Abstract
Background
Hidradenitis suppurativa is a complex and infrequent autoinflammatory disease that impacts on quality of life. Its pathogenesis is not fully understood, which limits the development of curative treatments.
Objectives
To evaluate clinical and quality of life aspects of hidradenitis suppurativa patients from a social group on the Internet.
Methods
A cross-sectional, Internet-based survey study among participants in a discussion group (Facebook) of individuals with hidradenitis suppurativa. Patients were asked to answer a questionnaire about clinical-demographic aspects and quality of life (DLQI-BRA).
Results
A total of 390 individuals agreed to participate in the study, 82% of them female, median age (p25-p75), of 31 (25-37) years old, disease onset at 15 (13-23) years, family member affected in 20% of cases, overweight (BMI 29 [25-33]) kg/m2 and severe impact on quality of life (DLQI 20 [13-25]). Regarding Hurley's classification, the participants provided information that enabled classification into: I (19%), II (52%) and III (29%). More severe cases were associated with males (OR = 1.69), higher weight (BMI: OR = 1.03) non-white color (OR = 1.43) and higher frequency of other autoinflammatory diseases (OR = 1.37).
Study limitations
Voluntary adherence survey with self-completion of the questionnaire by 390 from about 1600 group members.
Conclusions
Hidradenitis suppurativa patients who participated in a social network group had onset of the disease after puberty, with a predominance in females and overweight people, with great impact on the quality of life.
Keywords: Suppurative Hidradenitis, Quality of life, Crowdsourcing, Surveys and questionnaires
INTRODUCTION
Hidradenitis suppurativa (HS), acne inversa or Verneuil disease is an autoinflammatory, debilitating chronic disease that progresses with recurrent and hard-to-treat papules-nodules, pustules, abscesses and fistulas. The lesions are predominant in areas that are rich in apocrine glands such as axillae, inguinal and anogenital region.1,2 Its diagnosis is based in three mandatory clinical criteria: typical lesions, typical location, chronicity and recurrence.3,4
International estimates for the prevalence of HS range between 0.3% to 1% of the general population, being most frequent in women (2-3:1). In Brazil, the estimated incidence is of 0.41%. The first symptoms appear after puberty (90% after 11 years of age), with a higher incidence between 20 and 30 years of age.5-7
HS is associated to other comorbidities, such as obesity, metabolic syndrome, spondyloarthropathies, severe acne (follicular occlusion tetrad), cardiovascular disease and inflammatory bowel disease (Crohn's disease). Due to the symptoms, physical limitations imposed by the disease, frequent recurrence of the lesions and involvement of patients of childbearing age, HS greatly impacts in the quality of life, increasing suicide risk.8-10
There are few epidemiological studies in Brazil characterizing patients according to clinical and demographic aspects and to the impact on the quality of life. The territorial extension of the country and patient dispersion between multiple specialties (gynecology, plastic surgery, proctology, and dermatology) make the study of large series of cases difficult.11
The Internet enabled new methods of collection of multicentric clinical data, characterizing a way of knowledge and technology production where people from different locations can offer their services, observations, knowledge and intelligence for the development of products or the study of some areas of knowledge voluntarily.12-14
The objective of this study was to describe and analyze clinical and quality of life aspects of HS patients participating in a Brazilian discussion group about the disease on Facebook.
METHODS
It is a cross-sectional, social media-survey study with participants of a Facebook group destined to discussion and support for patients with HS (http://www.facebook.com/groups/hidradenitesupurativa/) between December 2016 and September 2017. The project was approved by the committee of ethics in research of the institution (CAAEE nº 61575116.4.0000.5411). There was no gender or age group restriction.
HS patients enrolled in the group were invited to access a specific online form (https://goo.gl/forms/24qbLy0T4VLIefd92), developed by the authors of this study, with questions about personal and clinical course characteristics and the Brazilian version of DLQI (Dermatology Life Quality Index).15 All registries were verified regarding duplicity and data consistency by redundant questions, included with this purpose.
