Abstract
Right ventricular outflow tract (RVOT) dysfunction is common following surgical repair of tetralogy of Fallot and other forms of complex congenital heart disease. This results in pulmonary stenosis or regurgitation and may ultimately lead to RV failure and dysrhythmias. Transcatheter valve technologies are now available to treat certain patients with RVOT dysfunction. Current devices include the Medtronic Melody valve and the Edwards Lifesciences SAPIEN XT. Although these valves are approved for use in dysfunctional circumferential RVOT conduits, they are increasingly being used off label for nonconduit outflow tracts. Procedural complications include but are not limited to conduit rupture and coronary compression. Longer-term complications include stent fracture and endocarditis. Outcomes with these valves have demonstrated durable relief of stenosis and regurgitation. The Medtronic Harmony valve and the Alterra Prestent from Edwards Lifesciences are investigational devices that are intended to treat the patulous RVOT that is too large to accommodate currently available valves. This review will focus on current indications to treat RVOT dysfunction, existing transcatheter valve technologies, and investigational devices undergoing clinical trials. Hopefully, within the not-too-distant future, transcatheter pulmonary valve implantation will be feasible in the vast majority of patients with RVOT dysfunction following surgical repair of congenital heart disease.
Keywords: right ventricular outflow tract dysfunction, RVOT, tetralogy of Fallot, congenital heart disease, ventricular septal defect
INTRODUCTION
According to the Baltimore-Washington Infant Study, tetralogy of Fallot (TOF) is the most common form of cyanotic congenital heart disease and accounts for 6.7% of all live babies born with congenital heart disease.1 Tetralogy of Fallot comprises four heart defects: a large ventricular septal defect (VSD), overriding aorta, right ventricular (RV) hypertrophy, and RV outflow tract obstruction (RVOTO). This latter abnormality determines the clinical course of the patient; for example, patients with minimal obstruction will have physiological signs, such as a VSD with a net left-to-right shunt and mild to no cyanosis (referred to as a “pink tet”), whereas those with severe obstruction will be severely cyanotic and require early intervention. Approximately 80% of patients with TOF will have degrees of pulmonary stenosis, and 20% will have pulmonary atresia.1
Patients with TOF typically undergo VSD closure and relief of the RVOTO within the first 6 months of life. The type of surgery to relieve the RVOTO will vary depending on the patient's individual anatomy: Patients with a relatively normal pulmonary valve annulus may undergo RVOT muscle bundle resection and an annular sparing approach, while those with severe pulmonary annular hypoplasia and infundibular stenosis will undergo a transannular patch (TAP) and muscle bundle resection. The patch is often carried out onto the branch pulmonary arteries as well. Patients with TOF/pulmonary atresia or those with an anomalous coronary artery crossing the RVOT may require placement of an RV-to-pulmonary-artery conduit.
As a result of the RVOT surgery, the patient is left with progressive RVOT dysfunction. RVOT dysfunction is manifested by severe pulmonary insufficiency that results in a volume-loaded RV (typically seen in patients with a TAP), progressive pulmonary stenosis that results in a pressure-loaded RV (seen in patients with an RVOT conduit), or mixed disease with both pulmonary insufficiency and stenosis. This leads to progressive RV dilation and/or hypertrophy and ultimately may lead to RV failure. Treatment for RVOT dysfunction has historically required surgical placement of a new conduit or bioprosthetic valve, both of which have a finite lifespan.2,3 As a result, patients are subjected to repeated conduit or valve replacement surgeries, each with the attendant risk of morbidity or mortality. In order to overcome the need for repeat surgeries, Bonhoeffer et al. developed a percutaneously implantable pulmonary valve, and in 2000, they ushered in the era of transcatheter valve implantation with the first human implant of a transcatheter pulmonary valve in Paris, France.4 This valve ultimately became the Melody valve (Medtronic). Since then, other transcatheter pulmonary valves have been developed.
This article will focus specifically on currently available valve technology and its application to patients with dysfunctional RVOTs—not only those with TOF but also those who require an RV-PA conduit for any type of congenital heart disease, such as a double outlet RV or truncus arteriosus, or patients undergoing a Ross procedure. In addition, we discuss indications for percutaneous pulmonary valve implantation, potential adverse events associated with the procedure or follow-up, and future developments in valve technology.
INDICATIONS FOR INTERVENTION
Optimal timing for percutaneous pulmonary valve implantation (PPVI) in patients with RVOT dysfunction remains challenging. This is well stated in recent review articles, and the reader is referred to them for a comprehensive discussion.5–7 The American Heart Association/American College of Cardiology,8 Canadian Cardiovascular Society,9 and European Society of Cardiology10 have guidelines to address this issue, each with minor variations. Pulmonary valve replacement (PVR) is generally recommended for all patients with RVOT dysfunction (> moderate pulmonary regurgitation [PR] and/or RV systolic pressure > 2/3 systemic) and symptoms attributable to this dysfunction. A variety of thresholds have been proposed for indexed RV end diastolic (RVEDVi) and end systolic dimensions (RVESVi) as well as other parameters of RV and LV function to determine when to intervene on asymptomatic patients.11–15 These indications are not nearly as clear and have been evolving as experience with PPVI and knowledge about the fate of the RV following PPVI increases. There is the need to balance an overly late PPVI intervention—when the RV dysfunction is too advanced and the RV won't recover—versus intervening too early and risking unintended consequences such as heart failure or arrhythmias.14 I consider PPVI if any of these criteria are met: (1) RVEDVi > 150 mL/m2, (2) RVESVi > 82 mL/m2, (3) significant RV dysfunction (RV ejection fraction < 45%), (4) progressive tricuspid regurgitation in the setting of a dilated RV, or (5) sustained arrhythmias with a dilated RV.
