Abstract
Purpose
Recent advances in head and neck cancer (HNC) treatment, such as increased use of organ-preserving advanced radiation treatments, the approval of cetuximab for HNC treatment, and the increase in human papillomavirus (HPV)-related HNC, have changed clinical approaches to HNC management. We sought to identify treatment trends in a population-based cohort of HNC patients.
Methods
The Surveillance, Epidemiology, and End Results Patterns of Care program collected additional treatment and HPV testing information on stratified random samples of HNC patients diagnosed in 1997 (n=473), 2004 (n=1,317), and 2009 (n=1,128). Rao-Scott chi-square tests were used to examine unadjusted associations between year of diagnosis and patient sociodemographic, tumor, and treatment characteristics. Cochran-Armitage tests for trend were used to examine the hypothesis that certain treatments were used increasingly (or decreasingly) over the time period, while logistic regression was used to examine factors associated with particular treatments.
Results
Use of radiation and chemotherapy without surgery significantly increased for all HNC sites between 1997 and 2009. Cetuximab and taxane use also showed a significantly increasing trend. Lack of insurance was associated with not receiving treatment in multivariate models. The majority (64%) of cases undergoing radiation in 2009 received an advanced treatment, with 55% receiving intensity modulated. The majority of oropharyngeal cases with known HPV status received chemotherapy and radiation only (62%) and nearly all were insured and had one or fewer co-morbidities.
Conclusions
Treatment patterns have changed for HNC, leading to increased incorporation of systemic therapy and newer radiation techniques. HPV testing should be targeted for more widespread use, especially in traditionally underserved groups.
Keywords: Head and Neck Cancer, Radiation, Chemotherapy, Cetuximab, Human Papillomavirus
Introduction
Treatment of head and neck cancer (HNC) has been evolving in recent decades under several concurrent influences. Treatment selection is based on the location and stage of the tumor. Early stage tumors are usually treated with surgery or radiation therapy (RT), while advanced tumors are usually treated with multiple modalities, often including systemic agents [1].
There has been increasing use of organ preservation therapy based on studies of tumor control, functional outcomes, and survival from RT and chemotherapy with RT [2–6]. However, RT can also result in conditions such as pharyngeal strictures, dysphagia and xerostomia [7–10]. Intensity-modulated RT (IMRT), an advanced form of external beam RT where beams are manipulated to better conform to the shape of the tumor and thus spare more normal tissue, was introduced in the early 2000s [11, 12]. Studies have shown that the use of IMRT in HNC management has increased between 2000 and 2005 [13, 14] and is associated with improved survival [15]. Hyperfractionated RT (HRT) entails delivery of radiation in a twice-daily schedule rather than once daily, and has been reported to lower toxicity and improve the effectiveness of RT over standard fractionation and other altered fractionation methods [16, 17]. HRT and IMRT are also being increasingly used together in HNC RT regimens. Small scale studies thus far have found HRT-IMRT to be well tolerated with marginally improved locoregional control in patients with locally advanced HNC [18–20]. Given the recent incorporation of HRT and IMRT into HNC treatment, more research is needed to determine factors affecting treatment selection, prognosis and survival associated with these forms of therapy.
Chemotherapy and other systemic therapies have also been increasingly incorporated in curative HNC therapy, with guidelines now recommending patients with locally advanced disease be offered chemotherapy and radiation either as first line treatment or following surgery, in order to preserve organ function and improve locoregional control [21, 22]. Induction chemotherapy with cisplatin/5-fluorouracil (5-FU) has been in use since the 1980s, with taxanes being added to these regimens with United States (US) Food and Drug Administration (FDA) approval in the mid-1990s after three phase III trials showed superior outcomes for those receiving a combination of cisplatin, 5-FU, and docetaxel or paclitaxel [23]. More recently, the epidermal growth factor receptor inhibitor cetuximab was FDA approved in 2006 as an initial treatment in combination with RT for locally or regionally advanced squamous cell HNC after being shown to improve locoregional control and overall survival with no increase in side effects beyond RT treatment alone [24, 25]. Furthermore, in a 2008 trial, cetuximab was shown to improve overall survival for patients with recurrent or metastatic squamous cell HNC when added to standard platinum/5-FU chemotherapy [26]. Dansky et al. (2012) predicted that the use of cetuximab and taxanes would increase due to research that has shown the benefits of these agents in the management of advanced stage HNC [27].
Another major shift in the landscape of HNC treatment is the recent increase in human papillomavirus (HPV) associated cancers [28–30]. HPV-positive oropharyngeal cancers are associated with improved survival compared to HPV-negative cancers [31, 32]. Recently updated National Comprehensive Cancer Network (NCCN) guidelines now require HPV testing for oropharyngeal cancers [23]. Saraiya et al. (2015) showed that approximately 70% of oropharyngeal cancers were HPV positive using cancer registry samples collected between 1993 and 2005 [33]. However, Surveillance, Epidemiology, and End Results (SEER) Patterns of Care (POC) data showed that few (14%) oropharyngeal cancer cases diagnosed in 2009 had known HPV status [34]. An audit of oropharyngeal squamous cell carcinoma patients from the Iowa Cancer Registry diagnosed 2010-2014 showed the rate of testing increased from 45% in 2010 to 55% in 2014 [35]. Results of a recent survey of otolaryngologists who were members of three professional associations in North America showed that 67% of respondents systematically test HNC patients for HPV or p16, and suggested that hospital characteristics such as presence of residency training programs were associated with receipt of HPV testing [36]. Previous studies have also shown that treatment selection is associated with hospital teaching status [37].
For patients with locally advanced disease, concurrent cisplatin and radiation is generally guideline recommended, with a body of evidence describing the use of taxanes and cetuximab in the primary setting as well as in recurrent, metastatic, and unresectable HNC. Accordingly, depending on site and stage, platinum-based therapy in combination with taxanes and/or cetuximab are also guideline-recommended clinical options. Radiation techniques such as IMRT or 3-D conformal are guideline preferred to minimize damage to nearby structures for most cancers,. While HRT is a recommended option in cases of high risk disease and/or where radiation is the definitive treatment, its use in combination with IMRT is also evolving, with modified dose plans incorporating HRT, such as the Concomitant Boost Accelerated schedule where for six weeks subclinical targets are delivered a once daily dose and then over the last twelve days of treatment a second daily fraction. HPV testing by p16 immunohistochemistry is guideline recommended in the work-up for oropharyngeal cases [[22]].
HNC treatment is further affected by patient and area-level sociodemographic factors. A study of Medicaid claims from 2002-2006 linked to cancer registry data in California and Georgia showed Medicaid patients (who generally qualify as low income) were less likely to receive a diagnosis at an early stage, while black patients were less likely to receive surgical treatment and have greater mortality [38]. Another study of newly diagnosed HNC patients from three hospitals in Michigan recruited between January 2003 and November 2008 and followed through April 1, 2009 found that black patients were less likely to receive chemotherapy, while having lower income and educational attainment was associated with poorer survival [39]. In addition, a 2017 study found HNC patients residing in the most socioeconomically deprived areas also had to travel the longest to reach a cancer treatment center, adding to the cost and overall burden of patients already experiencing baseline disadvantage [40]. Nevertheless, there is still a paucity of knowledge on current HNC patterns of treatment selection and care in the wider US HNC patient population, who are treated in both academic and community medical centers and vary in a wide array of sociodemographic factors.
