported as changes from baseline; 95% CIs could not be estimated in our analysis (Supplementary Methods section).
Results
Principal analysis
Matching baseline characteristics
Significant differences were found between patients treated with secukinumab and those receiving etanercept before matching, whereas no significant differences were observed after matching (Table 1).
ACR response rates
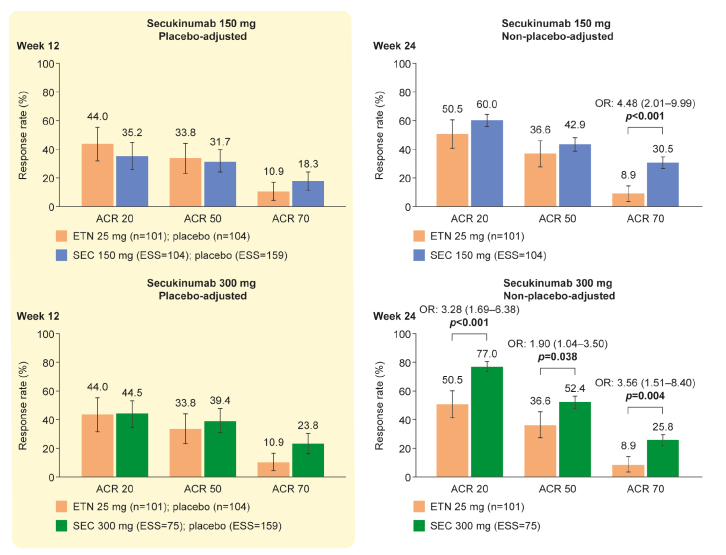
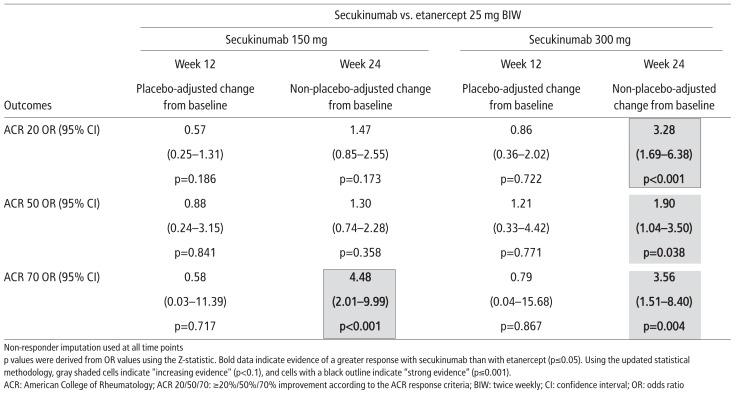
At week 12, placebo-adjusted ACR 20, 50, and 70 response rates for secukinumab (150 mg or 300 mg) and for etanercept were similar in the principal analysis (Figure 2 and Table 2). At week 24, ACR 20 and 50 response rates for secukinumab 150 mg and etanercept were not statistically different, but ACR 70 response rate was statistically higher in patients receiving secukinumab than in those receiving etanercept (OR (95% CI): 4.48 (2.01–9.99), p<0.001). At week 24, ACR 20, 50, and 70 response rates were statistically higher in patients treated with secukinumab 300 mg than in those receiving etanercept (OR (95% CI): ACR 20, 3.28 (1.69–6.38), p<0.001; ACR 50, 1.90 (1.04–3.50), p=0.038; and ACR 70, 3.56 (1.51–8.40), p=0.004). This equates to strong evidence for a statistical difference between secukinumab 150 mg and etanercept with respect to ACR 70 response rate and between secukinumab 300 mg and etanercept with respect to ACR 20 response rate at week 24 and increasing evidence for a difference between secukinumab 300 mg and etanercept with respect to ACR 50 and 70 response rates.
Figure 2.
ACR response rates for secukinumab 150 mg and secukinumab 300 mg compared with etanercept (principal analyses). ACR 20/50/70 response rates are the absolute mean response rate (NCT00317499) and the predicted mean response rate (FUTURE 2, 3, and 5). An OR <1 favors etanercept; an OR >1 favors secukinumab. p values are derived from the ORs and are set at a 5% significance level. The 95% CIs of the OR are shown in square brackets. Error bars show 95% CIs on the bar graph. Non-responder imputation was used to match NCT00317499.
ACR: American College of Rheumatology; ACR 20/50/70: ≥20%/50%/70% improvement according to the ACR response criteria; CI: confidence interval; ESS: effective sample size; ETN: etanercept; OR: odds ratio; SEC: secukinumab
Table 2.
ACR response rates for secukinumab 150 mg and secukinumab 300 mg compared with etanercept (principal analyses)
Changes in SF-36 PCS and MCS scores
As shown in Table 3, mean increase at week 24 in SF-36 PCS scores from baseline was numerically greater for patients receiving etanercept than for those receiving secukinumab 150 mg or 300 mg (etanercept, 9.3; secukinumab 150 mg, 6.58; and secukinumab 300 mg, 9.00). However, both doses of secukinumab were associated with numerically higher changes in mean SF-36 MCS scores from baseline than etanercept (etanercept, 2.7; secukinumab 150 mg, 6.03; and secukinumab 300 mg, 6.12).
Table 3.
Mean changes from baseline in SF-36 PCS and MCS scores (principal and sensitivity analyses)
| Outcomes |
NCT00317499 Etanercept 25 mg BIW |
FUTURE 2/3/5 Secukinumab 150 mg Principal analysis |
FUTURE 2/3/5 Secukinumab 150 mg Sensitivity analysis |
FUTURE 2/3/5 Secukinumab 300 mg Principal analysis |
FUTURE 2/3/5 Secukinumab 300 mg Sensitivity analysis |
|---|---|---|---|---|---|
| SF-36 PCS score, mean change from baseline (95% CI), week 24 | 9.3 | 6.58 (5.81–7.36) | 6.22 (5.50–6.95) | 9.00 (8.21–9.79) | 7.86 (7.13–8.58) |
| SF-36 MCS, score, mean change from baseline (95% CI), week 24 | 2.7 | 6.03 (5.06–7.00) | 5.62 (4.65–6.59) | 6.12 (4.91–7.34) | 4.28 (3.25–5.31) |
LOCF imputation used in all analyses. Data are shown as mean change from baseline (95% CI)
BIW: twice weekly; CI: confidence interval; LOCF: last observation carried forward; MCS: mental component summary; PCS: physical component summary; SF-36: 36-Item Short-Form Health Survey
Sensitivity analysis
Matching baseline characteristics
After matching, both secukinumab arms were well matched with the etanercept arm in the sensitivity analysis (Supplementary Table 1).
