Abstract
Steady advances in the diagnosis and management of congenital heart disease over the last few decades has resulted in a growing population of adults with congenital heart disease (ACHD). Consequently, there has been a parallel increase in the number of ACHD patients plagued with end-stage heart failure. Even so, the transplantation rate for these patients has remained low, at about 3% of all adult heart transplants. This review discusses the scope of transplantation for ACHD, including indications and contraindications, specific challenges and nuances, and post-transplant outcomes.
Keywords: adult congenital heart disease, ACHD, transplantation
INTRODUCTION
The population of adults who have survived with congenital heart disease (CHD) continues to increase due to steady advances in the diagnosis and management of simple and complex CHD. In fact, there are so many people with CHD who are living into adulthood that these adult survivors now outnumber the children with CHD. As a result, there has been a parallel increase in the prevalence of advanced heart failure among these adult patients, largely due to multiple interrelated factors such as aging, palliated circulations, and the impact of residual cardiac lesions. It is now estimated that heart failure accounts for up to 40% of deaths in adult CHD (ACHD).1 Unfortunately, the heterogeneous nature of this patient population complicates the universal application of typical heart failure therapies and transplant paradigms.
Heart transplantation in those with ACHD presents many unique challenges because these patients tend to have cumulative risk factors such as multiple prior surgeries, longstanding cardiac dysfunction or cyanosis with effects on other organs (ie, kidneys, liver, lungs), and elevated pulmonary vascular resistance. It is precisely these challenges that have put ACHD patients at a disadvantage when being considered for transplantation. Although the population with ACHD is rapidly increasing, the percentage undergoing heart transplantation has not significantly changed over time.2 The International Society of Heart and Lung Transplantation (ISHLT) 2018 registry reported that ACHD patients accounted for only 3% of adult heart transplants performed worldwide between 2009 and 2017 (Figure 1).2,3 This percentage is not significantly different from the prior period between 1982 and 2009. In addition, those ACHD patients who are candidates for heart transplantation are generally labeled as low-urgency status, have longer waiting list times, and are much less likely to have been bridged with mechanical circulatory support.4
Figure 1.
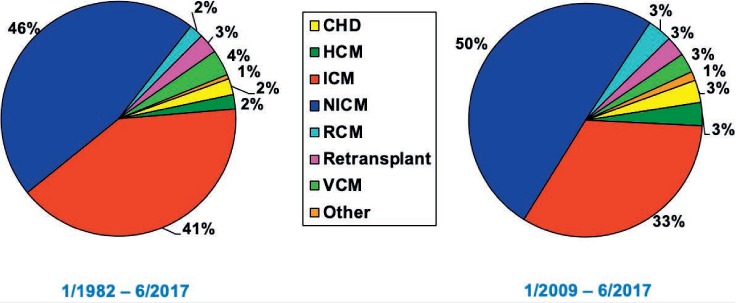
Adult heart transplants by diagnosis.2,3 CHD: congenital heart disease; HCM: hypertrophic cardiomyopathy; ICM: ischemic cardiomyopathy; NICM: nonischemic cardiomyopathy; RCM: restrictive cardiomyopathy; VCM: valvular cardiomyopathy
INDICATIONS
One of the first challenges presented by ACHD patients is how best to characterize the nature of their heart failure since the very definition of heart failure in this context is rather ill-defined. Every patient who underwent CHD repair or palliation in infancy or childhood can develop late myocardial dysfunction with elements of both systolic and diastolic failure. The mechanism of heart failure is often multifactorial5,6 and can result from (1) intrinsic myocardial dysfunction related to chronic cyanosis, (2) volume overload from residual intracardiac shunts and/or valvular regurgitation, (3) pressure overload from obstructive lesions, (4) pulmonary or systemic arterial hypertension, or (5) poorly controlled arrhythmias. In ACHD, certain diagnoses in particular are prone to the development of late ventricular dysfunction. For example, diagnoses that include the presence of a systemic right ventricle, congenitally corrected transposition, and other forms of univentricular hearts are most likely to be the cause of late cardiac dysfunction in ACHD.