Hurley's classification (clinical severity) was indicated based on conceptual elements and photographs of lesions with which the patients should identify.
The answers were tabulated and analyzed according to their frequency in percentage or to measurements of central tendency (mean or median) and dispersion (standard deviation and quartiles: p25-p75), according to the normality of data distribution, evaluated by the\Shapiro-Wilk test.16
The proportions between the subgroups were compared with Pearson's chi-square test.17 The median number of body regions involved was compared, according to Hurley's scale, by the Jonckheere-Terpstra test.18
Subsequently, the variations in the levels of clinical severity, quality of life, and age of onset of the disease were evaluated in a multivariate way according to clinical elements (cofactors) by generalized linear models, with estimation of robust covariance. The adjustment of the models was given by AIC (Akaike Information Criterion), being considered significant p<0.05.17
For the multivariate analysis, missing data in up to 5% of the sample were estimated with the technique of multiple imputation, with 10 interactions.19
Clinical and demographic variables and affected body regions were categorized and their multivariate correlations (simultaneous) were explored with the analysis of multiple correspondence of two dimensions. The dimension of the effect was estimated by the inertia in each dimension and the associations between the variables were represented by their geometrical proximities.20
The sample was initially planned to satisfy a multivariate model with up to 14 covariables according to the adapted formula of Freeman, with a minimum of 300 participants in total.21
The online form was developed with the web Google Forms (http://www.google.com/forms/) app and data were tabulated in MsExcel 2010 and analyzed in the software IBM SPSS 25.0.22
RESULTS
At the time of the study, the support group was composed by nearly 1600 participants, among which were HS patients, family members, physicians and friends. Of those, 390 HS patients were assessed and their clinical data are shown in table 1. We highlight the predominance of females, the age of onset of the disease in adolescence, the long time between disease onset and accurate diagnosis, high prevalence of current smokers, family history of HS, overweight/obesity and a marked impact in the quality of life.
Table 1.
Main clinical and quality of life data of the hidradenitis suppurativa patients evaluated (n=390)
| Variables | Value |
|---|---|
| Sex - N (%) | |
| Female | 318 (82) |
| Male | 72 (18) |
| Age (in years)* | 31 (25-37) |
| BMI (kg/m2)* | 29 (25-33) |
| Self-reported skin color - N (%) | |
| White | 218 (56) |
| Brown | 127 (33) |
| Black | 31 (8) |
| Yellow | 13 (3) |
| Current smoking - N (%) | 103 (26) |
| Region in Brazil - N (%) | |
| South | 48 (12) |
| Southeast | 238 (61) |
| Mid-West | 28 (7) |
| North | 10 (3) |
| Northeast | 55 (14) |
| First degree family member - N (%) | 79 (20) |
| Age of onset (years)* | 15 (13-22) |
| Time to diagnosis (years)* | 6 (2-12) |
| Time with the disease (years)* | 13 (8-20) |
| Body area affected - N (%) | |
| Inguinal | 295 (76) |
| Axilla | 291 (75) |
| Anogenital | 171 (44) |
| Mammary | 123 (32) |
| Cervical | 55 (14) |
| Buttocks | 23 (6) |
| Number of body areas affected* | 3 (2-4) |
| Outbreaks in the past six months* | 5 (3-10) |
| Hurley stage - N (%) | |
| I | 73 (19) |
| II | 199 (52) |
| III | 113 (29) |
| DLQI* | 20 (13-25) |
BMI=body mass index; DLQI=Dermatology life quality index;
median (p25-p75)
When considering body regions affected according to sex, females had significantly more lesions on the breasts (37% vs. 7%) and inguinal region (79% vs. 63%); however, less perianal lesions (41% vs. 56%). The median number of body regions affected was higher according to Hurley's grade (p<0.01): I (1), II (2) and III (3).