CURRENTLY AVAILABLE VALVES
Melody Valve
The Melody valve (Medtronic) is approved by the US Food and Drug Administration (FDA) for use in patients with a clinical indication for intervention and a dysfunctional RVOT conduit or bioprosthetic valve with ≥ moderate PR and/or a mean RVOT gradient > 35 mm Hg. The Melody valve (Figure 1) is a transcatheter pulmonary valve (TPV) consisting of a bovine jugular vein sutured inside of a platinum iridium stent. There are currently two available valve sizes: the TPV 20 and the TPV 22. The TPV 20 uses a 16-mm bovine jugular vein and is intended for implantation at no more than 20-mm diameter, whereas the TPV 22 is an 18-mm bovine jugular vein intended for implantation at sizes up to 22-mm diameter. The unexpanded valve height is 30 mm for the TPV 20 and 28 mm for the TPV 22. Both valves are deployed using the Medtronic Ensemble delivery system (Figure 2), a 22F delivery system using balloon-in-balloon technology. The Ensemble is available in three sizes: 18 mm, 20 mm, and 22 mm.
Figure 1.
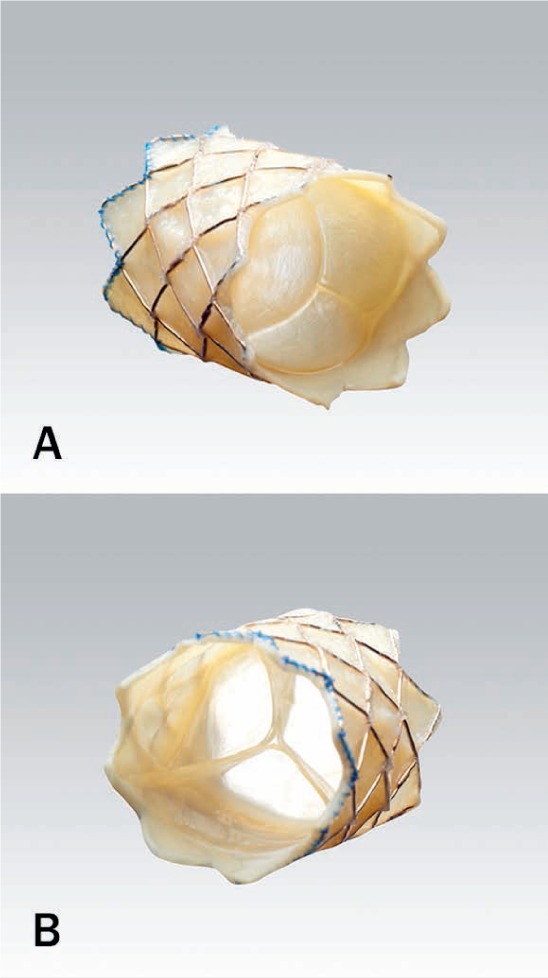
(A) View of Melody valve (Medtronic) from inflow (towards right ventricle) portion of valve. (B) View of valve from outflow (pulmonary) portion demonstrating natural venous valve with thin valve leaflets.
Figure 2.
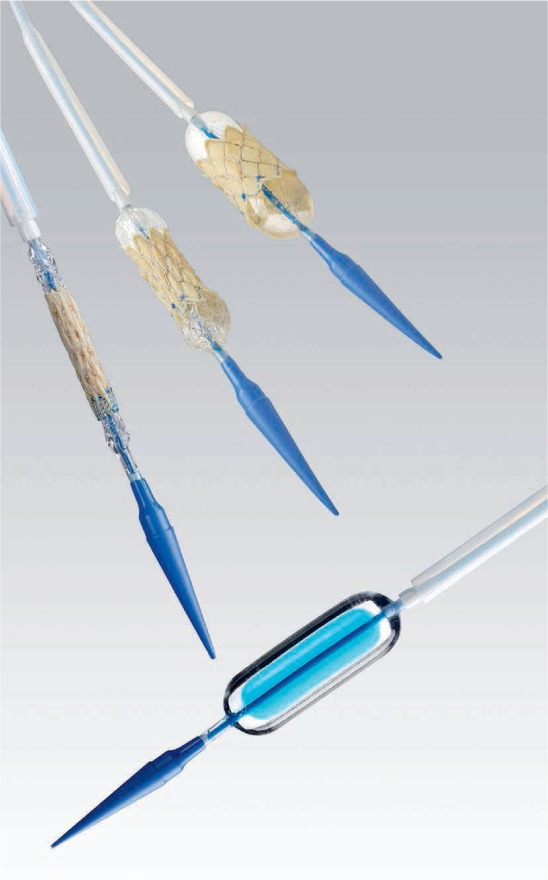
Ensemble delivery system with crimped Melody valve (Medtronic).
The Melody valve received the European CE mark in September 2006, and the first United States implant was performed in January 2007. The FDA granted the Melody valve approval under humanitarian device exemption guidelines for implantation in circumferential conduits in 2010 and full premarket approval in January 2015; FDA approval for implantation in failed bioprosthetic valves was received in 2017. To date, more than 13,000 patients worldwide have received a Melody valve.