ACR response rates
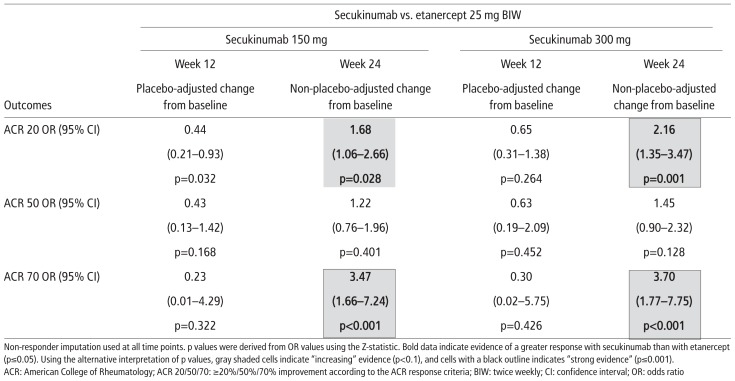
Placebo-adjusted ACR 20, 50, and 70 response rates were similar for secukinumab (150 mg or 300 mg) and etanercept at week 12 (Supplementary Figure 1 and Supplementary Table 2). At week 24, non-placebo-adjusted ACR 50 response rates were similar for either dose of secukinumab and etanercept, but ACR 20 and 70 response rates were higher in patients treated with secukinumab 150 mg than in those receiving etanercept (OR (95% CI): ACR 20, 1.68 (1.06–2.66), p=0.028 and ACR 70, 3.47 (1.66–7.24), p<0.001). The findings were similar for secukinumab 300 mg compared with etanercept (OR (95% CI): ACR 20, 2.16 (1.35–3.47), p=0.001 and ACR 70, 3.70 (1.77–7.75), p<0.001). Therefore, these data provide similarly strong evidence for secukinumab 150 mg and secukinumab 300 mg with respect to ACR 70 response rates, but stronger evidence for the 300 mg dose than for the 150 mg dose with respect to ACR 20 response rates.
Changes in SF-36 PCS and MCS scores
As in the principal analysis, mean increase in SF-36 PCS scores from baseline was numerically greater for patients receiving etanercept than for those receiving secukinumab 150 mg or 300 mg (etanercept, 9.3; secukinumab 150 mg, 6.22; and secukinumab 300 mg, 7.86; Table 3). However, both doses of secukinumab were associated with numerically higher changes in mean SF-36 MCS scores from baseline than etanercept (etanercept, 2.7; secukinumab 150 mg, 5.62; and secukinumab 300 mg, 4.28).
Discussion
The present study used MAIC to examine the comparative effectiveness of secukinumab and etanercept in biologic-naïve patients with active PsA. Our results suggest that secukinumab and etanercept have similar short-term efficacy with respect to ACR 20, 50, and 70 response rates; no differences between the two treatments were observed at week 12. At week 24, advantages were observed with secukinumab 150 mg with respect to increased ACR 70 response rates over etanercept and with secukinumab 300 mg with respect to increased ACR 20, 50, and 70 response rates over etanercept. Our findings for ACR 20 and 70 response rates are largely supported by the sensitivity analysis; however, other results are not replicated consistently in both scenarios and should be treated with caution. This may be because our principal analyses used as matching parameters a comparatively more complete set of potential characteristics that may influence treatment responses.
Data have been published from FIXTURE (NCT01358578), a head-to-head RCT of secukinumab and etanercept in patients with moderate-to-severe psoriasis, showing superiority for secukinumab over etanercept (24). A subgroup analysis of patients with concomitant PsA demonstrated greater improvements with respect to both skin signs and symptoms and physical function, assessed using the Health Assessment Questionnaire Disability Index (HAQ-DI), for secukinumab than for etanercept (25). Our findings are in line with this study, particularly as HAQ-DI scores are often used to assess physical disability and are part of the ACR response criteria.
A recently published network meta-analysis (NMA) compared secukinumab with other biologic therapies approved for treating PsA; however, secukinumab and etanercept were compared only with respect to PASI scores. In the NMA, secukinumab 150 mg and 300 mg demonstrated superiority over etanercept 25 mg twice weekly and etanercept 50 mg twice weekly or once weekly with respect to PASI 50, 75, or 90 response rates at 12–16 weeks (26).
Our SF-36 analyses examined the impact of secukinumab and etanercept on patients’ HRQoL, especially well-being and daily functioning. There was evidence of a greater improvement in SF-36 PCS scores with etanercept than with secukinumab 150 mg, but the improvement with secukinumab 300 mg was similar to that observed with etanercept at week 24. However, patients in the NCT00317499 trial had substantially lower SF-36 PCS scores at baseline than those in the FUTURE 2, 3, and 5 trials, which may impact the comparability of the results. Our findings are not in line with the previous head-to-head comparison of secukinumab 300 mg and etanercept, which indicated comparatively higher functional improvement associated with secukinumab 300 mg, assessed using the HAQ-DI (25). This may be influenced by the disparity in the outcome measures used; HAQ-DI measures functional ability only, whereas SF-36 PCS comprises a more comprehensive and holistic set of items. Improvements from baseline in SF-36 MCS scores were greater for patients receiving either dose of secukinumab than for those receiving etanercept in our study. However, standard deviations or standard errors were not reported in the NCT00317499 trial (16), meaning that it was not possible to calculate p values for these comparisons, and the results should be interpreted with caution.
Our study has some limitations. First, although efficacy outcomes after week 24 were reported in the NCT00317499 trial, times from randomization to the start of open-label treatment were different across individuals, and therefore the reported results included patients with different durations of therapy (27). Therefore, long-term efficacy outcomes were not available for use in our analysis, and no valid comparison could be drawn between trials. Owing to between-trial differences in the placebo arm designs and reporting at week 24 and the associated risk of bias, placebo could not be used for adjustment at this time point, and our comparison uses only the matched treatment arms as the adjustment method. Additionally, the use of stringent criteria to increase the accuracy of the matching process resulted in lower, but adequate, ESSs for secukinumab in our principal analyses, using pooled data from FUTURE 2, 3, and 5. An alternative to performing MAIC using pooled IPD would have been to perform identical MAICs based on each IPD population and then pool the relative effect estimates with standard meta-analysis methods (5); however, pooling of IPD was considered to be acceptable because of the similarities in study design and protocol. The large differences in pre- and post-matching sample sizes demonstrate the heterogeneity before matching and the need for MAIC rather than older methods, which compared data from different trials without any adjustment.
MAIC also has general limitations. Although non-placebo-adjusted comparisons are a legitimate means of comparing treatments using long-term data, adjustments cannot be made for unobserved differences between trial populations, variables reported in only one study, or certain aspects of study design. Furthermore, as in any study, our results are most applicable to the study population: the NCT00317499 trial population, to which the FUTURE 2, 3, and 5 populations were matched. Therefore, the results of our MAIC are most applicable to patients who are biologic-naïve, and cannot be used to draw conclusions about the relative efficacy of secukinumab and etanercept in patients who have experienced biologic therapy failure.
The NICE guidelines recommend quantification of the possible extent of any residual systematic error resulting from unobserved prognostic variables and effect modifiers in unanchored comparisons, although this area requires further research (8). The guidelines propose an out-of-sample method, comparing observed and predicted outcomes of the drug of interest in a range of different studies in the target population. This method was applied in our current study using MAICs (with covariate adjustments as in our principal analysis) to predict ACR 20 and 50 response rates for secukinumab in four different PsA trial populations (16, 28–30). However, the observed outcomes in these trials were found to be very similar, so that the between-study standard deviation estimated from the meta-analysis was zero. As a result, the ratio of the between-studies variance in predicted to observed outcomes could not be calculated. We note that the formula suggested by the NICE guidelines requires at least some heterogeneity of the trial populations, which may not be the case if trials use very similar inclusion and exclusion criteria.
