Abstract
Mycobacterium tuberculosis (Mtb) leads to approximately 1.5 million human deaths every year. In pulmonary tuberculosis (TB), Mtb must drive host tissue destruction to cause pulmonary cavitation and dissemination in the tissues. Matrix metalloproteinases (MMPs) are endopeptidases capable of degrading all components of pulmonary extracellular matrix (ECM). It is well established that Mtb infection leads to upregulation of MMPs and also causes disturbance in the balance between MMPs and tissue inhibitors of metalloproteinases (TIMPs), thus altering the extracellular matrix deposition. In TB, secretion of MMPs is mainly regulated by NF‐κB, p38 and MAPK signalling pathways. In addition, recent studies have demonstrated the immunomodulatory roles of MMPs in Mtb pathogenesis. Researchers have proposed a new regimen of improved TB treatment by inhibition of MMP activity to hinder matrix destruction and to minimize the TB‐associated morbidity and mortality. The proposed regimen involves adjunctive use of MMP inhibitors such as doxycycline, marimastat and other related drugs along with front‐line anti‐TB drugs to reduce granuloma formation and bacterial load. These findings implicate the possible addition of economical and well‐tolerated MMP inhibitors to current multidrug regimens as an attractive mean to increase the drug potency. Here, we will summarize the recent advancements regarding expression of MMPs in TB, their immunomodulatory role, as well as their potential as therapeutic targets to control the deadly disease.
Keywords: Host‐directed therapy, Immunopathology, MMPs, Mtb, Signalling pathways
1. INTRODUCTION
Tuberculosis (TB) is a major threat to public health worldwide, and it continues to kill more than 1.5 million people annually.1 Statistically, developing countries account for 90% of the tuberculosis cases worldwide.2 Mycobacterium tuberculosis (Mtb) is a successful intracellular pathogen. Once infected with Mtb, host innate immune response is activated and macrophages constitute an important part of the innate immune system.3 Importantly, they play a critical role in recognizing, responding and reacting to Mtb infection.4 Phagocytosis of Mtb by macrophages can be triggered by non‐specific pinocytosis or by the activation of specific receptors. Moreover, Mtb can also be recognized through pattern recognition receptors (PRRs) such as Toll‐like receptors (TLRs) and Nod‐like receptors (NLRs).5 This interaction of Mtb and macrophages eventually activates inflammatory response.6
Lungs are the primary site of Mtb infection, and pulmonary TB is characterized by granulomatous inflammation and destruction of lung parenchyma. The host immune response limits the spread of Mtb and walls off the bacteria in dense cellular masses known as granulomas or tubercular lesions.7, 8 Although host innate immune response is one of the important determinants of the disease, the possible outcome of the infection varies among susceptible individuals and the factors involved therein are not well understood.9, 10 Recent studies have suggested a new concept of TB immunopathology that directly involves inhibition of matrix metalloproteinase (MMP) activity to hinder matrix destruction and reduce the morbidity and mortality associated with TB.11, 12
Matrix metalloproteinases (MMPs, also known as matrixins) are secreted or membrane‐bound endopeptidases belonging to the metzincin superfamily, collectively capable of degrading all components of ECM. The prefix “metallo‐” refers to the reliance of these enzymes on zinc ions to carry out the hydrolysis of protein substrates, and their structure has been reviewed in detail.13, 14 The first MMP was reported by Gross and Lapiere in 1962 as a collagenase engaged in tail resorption during the tadpole metamorphosis.15 Currently, MMPs consist of 23 members in human and are expressed in almost all organs and tissues.16 These enzymes have key roles in inflammatory cell migration, tissue repair, chemokine and cytokine signalling, degradation of matrix and non‐matrix proteins, pathogenesis of various diseases and modulation of immune responses.17, 18, 19, 20, 21 MMPs can be broadly classified on the basis of substrate specificity into collagenases (MMP‐1, MMP‐8 and MMP‐13), gelatinases (MMP‐2 and MMP‐9), stromelysins (MMP‐3, MMP‐10 and MMP‐11), elastases (MMP‐7 and MMP‐12) and membrane‐type MMPs (MT‐MMPs; MMP‐14, MMP‐15, MMP‐16 and MMP‐17) which are surface anchored.22
Most of the MMPs are secreted as inactive zymogens called proMMPs which have a cysteine switch motif coordinating with Zn2+ in catalytic domain.23 In vitro, these proMMPs can be activated by chemical agents, such as sodium dodecyl sulphate, oxidized glutathione and thiol‐modifying agents24; however, in vivo activation of proMMPs is more complicated and is conducted by other MMPs or other classes of proteinases such as plasmin and neutrophil elastases.25 In healthy tissues, MMPs are occasionally expressed and their biological activity is tightly regulated by various mechanisms. Activity of activated MMPs is regulated by endogenous inhibitors called tissue inhibitors of metalloproteinases (TIMPs) that bind active and latent forms of MMPs.26
Matrix metalloproteinases activity is implicated in non‐infectious and chronic lung diseases such as asthma and COPD.27, 28, 29 Mtb infection leads to disturbance in the balance between MMPs and TIMPs, and also alters extracellular matrix deposition as well as the cell behaviour of monocyte‐microglial networks.30, 31 MMPs are secreted by Mtb‐infected macrophages and monocytes, and also by uninfected stromal cells stimulated through intercellular networks.32 Many studies have demonstrated the involvement of MMP‐1, the major human collagenase, and its activator MMP‐3 in driving pathology in pulmonary TB.30, 33, 34 In this review, we will focus on the recent studies demonstrating the immunomodulatory roles of MMPs and their potential as therapeutic targets to hamper the pulmonary matrix destruction and reduce the morbidity and mortality associated with TB.