This valve has a robust prospective data set, including the US investigational device exemption study,16,17 the US Postapproval Study,18 and the European and Canadian postmarket surveillance study.19 A total of 313 patients were enrolled in these three studies and followed up to 8 years. Procedural success rates were between 89% and 95%, with success defined as valve implantation at the intended site, RV-PA peak-to-peak gradient < 35 mm Hg post implant, < mild PR, and free of explant at 24 hours post implant. Mean RVOT gradients decreased from 32 to 38 mm Hg baseline to 15 to 19 mm Hg at 1 year, and this result was maintained out to 5 years of follow-up. In addition to sustained improvements in relief of RVOTO, there were significant improvements in the degree of PR. The majority of patients in all three studies had baseline moderate or severe PR, which decreased immediately to none or mild. This was maintained out to 5 years follow-up.19
Cheatham et al. reported outcomes of the US Investigational Device Exemption trial out to 7 years after PPVI in 150 patients who received a Melody valve implant.20 The authors showed that primary valve failure is rare, and TPV dysfunction is primarily manifested by stent fracture, loss of structural integrity, and recurrent stenosis. They also documented freedom from Melody valve reintervention of 76% at 5 years follow-up. Patients who did not receive a prestent—bare metal stent(s) implanted prior to valve implantation—had a preimplantation gradient > 35 mm Hg, and patients with a discharge gradient > 20 mm Hg had the shortest freedom from reintervention (HR 3.8; CI 1.7–8.7). Freedom from Melody valve explantation was 92% at 5 years.
Sapien Valve
The SAPIEN XT (Edwards Lifesciences) (Figure 3) was approved by the FDA in March 2016 for use in dysfunctional RVOT conduits using the same criteria as noted for the Melody valve. The SAPIEN XT consists of a trileaflet bovine pericardial valve inside a cobalt chromium frame and was originally designed for use in the aortic position, as was the Edwards Lifesciences Novaflex delivery system (Figure 4). Compared to the Melody valve, the SAPIEN XT is short, with valve heights varying between 14.3 mm for the 23-mm valve to 19.1 mm for the 29-mm valve.
Figure 3.
SAPIEN XT valve (Edwards Lifesciences).
Figure 4.
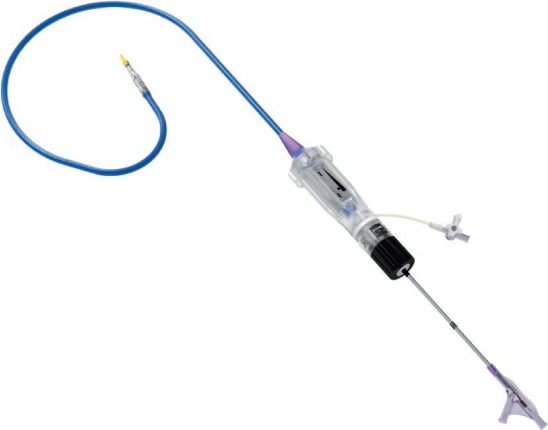
Novaflex delivery system (Edwards Lifesciences).
The SAPIEN S3 (Figure 5) is the latest generation of SAPIEN valve, and while it has not yet received FDA approval for implantation in the pulmonary position, it is currently undergoing a clinical trial. 21 There is also increasing experience with off-label use of this valve in centers outside of the clinical trial. Like the XT, the S3 is a trileaflet bovine pericardial valve sewn into a cobalt-chromium stent. However, the S3 has an additional polyethylene terephthalate skirt that is intended to decrease the incidence of paravalvular leaks in the aortic position. This valve is available in 20-, 23-, 26-, and 29-mm diameters and can be introduced through low-profile 14F and 16F expandable sheaths. The valve is delivered using the Commander delivery system, which has a lower profile and is less rigid than the Novaflex delivery system. The S3 has a slightly taller valve height than the XT, varying between 15.5 mm for the 20-mm valve up to 22.5 mm for the 29-mm valve.
Figure 5.
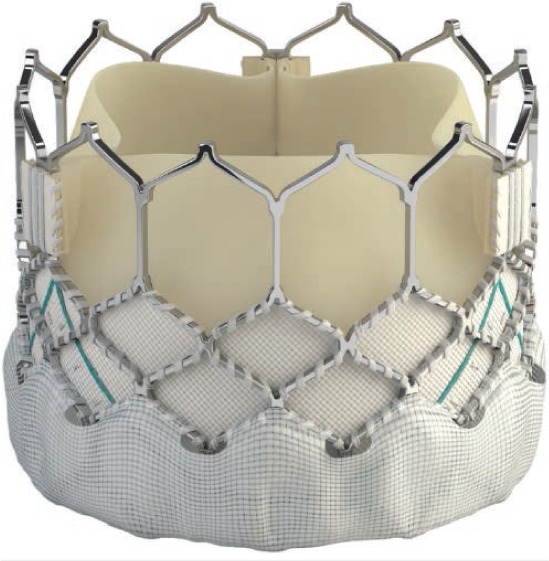
Sapien S3 valve (Edwards Lifesciences).
There is significantly less data available for the SAPIEN valves compared with the Melody valve, although early results are encouraging.22–24 The Congenital Multicenter Trial of Pulmonic Valve Regurgitation Studying the SAPIEN Interventional THV (COMPASSION) was a prospective, nonrandomized, multicenter trial that used the SAPIEN THV to treat dysfunctional RVOT conduits; 91% of patients received a prestent. The overall device success rate was 95.2%, and freedom from reintervention was 97.1% and 93.7% at 1 and 3 years, respectively. Freedom from major adverse cardiovascular and cerebrovascular events at 3 years was 87.5%. Similar to the Melody valve, the SAPIEN valve's peak stenosis gradient decreased from 37.5 mm Hg at baseline to 18.7 mm Hg at 1 month, and this was maintained out to 3 yrs with a peak gradient of 17.8 mm Hg. Likewise, the percent of patients with moderate or severe PR decreased dramatically from 89.9% at baseline to 8.8% at 3 years.23
Results of a French registry confirm the excellent early outcomes of the COMPASSION trial. The majority of patients (98.6%) also underwent prestenting, and the valve was successfully delivered in 95.8% of patients. The peak-to-peak systolic pressure gradient across the RVOT decreased from 34.5 mm Hg at baseline to 10.5 mm Hg following the procedure. Moderate or worse PR was present in 49% of patients prior to valve implantation and decreased to 0% immediately after the procedure and at 1 month.24
COMPLICATIONS
Stent Fracture
Stent fractures of the valve frame are a recognized complication of Melody valve implantation (Figure 6) and may lead to loss of structural integrity and recurrent obstruction requiring reintervention. To my knowledge, a stent fracture has never been reported with the SAPIEN valves, although the majority of patients in both the COMPASSION trial and the French registry received a prestent. Cabalka et al. analyzed data from the prospective North American and European Melody valve trials and reported stent fractures occurring in 81 of 251 (32%) implanted patients after a median follow-up of 5 years. Importantly, this study demonstrated that prestenting decreased the risk of stent fracture and that this risk was further reduced by the placement of multiple prestents. In this study, 53% of patients received multiple prestents and only 7% experienced a subsequent stent fracture, with 2 of these requiring reintervention.25 It has therefore become standard procedure when placing a Melody valve to prestent until there is no or minimal (< 1 mm) recoil of the stent.
