Abstract
Background
More than 20% of tuberculosis (TB) disease worldwide may be attributable to smoking and alcohol abuse. India is the second largest consumer of tobacco products, a major consumer of alcohol particularly among males, and has the highest burden of TB globally. The impact of increasing tobacco dose, relevance of alcohol misuse and past versus current or never smoking status on TB treatment outcomes remain inadequately defined.
Methods
We conducted a multi-centric prospective cohort study of newly diagnosed adult pulmonary TB patients initiated on TB treatment and followed for a minimum of 6 months to assess the impact of smoking status with or without alcohol abuse on treatment outcomes. Smokers were defined as never smokers, past smokers or current smokers. Alcohol Use Disorder Identification Test (AUDIT) scores were used to assess alcohol misuse. The association between smoking status and treatment outcomes was assessed in univariate and multivariate random effects poisson regression models.
Results
Of 455 enrolled, 129 (28%) had a history of smoking with 94 (20%) current smokers and 35 (8%) past smokers. Unfavourable treatment outcomes were significantly higher among past and current smokers as compared to never smokers. Specifically, the risk of treatment failure was significantly higher among past smokers (aIRR = 2.66, 95% CI: 1.41–4.90, p = 0.002), recurrent TB among current smokers (aIRR = 2.94, 95% CI: 1.30–6.67, p = 0.010) and death among both past (2.63, 95% CI: 1.11–6.24, p = 0.028) and current (aIRR = 2.59, 95% CI: 1.29–5.18, p = 0.007) smokers. Furthermore, the combined effect of alcohol misuse and smoking on unfavorable treatment outcomes was significantly higher among past smokers (aIRR: 4.67, 95% CI: 2.17–10.02, p<0.001) and current smokers (aIRR: 3.58, 95% CI: 1.89–6.76, p<0.001).
Conclusion
Past and current smoking along with alcohol misuse have combined effects on increasing the risk of unfavourable TB treatment outcomes. Innovative interventions that can readily address both co-morbidities are urgently needed.
Introduction
Tuberculosis (TB) is the ninth leading cause of death and the top infectious disease killer worldwide. The WHO Global Tuberculosis Report 2018 has reported that approximately 10 million people fell ill with TB in 2017 and India alone accounted for 27% (2.74 million) of the world’s TB case burden [1,2].
While there is effective treatment for TB, 10% to 30% of people with TB have composite outcomes of treatment failure, recurrence or death [2–6]. There are many important potentially modifiable risk factors associated with these unfavorable TB treatment outcomes, including tobacco smoking and alcohol use. Other risk factors include diabetes mellitus, HIV and low body mass index (BMI) [7,8]. Both tobacco smoking and alcohol use often occur together and use patterns are highly variable. While numerous studies have looked at these factors and have reported on the associations, an important shortcoming is the lack of collective, prospective ascertainment of these numerous risk factors as well as the assessment of impact of dose effect (e.g. past vs. current tobacco and amount of tobacco and alcohol consumed) and combined effects of tobacco smoking and alcohol use, adjusting for confounding effects.
We sought to assess the impact of smoking status (never, past and current) and its combined effect with alcohol use status given both are highly prevalent and likely have important interactions in influencing TB treatment outcomes in India.
Methodology
This study was conducted as part of the prospective ‘CTRIUMPh’ RePORT India cohort [9]. CTRIUMPh has been enrolling and following adult (≥18 years) pulmonary TB cases at the National Institute for Research in Tuberculosis (NIRT) in Chennai, India and the Byramjee-Jeejeebhoy Government Medical College–Sassoon General Hospitals (BJGMC-SGH) in Pune, India, since August 2014 through academic and operational partnership with the Johns Hopkins University (JHU), Baltimore, USA.
Pulmonary TB cases were diagnosed by the presence of acid-fast bacilli (AFB) on smear microscopy, Mycobacterium tuberculosis (Mtb) DNA on Xpert MTB/RIF assay, Mtb growth on liquid or solid culture, or based on clinical judgment in the absence of microbiological confirmation of TB. Cases with drug resistant disease, those with prior TB and pregnant women with TB were excluded. Participants were enrolled within 7 days of TB treatment initiation and prospectively followed for up to 24 months. Chest radiography evaluation at enrolment identified cavitary lung disease.
Standardized semi-structured interview schedules and operating procedures were used to collect socio-demographic, clinical and laboratory data. The Fagerström Test for Nicotine Dependence was used as a standard instrument for assessing the intensity of physical addiction to nicotine [10]. The Alcohol Use Disorder Identification Test (AUDIT), a 10-item screening questionnaire for hazardous and harmful alcohol consumption and alcohol related problems was used to assess alcohol use [11]. The Centre for Epidemiologic Studies Depression Scale (CES-D), a 20 item scale was used to measure symptoms of depression as defined by the American Psychiatric Association Diagnostic and Statistical Manual, fifth edition [12].
