Abstract
Silica-based lectin microcolumns were developed and optimized for the separation and analysis of glycoform fractions in alpha1-acid glycoprotein (AGP) based on both the degree of branching and level of fucosylation. Concanavalin A (Con A) and Aleuria Aurantia lectin (AAL) were immobilized onto HPLC-grade silica by reductive amination and packed into 2.1 mm i.d. × 5.0 cm microcolumns. Factors examined for these microcolumns include their protein content, binding capacity, binding strength and band-broadening under isocratic conditions (Con A) or step elution conditions (AAL) and in the presence of various flow rates or temperatures. These factors were examined by using experiments based on frontal analysis, zonal elution, peak profiling and peak decay analysis. Up to 200 μg AGP could be loaded onto a Con A microcolumn and provide linear elution conditions, and 100 μg AGP could be applied to an AAL microcolumn. The final conditions for separating retained and non-retained AGP glycoform fractions on a Con A microcolumn used a flow rate of 50 μL min−1 and a temperature of 50 °C, which gave a separation of these fractions within 20 min or less. The final conditions for an AAL microcolumn included a flow rate of 0.75 mL min−1, a temperature of 50 °C, and the use of 2.0 mM L-fucose as a competing agent for elution, giving a separation of non-retained and retained AGP glycoforms in 6 min or less. The inter-day precisions were ± 0.7–4.0% or less for the retention times of the AGP glycoforms and ± 2.2–3.0% or less for their peak areas.
Keywords: Lectin affinity chromatography, Alpha1-acid glycoprotein, Concanavalin A, Aleuria aurantia lectin, Affinity microcolumn, Glycoform analysis
Graphical Abstract
1. Introduction
Alpha1-acid glycoprotein (AGP) is an acute phase glycoprotein that has a molar mass of 41–43 kDa [1]. Human AGP contains a single polypeptide chain with 183 amino acids and two disulfide bonds. Complex-type asparagine linked glycans are attached to this sequence at five glycosylation sites and account for around 45% of the mass of AGP. The terminal sialic residues of these glycans result in AGP having a low pI of 2.8–3.8 [1].
There are several changes that can occur in AGP during disease. For instance, the normal plasma concentration of AGP in humans ranges from 0.5 to 1.0 mg mL−1 (or 12–24 μM); however, this concentration can increase by up to ten-fold during acute phase reactions [2]. In addition, an alteration in the glycosylation of AGP has been observed in a number of diseases, such as a change in the degree of branching during acute or chronic infections [3,4], liver disease [5,6], rheumatoid arthritis [7], and lung disease or lung cancer [8]. A change in glycan branching has also been found in women during their menstrual cycles or during estrogen-progesterone treatment [9]. An increase in fucosylation has been noted in pancreatic cancer [10], hepatocellular carcinoma, and cirrhosis [11]. Hypersialylation of AGP has been reported in ovarian cancer, lymphoma [12,13], hepatocellular carcinoma and cirrhosis [11].
The glycosylation or glycoforms of AGP have been examined by using capillary electrophoresis [14,15] or mass spectrometry [16]. A number of studies have also employed lectins (i.e., non-immune system carbohydrate-binding proteins) for such work[17–26]. One common lectin that has been used is concanavalin A (Con A) from Canavalia ensiformis. Con A can bind tightly to high-mannose type glycans or hybrid type glycans with mannose branching, with weaker binding to complex type biantennary glycans [27]. Aleuria aurantia lectin (AAL) is an alternative lectin that binds to α1–6, α1–3, and α1–4 linked fucose residues [27]. Examples of lectin-based methods that have been used for AGP analysis are crossed affinoimmunoelectrophoresis [4,7,17,18], affinity capillary electrophoresis [19,20], lectin enzyme-linked immunosorbent assay [21], and affinity-based surface plasma resonance [22]. Another such method is lectin affinity chromatography [23–26].
In lectin affinity chromatography, a lectin is immobilized onto a chromatographic support and used for the retention of given targets that contain complementary sugars within their structure, such as glycopeptides, glycoproteins and glycolipids. Elution of the targets is typically accomplished by adding a competing sugar to the mobile phase [28]. Lectin affinity chromatography is commonly used for the enrichment of low abundance glycoproteins and glycopeptides [29]. Prior work with lectin columns and AGP has primarily used low-performance supports such agarose or polyacrylamide-agarose composite beads [23–26], both which can be difficult to use directly as part of an HPLC system due to their limited pressure stability and slow mass transfer properties [30].
High performance affinity chromatography (HPAC) is a form of affinity chromatography in which an immobilized binding agent is attached to small, rigid particles or some other type of support that is capable of withstanding the high flow rates and operating pressures of HPLC. This method is more suitable than traditional, low-performance affinity chromatography for analytical applications due to its improved mass transfer properties, speed, precision, and ease of automation [31]. In recent work, HPAC has been explored for use with affinity microcolumns that have volumes in the low-to-mid microliter range [32]. Advantages that have been noted for these microcolumns are their need for only a minimal amount of immobilized binding agent and small sample sizes, as well as their low non-specific binding, fast analysis times and ease-of-use with on-line detectors or with other columns in multi-dimensional systems [32,33]. In contrast to this, all previous reports that have used Con A or AAL columns to bind and separate AGP glycoforms have involved long separation methods and the use of fraction collection prior to the measurement and study of the eluted AGP by other methods (e.g., mass spectrometry, capillary electrophoresis or protein assays) [23–26,34].
In this study, lectin microcolumns based on HPLC-grade porous silica will be developed and examined for their use in the separation and direct analysis of AGP glycoform fractions based on both the degree of branching and level of fucosylation. Both Con A and AAL will be immobilized onto HPLC-grade silica and used in affinity microcolumns for characterization by chromatographic techniques such as frontal analysis, zonal elution, peak decay analysis, and peak profiling. The protein content and binding/elution properties of these lectin microcolumns will be characterized during this process. The effect of altering the flow rate, column temperature and use of a competing agent for elution will also be considered. The final Con A and AAL microcolumns will be characterized in terms of their reproducibility in retaining and measuring their non-retained and retained AGP glycoform fractions. The properties and behavior of these methods will also be compared to those reported in prior studies using other types of Con or AAL columns and supports for the separation of AGP glycoform fractions [23–26,34].
2. Experimental section
2.1. Materials
The AGP (from pooled human plasma, product no. G9885, ≥ 99% pure), L-fucose (> 99%), (3-glycidoxypropyl)trimethoxysilane (≥ 98%), Con A (product C7275, type V, highly purified), human serum albumin (HSA, product A1887, 96%, fatty acid free), ovalbumin (≥ 98%), p-nitrophenyl α-D-mannopyranoside (p-NP-α-D-Man) and p-nitrophenyl α-L-fucopyranoside (p-NP-α-L-Fuc) were purchased from Sigma-Aldrich (St Louis, MO, USA). The AAL (product L-1390, homogeneous by SDS-PAGE) was purchased from Vector Laboratories (Burlingame, CA, USA). Nucleosil Si-300 silica (300 Å pore size, 5 μm particle diameter) was purchased from Macherey-Nagel (Duren, Germany). A micro BCA assay kit was obtained from Thermo Fisher Scientific (Waltham, MA, USA). All aqueous solutions and samples were prepared using water from a Milli-Q Advantage A10 Water Purification System (EMD Millipore Corporation, Billerica, MA, USA).
2.2. Apparatus
Work with the high performance lectin microcolumns was performed with a Jasco HPLC system (Tokyo, Japan) that contained two PU-2080 isocratic pumps, an AS-2057 autosampler, a CO-2067 column oven (i.e., for temperature control from 4 to 65 °C), and a UV-2075 UV detector. A two position/six port valve (MX Series II, IDEX Health & Science, Rohnert Park, CA, USA) was used to switch between the two isocratic pumps and their mobile phases. The HPLC system and valve were controlled with ChromNav software from Jasco. The chromatograms were analyzed with PeakFit 4.12 (Jandel Scientific Software, San Rafael, CA, USA). The microcolumns were packed by using an HPLC slurry packer from Chrom Tech (Apple Valley, MN, USA).
2.3. Preparation of lectin supports and microcolumns
AAL and Con A were immobilized onto Nucleosil Si-300 silica based on reductive amination [35]. Details of the immobilization procedures are provided in the Supplementary Material. The amount of immobilized lectin was determined by using a BCA protein assay [36]. For this assay, a small portion (i.e., around 4 mg) of each lectin support was washed with water three times (e.g., to remove any possible interferences from the storage buffer, such as Tris-HCl). The washed support was then dried at 50 °C prior to its use in the BCA assay. Con A was used as the standard in the assay for the Con A silica and HSA was used as a standard for the AAL support (note: HSA can be used as a general protein standard for the BCA assay and has a molar mass similar to AAL) [37,38]. A control support was used as the blank, and all measurements were made in triplicate. The total protein content in a microcolumn was determined from these results by combining them with the total internal volume of the microcolumn and the known packing density of the silica support (0.45 g/cm3).
