Abstract
Idiopathic pulmonary fibrosis (IPF) is a deadly disease with limited therapies. Tissue fibrosis is associated with type 2 immune response, although the causal contribution of immune cells is not defined. The AP-1 transcription factor Fra-2 is upregulated in IPF lung sections, and Fra-2 transgenic mice (Fra-2Tg) exhibit spontaneous lung fibrosis. Here, we show that bleomycin-induced lung fibrosis is attenuated upon myeloid inactivation of Fra-2 and aggravated in Fra-2Tg bone marrow chimeras. Type VI collagen (ColVI), a Fra-2 transcriptional target, is upregulated in 3 lung fibrosis models, and macrophages promote myofibroblast activation in vitro in a ColVI- and Fra-2–dependent manner. Fra-2 or ColVI inactivation does not affect macrophage recruitment and alternative activation, suggesting that Fra-2/ColVI specifically controls the paracrine profibrotic activity of macrophages. Importantly, ColVI-KO mice and ColVI-KO bone marrow chimeras are protected from bleomycin-induced lung fibrosis. Therapeutic administration of a Fra-2/AP-1 inhibitor reduces ColVI expression and ameliorates fibrosis in Fra-2Tg mice and in the bleomycin model. Finally, Fra-2 and ColVI positively correlate in IPF patient samples and colocalize in lung macrophages. Therefore, the Fra-2/ColVI profibrotic axis is a promising biomarker and therapeutic target for lung fibrosis and possibly other fibrotic diseases.
Keywords: Inflammation, Pulmonology
Keywords: Collagens, Fibrosis, Macrophages
Introduction
Fibrosis is the result of an abnormal healing process that can occur in every solid organ where hyperactive fibroblasts, called myofibroblasts, characterized by the expression of α-SMA, produce an excess of extracellular matrix (ECM). This accumulation of ECM ultimately alters organ structure and function (1, 2), and no treatment can halt or reverse fibrosis progression. Organ fibrosis is a leading cause of death worldwide and an important focus of research over the past years (3, 4). One dramatic example is idiopathic pulmonary fibrosis (IPF), a common manifestation of interstitial lung diseases (ILDs). IPF progression is very fast, with a median postdiagnosis survival of 2.5 years and a 20% to 30% 5-year survival rate (5). The current treatment options for IPF, including lung transplantation and the recently approved nintedanib and pirfenidone (6, 7), are limited, and there is an urgent need for new therapeutic targets.
While the participation of fibroblasts in disease progression is well accepted, whether inflammation contributes to IPF is debated. The inflammatory cell infiltrate is small compared with that of other inflammatory lung diseases, and antiinflammatory drugs are ineffective (8–10). A common feature of wound healing and fibrosis is the long-lasting presence of alternatively activated macrophages (AAMs), which participate in the resolution of inflammation and in the fibroblast-myofibroblast conversion (4, 11–14). Macrophages also contribute to ECM remodeling by producing matrix-related proteins, such as matrix-degrading enzymes and fibronectin (15–17). AAMs have an important role in chronic diseases as well as cancer and are therapeutically relevant in preclinical models (18, 19). IL-4 and IL-13 are the main inducers of alternative macrophage activation (20). These cytokines can lead to fibrosis by direct and indirect effects on fibroblasts together with or independently of the canonical profibrotic TGF-β1 (21, 22).
AP-1 proteins form dimers to activate or repress gene transcription in a context-dependent manner and are involved in causation of a variety of chronic diseases, from psoriasis to cancer, acting by regulating cellular processes, such as proliferation, differentiation, and inflammation (23, 24). Genetically engineered mice expressing the AP-1 transcription factor Fra-2, encoded by Fosl2, develop spontaneous systemic fibrosis (25). The accumulation of ECM is particularly prominent in the lungs of Fra-2 transgenic (Fra-2Tg) mice and causes premature death. Furthermore, fibrotic Fra-2Tg lungs display high IL-4 expression with eosinophilic and macrophage infiltration and vascular remodelling (25).
Using several preclinical models for lung fibrosis, we sought to define the cellular and molecular determinants of lung fibrosis and evaluate the therapeutic relevance of targeting AP-1 or any of these determinants. We show that Fra-2–expressing macrophages are important contributors to lung fibrosis, secreting fibroblast-activating factors, such as type VI collagen (ColVI). Importantly, while macrophage alternative activation and Th2 immunity are overall not affected, reduced bleomycin-induced lung fibrosis is observed in mice with broad or myeloid-specific Fra-2/AP-1 inactivation as well as in ColVI KO mice and ColVI BM chimeras.
Results
AAMs are prominent in lungs of Fra-2Tg mice.
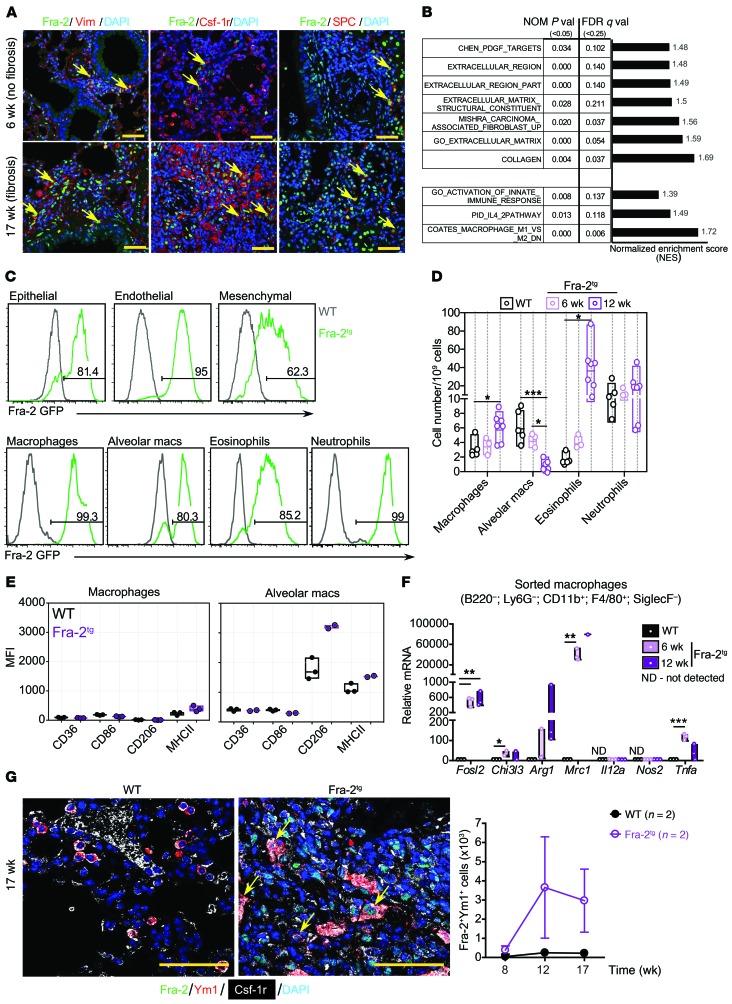
Immunohistological analysis of prefibrotic (before structural changes; at 6 weeks old) and fibrotic (17 weeks) Fra-2Tg lungs revealed that Fra-2 protein was detectable in mesenchymal cells expressing vimentin, in cells of the monocyte/macrophage lineage expressing colony-stimulating factor 1 receptor (Csf-1r), and in surfactant-associated protein C–positive (SPC-positive) alveolar epithelial type II (AEC2) cells (Figure 1A), a cell type implicated in fibrosis and lung tissue repair (26, 27). Consistent with the early occurrence of fibrosis-associated molecular changes, mRNA expression of ECM proteins, such as type I and III collagens (Col1a2 and Col3a1), or fibroblast-related genes, such as fibroblast-specific protein-1 (S100a4), was increased in prefibrotic (6 weeks) Fra-2Tg lungs (Supplemental Figure 1, A and B; supplemental material available online with this article; https://doi.org/10.1172/JCI125366DS1). In Fra-2Tg lung protein extracts, periostin, α-SMA, and osteopontin were increased at as early as 5, 9, and 12 weeks of age, respectively (Supplemental Figure 1C). Global gene expression analyses (RNA-Seq) and gene set enrichment analysis (GSEA) revealed positive correlation between prefibrotic Fra-2Tg lungs and published gene signatures related to ECM and collagen, but also with IL-4 response and alternative activation of macrophages (Figure 1B). TGF-β pathway activation was not apparent from RNA-Seq/GSEA (not shown) or immunoblot analyses (Supplemental Figure 1D), consistent with published studies (28). GSEA also confirmed Fra-1/2 pathway activation in Fra-2Tg lungs (Supplemental Figure 1E), and deconvolution into cell-specific subprofiles revealed enrichment in myeloid signatures, such as neutrophils, eosinophils, dendritic cells, and macrophages (Supplemental Figure 1F). Importantly, a significant increase in genes characteristic of AAMs, such as Ccl17, Ccl22, Chi3l3, Chi3l4, Mrc1, and Arg1, encoding for the chemokines Ccl17 and Ccl22, the chitinases Ym1 and Ym2, the mannose receptor 1/CD206, and arginase 1, respectively, was apparent in Fra-2Tg lungs (Supplemental Figure 1, G and H). In contrast, markers of classically activated macrophages (CAM), such as Cd86, Irf5, Il1b, and Il12a, were overall not consistently changed (Supplemental Figure 1, G and H). Macrophage activation is defined in vivo and in vitro by the organ source, the activator molecule, and the expression of a consensus collection of markers (29). According to this definition, gene expression profiling suggests that M(IL-4) macrophages are enriched in the lungs of young prefibrotic Fra-2Tg mice.
Figure 1. Alternative activation of macrophages in Fra-2Tg fibrotic lungs.
(A) Confocal microscopy images of IF for Fra-2 (green) and Vimentin (Vim), Csf-1r, or SPC (red). Arrows point to double-positive cells. Nuclei are counterstained with DAPI (blue). Scale bars: 25 μm. (B) GSEA on ECM- and type 2 immunity-related pathways in Fra-2Tg lungs compared with WT littermates (6 weeks, RNA-Seq, n = 3/3 from 1 experiment). Normalized enrichment score (NES), nominal (NOM) P values, FDR, and Q values are indicated. (C) Flow cytometry analysis on GFP expression in epithelial (CD45–EpCAM+), endothelial (CD45–EpCAM–CD31+), and mesenchymal (CD45–EpCAM–CD31–CD140a+) cells and in myeloid cell subpopulations in WT (black) and Fra-2Tg (green) mouse lungs at 12 weeks of age. Percentages of GFP-positive cells in the plots are shown. (D) Relative myeloid subpopulation cell numbers. Experiment was repeated twice. Mean, maximum, and minimum are plotted with individual values. *P < 0.05; ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. Group comparisons in each cell type are shown. macs, macrophages. (E) Expression of surface markers on nonalveolar (Siglec-F–CD11bhiF4/80+; left) and alveolar (Siglec-F+CD11blo; right) macrophages in the lungs of 12-week-old WT (black) and Fra-2Tg (purple) mice. Median fluorescence intensity (MFI) is plotted. (F) qRT-PCR analysis of lung macrophages isolated by FACS (n = 3; biological replicates from 1 experiment). Relative expression in WT is set to 1. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired 2-tailed t test. (G) Left, confocal microscopy images of Fra-2 (green), Csf-1r (white), and Ym1 (red) costaining. Nuclei are counterstained with DAPI (blue). Arrows point to triple-positive cells. Scale bars: 50 μm. Right, computational quantification of double Fra-2– and Ym1-positive cell numbers (n = 2; biological replicates from 1 experiment).
