The proper functioning of the physiological processes in living beings is regulated by numerous naturally occurring daily rhythms that have evolved to anticipate and prepare for environmental changes due to the solar day and year, allowing organisms to predict when environmental challenges are most likely to occur [1].
The recurrence of biological processes cycling at different frequency ranges enables healthy organisms to maintain homeostasis, that is the body’s ability to regulate stability in its inner environment in response to changing conditions in the outside environment. Rhythmic processes hallmarked by the same frequency may have the same phase or different phases, and usually show well-defined time-relationships to each other: functional disturbances and alteration of anatomic integrity may be caused by loss of this multi-frequency time structure leading to internal desynchronization [2].
Previous results show a transient change in timing and incidence of acute myocardial infarction after daylight saving time (increase in the spring and a decrease after returning to standard time in the fall) [3–5] In their study, Mafredini et al. [6] advocate an association between daylight saving time and a small increase of acute myocardial infarction occurrence, in particular after the spring shift and in male subjects, probably in relationship to sleep deprivation and circadian misalignment impacting cardiovascular health through increases in sympathetic tone and catecholamine levels.
Tissue functions and cellular processes in living beings show 24-h rhythms regulated by the biological clocks, present in every tissue and cell, and ticking through a molecular clockwork operated by a transcriptional/translational feedback loop hard-wired by core circadian genes and proteins (ARNTL–1/2, CLOCK/NPAS2, CRY1–2, PER1–3, NR1D1–2, RORA) driving the circadian oscillation of thousands of clock-controlled genes [2]. Circadian rhythmicity of the body is driven by environmental lighting conditions via a neural pathway including the retina and a specific retinohypothalamic tract projecting to the biological clocks of the suprachiasmatic nuclei, which in turn drive adrenal and pineal gland secretion of cortisol in the early morning and melatonin at nighttime, respectively [2]. The pineal gland modulates hypothalamus–pituitary–adrenal axis function, melatonin modulates the transcriptional activity of glucocorticoid receptors, and in turn adrenal corticosteroids modulate pineal melatonin synthesis [7]. Additionally, circadian fluctuations are the hallmark of other patterns of nocturnal neuro-endocrine hormone secretion, in particular of thyroid-stimulating hormone, as well as the total number of lymphocytes and percentages of different lymphocyte sub-populations in the peripheral blood [8].
We retrospectively took advantage of data collected for monitoring neuroendocrine hormones and the immune system of 11 healthy male subjects (ages 35–53 years) over a 24-h interval before and after daylight savings time. Six of the participants were sampled the first week of October (during daylight savings) and five were sampled the first week of November, directly after daylight savings. Our data display diurnal variation in lymphocyte abundance and hormone levels. All individuals were entrained to 07:00 (local time) lights-on and waking, and a 23:00 lights-off and sleep opportunity, for 1 week. Participants were then sampled for 24 h (in dim light during the night).
To combine data across participants, we standardize the data in two ways. First, we correct for the 1-h time change by representing the data in GMT as opposed to local time. Second, the data, Yt, i, j, from each individual (i) are standardized for each lymphocyte or hormone (j), at time (t), by dividing by the mean for that individual. Breakpoint regression is used to test for statistically significant diurnal variation. We observed significant variation in CD16+ (natural killer cells) and CD4+ (helper-inducer T lymphocytes) abundance, along with the concentration of melatonin, cortisol and thyroid stimulating hormone (Fig. 1). Variation is also observed in other parameters measured, such as CD8+dim (cytotoxic T lymphocytes) abundance, but the results are sensitive to edge-effects; thus, data from two or more consecutive days should be used to characterize circadian rhythms.
Fig. 1.