Each support was downward packed into a 2.1 mm i.d. × 50 mm stainless steel column (Note: other biocompatible materials for column construction can also be used; no problems or issues with the use of stainless steel were noted in this particular study). The pressure during this packing process was ramped at a rate of 400 psi per min, held at 4000 psi for 40 min and then reduced at a rate of 200 psi per min. A Con A microcolumn was packed by using a 10 mM Tris-HCl (pH 7.4) buffer that contained 0.15 M NaCl, 0.50 mM CaCl2, 0.50 mM MgCl2 and 0.50 mM MnCl2 [39]. An AAL microcolumn was packed using 10 mM Tris-HCl (pH 7.4) buffer containing 0.15 M NaCl [28]. These packing solutions were the same as the application buffers that were to be used later with these supports, thus allowing the immobilized ligands to maintain their binding activities throughout this process. Control microcolumns with identical dimensions were packed with the same procedure but using water as the packing solution. These microcolumns were stored in their corresponding packing buffers at 4 °C.
2.4. Frontal analysis
Frontal analysis was performed in triplicate to determine the binding capacities for the lectin microcolumns [31]. The mean point of each breakthrough curve was determined by obtaining the first derivative of this curve and using an exponentially-modified Gaussian fit for the resulting derivative peak [31]. Identical conditions to those used with each lectin microcolumn were used with a control microcolumn to correct for the void volume and non-specific binding to the support (Note: no significant interactions were noted between the control support and analytes that were used for frontal analysis).
The frontal analysis experiments for Con A were performed by first equilibrating a Con A microcolumn in 10 mM Tris-HCl (pH 7.4) buffer containing 0.15 M NaCl, 0.50 mM CaCl2, 0.50 mM MgCl2 and 0.50 mM MnCl2; this equilibration was done at 0.10 mL min−1 and 4 °C. A solution that contained 1.3 mM p-NP-α-D-Man, prepared in the same pH 7.4 application buffer, was then continuously applied to the microcolumn at 0.10 mL min−1 and 4 °C. This particular combination of temperature and mobile phase was used because the equilibrium constant for p-NP-α-D-Man with Con A has been previously determined under these conditions [40]. The given flow rate was selected to provide an easily-measured difference in breakthrough times between the Con A and control microcolumns (i.e., ~ 1 min; see Supplementary Material). The elution of p-NP-α-D-Man was monitored at 400 nm, which gave a response throughout the frontal analysis studies that was within the linear range of the detector.
Frontal analysis studies for AAL used an initial buffer for equilibration that consisted of 10 mM Tris-HCl (pH 7.4) containing 0.15 M NaCl and that was passed through an AAL microcolumn at 0.10 mL min−1 and 25 °C (Note: this temperature and mobile phase replicated previous conditions used to measure the equilibrium constant for p-NP-α-L-Fuc with AAL) [41]. This same buffer was used to prepare a 1.6 mM solution of p-NP-α-L-Fuc, which was then applied to the microcolumn continuously at 0.10 mL min−1 and 25 °C. The elution of p-NP-α-L-Fuc was monitored at 400 nm, which gave a response within the linear range of the detector throughout the frontal analysis study.
2.5. Chromatographic studies with AGP
Work with a Con A microcolumn and AGP was performed under isocratic conditions, due to the moderate binding strength that was observed for AGP to such a microcolumn under the conditions used in this report (see discussion provided later in Sections 3.2–3.3). The mobile phase was 10 mM Tris-HCl (pH 7.4) containing 0.15 M NaCl, 0.50 mM CaCl2, 0.50 mM MgCl2 and 0.50 mM MnCl2. This mobile phase has been used in prior with Con A and is known to allow good binding activity for this lectin [39]. A 20 μL portion was injected for each AGP sample, as prepared in this mobile phase. Flow rates ranging from 0.025–0.10 mL min−1 and temperatures of 10–50 °C were considered for this work (see Section 3.3 for final optimized conditions). The elution of AGP was monitored at 280 nm. The resulting peaks for AGP were analyzed by using the linear progressive baseline correction of PeakFit 4.12 and an exponentially-modified Gaussian fit.
Work with AGP on AAL microcolumns was performed using the step elution mode of affinity chromatography [42], due to the strong retention observed for some AGP glycoforms on this type of microcolumn (see Sections 3.4–3.6 for more details). The application buffer was 10 mM Tris-HCl (pH 7.4) containing 0.15 M NaCl. This mobile phase was also chosen based on prior studies with AAL and is known to allow good activity for this lectin [28]. This same buffer was used to prepare samples containing various concentrations of AGP. A 20 μL portion for each sample was injected. Flow rates of 0.20–0.75 mL min−1 and a temperature of 10–50 °C were examined for use in the separation of AGP glycoforms (see Section 3.6 for final conditions). L-Fucose was added into the application buffer as a competing agent for sample elution. L-Fucose concentrations ranging from 0.25 to 2.0 mM were considered. The AGP peaks were detected at 280 nm. The resulting peaks for AGP were analyzed by using the linear progressive baseline correction of PeakFit 4.12 and an exponentially-modified Gaussian fit.
3. Results and Discussion
3.1. Initial characterization of lectin microcolumns
Several factors were considered during the initial characterization of the lectin supports and microcolumns that were employed in this study. The first item examined was the total protein content of each support. This content was determined to be 88 (± 6) mg Con A per gram of silica and 29.4 (± 1.7) mg AAL per gram of silica, respectively. For a 2.1 mm i.d. × 5.0 cm microcolumn, these results corresponded to a total protein content of 6.9 mg (or 65 nmol) of Con A and 2.3 mg (or 32 nmol) of AAL (see Section 2.3 for more details on this type of calculation). These protein contents were 1.8- to 2.2-fold higher than previously-reported values of 40–50 mg Con A per gram of silica when using the same immobilization method [34,39] and about 1.5-fold larger than a content of 20 mg AAL per gram of silica that was obtained by using biotinylated AAL and silica containing immobilized avidin [34]. The higher protein content of the Con A support in this report versus Ref. [39] was probably due to the smaller pore size and larger surface area of the silica that was present (i.e., 1000 vs. 300 Å, and 20 vs. 100 m2 g−1), allowing more room for the immobilization of Con A [43], as well as the larger amount of Con A that was used for immobilization (i.e., 45 mg vs 8 mg per gram of silica). The Con A content of the support in this work was also higher than in Ref. [34], even though this prior study used more Con A for immobilization (78 mg per gram silica) and employed a non-conventional macroporous silica that had a two-fold higher surface area than in this study. A higher AAL content was also noted in this report than in Ref. [34], which was again probably due to the different immobilization conditions and supports that were employed (e.g., the use of biospecific adsorption [34] vs. covalent immobilization). One benefit of the higher protein contents that were achieved in this work is that it allowed a corresponding reduction in the size of columns that could be used with these supports to bind to AGP or other targets for Con A and AAL.
The amount of active Con A and AAL within these microcolumns was initially determined by using frontal analysis. In these experiments, p-NP-α-D-Man was used as a model solute with a known binding strength for Con A, having a dissociation constant (based on a single-site model) of 18 μM at 4 °C and in the presence of pH 7.4, 20 mM Tris-HCl buffer containing 0.15 M NaCl, 0.10 mM CaCl2, and 0.10 mM MnCl2 [40]. p-NP-α-L-Fuc was utilized as an equivalent solute for AAL, having a dissociation constant, based on a single-site model, of 9 μM at 25 °C and in the presence of pH 7.4, 10 mM Tris-HCl buffer containing 0.8% NaCl [41]. These studies were carried out using applied concentrations for both solutes that were 70- to 180-fold larger than their dissociation constants, resulting in near saturation (i.e., > 99%) of the Con A or AAL microcolumns [44]. Some typical chromatograms that were generated are shown in the Supplementary Material. It was found that the amount of active binding sites for p-NP-α-D-Man in a Con A microcolumn was 89 (± 2) nmol, and the amount of binding sites for p-NP-α-L-Fuc in an AAL microcolumn was 152 (± 5) nmol. If it is assumed that there were a maximum of four possible binding regions for p-NP-α-D-Man on Con A and up to ten binding sites for p-NP-α-L-Fuc on AAL [45,46], these results indicated that 34% of the sites on Con A and 48% of those on AAL were active after immobilization and/or available for binding to the given applied solutes [44].
The dynamic loading capacity for each type of lectin microcolumn was determined by injecting a fixed volume containing various concentrations of AGP. Because the injection volume directly contributed to band-broadening, the injection volume was selected to provide an area for the retained peak of AGP that could be easily resolved from the non-retained fraction and reliably measured. For a Con A microcolumn, an injection volume of 20 μL was employed under isocratic conditions; this value was 9.5% of the volume for the first eluting fraction of AGP and 0.9% of the volume for the more highly retained fraction of AGP under isocratic conditions, as calculated by using the known injection volume and the measured peak volumes. A sample volume of 100 μL was used for an AAL microcolumn under step elution conditions, which was 32% of the peak volume for the first eluting AGP fraction and 38% of the volume for the highly retained fraction.