2. EXPRESSION OF MMPS IN TB
The majority of MMPs are expressed in diseased conditions wherein the tissues are inflamed and undergo repair and remodelling, while some of the members such as MMP‐2, MMP‐19 and MMP‐28 are evident in normal tissues indicating their roles in homeostasis.35 Many immune cells express low levels of MMPs in the resting state, and expression of MMPs is upregulated by exogenous stimuli, cytokines and cell‐cell interaction.36 This regulation is mainly carried out by TIMPs as unstimulated human peripheral blood monocytes, B cells and T cells express higher levels of TIMP‐1, TIMP‐2 and TIMP‐4.37 Pulmonary epithelial cells are also a significant source of MMPs as they express many MMPs including MMP‐1, MMP‐2, MMP‐7 and MMP‐9.38 In many pathological conditions, cell migration is closely linked to degradation of the ECM and the activated MMPs are considered as a prerequisite for invasion and metastasis of cancerous cells.39
Many studies have analysed the expression of MMPs in the pathophysiology of TB (Table 1). Infection of THP‐1 cells with Mtb leads to increased expression of MMP‐9. This MMP‐9 induction is regulated by receptor‐mediated signalling pathways.40 In TB patients, plasma concentrations of various MMPs may vary between the genders and this expression may not associate with the severity of the disease. Sathyamoorthy et al found significantly higher plasma concentrations of MMP‐1 and MMP‐8 in male TB patients as compared to females. This increased concentration of the MMPs was inversely correlated with body mass index.41 Similarly, plasma MMP‐3 was also significantly higher in men as compared to women in a number of clinical conditions including both infectious and non‐infectious diseases.42 MMPs, like MMP‐1, cause lung extracellular matrix destruction, and MMP‐10 is known as a key activator of MMP‐1. In a recent study, MMP‐10 secretion was increased in Mtb‐infected macrophages while inhibition of MMP‐10 activity decreased collagen breakdown. MMP‐10 expression was also increased in both induced sputum and bronchoalveolar lavage fluid (BALF) as compared to control subjects and patients with other respiratory diseases.43 This Mtb‐driven MMP‐10 secretion was inhibited in a dose‐dependent manner by p38 and extracellular signal–related kinase mitogen‐activated protein kinase blockade. In vivo and in vitro, Mtb infection leads to increased expression and activity of MMP‐1, MMP‐2, MMP‐3 and MMP‐9.44 This study also reported the involvement of miR‐223 in MMP expression through BMAL1 modulation. Azikin et al evaluated the levels of MMP‐9 in children who lived in the same house with a person having active TB.45 There were no significant differences between the expression levels of MMP‐9 in the group of exposed and Mtb infected children, and the levels of MMP‐9 were not influenced by sex, age, nutritional status and the status of BCG immunization. In a related study, M avium also induced the secretion of MMP‐1 in duodenal biopsy tissues, as well as in blood samples as compared to negative controls.46 This induction of MMP‐1 by M avium in duodenal tissue suggests that mycobacteria might contribute to the epithelial disruption commonly seen in enteropathies. Systemic levels of various MMPs may reflect the severity of disease in TB patients. Kumar et al reported elevated levels of circulating MMP‐1, MMP‐2, MMP‐3, MMP‐7, MMP‐10 and MMP‐12 in TB patients having diabetes mellitus as compared to patients having TB only.47 Moreover, anti‐tuberculosis therapy with metformin was associated with a significant reduction in the levels of MMP expression. Gao et al compared the effect of minimally invasive operation and open surgery on the serum IL‐1β, MMP‐1 and MMP‐13 in patients with senile spinal tuberculosis.48 The serum values of IL‐1β, MMP‐1 and MMP‐13 after surgery were lower than those of before treatment.
Table 1.
Expression of MMPs and TIMPs in TB
| Species examined | Type of tissue/cells examined | Upregulation of MMPs/TIMPs | References |
|---|---|---|---|
| Human | THP‐1 cells | MMP‐9 | 40 |
| Human | Monocytes | MMP‐1 and MMP‐3 | 141 |
| Human | Blood | MMP‐1 and MMP‐8 | 41 |
| Human | Plasma | MMP‐3 | 42 |
| Human | Co‐culture model of the blood‐brain barrier | MMP‐9 | 43 |
| Human | Serum | MMP‐1 and MMP‐13 | 48 |
| Mouse | Blood and macrophages | MMP‐1, MMP‐2, MMP‐3 and MMP‐9 | 44 |
| Human | Lung tissue model | MMP‐1, MMP‐3, MMP‐9 and MMP‐12 | 12 |
| Human | Blood | MMP‐9 | 45 |
| Human | Duodenal biopsy tissues and blood | MMP‐1 | 46 |
| Human | Blood | MMP‐1, MMP‐2, MMP‐3, MMP‐7, MMP‐10 and MMP‐12 | 47 |
| Human | Cerebrospinal spinal fluid | MMP‐1 and MMP‐3 | 30, 51 |
| Human and mouse | Lung tissues | MMP‐8 | 165 |
| Human | Lung biopsies and macrophages | MMP‐8 | 101 |
| Human | Brain biopsies | MMP‐9 | 52 |
| Mouse | Brain biopsies | MMP‐9 | 53 |
| Human | Bronchial epithelial cells | MMP‐9 | 80 |
| Human | Sputum and bronchoalveolar lavage fluid (BALF) | MMP‐1 | 34 |
| Human | Pleural fluid | MMP‐2 and MMP‐9 TIMP‐1 | 62 |
| Human | Plasma and THP‐1 cells | TIMP‐1 | 65 |
| Human | Macrophages | MMP‐1 and MMP‐7 | 66 |
| Human | Monocyte‐derived Macrophages | MMP‐1 and MMP‐3 | 102 |
| Human | Sputum and macrophages | MMP‐14 | 140 |
| Human | Blood | MMP‐1, MMP‐2, MMP‐3, MMP‐8 and MMP‐9 and TIMP‐1, TIMP‐12 | 63 |
Like other tissues of the body, inflammation of central nervous system (CNS) also results in increased MMP secretion and it can also affect the permeability of blood brain barrier (BBB).49, 50 Increased expression of MMP‐1 and MMP‐3 has been reported in the patients with TB of CNS.30, 51 Similarly, in a recent study, MMP‐9 upregulation has been noticed in the brain biopsies of the patients having TB meningitis.52 This enhanced activity of MMP‐9 in the brain tissues may be involved in the damage of BBB, oedema and the inflammatory cell exudation. Li et al analysed the expression of MMP‐9 in the pathophysiological process of TB meningitis in a mouse model.53 The data exhibited elevated expression and activity of MMP‐9 as compared to control group. Although many studies have reported the differential expression of MMPs in TB, there is still a need for comprehensive MMP expression profiling in different immune cell lineages under resting and activated states, and in response to Mtb and other bacterial challenges.