MAIC analyses cannot replace an appropriately powered, head-to-head RCT, but can provide additional insights into comparative effectiveness. As well as comparing different modes of action, our analysis is also of interest because secukinumab and etanercept are the first recommended PsA treatments for which head-to-head data were published in a subpopulation of patients with psoriasis and PsA. Our findings indicate that biologic-naïve patients with active PsA have a similar probability of achieving short-term ACR responses (week 12) with secukinumab and with etanercept, but are comparatively more likely to achieve ACR responses after 24 weeks of treatment with secukinumab. Future direct or indirect comparisons, as well as real-world observations, will be valuable to provide further evidence on the relative treatment effects of these two therapies.
Supplementary Methods
Matching-adjusted indirect comparison
Identification of source data by SLR
An SLR with a cut-off of November 2015 was used to identify evidence for secukinumab and relevant comparators in the treatment of adult patients with PsA, with no restriction by disease severity; eligibility criteria were the same as those described previously (1). A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart is shown in Supplementary Figure 2. A total of 29 trials met the eligibility criteria, of which 20 included neither secukinumab nor etanercept, and 7 were excluded for other reasons (Supplementary Table 3). The two trials included in our analysis were FUTURE 2 (NCT01752634), a placebo-controlled study of secukinumab (2), and NCT00317499, a placebo-controlled trial of etanercept (3).
In April 2018, IPD from the FUTURE 3 (NCT01989468) (4) and FUTURE 5 (NCT02404350) (5) trials became available for inclusion in the present study. The FUTURE 2, 3, and 5 trials were similar with respect to inclusion and exclusion criteria, follow-up period, intervention, and setting, and included the approved 150 mg and 300 mg doses of secukinumab. This allowed us to pool IPD across the three trials, with the aim of increasing the potential ESS following matching, in accordance with the recommended methodology (6, 7).
A targeted literature search in PubMed and a search of the website (https://clinicaltrials.gov/) using the keywords “etanercept” and “psoriatic arthritis” were performed to identify any new published data for etanercept; however, no relevant data were identified.
Matching and adjusting IPD to published aggregate data
Pooled FUTURE 2, 3, and 5 IPD were weighted to match selected patient baseline characteristics using published aggregate data from the etanercept arm of NCT00317499 using SAS version 9.4 and R version 3.2.1.
The regression results were used to weigh patients in FUTURE 2, 3, and 5 using the method of moments (mean only). The method of moment was applied using the quasi-Newton optimization Broyden–Fletcher–Goldfarb–Shanno implemented in the R function optim, as recommended in the NICE guidelines (6). After this matching process, the weighted mean baseline characteristics of the pooled FUTURE 2, 3, and 5 populations matched those reported for NCT00317499, and the sample size of the pooled FUTURE 2, 3, and 5 data set was reduced to the ESS.
Analyses
Pairwise comparisons
For placebo-adjusted ACR response rate comparisons at week 12, ORs for the secukinumab arm versus the placebo arm in the matched FUTURE 2, 3, and 5 populations were derived from a logistic regression model by using generalized estimating equations (fitted using PROC GENMOD in SAS) with robust standard errors, following published guidelines (6, 8).
For non-placebo-adjusted comparisons at week 24, ORs were calculated as the odds of a response in the etanercept arm of NCT00317499 by the odds of a response in the secukinumab arm of the reweighted pooled FUTURE 2, 3, and 5 populations. Standard errors for ORs were estimated based on the information provided by a 2×2 contingency table.
Our principal and sensitivity analyses employed a standard p value threshold of ≤0.05, but we also acknowledge the recent American Statistical Association guidelines (9, 10) by interpreting our data using a more modern definition (11, 12). Therefore, we classified p values between 0.1 and 0.001 as “increasing evidence” and p≤0.001 as “strong evidence” against the null hypothesis.
A 95% CI around mean changes from baseline in SF-36 MCS and PCS scores for patients in the etanercept arm of NCT00317499 could not be estimated, as neither standard deviations nor standard errors were reported. Therefore, full statistical comparisons could not be performed.
PRISMA diagram.
The rules for this SLR were set a priori (as defined in Supplementary Table 3), yielding 29 trials. A second filter was then applied, i.e., studies that included either etanercept or secukinumab.
MAIC: matching-adjusted indirect comparison; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SLR: systematic literature review
ACR response rates for secukinumab 150 mg and secukinumab 300 mg compared with etanercept: sensitivity analyses.
ACR 20/50/70 responses are the absolute mean response rate (NCT00317499) and the predicted mean response rate (FUTURE 2, 3, and 5). An OR <1 favors etanercept; an OR >1 favors secukinumab. Error bars show 95% confidence intervals. The most appropriate imputation methods to match NCT00317499 were used.
ACR: American College of Rheumatology; ACR 20/50/70: ≥20/50/70% improvement according to the ACR response criteria; ESS: effective sample size; ETN: etanercept, OR: odds ratio; SEC: secukinumab
Supplementary Table 1.
Baseline characteristics before and after matching in the sensitivity analysis
| NCT00317499 trial | FUTURE 2/3/5 (before matching) | FUTURE 2/3/5 (after matching) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Baseline characteristics | ETN 25 mg BIW (n=101) | PBO (n=104) | SEC 150 mg (n=458) wk 12 | SEC 300 mg (n=461) wk 12 | PBO (n=567) | SEC 150 mg (ESS=276) | SEC 300 mg (ESS=253) | PBO (ESS=304) |
| Demographics | ||||||||
| Age (years), mean* | 47.6 | 47.3 | 48.5 (12.3) | 48.6 (12.8) | 49.4 (12.3) | 47.6 (10.0) | 47.6 (9.4) | 47.3 (9.4) |
| Weight (kg), median* | 88.4 | 88.4 | 85.0 | 82.5 | 82.3 | 88.1 | 88.4 | 88.6 |
| Female, n (%) | 43 (42.6) | 57 (54.8) | 231 (50.4) | 235 (51.0) | 308 (54.3) | (42.6)† | (42.6)† | (54.8)† |
| p=0.1525 | p=0.1261 | p=1.000 | p=1.000 | |||||
| White, n (%) | 91 (90) | 95 (91) | 397 (86.7) | 410 (88.9) | 501 (88.4) | (90.0)† | (90.0)† | (90.0)† |
| p=0.3505 | p=0.7339 | p=1.000 | p=1.000 | |||||
| Disease characteristics | ||||||||
| Methotrexate use at baseline, n (%) | 42 (42) | 43 (41) | 213 (46.5) | 227 (49.2) | 279 (49.2) | (42.0)† | (42.0)† | (41.0)† |
| p=0.3686 | p=0.1630 | p=1.000 | p=1.000 | |||||
| Psoriasis (=3% BSA) n (%) | 66 (65.3) | 62 (59.6) | 251 (54.8) | 213 (46.2) | 264 (46.6) | (65.3)† | (65.3)† | (59.6)† |
| p=0.0529 | p=0.0005 | p=1.000 | p=1.000 | |||||
| HAQ-DI at baseline, mean* | 1.1 | 1.1 | 1.2 (0.6) | 1.2 (0.6) | 1.1 (0.6) | 1.1 (0.5) | 1.1 (0.5) | 1.1 (0.5) |
| p=0.1882 | p=0.1876 | p=1.000 | p=1.000 | |||||
| TNFi-naïve, n (%) | 101 (100) | 104 (100) | 312 (68.1) | 316 (68.5) | 390 (68.7) | (100.0)† | (100.0)† | (100.0)† |
| p<0.0001 | p<0.0001 | p=1.000 | p=1.000 | |||||
| Variables not used for matching | ||||||||
| PsA duration (years), mean (SD)* | 9.0 | 9.2 | 7.0 (7.8) | 7.3 (8.4) | 6.7 (7.4) | 6.3 (6.5) | 5.6 (5.7) | 5.5 (5.6) |
| SJC, median* | 13.0 | 12.5 | 9.0 | 8.0 | 8.0 | 8.0 | 7.0 | 8.0 |
| TJC, median | 18.0 | 17.0 | 16.0 | 15.0 | 16.0 | 15.0 | 14.0 | 15.0 |
| CRP levels, median | 1.6 | 1.1 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 |
| SF-36 PCS, mean | 35.8 | 35.7 | 36.9 (8.0) | 37.7 (8.2) | 36.7 (8.4) | 38.7 (6.2) | 38.9 (6.6) | 38.5 (6.9) |
| SF-36 MCS, mean | 50.9 | 48.4 | 41.8 (10.8) | 44.1 (11.7) | 43.7 (11.2) | 41.9 (8.7) | 44.4 (9.0) | 44.9 (8.2) |
Bold data indicate the post-matching baseline characteristics in each group
p values are for secukinumab compared with etanercept.