Therefore, the purpose of this study is to analyze the patterns of care for HNC patients diagnosed in 1997, 2004, and 2009, with a particular focus on receiving advanced radiation therapy, taxanes, platinum-based regimens, and cetuximab among all HNC cases and HPV-testing among oropharyngeal cases. Treatment trends for surgery, radiation, and chemotherapy (alone and in combination) are examined along with patient sociodemographic and tumor characteristics associated with receiving any treatment, surgical vs. non-surgical treatment, particular forms of radiation, cetuximab, and HPV testing.
Methods
The SEER POC program collected additional information from 473, 1,317, and 1,128 HNC patients in 1997, 2004, and 2009, respectively (2,918 total), all of whom were included in this analysis. The National Cancer Institute’s Division of Cancer Control and Population Sciences and the Division of Cancer Treatment and Diagnosis decide annually which cancer sites to conduct POC studies on based on a need to determine dissemination of state of the art treatment across the US, especially in community practices [41]. With the beginning of the HNC POC studies in 1997, there was growing interest in the use of non-surgical organ preservation therapy and the increasing use of chemotherapy agents such as taxanes. In 2004, information on HRT and cetuximab was incorporated, while in 2009, advances in RT such as IMRT and 3-D conformal were incorporated into the survey along with HPV status with the growing body of literature showing it as an important prognostic factor [42].
SEER Registry areas included the states of California, Connecticut, Hawaii, Iowa, New Mexico, Utah, Kentucky, Louisiana, and New Jersey as well as the metropolitan areas of Detroit, Seattle, and Atlanta. SEER registries currently cover 28% of the US population, including 26% of African Americans, 44% of the Hispanic and 50% of the Asian American population [43].
SEER POC datasets are comprised of random samples of HNC patients selected within each registry stratified on age, sex, race/ethnicity, and stage for diagnosis years 1997, 2004, and 2009. In addition to the data available in the SEER public use data set, HNC POC datasets contain information on systemic therapies such as cetuximab, taxanes, 5-FU, and platinum-based drugs cisplatin and carboplatin. They also contain data on advanced radiation treatment including HRT, IMRT, image-guided, and 3-D conformal. Chemo- and immune-therapy sequence with surgery and radiation along with specific chemo- and immunotherapies used requires outpatient verification in POC datasets. SEER abstractors collect this information from patient medical records up to two years post-diagnosis. The patient’s treating physician is also mailed a form to verify all treatments abstracted and to report any treatments administered in an outpatient setting and not already included in the initial record abstraction [42]. Other variables included age at diagnosis, race/ethnicity, sex, insurance and marital status, Charlson co-morbidity score, HNC site, tumor grade, HPV status, smoking history, and treating hospital size (≤499 vs. ≥500 beds) and presence of a residency program.
HNC site was divided into four categories (oral cavity, oropharyngeal, laryngeal, and all other sites) as the number of cases was too small to subdivide into finer categories. Oral cavity was defined by International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) codes C02.0, C02.1, C02.2, C02.3, C02.8, C02.9, C03.0, C03.1, C03.9, C04.0, C04.1, C04.8, C04.9, C05.0, C06.0, C06.1, and C06.2. Oropharyngeal included ICD-O-3 codes C01.9, C02.4, C05.1, C05.2, C09.0, C09.1, C09.8, C09.9, C10.0, C10.1, C10.2, C10.3, C10.4, C10.8, and C10.9. Laryngeal was defined by ICD-O-3 codes C32.0, C32.1, and C32.2, while all other ICD-O-3 HNC codes were included in the all other sites category [44].
Probability sampling weights, the inverse of the sampling proportion for each stratum, were used in order to obtain results representative of the SEER HNC patient population while avoiding underestimation of the standard error. Analyses were conducted using SAS 9.4 (Cary, NC); weighted survey procedures were used for all analyses except the Cochran-Armitage trend analysis. Rao-Scott chi-square tests were used to examine unadjusted associations between year of diagnosis and patient sociodemographic, tumor, and treatment characteristics as well as for factors associated with cetuximab use and HPV-testing. Cochran-Armitage two-sided tests for trend were used to examine the hypothesis that certain treatments were used increasingly (or decreasingly) over the time period, and provide greater power to detect linear trends over Pearson chi-square tests for independence. Logistic regression was used to examine factors associated with receiving no treatment vs. any treatment among all cases and non-surgical vs. surgical treatment among those who received any treatment. Similarly, multinomial logistic regression was used to examine associations with receipt of advanced radiation treatments vs. usual external beam (referent) or no radiation therapy/unknown. The Human Subjects Office did not consider this study to be human subjects’ research and it was therefore exempt from review.
Results
The average age of patients across all three surveys was 61.1 years (95% Confidence Interval (CI): 60.4, 61.8). There were significant differences in patients’ race/ethnicity and insurance status over time, as well as in stage at diagnosis and treatments received (Table 1). Advanced RT, HPV status, and smoking history were only measured in 2009. Forty-two percent of patients received HRT, IMRT, image-guided RT (IGRT), and/or 3-D conformal RT in 2009. The majority (86%) of oropharyngeal cancer patients did not have a known HPV status in 2009, with a third being positive among those with known status. Sixty-nine percent (n=763) of HNC patients in 2009 were current or former smokers. Treating hospital size did not differ across the study years, though the presence of residency programs was marginally significant in unadjusted analysis (Table 1).
Table 1.