Figure 6.
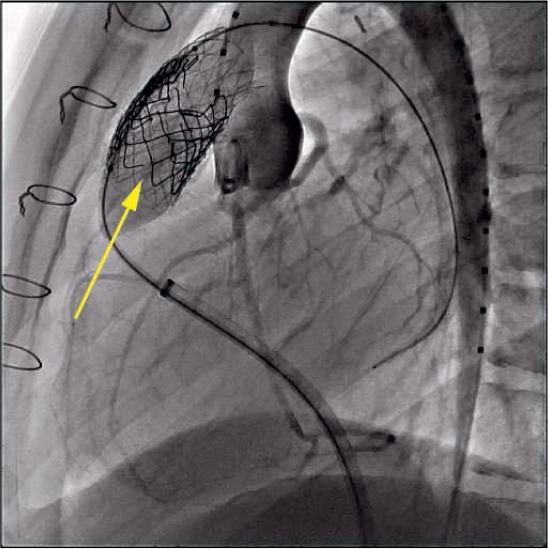
Multiple Melody valve (Medtronic) stent fractures (arrow) demonstrated during balloon dilation of right ventricular outflow tract and aortography for coronary assessment.
Conduit Rupture
Conduit rupture (Figures 7, 8) is a relatively common adverse event associated with rehabilitation of obstructed conduits and occurs in 19.5% to 22% of patients undergoing angioplasty.26,27 Fortunately, the majority of these ruptures are not clinically significant and do not lead to hemodynamic instability. There is, however, the potential for a life-threatening rupture, and the implanter must therefore be prepared to deal with this on an emergent basis by placing covered stents or referring for immediate surgery. The Pulmonary Artery Repair With Covered Stent trial was a prospective multicenter trial assessing the safety and efficacy of using the Covered CP Stent (CCPS; NuMED, Inc) to treat conduit injury in patients undergoing intended Melody valve implantation. This study demonstrated that conduit tears occurred in 19.5% of patients overall, with potentially life-threatening conduit tears in 1% of patients overall. Risk factors for conduit tears included smaller mean conduit diameter at implant (20 vs 22 mm), smaller angiographic conduit diameter before intervention (10.5 vs 14.6 mm), larger ratio of balloon diameter at time of injury to minimum angiographic diameter prior to any intervention (1.89 vs 1.57), smaller minimum angiographic diameter/implant diameter (0.54 vs 0.68), and higher baseline peak RVOT gradient (64.6 vs 57.6 mm Hg). The CCPS was effective in treating 95% of conduit tears. Of the six patients with severe conduit tears, four were successfully treated with the CCPS.26
Figure 7.
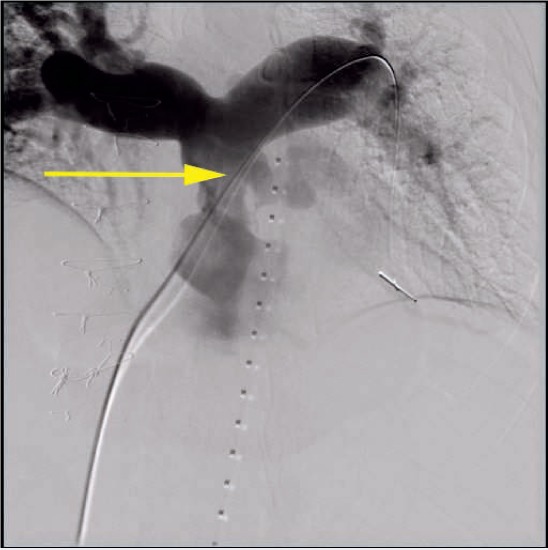
Digital subtraction angiogram of a large conduit rupture just inferior to the origin of the left pulmonary artery.
Figure 8.
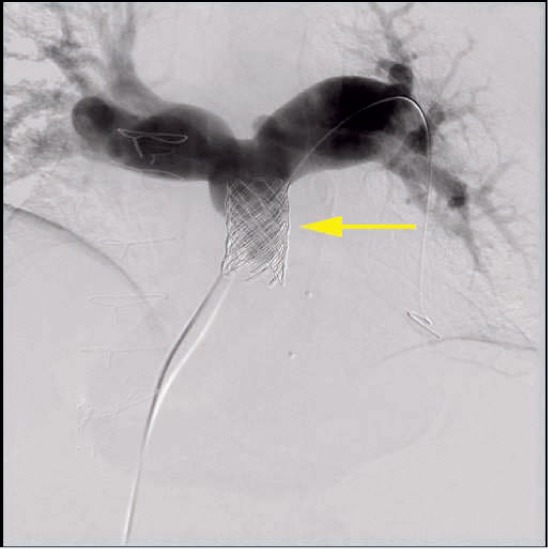
Digital subtraction angiogram showing sealed conduit tear following placement of two covered stents and a Melody valve (Medtronic).