Definitions
Tobacco smoke use
Tobacco smoking was defined as follows at study entry: never smokers were those who smoked <100 cigarettes in their lifetime and were not current smokers; past smokers were those who smoked ≥ 100 cigarettes in their lifetime and were not currently smoking; and current smokers were those who smoked ≥100 cigarettes in their lifetime and reported current smoking. Pack years was calculated by multiplying the number of years smoked with the average number of packs (20 smoked tobacco products/pack) per day.
Alcohol use
AUDIT scoring system was used to define levels of alcohol use, those with the minimum score being 8. Total scores of 8–15, 16, 17–19, and ≥20 indicate alcohol dependence, harmful use, alcohol abuse and hazardous use, respectively [13].
Treatment outcomes
Composite outcome that included death (all causes of mortality), treatment failure (microbiological evidence of TB during last 2 months of treatment (month 5 or 6) by culture or AFB microscopy or clinical judgement if microbiological evidence was unavailable) and recurrence (microbiological evidence of TB after the successful completion of treatment by culture or AFB microscopy or clinical judgement, if microbiological evidence unavailable).
Favourable outcomes were defined as cured with evidence of consecutive negative M.tb during the last 2 months of TB treatment and treatment completed as absence of bacteriological evidence and/or absence of symptoms suggestive of TB at completion of TB treatment.
Statistical approaches
The analysis included comparison of baseline characteristics against the smoking profile using Mann Whitney, Kruskal-Wallis with post-hoc test. We also analysed the association between baseline characteristics with TB treatment outcome using Fisher’s Exact test. Person-time was calculated in years from the time of initiation into TB treatment to the occurrence of the unfavourable TB treatment outcome or until the last observed time of visit. Furthermore, the incidence rate of outcome was calculated over the person time period.
Univariate and multivariate Poisson regression with person-time as offset was used to identify the risk for unfavourable TB treatment outcome due to smoking. For the multivariate analysis, variables known to be associated with composite and individual treatment outcomes were identified through a review of published literature. In addition, an exploratory data analysis was also done to identify the effect modifiers of the association between TB treatment outcome and smoking status using Breslow-Day test of homogeneity. From this analysis, alcohol consumption was considered as an effect modifier, which remained in the multivariate model as an interaction term with smoking status.
Sensitivity analysis was performed to assess the influence of gender on the association between smoking status and TB treatment outcome. The dose response analysis was done to assess the relationship between intensity, frequency and duration of smoking with TB treatment outcomes. A cigarette pack year was taken as a proxy for smoking intensity. Statistical significance was determined at p<0.05. The statistical analysis was done using Stata V.15.0 (StataCorp, USA).
Written informed consent from the study participants was obtained. Ethics approval for the project was obtained from Institutional Review Boards of NIRT, BJGMC–SGH and Johns Hopkins School of Medicine.
Results
General profile of study cohort
Of 455 participants enrolled, 295 (65%) were male. The median age was 38 years (IQR: 27–49), 78 (17%) had no formal education, 304 (67%) were employed, 261 (57.4%) had a BMI of <18.5 and 45 (10%) had an AUDIT ≥ 8. A total of 326 (72%) were never smokers, 35 (8%) were past smokers and 94 (20%) were current smokers. Based on the Fagerström scale for nicotine dependence, 56 (60%) of current smokers were classified as having “low-to-moderate” dependence and 21 (22%) had “moderate-to-high” dependence. Microbiology assessment at study entry identified 305 (67%) smear positive for AFB and 378 (83%) culture positive for Mtb (Table 1).
Table 1. Comparison of profile between smoker vs. never smokers.
| Factors | Never n = 326 |
Past n = 35 |
Current n = 94 |
P-value |
|---|---|---|---|---|
| Age (in years)* | 34 (25–45) | 45 (30–52) | 48.5 (39–54) | <0.001 |
| Gender | ||||
| Female | 159 (49%) | 0 (0%) | 1 (1%) | <0.001 |
| Male | 167 (51%) | 35 (100%) | 93 (99%) | |
| Alcohol use | ||||
| Audit < 8 | 312 (96%) | 24 (69%) | 74 (79%) | <0.001 |
| Audit ≥ 8 | 14 (4%) | 11 (31%) | 20 (21%) | |