3. REGULATION OF MMP EXPRESSION AND ACTIVITY
Since MMPs might cause significant damage to the host tissues, therefore, their expression is strictly regulated. This regulation involves several levels including gene expression, zymogen activation, compartmentalization and inhibition of active enzyme.35 Initially, MMPs are believed to be regulated at the transcriptional level by a variety of physiological factors including cytokines, tumour promoters, growth factors, hormones, chemokines and cell‐cell or cell‐ECM communications.54 MMP promoters contain cis‐acting elements that can be bound and regulated by several transcription factors such as activator protein 1 and NF‐kB.55 Expression of MMPs is further regulated at the post‐transcriptional level.56 Studies also have uncovered the contribution of epigenetic modifications in regulation of MMPs.57 MMP activity is also controlled through their compartmentalization in a certain intracellular or extracellular location.58 In addition, many intracellular pathways have been discovered which are actively involved in MMP regulation.
3.1. Role of TIMPs in MMP regulation in TB
Tissue inhibitors of metalloproteinases are well known to downregulate the activity of MMPs by binding their latent and active forms. TIMPs are constitutively expressed in many tissue fluids including cerebrospinal fluid (CSF).59, 60 There is a considerable connection in the biochemical properties of various TIMPs, although there are some MMPs having specificity to substrates.16 Expression of MMPs and TIMPs in TB has been reported in many studies. Residual pleural thickening (RPT) is the most commonly seen complication related to pleural TB, and it may happen even after successful anti‐tuberculosis medication. TIMP‐1 is an endogenous inhibitor of MMPs and regulates MMP activity by forming 1:1 complexes with MMPs.61 In a previous study, higher levels of TIMP‐1 in pleural fluid were found to be responsible for the development of RPT while expression of MMP‐2 and MMP‐9 had no significant correlation to RPT.62 This indicates the role of TIMP‐1 in RPT, and its expression level may predict the occurrence of RPT in pleural TB. Other studies have also unveiled that levels of MMP‐1, MMP‐2, MMP‐3, MMP‐8 and MMP‐9, as well as TIMP‐1 and TIMP‐12, were significantly higher in TB patients as compared with healthy controls.34, 63, 64 Recently, expression level of TIMP‐1 in plasma has been reported as a potential biomarker for the diagnosis of TB. Moreover, Bacillus Calmette‐Guérin (BCG) and M bovis infection of THP‐1 cells also significantly enhanced the TIMP‐1 mRNA expression in a time‐dependent manner.65 In contrast, Rand et al reported increased secretion of MMP‐1 and MMP‐7, and decreased expression of TIMP‐1 in primary human macrophages infected with Mtb. 66 Downregulation of TIMP‐1 could lead to increased activity of MMP‐1 and MMP‐7 and more tissue destruction which can be seen as an Mtb strategy to replicate and spread to other tissues. It is obvious that the balance between MMPs and TIMPs regulates matrix turnover, wherein either a surplus of MMPs or a scarcity of TIMPs may cause excessive ECM degradation and tissue damage.
3.2. Kallikrein‐kinin system in MMP regulation
Kallikrein‐related peptidases (KLKs) are a subgroup of serine proteases with either trypsin‐like or chymotrypsin‐like activity. Tissue KLKs are expressed in various tissues and consist of 15 proteases, KLK1 to KLK15. KLKs perform many physiological and pathological functions. The kallikrein‐kinin system appears to play a direct role in promoting anti‐fibrotic responses and collagen degradation.67, 68 As KLKs are able to convert kininogens into bradykinin, these kinins then bind to bradykinin receptor 1 (B1R) and bradykinin receptor 2 (B2R). B1R is generally latent, but it is upregulated in inflammation or by the members of cytokine family including IL‐1β and TNF‐α, while B2R is constitutively expressed in many tissues of the body.69 Bradykinins play a pivotal role in the modulation of airway inflammation by stimulation of cytokine expression and recruitment of inflammatory cells.70, 71
Many studies have investigated whether bradykinin stimulation induces release of MMPs in different tissues (Figure 1). For example, bradykinin treatment of isolated granulosa cells induced MMP‐3 and MMP‐20 expression explaining the role of bradykinin in ovulation in pigs.72 Pharmacological blockade or knockdown of B2R receptor (B2−/–) in mice and rats resulted in increased interstitial fibrosis, whereas transgenic mice expressing increased endogenous B2R showed reduced interstitial fibrosis. The increased interstitial fibrosis in B2–/– mice was associated with decreased activity of MMP‐2 suggesting the protective role of bradykinin and MMP‐2.73 Similarly, involvement of B2R in the release of MMP‐2 from tracheal smooth muscle cells of guinea pig has been reported.74 In a recent study, B1R agonist, Lys‐des[Arg9]‐bradykinin (LDBK), increased the proliferation of oestrogen‐sensitive breast cancer cells. B1R was also involved in the expression of MMP‐2 and MMP‐9 via ERK‐dependent pathway.75 Similarly, B2R has been reported to regulate MMP‐9 secretion via MAP kinases (ERK1/2) signalling in trabecular meshwork cells.76 On the other hand, MMPs can also regulate the function of KLKs.77 These studies suggest a potential interaction of KLKs and MMPs in various physiological and pathological conditions. It is likely that this B1R and B2R signalling is involved in the MMP secretion in TB but no study has been reported yet. Investigations of these signalling pathways may reveal bradykinin receptors as therapeutic targets in TB treatment.
Figure 1.
