The heart uses more free fatty acids (FFAs) and less glucose in individuals with obesity compared to normal-weight individuals.1 During ischemia, the myocardium typically shifts from β-oxidation of fatty acids to utilization of the more oxygen-efficient fuel glucose.2 By hindering glucose oxidation, obesity is expected to detrimentally affect the ability of the myocardium to adapt to ischemia.3
In humans, weight loss decreases myocardial FFA oxidation and total myocardial substrate oxidation.4 However, the effects of weight loss on intramyocardial glucose metabolism are unknown. We hypothesized that weight loss would increase myocardial glucose utilization, glucose utilization per unit of insulin, glycolysis, and glucose oxidation. We also hypothesized that these changes would be inversely related to changes in plasma FFA concentration. To test these hypotheses, we used 1-11C-glucose in conjunction with Positron Emission Tomography (PET) and kinetic modeling to quantify intramyocardial glucose metabolism in individuals with obesity who underwent diet-induced weight loss.
This was a prospective intervention study. Participants included sedentary individuals aged 21-49y with body mass index (BMI) ≥30.0 kg/m2. Exclusion criteria included: ejection fraction <50%; coronary artery disease; diabetes; heart, kidney or liver failure; weight >159 kg; pregnancy; untreated sleep apnea; postmenopausal status; and smoking. Written informed consent was obtained before enrollment in the study, which was approved by the Institutional Review Board of Washington University School of Medicine.
Subjects enrolled in a 20-week group behavior modification program with individualized calorie prescriptions and were provided with weight management skills. After 20 weeks, energy intake was adjusted so that subjects maintained a stable body weight for 2 weeks before follow-up assessments.
The night before the PET scans, subjects received a standardized dinner and then fasted. PET studies were performed at 0800h at rest with a Siemens tomograph (ECAT 962 HR+; Siemens Medical Systems, Iselin, NJ). After a transmission scan for attenuation correction, 15O-water was injected, a 5 min dynamic scan was performed to determine myocardial blood flow, 1-11C-glucose was injected, and a 30 min dynamic scan was performed.
The kinetic model developed by Herrero et al.5 was used to quantify myocardial glucose metabolism as follows:
fractional glucose uptake = glucose extraction fraction × myocardial blood flow
glucose utilization = fractional glucose uptake × plasma glucose concentration
glucose utilization = glucose undergoing glycolysis + glycogenesis
glycolysis = glucose undergoing full oxidation + lactate production
Plasma glucose, insulin, FFA, and lipid concentrations were measured and the homeostasis model assessment of insulin resistance (HOMA-IR) was computed.
Statistical analyses were performed using SAS version 9.4 and Excel 16.16.4. Changes in intramyocardial glucose metabolism in response to weight loss were evaluated using paired 2-tailed t tests. Pearson correlation analyses were performed to evaluate the univariate relationships between variables. Results are presented as mean±SE. P < 0.05 was considered statistically significant. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Twenty subjects achieved the pre-specified minimum weight loss of >3%, 13 of whom had adequate 11C-glucose PET images for inclusion in this analysis. Subjects were 54% female, 85% white and 34±2y of age. Weight loss averaged 11±5.3%. Body fat percentage, fasting plasma insulin, HOMA-IR, FFA, total cholesterol and triglycerides decreased after weight loss. There were no changes in LDL or HDL cholesterol levels, blood pressure, left ventricular mass, or ejection fraction.
