Abstract
Aim: The purpose of this study was to search for the influence of red LED light of oral squamous cell carcinoma cells (OSCC) Material & Methods: HSC-3 was irradiated with red LED light (630 nm · 3 J). Proliferative capacity was analyzed using WST-1. Transwell migration assay, real-time PCR, and ELISA method were also used to analyze migratory ability. Conclusions: HSC-3 cells irradiated with red LED light showed increased migration ability. Interestingly, we clarified that the expression of Interleukin-6 (IL-6), which promotes the migratory ability of cancer cells, is induced.
Keywords: Oral squamous cell carcinoma, LED, IL-6, migration, Low-level laser therapy
INTRODUCTION
LEDs (light emitting diodes) are known to be easier, safer, and cheaper to operate compared to conventional lasers, with various biological effects in accordance with variations in its wavelength. LEDs are applied to various medical fields, including uses in wound healing, sterilization, light sources of endoscopes, treatment for newborn jaundice, and sterilization of periodontal disease bacteria (1, 2, 3). However, at present, LEDs are rarely applied in the field of oral surgery, with few reports currently aiming to realize application thereto as far as we are aware. On the other hand, low-level laser therapy (LLLT) in the medical field has been reported to have biological effects such as analgesia, stimulation of the blood stream, stimulation of wound healing, and osteoplasty in the human body tissues (4, 5). In oral surgery, it has been reported to have a wound healing effect on refractory oral mucositis in addition to being useful for alleviating the symptoms of inferior alveolar nerve paralysis following the extraction of impacted wisdom teeth (6, 7, 8). At the same time, it has also been reported that LLLT promotes cancer cell proliferation (10). In some cases, it may be difficult to determine whether it is refractory mucositis or oral cavity cancer by clinical diagnosis, with a pathological diagnosis leading to a definite diagnosis. With this as a background, when considering the application of LEDs to diseases which are difficult to distinguish from oral cancer such as refractory stomatitis, we believe that the setting of safe irradiation conditions for LEDs is vital. In other words, it cannot be ruled out that the irradiation condition of LEDs, like lasers, may accelerate cancer. However, as of yet, there have been no reports on the effects of LEDs on cancer cells at the cellular/molecular level. In this study, in order to establish the safe clinical application thereof, we conducted an in vitro experiment using a cell strain HSC-3 of oral squamous-cell carcinoma to investigate the influence on HSC-3 of red LEDs with a wave-length close to that of LLLT.
MATERIALS AND METHODS
Cell culture
SCC (squamous cell carcinoma) cell line (HSC-3) were obtained from RIKEN Bio Resource Center (Tsukuba, Japan). SCC cell line was confirmed by short tandem repeat analysis at RIKEN and at the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University. HSC-3 cells were established from a metastatic deposit of poorly differentiated SCC of the tongue in a mid-internal jugular lymph node from a 64 year old man. We routinely maintained SCC cell lines in Dulbecco’s modified Eagle’s medium (DMEM; Wako) with 10% FBS (Sigma-Aldrich, Tokyo, Japan) and 1% antibiotic–antimycotic (gibco, USA). SCC were prepared by plating 3×105 tumor cells in 10 ml complete medium in dishes (ϕ 100mm, Falcon) for 24h. The cell cultures were incubated at 37°C with 5% carbon dioxide. Trypsin (0.25%) and ethylenediaminetetraacetic acid (0.02%; Giboco, Canada) solution was used to isolate cells for subculture. All experiments were performed on cell cultures passage 12 or less.
Irradiation with the red LED light
LED irradiation was carried out using a LED-based device (DAICO MFG CO., Ltd., Kyoto, Japan). Cells were exfoliated using trypsin EDTA by a conventional method right before the second-passage cultured cells reached confluency. After disseminating the HSC-3 cells into DMEM supplemented with 10% FBS and seeded into cell culture dishes, we allowed them to attach onto the culture dishes. 24 hours from the dissemination, red LED light (wavelength of 630 nm, power density of 20 mW/cm) was irradiated from 20 mm above the cell layer, as previously described (11).
Cell Proliferation Assay (WST-1 assay)
Cell proliferations were determined using the Pre- mix WST-1 Cell Proliferation Assay System (Takara Biochemicals, Tokyo, Japan) according to the manufacturer’s instructions. Briefly, each 1.0 × 104 cells (100 μl/well) were maintained in DMEM supplemented with 1% FBS and seeded into 96-well microtitre plates. After each 24h incubation, red LED light (wavelength of 630 nm, power density of 20 mW/cm) was irradiated. Twenty-four hours from the irradiation, 10 μl Premix WST-1 was added to each well, and the plates were incubated for 90 min at 37 °C; thereafter, absorbance at 450 nm was measured using a microplate reader (12).