Despite the heterogenous presentation of those with ACHD, the matrix used to determine when heart transplant therapy should be considered is often very similar to other adult patients. One main criterion is symptomatic ventricular or biventricular dysfunction resulting in multiple hospitalizations for congestive failure or low cardiac output despite being on maximal medical therapy. This applies to patients with the equivalent of New York Heart Association class III or IV with a major functional impairment, demonstrated by a VO2max < 12 mL/kg/min or a 6-minute walk test < 50% of predicted.7
CONTRAINDICATIONS
Many of the contraindications for heart transplantation in patients with ACHD are similar to those for other adult patients. Some are absolute, such as pulmonary vascular resistance > 5 Wood units (not responsive to vasodilator therapy), cancer, chronic infection, addiction, and psychiatric or behavioral problems that are likely to result in noncompliance. Other contraindications are relative and often program specific, such as obesity (body mass index > 30 kg/m2), chronic lung disease, chronic renal insufficiency with glomerular filtration rate < 30 mL/min, sensitization with a panel reactive antibody > 25%, and active smoking.7
TRANSPLANT EVALUATION
The goal of a pretransplant evaluation is very similar to that of other adult patients—that is, to verify the indication for transplantation and carefully rule out any possible contraindications. It typically includes a detailed review of cardiac catheterization data, cardiac angiography, or other forms of imaging such as computerized tomography or magnetic resonance imaging to evaluate individual anatomy and determine if additional procedures may be required during transplantation. The workup also includes a review of blood tests to assess end-organ function and an evaluation by a nutritionist, psychologists, and social workers. All of this information should be reviewed by a multidisciplinary team well versed in assessing risk factors in ACHD patients with the goal of being as complete and objective as possible.5 A recent review of the UNOS database by Menachem et al.8 found that the 30-day and 1-year post-transplant survival of ACHD patients at low-volume centers (< 6 ACHD transplants) was significantly lower compared to medium- and high-volume centers (6–20 ACHD transplants and > 20, respectively). This volume-related outcome is believed to be partly attributed to a dedicated and consistent multidisciplinary pre- and perioperative management team.
WAIT-LIST MANAGEMENT
Most people with ACHD have historically received low-priority status on the heart transplant waiting list compared to adults with heart failure related to other causes. This put ACHD patients at a disadvantage for a number of reasons. Mainly, priority within that status was based primarily on cumulative wait time, and most ACHD patients would have needed to be upgraded in status to have a realistic chance of being transplanted. Also, transplant candidates without ACHD had the advantage of being considered for ventricular assist devices or the routine use of Swanz-Ganz catheters, which provided the rationale for then upgrading to a higher status. These interventions are used less frequently in ACHD patients because of anatomic constraints or the finding that their condition is unlikely to benefit from hemodynamic monitoring.9
For ACHD patients with potentially reversible comorbidities such as renal failure or pharmacologically reversible pulmonary hypertension, the use of mechanical circulatory support (MCS) devices has been slow to be adopted.4 The belief has been that MCS is a risk factor for wait-list mortality in ACHD patients. VanderPluym et al. evaluated the outcomes of MCS use in ACHD versus non-ACHD patients from the International Registry of Mechanically Assisted Circulation (INTERMACS) and revealed several important findings.10 First, 21% of the ACHD patients were successfully bridged to transplant, 70% achieved a positive outcome over 6 months, and more than 50% remained alive on the device. Second, mortality in the ACHD group occurred predominantly in those receiving biventricular support or a total artificial heart; however, these patients were more often INTERMACS class 1 and 2, which implies that they were high-risk candidates for device implantation with more end-organ dysfunction. This report illustrates that with careful patient selection, the use of MCS in ACHD can result in a positive outcome particularly if used early before the patient's condition progresses (ie, INTERMACS 1).
The new adult heart allocation rules from the United Network for Organ Sharing (UNOS) were implemented in October 2018. This allocation system now has six statuses that stratify adult heart transplant candidates according to waiting list mortality. Other than a few ACHD patients with ventricular assist devices or a total artificial heart that boosts their priority, most ACHD patients will be assigned status 4, with 1 being the highest priority. Acknowledging the heterogeneity of ACHD candidates and the possibility of the new system putting them at a disadvantage due to a higher waiting list mortality, UNOS also created an exception request and review process to allow ACHD candidates to apply at any status that reflects their medical urgency.11,12
TRANSPLANT CONSIDERATIONS SPECIFIC TO ACHD
It is mission critical for the transplant surgeon to understand the specific needs of patients with ACHD, for these patients present a unique set of challenges that must be well understood, anticipated, and planned for in a deliberate manner to optimize post-transplant outcome and minimize morbidity. Planning should include a thorough review of the patient's surgical history and current anatomy to anticipate what additional surgical procedures will be needed at the time of implantation. Details about the systemic venous connections, pulmonary arteries, distal aortic arch, aortopulmonary collateral burden, patency of femoral vessels, and presence of intracardiac devices such as stents or occluders must be carefully assessed. This is particularly important for Fontan patients with palliated, univentricular circulations. This can often be accomplished using cardiac catheterization, computed tomography, or magnetic resonance angiography. The transplanting surgeon must anticipate the time needed for sternal reentry and mediastinal dissection as well as the need for additional reconstruction that may be required before implanting a structurally normal donor heart while trying to minimize the ischemic time.