| BMI (kg/m2) | ||||
| < 16.0 | 74 (23%) | 12 (34%) | 34 (36%) | 0.053 |
| 16.0–18.5 | 102 (31%) | 12 (34%) | 27 (29%) | |
| ≥ 18.5 | 150 (46%) | 11 (31%) | 33 (35%) | |
| Education | ||||
| Literate | 276 (85%) | 27 (77%) | 74 (79%) | 0.227 |
| Illiterate | 50 (15%) | 8 (23%) | 20 (21%) | |
| Occupation | ||||
| Non-Working | 104 (32%) | 17 (49%) | 30 (32%) | 0.142 |
| Working | 222 (68%) | 18 (51%) | 64 (68%) | |
| Family Income | ||||
| <15000 | 259 (79%) | 29 (83%) | 76 (81%) | 0.922 |
| >15000 | 67 (21%) | 6 (17%) | 18 (19%) | |
| Area | ||||
| Rural | 74 (23%) | 16 (46%) | 41 (44%) | <0.001 |
| Urban | 252 (77%) | 19 (54%) | 53 (56%) | |
| Diabetes | ||||
| Absence | 240 (74%) | 20 (57%) | 66 (70%) | 0.111 |
| Presence | 86 (26%) | 15 (43%) | 28 (30%) | |
| Smoke Age | NA | 18 (15–20) | 20 (16–21) | 0.110 |
| Smoke Duration | NA | 26 (13–37) | 30 (14–36) | 0.489 |
| Pack Year | NA | 8.6 (1.4–31) | 7.4 (2.6–22) | 0.481 |
| Centre for Epidemiologic Studies Depression Scale (CES) | ||||
| Normal | 169 (52%) | 16 (46%) | 54 (57%) | 0.452 |
| Depressed | 157 (48%) | 19 (54%) | 40 (43%) | |
| CES Score | n = 326; 9 (5–14) | n = 35; 10 (3–17) | n = 94; 8 (5–14) | 0.814 |
| Cavity | ||||
| Absence | 216 (66%) | 15 (43%) | 56 (60%) | 0.019 |
| Presence | 110 (34%) | 20 (57%) | 38 (40%) | |
| HIV | ||||
| Negative | 300 (92%) | 34 (97%) | 91 (97%) | 0.212 |
| Positive | 26 (8%) | 1 (3%) | 3 (3%) | |
| Smear | ||||
| Negative | 121 (37%) | 7 (20%) | 22 (23%) | 0.011 |
| Positive | 205 (63%) | 28 (80%) | 72 (77%) | |
| Culture | ||||
| Negative | 66 (20%) | 2 (6%) | 9 (10%) | 0.009 |
| Positive | 260 (80%) | 33 (94%) | 85 (90%) | |
Values were shown in n (%) and median (Inter-Quartile Range)
Fisher's Exact test was used to compare the categorical information and K-Wallis followed by Dunn post-test that was used to compare age and CES score at 5% level of significance
*"Never" is different from the other two groups
Comparison of profile of current smokers, past smokers and never smokers
The profile of smokers both past and current were similar and significantly different to never smokers. Specifically, the median age of current smokers, past smokers and never smokers was 48.5, 45 and 34 years respectively (p<0.001), living in urban areas (56% vs. 54% vs. 77%, p<0.001), BMI <16.0 (36% vs. 34% vs. 23%, p = 0.053), cavitary TB (40% vs. 57% vs. 34%, p = 0.019), smear positivity (77% vs. 80% vs. 63%, p = 0.011) and Mtb culture positivity (90% vs. 94% vs. 80%, p = 0.009). There was also a significant difference in AUDIT scores ≥8 by smoking status (current 21% vs past 31% vs never smokers 4%, p<0.001). Smoking and alcohol use among women was negligible; except for one woman, smoking was reported only among men (Table 1).
Smoking history
The median age of initiation of smoking among current smokers was 20 years (IQR: 16–21) with a median average smoking duration of 30 years (IQR: 14–36). Similarly, the age of initiation of smoking among past smokers was 18 years (IQR: 15–20) with an average smoking duration of 26 years (IQR: 13–37). The median number of packs smoked in a year by current smokers was 7.4 (IQR: 2.6–22.0) and by past smokers was 8.6 (IQR: 1.4–31.0) (Table 1). Among smokers, 83 (64%) were cigarette smokers, 79 (61%) smoked bidis and 28 (22%) smoked both (Not tabulated).
Overall TB treatment outcomes
The median follow-up time was 18 months, which constituted 623 person-years of risk. Overall, 81(18%) participants had composite (failure, recurrence and death) treatment outcomes with an incidence rate of 122 per 1000 person-years, of which 40 (9%) were failure with an incidence rate of 61 per 1000 person-years, 20 (4%) were recurrence with an incidence rate of 30 per 1000 person-years and 21 (5%) were death with an incidence rate of 45 per 1000 person-years (Not tabulated).
Risk factors associated with composite outcomes (failure, recurrence and death)
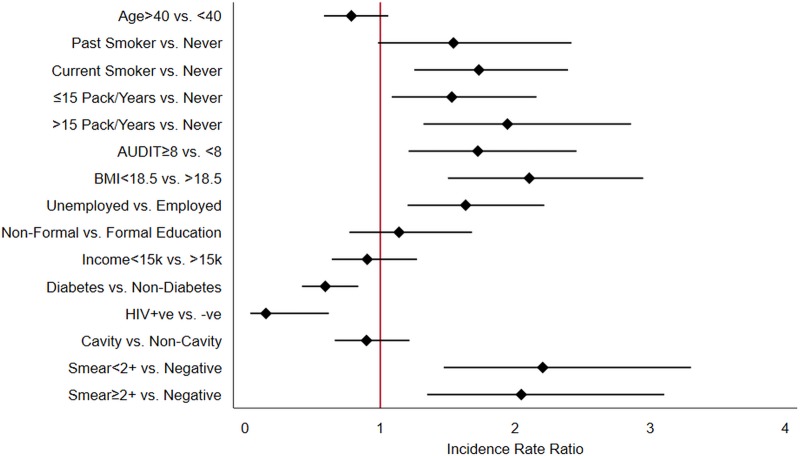
Females were excluded in further analysis of smoking and alcohol use given only one woman reported these behaviors. Univariate analysis shows that current smoking (IRR = 1.73, 95% CI: 1.25–2.39, p = 0.001), pack years of smoking >15 (IRR = 1.94, 95% CI: 1.32–2.86, p = 0.001), alcohol dependence i.e. AUDIT score ≥8 (IRR = 1.72, 95% CI: 1.21–2.45, p = 0.003), severe underweight i.e. BMI <18.5 (IRR = 2.10, 95% CI: 1.50–2.95, p<0.001), unemployment (IRR = 1.63, 95% CI: 1.20–2.21, p = 0.002) and higher AFB smear grade were risk factors associated with composite treatment outcomes (Fig 1).