Wound healing assay
Cell migration was monitored in a scratch wound assay. Briefly, a scratch was made with a sterile pipette tip in a confluent cell layer, washed twice in PBS, and then and then cultured in DEME with 1% FBS. LED light (wavelength of 630 nm) was irradiated from 20 mm above the cell layer. After each 12h incubation, we observed cell migration (12,13).
Transwell assay
The migration assay was performed using 24-well transwells units, each with an 8 μm pore size filter (BD FalconTM; Becton Dickinson, Lincoln Park, NY). Aliquots of 1.0 × 105 cells were placed in upper chamber with 1 ml medium containing 1% FBS, whereas the lower chamber was loaded with 1 ml medium containing 10% FBS. The cells were cultured for 24 h at 37°C in a CO2 incubator. The cells remaining in the upper inserts were completely removed with gently swabbing. The number of cells that had migrated through the membrane into lower chambers was counted using Diff-Quik (Sysmex, Kobe, Japan)(12,14).
Quantitative real-time PCR
The mRNA expression of GAPDH, Interleukin-6 (IL-6), VEGF-A, and TGF-β were analyzed by quantitative real-time PCR. Quantification of mRNA transcription was performed using an Applied Biosystems Step One Real-Time PCR System (Applied Biosystems). Real-Time PCR reactions (20 μL) contained 0.2 μM forward primer, 0.2μM reverse primer, and 2μL of cDNA template from RT reaction, and 10μL (2×) master mix for Power SYBR green master mix (Applied Biosystems). Reaction conditions included 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C. The level of each target gene was normalized to GAPDH level and expressed relative to the levels of the control group (ΔΔCT methods; Applied Biosystems)(11,14).
Design of primers
Primers for GAPDH, the housekeeping gene, were designed as follows: forward (5′-ACCACAGTCCATGCCATCAC-3′) and reverse (5′-TCCACCACCCTGTTGCTGTA-3′), for IL-6: forward (5′-TGCAATAACCACCCCTGACC-3′) and reverse (5′-GTGCCCATGCTACATTTGCC-3′), for VEGF-A: forward (5′-TGCTCTACCTCCACCATGCCAAGT-3′) and reverse (5′-GCGCAGAGTCTCCTCTTCCTTCAT-3′), for TGF-β: forward (5′-CATCAACGGGTTCACTACC-3′) and reverse (5′-CTCCGTGGAGCTGAAGCA-3′)(14,15,16). All primers were purchased from Hokkaido system science (Hokkaido, Japan).
Enzyme-linked immunosorbent assay
We measured IL-6 using the Quantikine ELISA Human IL-6 (R&D Systems, Minneapolis, MN) according to the manufacturer’s recommendations. A standard curve was generated for each plate and used to calculate the absolute concentration of IL-6.
Neutralizing antibody
In order to investigate the influence on the migration of IL-6, IL-6 neutralizing antibody was used. We used IL-6 neutralizing antibody / control IgG (R & D systems) according to the manufacturer’s instructions. Wound healing assay and Traswell assay were performed using these antibodies.
Statistical analysis
All experiments are done three times independently. Data are presented as the mean values ± standard error. The results of the two groups were analyzed using the T-test. The level of statistical significance was set at P < 0.05.
RESULTS
Red LED promotes cell migration
We evaluated the effect of HSC-3 on proliferation activity and migration ability by red LED light irradiation. Regarding the proliferative ability, WST-1 assay showed no significant difference in the proliferation activity but increased the 1.1-fold proliferation activity compared with the control group, and did not affect the proliferative capacity of HSC-3 (Fig. 1).Subsequently, we observed the migratory ability with a Wound healing assay. The photograph shows the state after 12 hours of LED irradiation (Fig. 2a). It can be seen that the decrease in the width of the red LED light irradiation group is larger than that of the control group. We also quantified the rate of decrease in width. A statistically significant difference was observed between the control group (53.9 ± 4.3%) and the irradiation group (74.2 ± 4.3%) (* P < 0.05, Fig. 2b). Furthermore, migratory ability was analyzed by Transwell assay. Compared with the control group, the migration of HSC-3 was enhanced about 2-fold (* P < 0.05, Fig. 2c) in the red LED light irradiation group.
Figure. 1.