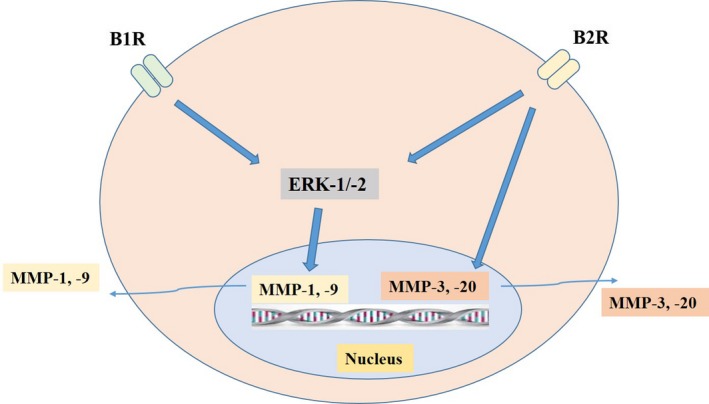
Role of bradykinin signalling in expression and regulation of MMPs in non‐TB conditions. Bradykinin stimulation can lead to activation of intracellular signalling pathways which in turn induces various MMP secretion. Both B1R and B2R can activate ERK1/2 kinases eventually leading to increased expression of MMP‐1 and MMP‐9 in breast cancer and tracheal smooth muscles, respectively. Similarly, exogenous bradykinin treatment of granulosa cells induced MMP‐3 and MMP‐20 explaining their role in ovulation
3.3. IL‐12 and IL‐17 cytokines mediated induction of MMPs
Cytokines are the important regulators of immunity and inflammation. These cytokines including TNF‐α and IFN‐γ generally upregulate the expression and activation of MMPs in monocytes and macrophages.78 For example, in neutrophils, MMP‐9 is stored as gelatinase granules and it is released following stimulation with IL‐8 or TNF.79 TNF‐α is essential for MMP‐1 and MMP‐9 expression by monocyte in bronchial epithelial cell networks.80, 81, 82 IL‐12 is an important cytokine involved in both innate and adaptive immune responses.83 It is mainly secreted by macrophages, monocytes, dendritic and B cells in response to bacterial infection.84 It stimulates T‐ and NK cells to produce IFN‐γ and promotes the Th1 response.85 Recently, researchers have investigated the role of IL‐12 in secretion of various MMPs. Miao and colleagues described that IL‐12 significantly increased the mRNA and protein level expressions of MMP‐1, MMP‐3 and MMP‐13, while it downregulated MMP‐2 and MMP‐9 in the human periodontal ligament fibroblasts.86 This IL‐12‐mediated regulation of MMPs was NF‐κB‐dependent. However, IL‐12 treatment had no significant effect on the mRNA and protein levels of TIMP‐1 and TIMP‐2. The other possible mechanisms of IL‐12‐mediated transcriptional regulation of MMPs in this study have also been discussed recently.87
Historically, Th1 cells have been believed to be essential in the control of Mtb infection but now Th17 cells have been recognized as critical players in Mtb control.88 Th17 cells produce IL‐17, a pro‐inflammatory cytokine that functions to induce the secretion of diverse cytokines, chemokines, anti‐microbial peptides and MMPs.89 In macaques, sterile granulomas had a higher frequency of T cells producing IL‐17 and pulmonary delivery of BCG vaccine triggers a mucosal immune response orchestrated by IL‐17.90, 91 In a study in the China involving Han population, genetic polymorphisms in IL‐17A and IL‐17F were related to host susceptibility to TB and infection with hypervirulent W‐Beijing strain HN878 required IL‐17 for early immunity.92, 93 Mice with genetically incapacitated IL‐17 receptor are more susceptible to Mtb, despite a normal Th1 response.94 Similarly, IL‐17 knockout mice failed to develop mature granulomas after BCG infection and showed diminished protection from virulent Mtb.95 So IL‐17 has a well‐established role in host defence against TB, but its role in TB‐driven tissue damage was unknown. A recent study by Singh et al discovered the role of this cytokine in regulation of MMP secretion by using biopsies from patients having pulmonary TB, patient's bronchoalveolar lavage fluid (BALF) and primary human airway epithelial cells.96 IL‐17 was expressed in TB patient granulomas, and MMP‐3 was expressed in adjacent pulmonary epithelial cell, while IL‐17 exhibited a concentration‐dependent effect on MMP‐3 secretion. On the other hand, IL‐17 decreased the secretion of MMP‐9. Moreover, this IL‐17‐driven MMP‐3 upregulation was p38 MAP kinase‐dependent.
3.4. NF‐κB and MAPK regulation of MMPs
In Mtb infection, multiple pathways are activated which together regulate MMP secretion (Figure 2). It has been reported that key transcriptional regulators of MMP expression in TB are NF‐κB and STAT3.30, 97, 98 TIMP‐1 lacks an NF‐κB promoter binding site, and so, NF‐kB signalling may regulate the MMP/TIMP expression.55 Recently, Miao and colleagues reported that IL‐12 significantly increases the mRNA and protein level expressions of MMP‐1, MMP‐3 and MMP‐13, and downregulates MMP‐2 and MMP‐9 in the human periodontal ligament fibroblasts.86 NF‐κB signalling was involved in this IL‐12‐mediated regulation of MMPs. Other studies have also reported the same findings.99 Intracellularly, MMP secretion is also regulated by the prostaglandin (PG) and mitogen‐activated protein kinase (MAPK) signal transduction pathways both in case of direct infection of Mtb or through intercellular networks.66, 98 p38 MAPK pathway activation has multiple downstream effects, its activation leads to COXII accumulation, prostaglandin (PG) E2 and cAMP activation and ultimately upregulates MMP‐1 secretion [66]. In addition, Mtb itself produces cAMP which can be utilized to undermine the host immune response.100 On the other hand, this pathogen‐derived cAMP may contribute to increased MMP secretion. In a recent study, p38 MAPK signalling was involved in IL‐17‐driven MMP‐3 upregulation in Mtb infection.96 AMPK also regulates neutrophil‐derived MMP‐8 secretion in TB.101 Besides these pathways, Moores et al investigated the role of histone acetylation changes in Mtb‐induced MMP secretion.102 Silencing of HDAC1 by using siRNA resulted in downregulation of MMP‐3 expression, but this silencing had no effect on MMP‐1, which shows epigenetic modification of histone acetylation also plays a role in expression of MMP‐3. Taken together, multiple pathways are involved in MMP regulation but it has yet to be established that which signalling pathway can be effectively targeted to minimize the TB pathology.
Figure 2.