Surveillance, Epidemiology, and End Results Head and Neck Patterns of Care Characteristics by Year of Diagnosis, Frequency (unweighted n, weighted %)
| Characteristic | Year | P-Value | |||
|---|---|---|---|---|---|
| 1997 (n=473) | 2004 (n=1,317) | 2009 (n=1,128) | |||
| Age at Diagnosis | Continuous (Mean, 95% Confidence Interval) | 60.9 (59.4-62.4) | 61.2 (60.2-62.2) | 61.2 (60.0-62.3) | 0.85a |
| Sex | Male | 350 (74%) | 766 (74%) | 641 (76%) | 0.47b |
| Female | 123 (26%) | 551 (26%) | 487 (24%) | ||
| Race/ethnicity | Non-Hispanic White | 224 (78%) | 504 (76%) | 401 (75%) | <0.0001b |
| Non-Hispanic Black | 164 (14%) | 352 (12%) | 297 (10%) | ||
| Other/Unknown | 85 (8%) | 461 (12%) | 430 (14%) | ||
| Insurance status | Any Insurancec | 430 (94%) | 1,205 (91%) | 1,504 (94%) | 0.07b |
| No insurance/Unknown | 43 (6%) | 112 (9%) | 74 (6%) | ||
| Marital Status | Married | 219 (50%) | 637 (53%) | 501 (51%) | 0.79b |
| Unmarried/Unknown | 254 (50%) | 680 (47%) | 627 (49%) | ||
| Charlson Comorbidity Score | 0 | 345 (77%) | 914 (69%) | 734 (67%) | 0.05b |
| ≥ 1 | 128 (23%) | 403 (31%) | 394 (33%) | ||
| Site | Oral Cavity | 111 (28%) | 350 (24%) | 315 (25%) | 0.07b |
| Oropharynx | 137 (30%) | 396 (35%) | 344 (36%) | ||
| Larynx | 110 (21%) | 342 (27%) | 277 (25%) | ||
| Other/Unknown | 115 (21%) | 229 (13%) | 192 (14%) | ||
| AJCC stage | I-III | 142 (33%) | 608 (49%) | 505 (47%) | <0.0001b |
| IVA-IVB | 158 (29%) | 439 (30%) | 444 (40%) | ||
| IVC | 33 (4%) | 58 (3%) | 60 (3%) | ||
| Unknown | 140 (34%) | 212 (18%) | 119 (10%) | ||
| Tumor grade | Well/Moderately differentiated (I/II) | 275 (61%) | 747 (58%) | 611 (54%) | 0.39b |
| Poor/Undifferentiated (III/IV) | 121 (25%) | 365 (27%) | 305 (27%) | ||
| Unknown | 77 (14%) | 205 (15%) | 212 (19%) | ||
| Treatment | Surgery with Adjuvant Radiation/Chemoradiation | 171 (40%) | 339 (27%) | 267 (23%) | <0.0001b |
| Surgery Only | 98 (22%) | 264 (19%) | 206 (19%) | ||
| Radiation Only | 88 (18%) | 191 (21%) | 130 (13%) | ||
| Radiation and Chemotherapy Only | 71 (13%) | 358 (25%) | 383 (36%) | ||
| All Other Treatment Combinations | 23 (4%) | 60 (3%) | 64 (4%) | ||
| No Treatment/Unknown | 22 (4%) | 105 (6%) | 78 (6%) | ||
| Advanced Radiotherapy | Usual External Beam Radiation, NOS | - | - | 307 (25%) | |
| Advanced Radiotherapyd | - | - | 491 (42%) | - | |
| No radiation/Other/Unknown | - | - | 330 (33%) | ||
| Cisplatin/Carboplatin | Received | 102 (19%) | 514 (35%) | 510 (44%) | <0.0001b |
| Didn’t receive | 371 (81%) | 803 (65%) | 618 (56%) | ||
| Fluorouracil | Received | 77 (14%) | 181 (12%) | 182 (16%) | 0.11b |
| Didn’t receive | 396 (86%) | 1,136 (88%) | 946 (84%) | ||
| Taxane | Received | 12 (2%) | 161 (12%) | 211 (19%) | <0.0001b |
| Didn’t receive | 461 (98%) | 1,156 (88%) | 917 (81%) | ||
| Cetuximab | Received | - | 11 (1%) | 184 (19%) | <0.0001b |
| Didn’t receive | - | 1,306 (99%) | 944 (81%) | ||
| Human Papillomavirus Statuse | Positive | - | - | 16 (5%) | - |
| Negative | - | - | 32 (9%) | ||
| No mention in record/Unknown | - | - | 296 (86%) | ||
| Smoking History | Current or Former Smoker | - | - | 763 (69%) | - |
| Never Smoker | - | - | 365 (31%) | ||
| Hospital Bed Size | ≤ 499f | 362 (78%) | 1,012 (79%) | 830 (76%) | 0.63b |
| ≥ 500 | 111 (22%) | 305 (21%) | 298 (24%) | ||
| Residency Program | Yes | 324 (63%) | 805 (55%) | 758 (62%) | 0.03b |
| No | 149 (37%) | 512 (45%) | 370 (38%) | ||
Weighted Anova,
Weighted Rao-Scott Chi-Square P-Value
Includes Private, Health Maintenance Organization, Veterans’ Affairs, Indian Health Service, Champus, Medicare, or Medicaid
Includes Hyperfractionated, Intensity-modulated, Image guided, and 3-D conformal radiation
Oropharyngeal cases only (n=344)
Includes Outpatient Facilities, Physician Offices & Unknown, - Not measured
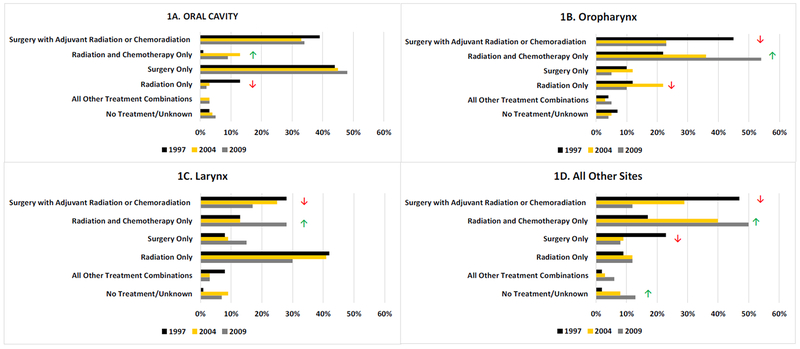
Figure 1 shows the weighted proportion of patients receiving specific treatment types by year and site. Among oral cavity patients (panel 1A), there was a significant trend of patients receiving radiation and chemotherapy without surgery, going from only <1% in 1997 up to 13% and 9% in 2004 and 2009, respectively (Cochran-Armitage test for trend p=0.02). With the increasing incorporation of chemotherapy into oral cavity tumor treatment, there was a concurrent decrease in patients receiving only RT, going from 13% in 1997 down to just 2% in 2009 (Cochran-Armitage test for trend p=0.0001). There was a significant decrease in the proportion of oropharyngeal patients (panel 1B) receiving surgery with adjuvant treatment, going from 45% in 1997 to 23% in 2004 and 2009 (Cochran-Armitage test for trend p=0.006). There was a decreases well in those receiving RT only, with 12% and 22% receiving in 1997 and 2004, respectively, and just 10% receiving in 2009 (Cochran-Armitage test for trend p=0.0002). Concurrent with the two aforementioned trends in oropharyngeal treatment, the proportion of oropharyngeal cases receiving RT and chemotherapy without surgery increased from 22% in 1997 to 54% in 2009. Similarly, the proportion of laryngeal cases (panel 1C) receiving surgery with adjuvant treatment showed a significantly decreasing trend, going from 28% in 1997 down to 17% in 2009 (Cochran-Armitage test for trend p=0.005). The proportion of laryngeal patients receiving RT and chemotherapy without surgery showed an increasing trend, going from 13% in 1997 to 28% in 2009 (Cochran-Armitage test for trend p=0.0001). For all other sites (panel 1D), there was a significant decreasing trend in the proportion of patients receiving surgery with adjuvant treatment (Cochran-Armitage test for trend p<0.0001) and surgery only (Cochran-Armitage test for trend p=0.0007). In contrast, the proportion receiving only RT and chemotherapy (Cochran-Armitage test for trend p<0.0001) and no treatment (Cochran-Armitage test for trend p=0.04) increased.