Fig 1. Univariate assessment of risk factors of composite TB treatment outcomes among males.
Smoking history and TB treatment outcomes
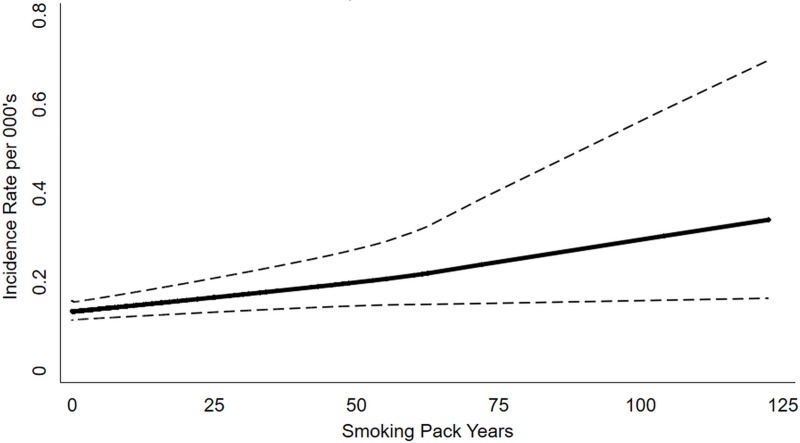
Multivariable analysis found that being a smoker i.e. past smoker (aIRR = 2.20, 95% CI: 1.27–3.81, p = 0.005) or current smoker (aIRR = 2.03, 95% CI: 1.33–3.08, p = 0.001) had a higher risk for composite TB treatment outcome after adjusting the potential confounders. Assessing individual treatment outcomes, the risk of failure (aIRR = 2.66, 95% CI: 1.41–4.90, p = 0.002) and recurrent TB (aIRR = 2.94, 95% CI: 1.30–6.67, p = 0.010) was significantly higher in past and current smokers respectively, compared to never smokers. The risk of death was higher among both current smokers (aIRR = 2.59, 95% CI: 1.29–5.18, p = 0.007) and past smokers (2.63, 95% CI: 1.11–6.24, p = 0.028) (Table 2). The dose response analysis reflects an increase in the risk of composite outcomes with increase in pack years (Fig 2).
Table 2. Risk of composite TB treatment outcomes due to smoking and alcohol among males.
| Smoking & Alcohol | Unadjusted | Adjusted2 | ||
|---|---|---|---|---|
| IRR (95%CI) | p-value | aIRR (95%CI) | p-value | |
| Composite1 | ||||
| Never & AUDIT <8 | Reference | Reference | ||
| Never & AUDIT ≥8 | 1.09 (0.48–2.51) |
0.834 | 1.01 (0.36–2.84) |
>0.950 |
| Past & AUDIT <8 | 1.30 (0.72–2.35) |
0.377 | 1.54 (0.76–3.13) |
0.227 |
| Past & AUDIT ≥8 | 1.95 (1.06–3.59) |
0.032 | 4.67 (2.17–10.02) |
<0.001 |
| Current & AUDIT <8 | 1.50 (1.04–2.17) |
0.031 | 1.75 (1.10–2.79) |
0.019 |
| Current & AUDIT ≥8 | 2.62 (1.62–4.21) |
<0.001 | 3.58 (1.89–6.76) |
<0.001 |
IRR = incidence rate ratio; aIRR = adjusted IRR
1Composite treatment outcome included failure, recurrence and death
2Adjusted for age, BMI, family income, HIV coinfection, diabetes, chest x-ray cavity and smear.
Fig 2. Dose-response relationship between tobacco smoking and the risk of composite TB treatment outcomes.
Combined effect of smoking and alcohol use on TB treatment outcomes
There was a significant change in the magnitude of the association between smoking and TB treatment outcomes when alcohol use was added into the model. Specifically, a synergistic effect of smoking and alcohol on TB treatment outcomes was observed. Compared to those who never smoked nor had alcohol use disorder, past smokers with alcohol dependence (4.67, 95% CI: 2.17–10.02, p<0.001) and current smokers with alcohol dependence (3.58, 95% CI: 1.89–6.76, p<0.001) had the highest observed risk of composite outcomes (Table 2).