OUTCOMES
Analyses have consistently demonstrated that perioperative mortality and morbidity are higher in ACHD-related transplants than in patients transplanted for other causes. Doumouras et al. recently performed a systematic review and meta-analysis of post-transplant outcomes in patients with ACHD versus those without ACHD and found that 30-day mortality was significantly higher in ACHD recipients than in non-ACHD recipients (17.4% vs 7.4%, respectively).13 However, at 10 years, there was a statistically significant mortality difference favoring ACHD patients (40.7% vs 49%). This finding was also highlighted in the 2018 ISHLT registry data (Figure 2), which showed a median survival of 15 years for patients with ACHD, 12 years for cardiomyopathy, and 9.7 years for coronary artery disease (CAD).2,3
Figure 2.
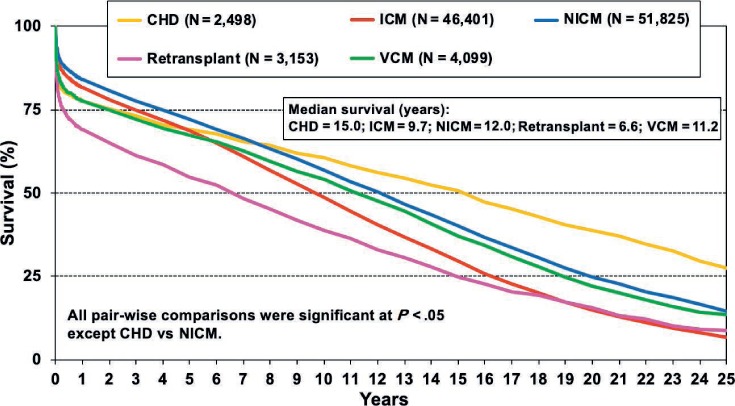
Adult heart transplant survival based on Kaplan-Meier Survival by Diagnosis (1982–2016).2,3 CHD: congenital heart disease; ICM: ischemic cardiomyopathy; NICM: nonischemic cardiomyopathy; RCM: restrictive cardiomyopathy; VCM: valvular cardiomyopathy
In the meta-analysis by Doumouras, the palliated single ventricle population (ie, Fontan/Glenn) represented a specifically high-risk population with increased early mortality after transplantation. As expected, the increased mortality was attributed to primary graft failure with longer ischemic times, stroke, and hemorrhage. This outcome is likely related to the complexity of the reconstruction at the time of implant, the higher incidence of right ventricular dysfunction of the graft due to elevated pulmonary vascular resistance, more bleeding, and lower systemic vascular resistance from comorbid liver disease.
While overall post-transplant death secondary to primary graft failure, stroke, and hemorrhage was higher in ACHD recipients, death caused by CAD, malignancy, infection, and rejection was lower than in non-CHD adults—likely reflecting the combined effects of younger age and absence of risk factors for coronary disease.6
SUMMARY
There has been universal reluctance to routinely consider transplantation in patients with ACHD due to a combination of factors, including an expanding adult heart transplant waiting list, limited donor availability, and significantly poorer early outcomes in ACHD recipients compared to non-ACHD recipients. Going forward, it is clear that the real challenge is to identify those ACHD patients who will benefit from transplantation versus those who are at high risk. ACHD encompasses a broad spectrum of disease, and risk stratification requires an ongoing effort at more nuanced and granular analysis. The new UNOS adult allocation rules will likely have an impact on waiting list management and mortality and on post-transplant outcomes in ACHD patients, and this impact will need to be carefully analyzed. However, with deliberate and evidence-driven patient selection coupled with carefully selected use of mechanical support and earlier patient referral, it reasonable to anticipate that transplant outcomes will continue to improve for adults with CHD.