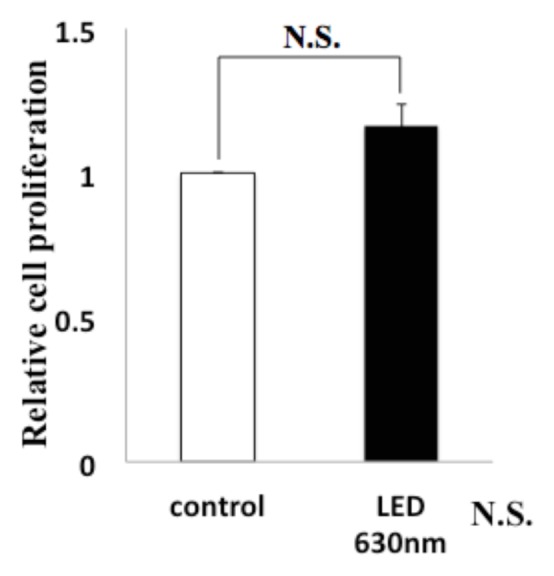
Relationship between Red LED Light and HSC-3 Proliferation Red LED irradiation did not affect the proliferative capacity of HSC-3.
Figure. 2a.
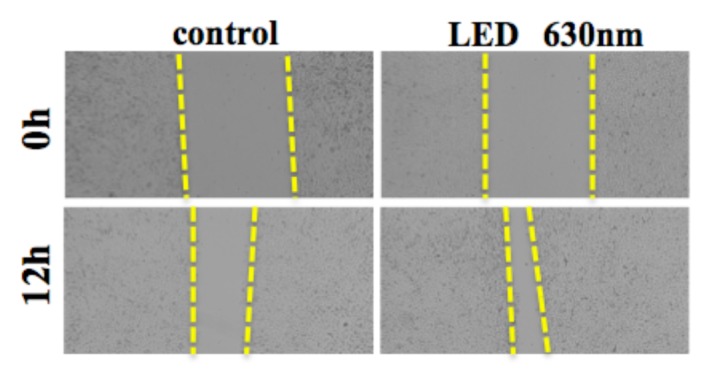
Relationship between red LED light and scratch width
Changes in scratch width after 12 hours of red LED irradiation are shown.
Figure. 2b.
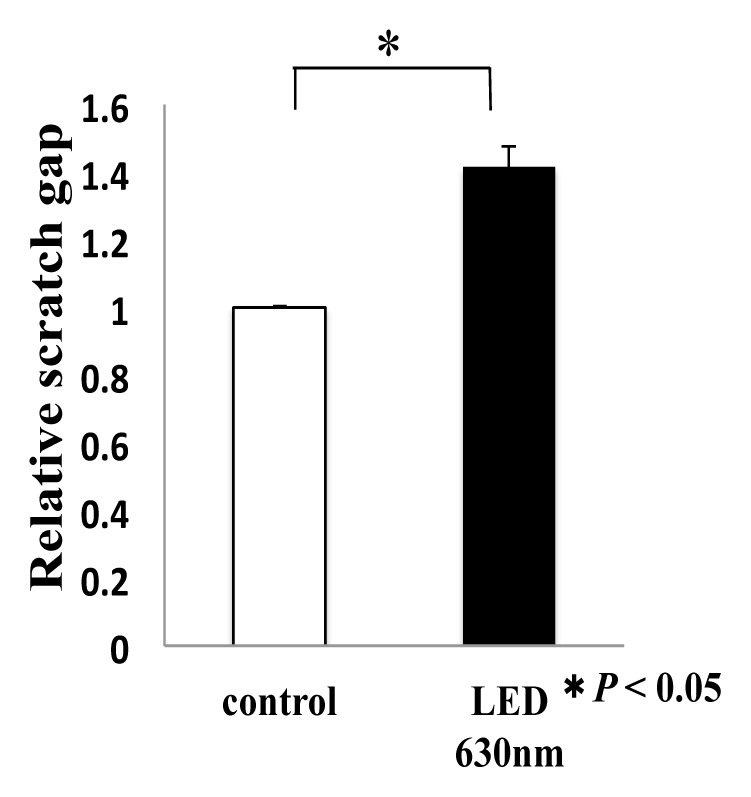
Relationship between red LED irradiation and scratch width reduction ratio
T-test was used. * indicated a significant difference of P < 0.05. N.S. means no significant difference.
Figure. 2c.