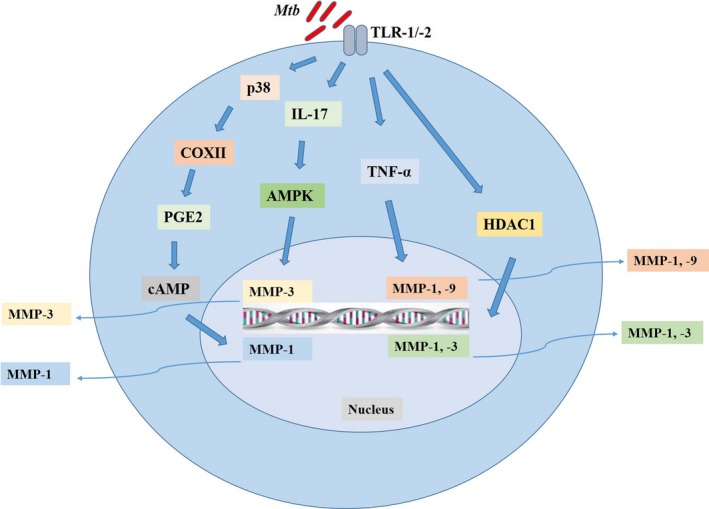
Expression and regulation of MMPs in TB. In Mtb infection, there is upregulation of many MMPs which are contributing to TB pathology. The expression and secretion of MMPs are strictly regulated as excess of these enzymes may cause tissue destruction. Many studies have investigated these signalling pathways. P38/COXII/PGE2/cAMP is well‐established signalling cascade involved in the regulation of MMP‐1. In a recent study, IL‐17‐mediated regulation of MMP‐3 was dependent on MAPK. In addition, TNF‐α and HDAC1 can also regulate MMP‐1 and MMP‐9, and MMP‐1 and MMP‐3, respectively. As the excess of these MMPs may be detrimental for host and may exaggerate the TB pathology, therefore, inhibition of these signalling pathways may provide a new avenue for host‐directed therapy in TB
4. ROLE OF MMPS IN TB IMMUNOPATHOLOGY
4.1. Role of MMPs in pulmonary granuloma and cavitation
Tuberculosis is primarily a disease of lung, and following infection with Mtb, pulmonary granulomas are formed in humans and develop heterogeneous microenvironments, often featuring hypoxia and central necrosis.103 The same lesions can be recapitulated in non‐human primate and rabbit models of the disease.104 Historically, pulmonary granuloma formation has been considered as a host protective response; however, recent studies suggest that Mtb uses secreted virulence factors to induce granuloma formation to create a favourable niche for its dissemination and replication.105, 106 Moreover, recent studies have demonstrated a wide variation in the distribution of drugs within TB granulomas, with very few agents able to penetrate the central regions of the granuloma.107 This differential ability of drugs to penetrate TB granulomas has been incorporated into modern TB drug development programmes to select a more efficient combination.108 However, the mechanisms contributing to this differential penetration of drugs are not fully understood yet, and novel approaches to improve TB drug delivery and efficacy are urgently needed. The standard treatment of TB has remained unchanged for many decades, and multidrug‐ and extensively drug‐resistant strains are emerging progressively, leading to high mortality rates among patients even after commencement of TB treatment.109, 110, 111, 112 Therefore, it urges the development of new drugs to accomplish the sustainable development goals, aiming to reduce 90% of TB incidence rate by 2030.113
The role of MMPs in pulmonary physiology and pathology is gaining attention, and several studies show that they are associated with lung tissue destruction and inflammatory lung disorders including chronic obstructive pulmonary disease (COPD) and emphysema. Mtb infection induces the production of MMPs both in vitro and in vivo.40, 114 Many MMPs, specifically MMP‐1, have been shown to contribute in TB pathology in human lungs.82 Although the primary function of MMPs is thought to be matrix cleavage and tissue remodelling, many evidences suggest these enzymes also play a major role in angiogenesis, cell motility, apoptosis, regulating immunity, inflammation and host defences.115 Moreover, several MMPs through proteolytic activity can modulate the functions of cytokines and chemokines including IFN‐γ, IL‐1β, TNF‐α, CXCL8 and CCL7. Thus, MMPs, besides tissue destruction, can regulate chemokine gradients and leucocyte recruitments to the sites of inflammation.115
Many studies have validated the role of MMPs in pulmonary granuloma formation. Mice treated with BB‐94, a broad‐spectrum inhibitor of MMPs, revealed either a delay in granuloma induction or form smaller granulomas with more collagen content.116, 117 These studies, for the first time, suggested that MMPs regulate cell migration and granuloma formation after Mtb infection. Consistent with these findings by using various MMP inhibitors, MMP‐9‐deficient mice also showed a reduction in macrophage recruitment to the lungs and developed comparatively smaller granulomas.21 Similarly, Volkman and colleagues discovered the molecular mechanisms that Mtb uses to induce granuloma formation.118 They showed that the 6‐kDa early secreted antigenic target (ESAT‐6) induces MMP‐9 in epithelial cells adjacent to infected macrophages. This upregulation of MMP‐9 is related to the recruitment of monocytes and macrophages essential for granuloma formation. These studies provided the basis for the emerging paradigm that MMPs remodel lung tissue and initiate granuloma formation. Upregulation of MMPs in Mtb infection may be considered as mycobacterial strategy to multiplicate and spread in the host lung tissues. Along with granuloma, pulmonary tissue destruction is a hallmark of TB pathology leading to morbidity, mortality and transmission of infection. ECM destruction allows necrosis and cavitation, and thereby creates an immune‐privileged site, wherein the bacilli can proliferate and eventually spread to new hosts.119 In contrast to MMP‐9 which plays a role in ECM deposition and granuloma formation, MMP‐1 is believed to degrade ECM as its primary function, thus contributing in spread of Mtb and cavitary disease development (Figure 3).22 However, the pathogenesis and role of other MMPs are incompletely understood.120 The understanding of MMP role is important for designing the rational therapies to minimize immune‐mediated host damage and improve outcomes in TB.
Figure 3.