Coronary Compression
Coronary compression is a potential complication preventing PPVI in approximately 5% of patients (Figure 9).28 This risk is present even for PPVI within bioprosthetic valves and therefore should always be assessed during the procedure.28,29 This is accomplished by simultaneously performing balloon angioplasty on the conduit (at the intended size of PPVI) while also assessing the coronary arteries with either ascending aortography or selective coronary angiography. In a study by Morray et al., there were 34 patients (71%) with TOF and abnormal coronary artery anatomy, 7 of whom experienced coronary compression upon testing.28
Figure 9.
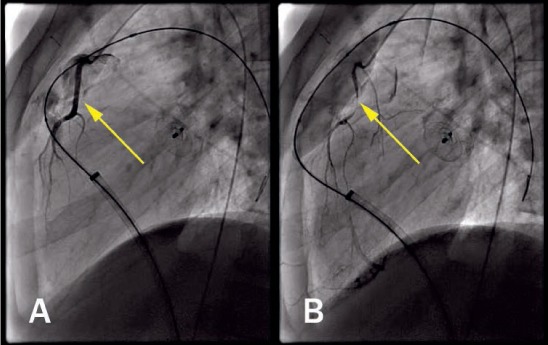
Lateral view of selective coronary injection (A) before and (B) after balloon inflation in right ventricular outflow tract demonstrating coronary compression (arrow).
Endocarditis
Endocarditis of implanted pulmonary valves has been reported for every type of bioprosthetic valve regardless of whether they are implanted surgically or percutaneously (Figure 10).30 Endocarditis is a temporally related phenomenon, so the longer that patients with a PPVI are followed, the higher the likelihood that an episode of endocarditis will be diagnosed. The annualized rate for endocarditis at any site in patients with a Melody valve is 2.4% per patient-year and 0.88% per patient-year for TPV-related endocarditis.31 More recently, McElhinney reviewed 76 unique cases of PPVI endocarditis that were reported through December 2016. The patients were predominantly male with a median time of 16 months from valve implant to endocarditis. A significant number of patients (37%) were diagnosed > 2 years from PPVI. Thirty-four percent of patients presented with severe RVOTO, whereas only 2.6% presented with significant PR. All patients received antibiotic therapy except one who was diagnosed on autopsy, and 10 had RVOTO treated via transcatheter procedures (balloon valvuloplasty, placement of a second valve, or a bare metal stent). The Melody valve was explanted in 43% of patients within 3 months of diagnosis.32
Figure 10.
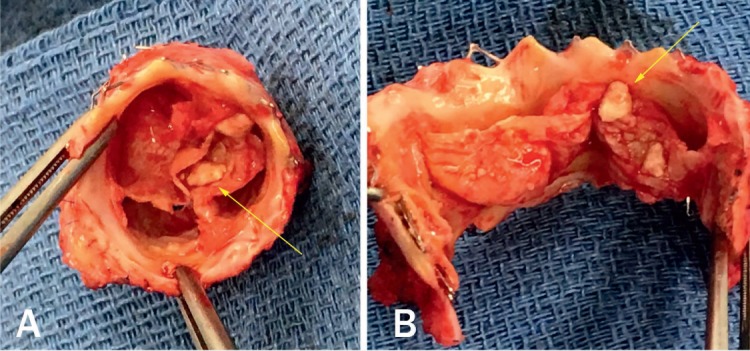
Explanted Melody valve (Medtronic) with thickened leaflets and vegetations (arrows). (A) Top view. (B) Open view.
Endocarditis has also been reported in patients receiving the SAPIEN valve. In the recently reported outcomes from the COMPASSION trial, the annualized rate of endocarditis was 1.04% per patient-year and 0.5% per patient-year for valve-related endocarditis, with freedom from endocarditis of 97.1% at up to 3 years of follow-up.23 Given that endocarditis is a time-related phenomenon, and that the Melody valve has much more extensive and long-term follow-up than the SAPIEN valve, it is premature to speculate whether one valve will have superiority over the other in terms of endocarditis risk. Ongoing vigilance and a high index of suspicion for endocarditis are paramount during the long-term follow-up of any patient receiving a PPVI.
FUTURE DIRECTIONS
PPVI is currently approved in the United States for implantation within circumferential conduits; however, the vast majority of patients with TOF have had surgical repairs with a transannular patch and not a conduit. These patients frequently have very large patulous outflow tracts, but it will nevertheless be feasible to treat some of them with currently available valve technology.33–36 The outer diameter of a 22-mm Melody valve deployed using a 22-mm delivery system will be slightly over 24 mm in diameter, allowing implantation in RVOTs up to approximately 22 mm to 23 mm in diameter. Alternatively, the 22-mm Melody valve may be deployed on a 24-mm balloon and placed in RVOTs up to approximately 25 mm in diameter.37 The largest available SAPIEN valve has an outer diameter of 29 mm, although it may be over-expanded to approximately 31 mm.38
The feasibility of this approach has been demonstrated in two recent reviews for the Melody and SAPIEN valves.35,36 Martin et al. reported a multi-institutional experience in which 229 patients were catheterized with the intention of placing a Melody valve in a nonconduit outflow tract. Valve implantation was successful in 58% of patients, and a prohibitively large RVOT was the primary reason for failure to implant. There was a 4% rate of stent embolization/malposition.36 Morgan et al. reported a multi-institutional experience using the SAPIEN S3 and XT valves without prestenting in 41 patients with native RVOTs. The study was not designed to assess the procedural success rates for patients with native RVOTs undergoing catheterization with the intention to implant a SAPIEN valve, but it does demonstrate the feasibility of this approach.35
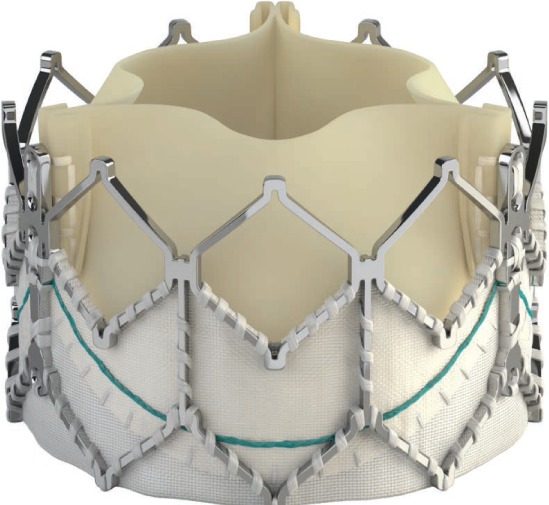
Despite the success of using currently available valve technology in patients with native outflow tracts, many patients will nonetheless be unsuitable candidates for this approach. To meet this need, the Medtronic Harmony TPV39 (Figure 11) and the Alterra Adaptive Prestent40 from Edwards Lifesciences (Figure 12) were developed and have undergone early clinical trials in the United States.