For continuous baseline characteristics in the NCT00317499 study, either only the median or the mean without SD was reported. Therefore, in both cases, it was not possible to perform a statistical test.
Integer population (n) values were not available because pooled SEC ESS values were calculated using the equation.
BIW: twice weekly; BSA: body surface area; CRP: C-reactive protein; ESS: effective sample size; ETN: etanercept; HAQ-DI: Health Assessment Questionnaire Disability Index; MCS: mental component summary; PBO: placebo; PCS: physical component summary; PsA: psoriatic arthritis; SD: standard deviation; SEC: secukinumab; SF-36: 36-Item Short-Form Health Survey; SJC: swollen joint count; TJC: tender joint count; TNFi: tumor necrosis factor inhibitor
Supplementary Table 2.
ACR response rates for secukinumab 150 mg and secukinumab 300 mg compared with etanercept (sensitivity analyses)
Supplementary Table 3.
Trials identified in the SLR and the reasons for their inclusion/exclusion in this MAIC
| Trial acronym | Intervention | Comparator | Population | Primary study reference | Secondary references | Study included in MAIC analysis? |
|---|---|---|---|---|---|---|
| CLEAR (subanalysis) | Secukinumab 300 mg (s.c.); weeks 0, 1, 2, and 3 and Q4W from week 4 | Ustekinumab 45 mg for individuals =100 kg and 90 mg for individuals >100 kg (s.c.); weeks 0, 4, and Q12W from week 16 | CLEAR included 676 patients with moderate-to-severe psoriasis whose disease was inadequately controlled by topical treatment, phototherapy, and/or previous systemic therapy. 123 had concomitant PsA | Gottlieb (13) | None | No—these trials were designed to assess dermatologic endpoints in psoriasis and consequently did not collect data on rheumatology disease characteristics at baseline or assess ACR response rates. This meant that matching and comparison of outcomes in our analysis would not have been possible. Furthermore, all patients had moderate-to severe psoriasis, which resulted in a discrepancy in baseline PASI scores between the population in this trial and the patients in other trials identified in the SLR |
| ERASURE (subanalysis) | Secukinumab 300 mg or 150 mg give at weeks 0, 1, 2, 3, and 4 and Q4W thereafter through week 48 | Placebo | ERASURE included patients with moderate-to-severe psoriasis whose disease was poorly controlled by topical treatment, phototherapy, and/o previous systemic therapy. 171 had concomitant PsA | Gottlieb (14) Gottlieb (15) |
Blauvelt (16) Gottlieb (17) Philipp (18) |
|
| FIXTURE (subanalysis) | Secukinumab 300 mg or 150 mg QW for 4 weeks | Placebo | FIXTURE included patients with moderate-to-severe psoriasis whose disease was poorly controlled by topical treatment, phototherapy, and/or previous systemic therapy. 196 had concomitant PsA | Gottlieb (15) | Gottlieb (19) Philipp (18) Sigurgeirsson (20) |
|
| FUTURE 1 | Secukinumab 10 mg/kg i.v. at weeks 0, 2, and 4 followed by 150 mg or 75 mg s.c. Q4W thereafter | Placebo: i.v. at weeks 0, 2, and 4 and s.c. Q4W thereafter | 606 patients with PsA and inadequate response or intolerance to NSAIDs | Mease (21) | Gottlieb (22) Mease (23) Mease (24) Mease (25) Strand (26) van der Heijde (27) |
No—intravenous loading for secukinumab |
| FUTURE 2 | Secukinumab 300 mg or 150 mg QW from baseline to week 4 and then Q4W | Placebo | 298 patients with active PsA despite treatment with NSAIDs, DMARDs, or TNFis | McInnes (2) | Gottlieb (28) Gottlieb (29) Kavanaugh (30) McInnes (31) Mease (32) Rahman (33) |
Yes |
| McInnes (2014) | Secukinumab 10 mg/kg i.v. at weeks 0 and 3 | Placebo | 42 patients with active PsA | McInnes (34) | McInnes (35) McInnes (36) |
No—intravenous loading for secukinumab |
| Mease (2000) | Etanercept 25 mg BIW | Placebo | 60 patients with active PsA and nadequate response to NSAIDs | Mease (37) | None | No—the study duration was only trial was a proof-of-concept study rather than a phase 3 trial 12 weeks, and this |
| NCT00317499 (2004) | Etanercept 25 mg BIW for 24 weeks | Placebo | 205 patients with active PsA and inadequate response to NSAIDs | Mease (3) | Atteno (38) Mease (39) Mease (40) |
Yes |
| PRESTA | Etanercept 50 mg BIW | Etanercept 50 mg QW | 748 patients with psoriasis and concomitant PsA | Sterry (41) | Gniadecki (42) Griffiths (43) Helliwell (44) Prinz (45) |
No—all patients had moderate-to-severe psoriasis, which resulted in a discrepancy in baseline PASI scores between and the patients in other trials identified in the SLR. Restriction of our analysis subgroup to account for this limited the available data set, restricted by psoriasis disease severity were excluded and consequently trials discrepancy would have only to patients in this the population in this trial |
Gray shaded rows indicate trials that were included in our analysis
ACR: American College of Rheumatology; BIW: twice weekly; DMARD: disease-modifying anti-rheumatic drug; i.v., intravenously; MAIC: matching-adjusted indirect comparison; NSAID: non-steroidal anti-inflammatory drug; PASI: Psoriasis Area and Severity Index; PsA: psoriatic arthritis; Q4W: every 4 weeks; Q12W: every 12 weeks; QW: once weekly; s.c.: subcutaneously; SLR: systematic literature review; TNFi: tumor necrosis factor inhibitor
References
- 1.Nash P, McInnes IB, Mease PJ, Thom H, Hunger M, Karabis A, et al. Secukinumab versus adalimumab for psoriatic arthritis: comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther. 2018;5:59–122. doi: 10.1007/s40744-018-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–46. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 3.Mease P, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–72. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 4.Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3) Arthritis Res Ther. 2018;20:47. doi: 10.1186/s13075-018-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77:890–7. doi: 10.1136/annrheumdis-2017-212687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submissions to NICE. 2016. Available from: http://nicedsu.org.uk/wp-content/uploads/2018/08/Population-adjustment-TSD-FINAL-ref-rerun.pdf.