Fig. 1.
Surveillance, Epidemiology, and End Results Head and Neck Patterns of Care Treatment by Site and Year of Diagnosis (weighted %)
 Significant increasing Cochran-Armitage Test for Trend
Significant increasing Cochran-Armitage Test for Trend
 Significant decreasing Cochran-Armitage Test for Trend
Significant decreasing Cochran-Armitage Test for Trend
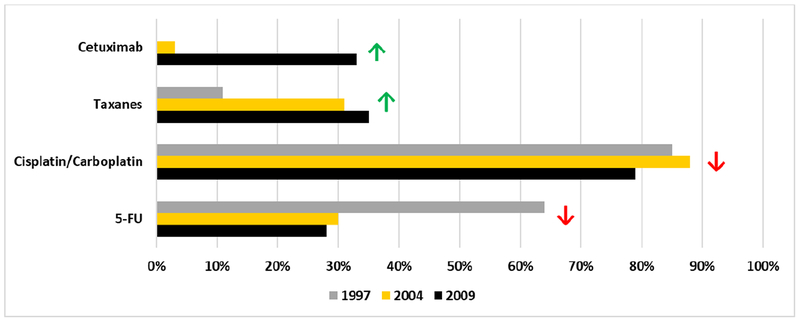
Figure 2 shows the proportion of those receiving cetuximab (not measured in 1997), taxanes, 5-FU, and cisplatin/carboplatin by year among those who received chemotherapy (n=1,222). While cetuximab and taxane use increased significantly over the study period, platinum-based therapy use decreased between 2004 and 2009 and 5-FU use decreased between 1997 and 2004 and 2004 to 2009 (all Cochran-Armitage trend tests p ≤ 0.01).
Fig. 2.
Surveillance, Epidemiology, and End Results Head and Neck Patterns of Care Specific Chemotherapies Received by Year of Diagnosis (weighted % among all who received any chemotherapy, unweighted n=1,222)
NOTE: Cetuximab not measured in 1997
 Significant increasing Cochran-Armitage Test for Trend
Significant increasing Cochran-Armitage Test for Trend
 Significant decreasing Cochran-Armitage Test for Trend
Significant decreasing Cochran-Armitage Test for Trend
Table 2 shows 2009 treatment by site and stage. A higher proportion (82%) of patients with oral cavity tumors received surgery with or without adjuvant treatment as compared to other sites. Fifty-four percent of oropharyngeal patients received radiation and chemotherapy only. A relatively high proportion of laryngeal cases also received non-surgical treatment, with 30% receiving radiation only and 28% receiving radiation and chemotherapy only. Half of cases diagnosed at other anatomical HNC sites received radiation and chemotherapy only, with 20% receiving surgery.
Table 2.
Surveillance, Epidemiology, and End Results Head and Neck Patterns of Care Treatment by Site and AJCC Stage, 2009 (unweighted n, weighted %)
| Site and Treatment | Stage I | Stage II | Stage III | Stage IVA-IVB | Stage IVC | Unknown | Total |
|---|---|---|---|---|---|---|---|
| Oral Cavity | |||||||
| No. of Patients | 99 | 37 | 44 | 91 | 9 | 35 | 315 |
| Surgery with Adjuvant Radiation or Chemoradiation | 9% | 56% | 47% | 58% | 25% | 6% | 34% |
| Surgery Only | 87% | 41% | 31% | 15% | 8% | 57% | 48% |
| Radiation Only | 1% | 3% | 1% | 3% | 0% | 1% | 2% |
| Radiation and Chemotherapy Only | 1% | 0% | 18% | 8% | 45% | 24% | 9% |
| All Other Treatment | 0% | 0% | 1% | 8% | 0% | 1% | 3% |
| No Treatment Received/Unknown | <1% | 0% | 2% | 9% | 22% | 10% | 5% |
| Oropharynx | |||||||
| No. of Patients | 11 | 16 | 57 | 203 | 19 | 38 | 344 |
| Surgery with Adjuvant Radiation or Chemoradiation | 17% | 42% | 40% | 18% | 5% | 3% | 23% |
| Surgery Only | 53% | 6% | 3% | 2% | 2% | 9% | 5% |
| Radiation Only | 28% | 18% | 7% | 10% | 7% | 5% | 10% |
| Radiation and Chemotherapy Only | 2% | 34% | 49% | 60% | 37% | 60% | 54% |
| All Other Treatment | 0% | 0% | 0% | 4% | 47% | 13% | 5% |
| No Treatment Received/Unknown | 0% | 0% | 1% | 5% | 2% | 10% | 4% |
| Larynx | |||||||
| No. of Patients | 85 | 35 | 46 | 78 | 13 | 20 | 277 |
| Surgery with Adjuvant Radiation or Chemoradiation | 14% | 9% | 16% | 18% | 12% | 36% | 17% |
| Surgery Only | 25% | 28% | 3% | 9% | 2% | 1% | 15% |
| Radiation Only | 51% | 29% | 11% | 8% | 10% | 36% | 30% |
| Radiation and Chemotherapy Only | 6% | 11% | 69% | 47% | 50% | 5% | 28% |
| All Other Treatment | <1% | 0% | 0% | 8% | 14% | 15% | 3% |
| No Treatment Received/Unknown | 3% | 23% | 1% | 10% | 12% | 7% | 7% |
| All Other Sites | |||||||
| No. of Patients | 10 | 23 | 42 | 72 | 19 | 26 | 192 |
| Surgery with Adjuvant Radiation or Chemoradiation | 24% | 18% | 13% | 14% | 0% | 1% | 12% |
| Surgery Only | 28% | 9% | 0% | 15% | 3% | 1% | 8% |
| Radiation Only | 6% | 8% | 3% | 5% | 5% | 44% | 12% |
| Radiation and Chemotherapy Only | 40% | 53% | 73% | 45% | 38% | 30% | 50% |
| All Other Treatment | 0% | 3% | 1% | 10% | 18% | 2% | 6% |
| No Treatment Received/Unknown | 3% | 8% | 11% | 10% | 36% | 22% | 13% |
Table 3 shows models examining factors associated with receiving no treatment (vs. any treatment) among all cases and non-surgical (vs. surgical) treatment among those who received any treatment. Both non-surgical treatment and no treatment were more likely to occur in 2004 and 2009. Those with insurance were less likely to receive no treatment (OR: 0.47, 95% CI: 0.23-0.96). Patients with oral cavity tumors were less likely to receive non-surgical treatment only (OR: 0.12, 95% CI: 0.08-0.19). Diagnosis at advanced or unknown stage increased the likelihood of receiving non-surgical treatment only or no treatment, as well as did having an unknown tumor grade.