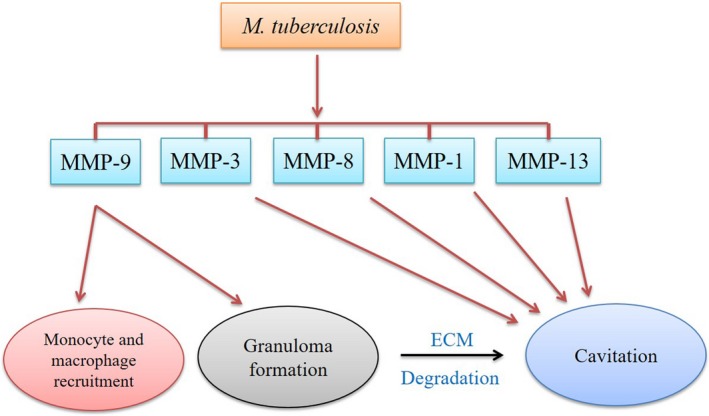
Role of MMPs in pulmonary granuloma and cavitation. Many studies have reported the increased expression of various MMPs in TB. Each of these MMPs has immunomodulatory role in the pathogenesis of TB. But MMP‐1, MMP‐3 and MMP‐9 are the most widely studied MMPs in TB, and their role in TB development has been well demonstrated. MMP‐9 upregulation is related to the recruitment of monocytes and macrophages at the site of infection, and these cells are main players of host innate immune response and are also crucial for granuloma formation. Upregulation of MMP‐9 in Mtb infection and as a result lung tissue remodelling is considered as mycobacterial strategy to create a suitable environment in host lung tissues. In contrast to MMP‐9, MMP‐1 degrades ECM as its primary function. ECM destruction leads to necrosis and cavitation thereby providing an immune‐privileged site for bacterial proliferation. MMPs such as MMP‐3, MMP‐8, MMP‐13 and others are also involved in tissue remodelling in TB. Detailed knowledge of these MMPs’ expression and function may help to devise a strategy to control the replication and spread of Mtb in host tissues
4.2. Animal models of pulmonary granuloma and cavitation
Mouse, rabbit and guinea pig are most commonly used animal models of TB.121, 122 These species of animals have contributed a lot to the understanding of TB immunopathology. In recent years, majority of in vivo Mtb investigations have been carried out by using mouse model, and different mouse strains and different infection methods have been created.123 Mtb infection has been reported to upregulate MMP‐9 expression in Mtb‐infected mouse.124 In another study, MMP‐9 knockout mice exhibited reduced cellular recruitment to the lung granuloma.125 These studies implicate that MMP‐9 is required for recruitment of macrophages and tissue remodelling in Mtb infection of mice. But, unfortunately, mouse model of TB fails to develop the well‐characterized granuloma structure and cavitation which is commonly seen in human TB.7, 126 Moreover, mice do not express human MMP‐1 orthologue which, along with other MMPs, causes tissue destruction and transmission in disseminated human granuloma.34 As MMP‐1 is considered as dominant collagenase driving matrix destruction, therefore, the C57BL6 mouse model of TB has limited use to dissect the role of MMPs in Mtb‐driven immunopathology. However, C3HeB/FeJ mice have been shown to develop granuloma and occasional cavitation. 127, 128 These mouse granuloma studies lack the information regarding expression of MMPs and their role in granuloma formation. In future, a thorough understanding of MMP expression in Mtb‐infected C3HeB/FeJ mice could increase the utility of this animal model.
Other animal models such as guinea pig, rabbits and non‐human primates (NHPs) are preferred which exhibit caseation necrosis as seen in human.129, 130, 131 Guinea pigs have contributed significantly to understand the immunopathology of pulmonary TB. Although the guinea pigs are relatively economical model and produce TB lesions more similar to human in terms of lung pathology, even then they lack some characteristics features of human TB.132 In this regard, rabbits develop cavitary TB same as seen in human and offer a good opportunity to study the factors causing this type of disease. Moreover, rabbits are also susceptible to bovine TB caused by M bovis.133 Rabbit model is suitable for the investigation related to drug penetration and distribution in the lung tissue, and also to evaluate the response to chemotherapy.134 Adjunctive host‐directed therapies have evolved as a new approach to enhance the efficacy of conventional anti‐microbials against TB. In a study on rabbit model, expression of MMP‐1, MMP‐12 and MMP‐14 was significantly reduced in the lungs of CC‐11050 (a phosphodiesterase‐4 inhibitor)‐treated rabbits as compared to the Mtb‐infected untreated animals.135 In a previous study by the same research group, INH treatment of Mtb‐infected rabbits significantly reduced the expression of MMP genes including MMP‐14, MMP‐12, MMP‐2, MMP‐3, MMP‐9, MMP‐1 and MMP‐13 as compared to the untreated infected controls. The expression of MMP‐1, MMP‐3 and MMP‐12 was further significantly reduced in the animals receiving combined treatment of INH and CC‐3052, a phosphodiesterase‐4 inhibitor, compared with the group receiving INH treatment alone.136
Zebrafish model has been used to study MMP expression and granuloma in TB. The role of MMP‐9 has been reported in modulating cellular recruitment to the granuloma, and reduced expression of MMP‐9 resulted in smaller granulomas.137 In a related study, virulent M marinum significantly upregulated the expression of MMP‐9, MMP‐13 and MMP‐14 as compared to an attenuated strain.138 These studies suggest the key role of MMP activity in the pathogenesis of mycobacterial infection. Besides these TB models, non‐human primates (NHPs) such as the cynomolgus macaque have been used to replicate human TB lesions.139 NHPs have a close evolutionary relationship with humans and produce TB disease with clinical findings and lesions very similar to those of humans. Macaques develop granuloma types as seen in humans with the presence of classical caseous pulmonary granuloma. Moreover, macaques also exhibit other types of lung lesions such as non‐necrotizing granulomas, calcification, cavitation, consolidations and interstitial fibrosis.140 These lesions have been reported recently in Mtb‐infected cynomolgus macaques of Chinese origin.141 In a previous study involving microarray analysis, multiple MMPs were upregulated in Mtb‐infected macaques. MMP‐1 was the most highly expressed, while MMP‐2, MMP‐7, MMP‐9, MMP‐14 and MMP‐25 expression was also induced by Mtb infection.142 Luckily, antigens of cynomolgus macaque give cross‐reactivity with immunologic reagents made for human cells and tissue, thus making the immunohistochemical investigation easier in these TB models. The main disadvantages of this TB model are high cost and more space requirement in BSL3 facilities.
4.3. Role of MMPs in TB/HIV co‐infection
It is believed that almost half of patients surviving pulmonary TB with apparent recovery suffer substantial pulmonary impairment.143, 144 Furthermore, cured pulmonary TB is a major cause of chronic lung disease globally.145 In 2017, TB caused about 1.3 million deaths among HIV‐negative people and there were additional 0.30 million deaths from TB among HIV‐positive people.146 Among HIV‐infected patients, the mechanisms leading to prolonged pulmonary morbidity after successful TB treatment are not well understood.143, 144 Previous reports show that the relative risk of active TB doubles during the first year of HIV infection even when the CD4 counts are still conserved. This risk of active TB continues to increase in the upcoming years as the CD4 number is decreased.147 These findings are also in line with the central role of cellular immune response in structural lung damage and cavity formation in TB.148 Mechanistically, MMPs are the main players in TB‐associated lung tissue destruction.18, 34 However, lower MMP levels and reduced TB‐associated lung damage were seen radiographically in TB/HIV co‐infection.64 Variable MMP activity has been shown in HIV‐1‐infected and HIV‐1‐uninfected TB individuals. HIV‐1 infection in TB patients leads to decreased pulmonary MMP concentrations and reduced cavitary lesions.63, 149
These findings suggest that patients having TB/HIV co‐infection may have less lung matrix destruction than those with TB infection alone, but other studies have reported conflicting results.145 Antiretroviral therapy (ART) is a critical part of HIV/TB co‐infection treatment. Early start of ART in patients having CD4+ T cell less than 50 per cubic millimetre increased the chances of AIDS‐free survival, while in another study the treatment was associated with pulmonary airway obstruction.150, 151 Immune response restoration in HIV may cause lung damage and consequently lead to immune reconstitution inflammatory syndrome (IRIS). IRIS usually occurs during the initial months of ART and is commonly associated with TB. This TB‐IRIS is associated with a distinct pattern of MMP gene expression and secretion. In a study, HIV/TB co‐infected adults, and in stimulated cultures, secretion of MMP‐1, MMP‐3, MMP‐7 and MMP‐10 was higher in TB‐IRIS than in controls. Corticosteroid therapy for two weeks resulted in non‐significant reduction in MMP‐7 in serum, while the secretion of other MMPs was not affected.152 While, in another study, ART induced the increased expression of MMP‐8,153 this increased expression of MMP‐8 might cause lung damage.