Fra-2–expressing myeloid cell populations were next analyzed by flow cytometry using the GFP reporter included in the Fra-2 transgene and by cell-surface marker expression (Supplemental Figure 1, I and J). In prefibrotic and fibrotic Fra-2Tg lungs, Fra-2 transgene expression was detected in all myeloid cell populations in proportions ranging from 70% to 90% and also in epithelial, endothelial, and mesenchymal cells (Figure 1C). A dramatic loss of resident alveolar macrophages was observed in Fra-2Tg mouse lungs at 12 weeks, while macrophages and eosinophils significantly increased (Figure 1D).
Flow cytometry analyses of macrophages revealed an upregulation of AAM marker CD206 (encoded by Mrc1) on Siglec-F+CD11blo alveolar macrophages in the lungs of 12-week-old Fra-2Tg mice. Although surface CD206 remained low on conventional, nonalveolar (Siglec-F–CD11bhiF4/80+) macrophages (Figure 1E), gene expression analyses of a panel of CAM and AAM markers in sorted lung macrophages revealed strong upregulation of Mrc1 transcription together with other AAM-associated genes (Figure 1F), consistent with alternative activation. Furthermore, the AAM marker Ym1 was coexpressed with Fra-2 in Csf-1r–positive macrophages in Fra-2Tg lung sections, and a progressive increase in Ym1-positive and Fra-2/Ym1 double-positive cells was observed over time (Figure 1G and Supplemental Figure 1K). Overall, these data indicate that AAMs, likely with a M(IL-4) phenotype, could be a relevant early contributor to lung fibrosis in the Fra-2Tg mouse model.
Fra-2 is upregulated during bleomycin-induced experimental fibrosis in mice.
Intratracheal administration of the antineoplastic drug bleomycin is a well-established ILD paradigm, with fibrotic lesions developing from 1 week and progressing during 21 days, followed by slow repair or progression to death (30). The lung function decline due to tissue stiffening can be followed by longitudinal plethysmography in bleomycin-treated mice, as lung resistance (LR) increases over time, while dynamic compliance (DC) decreases, resulting in a higher LR/DC ratio (Supplemental Figure 2A).
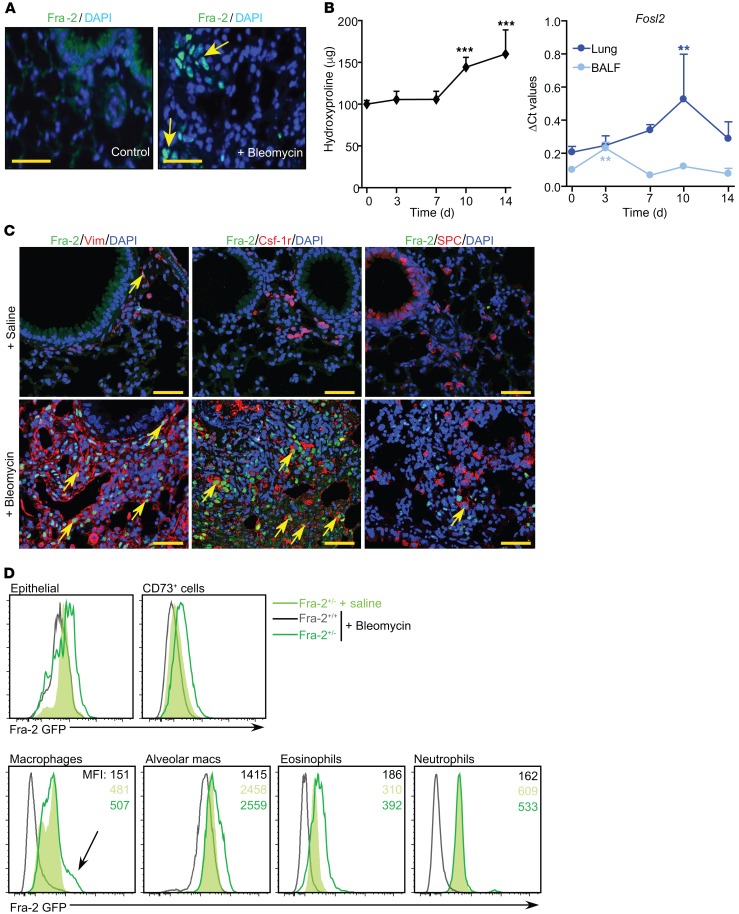
In WT mice, Fra-2 protein was detected in lung sections as early as 10 days after bleomycin instillation (Figure 2A and Supplemental Figure 2B). mRNA expression in the lung and the bronchioalveolar lavage fluid (BALF) was evaluated over time (Supplemental Figure 2C). Fra-2 mRNA expression peaked in BALF and lung at 3 and 10 days after bleomycin, respectively, and preceded collagen accumulation (Figure 2B). TGF-β1 and AAM marker gene expression increase was also detected in BALF cells and lung tissue (Supplemental Figure 2D). BALF contained high numbers of macrophages after bleomycin, while neutrophils and lymphocytes were barely detected (Supplemental Figure 2E). Fra-2 protein was detected in mesenchymal cells and macrophages by immunofluorescence (IF) in lung sections, a pattern reminiscent of that in Fra-2Tg lungs (Figure 1A), but not in AEC2 cells 14 days after bleomycin (Figure 2C). Fra-2+/– heterozygous mice (31) expressing a GFP reporter controlled by the endogenous Fra-2 regulatory elements were monitored by flow cytometry (Supplemental Figure 3, A and B). Under homeostatic conditions, the Fra-2 GFP reporter was barely detectable in lung epithelial and CD73+ cells (including fibroblast and endothelial cells), while it was expressed in alveolar and conventional macrophages, as well as in neutrophils and in eosinophils (Figure 2D). Ten days after bleomycin, Fra-2 GFP expression increased in epithelial cells, CD73+ cells, alveolar macrophages, eosinophils, and a subset of conventional macrophages, but not in neutrophils (Figure 2D). These results indicate that Fra-2–expressing macrophages might contribute to bleomycin-induced lung fibrosis.
Figure 2. Fra-2 expression in bleomycin-induced lung fibrosis.
(A) Fluorescence microscopy images of Fra-2 IF of lungs from WT mice 10 days after saline or bleomycin treatment. Nuclei are counterstained with DAPI. Arrows point to positive nuclei. Scale bars: 25 μm. (B) Time-course analysis of hydroxyproline accumulation and Fra-2 mRNA expression (qRT-PCR as Fosl2) in lungs and BALF from bleomycin-treated WT mice. ΔCt values are plotted. Sample size is n = 5 for lung tissue and n = 3 for BALF. **P < 0.01; ***P < 0.001, compared with the initial time point (0 days); 1-way ANOVA; Dunnet’s post test. (C) Confocal microscopy images on IF of fibrotic lungs from untreated and bleomycin-treated WT mice (14 days). Costaining for Fra-2 (green) and vimentin, Csf-1r, or SPC (red). Arrows point to double-positive cells. Nuclei are counterstained with DAPI (blue). Scale bars: 25 μm. (D) Flow cytometry analysis of lung GFP-expressing cells after 10 days of either saline or bleomycin (n = 3/group, from 2 independent experiments). MFI average values are presented in each plot. Arrow indicates a subpopulation of Fra-2–expressing macrophages present in bleomycin-treated mice that were absent from saline-treated controls.
Fra-2 is essential for lung fibrosis development.
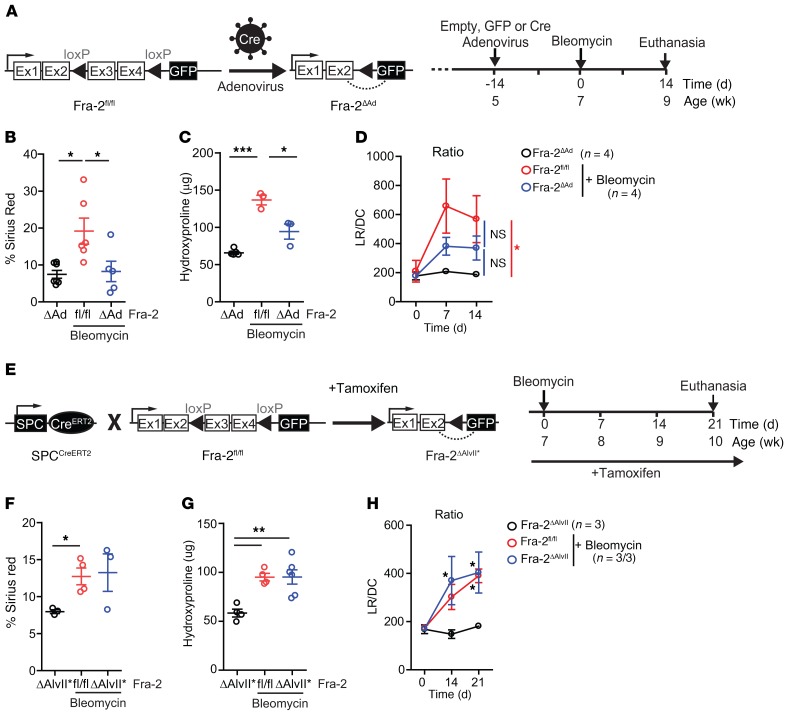
As germline inactivation of Fra-2 is lethal (31), Fra-2 was locally inactivated in the lung of mice homozygous for a Fra-2–floxed allele (Fra-2fl/fl) by intratracheal delivery of adenovirus expressing Cre recombinase (Fra-2ΔAd). This method is widely used to target lung epithelial cells (32), and the resulting Fra-2ΔAd mice were healthy with no lung phenotype for the duration of the experiment (Figure 3A). Flow cytometry analyses of mice treated with adenovirus expressing GFP and bleomycin revealed efficient targeting of epithelial cells, but also mesenchymal cells and all myeloid cells (Supplemental Figure 3C). Fra-2 inactivation resulted in less severe bleomycin-induced fibrosis, with decreased sirius red positivity (Figure 3B) and lower hydroxyproline content (Figure 3C) in the lungs of Fra-2ΔAd mice compared with Fra-2fl/fl littermates receiving empty adenovirus. Finally, longitudinal plethysmography revealed a somehow milder decline in lung function in Fra-2ΔAd mice 7 and 14 days after bleomycin, with a lower LR/DC ratio compared with that of empty virus littermates (Figure 3D).
Figure 3. Fra-2 expression is essential for bleomycin-induced lung fibrosis.