Discussion
Our cohort study has highlighted several key findings on the relationship between smoking status, dose and duration and treatment outcomes; the gender difference in prevalence of smoking and alcohol and the combined impact of smoking and alcohol misuse on composite outcomes. Our study highlights the critical need to address smoking and alcohol misuse with novel interventions given their significant negative sequel on TB treatment outcomes in India, the country with the greatest burden of TB.
Firstly, we observed that overall 18% of our cohort followed up for 18 months had an unfavorable outcome (failure, recurrence and death). More than 25% of TB patients reported history of smoking, either current or past smoking. Our study findings clearly indicate that being a smoker is a significant risk factor for composite treatment outcomes. Several studies have observed the association between smoking and TB treatment outcomes including studies from India, Georgia, Pakistan, Brazil and Armenia [14–17]. What has been less commented on is the dose and duration of smoking and TB treatment outcomes. We specifically assessed the impact of past and current smoking as well as dose response on TB treatment outcomes and observed that past and current smoking as well as cumulative exposure are associated independently with composite treatment outcomes. The risk of failure, recurrence and death was significantly higher among past and current smokers as compared to never smokers. Two cohort studies conducted in Malaysia and Morocco found that smoking increased 2–7 fold the odds of treatment failure [18,19]. This relationship between smoking and TB recurrence as well as smoking and death has also been observed [15,20–24]. A history of past smoking has prognostic importance for failure and dealth, and current smoking assessments would help provide timely intervention, which is important to prevent recurrence and failure. However, current smokers are the only focus of attention for any TB treatment intervention with the advice often being to quit smoking [25–28]. Our study findings also caution us not to confine to only an assessment of current smoking but to also give equal importance to eliciting the past history of smoking.
A dose response relationship in our study shows that an increase in the duration of smoking among participants with a history of smoking does not increase the risk of composite outcomes. This is contrary to another study in Congo, which reports that smoking was independently associated with TB treatment outcomes among those who reported smoking ≥10 years [29].
Secondly, only one female reported smoking, illustrating a clear sex-specific difference in risk factors contributing to unfavorable TB treatment outcomes. The GATS survey of TB tobacco smoking in India also found fewer women reported smoking compared to men, i.e., 19% of men and 2% of women reported smoking [30,31]. While the overall smoking prevalence among men and women has been growing in India, studies have reported that female smoking is growing at a faster rate than that of males but smoking prevalence among women is seldom reported [24,32]. This could be mainly because of the stigma attached to smoking among women considering the cultural norms [33,34]. This gap needs to be addressed and cannot be ignored while eliciting smoking history among TB patients.
Finally, we specifically assessed combined effect of past or current smoking and alcohol misuse on TB treatment outcomes. It was seen that males with past or current tobacco use along with alcohol misuse have the highest risk of unfavorable TB treament outcomes. This clearly highlights the importance of addressing both disorders together and initiating effective interventions for reducing tobacco and alcohol use during TB treatment. Prior studies have shown that alcohol consumption is associated with an increased risk of TB and is a major contributor to the TB burden of disease [35–40]. Two earlier studies have also reported that smoking and drinking habits tend to be linked together and are individually associated with unfavorable treatment outcomes [38,39]. However, it is suprising that there are relatively few data on the combined effect since these disorders are usually inextricably linked.
We also observed that being severely underweight was significantly associated with composite treatment outcomes. It was found that there was a significant difference in the profile of BMI between smokers and never smokers, which has also been reported in other studies [41–44]. Hence, low BMI along with smoking is an important history that health care providers need to be aware of in understanding treatment outcomes.
Limitations
Our study has some limitaitons. Since we have used self-reported smoking status and self reported AUDIT questionnaire and have not measured nicotine and alcohol or PEth levels, it is possible that we underestimated the smoking and alcohol prevalence and amount and their relationship with TB treatment outcomes. We also did not include smokeless tobacco use such as chewing tobacco which is often more prevalent among women.We may also have unmeasured confounders that contributed to unfavorable treatment outcomes. We did not measure Mtb strain types or host genetics that have been associated with TB outcomes. However, we systematically assessed multiple comorbidities and potential confounders in our prospective cohort; data which are often not readily available.