Studies have shown that ART causes virologic suppression, but, on the hand, it may lead to early immunologic failure which is associated with early mortality after ART initiation in advanced HIV/tuberculosis.154, 155 Therefore, interventions to decrease inflammation and promote cellular immune recovery during ART may be helpful in patients co‐infected with HIV/TB. A possible limitation of the studies addressing HIV‐mediated lung damage in TB is that the effects of TB treatment on cellular immune response have not been evaluated in details. Further studies would elaborate the association between lung damage and TB‐IRIS and also the mechanisms involved; whereby, immune response restoration impairs pulmonary function.
5. INHIBITION OF MMPS: UNLEASHING THEIR THERAPEUTIC ROLES
Host‐directed therapy with MMP inhibitors has been investigated in several inflammatory conditions such as multiple sclerosis.156 Encouraging results have been obtained in experimental models of meningococcal and pneumococcal meningitis, where MMP inhibition resulted in decreased morbidity and mortality.157, 158 Similarly, Oehlers et al showed that vascular endothelial growth factor (VEGF) inhibitors in combination with rifampin reduce M marinum burden in zebrafish.159
In TB, host‐directed therapies are evolving as a novel therapeutic paradigm and many research groups have used various MMP inhibitors to study the immunopathology of TB.160, 161 Doxycycline is an anti‐mycobacterial antibiotic, and it is the only FDA‐approved MMP inhibitor. It has also shown promising effects on TB treatment by inhibiting the mycobacterial growth in animal and in vitro models of the disease.64 In a previous study, doxycycline suppressed TB‐dependent MMP‐1 and MMP‐9 secretions from primary human macrophages and epithelial cells. Moreover, doxycycline treatment decreased MMP activity in a cellular model and suppressed mycobacterial growth in vitro and in guinea pigs.63 Neutrophil‐derived MMP‐8 may also drive lung cavitation, morbidity and death.101 Therefore, MMP‐8 inhibition may be a potential target to abolish excessive host tissue destruction as MMP‐8 inhibition in a murine model of lung injury improved the outcomes of the therapy.162
Marimastat (BB‐2516) is another specific MMP inhibitor, a collagen‐peptidomimetic drug that targets the active site zinc atom of several MMPs, thereby preventing their activity. Marimastat is well tolerated in in vivo and has been tested to prevent cancer metastasis.163 Though, the anti‐neoplastic properties of the drug are well known in clinical trials, however, it has not yet been approved for clinical use due to its side effects on musculoskeletal system.18 Recently, some research groups have used this and other related drugs to inhibit MMP expression in TB. Parasa and colleagues used lung tissue model of TB comprising of human lung‐derived cells and primary human monocyte‐derived macrophages.12 Inhibition of MMPs by marimastat reduced both granuloma formation and bacterial load in Mtb infection, suggesting that MMP‐targeting intervention could be considered as a supportive therapy in TB treatment. Administration of marimastat alone did not show protective response in Mtb‐infected C57BL/6J mice; however, when administered in combination with either rifampin or isoniazid as adjunctive treatment, it increased the drug exposure in infected lung tissues and caused a reduction in bacterial burden of lungs when compared with animals treated with rifampin or isoniazid alone.11 In contrast, administration of adjunctive cipemastat, an orally available potent inhibitor of MMP‐7, increased the frequency of cavitation, immunopathology and mortality in Mtb‐infected C3HeB/FeJ.164 The same research group evaluated the use of anti‐MMP‐9 antibody in combination with first‐line drugs of TB treatment and reported significantly reduced relapse rates of TB in C3HeB/FeJ mice as compared with the mice receiving standard therapy alone.165 Consequently, MMP inhibition has divergent effects when administered alone or in combination with first‐line TB treatment. The findings highlight the importance of exploiting strategies that improve the efficacy of existing drugs by increasing the effectiveness of the anti‐TB therapy.
6. CONCLUSIONS AND FUTURE PERSPECTIVES
Taken together, a promising model for the role of MMPs in TB is that Mtb induces lung tissue remodelling and granuloma formation through upregulation of MMPs. Intact granuloma is thought to be beneficial to the host as it keeps the pathogen under check and prevents its spread. Reactivation of the infection and increased secretion of MMP‐1 result in pulmonary matrix degradation and cavitation. However, regulation and role of specific MMPs during various stages of Mtb infection remain to be explored. Nonetheless, role of kallikrein‐kinin system in MMP regulation in Mtb infection remains completely unknown. Recently, most of the publications, using various animal models of TB, suggest MMPs as viable therapeutic targets. Adjunctive treatment with MMP inhibitors along with front‐line TB drugs including isoniazid and rifampin significantly reduces Mtb survival in the lungs by preventing maturation of granulomas and also minimizes the matrix degradation and cavitary lesions. Current TB therapeutic regimens need multiple drugs and have to be taken for long times; therefore, they impose other challenges such as non‐compliance and emergence of the drug‐resistant Mtb strains. Given these challenges, MMP targeting may provide a reliable approach to increase the potency of current anti‐TB drugs.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHORS' CONTRIBUTIONS
NS collected the data and wrote the manuscript. TH and MHM helped for figure and table compilation. XZ gave the idea behind the manuscript compilation. DZ reviewed the article before final submission. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This work was supported by "National Natural Science Foundation of China (Project No. 31572487, 31873005); "National Key Research and Development Program (Project No. 2017YFD0500901)"; "China Agriculture Research System (No. CARS‐36)"; "the MoSTRCUK International Cooperation Project (Project No. 2013DFG32500)" and "the High‐end Foreign Experts Recruitment Program (Project No. GDW20151100036, GDW20161100071)".