Figure 11.

Harmony valve (Medtronic) viewed from inflow portion.
Figure 12.
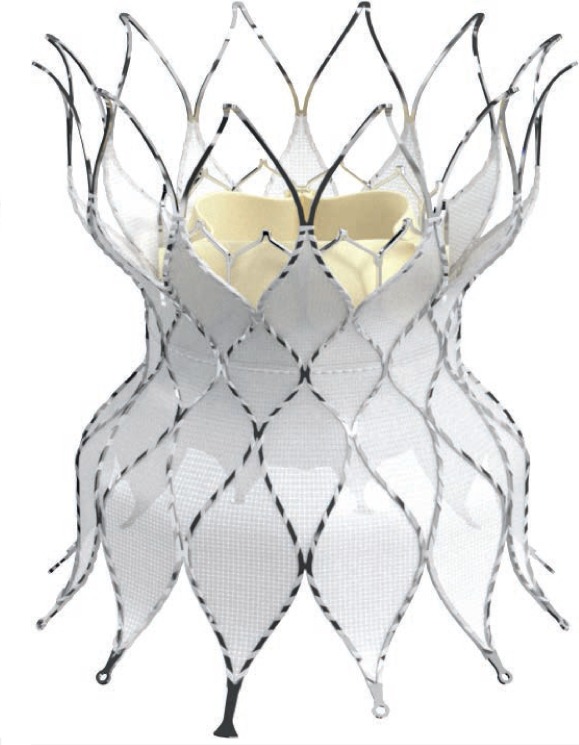
Alterra Adaptive Prestent (Edwards Lifesciences) side view. Open crowns will be oriented toward the pulmonary bifurcation.
The Harmony TPV is a porcine pericardial tissue valve mounted within a self-expanding covered nitinol frame. The device (TPV 22) originally came in only one size that was 55 mm long with an outflow/inflow diameters of 34 mm and 42 mm, respectively. This device underwent an early feasibility study and is currently in an ongoing clinical trial41 along with an additional device, the TPV 25. The TPV 25 is made of a larger-diameter valve and is shorter than the TPV 22, which should help to increase study enrollment. Both valves are delivered with a 25F delivery system. The 6-month outcomes of the early feasibility study were reported in 2017 by Bergersen et al., with 66 subjects enrolled in the study and 21 approved for implant. The main reason patients were not implanted was inappropriate anatomy. Twenty patients were implanted; in one patient, the device migrated distally within 24 hours and was explanted surgically. Another patient had the device explanted after the 1-month follow-up because of stent fracture with partial frame collapse. At 6 months follow-up, the device continued to function well in the remaining 18 patients, with no or trace PR in 94%, mild regurgitation in 6%, and a mean RVOT gradient of 15 + 6 mm Hg.39
The Alterra Adaptive Prestent is not a valve per se but rather is meant to function as a size reducer and docking station for a 29-mm SAPIEN S3 valve. The device consists of a self-expanding covered nitinol frame with designated inflow and outflow portions. It has a symmetric hourglass shape with inflow and outflow diameters of 40 mm and a central landing zone of 27-mm diameter. The total device length is 48 mm, but the covered portion is 30 mm long. The delivery system fits through a 16F eSheath (Edwards Life Sciences). The first human implant was recently reported, and the valve was functioning well 4 months after implant with trivial paradevice leak and a mean Doppler RVOT gradient of 5 mm Hg.40 This device recently underwent an early feasibility trial, but the 6-month outcomes have not been reported.42
CONCLUSION
RVOT dysfunction in patients with TOF is universal following surgical repair and manifests as either PR, stenosis, or both. The majority of patients have had a nonconduit (transannular) type of repair, but for those patients with either a conduit or a bioprosthetic valve, US-approved transcatheter options include the Melody valve and the SAPIEN XT. These valves have demonstrated very good outcomes—out to 7 years for the Melody valve and 3 years for the SAPIEN valve. However, these require vigilant monitoring for potential ongoing complications such as the development of endocarditis. To meet the needs of the majority of patients with nonconduit outflow tracts, off-label use of currently available valves is being performed, and newer devices such as the Harmony valve or the Alterra Adaptive Prestent are being developed. Hopefully, in the not-too-distant future, we will have multiple valve options available to treat the majority of patients with dysfunctional RVOTs following surgery for congenital heart disease.
KEY POINTS
Right ventricular outflow tract (RVOT) dysfunction is common following surgery for many forms of congenital heart disease and is manifest by pulmonary stenosis and/or regurgitation.
Currently available transcatheter valve technologies include the Melody valve and the SAPIEN XT and are approved for use in circumferential RVOTs. This is a relatively small percentage of patients (~ 20–25%) with RVOT dysfunction.
Off-label use of these valves for nonconduit RVOTs is feasible and increasing; however, there are still patients with an RVOT that is too large to accommodate currently existing valve technology.
