Abstract
Some vertebrates are able to regenerate the lens following its removal. This includes species in the genus Xenopus (i.e., X. laevis, X. tropicalis and X. borealis); the only anurans known to undergo lens regeneration. In Xenopus the regenerated lens is derived de novo from cells located within the basal-most layer of the larval cornea epithelium, and is triggered by factors provided by the neural retina. In larval frogs the cornea epithelium is underlain by an endothelium, which is separated from the cornea epithelium, except for a small central attachment (“stromal attracting center”). This connection grows larger as the stroma forms and the frogs approach metamorphosis. The capacity for regeneration is high in larval X. laevis and weaker in some other species due to the more rapid healing of the cornea endothelium, which prematurely cuts off the inducing factors (Freeman, 1963; Henry and Elkins, 2001; Filoni et al., 2006). Here we provide instructions for performing lentectomies (removal of the original lens) to study lens regeneration (Figure 1A–Q). The procedure has been described by Freeman (1963), Henry and Mittleman (1995) and Beck (2012).
MATERIALS
Lentectomies must be carried out using a dissecting microscope, ideally located in a clean hood. Sterile disposable plasticware is used and all work surfaces, tools and operating dishes are sanitized with 70% ethanol prior to use.
REAGENTS
1/20X NAM (“Normal Amphibian Media,” Slack, 1984, see recipes)
Anesthetic solution (1:2000 dilution of MS222 or tricaine methanesulfonate in 1/20X NAM. MS222, cat. no. A5040, Sigma-Aldrich, St Louis, MO)
Frog Culture water (De-chlorinated tap water or salt balanced RO water, autoclaved)
Penicillin (100 U/ml in the Modified L-15 Media, cat. no. P3032, Sigma-Aldrich, St Louis, MO)
Streptomycin (100 μg/ml in the Modified L-15 Media, cat. no. S1277, Sigma-Aldrich, St Louis, MO)
EQUIPMENT
Clay-lined 60mm plastic operating dish (Plastalina clay, dark green, cat no. 10119, Van Aken International, North Charleston, SC)
Spread the clay evenly using one’s finger tips. The clay layer should be about 7–8mm thick. Following use, rinse the dish with water followed by 70% ethanol.
Forceps
Standard Inox number 5 Dumont forceps (cat no. 07379, Polysciences, Inc., Warrington, PA) must be sharpened (EZE-LAP Pocket Diamond Fish Hook Sharpener, Model S, Carson City, NV). To sharpen, use a small rubber band to hold the tips together in alignment. The cutting action of diamonds is very aggressive, so proceed carefully to avoid shortening the forceps and making them too blunt (see before and after images in the inset of Figure 1O). The tips must be sharp and match perfectly to grasp the lens capsule
Figure 1.
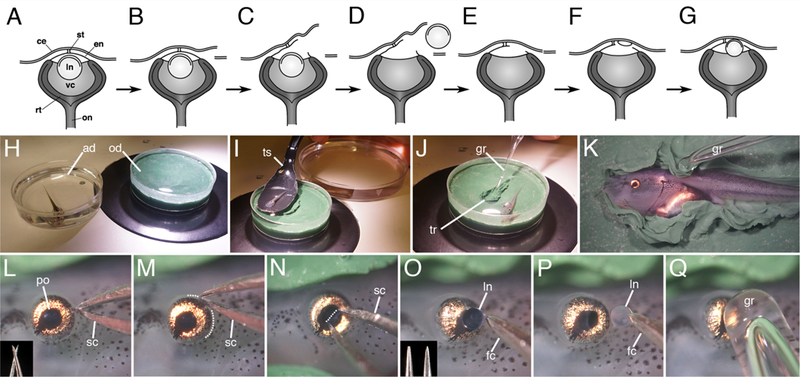
Lentectomy in Xenopus laevis larvae. (A-G) Simple lentectomy diagram. H) Tadpole is anesthetized in a dish containing MS222 solution, transferred to the operating dish (I) following sedation, and immobilized on its side in a clay trough (J). Flaps of clay are used to secure the tadpole (K), and lentectomy begins with an incision on the posterior side of the eye (L-M; microscissors are shown in the inset, dotted line outlines the incision). N) An incision is made across the pupillary opening in the cornea endothelium and the intact lens capsule containing the lens cells is removed (O-P). Inset in O shows forceps prior to (left) and after sharpening (right). (Q). The cornea epithelium is smoothed over the eye with a glass rod tool. ad, anesthetic dish; ce, cornea epithelium; e, eye; en, cornea endothelium; fc, forceps; gr, glass rod; ln, lens; od, operating dish; on, optic nerve; po, pupillary opening; rt, retina; sc, scissors; st, connecting stalk; ts, transfer spoon; tr, trough; vc, vitreous chamber.