The main comorbidities reported by those interviewed are described in table 2. Conditions associated to hyperinsulinemia, such as diabetes, polycystic ovaries and obesity, besides other autoinflammatory diseases such as acne, arthritis, ankylosing spondylitis, inflammatory bowel disease and psoriasis were reported.
Table 2.
Main comorbidities reported by hidradenitis suppurativa patients evaluated (n=390)
| Comorbidities | N (%) | |
|---|---|---|
| Obesity | 127 (33) | |
| Polycystic ovaries | 88 (28)* | |
| Acne (scarring) | 105 (27) | |
| Lumbar morning pain/stiffness/spondylitis | 69 (18) | |
| Diabetes | 21 (5) | |
| Morning arthritis (>2 joints) | 17 (4) | |
| Crohn's disease/ulcerative colitis | 6 (2) | |
| Psoriasis | 3 (1) |
percentage of 318 females
The factors associated to worsening HS, identified and reported by the patients, are shown in table 3. Hormonal, climate and behavioral aspects were mentioned. The treatments used by the patients are shown in table 4. Of note, the extensive use of antibiotics, surgery and retinoids.
Table 3.
Main worsening factors for hidradenitis suppurativa reported by the patients evaluated (n=390)
| Worsening factors | N (%) | |
|---|---|---|
| Menstrual period | 299 (94)* | |
| Tight clothing | 279 (72) | |
| Heat/summer | 277 (71) | |
| Shaving | 270 (69) | |
| Sweat | 269 (69) | |
| Psychological stress | 264 (68) | |
| Certain foods | 28 (7) |
percentage of 318 females
Table 4.
Main treatments reported by hidradenitis suppurativa patients evaluated (n=390)
| Reported treatments | N (%) |
|---|---|
| Oral or injectable antibiotics | 293 (75) |
| Topical antibiotics | 270 (69) |
| Surgery | 153 (39) |
| Oral isotretinoin/acitretin | 117 (30) |
| Laser hair removal | 53 (14) |
| Metformin | 48 (12) |
| Dapsone | 29 (6) |
| Antiandrogens (cyproterone, spironolactone) | 21 (7)* |
| Adalimumab/infliximab | 16 (4) |
| Oral or injectable steroids | 10 (3) |
| Finasteride | 10 (3) |
| Radiotherapy | 3 (1) |
percentage of 318 females
With the multivariate analysis, the earlier onset of the disease was directly and independently associated to female sex and to more severe disease (Hurley in the number of affected regions) (Table 5).
Table 5.
Generalized linear model, with distribution of probability type gamma and identity function for the age of onset of the symptoms (n=390)
| Cofactors | β coefficient of regression (IC 95%) |
p-value |
|---|---|---|
| Female gender | -1.74 (-3.51 to 0.00) | <0.01 |
| Self-reported
white skin color |
0.19 (-1.12 to 1.50) | 0.45 |
| Other
auto-inflamma- tory diseases |
-0.05 (-1.42 to 1.32) | 0.15 |
| Hyperinsulinemia | 0.23 (-1.62 to 2.08) | 0.77 |
| First degree
relative affected |
-0.57 (-2.30 to 1.6) | 0.20 |
| Body mass index | 0.06 (-0.0 to 0.21) | 0.70 |
| Number of
body regions affected |
<0.01 | |
| Severity (Hurley) | <0.01 | |
| I | 0.00 (-) | |
| II | -3.45 (-5.68 to -1.22) | |
| III | -0.87 (-3.36 to 1.62) |
AIC=2562; p(model)<0.01; p(constant)<0.01
Table 6 shows the factors associated to more severe HS. There was a significant and independent association with male gender, self-reported nonwhite skin color, overweight and other auto-inflammatory diseases.
Table 6.