Investigational devices including the Harmony valve and the Alterra Prestent (with SAPIEN S3) are in ongoing trials. The approval of these or similar devices should greatly increase the number of patients who can successfully be treated for RVOT dysfunction following repair of complex congenital heart disease.
Footnotes
Conflict of Interest Disclosure: Dr. Balzer serves as a proctor for Edwards Lifesciences Corp, Medtronic, and Abbott Medical.
REFERENCES
- 1.Perry LW, Neill CA, Ferencz C . Infants with congenital heart disease: the cases. Perspectives in pediatric cardiology. In: Ferencz C, Rubin JD, Loffredo CA, editors. Epidemiology of congenital heart disease, the Baltimore-Washington Infant Study 1981–1989. Armonk, NY: Futura; 1993. pp. 33–62. p. [Google Scholar]
- 2.Tweddell JS, Pelech AN, Frommelt PC et al. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation. 2000 Nov 7;102(19 Suppl 3):III, 130–5. doi: 10.1161/01.cir.102.suppl_3.iii-130. [DOI] [PubMed] [Google Scholar]
- 3.Batlivala SP, Emani S, Mayer JE, McElhinney DB. Pulmonary valve replacement function in adolescents: a comparison of bioprosthetic valves and homograft conduits. Ann Thorac Surg. 2012 Jun;93(6):2007–16. doi: 10.1016/j.athoracsur.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Bonhoeffer P, Boudjemline Y, Saliba Z et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000 Oct 21;356(9239):1403–5. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 5.de Torres-Alba F, Kaleschke G, Baumgartner H. Impact of Percutaneous Pulmonary Valve Implantation on the Timing of Reintervention for Right Ventricular Outflow Tract Dysfunction. Rev Esp Cardiol (Engl Ed) 2018 Oct;71(10):838–46. doi: 10.1016/j.rec.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Bhagra CJ, Hickey EJ, Van De Bruaene A, Roche SL, Horlick EM, Wald RM. Pulmonary Valve Procedures Late After Repair of Tetralogy of Fallot: Current Perspectives and Contemporary Approaches to Management. Can J Cardiol. 2017 Sep;33(9):1138–49. doi: 10.1016/j.cjca.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011 Jan 20;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warnes CA, Williams RG, Bashore TM et alDeveloped in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008 Dec 2;52(23):e143–263. doi: 10.1016/j.jacc.2008.10.001. . ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease) [DOI] [PubMed] [Google Scholar]
- 9.Silversides CK, Salehian O, Oechslin E et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: complex congenital cardiac lesions. Can J Cardiol. 2010 Mar;26(3):e98–117. doi: 10.1016/s0828-282x(10)70356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner H, Bonhoeffer P, De Groot NM et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010 Dec;31(23):2915–57. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 11.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005 Mar 15;95(6):779–82. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Oosterhof T, Van Straten A, Vliegen HW et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007 Jul 31;116(5):545–51. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Kim YM, Lee CH et al. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: implications for optimal timing of pulmonary valve replacement. J Am Coll Cardiol. 2012 Sep 11;60(11):1005–14. doi: 10.1016/j.jacc.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 14.Bokma JP, Winter MM, Oosterhof T et al. Preoperative thresholds for mid-to-late haemodynamic and clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Eur Heart J. 2016 Mar 7;37(10):829–35. doi: 10.1093/eurheartj/ehv550. [DOI] [PubMed] [Google Scholar]
- 15.Heng EL, Gatzoulis MA, Uebing A et al. Immediate and Midterm Cardiac Remodeling After Surgical Pulmonary Valve Replacement in Adults With Repaired Tetralogy of Fallot: A Prospective Cardiovascular Magnetic Resonance and Clinical Study. Circulation. 2017 Oct 31;136(18):1703–13. doi: 10.1161/CIRCULATIONAHA.117.027402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the U.S. clinical trial. J Am Coll Cardiol. 2009 Oct 27;54(18):1722–9. doi: 10.1016/j.jacc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 17.McElhinney DB, Hellenbrand WE, Zahn EM et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010 Aug 3;122(5):507–16. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong AK, Balzer DT, Cabalka AK et al. One-year follow-up of the Melody transcatheter pulmonary valve multicenter post-approval study. JACC Cardiovasc Interv. 2014 Nov;7(11):1254–62. doi: 10.1016/j.jcin.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov [Internet] Bethesda, MD: U.S. National Library of Medicine; c2008. Melody Transcatheter Pulmonary Valve (TPV) Post-Market Surveillance Study. 2015 Oct 29 [cited 2019 Feb 7]. Available at: https://clinicaltrials.gov/ct2/show/NCT00688571. [Google Scholar]
- 20.Cheatham JP, Hellenbrand WE, Zahn EM et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015 Jun 2;131(22):1960–70. doi: 10.1161/CIRCULATIONAHA.114.013588. [DOI] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov [Internet] Bethesda, MD: US National Library of Medicine; c2018. COMPASSION S3 - Evaluation of the SAPIEN 3 Transcatheter Heart Valve in Patients With Pulmonary Valve Dysfunction; 2018 Sep 6 [cited 2019 Feb 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT02744677. [Google Scholar]