Conclusion
Tobacco smoking is an important risk factor that needs to be urgently addressed to decrease adverse composite TB treatment outcomes. We further highlight the importance of eliciting a history of both past and current smoking, given the impact on mortality. We show that current smoking is associated with TB recurrence and the synergistic negative sequalae of combined smoking and alcohol misuse. Health care providers need to be equipped to use standardised measurements such as Ferguson and AUDIT to ascertain smoking and alcohol misuse for all TB patients. Lastly, a holistic approach to smoking cessation that addresses multimorbidity, particularly alcohol misuse, and undernutrion is crtitical for improving
Acknowledgments
We want to acknowledge all the members of the CTRIUMPh team listed in alphabetical order–Aarti Kinikar1, Akshay Gupte2, Amita Gupta2,3 (agupta25@jhmi.edu), Amita Nagraj1, Anand Kumar B4, Andrea DeLuca3, Anita More1, Anju Kagal1, Archana Gaikwad1, Ashwini Nangude1, Balaji S4, Beena Thomas4, Bency Joseph4, Bharath TK4, Brindha B4, Chandrasekaran P4, David Dowdy2,3, Deepak Pole1, Devanathan A4, Devi Sangamithrai M4, Dileep Kadam1, Divyashri Jain1, Dolla CK4, Gabriela Smit2,3, Gangadarsharma R4, Geetha Ramachandran4, Hanumant Chaugule1, Hari Koli1, Hemanth Kumar4, Jeeva J4, Jessica Elf2,3, Jonathan Golub2,3, Jyoti Chandane1, Kanade Savita1, Kannan M4, Kannan Thiruvengadam4, Karthikesh M4, Karunakaran S4, Kelly Dooley2,3, Krithiiga Sekar4, Lakshmi Murali5, Lavanya M4, Luke E. Hannah4, Madasamy S4, Madeshwaran A4, Mageshkumar M4, Mangaiyarkarasi S4, Mahesh Gujare1, Manoharan S4, Michel Premkumar M4, Munivardhan P4, Murugesan S4, Gomathy NS4, Neeta Pradhan1, Nikhil Gupte1, Nishi Suryavanshi1, Ponnuraja C4, Premkumar N4, Rahul Lokhande1, Rajkumar S4, Ranganathan K4, Rani S4, Rani V4, Renu Bharadwaj1, Renu Madewar1, Rengaraj R6, Rewa Kohli1, Robert Bollinger2,3, Rosemarie Warlick2,3, Rupak Shivakoti2,3, Sahadev Javanjal1, Sameer Joshi1, Sandhya Khadse1, Sathyamurthi P4, Shalini Pawar1, Shashank Hande1, Shital Muley1, Shital Sali1, Shri Vijay Bala Yogendra Shivakumar6, Suba Priya K4, Shrinivas B.M4, Shyam Biswal1, Silambu Chelvi K4, Smita Nimkar1, Soumya Swaminathan7, Sriram Selvaraj4, Sundeep Salvi1, Sushant Meshram1, Surendhar S6, Swapnil Raskar1, Uma Devi4, Vandana Kulkarni1, Vidula Hulyalkar1, Vidya Mave1, Vinod Tayawade1, Vrinda Bansode1, Yogesh Daware1.
1 Byramjee Jeejeebhoy Government Medical College—Johns Hopkins University Clinical Research site, Pune, Maharashtra, India
2 Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA
3 Johns Hopkins School of Medicine, Baltimore, Maryland, USA
4 Indian Council of Medical Research–National Institute for Research in Tuberculosis, Chennai, Tamil Nadu, India
5 State Tuberculosis Office, Tamil Nadu, India
6 Johns Hopkins University–India office, Pune, Maharashtra, India
7 Indian Council of Medical Research, New Delhi, India
Data Availability
The data contain potentially sensitive information and data access is governed by the larger RePORT Indian consortium. Data are therefore made available to all interested researchers upon request. A RePORT India consortium data access request form needs to be completed and reviewed. The form can be requested from RePORT India, dgnanadason@crdfglobal.org.
Funding Statement
Data in this manuscript were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium. This project has been funded in whole or in part with Federal funds from the Government of India's (GOI), Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the USA National Institutes of Health (NIH), the National Institute of Allergy and Infectious Diseases (NIAID), the Office of AIDS Research (OAR), and distributed in part by CRDF Global. This work was also supported by the National Institutes of Health (NIH R01AI097494 to Golub), the NIH funded Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks [UM1AI069465 to VM, NG, AG], Ujala foundation, Wyncote foundation and Gilead foundation. The authors also acknowledge support from Persistent Systems in kind. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the ICMR, the NIH, or CRDF Global. Any mention of trade names, commercial projects or organizations does not imply endorsement by any of the sponsoring organizations. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018. April 1;6(4):299–314. 10.1016/S2213-2600(18)30057-2 [DOI] [PubMed] [Google Scholar]
- 2.Global Tuberculosis Report 2017. Geneva; 2017.