Generalized linear model (ordinal logistic regression) for clinical severity, according to Hurley's classification (n=390)
| Variables | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Female gender | 1.03 (1.00-1.6) | 0.02 |
| Self-reported white
skin color |
1.43 (1.13-1.80) | <0.01 |
| Other
auto- inflammatory diseases |
1.37 (1.08-1.73) | <0.01 |
| Hyperinsulinemia | 0.84 (0.63-1.14) | 0.28 |
| First degree
relative affected |
0.97 (0.72-1.29) | 0.82 |
| Body mass index | 1.03 (1.01-1.06) | 0.02 |
| Current smoking | 0.93 (0.72-1.21) | 0.59 |
| Age of onset | 0.99 (0.97-1.00) | 0.14 |
AIC=791; p(model)<0.01
A higher impact in the quality of life was independently identified among patients who self-reported as nonwhite, having other autoinflammatory diseases, family history of HS, smokers, large number of affected body regions and more severe clinical lesions (Table 7).
Table 7.
Generalized linear model, with distribution of probability type gamma and identity function for DLQI-BRA score (n=390)
| Cofactors | β coefficient
of regression (IC 95%) |
p-value |
|---|---|---|
| Female gender | 0.39 (-1.93-2.71) | 0.30 |
| Self-reported white
skin color |
1.82 (0.30-3.33) | <0.01 |
| Other
auto-inflammato- ry diseases |
0.53 (-1.07-2.14) | <0.01 |
| Hyperinsulinemia | 1.14 (-1.17-3.44) | 0.71 |
| First degree
relative affected |
1.41 (-0.44-3.26) | 0.04 |
| Current smoking | 1.88 (0.29-3.47) | 0.02 |
| Body mass index | -0.09 (-0.28-0.10) | 0.12 |
| Time with the
disease (years) |
0.00 (-0.12-0.11) | 0.16 |
| Number of body
re- gions affected |
0.84 (0.12-1.56) | <0.01 |
| Severity (Hurley) | <0.01 | |
| I | 0.00 (-) | |
| II | 1.40 (-0.73-3.52) | |
| III | 5.05 (2.78-7.31) |
AIC=2618; p(model)<0.01; p(constant)<0.01
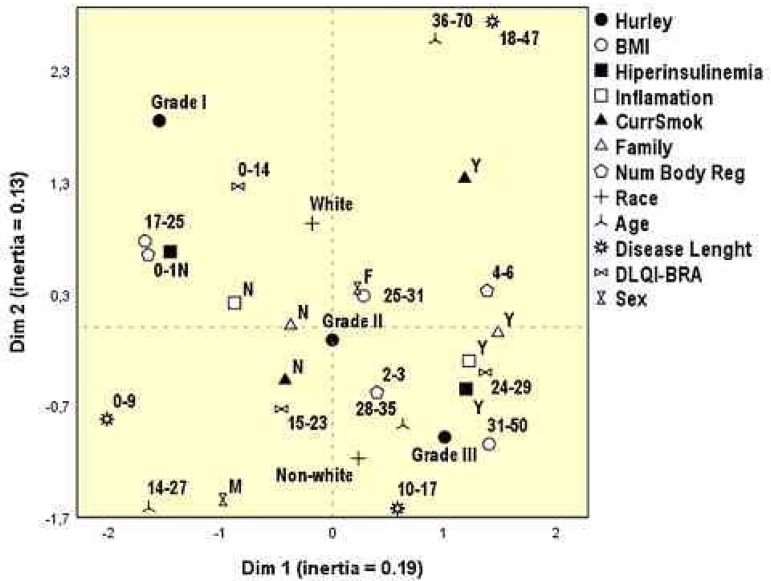
The perceptual map of figure 1 integrates and supports the results from tables 5 to 7. We highlight the geometrical proximities of the higher Hurley grade (III) and nonwhite patients, hyperinsulinemia, larger number of affected regions, inflammatory comorbidities, positive family history, and higher BMI. The mildest grade (I) approximates lower BMIs, self-reported white skin color, lower number of affected regions, absence of hyperinsulinemia, of family history and inflammatory comorbidities. Furthermore, quality of life scores follow the degree of inflammation.
Figure 1.