KEY POINTS
Heart transplantation in patients with adult congenital heart disease (ACHD) is a complicated, high-risk undertaking characterized by early morbidity and mortality but better long-term survival than patients undergoing heart transplantation without CHD.
Waiting list and post-transplant management of ACHD requires the involvement of a dedicated, multidisciplinary clinical team.
Surgeons performing heart transplants in patients with ACHD should have deep knowledge of congenital heart defects and must be equipped to assess the reconstructive and technical needs of individual patients.
Footnotes
Conflict of Interest Disclosure: The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Diller GP, Kempny A, Alonso-Gonzalez R et al. Survival Prospects and Circumstances of Death in Contemporary Adult Congenital Heart Disease Patients Under Follow-Up at a Large Tertiary Centre. Circulation. 2015 Dec 1;132(22):2118–25. doi: 10.1161/CIRCULATIONAHA.115.017202. [DOI] [PubMed] [Google Scholar]
- 2.Khush KK, Cherikh WS, Chambers DC et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multi-organ Transplantation. J Heart Lung Transplant. 2018 Oct;37(10):1155–68. doi: 10.1016/j.healun.2018.07.022. International Society for Heart and Lung Transplantation. [DOI] [PubMed] [Google Scholar]
- 3.The International Society for Heart & Lung Transplantation [Internet] Addison, TX: International Society for Heart & Lung Transplantation; c2019. International Thoracic Organ Transplant (TTX) Registry Data Slides. Adult Heart Transplantation Statistics; 2018 Oct [cited 2019 Mar 14]. Available from: https://ishltregistries.org/registries/slides.asp. [Google Scholar]
- 4.Ross HJ, Law Y, Book WM et al. Transplantation and mechanical circulatory support in congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2016 Feb 23;133(8):802–20. doi: 10.1161/CIR.0000000000000353. American Heart Association Adults With Congenital Heart Disease Committee of the Council on Clinical Cardiology and Council on Cardiovascular Disease in the Young, the Council on Cardiovascular Radiology and Intervention, and the Council on Functional Genomics and Translational Biology. [DOI] [PubMed] [Google Scholar]
- 5.Bryant R, 3rd, Morales D. Overview of adult congenital heart transplants. Ann Cardiothorac Surg. 2018 Jan;7(1):143–151. doi: 10.21037/acs.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canter CE. Fitting Heart Transplantation to Adults with Congenital Heart Disease: Square Peg in a Round Hole? J Am Coll Cardiol. 2016 Aug 30;68(9):918–20. doi: 10.1016/j.jacc.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Houyel L, To-Dumortier NT, Lepers Y et al. Heart Transplantation in adults with congenital heart disease. Arch Cardiovasc Dis. 2017 May;110(5):346–353. doi: 10.1016/j.acvd.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Menachem JN, Lindenfeld J, Sclendorf K et al. Center volume and post-transplant survival among adults with congenital heart disease. J Heart Lung Transplantation. 2018 Nov;37(11):1351–60. doi: 10.1016/j.healun.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Alshawabkeh LI, Hu N, Carter KD et al. Wait-List Outcomes for Adults With Congenital Heart Disease Listed for Heart Transplantation in the U.S. J Am Coll Cardiol. 2016 Aug 30;68(9):908–17. doi: 10.1016/j.jacc.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 10.VanderPluym CJ, Cedars A, Eghtesady P et al. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: An analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) J Heart Lung Transplant. 2018 Jan;37(1):89–99. doi: 10.1016/j.healun.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Stefanescu Schmidt AC, Opotowsky AR. Obstacles to improving outcomes of heart transplantation for adults with congenital heart disease. J Heart Lung Transplant. 2019 Jan;38(1):107–109. doi: 10.1016/j.healun.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Organ Procurement and Transplantation Network [Internet] Rockville, MD: Health Resources and Services Administration, U.S. Department of Health & Human Services.; c2019. Policy 6: Allocation of Hearts and Heart-Lungs; 2018 Jan 17 [cited 2019 Feb 21]. Available at: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. [Google Scholar]
- 13.Doumouras BS, Alba AC, Foroutan F, Burchill LJ, Dipchand AI, Ross HJ. Outcomes in adult congenital heart disease patients undergoing heart transplantation: A systematic review and meta-analysis. J Heart Lung Transplant. 2016 Nov;35(11):1337–1347. doi: 10.1016/j.healun.2016.06.003. [DOI] [PubMed] [Google Scholar]