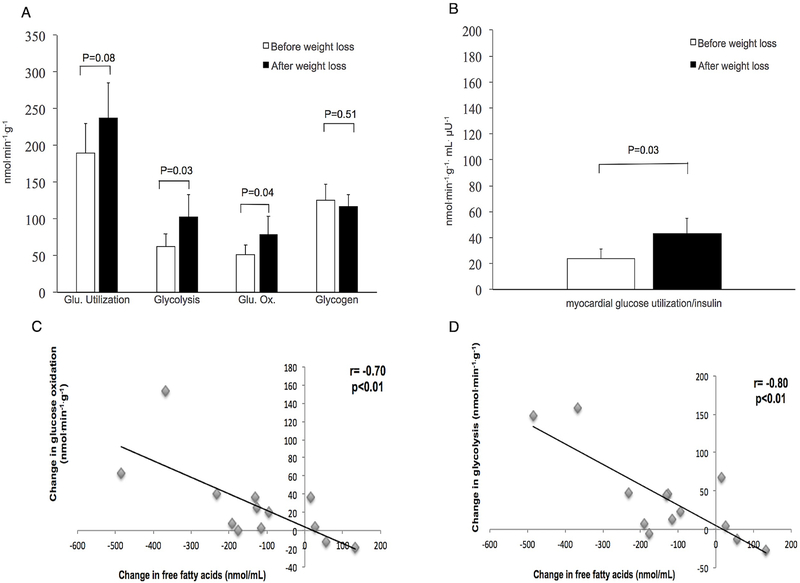
Weight loss did not change fasting plasma glucose (data not shown) or myocardial blood flow (1.16±0.27 to 1.07±0.07 mL/g/min, P = 0.37). Myocardial glycolysis increased 64% after weight loss (Figure [A]). Myocardial glucose oxidation and glucose utilization per unit of insulin increased in parallel with myocardial glycolysis (Figure [A]and [B]). Myocardial glucose utilization showed a trend toward an increase; glycogen synthesis did not change (Figure [A]).
Figure.
Myocardial glucose metabolism and free fatty acid changes with weight loss A, Myocardial glucose utilization, glycolysis, oxidation, and glycogenesis before and after weight loss; B, Myocardial glucose utilization per serum insulin level before and after weight loss; C, Change in glucose oxidation as a function of change in free fatty acid; D, Change in glycolysis as a function of change in free fatty acid
Changes in myocardial glucose oxidation and glycolysis were inversely related to plasma FFA changes (Figure [C] and [D]). Myocardial glucose utilization and glucose utilization/plasma insulin were not significantly related to FFA changes. The glucose utilization and metabolism changes were not related to BMI or HOMA-IR.
The major finding of this study was that diet-induced weight loss led to significant increases in myocardial glycolysis, myocardial glucose oxidation, and myocardial glucose utilization per unit of insulin, with a trend towards increased myocardial glucose utilization. Glycogen synthesis did not change. This alteration in myocardial glucose metabolism cannot be attributed to significant changes in glucose delivery because plasma glucose levels and myocardial blood flow rates did not change significantly. Rather, the striking changes in myocardial glycolysis and glucose oxidation appear to be related to the changes in plasma FFA levels. These findings, combined with those of Lin et al.4 imply that the left ventricle myocardium decreases its oxygen consumption by decreasing fatty acid utilization and increasing glycolysis and complete glucose oxidation. Because glucose is a more efficient fuel to oxidize than fatty acids, this shift would theoretically be beneficial in the setting of ischemia.
Acknowledgments
SOURCES OF FUNDING: This work was funded by grants R01-HL073120, UL1RR024992(CTSA), P60 DK020579, and P30 DK56341 from the National Institutes of Health, Bethesda, MD; and grants from the Mentors in Medicine Program of WUSM and the Foundation for Barnes-Jewish Hospital, St. Louis, Missouri.
Footnotes
DISCLOSURES:
None
REFERENCES
- 1.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Gropler RJ. Effect of Obesity and Insulin Resistance on Myocardial Substrate Metabolism and Efficiency in Young Women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.cir.0000127959.28627.f8 [DOI] [PubMed] [Google Scholar]
- 2.Taegtmeyer H. Ischemia and glucose metabolism. Circulation. 1997;96:3810–3811. [PubMed] [Google Scholar]
- 3.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. [DOI] [PubMed] [Google Scholar]
- 4.Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, Peterson LR. Myocardial Oxygen Consumption Change Predicts Left Ventricular Relaxation Improvement in Obese Humans After Weight Loss. Obesity. 2011;19:1804–1812. doi: 10.1038/oby.2011.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrero P, Kisrieva-Ware Z, Dence CS, Patterson B, Coggan AR, Han D, Gropler RJ. PET Measurements of Myocardial Glucose Metabolism with 1-11C-Glucose and Kinetic Modeling. J Nucl. Med. 2007;48:955–964. doi: 10.2967/jnumed.106.037598 [DOI] [PubMed] [Google Scholar]