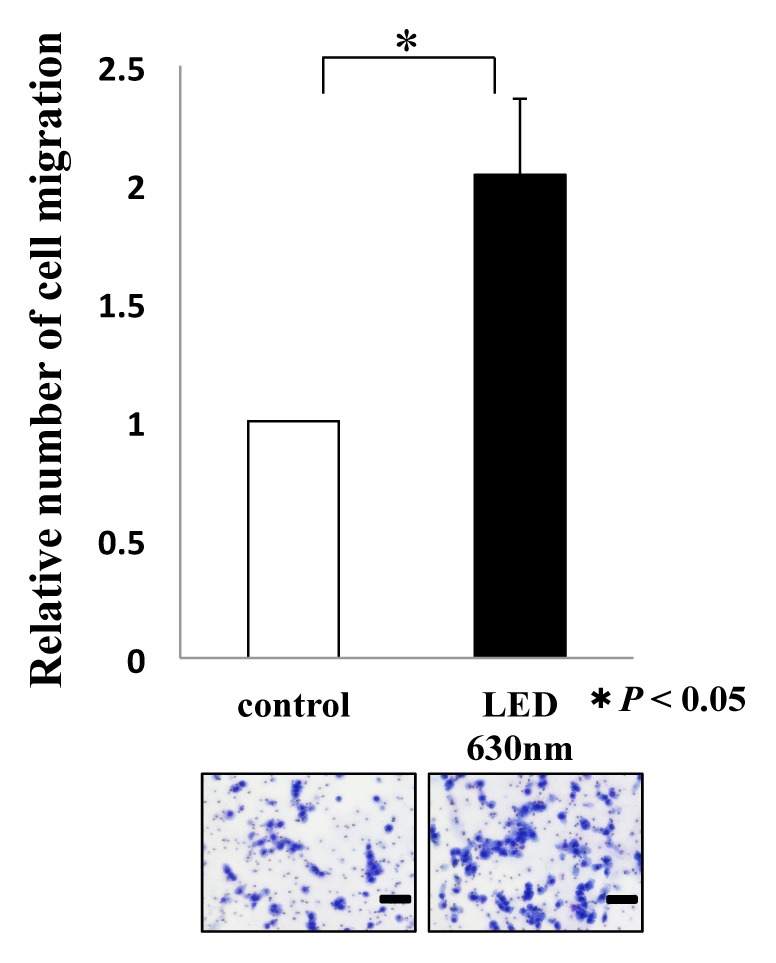
Relationship between red LED light and migration ability of HSC-3
The photograph shows HSC-3 that passed through the membrane. Scale bar indicates 100 μm. T-test was used. * indicated a significant difference of P < 0.05. N.S. means no significant difference.
IL-6 promote cell migration
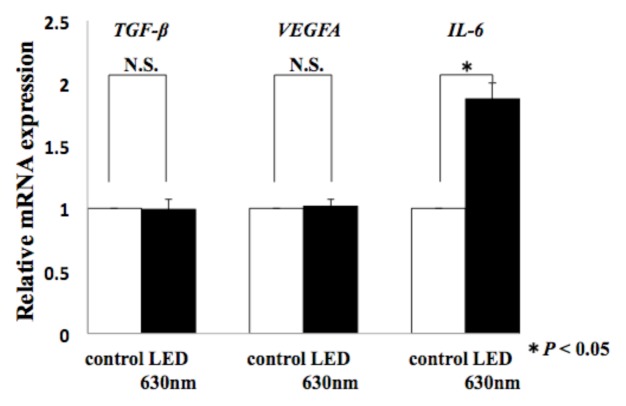
We analyzed the molecular mechanism of HSC-3 migration at the mRNA level using Real time-PCR, focusing on humoral factors that have been reported to be involved in cancer cell migration in the past. The expression of TGF-β, VEGF and IL-6 was 0.99-fold, 1.01-fold, and 1.87-fold, respectively, compared to the control group. In the red LED light irradiation group, only the induced expression level of IL-6 had a statistically significant difference (*P < 0.05, Fig. 3). Moreover, we analyzed the expression level of IL-6, which indicated a significant difference in expression at the mRNA level, at the serum-secretory protein level using ELISA. The control group was 1476.3 pg/ml, whereas the red LED irradiated group was 2697.5 pg/ml, indicating that the expression of IL-6 was actually induced even at the protein level (*P < 0.05, Fig. 4).
Figure. 3.
Effect of red LED light on gene expression of HSC-3
T-test was used. * indicated a significant difference of P < 0.05.N.S. means no significant difference.
Figure. 4.