(A) Schematic for genetic Cre/LoxP inactivation of Fra-2 (encoded by fosl2) using intratracheal adenovirus-based Cre delivery (Fra-2ΔAd) and experimental time line. Data come from 2 independent experiments. (B) Quantification of sirius red–positive areas 14 days after bleomycin treatment. Saline-treated Fra-2ΔAd mice were used as controls. *P < 0.05, 1-way ANOVA; Bonferroni’s post test. (C) Hydroxyproline content in lungs (left lobe) 14 days after bleomycin treatment. Saline-treated Fra-2ΔAd mice were used as controls. *P < 0.05; ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. (D) LR (mmHg/mL × s–1) and DC (mL/mmHg) were measured by plethysmography in the same animals over time, and mean ± SEM of the LR/DC ratios were plotted. *P < 0.05, 2-way ANOVA; Bonferroni’s post test. Statistics relative to animals receiving saline. (E) Schematic for genetic Cre/LoxP inactivation of Fra-2 (encoded by Fosl2) using the tamoxifen-inducible, lung alveolar type II cell–specific SPCCreERT2 knockin allele (Fra-2ΔAlvII*) and experimental time line. Experiment was repeated 3 times. (F) Quantification of lung sirius red–positive areas 21 days after bleomycin. *P < 0.05, unpaired 2-tailed t test. Fibrosis was assessed in 2 independent experiments. (G) Lung hydroxyproline content 21 days after bleomycin treatment. **P < 0.01, 1-way ANOVA; Bonferroni’s post test. Fibrosis was assessed in 2 independent experiments. (H) Respiratory function of bleomycin-treated Fra-2fl/fl and Fra-2ΔAlvII* mice and saline-treated Fra-2ΔAlvII* mice. *P < 0.05, 2-way ANOVA; Bonferroni’s post test. Statistics are relative to mice receiving saline.
Next, Fra-2 was genetically inactivated in AEC2 lung epithelial cells by combining Fra-2 floxed alleles with the tamoxifen-inducible SPC-CreERT2 knockin allele (33). The resulting Fra-2ΔAlvII*mice were healthy on a tamoxifen diet with no obvious lung alterations. The GFP reporter included in the Fra-2 floxed allele and expressed instead of Fra-2 upon Cre-mediated gene deletion (Figure 3E) was only detected in a small number of epithelial cells (CD45–EpCAM+) in the lungs of Fra-2ΔAlvII* mice (Supplemental Figure 3D). This is consistent with the limited expression of Fra-2 in SPC-positive cells after bleomycin (Figure 2, C and D) and confirms that SPC-CreERT2 does not target Fra-2–expressing hematopoietic cells (CD45+). Importantly, Fra-2ΔAlvII* mice and Fra-2–proficient littermates developed similar lung fibrosis upon bleomycin instillation with comparable sirius red positivity (Figure 3F), hydroxyproline accumulation (Figure 3G), and LR/DC ratio (Figure 3H). These data unequivocally demonstrate that Fra-2 expression in AEC2 cells is not required for lung fibrosis development and that the milder fibrosis observed in Fra-2ΔAd mice is likely due to Fra-2 inactivation in nonepithelial cells.
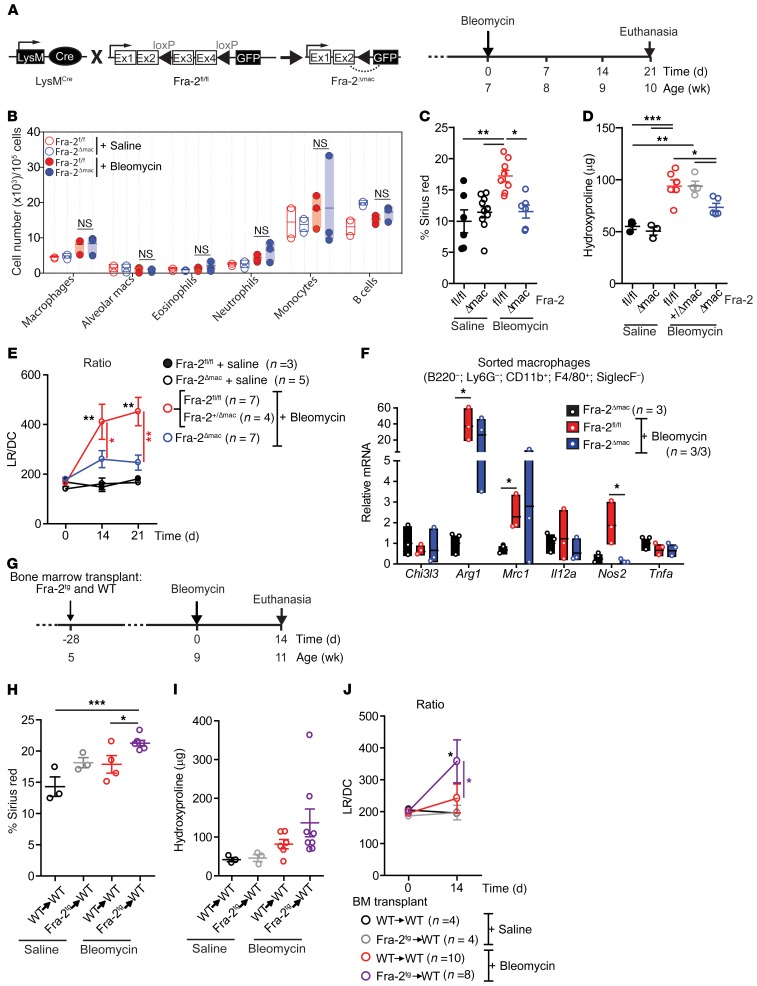
Myeloid-specific Fra-2 inactivation reduces, while Fra-2Tg BM potentiates, lung fibrosis.
The constitutively expressed Lyz2-Cre knockin allele (34), also called LysM, was used to inactivate Fra-2 in the myeloid lineage (Figure 4A). Fra-2Δmac mice were healthy, and flow cytometry analyses using the GFP reporter revealed efficient and exclusive recombination of the floxed Fra-2 allele in lung myeloid cells, both in homeostasis and upon bleomycin administration. Increased Fra-2–reporter expression after bleomycin was observed in a large fraction of macrophages and a small fraction of eosinophils, while expression in neutrophils, monocytes, and alveolar macrophages was comparable to that in untreated Fra-2Δmac mice (Supplemental Figure 4A). Efficient Fra-2 recombination was further confirmed by quantitative reverse-transcription PCR (qRT-PCR) analyses of sorted macrophages, with 85.6% reduction of Fra-2 mRNA in bleomycin-treated Fra-2Δmac mice (Supplemental Figure 4B), and by immunoblot of lung extracts (Supplemental Figure 4C).
Figure 4. Bleomycin-induced lung fibrosis requires Fra-2 expression in macrophages.
(A) Schematic for genetic Cre/LoxP inactivation of Fra-2 (encoded by Fosl2) using the myeloid cell-specific Lyz2-Cre knockin allele (Fra-2Δmac) and experimental time line. Experiment was repeated 6 times. (B) Relative myeloid subpopulation cell and B cell numbers in Fra-2fl/fl and Fra-2Δmac mice 10 days after saline or bleomycin treatment. (C) Quantification of sirius red–positive lung after either saline or bleomycin for 21 days. *P < 0.05; **P < 0.01, 1-way ANOVA; Bonferroni’s post test. (D) Lung hydroxyproline content 21 days after bleomycin treatment. *P < 0.05; **P < 0.01; ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. (E) Respiratory function of saline-treated Fra-2fl/fl and Fra-2Δmac mice and bleomycin-treated Fra-2fl/fl (including Fra-2+/Δmac) and Fra-2Δmac mice. *P < 0.05; **P < 0.01, 2-way ANOVA; Bonferroni’s post test. (F) qRT-PCR analysis in isolated lung macrophages (as B220–, Ly6G–, CD11b+, F4/80+, Siglec-F– cells) 10 days after bleomycin treatment (n = 3/group; 1 experiment). Average gene expression in saline-treated Fra-2Δmac sorted cells is set to 1. *P < 0.05, unpaired 2-tailed t test. (G) Schematic for experimental design and time line of saline- and bleomycin-treated WT mice transplanted with either WT BM (WT→WT) or Fra-2Tg BM (Fra-2Tg→WT). Bleomycin was injected into 2 independent sets of transplanted mice. (H) Quantification of sirius red–positive area in lung sections 14 days after bleomycin. Data from 2 independent experiments are plotted. *P < 0.05, ***P < 0.001, unpaired 1-tailed t test. (I) Lung hydroxyproline content 14 days after bleomycin treatment. (J) Respiratory function at 0 and 14 days after bleomycin treatment. *P < 0.05 compared with either saline control or WT→WT bleomycin-treated group. Two-way ANOVA; Bonferroni’s post test.
No significant differences in the total number of pulmonary myeloid cells were observed between Fra-2Δmac and Fra-2fl/fl mice 10 days after bleomycin treatment (Figure 4B), indicating that Fra-2 is not essential for the increase in myeloid cells during fibrosis. Lung vessel leakage during the acute inflammatory response to bleomycin was also not different between genotypes (Supplemental Figure 4D). However, bleomycin-treated Fra-2Δmac mice developed significantly less severe lung fibrosis, with decreased sirius red positivity (Figure 4C), lower collagen packing density (Supplemental Figure 4E), and reduced lung hydroxyproline content in the lung (Figure 4D). As a result, pulmonary function was significantly improved in Fra-2Δmac mice (Figure 4E). Saline-treated Fra-2Δmac mice were comparable to saline-treated Fra-2fl/fl mice in all measured fibrosis-related parameters (Figure 4, C–E), and myeloid-specific deletion of a single Fra-2 allele (Fra-2+/fl; LysM-Cre+ mice: Fra-2+/Δmac) was not sufficient to affect collagen accumulation (Figure 4D) or lung function (Supplemental Figure 4F), additionally ruling out that the changes observed in Fra-2Δmac mice are due to the Lys2-Cre allele. Detailed analyses further revealed decreased induction of ECM genes, such as Col3a1 and Fn1 (encoding for fibronectin), and the ECM proteins procollagen type I and fibronectin in Fra-2Δmac lungs (Supplemental Figure 4, G and H). Interestingly, while total lung mRNA expression of AAM marker genes was decreased 21 days after bleomycin in Fra-2Δmac mice compared with Fra-2fl/fl mice (Supplemental Figure 4I), no difference was observed between these 2 groups in lung macrophages sorted at day 14, except for Nos2 (Figure 4F). Arg1 was significantly upregulated after bleomycin compared with saline, but the increase was not affected by Fra-2 deletion (Figure 4F). These data indicate that, at least in the early phases of fibrosis, Fra-2 expression in myeloid cells is not crucial for the relative number or distribution of macrophage subpopulations or for the polarization of AAM, while it is essential to their fibrogenic activity in vivo.