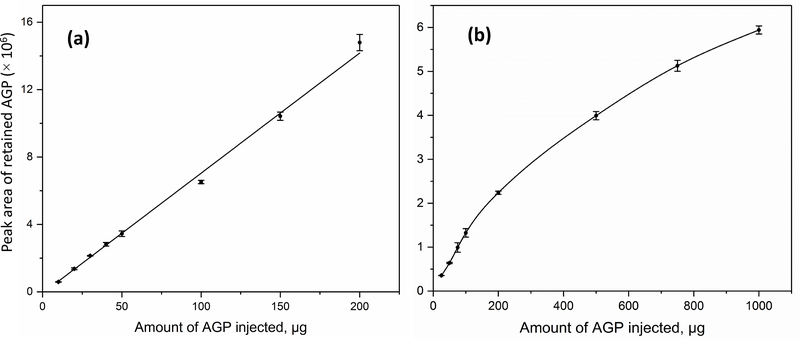
The results of the dynamic loading capacity studies are shown in Figure 1. The area of the retained peak for AGP on a 2.1 mm i.d. × 5.0 cm Con A microcolumn (the second peak in Figure 2), as measured at 0.10 mL min−1 (i.e., a flow rate within the range considered in Section 3.2 for use with this type of microcolumn), gave a linear increase up to at least a dynamic loading capacity of 200 μg (or 4.8 nmol) AGP. This value was 5.4% of the total loading capacity that had been measured by frontal analysis and was at least 2.7-fold higher than a binding capacity that has been reported for another glycoprotein (i.e., horseradish peroxidease) on a 1.0 mm i.d. × 5.0 cm Con A microcolumn that was based on non-conventional macroporous silica [34]. The area of the retained peak for AGP on a 2.1 mm i.d. × 5.0 cm AAL microcolumn (the second peak in Figure 5), as determined at 0.20 mL min−1 (i.e., a flow rate within the range considered later in Section 3.4), increased in a similar linear fashion up to a dynamic loading capacity of 100 μg (or 2.4 nmol) AGP; only slight deviations from linearity were seen up to 200 μg AGP and even 1000 μg could be applied to this type of column under non-linear conditions. A value of 100 μg was 1.6% of the total measured loading capacity that was acquired by frontal analysis for an AAL microcolumn. Based on these data, all later studies used a sample load of less than 4.8 nmol AGP for the Con A microcolumn and less than 2.4 nmol AGP for the AAL microcolumn, with less than half of these amounts typically being used in the following sections.
Figure 1.
Characterization of the dynamic loading capacity for AGP on 2.1 mm i.d. × 50 mm microcolumns containing (a) Con A or (b) AAL. The error bars represent a range of ± 1 S.D. (n = 3), and peak areas are expressed in relative units. The conditions used in (a) for the Con A microcolumn were as follows: injection volume, 20 μL; flow rate, 0.10 mL min−1; temperature, 25 °C; mobile phase used for isocratic elution, 10 mM Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl, 0.50 mM CaCl2, 0.50 mM MgCl2 and 0.50 mM MnCl2. The conditions in (b) for the AAL microcolumn were as follows: injection volume, 100 μL; flow rate, 0.20 mL min−1; temperature, 25 °C; application buffer, 10 mM Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl; elution buffer, same composition as the application buffer plus 1 mM L-fucose.
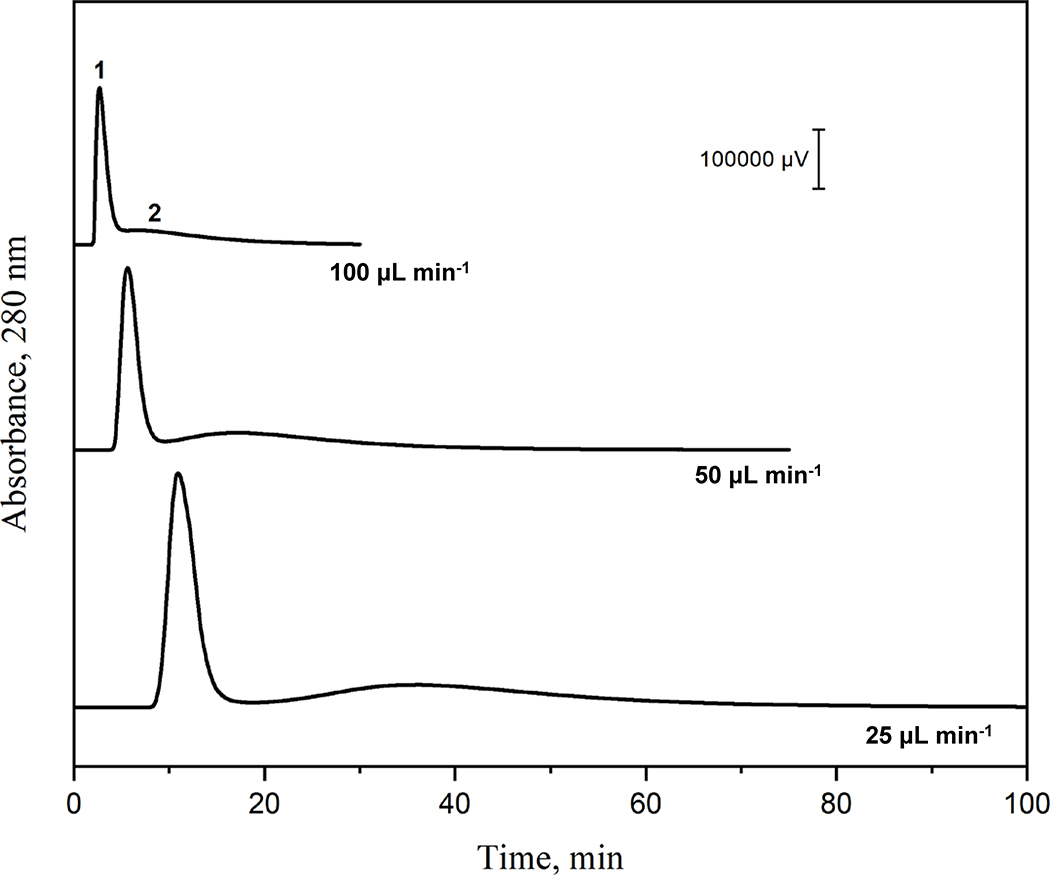
Figure 2.
Chromatograms obtained for AGP on a 2.1 mm i.d. × 50 mm Con A microcolumn at various flow rates. Conditions: amount of injected AGP, 200 μg; temperature, 25 °C. Other conditions were the same as in Figure 1(a).
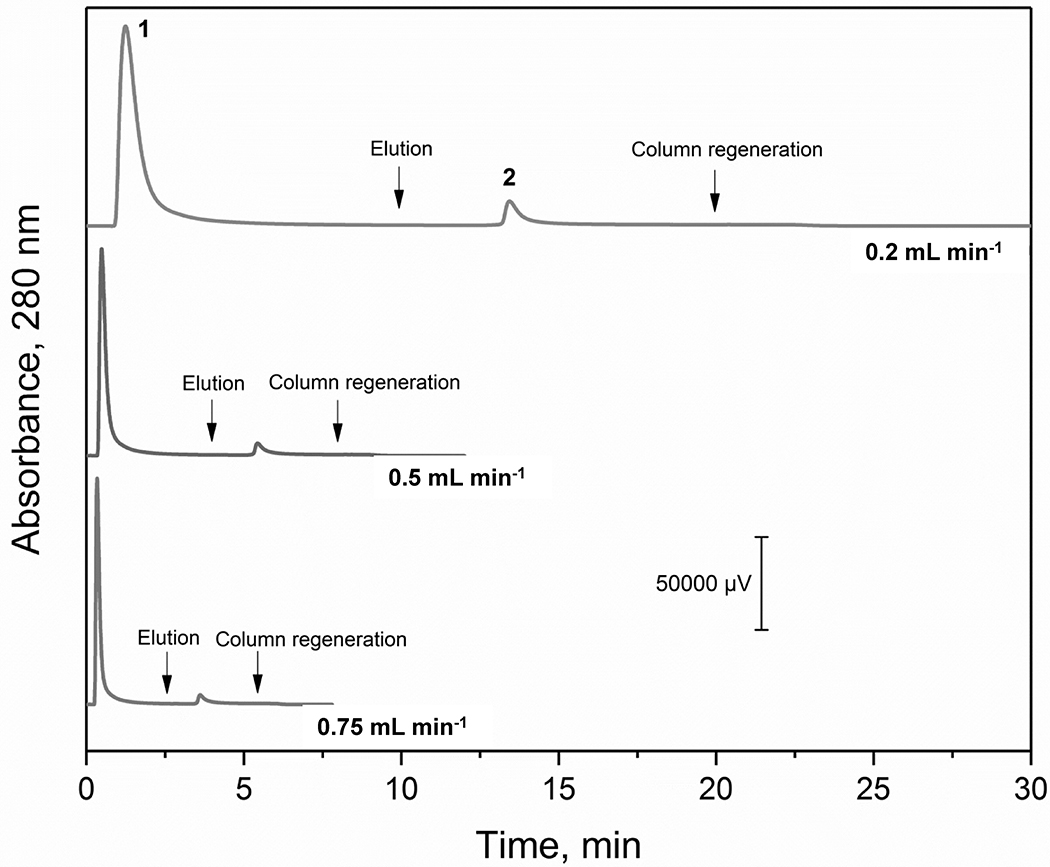
Figure 5.
Chromatograms obtained for AGP on a 2.1 mm × 50 mm AAL microcolumn at various flow rates. Conditions: injection volume, 20 μL of 1.5 mg mL−1 AGP (30 μg); column temperature, 25 °C; application buffer, 10 mM Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl; elution buffer, same as the application buffer but with the addition of 1 mM L-fucose.