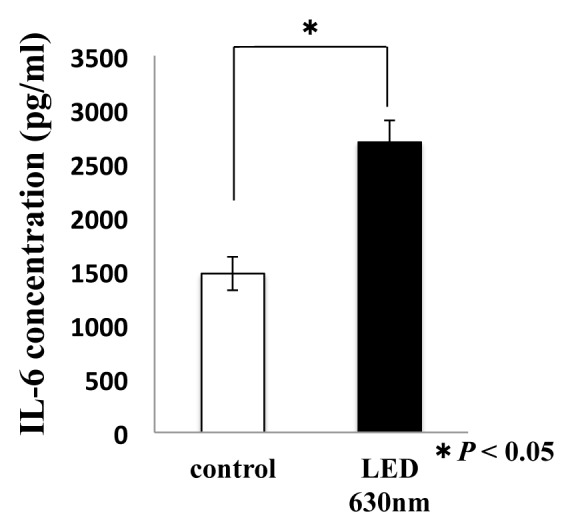
Red LED irradiation on HSC-3 promotes the secretion of IL-6
T-test was used. * indicated a significant difference of P < 0.05. N.S. means no significant difference.
Inhibited tumor migration
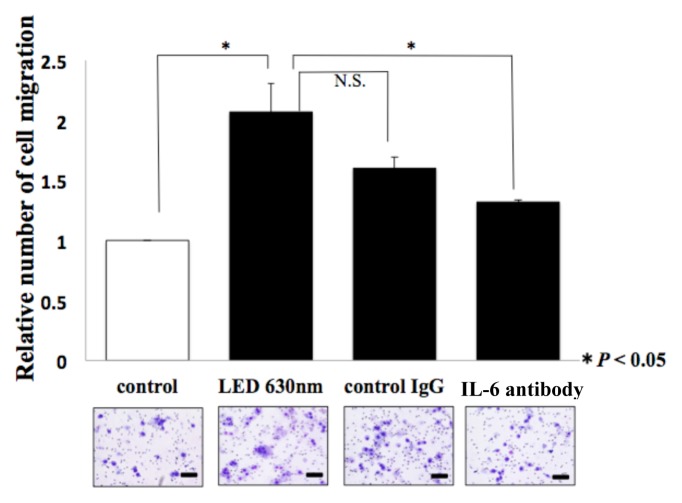
A wound healing assay and Transwell assay were performed again in a cell culture medium containing neutralizing antibodies of IL-6. In the wound healing assay, regarding the reduction rate of the width compared to the control group (no antibodies, no LED irradiation), the group of red LED irradiation on cells cultured in the medium without antibodies indicated 1.39-fold, the group of LED irradiation on the medium with control IgG antibodies added indicated 1.28-fold, and the group of LED irradiation on the medium with IL-6 neutralizing antibodies added indicated 1.1-fold (*P < 0.05, Fig. 5a). Moreover, in the Transwell assay, compared to the control group, the group of red LED irradiation without antibodies added indicated 2.07-fold, the group of LED irradiation on the medium with control IgG antibodies added indicated 1.6-fold, and the group of LED irradiation on the medium with IL-6 neutralizing antibodies added indicated 1.32-fold (*P < 0.05, Fig. 5b).
Figure 5a.
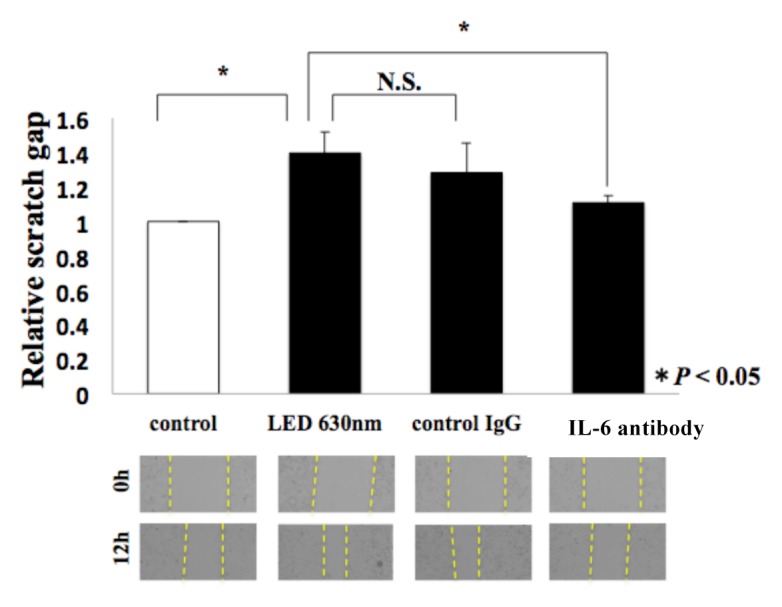
The picture of the scratch assay is shown below the graph. T-test was used. * indicated a significant difference of P < 0.05. N.S. means no significant difference.
Figure. 5b.