- 22.Kenny D, Hijazi ZM, Kar S et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011 Nov 15;58(21):2248–56. doi: 10.1016/j.jacc.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Kenny D, Rhodes JF, Fleming GA et al. 3-Year Outcomes of the Edwards SAPIEN Transcatheter Heart Valve for Conduit Failure in the Pulmonary Position From the COMPASSION Multicenter Clinical Trial. JACC Cardiovasc Interv. 2018 Oct 8;11(19):1920–9. doi: 10.1016/j.jcin.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Plessis J, Hascoët S, Baruteau A et al. Edwards SAPIEN Transcatheter Pulmonary Valve Implantation: Results From a French Registry. JACC Cardiovasc Interv. 2018 Oct 8;11(19):1909–16. doi: 10.1016/j.jcin.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 25.Cabalka AK, Hellenbrand WE, Eicken A et al. Relationships Among Conduit Type, Pre-Stenting, and Outcomes in Patients Undergoing Transcatheter Pulmonary Valve Replacement in the Prospective North American and European Melody Valve Trials. JACC Cardiovasc Interv. 2017 Sep 11;10(17):1746–59. doi: 10.1016/j.jcin.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Delaney JW, Goldstein BH, Bishnoi R et al. Covered CP Stent for Treatment of Right Ventricular Conduit Injury During Melody Transcatheter Pulmonary Valve Replacement. Circ Cardiovasc Interv. 2018 Oct;11(10):e006598. doi: 10.1161/CIRCINTERVENTIONS.118.006598. PARCS Investigators. [DOI] [PubMed] [Google Scholar]
- 27.Hainstock MR, Marshall AC, Lock JE, McElhinney DB. Angioplasty of obstructed homograft conduits in the right ventricular outflow tract with ultra-noncompliant balloons: assessment of therapeutic efficacy and conduit tears. Circ Cardiovasc Interv. 2013 Dec;6(6):671–9. doi: 10.1161/CIRCINTERVENTIONS.112.000073. [DOI] [PubMed] [Google Scholar]
- 28.Morray BH, McElhinney DB, Cheatham JP et al. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013 Oct 1;6(5):535–42. doi: 10.1161/CIRCINTERVENTIONS.113.000202. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie MJ, Rome JJ, Levi DS et al. Melody valve implant within failed bio-prosthetic valves in the pulmonary position: a multicenter experience. Circ Cardiovasc Interv. 2012 Dec;5(6):862–70. doi: 10.1161/CIRCINTERVENTIONS.112.972216. [DOI] [PubMed] [Google Scholar]
- 30.Khambadkone S, Coats L, Taylor A et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005 Aug 23;112(8):1189–97. doi: 10.1161/CIRCULATIONAHA.104.523266. [DOI] [PubMed] [Google Scholar]
- 31.McElhinney DB, Benson LN, Eicken A et al. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circ Cardiovasc Interv. 2013 Jun;6(3):292–300. doi: 10.1161/CIRCINTERVENTIONS.112.000087. [DOI] [PubMed] [Google Scholar]
- 32.McElhinney DB. Reflection and Rationalization: Making Sense of the Literature on Endocarditis After Transcatheter Pulmonary Valve Replacement. Circ Cardiovasc Interv. 2017 Feb;10(2) doi: 10.1161/CIRCINTERVENTIONS.117.004983. [DOI] [PubMed] [Google Scholar]
- 33.Boshoff DE, Cools BL, Heying R et al. Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv. 2013 May;81(6):987–95. doi: 10.1002/ccd.24594. [DOI] [PubMed] [Google Scholar]
- 34.Malekzadeh-Milani S, Ladouceur M, Cohen S, Iserin L, Boudjemline Y. Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch Cardiovasc Dis. 2014 Nov;107(11):592–8. doi: 10.1016/j.acvd.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Morgan GJ, Sadeghi S, Salem M et al. SAPIEN valve for percutaneous transcatheter pulmonary valve replacement without “pre-stenting”: A multi-institutional experience. Catheter Cardiovasc Interv. 2019 Feb 1;93(2):324–9. doi: 10.1002/ccd.27932. [DOI] [PubMed] [Google Scholar]
- 36.Martin MH, Meadows J, McElhinney DB et al. Safety and Feasibility of Melody Transcatheter Pulmonary Valve Replacement in the Native Right Ventricular Outflow Tract: A Multicenter Pediatric Heart Network Scholar Study. JACC Cardiovasc Interv. 2018 Aug 27;11(16):1642–50. doi: 10.1016/j.jcin.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Cheatham SL, Holzer RJ, Chisolm JL, Cheatham JP. The Medtronic Melody® transcatheter pulmonary valve implanted at 24-mm diameter–it works. Catheter Cardiovasc Interv. 2013 Nov 1;82(5):816–23. doi: 10.1002/ccd.24821. [DOI] [PubMed] [Google Scholar]
- 38.Shivaraju A, Kodali S, Thilo C et al. Overexpansion of the SAPIEN 3 Transcatheter Heart Valve: A Feasibility Study. JACC Cardiovasc Interv. 2015 Dec 28;8(15):2041–3. doi: 10.1016/j.jcin.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Bergersen L, Benson LN, Gillespie MJ et al. Harmony Feasibility Trial: Acute and Short-Term Outcomes With a Self-Expanding Transcatheter Pulmonary Valve. JACC Cardiovasc Interv. 2017 Sep 11;10(17):1763–73. doi: 10.1016/j.jcin.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 40.Zahn EM, Chang JC, Armer D, Garg R. First human implant of the Alterra Adaptive PrestentTM: A new self-expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv. 2018 May 1;91(6):1125–9. doi: 10.1002/ccd.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov [Internet] Bethesda, MD: US National Library of Medicine; c2018. The Medtronic Harmony™ Transcatheter Pulmonary Valve Clinical Study; 2018 Dec 21 [cited 2019 Feb 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT02979587. [Google Scholar]
- 42.ClinicalTrials.gov [Internet] Bethesda, MD: US National Library of Medicine; c2018. Multicenter Trial of Congenital Pulmonic Valve Dysfunction Studying the SAPIEN 3 THV With the Alterra Adaptive Prestent (ALTERRA); 2018 Oct 8 [cited 2019 Feb 15]. Available from: https://clinicaltrials.gov/ct2/show/NCT03130777. [Google Scholar]