3.2. Effect of flow rate on separation of AGP glycoforms by Con A microcolumns
Con A microcolumns have been used in a number of studies with step elution methods and strongly-retained glycoproteins such as horseradish peroxidase, ribonuclease B and various agents from serum [34,39,47]. However, it was found in this report that the binding of Con A to AGP was sufficiently weak to allow the separation of AGP glycoforms into two peaks under isocratic conditions (Note: no additional elution of AGP was observed when a competing agent such as methyl α-D-mannopyranoside was then added to the mobile phase, as has been employed in some prior work with AGP and Con A columns) [24]. The isocratic separation of AGP glycoform fractions is illustrated in Figure 2 at 25 °C when using a mobile phase that consisted of 10 mM Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl, 0.50 mM CaCl2, 0.50 mM MgCl2 and 0.50 mM MnCl2. The isocratic elution of the retained fraction for AGP (i.e., peak 2 in Figure 2), which contained biantennary complex-type N-glycans, was possible due to the moderate binding strength of Con A towards such groups [27] (Note: the presence of five glycosylation sites on AGP means that some of the retained AGP also contained tri- and tetra-antennary N-glycans in addition to bi-antennary glycans) [24]. The average retention factor for this retained fraction, and at the flow rates used in Figure 2, was 6.43 (± 0.08). The other glycoform fraction for AGP (peak 1) had only weak or negligible retention, with an average retention factor of 0.19 (± 0.01). The separation factor (α, or the ratio of the retention factors) for these two peaks was 33.8 (± 1.8). The ~45% portion of normal AGP that was found to be retained by the Con A microcolumn in this example was consistent with a range 40–55% that has been reported in previous studies for normal AGP when using Con A columns based on low-performance media [23–26].
Figure 2 also shows the effect of flow rate on the separation of these two AGP fractions on a Con A microcolumn. Reducing the flow rate over a representative range of 100 to 50 μL min−1 gave a 1.3-fold increase in resolution approaching a baseline separation; using 25 μL min−1 gave a further 1.1-fold increase in resolution but also resulted in longer analysis times. This increase in resolution was the result of an increase in efficiency for both the first eluting AGP peak (i.e., plate numbers of 18.6, 34 and 49 at 100, 50 and 25 μL min−1, respectively) and the second, more strongly retained peak (i.e., plate numbers of 4.8, 6.6 and 8.2). The decrease in flow rate also produced a lower back pressure (i.e., 435, 290 or 130 psi at 100, 50 or 25 μL min−1) and a corresponding increase in the separation time (from ~18 to 35 or 70 min).
Because the apparent peak width when shown on a time scale is affected by flow rate, the peak widths in terms of volume were also used to compare the two AGP fractions [48]. It was found that the peak volume for AGP peak 1 decreased by up to 1.8-fold over the flow range that was examined (i.e., from 0.29 to 0.21 or 0.16 mL when the flow rate was decreased from 100 to 50 or 25 μL min−1). The peak volume for AGP peak 2 was lowered by up to 1.3-fold under the same conditions (i.e., from 2.26 to 2.06 or 1.68 mL), as is reflected by the observed increase in resolution between the AGP peaks in Figure 2. Based on all of these results, a flow rate of 50 μL min−1 was used with Con A microcolumns in the following sections as a compromise between the analysis time and resolution that were obtained in the separation of the retained and non-retained AGP glycoform fractions.
3.3. Effect of temperature on separation of AGP glycoforms by Con A microcolumns
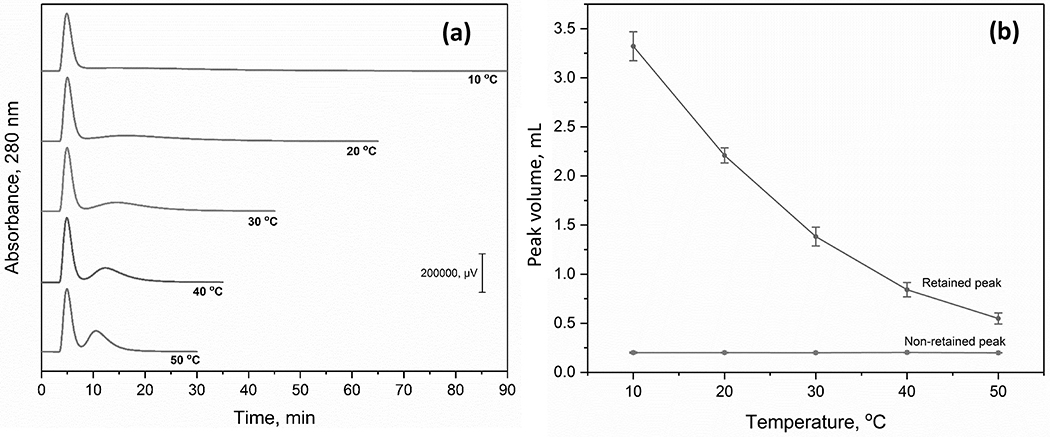
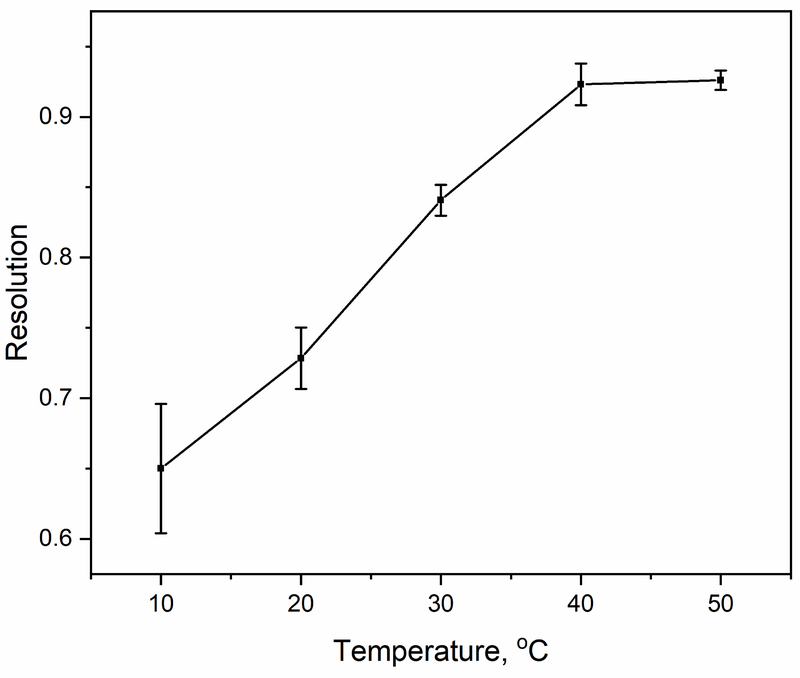
The effect of temperature on the retention of AGP fractions by Con A was also evaluated. Temperatures spanning up to 50 °C were considered, which are conditions at which the conformation of AGP has been shown to be stable for up to 6 days [53]. An increase in temperature from 10 to 50 °C reduced the average retention time of the retained peak for AGP from 29 to 12 min, as is shown in Figure 3(a) and the Supplementary Material. The elution time of peak 1 for AGP, which was non-retained or only weakly retained, remained essentially constant under the same conditions. Overall, a change in temperature from 10 to 50 °C resulted in a 1.4-fold increase in resolution for the two AGP peaks (i.e., going from a resolution of 0.65 to 0.93) and a 3-fold decrease in separation time (i.e., from 60 to 20 min). This change in binding with temperature was at least partly due to the change in the free energy from entropy but may have also been affected by alterations in the structure/conformation of Con A or AGP [51–53]. There was also a decrease in the back pressure from 2.0 to 1.2 MPa (290 to 174 psi) due to the lower viscosity of the mobile phase as the temperature was increased.
Figure 3.
(a) Chromatograms obtained for AGP on a 2.1 mm i.d. × 50 mm Con A microcolumn at various temperatures and (b) the volume of peaks 1 and 2 for AGP on the Con A microcolumn at the tested temperatures. Conditions: amount of injected AGP, 100 μg; and flow rate, 50 μL min−1. Other conditions were the same as in Figure 1(a). The error bars represent a range of ± 1 S.D. (n = 3).
The observed change in the retention factor for AGP peak 2 was used to estimate the global affinity constant (nKa’) between Con A and this glycoform fraction over the temperature range that was studied. This was accomplished by using Eq. (1) along with the measured retention factor (k) for peak 2, the void volume of the column (VM) and the total moles of active binding sites for AGP in the column (mL, as estimated by using data for ovalbumin - see Supplementary Material) [31].
| (1) |
The results are summarized in Table 1. It was assumed in these calculations that the value of mL did not change significantly over a temperature range of 10 to 50 °C. It was found through this approach that the global affinity constant for the retained AGP fraction on the Con A microcolumn decreased by 3.5-fold from ~1.8 × 105 M−1 to 5.0 × 104 M−1 as the temperature was increased from 10 to 50 °C. A similar change in binding strength (i.e., a 4.5-fold decrease) has been observed between Con A and p-aminophenyl β-D-glucoside in going from a temperature of 5 to 37 °C [49].
Table 1.