BM transplantation experiments were next performed to assess whether increased Fra-2 expression in immune cells, particularly in the myeloid lineage, would potentiate fibrosis. WT or Fra-2Tg BM was transplanted into lethally irradiated WT mice, which were subsequently subjected to bleomycin (Figure 4G). In this setting, no fibrosis was observed in the saline groups, and regardless of the BM genotype, a milder fibrosis developed 14 days after bleomycin, compared with our previous experiments using nontransplanted mice, in which fibrosis was severe. Importantly, mice reconstituted with Fra-2Tg BM accumulated more collagen (Figure 4, H and I) and had worsened lung function (Figure 4J) after bleomycin treatment compared with mice transplanted with WT BM. This demonstrates that ectopic Fra-2 expression in immune cells promotes bleomycin-induced fibrosis.
Overall, these data strongly support an important functional contribution of Fra-2–expressing macrophages to lung fibrosis and suggest a crosstalk between activated macrophages recruited to the lung upon injury and ECM-producing cell types, such as pulmonary fibroblasts.
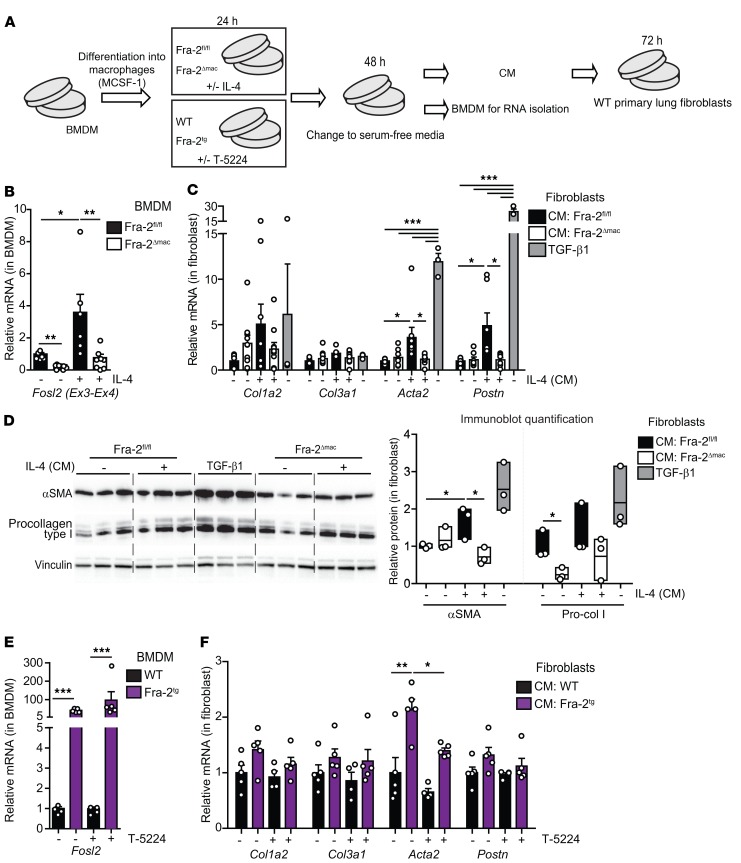
Macrophages release profibrotic factors in a Fra-2/AP-1–dependent manner.
Monocyte-derived macrophages recruited to the lung are major contributors to murine lung fibrosis (35, 36). BM monocytes were therefore isolated from Fra-2Δmac and Fra-2Tg mice, differentiated into macrophages (BM-derived macrophages [BMDMs]), and assessed for their ability to induce a fibrogenic response in WT primary lung fibroblasts. BMDMs were differentiated and expanded using macrophage CSF-1 (MCSF-1) and either left untreated or exposed to the AAM-inducing cytokine IL-4 or to the AP-1 activity inhibitor T-5224 (37). Medium was next changed to serum- and factor-free medium, and the cells and their conditioned media (CM) were harvested 48 hours later (Figure 5A). IL-4 pretreatment increased Fra-2 mRNA expression in Fra-2fl/fl, but not in Fra-2Δmac, BMDMs (Figure 5B). Consistent with our in vivo observations, no difference in the expression of M(IL-4) marker genes was observed between the 2 genotypes (Supplemental Figure 5A), and the mRNA induction of Tgfb1, Timp1, and Mmp12 in BMDMs was also comparable between genotypes (Supplemental Figure 5B). These data indicate that macrophage polarization in vitro in response to IL-4 is not substantially affected by Fra-2 inactivation. In an independent experiment, the Fra-2Δmac BMDM expression of CAM markers in response to LPS was also largely comparable to that in WT, with the notable exception of higher Il12a induction (Supplemental Figure 5C).
Figure 5. CM from Fra-2–expressing BM-derived macrophages induces lung fibroblast activation.
(A) Experimental design to assess the effect of BMDM conditioned medium on WT primary lung fibroblasts. Experiment was repeated 5 and 2 times for the Fra-2 loss and gain of function, respectively. Each individual value represents a biological replicate, since each BMDM culture originates from 1 individual mouse. IL-4 was added at 20 ng/mL, T-5224 at 3 μM, and TGF-β1 at 0.5 ng/mL. (B) Fra-2 expression in BMDMs when CM was collected (qRT-PCR). Note that specific primers located in the floxed/deleted exons (Ex3-Ex4) are used. *P < 0.05; **P < 0.01, 1-way ANOVA; Bonferroni’s post test. (C) qRT-PCR analysis of fibroblast marker genes in primary WT lung fibroblasts cultured with CM and TGF-β1 (positive control). *P < 0.05; ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. Group analysis for each gene. TGF-β1, n = 3; other groups, n ≥ 7. (D) Immunoblot analysis of procollagen I and α-SMA in primary lung fibroblast lysates. Relative densitometry quantification for each protein is shown as a ratio to vinculin density (loading control). Individual values and mean ± SEM from 1 experiment are plotted. *P < 0.05, unpaired t test; 1-tailed. Pro-col I, procollagen I. (E) Fra-2 expression in WT and Fra-2Tg BMDMs at the time the CM was collected (qRT-PCR). ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. (F) qRT-PCR analysis of fibroblast marker genes in primary WT lung fibroblasts cultured with WT and Fra-2Tg BMDM-CM. *P < 0.05; **P < 0.01, paired 2-tailed t test. In all panels, bars represent mean ± SD/SEM. Relative mRNA and protein expression in untreated Fra-2fl/fl BMDMs and derived CM is set to 1.
Incubation of primary lung fibroblasts with CM from IL-4–pretreated BMDM increased cell viability independently of the BMDM genotype (Supplemental Figure 5D). However, increased mRNA expression of the myofibroblast markers Acta2 and Postn, encoding α-SMA and periostin, respectively, was exclusively observed when lung fibroblasts were exposed to CM from IL-4–pretreated Fra-2–proficient BMDMs (Figure 5C). The reduced fibrogenic potential of Fra-2Δmac BMDM CM compared with that in WT was also apparent by α-SMA immunoblot (Figure 5D). The profibrogenic activity of macrophages expressing Fra-2 was next assessed using nonpolarized Fra-2Tg BMDMs treated with the AP-1 inhibitor T-5224 (38). Transgene expression was confirmed by qRT-PCR and GFP positivity (Figure 5E and data not shown). Interestingly, mRNA expression of the M(IL-4) macrophage marker Mrc1 was decreased in Fra-2Tg BMDMs and only affected by T-5224 pretreatment in control BMDMs. Chi3l3 (Ym1) tended to increase in Fra-2Tg BMDMs, while the CAM marker Il12a was undetectable (Supplemental Figure 5E). Expression of Tfgb1 and Timp1 was not significantly altered, whereas increased Mmp12 mRNA in Fra-2Tg BMDMs was attenuated by T-5224 (Supplemental Figure 5F). Importantly, mirroring the Fra-2 loss-of-function setting, exposure to Fra-2Tg BMDM CM induced fibrotic gene expression in lung fibroblasts, such as Acta2, which was prevented by AP-1 inhibitor pretreatment (Figure 5F). Collectively, these data indicate that in macrophages, Fra-2 controls the production of profibrogenic factors, but does not substantially alter their polarization.
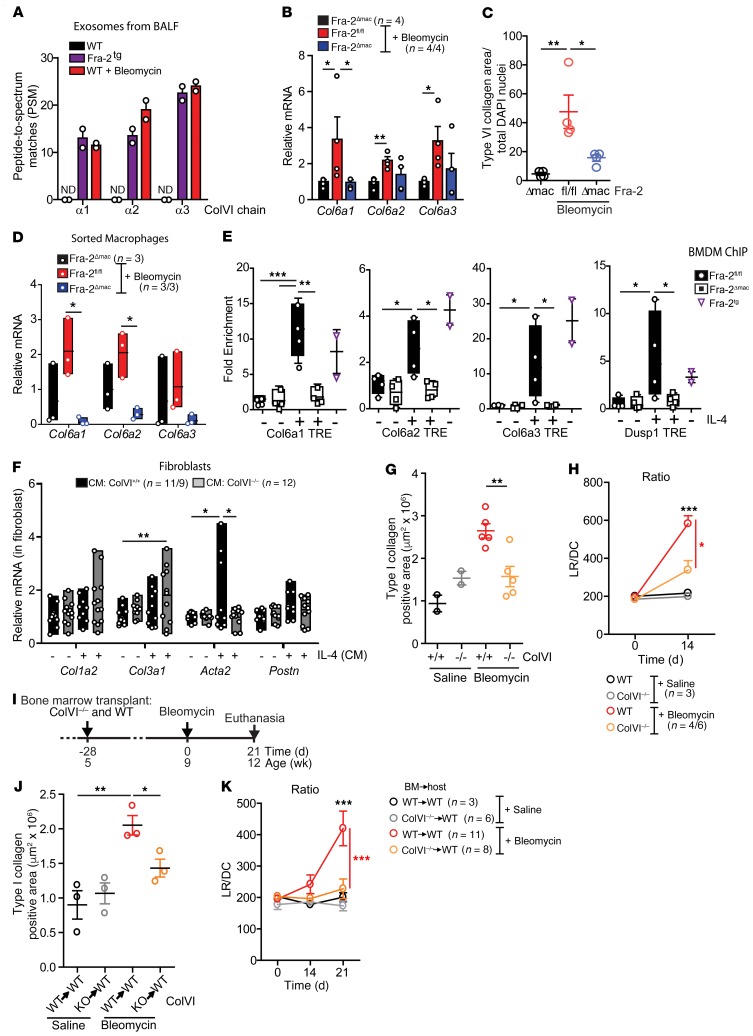
ColVI chains are secreted by macrophages and are direct Fra-2 transcriptional targets.