Perceptual map of the analysis of multiple correspondence of clinical variables and quality of life of the participants evaluated (n=390)
DISCUSSION
The group of patients studied had similar features to many series of cases described in the literature: predominance of females, age of onset after puberty, high prevalence of smoking, obesity, inflammatory diseases, family history of HS, diseases coursing with hyperinsulinemia and a marked impact in the quality of life.23,24
The factors related to worsening of the condition referred mainly to hormonal changes of the menstrual cycle, occlusion and heat. Aspects related to diet were reported by 7% of the patients, what indicates a possibility for the investigation of dietary aspects, still little explored in international studies.25,26
A great variety of treatments was reported by patients; in the great majority antibiotics were prescribed. A small portion reported surgical treatment and even less patients reported the use of biologics, what reflects a still limited access to treatments of the most severe forms.27
Since the patients studied came from a help and discussion group about the disease, it is plausible that severe cases are more representative in the sample (Hurley II and III) than what would be expected in the general population, as what happens in series of cases from tertiary services. However, the higher proportion of severe cases favors a comparison between the subgroups and the exploration of factors associated to increased clinical severity, in this case: male gender, self-reported nonwhite skin color, overweight and other auto-inflammatory diseases.23,28
The largest epidemiological studies in HS come from hospital cases, subject to selection bias. Our results support the literature regarding the factors associated to increased severity, and also highlight the importance of early diagnosis and treatment, in order to minimize sequelae.11,29,30
Social media users can present selection bias for better financial conditions and education. For this reason, the survey did not evaluate the aspects related to socioeconomic condition, although increased severity and prevalence of HS among less favored groups are reported.31 In Brazil, low family income and schooling are also associated to nonwhite populations.32-35 Specific designs should be developed to explore these aspects linked to HS.
HS clinical manifestations appear after puberty, usually between the second and third decades of life. Cases of earlier onset were associated to female gender and to more severe disease (Hurley and number of affected body regions). These data confirm the results of international case series, except for family history of HS which, in our sample, was not associated to an earlier onset of the disease.36-39
Many variables indicated the influence in the perception of quality of life ((QoL) in HS patients, suggesting that different life dimensions could be involved in the process of perception of the distress caused by the disease, besides clinical and symptomatic representation. QoL scores in patients with severe HS are usually higher than in other skin conditions and also higher compared to patients with clinical conditions such as malignant neoplasms, Parkinson's disease and stroke.40-44
The multivariate spatial exploration allowed for a broad evaluation of the correlations between the different variables studied. The perception of impact in the quality of life followed the grades of severity of HS which, in turn, were correlated to inflammatory aspects, hyperinsulinemia, family history and race (nonwhite). These findings reinforce the concept that HS is a multifactorial disease, dependent of the genetic background and triggered by endocrine and inflammatory aspects.45-48
Studies with nonrandomized samples disfavors the estimation of the prevalence of the disease in the population or even of the variables studied, since the proportion of cases with different severities might not represent the fractions found in the population. Moreover, social media-recruited surveys can inflate severe cases of the disease. However, as with other cross-sectional studies, internal comparisons among subtype variables can be estimated, with limited inference in the population.
Self-reported surveys are also susceptible to the loss of accuracy of information and the inability of confirming their authenticity. It was up to the authors to develop an intuitive questionnaire and, at the end, identify cases of duplicate or inconsistent forms that could lead to loss of quality of the analysis. Still, it is one of the largest series of cases of HS ever studied, whose observed clinical features and associations are compatible with the literature.2,5,7,11,40,49,50
The formation of patient groups for support, information and discussion is made easier by the Internet and social media. Such grouping favors the collection of clinical-epidemiological information or even the recruitment of patients to clinical trials or pathophysiology studies. Recruiting techniques in virtual groups such as crowdsourcing can have a multicentric characteristic and, particularly in less prevalent diseases, encourage science progress quicker than what would happen with individualized study groups.
CONCLUSIONS
Hidradenitis suppurativa patients that participate in a social media group developed the disease after puberty, had a predominance of females, overweight and a marked impact in the quality of life. Recruitment of participants in social media can be a source of relevant scientific information, particularly in less frequent diseases.