- 3.Daniel Tarekegne MJ. Treatment Outcomes of Tuberculosis Patients in Metema Hospital, Northwest Ethiopia: A Four Years Retrospective Study. Mycobact Dis. 2014. July 25;05(04):1–7. [Google Scholar]
- 4.Manissero D, Hollo V, Huitric E, Ködmön C, Amato-Gauci A. Analysis of tuberculosis treatment outcomes in the European Union and European Economic Area: Efforts needed towards optimal case management and control. Eurosurveillance. 2010;15(11):21–9. [PubMed] [Google Scholar]
- 5.Ditah IC, Reacher M, Palmer C, Watson JM, Innes J, Kruijshaar ME, et al. Monitoring tuberculosis treatment outcome: Analysis of national surveillance data from a clinical perspective. Thorax. 2008. May 1;63(5):440–6. 10.1136/thx.2006.073916 [DOI] [PubMed] [Google Scholar]
- 6.Vasankari T, Holmström P, Ollgren J, Liippo K, Kokki M, Ruutu P. Risk factors for poor tuberculosis treatment outcome in Finland: A cohort study. BMC Public Health. 2007. December 14;7(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marais BJ, Lönnroth K, Lawn SD, Migliori GB, Mwaba P, Glaziou P, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: Integrating health services and control efforts. Vol. 13, Lancet Infectious Diseases. Elsevier; 2013. p. 436–48. 10.1016/S1473-3099(13)70015-X [DOI] [PubMed] [Google Scholar]
- 8.Bates M, Marais BJ, Zumla A. Tuberculosis comorbidity with communicable and noncommunicable diseases. Cold Spring Harb Perspect Med. 2015. February 6;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupte A, Padmapriyadarsini C, Mave V, Kadam D, Suryavanshi N, Shivakumar SVBY, et al. Cohort for Tuberculosis Research by the Indo-US Medical Partnership (CTRIUMPH): Protocol for a multicentric prospective observational study. BMJ Open. 2016. February 25;6(2):e010542 10.1136/bmjopen-2015-010542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991. September;86(9):1119–27. [DOI] [PubMed] [Google Scholar]
- 11.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Geneva, Switzerland; 2001. 1–41 p.
- 12.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977. June 26;1(3):385–401. [Google Scholar]
- 13.Rehm J, Baliunas D, Borges GLG, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction. 2010. May;105(5):817–43. 10.1111/j.1360-0443.2010.02899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gegia M, Magee MJ, Kempker RR, Kalandadze I, Chakhaia T, Golub JE, et al. Tobacco smoking and tuberculosis treatment outcomes: A prospective cohort study in Georgia. Bull World Health Organ. 2015. June 1;93(6):390–9. 10.2471/BLT.14.147439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AH, Israr M, Khan A, Aftab RA, Khan TM. Smoking on treatment outcomes among tuberculosis patients. Am J Med Sci. 2015;349(6):505–9. 10.1097/MAJ.0000000000000473 [DOI] [PubMed] [Google Scholar]
- 16.Cailleaux-Cezar M, Loredo C, Silva JRL e, Conde MB. Impact of smoking on sputum culture conversion and pulmonary tuberculosis treatment outcomes in Brazil: a retrospective cohort study. J Bras Pneumol. 2018;44(2):99–105. 10.1590/s1806-37562017000000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balian DR, Davtyan K, Balian A, Grigoryan A, Hayrapetyan A, Davtyan H. Tuberculosis treatment and Smoking, Armenia, 2014–2016. J Clin Tuberc Other Mycobact Dis. 2017. August 1;8:1–5. 10.1016/j.jctube.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tachfouti N, Nejjari C, Benjelloun MC, Berraho M, Elfakir S, Rhazi K El, et al. Association between smoking status, other factors and tuberculosis treatment failure in Morocco. Int J Tuberc Lung Dis. 2011;15(6):838–43. 10.5588/ijtld.10.0437 [DOI] [PubMed] [Google Scholar]
- 19.Dujaili JA, Sulaiman SAS, Awaisu A, Muttalif AR, Blebil AQ. Outcomes of tuberculosis treatment: a retrospective cohort analysis of smoking versus non-smoking patients in Penang, Malaysia. J Public Health (Bangkok). 2010. April 25;19(2):183–9. [Google Scholar]
- 20.d’Arc Lyra Batista J, de Fátima Pessoa Militão de Albuquerque M, de Alencar Ximenes RA, Rodrigues LC. Smoking increases the risk of relapse after successful tuberculosis treatment. Int J Epidemiol. 2008;37(4):841–51. 10.1093/ije/dyn113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yen YF, Yen MY, Lin YS, Lin YP, Shih HC, Li LH, et al. Smoking increases risk of recurrence after successful anti-tuberculosis treatment: A population-based study. Int J Tuberc Lung Dis. 2014;18(4):492–8. 10.5588/ijtld.13.0694 [DOI] [PubMed] [Google Scholar]
- 22.Jee SH, Golub JE, Jo J, Park IS, Ohrr H, Samet JM. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol. 2009. December 15;170(12):1478–85. 10.1093/aje/kwp308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gajalakshmi V, Peto R, Kanaka TS, Jha P. Smoking and mortality from tuberculosis and other diseases in India: Retrospective study of 43 000 adult male deaths and 35 000 controls. Lancet. 2003. August 16;362(9383):507–15. 10.1016/S0140-6736(03)14109-8 [DOI] [PubMed] [Google Scholar]
- 24.Mahishale V, Patil B, Lolly M, Eti A, Khan S. Prevalence of Smoking and Its Impact on Treatment Outcomes in Newly Diagnosed Pulmonary Tuberculosis Patients: A Hospital-Based Prospective Study. Chonnam Med J. 2015;51(2):86 10.4068/cmj.2015.51.2.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Shen H. Review of cigarette smoking and tuberculosis in China: Intervention is needed for smoking cessation among tuberculosis patients. BMC Public Health. 2009. August 12;9:292 10.1186/1471-2458-9-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang C-Y, Bam TS. Should tobacco control intervention be implemented into tuberculosis control program? Expert Rev Respir Med. 2018. July 3;12(7):541–3. 10.1080/17476348.2018.1481394 [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Wang L-X, Qiu L-X, Huang Q, Shu Q, Lin H-X, et al. A smoking cessation intervention among tuberculosis patients in rural China. Public Heal Action. 2015;5(3):183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng N, Padmawati RS, Prabandari YS, Nichter M. Smoking behavior among former tuberculosis patients in Indonesia: Intervention is needed. Int J Tuberc Lung Dis. 2008;12(5):567–72. [PubMed] [Google Scholar]
- 29.Vanden Driessche K, Patel MR, Mbonze N, Tabala M, Yotebieng M, Behets F, et al. Effect of smoking history on outcome of patients diagnosed with TB and HIV. Eur Respir J. 2015. March 1;45(3):839–42. 10.1183/09031936.00160714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global Adult Tobacco Survey Fact Sheet (India 2016–17). 2018.