The corresponding picture is shown below the graph. The picture shows HSC-3 which passed through the membrane. The scale bar indicates 100 μm. T-test was used. * indicated a significant difference of P < 0.05. N.S. means no significant difference.
DISCUSSION
The number of patients with oral cancer accounts for 1 to 3% of the total number of cancer patients, with the number of patients in Japan increasing. The method of treatment includes not only surgical resection but also chemoradiotherapy, which may be used in combination. Postoperative chemoradiotherapy has achieved good outcomes in the local control and survival rate of patients with head and neck carcinomas (17). On the other hand, it has problems such as causing refractory oral mucositis as a side effect and significantly lowering the patient’s QOL. Currently, LLLT has been conducted as one symptomatic treatment for oral mucositis. However, as the use of LEDs has been expanding in the medical field, we believe that further studies are necessary on the safety of introducing LED treatment in the field of oral surgery.
In an experiment involving irradiating lasers used for LLLT on cancer cells, Rhee et al. revealed that irradiation of a 650 nm diode laser on undifferentiated thyroid cancer cells enhances their ability to proliferate (10). On the other hand, Schalch et al. reported that irradiation of a 660 or 780 nm semiconductor laser on oral squamous-cell carcinoma cells inhibited their migratory ability (18). These results indicated conflicts regarding cancer cell proliferation and development into surrounding tissues. Furthermore, Basso et al. reported that irradiation of a 780 nm diode laser on gingival fibroblasts as normal cells enhanced the proliferation and migration of cells. Tsai et al. reported that irradiation of a 660 nm semiconductor laser on fibroblasts enhanced the migration ability of cells (19). These study results using normal cells suggest that enhancing cellular activity is effective for wound healing.
According to past reports on LED irradiation of cancer cells, the irradiation of 450 nm blue LED light with different wavelengths on colon cancer cells in mice resulted in inhibition of the migration of cancer cells (20). Moreover, Yoshimoto et al. reported that an experiment involving the irradiation of 465 nm blue LED light on human colon cancer cells resulted in a decline in the survival rate of cancer cells. These reports indicate an inhibitory function on cancer cells and affirm the possibility of applying blue LEDs to cancer cells. In this study, we investigated the possibility of the clinical application of red LEDs with different wavelengths from blue LEDs. There have been few studies using red LEDs as of yet. Yamauchi et al. reported that the irradiation of 650 nm red LEDs on teeth root stem cells promoted proliferation, osteogenesis, and mineralization of cells and was effective for tissue repair (21). However, no experiments involving the irradiation of red LED light with a near wavelength on oral squamous-cell carcinoma cells have been conducted. In this study, we irradiated near-wavelength red LEDs (630 nm, 3J) on oral squamous-cell carcinoma cell strain HSC-3, resulting in enhanced migration ability without an inhibitory function. As seen from the above, laser and LED devices have various effects on cells. One reason for this is that different phenotypes may be induced by the differences in used devices, their wavelengths, the cells to be irradiated, and the photosensitivity of each cell. From the above, lasers and LEDs may be expected to have effects such as wound healing of tissues, angiogenesis, and improved circulation, depending on the conditions under which they are used. However, as this study indicated enhanced migratory ability, we think further investigation is necessary for the application of red LEDs to cancer cells.
In order to reveal the molecular mechanism of migration, we focused on TGF-β (22), VEGFA (23) and IL-6 (24), which have been reported as humoral factors related to the enhanced migration abilities of tumor cells. In this experiment, we found that only the expression of IL-6 was significantly enhanced at the mRNA level in the red LED irradiation group. At the serum protein level as well, the secretion of IL-6 is enhanced. This fact suggested the existence of some pathway promoting the transcription of IL-6 genes after HSC-3 sensed red LED light.
IL-6 is a molecule that in 1986 was found to be a cytokine that differentiates activated B cells into antibody-producing cells. It is produced by T cells, B cells, monocytes, fibroblasts, keratinocytes, endothelial cells, adipocytes, and some types of tumor cells (25). Furthermore, IL-6 binds to two kinds of molecules, interleukin 6 receptor (IL-6R) and gp130, and induces various biological activities via the JAK/STAT (26). In this experiment, we confirmed the promotion of the secretion of IL-6, suggesting that LED light affects the expression of these receptors as well as the signals at downstream thereof. In general, the functions of IL-6 include acute phase reactions associated with trauma and infection, angiogenesis (27), migrating of neutrophilic leukocyte (28) and bone metabolism. In recent years, it has been reported that IL-6 significantly increases the blood concentration of cancer cells in patients with oral cavity cancer, carcinoma of the esophagus, and lung cancer, suggesting a relationship with malignant tumors (29). As further evidence to support this, past studies have reported that IL-6 affects the proliferation (30) and metastasis (31) of cancer cells and angiogenesis. IL-6 has been investigated as an important cytokine.