Footnotes
Work conducted at the Department of Dermatology and Radiotherapy, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista, Botucatu (SP), Brazil.
Financial support: None.
Conflict of interest: None.
AUTHORS’CONTRIBUTIONS
Isabel Greco Tavora
0000-0002-6476-7556
Approval of the final version of the manuscript; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript
Gabriela Cabral Bissoli
0000-0002-6730-3327
Approval of the final version of the manuscript; Conception and planning of the study; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Critical review of the literature; Critical review of the manuscript
Hélio Amante Miot
0000-0002-2596-9294
Statistical analysis; Approval of the final version of the manuscript; Conception and planning of the study; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Effective participation in research orientation; Intellectual participation in propaedeutic and/or therapeutic conduct of the cases studied; Critical review of the literature; Critical review of the manuscript
Juliano Vilaverde Schmitt
0000-0002-7975-2429
Statistical analysis; Approval of the final version of the manuscript; Conception and planning of the study; Elaboration and writing of the manuscript; Obtaining, analyzing and interpreting the data; Effective participation in research orientation; Intellectual participation in propaedeutic and/or therapeutic conduct of the cases studied; Critical review of the literature; Critical review of the manuscript
REFERENCES
- 1.Sá DC, Festa C Neto. Inflammasomes and dermatology. An Bras Dermatol. 2016;91:566–578. doi: 10.1590/abd1806-4841.20165577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt JV, Bombonatto G, Martin M, Miot HA. Risk factors for hidradenitis suppurativa: a pilot study. An Bras Dermatol. 2012;87:936–938. doi: 10.1590/S0365-05962012000600024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: Clinical presentations and phenotypes. J Am Acad Dermatol. 2015;73(Suppl 1):S23–S26. doi: 10.1016/j.jaad.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Revuz JE, Jemec GB. Diagnosing Hidradenitis Suppurativa. Dermatol Clin. 2016;34:1–5. doi: 10.1016/j.det.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Jemec GB, Kimball AB. Hidradenitis suppurativa: Epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(Suppl 1):S4–S7. doi: 10.1016/j.jaad.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Ianhez M, Schmitt JV, Miot HA. Prevalence of hidradenitis suppurativa in Brazil: a population survey. Int J Dermatol. 2018;57:618–620. doi: 10.1111/ijd.13937. [DOI] [PubMed] [Google Scholar]
- 7.Micheletti RG. Natural history, presentation, and diagnosis of hidradenitis suppurativa. Semin Cutan Med Surg. 2014;33(Supp l):S51–S53. doi: 10.12788/j.sder.0092. [DOI] [PubMed] [Google Scholar]
- 8.von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809–813. doi: 10.1046/j.1365-2133.2001.04137.x. [DOI] [PubMed] [Google Scholar]
- 9.Thorlacius L, Cohen AD, Gislason GH, Jemec GBE, Egeberg A. Increased Suicide Risk in Patients with Hidradenitis Suppurativa. J Invest Dermatol. 2018;138:52–57. doi: 10.1016/j.jid.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Tzellos T, Zouboulis CC, Gulliver W, Cohen AD, Wolkenstein P, Jemec GB. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015;173:1142–1155. doi: 10.1111/bjd.14024. [DOI] [PubMed] [Google Scholar]
- 11.Andrade TCPC, Vieira BC, Oliveira AMN, Martins TY, Santiago TM, Martelli ACC. Hidradenitis suppurativa: epidemiological study of cases diagnosed at a dermatological reference center in the city of Bauru, in the Brazilian southeast State of Sao Paulo, between 2005 and 2015. An Bras Dermatol. 2017;92:196–199. doi: 10.1590/abd1806-4841.20175588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong AW, Harskamp CT, Cheeney S, Schupp CW. Crowdsourcing in eczema research: a novel method of data collection. J Drugs Dermatol. 2012;11:1153–1155. [PubMed] [Google Scholar]