Macrophages are a major cellular component of the BALF in fibrotic mice (Supplemental Figure 2E), and exosomes often carry disease-specific proteins that could serve as biomarkers and therapeutic targets (39). We isolated exosomes from 2 experimental lung fibrosis paradigms, the spontaneous Fra-2Tg and the bleomycin-induced WT mouse models, to find an unbiased representation of molecules secreted by cells in the local microenvironment of fibrotic lungs, including macrophages and possibly other cell types. Exosome-like vesicles were purified from the BALF of fibrotic Fra-2Tg (12 weeks) and bleomycin-treated WT mice (12 days; Supplemental Figure 6A). NanoSight analyses revealed a reduced number of vesicles in both fibrosis models compared with nonfibrotic controls, while vesicles were larger in bleomycin-treated mice (Supplemental Figure 6B). Exosomal proteome was analyzed by label-free liquid chromatography–tandem mass spectrometry (LC-MS/MS), and around 400 proteins were identified in the BALF of WT mice with or without bleomycin, while more than 600 proteins were found in fibrotic Fra-2Tg mice (Supplemental Figure 6C). Enrichment in extracellular- and vesicle-related protein profiles was confirmed by Gene Ontology analysis (Supplemental Figure 6D) with exosome markers, such as CD63, CD81, Alix, or Hsp90, present in all groups (data not shown). Importantly, the list of protein peptides identified in BALF exosomes from fibrotic, Fra-2Tg, and bleomycin-treated WT mice, but not in healthy controls, was enriched in macrophage signatures (Supplemental Figure 6E), suggesting an increase of vesicles of myeloid origin during lung fibrosis. We next compared this list of exosome-derived potential fibrosis biomarkers to the genes found significantly changed in Fra-2Tg prefibrotic lungs by RNA-Seq. Strikingly, peptides from all 3 ColVI chains, α1, α2, and α3, were present in BALF exosomes from fibrotic mice (Figure 6A and Supplemental Figure 6F), and their respective mRNAs, Col6a1, Col6a2, and Col6a3, were upregulated in prefibrotic Fra-2Tg lungs (Supplemental Figure 6G). In the bleomycin model, Col6a1, Col6a3, and to a lesser extent, Col6a2 mRNA were increased in the lungs of Fra-2fl/fl mice at 21 days, but not in Fra-2Δmac mice (Figure 6B). ColVI is a nonfibrillar collagen formed by trimers of α1, α2, and α3 chains that can promote fibroblast migration (40) and be secreted by macrophages (41). ColVI was coexpressed with Fra-2 in Ym1-positive, alternatively activated macrophages in fibrotic lungs of bleomycin-treated mice and also in fibrotic lungs sections of an independent experimental model of fibrosis (42), γ-herpesvirus-infected (MHV68) IFN-γ receptor KO (IFNγR–/–) mice (Supplemental Figure 6H). Additionally, ColVI protein expression in the lungs was significantly induced by bleomycin in WT mice compared with Fra-2Δmac mice (Figure 6C) as well as in Fra-2Tg BM chimeras compared with WT BM chimeras (Supplemental Figure 6I). Comparing Col6a gene expression in macrophages and mesenchymal and epithelial cells FACS sorted 14 days after bleomycin revealed that, although PDGFRα-positive mesenchymal cells have the highest relative ColVI expression when normalized to cell number, F4/80-positive macrophages are the only cell type in which the 3 ColVI genes are robustly induced by bleomycin (Supplemental Figure 7A). Furthermore, the increase in Col6a gene expression in lung macrophages FACS sorted 10 days after bleomycin (Figure 6D) or in IL-4–pretreated BMDMs (Supplemental Figure 7B) was largely Fra-2 dependent, as it was not observed in Fra-2Δmac cells. Interestingly, Fra-2 did not appear to be essential for the in vitro induction of Col6a genes by TGF-β1 in primary lung (Supplemental Figure 7C) or embryonic (Supplemental Figure 7D) fibroblasts.
Figure 6. ColVI expression contributes to lung fibrosis.
(A) Peptide-spectrum match (PSM) identified for ColVI fragments by LC-MS/MS of BALF exosomes extracted from 12-week-old WT mice, Fra-2Tg mice, and WT mice after 12 days of bleomycin treatment. ND, not detected. (B) ColVI gene expression in lungs 21 days after treatment. Relative expression in saline-treated Fra-2fl/fl is set to 1. *P < 0.05; **P < 0.01, unpaired 1-tailed t test. (C) ColVI-positive area in lungs 21 days after treatment. Control group was treated with saline. Data were normalized to nuclei number. *P < 0.05; **P < 0.01, 1-way ANOVA; Bonferroni’s post test. (D) ColVI gene expression in sorted nonalveolar lung macrophages 14 days after bleomycin treatment. Expression in sorted cells from saline-treated lungs is set to 1. *P < 0.05, unpaired 2-tailed t test. (E) Fra-2 ChIP assay in BMDM. Experiment was repeated twice. Relative expression in untreated Fra-2fl/fl cells is set to 1. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired 1-tailed t test. (F) Fibroblast marker gene expression in WT lung fibroblasts (CM, BMDM CM). Experiment was repeated twice. *P < 0.05; **P < 0.01, 1-way ANOVA; Bonferroni’s post test. (G) Type I collagen area of lungs 14 days after saline or bleomycin treatment. Data from 2 independent experiments are plotted. **P < 0.01, unpaired 2-tailed t test. (H) Respiratory function 14 days after saline or bleomycin treatment. Two independent sets of mice were treated. *P < 0.05; ***P < 0.001, 2-way ANOVA; Bonferroni’s post test. (I) Schematic for experimental design and time line of saline- and bleomycin-treated WT mice transplanted with either WT BM (WT→WT) or ColVI–/– BM (KO→WT). Bleomycin was injected into 2 independent sets of mice. (J) Type I collagen area at the end of the experiment. *P < 0.05; **P < 0.01, unpaired 1-tailed t test. (K) Respiratory function 21 days after bleomycin. ***P < 0.001, 2-way ANOVA; Bonferroni’s post test.
Collectively, these data indicate that, under fibrotic conditions, ColVI expression is increased in macrophages in 3 independent lung fibrosis paradigms in a Fra-2–dependent manner.
Inspection of the murine Col6a genes revealed several Fra-2/AP-1 dimer binding (TRE) elements in regulatory regions (Supplemental Figure 7E). ChIP-qPCR assays using in vitro–cultured BMDMs and specific Fra-2 antibodies demonstrated Fra-2 binding to TRE-containing promoter regions of Col6a1, Col6a2, and Col6a3 in Fra-2Tg and in Fra-2fl/fl BMDMs pretreated with IL-4, but not in Fra-2–deficient BMDMs (Figure 6E), indicating that Col6a genes are direct Fra-2/AP-1 target genes in myeloid cells, implicated in the profibrogenic action of macrophages.
ColVI deficiency decreases fibrosis in vitro and in vivo.
WT primary lung fibroblasts express higher Acta2 when plated on ColVI (Supplemental Figure 8A). Primary lung fibroblasts exposed to CM from IL-4–pretreated Col6a1-KO (43) BMDMs (ColVI–/–) failed to induce Acta2 compared with WT BMDMs (Figure 6F), while M(IL-4) marker genes were similarly induced (Supplemental Figure 8B). Interestingly, CM from IL-4–treated ColVI–/– BMDMs induced Col3a1 mRNA in fibroblasts (Figure 6F), while the alterations in Col1a2 mRNA and procollagen type I proteins were not significant (Figure 6F and Supplemental Figure 8C). Nevertheless, these in vitro data suggest that, similarly to what occurs in Fra-2–deficient cells, IL-4–polarization of ColVI-deficient macrophages is unaltered, while their profibrotic paracrine potential is impaired.
To evaluate the profibrotic role of ColVI in vivo, ColVI–/– mice were subjected to the bleomycin lung fibrosis paradigm. qRT-PCR analyses confirmed increased Col6a1 gene expression in bleomycin-treated WT controls, which was undetectable in ColVI–/– mutants (Supplemental Figure 8D). Importantly, while macrophage numbers were not overtly affected (data not shown), the ECM components type I collagen and fibronectin accumulated significantly less in ColVI-deficient lungs, and Fra-2 expression was reduced (Figure 6G and Supplemental Figure 8, E and F). Furthermore, lung function was significantly improved in ColVI-deficient mice 14 days after bleomycin (Figure 6H). We next treated lethally irradiated WT mice, reconstituted with either WT or ColVI-deficient BM, with bleomycin (Figure 6I and Supplemental Figure 8G). Mice transplanted with ColVI–/– BM were protected from bleomycin-induced lung fibrosis compared with mice that received WT BM, with decreased type I collagen accumulation (Figure 6J), decreased sirius red positivity (Supplemental Figure 8H), and improved respiratory function 21 days after bleomycin treatment (Figure 6K). Taken together, these experiments clearly demonstrate a critical functional role of ColVI expression in immune cells, such as macrophages, during lung fibrosis.
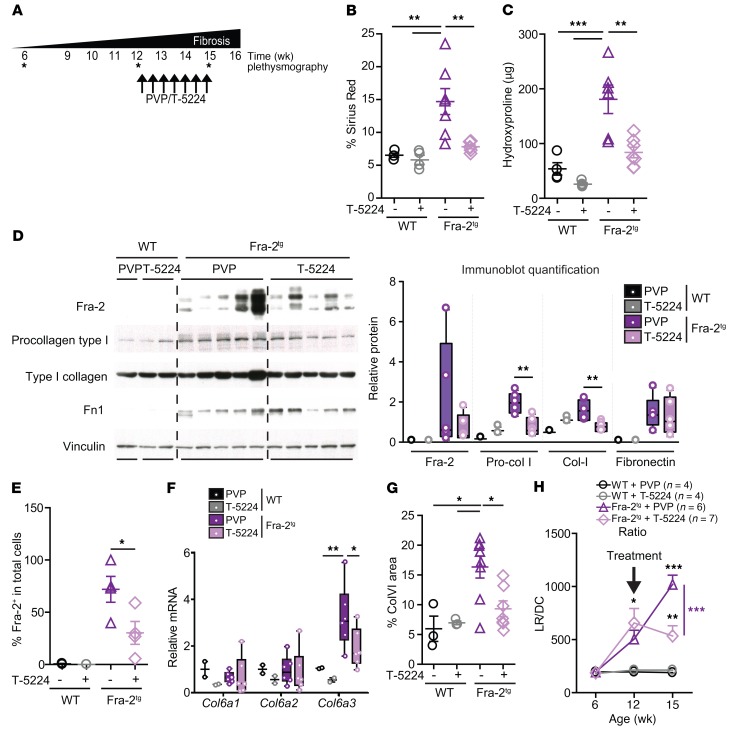
Pharmacological AP-1 inhibition ameliorates fibrosis in the bleomycin model and in Fra-2Tg mice.
We next tested the therapeutic potential of the AP-1 inhibitor T-5224 (37). Saline- or bleomycin-treated WT mice were randomized at day 14, when the acute inflammation phase had subsided and fibrosis has developed, for daily T-5224 or vehicle (polyvinylpyrrolidone [PVP]) oral gavage over 1 week (Supplemental Figure 9A). Under these conditions and consistent with the previously reported benefits of T-5224 when supplied from day 10 (44), bleomycin-induced lung hydroxyproline was significantly lower in T-5224–treated mice than the vehicle-treated cohort (Supplemental Figure 9B). Pulmonary ColVI expression was also reduced (Supplemental Figure 9C), and most importantly, longitudinal plethysmography revealed a significant improvement in lung function with T-5224, with an LR/DC ratio almost reaching the values measured in mice that did not receive bleomycin (Supplemental Figure 9D).