Estimated dissociation rate constants and global affinity constants for retained AGP glycoforms on a Con A microcolumna
| T, °C | HR, cm | Hk, cm | k | kd, s−1 | nKa’, × 104 M−1 |
|---|---|---|---|---|---|
| 10 | 2.00 (± 0.07) | 1.89 (± 0.07) | 8.95 (± 0.32) | 0.0030 (± 0.0001) | 17.6 (± 1.7) |
| 20 | 1.53 (± 0.09) | 1.42 (± 0.09) | 6.56 (± 0.07) | 0.0051 (± 0.0003) | 12.9 (± 1.2) |
| 30 | 0.97 (± 0.05) | 0.86 (± 0.05) | 4.94 (± 0.27) | 0.0103 (± 0.0007) | 9.7 (± 1.0) |
| 40 | 0.61 (± 0.06) | 0.50 (± 0.06) | 3.55 (± 0.19) | 0.0216 (± 0.0025) | 7.0 (± 0.7) |
| 50 | 0.43 (± 0.04) | 0.32 (± 0.04) | 2.52 (± 0.20) | 0.0397 (± 0.0051) | 5.0 (± 0.6) |
The values in the parentheses represents ± 1 S.D. The value of HM that was used with Eq. (2) to obtain the dissociation rate constant (kd) was equal to 0.111 (± 0.003) cm, and the value of v was 0.032 cm s−1. The globe affinity constant (nKa’) was calculated by using Eq. (1) along with an mL value of 6.7 (± 0.6) nmol and a value for VM of 0.132 mL for the Con A microcolumn.
The effect of column temperature on AGP band-broadening was next evaluated. As shown in Figure 3(b), the volume of the retained AGP peak was reduced by 6.1-fold in going from 10 to 50 °C, while the volume of the weakly or non-retained AGP fraction remained essentially constant. This effect was examined more closely by using the method of peak profiling and Eq. (2) to estimate the net dissociation rate constant (kd) for Con A and the retained AGP glycoforms under these temperature conditions [50].
| (2) |
In this equation, HR is the total plate height measured for the retained target (i.e., the AGP glycoforms in peak 2) on immobilized binding agent (i.e., Con A), and HM is the plate height measured on the same column and under the identical conditions for a non-retained solute (e.g., sodium nitrate in this study). The term Hk is the plate height contribution due to stationary phase mass transfer, v is the linear velocity, and k is the retention factor for the analyte [50].
As shown in Table 1, an increase in temperature from 10 to 50 °C resulted in a 13-fold increase in the apparent dissociation rate constant (i.e., from 0.003 to 0.040 s−1) for the retained AGP glycoforms on a Con A microcolumn. A similar trend has been seen for p-nitrophenyl-α-D-glucopyranoside and p-nitrophenyl-α-D-mannopyranoside on larger Con A columns, which gave a 3- to 5-fold increase in their dissociation rate constants as the temperature increased from 25 to 50 °C [51]. This change in the dissociation rate, combined with the 3.6-fold decrease in retention seen for the retained AGP fraction under the same conditions, corresponded to a 6-fold decrease in band-broadening due to stationary phase mass transfer and a 5-fold decrease in the total plate height (i.e., from 2.0 to 0.43 cm) when going from 10 to 50 °C.
The net result of these temperature effects was that the overall resolution between the AGP fractions increased by 1.4-fold (from 0.65 to 0.93) on a Con A microcolumn as the temperature was increased from 10 to 50 °C. This is illustrated in Figure 4 and occurred because the reduction in band-broadening overcame the decrease in retention for AGP that was observed under such conditions. Based on the combined observations that were made for Con A and AGP, a flow rate of 50 μL min−1 and a column temperature of 50 °C were used in all later experiments as optimized conditions for separating AGP glycoforms. The two AGP fractions were separated under these conditions, and monitored by an on-line absorbance detector, within 20 min or less on a 2.1 mm i.d. × 5.0 cm Con A microcolumn, as demonstrated at the bottom of Figure 3(a). This time corresponded to a total elution volume of 1.0 mL or less to acquire both the non-retained and retained AGP fractions. In contrast to this, prior methods for the separation of AGP glycoforms by Con A columns, including work with both low-performance media and non-conventional macroporous silica, have required much longer total elution times for the non-retained and retained fractions (80 min) [34] or much larger elution volumes (i.e., 60–75 mL up to more than 120–600 mL) [23–26].
Figure 4.
Resolution measured between the non-retained and retained peaks for AGP on a 2.1 mm × 50 mm Con A microcolumn at various temperatures. Other conditions are the same as in Figure 3. The error bar represent ± 1 S.D. (n = 3).
3.4. Effects of flow rate on separation of AGP glycoforms by AAL microcolumns
Previous work by others has shown that AAL columns can be used to enrich AGP [34]. However, no prior reports have used AAL to separate AGP glycoforms. It was found in this current study that an AAL microcolumn could separate AGP glycoforms into two peaks when using step elution based on the addition of L-fucose as a competing sugar, as is illustrated in Figure 5. It was possible to use step elution in this situation due to the relatively strong binding of AAL to fucose residues [41], as are present in some AGP glycoforms [1]. The step elution buffer and competing agent were applied after the non-retained peak had been completely eluted from the microcolumn. An advantage of using step elution is that it allowed high resolution (i.e., Rs > 1.5, and typically 6.1 or greater) to be achieved between the retained and non-retained AGP glycoforms through control of the time at which the competing agent was applied. This effect also made it possible to use much higher flow rates for the AAL microcolumns than were needed under isocratic conditions with the Con A microcolumn to provide good resolution between retained and non-retained AGP glycoforms.
The flow rate employed with an AAL microcolumn affected both the analysis time and broadening of the AGP peaks. For instance, as shown in Figure 5, an increase in the flow rate from 0.2 to 0.75 mL min−1 decreased the separation time from 15 to 5 min on a 2.1 mm i.d. × 50 mm AAL microcolumn, with a corresponding increase in the column back pressure from 1.9 to 7.0 MPa (275 to 1000 psi). The effect of the flow rate on band-broadening was examined by looking at the corresponding change in volume for the AGP peaks. This peak volume increased by 3.3-fold from 0.33 to 1.09 mL for the retained AGP glycoforms (peak 2), while the volume of the non-retained AGP fraction (peak 1) remained essentially constant at ~0.3 mL. The peak volume ratio for these retained vs. non-retained fractions of AGP was 1.1 at 0.2 mL min−1 and increased to 4.5 at 0.75 mL min−1. These results indicated that the retained AGP fraction was much more sensitive than the non-retained fraction to the effects of flow rate on band-broadening. This was expected because these two fractions had similar band-broadening processes in the microcolumn except for the stationary phase mass transfer (i.e., adsorption-desorption kinetics), which was present for only the retained AGP glycoforms [54]. Based on these results, a flow rate of 0.75 mL min−1 was used in all further studies with AGP and an AAL microcolumn as a compromise between separation speed and efficiency.
3.5. Effects of competing agent levels on separation of AGP glycoforms by AAL microcolumns
A previous report has shown that L-fucose can be employed as a competing agent for the step elution of retained AGP from a small AAL column [34]. This general type of step elution is known to be dependent on the concentration of competing agent that is passed through the column. For instance, Eq. (3) shows how the observed retention factor (k) for an analyte (A) is affected by the presence of a competing agent (I) for an immobilized binding agent that has single-site interactions for both A and I [31].
| (3) |
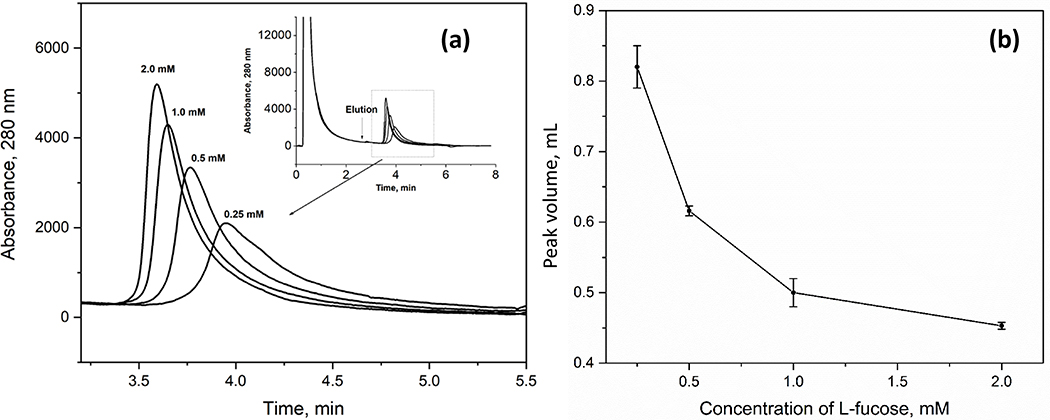
In this equation, Ka,I is the association equilibrium constant for the competing agent at its site of competition with A for the immobilized binding agent, and Ka,A is the association equilibrium constant for A at this same site. The term VM is again the void volume, [I] is the mobile phase concentration of the competing agent, and mL is the total moles of common binding sites for A and I in the column. Eq. (3) indicates that the value of k for A will decrease as [I] is increased, thus resulting in faster elution of A. This effect is illustrated in Figure 6(a) for the elution of retained AGP from an AAL microcolumn as the fucose concentration in the mobile phase is increased from 0.25 to 2.0 mM.