Table 3.
Weighted Logistic Regression Models Examining No Treatment (vs. Any Treatment) among all cases (unweighted n=2,918) and Non-Surgical (vs. Surgical) Treatment among those who received any treatment (unweighted n=2,713) in Surveillance, Epidemiology, and End Results Head and Neck Patterns of Care Survey Data: 1997, 2004, 2009
| Characteristic | Odds Ratioc (95% Confidence Interval) | ||
|---|---|---|---|
| No Treatment (vs. Any Treatment) | Non-Surgical (vs. Surgical) Treatment | ||
| Year of Diagnosis | 1997 | 1.00 (referent) | 1.00 (referent) |
| 2004 | 2.08 (1.08-4.01) | 2.39 (1.59-3.58) | |
| 2009 | 2.07 (1.00-4.29) | 3.16 (2.11-4.74) | |
| Age at Diagnosis | Continuous | 1.05 (1.03-1.07) | 1.01 (0.99-1.02) |
| Sex | Male | 1.00 (referent) | 1.00 (referent) |
| Female | 0.71 (0.44-1.14) | 0.85 (0.64-1.12) | |
| Race/ethnicity | Non-Hispanic (NH) White | 1.00 (referent) | 1.00 (referent) |
| Non-Hispanic (NH) Black | 1.49 (0.88-2.51) | 1.27 (0.95-1.69) | |
| Other/Unknown | 1.41 (0.86-2.34) | 0.97 (0.75-1.27) | |
| Insurance status | No insurance/Unknown | 1.00 (referent) | 1.00 (referent) |
| Any Insurancea | 0.47 (0.23-0.96) | 0.68 (0.39-1.16) | |
| Marital Status | Married | 1.00 (referent) | 1.00 (referent) |
| Unmarried/Unknown | 1.41 (0.84-2.38) | 1.11 (0.83-1.50) | |
| Charlson Comorbidity Score | 0 | 1.00 (referent) | 1.00 (referent) |
| ≥ 1 | 1.34 (0.82-2.20) | 0.98 (0.70-1.38) | |
| Site | Oropharynx | 1.00 (referent) | 1.00 (referent) |
| Oral Cavity | 0.88 (0.40-1.95) | 0.12 (0.08-0.19) | |
| Larynx | 1.44 (0.71-2.93) | 1.14 (0.76-1.71) | |
| Other/Unknown | 1.64 (0.80-3.37) | 0.85 (0.57-1.26) | |
| AJCC stage | I-III | 1.00 (referent) | 1.00 (referent) |
| IVA-IVB | 2.43 (1.29-4.57) | 1.73 (1.22-2.47) | |
| IVC | 4.29 (1.96-9.39) | 3.58 (1.87-6.85) | |
| Unknown | 2.98 (1.54-5.76) | 2.11 (1.37-3.27) | |
| Tumor grade | Well/Moderately differentiated (I/II) | 1.00 (referent) | 1.00 (referent) |
| Poor/Undifferentiated (III/IV) | 0.86 (0.46-1.60) | 1.20 (0.85-1.69) | |
| Unknown | 1.52 (0.73-3.16) | 2.66 (1.71-4.15) | |
| Hospital Size | ≤ 499 bedsb | 1.00 (referent) | 1.00 (referent) |
| ≥ 500 beds | 1.18 (0.61-2.30) | 0.91 (0.64-1.29) | |
| Hospital Residency Program | No program | 1.00 (referent) | 1.00 (referent) |
| Has program | 0.82 (0.45-1.47) | 0.74 (0.53-1.04) | |
Includes Private, Health Maintenance Organization, Veterans’ Affairs, Indian Health Service, Champus, Medicare, or Medicaid
Includes Outpatient Facilities, Physician Offices & Unknown
Adjusted for all other variables in this table
HRT was measured beginning in 2004, while IMRT, IGRT, and 3-D conformal RT were measured only in 2009. Among those receiving radiation diagnosed in 2004 and 2009, 3% underwent HRT, while among those diagnosed in 2009, the majority (55%) received IMRT, with 2% receiving IGRT, 4% 3D conformal, 3% IMRT and IGRT, 1% other/unknown, and 35% receiving usual external beam not otherwise specified (EB, NOS). HRT can be given as standard EB or using more advanced techniques such IMRT, IGRT, or 3D-conformal RT. Among those receiving hyperfractionated radiation in 2009, 49% received IMRT, 2% received IGRT, 12% received 3D-conformal, and 36% received EB, NOS.
Receiving advanced RT techniques such as HRT, IMRT, IGRT, and/or 3-D conformal RT (2009 only) vs. usual external beam radiation was more likely in female patients (OR: 1.65, 95% CI: 1.03-2.65) and those who received chemotherapy (OR: 2.52, 95% CI: 1.34-4.73) and less likely in laryngeal cases (OR:0.49, 95% CI: 0.25-0.96). Non-Hispanic Black patients were less likely to receive no radiation (OR: 0.41, 95% CI: 0.23-0.74) as were those who received chemotherapy (OR: 0.24, 95% CI: 0.11-0.52), while stage IVC patients were more likely to receive no radiation (OR: 4.50, 95% CI: 1.15-17.54) (multinomial logistic regression results not shown).
Cetuximab use was included on the 2004 and 2009 surveys. Of those receiving cetuximab, 26% also received 5-FU, 48% received a platinum-based drug, and 31% received a taxane, with only 2% of patients receiving cetuximab with no chemotherapeutic agents. The majority of those receiving cetuximab received RT and chemotherapy with no surgery (73%). Of those receiving cetuximab, the majority (66%) were married (Rao-Scott Chi-Square p=.006), had oropharyngeal cancer (57%, Rao-Scott Chi-Square p=.001), and were diagnosed at stage III (30%) or IVA-B (50%, Rao-Scott Chi-Square p<.0001).
There was no significant difference in average age between those who received (62.5 years) vs. those who did not receive (61.0 years) cetuximab. T-tests showed those receiving taxanes, cisplatin or carboplatin, and 5-FU were significantly younger than those who did not receive these agents (p<.0001 for all). The average age of those who received taxanes was six years younger than those who did not (55.9 years vs. 61.9 years), with a similar difference seen for platinum-based drugs (57.3 years vs. 63.2 years) and 5-FU (55.1 years vs. 62.1 years).
Among oropharyngeal cases diagnosed in 2009, the average age of those with known HPV status was 58.1 (95% CI: 56.0-59.6), with no significant difference in age between those with known and unknown HPV status. Of the oropharyngeal cases diagnosed in 2009 with known HPV status, 62% received radiation and chemotherapy only and 31% received surgery with adjuvant RT or chemoradiation, with the rest falling into other treatment categories. Almost all (99.6%) of those with known HPV status had some form of insurance (Rao-Scott Chi-Square p=.005) and a Charlson co-morbidity score ≤1 (99.5%, Rao-Scott Chi-Square p=.007).