- 13.Armstrong AW, Harskamp CT, Cheeney S, Schupp CW. Crowdsourcing for research data collection in rosacea. Dermatol Online J. 2012;18:15–15. [PubMed] [Google Scholar]
- 14.Behrend TS, Sharek DJ, Meade AW, Wiebe EN. The viability of crowdsourcing for survey research. Behav Res Methods. 2011;43:800–813. doi: 10.3758/s13428-011-0081-0. [DOI] [PubMed] [Google Scholar]
- 15.Silvares MR, Fortes MR, Miot HA. Quality of life in chronic urticaria: a survey at a public university outpatient clinic, Botucatu (Brazil) Rev Assoc Med Bras (1992) 2011;57:577–582. doi: 10.1590/s0104-42302011000500018. [DOI] [PubMed] [Google Scholar]
- 16.Miot HA. Assessing normality of data in clinical and experimental trials. J Vasc Bras. 2017;16:88–91. [Google Scholar]
- 17.Norman GR, Streiner DL. Biostatistics: the bare essentials. 4th ed. Shelton (CT): PMPH-USA; 2014. [Google Scholar]
- 18.Weller EA, Ryan LM. Testing for trend with count data. Biometrics. 1998;54:762–773. [PubMed] [Google Scholar]
- 19.Mackinnon A. The use and reporting of multiple imputation in medical research-a review. J Intern Med. 2010;268:586–593. doi: 10.1111/j.1365-2796.2010.02274.x. [DOI] [PubMed] [Google Scholar]
- 20.Watts DD. Correspondence analysis: a graphical technique for examining categorical data. Nurs Res. 1997;46:235–239. doi: 10.1097/00006199-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH. Multivariable analysis: a practical guide for clinicians: 2nd ed. New York: Cambridge university press; 2006. [Google Scholar]
- 22.IBM Corp 2017 . IBM SPSS Statistics for Windows, Version 25.0. Chicago (IL): SPSS Incorporation; 2017. [Google Scholar]
- 23.Parulkar I, Haleem H, Paek SY. Epidemiologic and clinical features of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:42–46. doi: 10.12788/j.sder.2017.019. [DOI] [PubMed] [Google Scholar]
- 24.Egeberg A, Jemec GBE, Kimball AB, Bachelez H, Gislason GH, Thyssen JP, et al. Prevalence and Risk of Inflammatory Bowel Disease in Patients with Hidradenitis Suppurativa. J Invest Dermatol. 2017;137:1060–1064. doi: 10.1016/j.jid.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 25.Danby FW. Diet in the prevention of hidradenitis suppurativa (acne inversa) J Am Acad Dermatol. 2015;73(Suppl 1):S52–S54. doi: 10.1016/j.jaad.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 26.Brocard A, Knol AC, Khammari A, Dréno B. Hidradenitis suppurativa and zinc: a new therapeutic approach. A pilot study. Dermatology. 2007;214:325–327. doi: 10.1159/000100883. [DOI] [PubMed] [Google Scholar]
- 27.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 28.Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology. 2015;231:184–190. doi: 10.1159/000431175. [DOI] [PubMed] [Google Scholar]
- 29.Schrader AM, Deckers IE, van der Zee HH, Boer J, Prens EP. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460–467. doi: 10.1016/j.jaad.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Canoui-Poitrine F, Revuz JE, Wolkenstein P, Viallette C, Gabison G, Pouget , et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51–57. doi: 10.1016/j.jaad.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Deckers IE, Janse IC, van der Zee HH, Nijsten T, Boer J, Horváth B, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): A cross-sectional reference study. J Am Acad Dermatol. 2016;75:755–759.e1. doi: 10.1016/j.jaad.2016.04.067. [DOI] [PubMed] [Google Scholar]