Figure 6.
(a) Chromatograms for AGP on a 2.1 mm × 50 mm AAL microcolumn and (b) volume of the retained AGP peak in the presence of various concentrations of L-fucose as a competing agent. Chromatographic condition: flow rate, 0.75 mL min−1; application buffer: 10 mM Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl; elution buffer, same as the application buffer but with the addition of 0.25 to 2.0 mM L-fucose. Other conditions are the same as in Figure 5. The error bar stands for ± 1 S.D. (n = 3).
The effect of altering the fucose concentration on band-broadening for the retained AGP glycoforms (peak 2) was also examined. The results are shown in Figure 6(b), as obtained on a 2.1 mm i.d. × 50 mm AAL microcolumn at 0.75 mL min−1. The profile for the elution peak became sharper as the concentration of L-fucose increased from 0.25 to 2.0 mM, as is illustrated in Figure 6(a). In addition, the peak volume for the retained AGP fraction decreased by 1.8-fold under these conditions, from 0.82 to 0.45 mL, as is shown Figure 6(b); it was also determined from these data that fucose concentrations above 2.0 mM would provide only a moderate further decrease in this peak volume. Based on these results, a final concentration of 2.0 mM L-fucose was used for the elution of retained AGP from AAL microcolumns in all further studies based on these results.
3.6. Effect of temperature on separation of AGP glycoforms by AAL microcolumns
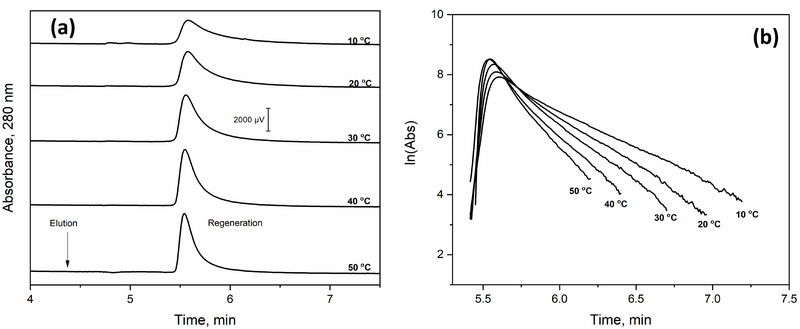
The effect of temperature on band-broadening was also evaluated for the AGP glycoforms on an AAL microcolumn. The results are shown in Figure 7 for a 2.1 mm i.d. × 50 mm AAL microcolumn that was used at 0.75 mL min−1 and with 2.0 mM fucose being employed as a competing agent for step elution. An increase in temperature from 10 to 50 °C reduced the peak volume for the retained AGP fractions by 2.6-fold, or from 0.93 to 0.36 mL, as indicated in Figure 7(a). This change was probably due to an increase in the dissociation rate of the retained AGP glycoforms from the AAL microcolumn as the temperature was increased.
Figure 7.
(a) Chromatograms and (b) natural logarithm of elution profile of AGP on a 2.1 mm × 50 mm AAL microcolumn at various temperatures. Conditions: sample, 20 μL AGP with a concentration of 1.5 mg mL−1; flow rate, 0.75 mL min−1; application buffer: 10 mM Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl; elution buffer, same as the application buffer but with the addition of 2.0 mM L-fucose.
This change in the rate of AGP dissociation from AAL with temperature was examined in more detail by using peak decay analysis [55–57]. In this method, the apparent dissociation rate constant (kd) for the retained AGP from an AAL microcolumn was obtained from the slope of the natural logarithm of the elution profile for AGP, as represented by Eq. (4) [55–57].
| (4) |
In this equation, t is the time after initiation of step elution, mE is the moles of analyte that has been eluted at time t, and is the moles of analyte that were initially bound to the microcolumn. As has been discussed for other systems, this equation assumes that no re-association of the analyte (i.e., AGP) occurs after it has dissociated from the microcolumn during the elution step and that this dissociation is the rate limiting step for elution of the analyte [55–57]. In this study, a flow rate of 0.75 mL min−1 was used during the peak decay measurements to prevent AGP from re-entering the stagnant mobile phase of the support and re-associating with AAL during the elution step. In addition, 2 mM L-fucose was present in the elution buffer and used to block the binding sites of AAL as AGP dissociated from the microcolumn.
The results that were obtained through the peak decay studies are shown in Figure 7(b) and Table 2. It was found that the apparent dissociation rate constant for the retained AGP glycoforms during the elution step increased by 2.3-fold (i.e., from 0.040 to 0.091 s−1) as the temperature was increased from 10 to 50 °C. These apparent dissociation rate constants for AGP from AAL during step elution were 2- to 13-fold higher than those observed in Section 3.3 for AGP glycoforms bound to Con A at the same temperatures but eluted under isocratic conditions.
Table 2.
Dissociation rate constants (kd) estimated by the peak decay method for retained AGP glycoforms on an AAL microcolumna
| T, °C | kd, s−1 |
|---|---|
| 10 | 0.040 (± 0.001) |
| 20 | 0.050 (± 0.001) |
| 30 | 0.061 (± 0.002) |
| 40 | 0.075 (± 0.001) |
| 50 | 0.091 (± 0.002) |
The values in the parentheses represent ± 1 S.D.
The overall binding activity between the immobilized AAL and AGP was also evaluated at these temperatures. The area for the retained AGP glycoforms remained at a relatively constant value over this temperature range, with a maximum value being obtained at 30 °C and only a slightly decrease of 5.2 to 16% occurring when increasing the temperature further to 40 or 50 °C, respectively. This indicated that the activity of the immobilized AAL and its ability to bind to AGP were relatively consistent up to 30 °C and had only a modest decrease up to 40 or 50 °C (e.g., due to change in the free energy due to entropy or changes in the conformation of either AAL or AGP with temperature). An increase in temperature from 10 to 50 °C also reduced the back pressure from 8.3 to 5.4 MPa (1200 to 780 psi) for such a microcolumn.
The observations made in this section and the previous sections in terms of the role played by temperature, flow rate and fucose concentration were used to select final conditions for the AAL microcolumns when separating AGP glycoforms. These conditions included a flow rate of 0.75 mL min−1, a column temperature of 50 °C, and an elution buffer that contained 2 mM L-fucose as a competing agent. Under these conditions, AGP glycoforms were resolved into two peaks within 6 min or less (a total elution volume of 4.5 mL or less), as shown in Figure 7(a). Although little prior work has been done in separating AGP glycoform fractions by AAL columns, this current method was significantly faster than a previous method that used a total elution time of 80 min for this glycoprotein [34]. In addition, it was found that ~9% of normal AGP was retained by the AAL microcolumn under the final optimized conditions. This result was consistent with an estimated binding level of AAL for AGP from standard serum or healthy control subjects of 18–20% (S.D., ± 3–4%), as determined in a lower-resolution separation method based on crossed affinoimmunoelectrophoresis [18].
3.7. Con A and AAL microcolumn stability and reproducibility
In the last stage of this work, the Con A and AAL microcolumns were used under their final selected conditions and evaluated for their stability and reproducibility. As shown in Table 3, a Con A microcolumn had within-day precisions (n = 3) for the retention times of the non-retained and retained AGP peaks of ± 0.34% and ± 4.7%. The day-to-day precisions (n = 9, as representing three measurements made per day over three sequential days) for the same peaks and their retention times were ± 1.1% and ± 4.0%. The within-day precisions for the areas of these peaks were ± 0.49% and ± 3.5%, respectively, and their day-to-day precisions were ± 0.42% and ± 3.0%. The day-to-day precisions for the peak areas agree with a previously-reported variation for other small Con A columns of less than 5% during the separation of glycoforms for ribonuclease B [39]. These results indicated that a Con A microcolumn could provide good reproducibility and stability for the separation and analysis of AGP glycoforms under the conditions that were used in this study.
Table 3.
Precisions of retention times and areas for retained and non-retained AGP peaks on Con A and AAL microcolumnsa
| Precision of retention time |
Precision of peak area |
|||
|---|---|---|---|---|
| Within-day (n = 3) | Day-to-day (n = 9) | Within-day (n = 3) | Day-to-day (n = 9) | |
| Con A microcolumn | ||||
| Non-retained AGP glycoforms (peak 1) | ± 0.34% | ± 1.1% | ± 0.49% | ± 0.42% |
| Retained AGP glycoforms (peak 2) | ± 4.7% | ± 4.0% | ± 3.5% | ± 3.0% |
| AAL microcolumn | ||||
| Non-retained AGP glycoforms (peak 1) | ± 0.68% | ± 0.68% | ± 3.0% | ± 2.2% |
| Retained AGP glycoforms (peak 2) | ± 0.27% | ± 0.18% | ± 1.7% | ± 1.5% |
The listed values are ranges of ± 1 R.S.D. The day-to-day precisions represent three measurements made per day over three days.
Similar levels of precision were obtained with conditions that were used to separate AGP glycoforms on an AAL microcolumn. The retention time of retained AGP glycoforms was controlled by the time at which the elution buffer was applied to the column, which occurred in these experiments at 2.7 min. In this case, the within-day precisions (n = 3) of the retention times for the non-retained and retained AGP glycoforms were ± 0.68% and ± 0.27%, and the day-to-day precisions (n = 9) were ± 0.68% and ± 0.18%. The within-day precisions for the peak areas were ± 3.0% and ± 1.7%, and the day-to-day precisions for these peak areas were ± 2.2% and ± 1.5%.