Although HPV status was only available for 2009, we examined base of tongue and tonsil subsite (ICD-O-3 codes [39] C01.9, C02.4, C09.0, C09.1, C09.8, and C09.9) across the three survey years as a surrogate for HPV positive status. The weighted percent of base of tongue and tonsil cancers increased from 19% in 1997 to 30% in 2004 and 31% in 2009 (Cochhran-Armitage test for trend=.03).
Discussion
SEER POC data provide a description of the recent state of practice across the population. There have been significant changes in HNC treatment including introduction of systemic therapy and advanced RT techniques, as well as the growing etiologic role of HPV. Starting with the 1997 HNC POC study, there was growing interest in the use of non-surgical approaches with state of the art chemoradiation agents and techniques, specifically taxanes in combination with platinum based agents, in order to preserve organ function. With the follow-up in 2004, HRT and the EGFR inhibitor cetuximab were increasingly being incorporated into HNC treatment, while the 2009 survey sought to quantify the increasing incorporation of HPV testing and targeted radiation techniques such as IMRT and 3-D conformal into HNC treatment [42]. Practice patterns at academic centers are described in the literature and are reflected in guidelines, but it is also important to understand how practices have changed across the entire US population, and these data provide a representative assessment of the recent state of HNC treatment across all US cancer patients [45].
Although guidelines and formal benchmarks for quality HNC care are evolving, a recent study found utility in using NCCN guidelines as quality indicators during multidisciplinary cancer treatment conference. This single institution study found generally high compliance with NCCN guidelines, with 99.1% of conference recommendations compliant for laryngeal cancer, 93.8% for oropharyngeal, 86.6% for oral cavity, and 84.2% for nasopharyngeal [46]. For the current population-based nationwide study, there was wider range in treatment protocols although a high proportion of cases in 2009 also met NCCN guideline-recommended treatment, with the majority of early stage laryngeal cases receiving radiation only and the majority of early stage oral cavity and oropharyngeal patients receiving surgery. Later stage laryngeal cases most often received chemoradiation only, while the majority of later stage oral cavity patients received surgery with adjuvant radiation or chemoradiation and oropharyngeal receiving a combination of surgery with adjuvant radiation or chemoradiation, or chemoradiation only, with all being options under NCCN guidelines.
Close to 70% of HNC patients received RT in 2009, and of those, over 60% received more advanced form(s) of RT beyond usual external beam such as HRT, IMRT, IGRT, and/or 3-D conformal. These techniques have shown improved local-regional control and overall- and cancer-specific survival while causing fewer or equivalent side effects [3–4, 15–17]. While guidelines are still evolving, the NCCN generally recommends more targeted therapies such as IMRT in order to reduce long-term toxicity in oropharyngeal, paranasal sinus, and nasopharyngeal cancers, while HRT or other altered fractionation schedules can be used for high risk tumors and/or where RT is the definitive treatment [22]. IMRT in particular was shown previously to have rapid uptake between 2000 and 2005 in the SEER and SEER-Medicare database [13–14]. While these previous studies found there was geographic variation in uptake and those in lower socioeconomic census tracts were less likely to receive IMRT, in our study of 2009 SEER POC HNC patients incorporating multiple advanced RT modalities, only male patients, those not receiving chemotherapy, and laryngeal cases were less likely to receive advanced RT, likely reflecting wider diffusion of these RT modalities in the proceeding years.
This study demonstrates the increase in utilization of chemotherapy after publication of several large studies in the 2000s showing concomitant chemoradiation reduces locoregional failure rates and improves organ preservation [21, 27]. Chemoradiation is guideline recommended for locally advanced tumors, with various platinum-based regimens incorporating taxanes and cetuximab included as clinical options depending on site and stage [22]. Expanding systemic therapy use has allowed for cancer treatment to take place in the absence of surgical specialists, and possibly across a wider range of practice sites. The data also show that nearly 20% of HNC patients received cetuximab in 2009, just three years after its FDA approval for use in HNC [25], indicating the strength of its appeal in selected patients.
Also of interest is the difference in the direction of association between age and use of chemotherapy agents versus cetuximab. Platinum agents, 5-FU, and taxanes were all significantly less likely to be used in older patients, while cetuximab was used more in older patients, though this difference was not significant. This practice likely reflects the significant toxicities of chemotherapy and evidence that cetuximab potentiates the effect of RT while being better tolerated than chemotherapy [21, 24, 26].
It should be noted that the introduction of immunotherapy, specifically PD-1 checkpoint inhibitors, in the treatment of recurrent or metastatic HNC has shown promise in improving overall survival while being generally well tolerated by patients as compared to standard treatment [47]. Pembrolizumab and nivolumab, two anti-PD-1 antibodies, are both guideline recommended as second-line treatments in patients with recurrent or metastatic squamous cell HNC where platinum-based chemotherapy fails to stop progression [22]. Both of these agents are also currently being examined in trials for use in earlier stage HNC at high risk of recurrence [47].
The International Agency for Research on Cancer first listed HPV type 16 as a cause of oropharyngeal and oral cavity cancer in 2005 [48]. However, due to findings of better response to treatment and prognosis for HPV-positive patients, it is only recently that major guidelines require or recommend routine HPV-testing via p16 immunohistochemistry for all newly diagnosed oropharyngeal squamous cell carcinomas [22, 49]. As we show in this and our previous analysis [34], in 2009 (first year HPV testing measured in SEER HNC POC data) a small proportion (14%) of oropharyngeal patients received testing. Given the wide availability of clinical tests for p16 expression [22] and new lines of research into HPV specific treatment regimens, especially de-intensifying therapies to better preserve function and quality of life in younger oropharyngeal patients more likely to be diagnosed with HPV-positive cancer [50], it is likely the proportion of patients tested will significantly increase.
While not statistically significant, our results suggest Non-Hispanic Black HNC patients are more likely to receive no treatment as well as non-surgical treatment. Previous studies have reported US Black patients have higher age-adjusted incidence and are more likely to present with advanced disease and have lower survival [38, 51]. They are also less likely to receive surgery and chemotherapy [38, 39]. Alternatively, being of Hispanic or Chinese race/ethnicity has been shown to confer improved survival [52].
Despite the aforementioned strengths of the SEER HNC POC data, a few limitations should be noted. While SEER POC data includes information beyond what is included in the SEER public use database, such as detailed chemotherapy/systemic treatment data, it does not include patient preference information, so it is unable to fully explain if trends such as increasing use of organ-preserving therapies are due to physician recommendation, patient preference, or a combination of both. HNC POC data for 2016 (most recent data collection year) are not yet available for analysis, so we are unable to include a more recent data point, though it is likely the use of systemic therapies such as cetuximab and advanced RT techniques have continued in an upward trend. Furthermore, our data showing increased usage of more advanced, targeted RT, cetuximab, especially among older patients, and the advent of HPV testing for HNC, point to more individualized approaches to treatment taking into account each patient’s unique characteristics and preferences as well as the traits of their particular tumor. Advances in classification of HNC tumors combined with information on HPV status will lead to increasingly personalized treatments that target the underlying anatomy, genetics, and histology of individual tumors. Personalized HNC therapy will affect how clinicians not only approach first-line, but also secondary treatment and drug resistance as more knowledge regarding specific tumor and pharmacologic pathways is gathered and incorporated into multistage treatment regimens [53, 54].