Glass rod tool
Use a gas burner to melt the tip of a short glass Pasteur pipette. Heat only the tip and rotate the glass for even heating. The open end should seal completely and a small rounded ball will form at the tip, approximately 1–2mm in diameter.
Microscissors
Lumsden BioScissors (Oban BioScissors, Oban Precision Instruments, Oban, Argyll, Scotland) are ideal for these surgeries. Similar scissors can be made from number 5 watchmaker’s forceps. These microscissors have a pincher type action that offers a tremendous advantage over traditional scissors. As such, they grip the cornea tissue just before the cut is made. Standard scissors tend to push tissues out and away from the cutting edges, making these operations more difficult.
Transfer scoop
A small stainless steel or plastic spoon used to handle the animals.
METHOD
Lentectomy operation
Anesthetize the animal in a small petri dish containing MS222/antibiotic solution for 2–3 minutes, until it is unresponsive to touch and has lost the righting reflex (Figure 1H). To limit the animal’s time of exposure, no more then two animals should be anesthetized together in this dish. Typically it takes only 1–3 minutes to complete each surgery. Ideally anesthesia should be limited to no longer than 10 minutes (see Hamilton and Henry, 2014). Stage 48–52 or even younger larvae are typically used for these experiments, as both the rate of regeneration (growth of the new lens) and the number of animals that can undergo regeneration gradually declines as frogs approach metamorphosis (Freeman, 1963). Freeman reports that animals can no longer regenerate a lens after metamorphosis (stage 66).
Transfer the anesthetized animal to the clay-lined operating dish using the transfer scoop (Figure 1I), Animals should remain covered with anesthetic solution.
Using the glass rod tool, form a small trough in the clay the same size and depth as the tadpole (Figure 1J). This recess can be used for subsequent operations. Raise up a few folds of clay along the sides of the trough to use as flaps to restrain the animal.
Secure the animal in the trough on its side with one eye upwards for surgery (Figure 1K). Use the glass ball tool to move the animal in place and gently push the flaps of clay down along the sides of the abdomen to secure the animal. Avoid compression in the heart region, which will continue to beat under anesthesia.
Use microscissors to cut along one edge of the cornea epithelium (Figures 1C,L). Make a series of continuous cuts around 1/3 of the circumference of the eye along the posterior edge (Figure 1M). If the cut is too big the cornea may flap open and the wound may not heal properly.
Reach under the cut epithelium with microscissors to pierce the deeper cornea endothelium (Figures 1C,N). This is the more difficult step, as the eyeball tends to move. Make a straight cut through the cornea endothelium across the center of the pupillary opening. Approach the eye from straight above, at a high angle, with the tips of the scissors initially straddling the pupillary opening, to steady the eyeball. If you approach the eye from a lower angle the scissors will not cut the tissue, and will tend to slide off the slippery surface. Some force is required, but not enough to damage the deeper lens tissues. Care must be taken not to cut the iris or retinal tissue at the edge of the pupillary opening, as there are large blood vessels located there.
Use sharpened forceps to grab the lens capsule and remove it (Figures 1D,O–P). The intact lens is completely spherical and transparent. Only the exposed, outer surface (i.e., lens capsule) should be grasped. Sharp forceps are essential to grasp the lens capsule. The lens should be removed intact and undamaged. An undamaged lens will appear uniformly spherical and completely transparent and held by only the very tips of the forceps. If the lens is irregular in shape or cloudy, it has been damaged, and it is more likely that you will leave some lens cells inside the vitreous chamber. Wipe the forceps clean before and after each use to remove any lens debris from previous surgeries. Any remaining lens epithelial tissues can proliferate and reform a lens (an alternative form of “lens regeneration”). If the openings made within the inner and outer corneas are too small it will be difficult to remove the lens. It can be difficult to determine if residual lens cells remain inside the eye. Therefore, one should assume that if the lens has not been removed intact, as described above, lens cells are likely to be present, and those animals should be discarded.
Smooth the cornea back into place with the glass rod tool to help close the wound (Figures 1E,Q).
Release the clay flaps with the glass rod tool and remove the animal from the recess.
-
Transfer the animal through three washes in 1/20× NAM with antibiotics over the course of 10 minutes to remove the anesthetic before transferring it to a larger recovery bowl (containing 1/20× NAM). Gentle agitation on a rocker will hasten recovery from the anesthetic (Hamilton and Henry, 2014). The cornea heals rapidly within 12–24 hours. Antibiotics should continue to be used through this time period. After 24 hours, frog culture water may be used instead of 1/20× NAM. Antibiotics are not required once the cornea has healed. Lens regeneration will occur whether or not antibiotics are used.