The type of Con A microcolumn that was prepared and used in this study was found to provide consistent binding to AGP glycoforms containing bi-antennary N-glycans. These AGP glycoforms had a measured abundance of 45–47% in a standard sample of normal AGP that was monitored by a Con A microcolumn over the course of fifteen months and at least 90 sample injection cycles. It was found that the type of AAL microcolumn employed in this work also gave consistent binding to AGP glycoforms containing fucosylated N-glycans. In this case, these AGP glycoforms were determined on a single AAL microcolumn to be present at a measured level of 9–10% in a standard sample of normal AGP, as determined over the course of at least seven months of use and more than 80 sample injections. These results fit with a previous report that has found small Con A columns could be employed without any major change in their capture efficiency for ribonuclease B over 30 injections and more than 6 months of operation [39].
4. Conclusion
In this report, affinity microcolumns containing Con A or AAL were developed, optimized and evaluated for the separation of non-retained and retained AGP glycoform fractions. Factors that were considered in this process include the binding capacity of these microcolumns and the effects of flow rate, temperature and elution method on these separations. Techniques that used for this analysis ranged from frontal analysis to zonal elution, peak profiling, and peak decay analysis. The final conditions selected for the separation of AGP glycoforms by a Con A microcolumn involved the use of a flow rate of 0.05 mL min−1 and a temperature of 50 °C under isocratic elution conditions, which gave a separation of non-retained and retained AGP glycoform fractions in less than 20 min. A separation of non-retained and retained AGP glycoform fractions was obtained in less than 6 min on an AAL microcolumn by using a flow rate of 0.75 mL min−1, a temperature of 50 °C, and 2 mM L-fucose as a competing agent. These separations were significantly faster than those that have been previously been reported for AGP with other Con A or AAL columns [23–26,34] and could be followed directly with on-line absorbance detector.
Both types of lectin microcolumns had good reproducibility in terms of their retention times and peak areas for the AGP glycoform fractions. For instance, the inter-day precision was less than 4.0% for the retention times and less than 3.0% for the peak areas on a Con A microcolumn. For AAL microcolumn, the inter-day precision was less than 0.7% for the retention time and less than 2.2% for the peak areas. It was also found that these microcolumns had good long term stabilities and reasonably low back pressures. All of these features should make microcolumns valuable in future work to examine structural variations in AGP that has been isolated from natural samples representing various disease states (e.g., by immunoaffinity chromatography) [3–12] and in use with other approaches, such as off-line coupling with capillary electrophoresis or mass spectrometry [26,34], to allow the multi-dimensional analysis of AGP glycoforms. The same methods and microcolumns should also be useful in examining other glycoproteins that may have glycans that can bind to Con A or AAL [34].
Supplementary Material
Highlights.
Lectin microcolumns were created to separate glycoforms of alpha1-acid glycoprotein.
Concanavalin A (Con A) and Aleuria Aurantia lectin (AAL) were used as binding agents.
Frontal analysis, peak decay analysis and peak profiling were used for column evaluation.
Glycoforms were separated in less than 20 min by Con A and less than 6 min by AAL.
An increase in column temperature reduced band-broadening and overall analysis times.
Acknowledgements
This work was supported by the National Institutes of Health under grant R01 GM044931.
Footnotes
Declaration of Interest Statement
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fournier T, Medjoubi N, Porquet D, Alpha-1-acid glycoprotein, Biochim. Biophys. Acta 1482 (2000) 157–171. [DOI] [PubMed] [Google Scholar]
- [2].Ceciliani F, Pocacqua V, The acute phase protein α1-acid glycoprotein: a model for altered glycosylation during diseases, Curr. Protein Pept. Sci. 8 (2007) 91–108. [DOI] [PubMed] [Google Scholar]
- [3].Fassbender K, Zimmerli W, Kissling R, Sobieska M, Aeschlimann A, Kellner M, Müller W, Glycosylation of α1-acid glycoprotein in relation to duration of disease in acute and chronic infection and inflammation, Clin. Chim. Acta 203 (1991) 315–327. [DOI] [PubMed] [Google Scholar]
- [4].Mackiewicz A, Marcinkowska-Pieta R, Ballou S, Mackiewicz S, Kushner I, Microheterogeneity of alpha1-acid glycoprotein in the detection of intercurrent infection in systemic lupus erythematosus, Arthritis Rheum. 30 (1987) 513–518. [DOI] [PubMed] [Google Scholar]
- [5].Serbource-Goguel Seta N, Durand G, Corbic M, Agneray J, Feger J, Alterations in relative proportions of microheterogenous forms of human α1-acid glycoprotein in liver disease, J. Hepatol 2 (1986) 245–252. [DOI] [PubMed] [Google Scholar]
- [6].Jezequel M, Seta NS, Corbic MM, Feger JM, Durand GM, Modifications of concanavalin A patterns of α1-acid glycoprotein and α2-HS glycoprotein in alcoholic liver disease, Clin. Chim. Acta 176 (1988) 49–57. [DOI] [PubMed] [Google Scholar]
- [7].Mackiewicz A, Pawo̵wski T, Mackiewicz-Pawłowska A, Wiktorowicz K, Mackiewicz S, Microheterogeneity forms of alpha1-acid glycoprotein as indicators of rheumatoid arthritis activity, Clin. Chim. Acta 163 (1987) 185–190. [DOI] [PubMed] [Google Scholar]
- [8].Hansen J-ES, Larsen VA, Bøg-Hansen TC, The microheterogeneity of α1-acid glycoprotein in inflammatory lung disease, cancer of the lung and normal health, Clin. Chim. Acta 138 (1984) 41–47. [DOI] [PubMed] [Google Scholar]
- [9].Succari M, Foglietti M-J, Percheron F, Microheterogeneity of α1-acid glycoprotein: variation during the menstrual cycle in healthy women, and profile in women receiving estrogen-progestogen treatment, Clin. Chim. Acta 187 (1990) 235–241. [DOI] [PubMed] [Google Scholar]
- [10].Balmaña M, Giménez E, Puerta A, Llop E, Figueras J, Fort E, Sanz-Nebot V, de Bolós C, Rizzi A, Barrabés S, de Frutos M, Peracaula R, Increased α1–3 fucosylation of α−1-acid glycoprotein (AGP) in pancreatic cancer, J. Proteomics 132 (2016) 144–154. [DOI] [PubMed] [Google Scholar]
- [11].Zhang D, Huang J, Luo D, Feng X, Liu Y, Liu Y, Glycosylation change of alpha-1-acid glycoprotein as a serum biomarker for hepatocellular carcinoma and cirrhosis, Biomark. Med 11 (2017) 423–430. [DOI] [PubMed] [Google Scholar]
- [12].Lacunza I, Sanz J, Diez-Masa JC, De Frutos M, CZE of human alpha-1-acid glycoprotein for qualitative and quantitative comparison of samples from different pathological conditions, Electrophoresis 27 (2006) 4205–14. [DOI] [PubMed] [Google Scholar]
- [13].Lacunza I, Sanz J, Diez-Masa JC, de Frutos M, Erratum: CZE of human alpha-1-acid glycoprotein for qualitative and quantitative comparison of samples from different pathological conditions, Electrophoresis 28 (2007) 492. [DOI] [PubMed] [Google Scholar]
- [14].Zhang C, Hage DS, Glycoform analysis of alpha1-acid glycoprotein by capillary electrophoresis, J. Chromatogr. A 1475 (2016) 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang C, Bi C, Clarke W, Hage DS, Glycoform analysis of alpha1-acid glycoprotein based on capillary electrophoresis and electrophoretic injection, J. Chromatogr. A 1523 (2017) 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Balaguer E, Neusüss C, Glycoprotein characterization combining intact protein and glycan analysis by capillary electrophoresis-electrospray ionization-mass spectrometry, Anal. Chem 78 (2006) 5384–5393. [DOI] [PubMed] [Google Scholar]
- [17].Kratz E, Poland DCW, van Dijk W, Kątnik-Prastowska I, Alterations of branching and differential expression of sialic acid on alpha-1-acid glycoprotein in human seminal plasma, Clin. Chim. Acta 331 (2003) 87–95. [DOI] [PubMed] [Google Scholar]
- [18].Hashimoto S, Asao T, Takahashi J, Yagihashi Y, Nishimura T, Saniabadi AR, Poland DCW, van Dijk W, Kuwano H, Kochibe N, Yazawa S, α1-Acid glycoprotein fucosylation as a marker of carcinoma progression and prognosis, Cancer 101 (2004) 2825–2836. [DOI] [PubMed] [Google Scholar]
- [19].Bergström M, Nilsson M, Isaksson R, Rydén I, Påhlsson P, Ohlson S, Lectin affinity capillary electrophoresis in glycoform analysis applying the partial filling technique, J. Chromatogr. B 809 (2004) 323–329. [DOI] [PubMed] [Google Scholar]