In order to investigate the involvement of IL-6 in HSC-3 migration, we conducted an experiment using IL-6 neutralizing antibodies. In the medium with IL-6 neutralizing antibodies added, the migration of HSC-3 was significantly inhibited despite the irradiation of red LED, strongly suggesting the involvement of IL-6 in migration. However, it should be noted that migration was not inhibited in the IL-6 neutralizing antibody-added group as much as in the control group, as illustrated in Figure 5a and 5b. One reason for this may be that, when irradiating red LED light onto HSC-3, migration factors other than IL-6 such as TNF-α and IL-1α were expressed and induced, involving migration. Going forward, we believe that comprehensive analyses are necessary to identify other factors involved in migration.
This study revealed that the irradiation of red LEDs onto the oral squamous-cell carcinoma cell strain HSC-3 enhances the migration ability by secreting IL-6. We believe that application to the field of oral surgery, especially oral cavity cancer, requires further study and a cautious stance are desirable.
ACKNOWLEDGEMENTS
None of the authors has any conflicts of interest or any financial ties to disclose.
REFERENCES
- 1.Rohringer S, Holnthoner W, Dungel P, et al. The impact of wavelengths of LED light-therapy on endothelial cells. SciRep. 2017;6 doi: 10.1038/s41598-017-11061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asnaashari M, Mojahedi SM, Maleki A, et al. A comparison of the antibacterial activity of the two methods of photodynamic therapy (using diode laser 810 nm and LED lamp 630 nm) against Enterococcus faecalis in extracted human anterior teeth. Photodiagnosis Photodyn Ther. 2015;13:233–7. doi: 10.1016/j.pdpdt.2015.07.171. [DOI] [PubMed] [Google Scholar]
- 3.Majid M, Fereshteh KE, Zohreh B. Is the light-emitting diode a better light source than fluorescent tube for phototherapy of neonatal jaundice in preterm infants? Adv Biomed Res. 2012;1:51. doi: 10.4103/2277-9175.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahimipour F, Mahdian M, Khojasteh A, et al. The effect of He-Ne and Ga-Al-As laser light on the healing of hard palate mucosa of mice. Lasers Med Sci. 2013;28(1):93–100. doi: 10.1007/s10103-012-1060-0. [DOI] [PubMed] [Google Scholar]
- 5.Fahimeh R, Khadijeh H, Soraya P, et al. Comparison of the Effects of Transcutaneous Electrical Nerve Stimulation and Low-Level Laser Therapy on Drug-Resistant Temporomandibular Disorders. J Dent. 2017;18(3):187–192. [PMC free article] [PubMed] [Google Scholar]
- 6.Agha-Hosseini F, Moslemi E, Mirzaii-Dizgah I, et al. Comparative evaluation of low-level laser and CO2 laser in treatment of patients with oral lichen planus. Int J Oral Maxillofac Surg. 2017;41:1265–1269. doi: 10.1016/j.ijom.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Hanaa ME, Amany ME, Mohamed A, et al. Clinical Assessment of the Efficiency of Low Level Laser Therapy in the Treatment of Oral Lichen Planus. Open Access Maced J Med Sci. 2015;3(4):717–721. doi: 10.3889/oamjms.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozen T, Orhan K, Gorur I, Ozturk A. Efficacy of low level laser therapy on neurosensory recovery after injury to the inferior alveolar nerve. Head Face Med. 2006;2:3. doi: 10.1186/1746-160X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer S, Kazancioglu HO, Acar AH, Demirtas N, Kandas NO, et al. Comparison of laser and ozone treatments on oral mucositis in an experimental model. Lasers Med Sci. 2017;32(3):673–677. doi: 10.1007/s10103-017-2166-1. [DOI] [PubMed] [Google Scholar]
- 10.Rhee YH, Moon JH, Choi SH, Ahn JC. Low-Level Laser Therapy Promoted Aggressive Proliferation and Angiogenesis Through Decreasing of Transforming Growth Factor-b1 and Increasing of Akt/Hypoxia Inducible Factor-1a in Anaplastic Thyroid Cancer. Photomedicine and Laser Surgery. 2016;34:229–235. doi: 10.1089/pho.2015.3968. [DOI] [PubMed] [Google Scholar]
- 11.Asai T, Suzuki H, Kitayama M, Komori T, et al. The Long-term Effects of Red Light-emitting Diode Irradiation on the Proliferation and Differentiation of Osteoblast-like MC3T3-E1 Cells. Kobe J Med Sci. 2014;60(1):E12–E18. [PubMed] [Google Scholar]
- 12.Usami Y, Satake S, Yokozaki H, et al. Snail-associated epithelial–mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol. 2008;215:330–339. doi: 10.1002/path.2365. [DOI] [PubMed] [Google Scholar]