- 7.Belger M, Brnabic A, Kadziola Z, Petto H, Faries D. Alternative weighting approaches for matching adjusted indirect comparisons (MAIC) Value Health. 2015;18:A31–A2. doi: 10.1016/j.jval.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28:935–45. doi: 10.2165/11538370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat. 2016;70:129–33. [Google Scholar]
- 10.Best AM, Greenberg BL, Glick M. From tea tasting to t test: a P value ain’t what you think it is. J Am Dent Assoc. 2016;147:527–9. doi: 10.1016/j.adaj.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JA, Davey Smith G. Sifting the evidence – what’s wrong with significance tests? BMJ. 2001;322:226–31. doi: 10.1136/bmj.322.7280.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman S, Greenland S. Why most published research findings are false: problems in the analysis. PLoS Med. 2007;4:e168. doi: 10.1371/journal.pmed.0040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb A, Thaci D, Blauvelt A, Milutinovic M, Mpofu S. Secukinumab improves skin symptoms and physical functioning compared with ustekinumab in patients with moderate to severe psoriasis with concomitant psoriatic arthritis: Subanalysis of a randomized, double blind, parallel-group, active comparator-controlled phase 3b trial. ACR/ARHP Annual Meeting; 6–11 November 2015; San Francisco, CA, USA. [Google Scholar]
- 14.Gottlieb A, Sigurgeirsson B, Blauvelt A, Mpofu S, Martin R, Papavassilis C. Secukinumab shows substantial improvement in both psoriasis symptoms and physical functioning in moderate-to-severe plaque psoriasis patients with psoriatic arthritis: a subanalysis of a phase 3, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 2013;65:S136–S7. [Google Scholar]
- 15.Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. J Drugs Dermatol. 2015;14:821–33. [PubMed] [Google Scholar]
- 16.Blauvelt A, Gottlieb A, Sigurgeirsson B, Papavassilis C, Martin R. Secukinumab efficacy in subjects with moderate-to-severe plaque psoriasis and concomitant psoriatic arthritis: a subanalysis of the ERASURE study. J Am Acad Dermatol. 2014;70:AB2. [Google Scholar]
- 17.Gottlieb A, Sigurgeirsson B, Blauvelt A, Gong Y, Papavassilis C, Mpofu S. Secukinumab reduces hsCRP Levels in subjects with moderate-to-severe plaque psoriasis and concomitant psoriatic arthritis: a sub-analysis from the phase 3 ERASURE Study. Ann Rheum Dis. 2014;73:1047–8. [Google Scholar]
- 18.Philipp S, Gottlieb A, Langley RG, Sigurgeirsson B, Blauvelt A, Gong Y, et al. Secukinumab decreases inflammation as measured by a biomarker hsCRP in subjects with moderate-to-severe plaque psoriasis and concomitant psoriatic arthritis: subanalyses from two phase 3 studies. Exp Dermatol. 2015;24:E27. [Google Scholar]
- 19.Gottlieb A, Langley RG, Philipp S, Martin R, Papavassilis C, Mpofu S. Improvement in psoriasis symptoms and physical functioning with secukinumab compared with placebo and etanercept in subjects with moderate-to-severe plaque psoriasis and psoriatic arthritis. Arthritis Rheum. 2013;65:3319–29. [Google Scholar]
- 20.Sigurgeirsson B, Gottlieb A, Langley RG, Philipp S, Martin R, Papavassilis C. Effect of secukinumab on psoriasis symptoms and physical functioning compared with placebo and etanercept in subjects with moderate-to-severe plaque psoriasis and concomitant psoriatic arthritis: a subanalysis from the phase 3 FIXTURE study. Scand J Rheumatol. 2014;43:64–5. [Google Scholar]
- 21.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373:1329–39. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb A, Mease P, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, significantly reduces psoriasis burden in patients with psoriatic arthritis: results from a phase 3 randomized controlled trial. Arthritis Rheumatol. 2014;66:S233. [Google Scholar]
- 23.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, Van der Heijde D, et al. Secukinumab, a human anti-interleukin-17a monoclonal antibody, improves active psoriatic arthritis and inhibits radiographic progression: efficacy and safety data from a phase 3 randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheumatol. 2014;66:S423–4. [Google Scholar]
- 24.Mease P, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, Van der Heijde D, et al. Secukinumab provides sustained improvements in psoriatic arthritis: 2-year efficacy and safety results from a phase 3 randomized, double-blind, placebo-controlled trial. ACR/ARHP Annual Meeting; 6–11 November 2015; San Francisco, CA, USA. [Google Scholar]
- 25.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, Van der Heijde D, et al. Secukinumab improves active psoriatic arthritis and inhibits radiographic progression: results of a phase 3 randomized, multicenter, double-blind, placebo-controlled study. J Invest Dermatol. 2015;135 [Google Scholar]
- 26.Strand V, Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, et al. Secukinumab, an anti-interleukin-17a monoclonal antibody, improves physical function, quality of life and work productivity in patients with active psoriatic arthritis: results from a phase 3, randomized, controlled trial. Arthritis Rheumatol. 2014;66:S240–1. [Google Scholar]
- 27.van der Heijde D, Landewe R, Mease PJ, McInnes IB, Conaghan PG, Pricop L, et al. Secukinumab, a monoclonal antibody to interleukin-17a, provides significant and sustained inhibition of joint structural damage in active psoriatic arthritis regardless of prior TNF inhibitors or concomitant methotrexate: a phase 3 randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2014;66:S424–5. [Google Scholar]
- 28.Gottlieb A, McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ligozio G, et al. Secukinumab improves signs and symptoms of active psoriatic arthritis: results from a phase 3 randomized, multicenter, double-blind, placebo-controlled study using a subcutaneous dosing regimen (FUTURE 2) J Invest Dermatol. 2015;135:S1–S37. [Google Scholar]
- 29.Gottlieb A, Strand V, McInnes IB, Marzo-Ortega H, Kavanaugh A, Kandala S, et al. Secukinumab improves physical function, quality of life, fatigue and work productivity in patients with active psoriatic arthritis: results of a randomized, double-blind, placebo-controlled phase 3 trial (FUTURE 2) J Invest Dermatol. 2015;135 [Google Scholar]
- 30.Kavanaugh A, McInnes IB, Mease P, Hall S, Chinoy H, Kivitz AJ, et al. Secukinumab provides sustained improvements in the signs and symptoms of active psoriatic arthritis in anti-TNF-naive patients and those previously exposed to anti-TNF therapy: 52-week results from a randomized, double-blind, placebo-controlled phase 3 trial with subcutaneous dosing. Arthritis Rheumatol. 2015;67 [Google Scholar]