- 32.D’Elia MP, Brandão MC, de Andrade Ramos BR, da Silva MG, Miot LD, Dos Santos SE, et al. African ancestry is associated with facial melasma in women: a cross-sectional study. BMC Med Genet. 2017;18:17–17. doi: 10.1186/s12881-017-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch JW, Smith GD, Kaplan GA, House JS. Income inequality and mortality: importance to health of individual income, psychosocial environment, or material conditions. BMJ. 2000;320:1200–1204. doi: 10.1136/bmj.320.7243.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bairros FS, Meneghel SN, Dias-da-Costa JS, Bassani DG, Menezes AM, Gigante DP, et al. Racial inequalities in access to women’s health care in southern Brazil. Cad Saude Publica. 2011;27:2364–2372. doi: 10.1590/s0102-311x2011001200008. [DOI] [PubMed] [Google Scholar]
- 35.Heringer R. Racial inequalities in Brazil: a synthesis of social indicators and challenges for public policies. Cad Saude Publica. 2002;(Supp l):S57–S65. [PubMed] [Google Scholar]
- 36.Micheletti RG. Natural history, presentation, and diagnosis of hidradenitis suppurativa. Semin Cutan Med Surg. 2014;33(Supp):S51–S53. doi: 10.12788/j.sder.0092. [DOI] [PubMed] [Google Scholar]
- 37.Kromann CB, Deckers IE, Esmann S, Boer J, Prens EP, Jemec GB. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819–824. doi: 10.1111/bjd.13090. [DOI] [PubMed] [Google Scholar]
- 38.Gatek J, Dudesek B, Krátká A, Vrána D, Duben J. Severe hidradenitis suppurativa. Rozhl Chir. 2014;93:468–471. [PubMed] [Google Scholar]
- 39.Deckers IE, van der Zee HH, Boer J, Prens EP. Correlation of early-onset hidradenitis suppurativa with stronger genetic susceptibility and more widespread involvement. J Am Acad Dermatol. 2015;72:485–488. doi: 10.1016/j.jaad.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539–561. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 41.Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264–268. doi: 10.2340/00015555-0866. [DOI] [PubMed] [Google Scholar]
- 42.Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol. 2010;62:706–708. doi: 10.1016/j.jaad.2009.09.021. 708.e1. [DOI] [PubMed] [Google Scholar]
- 43.Wolkenstein P, Loundou A, Barrau K, Auquier P, Revuz J, Quality of Life Group of the French Society of Dermatology Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621–623. doi: 10.1016/j.jaad.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 44.Alavi A, Anooshirvani N, Kim WB, Coutts P, Sibbald RG. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61–65. doi: 10.1007/s40257-014-0105-5. [DOI] [PubMed] [Google Scholar]
- 45.Fitzsimmons JS, Guilbert PR, Fitzsimmons EM. Evidence of genetic factors in hidradenitis suppurativa. Br J Dermatol. 1985;113:1–8. doi: 10.1111/j.1365-2133.1985.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 46.Karagiannidis I, Nikolakis G, Sabat R, Zouboulis CC. Hidradenitis suppurativa/Acne inversa: an endocrine skin disorder? Rev Endocr Metab Disord. 2016;17:335–341. doi: 10.1007/s11154-016-9366-z. [DOI] [PubMed] [Google Scholar]
- 47.Kurayev A, Ashkar H, Saraiya A, Gottlieb AB. Hidradenitis Suppurativa: Review of the Pathogenesis and Treatment. J Drugs Dermatol. 2016;15:1017–1022. [PubMed] [Google Scholar]
- 48.Micheletti RG. Hidradenitis suppurativa: current views on epidemiology, pathogenesis, and pathophysiology. Semin Cutan Med Surg. 2014;33(Supp l):S48–S50. doi: 10.12788/j.sder.0091. [DOI] [PubMed] [Google Scholar]
- 49.Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97–103. doi: 10.1038/jid.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jemec GB, Kimball AB. Hidradenitis suppurativa: Epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(Suppl 1):S4–S7. doi: 10.1016/j.jaad.2015.07.052. [DOI] [PubMed] [Google Scholar]