- 31.Mishra S, Joseph RA, Gupta PC, Pezzack B, Ram F, Sinha DN, et al. Trends in bidi and cigarette smoking in India from 1998 to 2015, by age, gender and education. BMJ Glob Heal. 2016. April 1;1(1):e000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goel S, Tripathy JP, Singh RJ, Lal P. Smoking trends among women in India: Analysis of nationally representative surveys (1993–2009). South Asian J Cancer. 2014. October;3(4):200–2. 10.4103/2278-330X.142958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kathirvel, Thakur J, Sharma S. Women and tobacco: A cross sectional study from North India. Indian J Cancer. 2014. December;51(5):78. [DOI] [PubMed] [Google Scholar]
- 34.Venkateshan M, Panwar R, Chamoli P, Rawat P, Dhiman P, Benson R, et al. Attitude and determinants of female smoking among older female subjects in the selected rural areas of Uttarakhand, India. Int J Med Sci Public Heal. 2015;5(08):1. [Google Scholar]
- 35.Imtiaz S, Shield KD, Roerecke M, Samokhvalov A V, Lönnroth K, Rehm J. Alcohol consumption as a risk factor for tuberculosis: meta-analyses and burden of disease. Eur Respir J. 2017. July;50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suhadev M, Thomas BE, Raja Sakthivel M, Murugesan P, Chandrasekaran V, Charles N, et al. Alcohol use disorders (AUD) among tuberculosis patients: A study from Chennai, South India. PLoS One. 2011;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soh AZ, Chee CBE, Wang Y-T, Yuan J-M, Koh W-P. Alcohol drinking and cigarette smoking in relation to risk of active tuberculosis: prospective cohort study. BMJ Open Respir Res. 2017. October 13;4(1):e000247 10.1136/bmjresp-2017-000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown KE, Campbell AH. Tobacco, alcohol and tuberculosis. Br J Dis Chest. 1961. July 1;55(3):150–8. [Google Scholar]
- 39.Lewis JG, Chamberlain DA. Alcohol Consumption and Smoking Habits in Male Patients with Pulmonary Tuberculosis. Br J Prev Soc Med. 1963. July;17(3):149–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas B, Suhadev M, Mani J, Ganapathy BG, Armugam A, Faizunnisha F, et al. Feasibility of an alcohol intervention programme for TB patients with Alcohol Use Disorder (AUD)—a qualitative study from Chennai, South India. PLoS One. 2011;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pednekar MS, Hakama M, Gupta PC. Tobacco Use or Body Mass–Do They Predict Tuberculosis Mortality in Mumbai, India? Results from a Population-Based Cohort Study. Pai M, editor. PLoS One. 2012. July 27;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patra J, Jha P, Rehm J, Suraweera W. Tobacco smoking, alcohol drinking, diabetes, low body mass index and the risk of self-reported symptoms of active tuberculosis: Individual Participant Data (IPD) meta-analyses of 72,684 individuals in 14 high tuberculosis burden countries. Pai M, editor. PLoS One. 2014. May 2;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirenga BJ, Ssengooba W, Muwonge C, Nakiyingi L, Kyaligonza S, Kasozi S, et al. Tuberculosis risk factors among tuberculosis patients in Kampala, Uganda: implications for tuberculosis control. BMC Public Health. 2015. January 21;15:13 10.1186/s12889-015-1376-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong B, Ge N, Zhou Y. Smoking and alcohol consumption as risk factors of pulmonary tuberculosis in Chengdu: a matched case-control study. Hua xi yi ke da xue xue bao/ J West China Univ Med Sci. 2001. March;32(1):104–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data contain potentially sensitive information and data access is governed by the larger RePORT Indian consortium. Data are therefore made available to all interested researchers upon request. A RePORT India consortium data access request form needs to be completed and reviewed. The form can be requested from RePORT India, dgnanadason@crdfglobal.org.