- 31.McInnes IB, Mease P, Kirkham B, Kavanaugh A, Ritchlin C, Rahman P, et al. Secukinumab, a human anti-interleukin-17a monoclonal antibody, improves active psoriatic arthritis: 24-week efficacy and safety data from a phase 3 randomized, multicenter, double-blind, placebo-controlled study using subcutaneous dosing. ACR/ARHP Annual Meeting; 14–19 November 2014; Boston, MA, USA. [Google Scholar]
- 32.Mease P, Kirkham B, McInnes IB, Kremer JM, Kandala S, Pricop L, et al. Secukinumab is effective in reducing dactylitis and enthesitis using multiple measures in patients with psoriatic arthritis: results of a phase 3 randomized, multicenter, double-blind, placebo-controlled study (FUTURE 2) J Invest Dermatol. 2015;135:S30. [Google Scholar]
- 33.Rahman P, Strand V, McInnes IB, Marzo-Ortega H, Dokoupilová E, Churchill M, et al. Secukinumab improves physical function, quality of life, fatigue and work productivity in patients with active psoriatic arthritis in FUTURE 2, a phase 3 trial. Ann Rheum Dis. 2015;74:356. [Google Scholar]
- 34.McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349–56. doi: 10.1136/annrheumdis-2012-202646. [DOI] [PubMed] [Google Scholar]
- 35.McInnes IB, Sieper J, Braun J, Emery P, Van der Heijde D, Isaacs J, et al. Secukinumab, a fully human anti-interleukin-17A antibody, improves signs and symptoms of psoriatic arthritis: a 24-week, double-blind, placebo-controlled, multicenter trial. Dermatol Ther. 2012;2:10. [Google Scholar]
- 36.McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs J, et al. Effect of secukinumab on signs and symptoms of psoriatic arthritis: results of a 24-week multicentre, double-blind, randomized, placebo-controlled trial. Rheumatology. 2012;51:90–1. [Google Scholar]
- 37.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–90. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 38.Atteno M, Peluso R, Costa L, Padula S, Iervolino S, Caso F, et al. Comparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugs. Clin Rheumatol. 2010;29:399–403. doi: 10.1007/s10067-009-1340-7. [DOI] [PubMed] [Google Scholar]
- 39.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol. 2006;33:712–21. [PubMed] [Google Scholar]
- 40.Mease PJ, Woolley MJ, Singh A, Tsuji W, Dunn M, Chiou CF. Patient-reported outcomes in a randomized trial of etanercept in psoriatic arthritis. J Rheumatol. 2010;37:1221–7. doi: 10.3899/jrheum.091093. [DOI] [PubMed] [Google Scholar]
- 41.Sterry W, Ortonne JP, Kirkham B, Brocq O, Robertson D, Pedersen RD, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ. 2010;340:c147. doi: 10.1136/bmj.c147. [DOI] [PubMed] [Google Scholar]
- 42.Gniadecki R, Robertson D, Molta CT, Freundlich B, Pedersen R, Li W, et al. Self-reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. J Eur Acad Dermatol Venereol. 2012;26:1436–43. doi: 10.1111/j.1468-3083.2011.04308.x. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths CE, Sterry W, Brock F, Dilleen M, Stefanidis D, Germain JM, et al. Pattern of response in patients with moderate-to-severe psoriasis treated with etanercept. Br J Dermatol. 2015;172:230–8. doi: 10.1111/bjd.13139. [DOI] [PubMed] [Google Scholar]
- 44.Helliwell P, Mease PJ, Bananis E, Jones H, Szumski A, Marshall L. Baseline characteristics and treatment outcomes in psoriasis patients with polyarthritis or oligoarthritis. Arthritis Rheum. 2013;65:S147. [Google Scholar]
- 45.Prinz JC, FitzGerald O, Boggs RL, Foehl J, Robertson D, Pedersen R, et al. Combination of skin, joint and quality of life outcomes with etanercept in psoriasis and psoriatic arthritis in the PRESTA trial. J Eur Acad Dermatol Venereol. 2011;25:559–64. doi: 10.1111/j.1468-3083.2010.03838.x. [DOI] [PubMed] [Google Scholar]
Acknowledgements
The authors would like to acknowledge Christian Eichinger, Alistair Ray, and Caroline Freeman of Oxford PharmaGenesis, Oxford, UK, who provided medical writing support during the development of this manuscript.
Footnotes
Ethics Committee Approval: This study was of non-interventional nature and did not include primary data collection (i.e. was based on published secondary data only). Therefore, ethic committee or institutional review board approval was not required. Data used were taken from published randomized controlled trials, which were conducted according to the principles of the Declaration of Helsinki and with informed consent from participants.
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - P.M., P.N., E.C., S.M.J., K.K.G., L.P., H.T.; Design - P.M., SM.J., K.K.G., M.H., C.K., H.T., E.C.; Supervision - P.M., S.M.J., K.K.G., L.P., H.T.; Resources - S.M.J.; Materials - N/A; Data Collection and/or Processing - M.H., C.K., H.T., S.M.J.; Analysis and/or Interpretation - P.M., E.C., P.N., C.K., M.H., L.P., K.K.G., S.M.J., H.T.; Literature Search - S.M.J., K.K.G., L.P., H.T.; Writing Manuscript - P.M., E.C., P.N., C.K., M.H., L.P., K.K.G., S.M.J., H.T.; Critical Review - P.M., E.C., P.N., C.K., M.H., L.P., K.K.G., S.M.J., H.T.
Conflict of Interest: P.M. has received research grants from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sun and UCB. He has also received consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Eli Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Sun, and UCB, and has been a speaker for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Novartis, Pfizer, and UCB. E.C. has received research grants from Bio-Cancer, Biogen, Novartis, Pfizer, Roche, Sanofi, and UCB; and has received consultancy fees from Abbvie, Chugai Pharma, Eli Lilly, Janssen, Novartis, ObsEva, Pfizer, Regeneron, Roche, R-Pharm, Sanofi, and Syn-Act Pharma and Tonix. P.N. has received funding for research and clinical trials, and honoraria for lectures and advice from Abbvie, BMS, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, and UCB. C.K. was a paid employee of Mapi Group, an ICON plc company at the time of this study. M.H. is a paid employee of Mapi Group, an ICON plc company. Mapi Group received funding from Novartis Pharma AG for this study. L.P. and K.K.G. are paid employees of Novartis Pharmaceuticals Corporation East Hanover, NJ, USA and own Novartis stock. S.M.J. is a paid employee of Novartis Pharma AG, Basel, Switzerland and owns Novartis stock. H.T. has received consultancy fees from Bayer, Eli Lilly, F. Hoffmann-La Roche AG, Novartis Pharma AG, and Pfizer.
Financial Disclosure: Editorial support was provided by Oxford PharmaGenesis and was funded by Novartis Pharma AG. The matching-adjusted indirect comparison and associated report completed by MAPI Group were funded by Novartis Pharma AG. The original systematic literature review was performed by RTI Health Solutions (Research Triangle Park, NC, USA) and the update was performed by Costello Medical Consulting Ltd (Cambridge, UK), both funded by Novartis Pharma AG. RTI Health Solutions conducted the regression analysis of baseline parameters which was funded by Novartis Pharma AG. The FUTURE 2 (NCT01752634), FUTURE 3 (NCT01989468), and FUTURE 5 (NCT02404350) trials were funded by Novartis Pharma AG.