Unfortunately, HPV status was only recorded for the 2009 data, and the majority of patients at that time (86%) had an unknown status. Going forward, it is likely testing will become more widespread as HPV status is increasingly used as a prognostic marker and incorporated into treatment plans. Despite these limitations, SEER HNC POC data are a representative sample of the US HNC patient population, including those treated in both academic and community centers and among younger patients not included in SEER-Medicare datasets, and thus are a valuable tool for examining population-based treatment trends [27, 45].
Our results extend those of Dansky-Ullmann et al. [27] by adding a data point five years after their description. Their expectation that use of taxanes and cetuximab would continue to increase [27] is shown in the present data. As newer, more refined and targeted RT modalities and systemic treatments are incorporated into clinical practice, along with the increase in HPV-related HNC, the treatment of HNC is likely to even more rapidly evolve over the next decade. In addition to developing more refined, less toxic treatment regimens to both improve disease-free survival and quality of life, future studies should examine how to make sure HPV testing and advanced therapies reach all populations, including those traditionally underserved.
Acknowledgments
Funding This work was supported in part under NIH/NCI contract number HHSN26120130000201/HHSN26100006 with University of Iowa (MEC, CC, JAS). This work was also supported by the University of Iowa Holden Comprehensive Cancer Center, which is funded in part by NIH/NCI P30 CA086862.
References
- 1.National Cancer Institute (2018) Oropharyngeal Cancer Treatment (Adult) (PDQ®)–Health Professional Version. https://www.cancer.gov/types/head-and-neck/hp/adult/oropharyngeal-treatment-pdq. Accessed 17 September 2018
- 2.Allal AS, Nicoucar K, Mach N, Dulguerov P (2003) Quality of life in patients with oropharynx carcinomas: assessment after accelerated radiotherapy with or without chemotherapy versus radical surgery and postoperative radiotherapy. Head Neck 25:833–839 [DOI] [PubMed] [Google Scholar]
- 3.Argiris A, Karamouzis MV, Raben D, Ferris RL (2008) Head and neck cancer. Lancet 371:1695–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisbruch A, Harris J, Garden AS, Chao CK, Straube W, Harari PM, Sanguineti G, Jones CU, Bosch WR, Ang KK (2010) Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J of Radiat Oncol Biol Phys 76:1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryzek DF, Mantsopoulos K, Kunzel J, Grundtner P, Zenk J, Iro H, Psychogios G (2014) Early stage oropharyngeal carcinomas: comparing quality of life for different treatment modalities. Biomed Res Int 2014: Article ID 421964, 7 pages [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber RS, Gidley P, Morrison WH, Peters LJ, Hankins P, Wolf P, Guillamondegui O (1990) Treatment selection for carcinoma of the base of the tongue. Am J Surg 160:415–419 [DOI] [PubMed] [Google Scholar]
- 7.Chambers MS, Garden AS, Kies MS, Martin JW (2004) Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck 26:796–807 [DOI] [PubMed] [Google Scholar]
- 8.Chambers MS, Rosenthal DI, Weber RS (2007) Radiation-induced xerostomia. Head Neck 29:58–63 [DOI] [PubMed] [Google Scholar]
- 9.Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P (2001) Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 23:389–398 [DOI] [PubMed] [Google Scholar]
- 10.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ (2008) Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 26:3770–3776 [DOI] [PubMed] [Google Scholar]
- 11.Webb S (2001) Intensity-modulated radiation therapy. CRC Press, Taylor & Francis Group, Abingdon, UK [Google Scholar]
- 12.Intensity Modulated Radiation Therapy Collaborative Working Group (2001) Intensity-modulated radiotherapy: current status and issues of interest. Int J of Radiat Oncol Biol Phys 51:880–914 [DOI] [PubMed] [Google Scholar]
- 13.Guadagnolo BA, Liu CC, Cormier JN, Du XL (2010) Evaluation of trends in the use of intensity-modulated radiotherapy for head and neck cancer from 2000 through 2005: socioeconomic disparity and geographic variation in a large population-based cohort. Cancer 116:3505–3512 [DOI] [PubMed] [Google Scholar]
- 14.Sher DJ, Neville BA, Chen AB, Schrag D (2011) Predictors of IMRT and conformal radiotherapy use in head and neck squamous cell carcinoma: a SEER-Medicare analysis. Int J Radiat Oncol Biol Phys 81:e197–206 [DOI] [PubMed] [Google Scholar]
- 15.Beadle BM, Liao KP, Elting LS, Buchholz TA, Ang KK, Garden AS, Guadagnolo BA (2014) Improved survival using intensity-modulated radiation therapy in head and neck cancers: a SEER-Medicare analysis. Cancer 120:702–710 [DOI] [PubMed] [Google Scholar]
- 16.Beitler JJ, Zhang Q, Fu KK, Trotti A, Spencer SA, Jones CU, Garden AS, Shenouda G, Harris J, Ang KK (2014) Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 89:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot J-C, Le Maître A, Pajak TF, Poulsen MG (2006) Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 368:843–854 [DOI] [PubMed] [Google Scholar]
- 18.Balukrishna S, Pilaka VK, Michael RC, Samuel P, Ravindran PB (2015) Hyper-fractionated Intensity Modulated Radiation Therapy (HF-IMRT) in Head and Neck Cancer: The Technical Feasibility and Results of a Short Clinical Series. J Clin Diagn Res 9:XR01–XR04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao J, Genden EM, Gupta V, Policarpio EL, Burri RJ, Rivera M, Gurudutt V, Som PM, Teng M, Packer SH (2011) Phase 2 trial of concurrent 5-fluorouracil, hydroxyurea, cetuximab, and hyperfractionated intensity-modulated radiation therapy for locally advanced head and neck cancer. Cancer 117:318–326 [DOI] [PubMed] [Google Scholar]
- 20.Lee VH, Kwong DL, Leung TW, Ng SC, Lam KO, Tong CC, Sze CK (2017) Hyperfractionation compared to standard fractionation in intensity-modulated radiation therapy for patients with locally advanced recurrent nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol 274:1067–1078 [DOI] [PubMed] [Google Scholar]
- 21.Cohen EE, Lingen MW, Vokes EE (2004) The expanding role of systemic therapy in head and neck cancer. J Clin Oncol 22:1743–1752 [DOI] [PubMed] [Google Scholar]
- 22.NCCN Guidelines Version 2.2018 Panel Members: Head and Neck Cancers (2018) NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Version 2.2018 - June 20, 2018. National Comprehensive Cancer Network; https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 17 September 2018 [Google Scholar]