We next treated 12-week-old already fibrotic Fra-2Tg and WT littermates with T-5224 or PVP over 4 weeks (Figure 7A). As previously reported (38), extended T-5224 treatment was well tolerated and did not alter body weight (Supplemental Figure 9E). Importantly, T-5224 resulted in significantly less collagen accumulation in the lungs of Fra-2Tg mice as measured at end point by sirius red positivity (Figure 7B and Supplemental Figure 9F) and hydroxyproline content (Figure 7C). Gene and protein analyses further confirmed ECM components, such as fibrillar collagens I and III and fibronectin, were less expressed in the lungs of Fra-2Tg mice treated with T-5224 (Figure 7D and Supplemental Figure 9G). In T-5224–treated lungs, Fra-2 mRNA, protein, and the number of Fra-2–positive cells were decreased, while IL-4 appeared unaffected (Figure 7, D and E, and Supplemental Figure 9, H and I). With the notable exception of decreased Arg1 and Nos2, end-point mRNA expression of AAM and CAM marker genes was also minimally affected by T-5224 (Supplemental Figure 9J). Increased ColVI protein and Col6a3 mRNA was still apparent in the lungs of Fra-2Tg mice at end-stage disease, but was attenuated by T-5224 treatment (Figure 7, F and G). Most strikingly, longitudinal plethysmography revealed a clear functional benefit of T-5224, as it halted the progressive decline in Fra-2Tg lungs. LR/DC remained stable in T-5224–treated mutants, while it further worsened in vehicle-treated Fra-2Tg mice (Figure 7H). These data demonstrate that lung fibrosis is substantially ameliorated by therapeutic AP-1 inhibition in 2 experimental models of fibrosis and that AP-1 pharmacological inhibitors such as T-5224 should therefore be considered for lung fibrosis therapy.
Figure 7. AP-1 inhibition reverts lung fibrosis in Fra-2Tg mice.
(A) Schematic for AP-1 inhibitor T-5224 therapeutic protocol in Fra-2Tg mice. All data originate from 3 independent experiments designed as randomized blocks. Asterisks indicate when the plethysmography was performed within the experiment timeline. (B) Quantification of sirius red–positive areas in lungs at the end of the experiment. **P < 0.01, 1-way ANOVA; Bonferroni’s post test. (C) Hydroxyproline content in lungs (left lobe). **P < 0.01; ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. (D) Immunoblot analysis of Fra-2, procollagen I, type I collagen, and fibronectin in lung lysates at the end of the experiment. Band density quantification is shown in the graph, with individual values relative to vinculin (loading control). **P < 0.01, unpaired 2-tailed t test. (E) Quantification of Fra-2–positive nuclei in lung IF relative to total cells. *P < 0.05, unpaired 2-tailed t test. (F) qRT-PCR analysis of ColVI genes in the lungs of Fra-2Tg and WT controls treated with T-5224 or PVP at the end of the experiment. Relative expression in WT+PVP is set to 1. *P < 0.05; **P < 0.01, 1-way ANOVA; Bonferroni’s post test. (G) Quantification of lung ColVI-positive area in IHC staining. *P < 0.05, 1-way ANOVA; Bonferroni’s post test. (H) Longitudinal analyses of respiratory function of Fra-2Tg and WT controls with T-5224 or PVP. Note that the T-5224 or vehicle treatment started around 12 weeks of age, when animal respiratory function was already compromised (arrow). *P < 0.05; **P < 0.01; ***P < 0.001, 2-way ANOVA; Bonferroni’s post test.
ColVI and Fra-2 are coexpressed in human fibrotic lungs and macrophages.
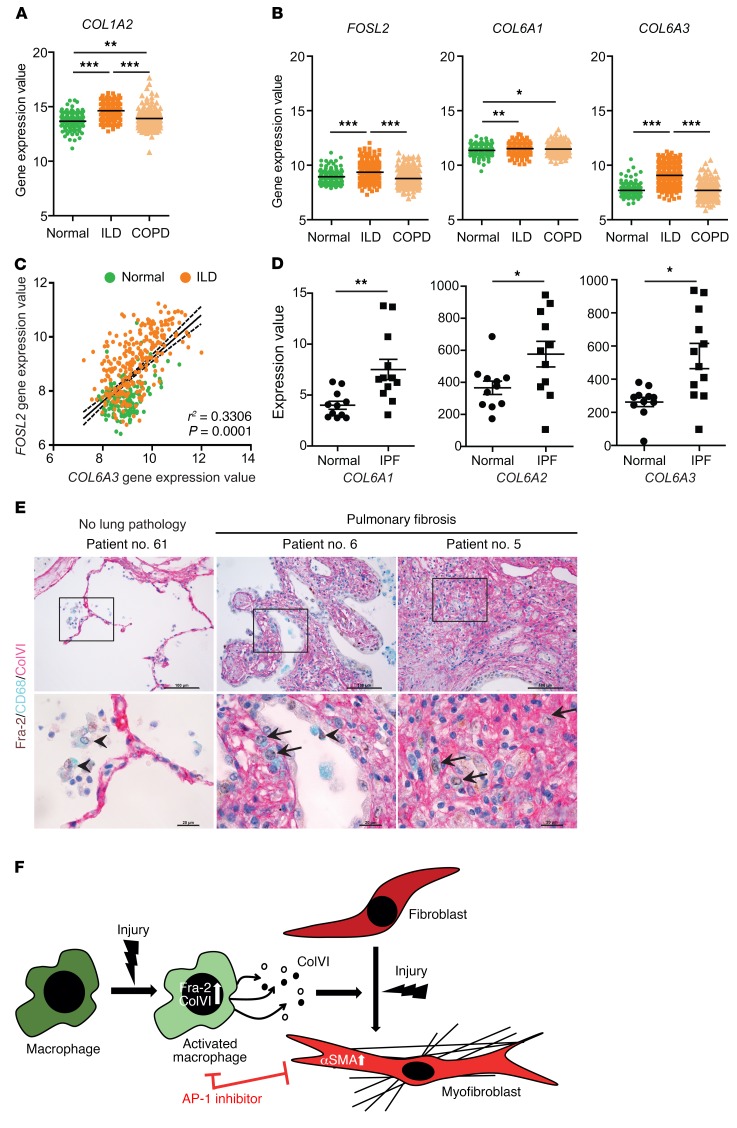
Finally, we assessed Fra-2 and collagen gene expression in human disease by computational analysis of the Lung Genomics Research Consortium (LGRC) database, with gene expression data from diseased lung tissue from 255 ILD and 219 chronic obstructive pulmonary disease (COPD) patients and normal lung tissue from 173 thoracic surgery patients (GSE47460). The fibrosis marker COL1A2 (type I collagen) was increased in both diseased cohorts (Figure 8A). Importantly, while COL6A1 was higher in patients with both ILD and COPD compared with healthy individuals, FOSL2 and COL6A3 were specifically increased in ILD samples, but not in COPD samples (Figure 8B). Increased FRA-2 and COLVI were observed in lung protein extracts from an independent patient cohort (data not shown). Furthermore, the expression of FOSL2 and COL6A3 positively correlated across ILD and healthy samples (Figure 8C). A similar analysis using an independent data set for IPF (GDS1252) also showed increased mRNA expression of COL6A1 and COL6A3 and indicated that COL6A2 is also increased in IPF lung samples compared with healthy lungs (Figure 8D and ref. 45). Importantly, both alveolar and interstitial macrophages highly express Fra-2 in lung sections from fibrosis patients when compared with healthy tissues. Furthermore, Fra-2, ColVI, and CD68 (human macrophage marker) triple immunohistochemistry indicated that interstitial macrophages expressing Fra-2 also express ColVI (Figure 8E). These results indicate that Fra-2 expression in AAMs contributes to mouse and human lung fibrosis, possibly by controlling the expression of secreted factors, such as ColVI.
Figure 8. Fra-2 and ColVI expression in human lung fibrosis.
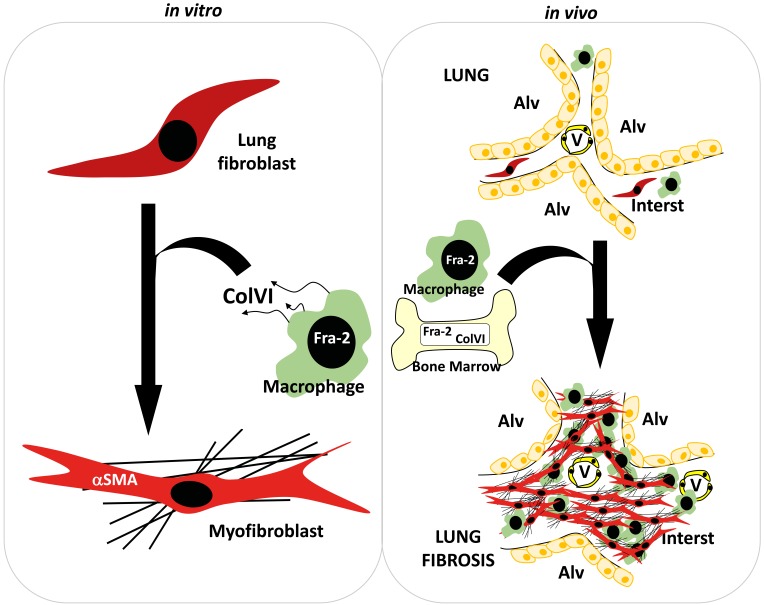
(A) Gene expression values of COL1A2 in lungs from human patients with normal histology (normal; n = 173) and diagnosed ILD (n = 255) and COPD (n = 219). Expression values were obtained from the public gene expression database of the LGRC (GSE47460). **P < 0.01; ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. (B) Gene expression values of FOSL2 (Fra-2), COL6A1 (ColVI chain α1), and COL6A3 (ColVI chain α3) genes in lungs from same cohort. Note that expression values for COL6A2 (ColVI chain α2) were absent in the data set. *P < 0.05; **P < 0.01; ***P < 0.001, 1-way ANOVA; Bonferroni’s post test. (C) Expression values for FOSL2 are plotted against COL6A3 for linear regression and Pearson’s correlation analysis of normal and ILD samples (r2 and P values are indicated). (D) Gene expression values of ColVI genes in lungs from human patients with normal histology (normal) and diagnosed IPF. Expression values were obtained from a public curated data set (GDS1252). *P < 0.05; **P < 0.01, 2-tailed paired t test. (E) Triple IHC for Fra-2 (brown-nuclear), CD68 (blue-cytoplasmic), and ColVI (purple-extracellular) of human lungs from healthy and fibrosis patients. Nuclei are counterstained with hematoxylin. Arrows point to interstitial macrophages expressing Fra-2 and colocalizing with ColVI (triple positive); arrowheads point to Fra-2–positive alveolar macrophages. Scale bars: 100 μm (low magnification); 20 μm (high magnification). (F) Working model for the role of Fra-2/ColVI in macrophages during lung fibrosis.