References
- 1.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, Smolen JS, Gaujoux-Viala C, Ash Z, Marzo-Ortega H, van der Heijde D, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis. 2012;71:4–12. doi: 10.1136/annrheumdis-2011-200350. [DOI] [PubMed] [Google Scholar]
- 3.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–71. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 4.Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940–7. doi: 10.1016/j.jval.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submissions to NICE. 2016. Available from: URL: http://nicedsu.org.uk/wp-content/uploads/2018/08/Population-adjustment-TSD-FINAL-ref-rerun.pdf.
- 6.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–17. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 8.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38:200–11. doi: 10.1177/0272989X17725740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thom H, Jugl S, Palaka E, Jawla S. Matching adjusted indirect comparisons to assess comparative effectiveness of therapies: usage in scientific literature and health technology appraisals (ID 4483) Value Health. 2016;19:A100–A1. doi: 10.1016/j.jval.2016.03.1723. [DOI] [Google Scholar]
- 10.Kirson NY, Rao S, Birnbaum HG, Kantor E, Wei RS, Cifaldi M. Matching-adjusted indirect comparison of adalimumab vs etanercept and infliximab for the treatment of psoriatic arthritis. J Med Econ. 2013;16:479–89. doi: 10.3111/13696998.2013.768530. [DOI] [PubMed] [Google Scholar]
- 11.Nash P, McInnes IB, Mease PJ, Choy EH, Thom H, Kalyvas C, et al. Secukinumab for the treatment of psoriatic arthritis: comparative effectiveness versus adalimumab using a matching-adjusted indirect comparison. Arthritis Rheumatol. 2016;68(suppl 10):1738. [Google Scholar]
- 12.Strand V, Betts KA, Mittal M, Song J, Skup M, Joshi A. Comparative effectiveness of adalimumab versus secukinumab for the treatment of psoriatic arthritis: a matching-adjusted indirect comparison. Rheumatol Ther. 2017;4:349–62. doi: 10.1007/s40744-017-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–46. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 14.Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3) Arthritis Res Ther. 2018;20:47. doi: 10.1186/s13075-018-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77:890–7. doi: 10.1136/annrheumdis-2017-212687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mease P, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–72. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 17.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 18.Nash P, McInnes IB, Mease PJ, Thom H, Hunger M, Karabis A, et al. Secukinumab versus adalimumab for psoriatic arthritis: comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther. 2018;5:59–122. doi: 10.1007/s40744-018-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28:935–45. doi: 10.2165/11538370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Signorovitch J, Swallow E, Kantor E, Wang X, Klimovsky J, Haas T, et al. Everolimus and sunitinib for advanced pancreatic neuroendocrine tumors: a matching-adjusted indirect comparison. Exp Hematol Oncol. 2013;2:32. doi: 10.1186/2162-3619-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gladman DD, Mease PJ, Strand V, Healy P, Helliwell PS, Fitzgerald O, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167–70. [PubMed] [Google Scholar]
- 22.Tillett W, Eder L, Goel N, De Wit M, Gladman DD, FitzGerald O, et al. Enhanced patient involvement and the need to revise the core set - report from the psoriatic arthritis working group at OMERACT 2014. J Rheumatol. 2015;42:2198–203. doi: 10.3899/jrheum.141156. [DOI] [PubMed] [Google Scholar]
- 23.Orbai AM, Mease PJ, de Wit M, Kalyoncu U, Campbell W, Tillett W, et al. Report of the GRAPPA-OMERACT Psoriatic Arthritis Working Group from the GRAPPA 2015 Annual Meeting. J Rheumatol. 2016;43:965–9. doi: 10.3899/jrheum.160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis - results of two phase 3 trials. N Engl J Med. 2014;371:326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. J Drugs Dermatol. 2015;14:821–33. [PubMed] [Google Scholar]
- 26.McInnes IB, Nash P, Ritchlin C, Choy EH, Kanters S, Thom H, et al. Secukinumab for psoriatic arthritis: comparative effectiveness versus licensed biologics/apremilast: a network meta-analysis. J Comp Eff Res. 2018;7:1107–23. doi: 10.2217/cer-2018-0075. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb A, Mease P, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, significantly reduces psoriasis burden in patients with psoriatic arthritis: results from a phase 3 randomized controlled trial. Arthritis Rheumatol. 2014;66:S233. [Google Scholar]
- 28.Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150–7. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 30.Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA diagram.
The rules for this SLR were set a priori (as defined in Supplementary Table 3), yielding 29 trials. A second filter was then applied, i.e., studies that included either etanercept or secukinumab.
MAIC: matching-adjusted indirect comparison; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SLR: systematic literature review
ACR response rates for secukinumab 150 mg and secukinumab 300 mg compared with etanercept: sensitivity analyses.
ACR 20/50/70 responses are the absolute mean response rate (NCT00317499) and the predicted mean response rate (FUTURE 2, 3, and 5). An OR <1 favors etanercept; an OR >1 favors secukinumab. Error bars show 95% confidence intervals. The most appropriate imputation methods to match NCT00317499 were used.
ACR: American College of Rheumatology; ACR 20/50/70: ≥20/50/70% improvement according to the ACR response criteria; ESS: effective sample size; ETN: etanercept, OR: odds ratio; SEC: secukinumab
Supplementary Table 1.
Baseline characteristics before and after matching in the sensitivity analysis
| NCT00317499 trial | FUTURE 2/3/5 (before matching) | FUTURE 2/3/5 (after matching) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Baseline characteristics | ETN 25 mg BIW (n=101) | PBO (n=104) | SEC 150 mg (n=458) wk 12 | SEC 300 mg (n=461) wk 12 | PBO (n=567) | SEC 150 mg (ESS=276) | SEC 300 mg (ESS=253) | PBO (ESS=304) |
| Demographics | ||||||||
| Age (years), mean* | 47.6 | 47.3 | 48.5 (12.3) | 48.6 (12.8) | 49.4 (12.3) | 47.6 (10.0) | 47.6 (9.4) | 47.3 (9.4) |
| Weight (kg), median* | 88.4 | 88.4 | 85.0 | 82.5 | 82.3 | 88.1 | 88.4 | 88.6 |
| Female, n (%) | 43 (42.6) | 57 (54.8) | 231 (50.4) | 235 (51.0) | 308 (54.3) | (42.6)† | (42.6)† | (54.8)† |
| p=0.1525 | p=0.1261 | p=1.000 | p=1.000 | |||||
| White, n (%) | 91 (90) | 95 (91) | 397 (86.7) | 410 (88.9) | 501 (88.4) | (90.0)† | (90.0)† | (90.0)† |
| p=0.3505 | p=0.7339 | p=1.000 | p=1.000 | |||||
| Disease characteristics | ||||||||
| Methotrexate use at baseline, n (%) | 42 (42) | 43 (41) | 213 (46.5) | 227 (49.2) | 279 (49.2) | (42.0)† | (42.0)† | (41.0)† |
| p=0.3686 | p=0.1630 | p=1.000 | p=1.000 | |||||
| Psoriasis (=3% BSA) n (%) | 66 (65.3) | 62 (59.6) | 251 (54.8) | 213 (46.2) | 264 (46.6) | (65.3)† | (65.3)† | (59.6)† |
| p=0.0529 | p=0.0005 | p=1.000 | p=1.000 | |||||
| HAQ-DI at baseline, mean* | 1.1 | 1.1 | 1.2 (0.6) | 1.2 (0.6) | 1.1 (0.6) | 1.1 (0.5) | 1.1 (0.5) | 1.1 (0.5) |
| p=0.1882 | p=0.1876 | p=1.000 | p=1.000 | |||||
| TNFi-naïve, n (%) | 101 (100) | 104 (100) | 312 (68.1) | 316 (68.5) | 390 (68.7) | (100.0)† | (100.0)† | (100.0)† |
| p<0.0001 | p<0.0001 | p=1.000 | p=1.000 | |||||
| Variables not used for matching | ||||||||
| PsA duration (years), mean (SD)* | 9.0 | 9.2 | 7.0 (7.8) | 7.3 (8.4) | 6.7 (7.4) | 6.3 (6.5) | 5.6 (5.7) | 5.5 (5.6) |
| SJC, median* | 13.0 | 12.5 | 9.0 | 8.0 | 8.0 | 8.0 | 7.0 | 8.0 |
| TJC, median | 18.0 | 17.0 | 16.0 | 15.0 | 16.0 | 15.0 | 14.0 | 15.0 |
| CRP levels, median | 1.6 | 1.1 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 |
| SF-36 PCS, mean | 35.8 | 35.7 | 36.9 (8.0) | 37.7 (8.2) | 36.7 (8.4) | 38.7 (6.2) | 38.9 (6.6) | 38.5 (6.9) |
| SF-36 MCS, mean | 50.9 | 48.4 | 41.8 (10.8) | 44.1 (11.7) | 43.7 (11.2) | 41.9 (8.7) | 44.4 (9.0) | 44.9 (8.2) |
Bold data indicate the post-matching baseline characteristics in each group
p values are for secukinumab compared with etanercept.