- 23.Pfister DG, Ang K-K, Brizel DM, et al. (2011) Head and Neck Cancers, Clinical Practice Guidelines in Oncology. JNCCN 9:596–650 [DOI] [PubMed] [Google Scholar]
- 24.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578 [DOI] [PubMed] [Google Scholar]
- 25.Kreeger K (2006) News: Cetuximab approved by FDA for treatment of head and neck squamous cell cancer. Cancer Biol Ther 5:340–342 [PubMed] [Google Scholar]
- 26.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer H-R, Cupissol D (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127 [DOI] [PubMed] [Google Scholar]
- 27.Dansky Ullmann C, Harlan LC, Shavers VL, Stevens JL (2012) A population‐based study of therapy and survival for patients with head and neck cancer treated in the community. Cancer 118:4452–4461 [DOI] [PubMed] [Google Scholar]
- 28.Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, Wu X, Chaturvedi AK, Kawaoka K (2008) Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998-2003. Cancer 113(10 Suppl):2901–2909 [DOI] [PubMed] [Google Scholar]
- 29.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356:1944–1956 [DOI] [PubMed] [Google Scholar]
- 30.Nguyen NP, Chi A, Nguyen LM, Ly BH, Karlsson U, Vinh-Hung V (2010) Human papillomavirus-associated oropharyngeal cancer: a new clinical entity. Q J Med 103:229–236 [DOI] [PubMed] [Google Scholar]
- 31.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269 [DOI] [PubMed] [Google Scholar]
- 33.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, Copeland G, Cozen W, Peters ES, Huang Y, Saber MS, Altekruse S, Goodman MT - HPV Typing of Cancers Workgroup (2015) US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagedar NA, Chioreso C, Schlichting JA, Lynch CF, Charlton ME (2017) Treatment selection in oropharyngeal cancer: a surveillance, epidemiology, and end results (SEER) patterns of care analysis. Cancer Causes Control 28:1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahl AR, Charlton ME, Pagedar NA, Sperry SM, Matt B, Platz C, Lynch CF (2018) Accuracy of the HPV status site-specific factor 10 (SSF-10) variable for patients with oropharyngeal cancers in the Iowa Cancer Registry, 2010-2014. Head Neck 40:2199–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniakas A, Moubayed SP, Ayad T, Guertin L, Nguyen-Tan PF, Gologan O, Soulieres D, Christopoulos A (2014) North-American survey on HPV-DNA and p16 testing for head and neck squamous cell carcinoma. Oral Oncol 50:942–946 [DOI] [PubMed] [Google Scholar]
- 37.Chen AY, Schrag N, Hao Y, Stewart A, Ward E (2007) Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope 117:16–21 [DOI] [PubMed] [Google Scholar]
- 38.Subramanian S, Chen A (2013) Treatment patterns and survival among low-income medicaid patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg 139:489–495 [DOI] [PubMed] [Google Scholar]
- 39.Choi SH, Terrell JE, Fowler KE, McLean SA, Ghanem T, Wolf GT, Bradford CR, Taylor J, Duffy SA (2016) Socioeconomic and Other Demographic Disparities Predicting Survival among Head and Neck Cancer Patients. PLoS One 11:e0149886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker BB, Schuurman N, Auluck A, Lear SA, Rosin M (2017) Socioeconomic disparities in head and neck cancer patients’ access to cancer treatment centers. Rural Remote Health 17:4210. [DOI] [PubMed] [Google Scholar]
- 41.National Cancer Institute, Division of Cancer Control & Population Sciences, Healthcare Delivery Research Program (2019) Patterns of Care Studies. https://healthcaredelivery.cancer.gov/poc/. Accessed 13 May 2019
- 42.National Cancer Institute, Division of Cancer Control and Population Sciences and Division of Cancer Treatment and Diagnosis, Surveillance, Epidemiology, and End Results Program (1997, 2004, 2009) Head and Neck Cancer Data Set. POC Data Acquisition Manual [Google Scholar]
- 43.Surveillance, Epidemiology, and End Results Program (2018) Overview of the SEER Program. National Cancer Institute. https://seer.cancer.gov/about/overview.html. Accessed 17 September 2018
- 44.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, eds. (2013) International Classification of Diseases for Oncology, 3rd edn World Health Organization, Geneva, Switzerland [Google Scholar]
- 45.Yu JB, Gross CP, Wilson LD, Smith BD (2009) NCI SEER public-use data: applications and limitations in oncology research. Oncology 23:288–295 [PubMed] [Google Scholar]
- 46.Lewis C, Nurgalieva Z, Lai S, Weber R (2014) Improving the quality of multidisciplinary care for head and neck cancer. J Clin Oncol 32:232 [Google Scholar]
- 47.Dogan V, Rieckmann T, Munscher A, Busch CJ (2018) Current studies of immunotherapy in head and neck cancer. Clin Otolaryngol 43:13–21 [DOI] [PubMed] [Google Scholar]
- 48.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, World Health Organization International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans (2005) Policy Watch: Carcinogenicity of human papillomaviruses. Lancet 6: 204. [DOI] [PubMed] [Google Scholar]
- 49.Fakhry C, Lacchetti C, Rooper LM, Jordan RC, Rischin D, Sturgis EM, Bell D, Lingen MW, Harichand-Herdt S, Thibo J et al. (2018) Human Papillomavirus Testing in Head and Neck Carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J Clin Oncol 36:3152–3161 [DOI] [PubMed] [Google Scholar]
- 50.Bath C (2017) Deintensifiying Treatment of HPV-Positive Oropharyngeal Cancer Could Reduce Toxicity While Maintaining Function and Survival. The ASCO Post, American Society of Clinical Oncology; http://www.ascopost.com/issues/april-25-2017/deintensifiying-treatment-of-hpv-positive-oropharyngeal-cancer-could-reduce-toxicity-while-maintaining-function-and-survival/. Accessed 17 September 2018 [Google Scholar]
- 51.Daraei P, Moore CE (2015) Racial Disparity Among the Head and Neck Cancer Population. J Canc Educ (30):546–551 [DOI] [PubMed] [Google Scholar]
- 52.Xu C, Chen YP, Liu X, Tang LL, Chen L, Mao YP, Zhang Y, Guo R, Zhou GQ, Li WF, et al. (2017) Socioeconomic factors and survival in patients with non-metastatic head and neck squamous cell carcinoma. Cancer Sci 108:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross AM, Cohen EE (2015) Towards a personalized treatment of head and neck cancer. Head and Neck Cancer, Am Soc Clin Oncol Educ Book 28–32 [DOI] [PubMed] [Google Scholar]
- 54.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, Brown C, Pugh TJ, Stojanov P, Cho J, et al. (2015) Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res 21:632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]