Discussion
The role of the immune response in lung fibrosis and its potential as therapeutic target are not clearly established. Here, we provide evidence for a functional contribution of Fra-2–expressing macrophages to the paracrine activation of fibroblasts and to lung fibrosis (Figure 8F). We identify ColVI as a Fra-2 transcriptional target in macrophages and unravel a profibrogenic role for ColVI in vitro and in vivo. Importantly, inhibiting Fra-2/AP-1 or ColVI is therapeutically relevant in mouse models of lung fibrosis.
The fibrotic phenotype in the Fra-2Tg model of fibrosis is reminiscent of a type 2 cytokine–driven disease with enhanced IL-4 expression, IL-4 pathway signature, eosinophil/neutrophil infiltration, and M(IL-4) macrophage enrichment (25, 28, 46). The contribution of type 2 cytokines, IL-4 and IL-13, to macrophage activation and fibrosis development in different organs is well accepted (4, 47, 48). Lung-specific expression of these cytokines increases after bleomycin treatment, although their importance in this particular lung fibrosis model is controversial (49–51). While type 2 cytokines seem dispensable or even detrimental in the early phases after bleomycin treatment, these likely promote subsequent fibrotic events (52, 53). IL-4 and IL-13 induce Fra-2 expression in macrophages (ref. 54 and our in vitro data) and promote profibrotic macrophage activation and myofibroblast differentiation (14, 55, 56). We demonstrate that Fra-2 expression in macrophages is not essential for the early inflammatory phase of bleomycin-induced fibrosis nor for macrophage recruitment or phenotypic switch, but modulates the expression of molecules produced by M(IL-4) macrophages to promote the differentiation of fibroblasts to myofibroblasts and lung fibrosis. This is particularly relevant, as clinical trials for IPF and other type 2 immunity diseases using blocking antibodies against type 2 cytokines had limited success, likely due to exacerbation of type 1 (Th1) inflammation or impaired tissue regeneration (57, 58). New strategies decreasing the profibrotic arm of type 2 (Th2) inflammation and/or targeting AAMs secretory/paracrine activity without affecting tissue repair and Th1 inflammation are therefore urgently needed.
Macrophage-secreted factors, such as platelet-derived growth factor (PDGF), TGF-β1, MMPs, and tissue-inhibitor metalloproteinases (TIMPs), can induce and sustain fibroblast-to-myofibroblast activation (59–63). This and the recently proposed direct transition from macrophages to myofibroblasts (64) defines the current understanding of macrophage-fibroblast crosstalk in wound healing and fibrosis. PDGF and TGF-β signaling modulate vascular remodeling in Fra-2Tg mice, but appear largely dispensable for lung fibrogenesis (28, 65). No MMPs/TIMPs were found consistently deregulated in the lung, macrophage, and/or BMDM cultures across the different experimental models used in the current study. Instead, we unraveled an important contribution of ColVI, a direct transcriptional target of Fra-2/AP-1 in macrophages, in modulating fibroblast activation in vitro and fibrogenesis in vivo. We provide experimental evidence for this Fra-2/ColVI connection in 3 independent experimental models of lung fibrosis — chemical, virus, and transgene induced (25, 42, 66) — as well as in patient samples. In the bleomycin model, we show for what we believe is the first time that ectopic Fra-2 expression in BM promotes lung fibrosis, while myeloid-specific Fra-2 inactivation as well as complete or BM-derived ColVI deficiency is protective. While the contribution of other BM-derived cell types cannot be excluded in the transplantation experiments, our collective data in vitro and in vivo suggest that monocyte/macrophages expressing Fra-2 are a relevant functional source of profibrotic ColVI. A more definitive assessment of the contribution of ColVI-expressing macrophages in vivo would require the generation of a new mouse model with conditional ColVI alleles and/or efficient and long-term engraftment of adoptively transferred ColVI-deficient monocytes. Both experimental strategies are challenging and time consuming. As Fra-2 and ColVI are broadly expressed in other fibrosis-relevant lung cell types, such as in mesenchymal cells, future investigations using the appropriate cell-specific genetic tools are certainly warranted for exploring and fully determining the functional role of these 2 genes in the lung and also in tissue fibrosis.
Monocyte-derived macrophages are key drivers of lung fibrosis, replenishing alveolar macrophages immediately lost upon injury (35, 36, 67). While macrophage depletion during wound healing resulted in antagonistic phase-dependent outcomes (68), it prevented fibrosis in several models (69, 70), including bleomycin-induced lung fibrosis (11, 63, 71, 72). Nintedanib, the first-line treatment for IPF, is a potent inhibitor of several growth factor receptors, including the macrophage-survival cytokine M-CSF-R. Nintedanib reduces circulating M-CSF and skin AAMs in Fra-2Tg mice and ameliorates skin and lung fibrosis (46). ColVI expression by macrophages promotes lung fibroblast activation, but similarly to Fra-2, is not essential for the macrophage phenotype switch. Consistent with the implication of AP-1 proteins Fra-2 and c-Jun and the AP-1–activating JNK kinases in lung fibrosis (73, 74), pharmacological AP-1 inhibition decreased ColVI expression and substantially ameliorated bleomycin- and Fra-2Tg–induced fibrosis. Therefore, unlike conventional strategies aiming at depleting macrophages or blocking IL-4/IL13/Th2 response, the more restricted outcome of targeting Fra-2/ColVI using compounds such as T-5224 would provide a therapeutic opportunity to block the profibrogenic arm of chronic Th2-associated diseases without affecting proregenerative effectors.
Increased ColVI in IPF patient lung sections was reported (75). We now show that Fra-2 and ColVI are coexpressed in human IPF lung macrophages and specifically increased and correlated in ILD, but not inflammatory COPD, indicating that coexpression of Fra-2 and ColVI could be a better biomarker for lung fibrosis than type I collagen, which is increased in both diseases. Whether, similarly to what occurs with Fra2Tg and bleomycin-treated mice, ColVI peptides are detectable in BALF exosome-like vesicles from IPF patients undergoing diagnostic bronchoscopy awaits experimental evaluation. In conclusion, Fra-2–expressing macrophages and ColVI are two therapeutically relevant determinants of paracrine fibroblast activation and tissue fibrogenesis. Further work will likely identify additional Fra-2/AP-1–regulated molecules that could be targeted therapeutically and stimulate the development of specific drugs for these largely untreatable human diseases.
Methods
Mouse procedures.
H2kb-fosl2-LTR, Lyz2-Cre, sftpc-CreERT2, col6a1KO, and fosl2Δ alleles are described elsewhere (25, 31, 43, 76). All mouse lines were maintained on a C57BL/6 background and housed in a specific pathogen–free facility accredited by the American Association for Laboratory Animal Care (AALAC), with food and water ad libitum. Male mice were anesthetized by i.p. injection of medetomidine (Domtor) and ketamine (Imalgene), intubated with a 24-gauge catheter (BD), and instilled with a single dose of 1.5 U/kg of bleomycin (MilliporeSigma). Anesthesia was reverted with atipamezole (Antisedan) and mice euthanized at different time points using carbon dioxide. Bleomycin-treated and Fra-2Tg mice were monitored by body weight control and lung mechanics measurement (plethysmography). When indicated, 2.5 × 108 PFU of either Cre-expressing, GFP-expressing, or empty adenovirus were delivered intratracheally after anesthesia. Tamoxifen was injected i.p. (10 mg/d) for 5 consecutive days to trigger Cre recombinase activity in Fra-2ΔalvII* mice. At the experimental end point, blood was collected by cardiac puncture after euthanasia. In all experiments, sex-matched littermates were used as controls. IFNγR–/– C57BL/6 mice were bred in house. Gamma-herpes virus-induced lung fibrosis was performed as previously described (42). In brief, mice were infected at 8 to 12 weeks of age. Prior to infection, mice were sedated with isofluorane and intranasally infected with 5 × 105 PFU in 20 μl of DMEM. For the transplantation experiments, lethally irradiated WT mice (14 Gy) were transplanted i.v. with 2 × 106 BM cells from donor mice.
Lung plethysmography.
Mice were anesthetized by i.p. injection of medetomidine (Domtor) and ketamine (Imalgene, Merial Laboratories), intubated with a 24-gauge catheter (BD), and introduced in the chamber of a plethysmograph (EMKA). A MiniVent (Harvard Apparatus) was connected to the plethysmograph and the tracheal cannula for animal ventilation at 10 ml/kg of tidal volume and 150 breaths per minute. Data were measured by 2 pressure transducers that detect pressure variations in the chamber (flow) and in the tracheal cannula (pressure). This allows for measurements of LR (mmHg/mL × s–1) and DC (mL/mmHg) in addition to other lung function parameters. Lung function measurement was repeated at least 3 times and the data averaged for each mouse. Fibrotic lungs show increased LR and decreased DC (reduced tissue elasticity).
Cell culture.
Primary mouse lung fibroblasts were isolated from adult mice. Lungs were perfused with saline to eliminate blood cells and lung tissue was minced and incubated for 45 minutes in serum-free media with 0.14 Wunsch units/mL Liberase Blendzyme 3 (Roche). After centrifugation, the pellet was resuspended in 20% FBS and 1% penicillin-streptomicin–supplemented DMEM/F12 culture media (Lonza). Cells attaching from the tissue pieces were trypsinized and cultured in monolayer. When confluent, CD45-negative cells were sorted to purify the cell culture from possible hematopoietic cell contamination. All lung fibroblasts were used between passages 3 and 5.
Primary BMDMs were isolated from adult mice by differentiation of BM-derived monocytes with incubation of 3 to 5 days with 50 ng/mL of mouse MCSF-1 (R&D and Prepotech) in bacteria plates. Before confluence, cells were trypsinized, counted, and plated again at a similar number for the experiments. Mouse IL-4 (Prepotech) was added at a concentration of 20 ng/mL, while the AP-1 inhibitor T-5224 was added at 3 μM. LPS was added at 1 μg/mL. For CM experiments, cells were cultured in serum- and phenol red–free media. When collected, supernatants were centrifuged, filtered through a 45 μm filter, and aliquoted before storage. Fibronectin, ColI, and ColVI were used to precoat culture dishes (20 μg/mL). Primary lung fibroblasts were harvested for RNA analysis 24 hours after plating. Fra-2Tg mouse lung RNA-Seq data were deposited in the NCBI’s Gene Expression Omnibus database (GEO GSE103355).
For further information, see Supplemental Methods. See complete unedited blots in the supplemental material.
Statistics.
Unless otherwise specified, data are expressed as mean ± SEM and individual values are plotted. Statistical significance was determined using either paired or unpaired t test (1 or 2 tailed) or Mann-Whitney U test according to sample distribution for comparing 2 groups of samples. One-way ANOVA or two-way ANOVA was performed for grouped or multivariate analysis, as appropriate. For all experiments, P < 0.05 was considered statistically significant.
Study approval.