At the conclusion of the experiments, or in cases where the lens cannot be completely removed, the animals should be euthanized following accepted standards. An overdose of MS222 (2–3gm/liter) is suitable for larvae, and benzocaine HCl (250mg/liter) is suitable for adults.
DISCUSSION
The stages of lens regeneration have been thoroughly described by Freeman (1963, see also Henry 2003; Henry et al., 2008; Henry and Tsonis, 2010). Distinct cellular changes occur within the first 24 hours following lens removal (cells of the basal cornea epithelium become cuboidal and the nuclei in a significant percentage of these cells will possess only one nucleolus, rather than two). A small lentoid (placode) that expresses lens crystallin proteins generally appears within 5 days, which can be recognized in histological sections (Figure 1F). At that time the new lens cells begin to express lens crystallins, which can be detected by antibodies (Henry and Mittleman, 1995). These lentoids will continue to grow larger over time to form spherical lenses within one to two weeks. These lenses will become visible through the pupillary opening in live animals (Figure 1G). One can also visualize lens regeneration using live, transgenic animals that express GFP driven by the γ-crystallin enhancer/promoter (Henry and Elkins, 2001). The regenerated lens will eventually grow to reach the original size, which may take a few weeks to a month.
RECIPES
1/20X NAM, (“Normal Amphibian Media,” Slack, 1984, 1L)
5ml 10X NAM salts solution (see below)
20ml 0.1 M NaPO4 pH 7.5 (see below)
0.5ml 0.1M NaHCO3 (from frozen aliquots, see below)
Bring up to 1L with autoclaved dH20
(note: make up 1/20 NAM with the stock solutions listed below using sterile technique, and do not re-sterilize this working solution).
10X NAM salts solution, (“10X Normal Amphibian Media salts,” Slack, 1984, 1L)
65.0gm NaCl (110 mM)
1.5gm KCl (2mM)
2.4gm Ca(NO3)2•4H2O (1mM)
2.5gm MgSO4•7H20 (1mM)
Na2EDTA•2H2O (1mM)
Bring up to 1L with dH20, autoclave
0.1M NaPO4, pH 7.5 (1L)
22.5gm Na2HPO4•7H20 (84mM)
2.2gm NaH2PO4•H20 (16mM)
Bring up to 1L with dH20, adjust pH to 7.5, autoclave
0.1M NaHCO3 (50ml)
0.42gm 0.1M NaHCO3
Bring up to 50mL with dH20
Filter sterilize with 0.2µm SFCA membrane filter unit
Freeze as 0.5ml aliquots
ACKNOWLEDGEMENTS
This research is funded by NIH/NEI grant EYO23979 to JJH.
REFERENCES
- Beck CW (2012). Studying regeneration in Xenopus. In “Xenopus Protocols,” (Stefan Hoppler and Peter Vise, ed.). Methods in Molecular Biology 917: 525–539. [DOI] [PubMed] [Google Scholar]
- Freeman G (1963). Lens regeneration from the cornea in Xenopus laevis. J. Exp. Zool 154: 39–66. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, and Cannata SM. (2006). Experimental analysis of lens-forming capacity in Xenopus borealis larvae. J Exp Zoolog A Comp Exp Biol 305:538–550. [DOI] [PubMed] [Google Scholar]
- Filoni S Bernardini S Cannata SM and D’Alessio A (1997). Lens regeneration in larval Xenopus laevis: experimental analysis of the decline in the regenerative capacity during development. Dev. Biol, 187: 13–24. [DOI] [PubMed] [Google Scholar]
- Hamilton P and Henry JJ (2014). Prolonged in vivo imaging of Xenopus laevis. Dev. Dynamics 243: 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ (2003). Cell and Molecular Biology of Lens Regeneration. Int. Review of Cytology: A Survey of Cell Biology 228: 195–264. [DOI] [PubMed] [Google Scholar]
- Henry JJ, and Elkins ME (2001). Cornea-lens transdifferentiation in Xenopus tropicalis. Development Genes & Evolution 211: 377–387. [DOI] [PubMed] [Google Scholar]
- Henry JJ and Mittleman J (1995). The matured eye of Xenopus laevis tadpoles produces factors that elicit a lens-forming response in embryonic ectoderm. Developmental Biology 171: 39–50. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Wever JA, Veragara MN and Fukui L (2008). Ch. 6. Xenopus, An Ideal Vertebrate System for Studies of Eye Development and Regeneration. In: “Animal Models for Eye Research” (Tsonis PA, ed). Academic Press. [Google Scholar]
- Henry JJ and Tsonis PA (2010). Molecular and Cellular Aspects of Amphibian Lens Regeneration. Progress in Retinal and Eye Research 29: 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JMW (1984). Regional biosynthetic markers in the early amphibian embryo. J Embryol Exp Morphol 80, 289–319. [PubMed] [Google Scholar]