For continuous baseline characteristics in the NCT00317499 study, either only the median or the mean without SD was reported. Therefore, in both cases, it was not possible to perform a statistical test.
Integer population (n) values were not available because pooled SEC ESS values were calculated using the equation.
BIW: twice weekly; BSA: body surface area; CRP: C-reactive protein; ESS: effective sample size; ETN: etanercept; HAQ-DI: Health Assessment Questionnaire Disability Index; MCS: mental component summary; PBO: placebo; PCS: physical component summary; PsA: psoriatic arthritis; SD: standard deviation; SEC: secukinumab; SF-36: 36-Item Short-Form Health Survey; SJC: swollen joint count; TJC: tender joint count; TNFi: tumor necrosis factor inhibitor
Supplementary Table 2.
ACR response rates for secukinumab 150 mg and secukinumab 300 mg compared with etanercept (sensitivity analyses)
Supplementary Table 3.
Trials identified in the SLR and the reasons for their inclusion/exclusion in this MAIC
| Trial acronym | Intervention | Comparator | Population | Primary study reference | Secondary references | Study included in MAIC analysis? |
|---|---|---|---|---|---|---|
| CLEAR (subanalysis) | Secukinumab 300 mg (s.c.); weeks 0, 1, 2, and 3 and Q4W from week 4 | Ustekinumab 45 mg for individuals =100 kg and 90 mg for individuals >100 kg (s.c.); weeks 0, 4, and Q12W from week 16 | CLEAR included 676 patients with moderate-to-severe psoriasis whose disease was inadequately controlled by topical treatment, phototherapy, and/or previous systemic therapy. 123 had concomitant PsA | Gottlieb (13) | None | No—these trials were designed to assess dermatologic endpoints in psoriasis and consequently did not collect data on rheumatology disease characteristics at baseline or assess ACR response rates. This meant that matching and comparison of outcomes in our analysis would not have been possible. Furthermore, all patients had moderate-to severe psoriasis, which resulted in a discrepancy in baseline PASI scores between the population in this trial and the patients in other trials identified in the SLR |
| ERASURE (subanalysis) | Secukinumab 300 mg or 150 mg give at weeks 0, 1, 2, 3, and 4 and Q4W thereafter through week 48 | Placebo | ERASURE included patients with moderate-to-severe psoriasis whose disease was poorly controlled by topical treatment, phototherapy, and/o previous systemic therapy. 171 had concomitant PsA | Gottlieb (14) Gottlieb (15) |
Blauvelt (16) Gottlieb (17) Philipp (18) |
|
| FIXTURE (subanalysis) | Secukinumab 300 mg or 150 mg QW for 4 weeks | Placebo | FIXTURE included patients with moderate-to-severe psoriasis whose disease was poorly controlled by topical treatment, phototherapy, and/or previous systemic therapy. 196 had concomitant PsA | Gottlieb (15) | Gottlieb (19) Philipp (18) Sigurgeirsson (20) |
|
| FUTURE 1 | Secukinumab 10 mg/kg i.v. at weeks 0, 2, and 4 followed by 150 mg or 75 mg s.c. Q4W thereafter | Placebo: i.v. at weeks 0, 2, and 4 and s.c. Q4W thereafter | 606 patients with PsA and inadequate response or intolerance to NSAIDs | Mease (21) | Gottlieb (22) Mease (23) Mease (24) Mease (25) Strand (26) van der Heijde (27) |
No—intravenous loading for secukinumab |
| FUTURE 2 | Secukinumab 300 mg or 150 mg QW from baseline to week 4 and then Q4W | Placebo | 298 patients with active PsA despite treatment with NSAIDs, DMARDs, or TNFis | McInnes (2) | Gottlieb (28) Gottlieb (29) Kavanaugh (30) McInnes (31) Mease (32) Rahman (33) |
Yes |
| McInnes (2014) | Secukinumab 10 mg/kg i.v. at weeks 0 and 3 | Placebo | 42 patients with active PsA | McInnes (34) | McInnes (35) McInnes (36) |
No—intravenous loading for secukinumab |
| Mease (2000) | Etanercept 25 mg BIW | Placebo | 60 patients with active PsA and nadequate response to NSAIDs | Mease (37) | None | No—the study duration was only trial was a proof-of-concept study rather than a phase 3 trial 12 weeks, and this |
| NCT00317499 (2004) | Etanercept 25 mg BIW for 24 weeks | Placebo | 205 patients with active PsA and inadequate response to NSAIDs | Mease (3) | Atteno (38) Mease (39) Mease (40) |
Yes |
| PRESTA | Etanercept 50 mg BIW | Etanercept 50 mg QW | 748 patients with psoriasis and concomitant PsA | Sterry (41) | Gniadecki (42) Griffiths (43) Helliwell (44) Prinz (45) |
No—all patients had moderate-to-severe psoriasis, which resulted in a discrepancy in baseline PASI scores between and the patients in other trials identified in the SLR. Restriction of our analysis subgroup to account for this limited the available data set, restricted by psoriasis disease severity were excluded and consequently trials discrepancy would have only to patients in this the population in this trial |
Gray shaded rows indicate trials that were included in our analysis
ACR: American College of Rheumatology; BIW: twice weekly; DMARD: disease-modifying anti-rheumatic drug; i.v., intravenously; MAIC: matching-adjusted indirect comparison; NSAID: non-steroidal anti-inflammatory drug; PASI: Psoriasis Area and Severity Index; PsA: psoriatic arthritis; Q4W: every 4 weeks; Q12W: every 12 weeks; QW: once weekly; s.c.: subcutaneously; SLR: systematic literature review; TNFi: tumor necrosis factor inhibitor