All animal studies were approved by the CNIO IACUC, by the ethics and animal welfare committee of the the Instituto de Salud Carlos III (Madrid, Spain) and by the Comunidad de Madrid (Madrid, Spain), in accordance with National and European regulations. In the case of the MHV68-induced lung fibrosis mouse model, the protocol was approved by the Emory University Institutional Animal Care and Use Committee and in accordance with established guidelines and policies at Emory University School of Medicine (protocol YER-2002245-031416GN). The protocol was also carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011). The project design to obtain human samples was approved by the ethical committee of the Instituto de Salud Carlos III (Madrid, Spain). In addition, samples and/or data from patients included in this study were provided by the Biobanco i+12 in the Hospital 12 de Octubre integrated in the Spanish Hospital Biobanks Network (RetBioH; www.redbiobancos.es) following standard operation procedures, with appropriate approval of the Ethical and Scientific Commitees, Madrid Spain. Paraffin-embedded tissue sections and OCT-embedded fresh tissue from 11 pulmonary fibrosis patients and 3 controls without any lung pathology were obtained. All patients provided informed consent.
Author contributions
ACU designed and performed experiments and wrote the paper. LB contributed to mouse colony management, experimental design, and manuscript writing. MS contributed to experimental design and provided the data for the bleomycin experiment in WT mice. BR designed and analyzed flow cytometry experiments and contributed to manuscript writing. MJ contributed to experimental design and performed some of the experiments. CFT analyzed the RNA-Seq data. PXE acquired and analyzed LC-MS/MS data. AIH synthesized the T-5224 inhibitor. DM acquired and analyzed confocal microscopy images. P Braghetta and P Bonaldo provided the ColVI-deficient mice and bones for in vitro and in vivo experiments. PM provided data and samples from the MVH68 model. LPA provided access to the human lung samples. EFW directed the study, approved the data, and edited the paper.
Supplementary Material
Acknowledgments
We thank Brigid L.M. Hogan, Irmgard Förster, and Harold L. Moses for the Sftpc-CreERT2, Lys2-Cre, and S100a4-Cre mouse lines and Chiara Cianciaruso and Dario Bizzotto for helping with the ColVI-deficient samples and mice. We are grateful to the members of the Wagner laboratory for valuable suggestions; V. Bermeo and F. Montes for technical help; S. Leceta, G. Medrano, and P. García for assisting with mouse experiments, and the staff at the Histopathology Unit at CNIO for optimizing triple human IHC. We are thankful to the Biobanco i+12 at the Hospital 12 de Octubre, integrated in the Spanish Hospital Biobanks Network (RetBioH) and supported by the Instituto de Salud Carlos III, to Ana Belén Enguita for human samples selection, and above all, to the patients for informed consent. The EFW laboratory is supported by grants from the Spanish Ministry of Economy (BFU2012-40230 and SAF2015-70857), cofunded by the ERDF-EU, the Daiichi Sankyo Company, and a Jesus Serra Visiting Scientist Grant to Wolfgang Weninger/BR.
Version 1. 05/28/2019
In-Press Preview
Version 2. 07/15/2019
Electronic publication
Version 3. 08/01/2019
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(8):3293–3309.https://doi.org/10.1172/JCI125366.
Contributor Information
Alvaro C. Ucero, Email: aucero@cnio.es.
Latifa Bakiri, Email: lbakiri@cnio.es.
Ben Roediger, Email: b.roediger@centenary.org.au.
Masakatsu Suzuki, Email: suzuki.masakatsu.mr@daiichisankyo.co.jp.
Maria Jimenez, Email: mjimenez@cnio.es.
Pratyusha Mandal, Email: pratyusha.mandal@emory.edu.
Paola Braghetta, Email: paola.braghetta@unipd.it.
Paolo Bonaldo, Email: paolo.bonaldo@unipd.it.
Luis Paz-Ares, Email: lpazaresr@seom.org.
Coral Fustero-Torre, Email: cfustero@cnio.es.
Pilar Ximenez-Embun, Email: mpximenez@cnio.es.
Ana Isabel Hernandez, Email: aihernandez@cnio.es.
Diego Megias, Email: dmegias@cnio.es.
Erwin F. Wagner, Email: ewagner@cnio.es.
References
- 1.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol, Cell Physiol. 2013;304(3):C216–C225. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richeldi L. Idiopathic pulmonary fibrosis: moving forward. BMC Med. 2015;13:231. doi: 10.1186/s12916-015-0481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gieseck RL, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 5.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 7.Noble PW, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 8.Luppi F, Cerri S, Beghè B, Fabbri LM, Richeldi L. Corticosteroid and immunomodulatory agents in idiopathic pulmonary fibrosis. Respir Med. 2004;98(11):1035–1044. doi: 10.1016/j.rmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Wuyts WA, et al. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J. 2013;41(5):1207–1218. doi: 10.1183/09031936.00073012. [DOI] [PubMed] [Google Scholar]
- 10.Balestro E, et al. Immune inflammation and disease progression in idiopathic pulmonary fibrosis. PLoS ONE. 2016;11(5):e0154516. doi: 10.1371/journal.pone.0154516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons MA, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184(5):569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 12.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30(3):245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Linthout S, Miteva K, Tschöpe C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102(2):258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 15.Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55(4–5):495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 16.Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi K, Martinet Y, Crystal RG. Modulation of fibronectin gene expression in human mononuclear phagocytes. J Clin Invest. 1987;80(6):1720–1727. doi: 10.1172/JCI113263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ, Carter AB. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity. 2016;44(3):582–596. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Kaviratne M, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173(6):4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 22.Doucet C, Brouty-Boyé D, Pottin-Clémenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest. 1998;101(10):2129–2139. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zenz R, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437(7057):369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 24.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 25.Eferl R, et al. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc Natl Acad Sci U S A. 2008;105(30):10525–10530. doi: 10.1073/pnas.0801414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sisson TH, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(3):254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkauskas CE, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsujino K, Reed NI, Atakilit A, Ren X, Sheppard D. Transforming growth factor-β plays divergent roles in modulating vascular remodeling, inflammation, and pulmonary fibrosis in a murine model of scleroderma. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L22–L31. doi: 10.1152/ajplung.00428.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng R, et al. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for “active” disease. PLoS ONE. 2013;8(4):e59348. doi: 10.1371/journal.pone.0059348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eferl R, Zenz R, Theussl HC, Wagner EF. Simultaneous generation of fra-2 conditional and fra-2 knock-out mice. Genesis. 2007;45(7):447–451. doi: 10.1002/dvg.20311. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland KD, Song JY, Kwon MC, Proost N, Zevenhoven J, Berns A. Multiple cells-of-origin of mutant K-Ras-induced mouse lung adenocarcinoma. Proc Natl Acad Sci U S A. 2014;111(13):4952–4957. doi: 10.1073/pnas.1319963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock JR, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 35.Satoh T, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541(7635):96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 36.Misharin AV, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214(8):2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchida K, et al. Design, synthesis, and biological evaluation of new cyclic disulfide decapeptides that inhibit the binding of AP-1 to DNA. J Med Chem. 2004;47(17):4239–4246. doi: 10.1021/jm049890+. [DOI] [PubMed] [Google Scholar]
- 38.Aikawa Y, et al. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat Biotechnol. 2008;26(7):817–823. doi: 10.1038/nbt1412. [DOI] [PubMed] [Google Scholar]
- 39.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knüppel L, et al. FK506-binding protein 10 (FKBP10) regulates lung fibroblast migration via collagen VI synthesis. Respir Res. 2018;19(1):67. doi: 10.1186/s12931-018-0768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnoor M, et al. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol. 2008;180(8):5707–5719. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- 42.O’Flaherty BM, et al. CD8+ T cell response to gammaherpesvirus infection mediates inflammation and fibrosis in interferon gamma receptor-deficient mice. PLoS ONE. 2015;10(8):e0135719. doi: 10.1371/journal.pone.0135719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet. 1998;7(13):2135–2140. doi: 10.1093/hmg/7.13.2135. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, et al. Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat Commun. 2016;7:12564. doi: 10.1038/ncomms12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardo A, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2(9):e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J, et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann Rheum Dis. 2017;76(11):1941–1948. doi: 10.1136/annrheumdis-2016-210823. [DOI] [PubMed] [Google Scholar]
- 47.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–282. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 48.Vannella KM, et al. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci Transl Med. 2016;8(337):337ra65. doi: 10.1126/scitranslmed.aaf1938. [DOI] [PubMed] [Google Scholar]
- 49.Wilson MS, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakubzick C, et al. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4– and IL-13-responsive cells. J Immunol. 2003;171(5):2684–2693. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- 51.Gharaee-Kermani M, Nozaki Y, Hatano K, Phan SH. Lung interleukin-4 gene expression in a murine model of bleomycin-induced pulmonary fibrosis. Cytokine. 2001;15(3):138–147. doi: 10.1006/cyto.2001.0903. [DOI] [PubMed] [Google Scholar]
- 52.Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003;170(4):2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- 53.Singh B, Kasam RK, Sontake V, Wynn TA, Madala SK. Repetitive intradermal bleomycin injections evoke T-helper cell 2 cytokine-driven pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2017;313(5):L796–L806. doi: 10.1152/ajplung.00184.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 55.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol. 2001;107(6):1001–1008. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- 57.Gieseck RL, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 58.Sheridan C. Drugmakers cling to dual IL-13/IL-4 blockbuster hopes. Nat Biotechnol. 2018;36(1):3–5. doi: 10.1038/nbt0118-3. [DOI] [PubMed] [Google Scholar]
- 59.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832(7):989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagaoka I, Trapnell BC, Crystal RG. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest. 1990;85(6):2023–2027. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7(2):193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Z, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22(2):154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sinha M, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun. 2018;9(1):936. doi: 10.1038/s41467-018-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maurer B, et al. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann Rheum Dis. 2012;71(8):1382–1387. doi: 10.1136/annrheumdis-2011-200940. [DOI] [PubMed] [Google Scholar]
- 66.Ji WJ, et al. Temporal and spatial characterization of mononuclear phagocytes in circulating, lung alveolar and interstitial compartments in a mouse model of bleomycin-induced pulmonary injury. J Immunol Methods. 2014;403(1–2):7–16. doi: 10.1016/j.jim.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 67.Chakarov S, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363(6432):eaau0964. doi: 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- 68.Lucas T, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 69.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol Dis. 2015;74:114–125. doi: 10.1016/j.nbd.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gwinn WM, Kapita MC, Wang PM, Cesta MF, Martin WJ. Synthetic liposomes are protective from bleomycin-induced lung toxicity. Am J Physiol Lung Cell Mol Physiol. 2011;301(2):L207–L217. doi: 10.1152/ajplung.00149.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borthwick LA, et al. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 2016;9(1):38–55. doi: 10.1038/mi.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wernig G, et al. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci U S A. 2017;114(18):4757–4762. doi: 10.1073/pnas.1621375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Velden JL, et al. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin Transl Med. 2016;5(1):36. doi: 10.1186/s40169-016-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Specks U, Nerlich A, Colby TV, Wiest I, Timpl R. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med. 1995;151(6):1956–1964. doi: 10.1164/ajrccm.151.6.7767545. [DOI] [PubMed] [Google Scholar]
- 76.Xu X, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109(13):4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.