- 13.Jung DW, Che ZM, Kim J, et al. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer. 2010;15(127)(2):332–44. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 14.Shigeoka M, Urakawa N, Yokozaki H, et al. Cyr61 promotes CD204 expression and the migration of macrophages via MEK/ERK pathway in esophageal squamous cell carcinoma. Cancer Med. 2010;4(3):437–46. doi: 10.1002/cam4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao H, Huang Y. Leptin promotes migration and invasion of breast cancer cells by stimulating IL-8 production in M2 macrophages. Oncotarget. 2016;7(40):65441–65453. doi: 10.18632/oncotarget.11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigeoka M, Urakawa N, Yokozaki H, et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104(8):1112–9. doi: 10.1111/cas.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argiris A, Karamouzis MV, Ferris RL, et al. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schalch TD, Fernandes MH, Fernandes KPS, et al. Photobiomodulation is associated with a decrease in cell viability and migration in oral squamous cell carcinoma. Lasers Med Sci. 2018 doi: 10.1007/s10103-018-2640-4. [DOI] [PubMed] [Google Scholar]
- 19.Tsai WC, Hsu CC, Liang FC, et al. Low-level laser irradiation stimulates tenocyte migration with up-regulation of dynamin II expression. PLoS One. 2012;7(5):e38235. doi: 10.1371/journal.pone.0038235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh PS, Kim HS, Jeong HJ, et al. Inhibitory effect of blue light emitting diode on migration and invasion of cancer cells. J Cell Physiol. 2017;232(12):3444–3453. doi: 10.1002/jcp.25805. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi N, Umeda M, et al. High-power, red-light-emitting diode irradiation enhances proliferation, osteogenic differentiation, and mineralization of human periodontal ligament stem cells via ERK signaling pathway. J Periodontol. 2018;89(3):351–360. doi: 10.1002/JPER.17-0365. [DOI] [PubMed] [Google Scholar]
- 22.Chiba T, Ishisaki A, Kamo M, et al. Transforming growth factor-β1 suppresses bone morphogenetic protein-2-induced mesenchymal-epithelial transition in HSC-4 human oral squamous cell carcinoma cells via Smad1/5/9 pathway suppression. Oncol Rep. 2017;37(2):713–720. doi: 10.3892/or.2016.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Yu J, Shao Y, et al. Xanthatin, a novel potent inhibitor of VEGFR2 signaling, inhibits angiogenesis and tumor growth in breast cancer cells. Int J ClinExpPathol. 2015;8(9):10355–64. [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang JY, Huang YL, Tang CH, et al. Syk/JNK/AP-1 signaling pathway mediates interleukin-6-promoted cell migration in oral squamous cell carcinoma. Int J Mol Sci. 2014;15(1):545–59. doi: 10.3390/ijms15010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose-John S, Scheller J, Jones SA, et al. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto T. IL-6: from its discovery to clinical applications. IntImmunol. 2010;22(5):347–52. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 27.Hashizume M, Hayakawa N, Mihara M, et al. Rheumatol Int. IL-6/sIL-6R trans-signalling, but not TNF-alpha induced angiogenesis in a HUVEC and synovial cell co-culture system. Rheumatol Int. 2009;29(12):1449–54. doi: 10.1007/s00296-009-0885-8. [DOI] [PubMed] [Google Scholar]
- 28.Rabe B, Chalaris A, Scheller J, et al. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111(3):1021–8. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- 29.Vinocha A, Grover RK, Deepak R. Clinical significance of interleukin-6 in diagnosis of lung, oral, esophageal, and gall bladder carcinomas. J Cancer Res Ther. 2018;14(10):S758–S760. doi: 10.4103/0973-1482.183217. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, Gupta K, Li R, et al. Tumor-extrinsic discoidin domain receptor 1 promotes mammary tumor growth by regulating adipose stromal interleukin 6 production in mice. J Biol Chem. 2014;293(8):2841–2849. doi: 10.1074/jbc.RA117.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross AC, Cam H, Roberts RD, et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight. 2018;3(16) doi: 10.1172/jci.insight.99791. pii: 99791. [DOI] [PMC free article] [PubMed] [Google Scholar]