- [20].Shimura K, Tamura M, Toda T, Yazawa S, Kasai K, Quantitative evaluation of lectin-reactive glycoforms of α1-acid glycoprotein using lectin affinity capillary electrophoresis with fluorescence detection, Electrophoresis 32 (2011) 2188–2193. [DOI] [PubMed] [Google Scholar]
- [21].Rydén I, Lundblad A, Påhlsson P, Lectin ELISA for analysis of α1-acid glycoprotein fucosylation in the acute phase response, Clin. Chem 45 (1999) 2010–2012. [PubMed] [Google Scholar]
- [22].Liljeblad M, Rydén I, Ohlson S, Lundblad A, Påhlsson P, A lectin immunosensor technique for determination of α1-acid glycoprotein fucosylation, Anal. Biochem 288 (2001) 216–224. [DOI] [PubMed] [Google Scholar]
- [23].Nicollet I, Lebreton J-P, Fontaine M, Hiron M, Evidence for alpha-1-acid glycoprotein populations of different pI values after concanavalin a affinity chromatography study of their evolution during inflammation in man, Biochim. Biophys. Acta - Protein Struct 668 (1981) 235–245. [DOI] [PubMed] [Google Scholar]
- [24].Bierhuizen MFA, De wit M, Govers CARL, Ferwerda W, Koeleman C, Pos O, Van dijk W, Glycosylation of three molecular forms of human α1-acid glycoprotein having different interactions with concanavalin A, Eur. J. Biochem 175 (1988) 387–394. [DOI] [PubMed] [Google Scholar]
- [25].Hervé F, Gomas E, Duché J-C, Tillement J-P, Fractionation of the genetic variants of human α1-acid glycoprotein in the native form by chromatography on an immobilized copper(II) affinity adsorbent: heterogeneity of the separate variants by isoelectrofocusing and by concanavalin A affinity chromatography, J. Chromatogr. B 615 (1993) 47–57. [PubMed] [Google Scholar]
- [26].Kakehi K, Kinoshita M, Kawakami D, Tanaka J, Sei K, Endo K, Oda Y, Iwaki M, Masuko T, Capillary electrophoresis of sialic acid-containing glycoprotein. Effect of the heterogeneity of carbohydrate chains on glycoform separation using an α1-acid glycoprotein as a model, Anal. Chem 73 (2001) 2640–2647. [DOI] [PubMed] [Google Scholar]
- [27].Cummings RD, Darvill AG, Etzler ME, Hahn MG, Glycan-recognizing probes as tools, in: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH (Eds.), Essentials of Glycobiology, 3rd ed., Cold Spring Harbor, New York, 2017: pp. 611–626. [Google Scholar]
- [28].Kobayashi Y, High-performance lectin affinity chromatography, in: Hirabayashi J (Ed.), Lectins - Methods and Protocols, Springer, New York, 2014: pp. 69–78. [DOI] [PubMed] [Google Scholar]
- [29].Alley WR, Mann BF, V Novotny M, High-sensitivity analytical approaches for the structural characterization of glycoproteins, Chem. Rev 113 (2013) 2668–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moser AC, Hage DS, Immunoaffinity chromatography: an introduction to applications and recent developments, Bioanalysis 2 (2010) 769–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hage DS, High-performance affinity chromatography: a powerful tool for studying serum protein binding, J. Chromatogr. B 768 (2002) 3–30. [DOI] [PubMed] [Google Scholar]
- [32].Pfaunmiller EL, Anguizola JA, Milanuk ML, Papastavros E, Carter N, Matsuda R, Zheng X, Hage DS, Development of microcolumn-based one-site immunometric assays for protein biomarkers, J. Chromatogr. A 1366 (2014) 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vissers JPC, Recent developments in microcolumn liquid chromatography, J. Chromatogr. A 856 (1999) 117–143. [DOI] [PubMed] [Google Scholar]
- [34].Mann BF, Mann AKP, Skrabalak SE, V Novotny M, Sub 2-μm macroporous silica particles derivatized for enhanced lectin affinity enrichment of glycoproteins, Anal. Chem 85 (2013) 1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Emerson WS, The preparation of amines by reductive alkylation, Org. React 4 (1948) 174–255. [Google Scholar]
- [36].Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC, Measurement of protein using bicinchoninic acid, Anal. Biochem 150 (1985) 76–85. [DOI] [PubMed] [Google Scholar]
- [37].Wimmerova M, Mitchell E, Sanchez J-F, Gautier C, Imberty A, Crystal structure of fungal lectin: six-bladed β-propeller fold and novel fucose recognition mode for aleauria aurantia lectin, J. Biol. Chem 278 (2003) 27059–27067. [DOI] [PubMed] [Google Scholar]
- [38].Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P, Human serum albumin: from bench to bedside, Mol. Aspects Med 33 (2012) 209–290. [DOI] [PubMed] [Google Scholar]
- [39].Madera M, Mechref Y, V Novotny M, Combining lectin microcolumns with high-resolution separation techniques for enrichment of glycoproteins and glycopeptides, Anal. Chem 77 (2005) 4081–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ohyama Y, Kasai K, Nomoto H, Inoue Y, Frontal affinity chromatography of ovalbumin glycoasparagines on a concanavalin A-sepharose column. A quantitative study of the binding specificity of the lectin, J. Biol. Chem 260 (1985) 6882–6887. [PubMed] [Google Scholar]
- [41].Matsumura K, Higashida K, Hata Y, Kominami J, Nakamura-Tsuruta S, Hirabayashi J, Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography, Anal. Biochem 386 (2009) 217–221. [DOI] [PubMed] [Google Scholar]
- [42].Zhang C, Rodriguez E, Bi C, Zheng X, Suresh D, Suh K, Li Z, Elsebaei F, Hage DS, High performance affinity chromatography and related separation methods for the analysis of biological and pharmaceutical agents, Analyst 143 (2018) 374–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Clarke W, Beckwith JD, Jackson A, Reynolds B, Karle EM, Hage DS, Antibody immobilization to high-performance liquid chromatography supports: characterization of maximum loading capacity for intact immunoglobulin G and Fab fragments, J. Chromatogr. A 888 (2000) 13–22. [DOI] [PubMed] [Google Scholar]
- [44].Hage DS, Chen J, Quantitative affinity chromatography: practical aspects, in: Hage DS (Ed.), Handbood of Affinity Chromatography, 2nd ed., CRC Press, Boca Raton, 2005: pp. 595–628. [Google Scholar]
- [45].Sumner JB, Gralën N, Eriksson-Quensel I-B, The molecular weights of urease, canavalin, concanavalin A and concanavalin B, Science 87 (1938) 395–396. [DOI] [PubMed] [Google Scholar]
- [46].Olausson J, Tibell L, Jonsson B-H, Påhlsson P, Detection of a high affinity binding site in recombinant Aleuria aurantia lectin, Glycoconj. J 25 (2008) 753. [DOI] [PubMed] [Google Scholar]
- [47].Kullolli M, Hancock WS, Hincapie M, Preparation of a high-performance multi-lectin affinity chromatography (HP-M-LAC) adsorbent for the analysis of human plasma glycoproteins, J. Sep. Sci 31 (2008) 2733–2739. [DOI] [PubMed] [Google Scholar]
- [48].Huang X, Coleman WF, Zare RN, Analysis of factors causing peak broadening in capillary zone electrophoresis, J. Chromatogr. A 480 (1989) 95–110. [Google Scholar]
- [49].Oda Y, Kasai K, Ishii S, Studies on the specific interaction of concanavalin A and saccharides by affinity chromatography. Application of quantitative affinity chromatography to a multivalent system, J. Biochem 89 (1981) 285–296. [DOI] [PubMed] [Google Scholar]
- [50].Schiel JE, Ohnmacht CM, Hage DS, Measurement of drug−protein dissociation rates by high-performance affinity chromatography and peak profiling, Anal. Chem 81 (2009) 4320–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Muller AJ, Carr PW, Chromatographic study of the thermodynamic and kinetic characteristics of silica-bound concanavalin A, J. Chromatogr. A 284 (1984) 33–51. [Google Scholar]
- [52].Huet C, Lonchampt M, Huet M, Bernadac A, Temperature effects on the concanavalin molecule and on concanavalin A binding, Biochim. Biophys. Acta - Protein Struct 365 (1974) 28–39. [DOI] [PubMed] [Google Scholar]
- [53].Fukuda J, Yamazaki K, Iwura T, Arisaka F, Conformational stability, reversibility and heat-induced aggregation of α−1-acid glycoprotein, J. Biochem 156 (2014) 345–352. [DOI] [PubMed] [Google Scholar]
- [54].Giddings JC, Dynamics of Chromatography: Principles and Theory, Marcel Dekker, New York, 1965. [Google Scholar]
- [55].Moore RM, Walters RR, Peak-decay method for the measurement of dissociation rate constants by high-performance affinity chromatography, J. Chromatogr. A 384 (1987) 91–103. [Google Scholar]
- [56].Anguizola JA, Pfaunmiller EL, Milanuk ML, Hage DS, Peak decay analysis and biointeraction studies of immunoglobulin binding and dissociation on protein G affinity microcolumns, Methods 146 (2018) 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yoo MJ, Hage DS, Use of peak decay analysis and affinity microcolumns containing silica monoliths for rapid determination of drug–protein dissociation rates, J. Chromatogr 1218 (2011) 2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.