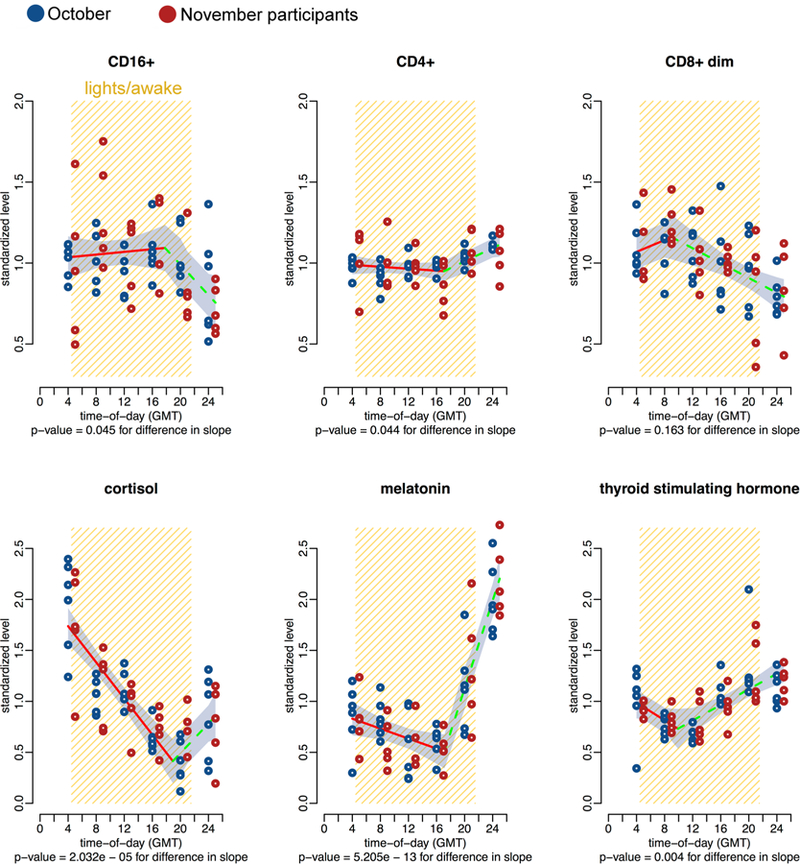
x–y Plots showing 24-h time-qualified profiles of 4 h serum levels for cortisol, melatonin, thyroid stimulating hormone, and lymphocyte subpopulations from 11 healthy men (mean age 43 ± 5 years). Values of y expressed as normalized percent of mean ± SE. Original levels assigned to sampling time
As for daylight savings, the effect of changing the clocks back in fall is twofold in our study population. First, by setting the clock back at the end of October, it perturbs the natural autumnal progression of the delay of sunrise, relative to wake-up at 07:00. Without daylight savings, as autumn progresses, the sun should come up later after waking. Setting the clock back, however, results in sunrise-after-waking in October and sunrise-prior-to-waking in November. Second, setting the clock back extends the time span between sunset and bedtime at 23:00. As autumn progresses, sunset will occur earlier as the photoperiod shortens; however, setting the clock back exacerbates this phenomenon, and has the potential to increase light-at-night exposure.
Overall, the practice of setting the clocks back in the fall, to make better use of natural daylight, might impact circadian rhythmicity in the neuro-endocrine-immune system, possibly also the body’s physiology, and could interact with pathophysiological mechanisms involved in cardiovascular acute events. As well, the distorted functioning of the biological clock is importantly engaged in the pathogenesis of non-alcoholic fatty liver disease, which is associated with augmented cardiovascular risk [9, 10].
Both fall and spring daylight savings shifts have the potential to cause chronodisruption by way of subtle changes of the complex system sustaining internal timing coordination, and may be involved in the seasonality of timing and incidence of cardiovascular disease-related acute events.
Acknowledgements
The work in GM’s laboratory was supported by the “5 × 1000” voluntary contribution, and by a Grant from the Italian Ministry of Health through Department of Medical Sciences, Division of Internal Medicine and Laboratory of Chronobiology (RC1803ME40), Fondazione IRCCS “Casa Sollievo della Sofferenza”, Opera di Padre Pio da Pietrelcina, San Giovanni Rotondo (FG), Italy.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Research involving in human and animal rights This article does not contain any studies with animals performed by any of the authors. Subjects in the study participated willingly.
Informed consent Human participants gave written informed consent.
References
- 1.Aschoff J (ed) (1981) Biological rhythms. Handbook of behavioural neurobiology, vol 4 Plenum Publishing Corporation, New York, p 4 [Google Scholar]
- 2.Vinciguerra M, Tevy MF, Mazzoccoli G (2014) A ticking clock links metabolic pathways and organ systems function in health and disease. Clin Exp Med 14(2):133–140 [DOI] [PubMed] [Google Scholar]
- 3.Janszky I, Ljung R (2008) Shifts to and from daylight savings time and incidence of myocardial infarction. N Engl J Med 359:1966–1968 [DOI] [PubMed] [Google Scholar]
- 4.Jiddou MR, Pica M, Boura J, Qu L, Franklin BA (2013) Incidence of myocardial infarction with shifts to and from daylight savings time. Am J Cardiol 111:631–635 [DOI] [PubMed] [Google Scholar]
- 5.Sipilä JO, Rautava P, Kytö V (2016) Association of daylight saving time transitions with incidence and in-hospital mortality of myocardial infarction in Finland. Ann Med 48(1–2):10–16 [DOI] [PubMed] [Google Scholar]
- 6.Manfredini R, Fabbian F, Cappadona R, Modesti PA (2018) Daylight saving time, circadian rhythms, and cardiovascular health. Intern Emerg Med 13(5):641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzoccoli G (2011) The timing clockwork of life. J Biol Regul Homeost Agents 25(1):137–143 [PubMed] [Google Scholar]
- 8.Mazzoccoli G, De Cata A, Greco A, Carughi S, Giuliani F, Tarquini R (2010) Circadian rhythmicity of lymphocyte subpopulations and relationship with neuro-endocrine system. J Biol Regul Homeost Agents 24(3):341–350 [PubMed] [Google Scholar]
- 9.Tarquini R, Clock Mazzoccoli G, Genes Metabolism, Risk Cardiovascular (2017) Clock genes, metabolism, and cardiovascular risk. Heart Fail Clin 13(4):645–655 [DOI] [PubMed] [Google Scholar]
- 10.Mazzoccoli G, De Cosmo S, Mazza T (2018) The biological clock: a pivotal hub in non-alcoholic fatty liver disease pathogenesis. Front Physiol 15(9):193. [DOI] [PMC free article] [PubMed] [Google Scholar]