Sabir N, Hussain T, Mangi MH, Zhao D, Zhou X. Matrix metalloproteinases: Expression, regulation and role in the immunopathology of tuberculosis. Cell Prolif. 2019;52:e12649 10.1111/cpr.12649
REFERENCES
- 1. Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856‐861. [DOI] [PubMed] [Google Scholar]
- 2. Sanou A, Bañuls A‐L, Van Anh NT, Godreuil S. Mycobacterium tuberculosis: Ecology and evolution of a human bacterium. J Med Microbiol. 2015;64:1261‐1269. [DOI] [PubMed] [Google Scholar]
- 3. Bosedasgupta S, Pieters J. Inflammatory stimuli reprogram macrophage phagocytosis to macropinocytosis for the rapid elimination of pathogens. PLoS Pathog. 2014;10:e1003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cambier CJ, Takaki KK, Larson RP, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hmama Z, Pena‐Diaz S, Joseph S, et al. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol Rev. 2015;264:220‐232. [DOI] [PubMed] [Google Scholar]
- 6. Mahajan S, Dkhar HK, Chandra V, et al. Mycobacterium tuberculosis modulates macrophage lipid sensing nuclear receptors PPAR gamma and TR4 for survival. J Immunol. 2012;188:5593‐5603. [DOI] [PubMed] [Google Scholar]
- 7. Russell DG, Barry CE, Flynn JL. Tuberculosis: What we don’t know can, and does, hurt us. Science. 2010;328:852‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39‐47. [DOI] [PubMed] [Google Scholar]
- 9. Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80:3343‐3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGarry HA. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44–46:167‐174. [DOI] [PubMed] [Google Scholar]
- 11. Xu Y, Wang L, Zimmerman MD, et al. Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathog. 2018;14:e1006974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parasa VR, Muvva JR, Rose JF, et al. Inhibition of tissue matrix metalloproteinases interferes with mycobacterium tuberculosis‐induced granuloma formation and reduces bacterial load in a human lung tissue model. Front Microbiol. 2017;8:2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein T, Bischoff R. Active metalloproteases of the disintegrin and metalloprotease (ADAM) family: biological function and structure. J Proteome Res. 2011a;10:17‐33. [DOI] [PubMed] [Google Scholar]
- 14. Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011b;41:271‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc Natl Acad Sci USA. 1962;48:1014‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13:649‐665. [DOI] [PubMed] [Google Scholar]
- 17. Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53:585‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190:9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dobaczewski M, Gonzalez‐Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheen P, O’Kane CM, Chaudhary K, et al. High MMP‐9 activity characterises pleural tuberculosis correlating with granuloma formation. Eur Respir J. 2009;33:134‐141. [DOI] [PubMed] [Google Scholar]
- 21. Taylor JL, Hattle JM, Dreitz SA, et al. A role for matrix metalloproteinase‐9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006;74:6135‐6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207‐214. [DOI] [PubMed] [Google Scholar]
- 23. VanWart HE, Birkedal‐Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578‐5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151‐160. [PubMed] [Google Scholar]
- 25. English WR, Holtz B, Vogt G, Knäuper V, Murphy G. Characterization of the role of the “MT‐loop”: an eight‐amino acid insertion specific to progelatinase a (MMP2) activating membrane‐type matrix metalloproteinases. J Biol Chem. 2001;276:42018‐42026. [DOI] [PubMed] [Google Scholar]
- 26. Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602‐608. [DOI] [PubMed] [Google Scholar]
- 27. Chernov AV, Strongin AY. Epigenetic regulation of matrix metalloproteinases and their collagen substrates in cancer. Biomol Concepts. 2011;2:135‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38:191‐208. [DOI] [PubMed] [Google Scholar]
- 29. Mortaz E, Masjedi MR, Barnes PJ, et al. Epigenetics and chromatin remodeling play a role in lung disease. Tanaffos. 2011;10:7‐16. [PMC free article] [PubMed] [Google Scholar]
- 30. Green JA, Elkington PT, Pennington CJ, et al. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase‐1 and ‐3 expression and secretion via NF‐kappa B and Activator Protein‐1‐dependent monocyte networks. J Immunol. 2010;184:6492‐6503. [DOI] [PubMed] [Google Scholar]
- 31. Salgame P. MMPs in tuberculosis: Granuloma creators and tissue destroyers. J Clin Invest. 2011;121:1686‐1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh S, Saraiva L, Elkington PT, et al. Regulation of matrix metallo‐proteinase‐1, ‐3, and ‐9 in Mycobacterium tuberculosis‐dependent respiratory networks by the rapamycin‐sensitive PI3K/p70(S6K) cascade. FASEB J. 2014;28(1):85‐93. [DOI] [PubMed] [Google Scholar]
- 33. Al Shammari B, Shiomi T, Tezera L, et al. The extracellular matrix regulates granuloma necrosis in tuberculosis. J Infect Dis. 2015;212:463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elkington P, Shiomi T, Breen R, et al. MMP‐1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121(5):1827‐1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murthy A, Shao YW, Defamie V, Wedeles C, Smookler D, Khokha R. Stromal TIMP3 regulates liver lymphocyte populations and provides protection against Th1 T cell‐driven autoimmune hepatitis. J Immunol. 2012;188:2876‐2883. [DOI] [PubMed] [Google Scholar]
- 37. Bar‐Or A. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738‐2749. [DOI] [PubMed] [Google Scholar]
- 38. Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is upregulated in pulmonary emphysema and mediates matrix metalloproteinase‐1 induction by cigarette smoke. J Biol Chem. 2004;279:17690‐17696. [DOI] [PubMed] [Google Scholar]
- 39. Svineng G, Ravuri C, Rikardsen O, Huseby N‐E, Winberg J‐O. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Conn Tissue Res. 2008;49:197‐202. [DOI] [PubMed] [Google Scholar]
- 40. Rivera‐marrero CA, Schuyler W, Roser S, et al. tuberculosis induction of matrix metalloproteinase‐ 9: the role of mannose and receptor‐mediated mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;282:L546‐L555. [DOI] [PubMed] [Google Scholar]
- 41. Sathyamoorthy T, Sandhu G, Tezera LB, et al. Gender dependent differences in plasma matrix metalloproteinase‐8 elevated in pulmonary tuberculosis. PLoS ONE. 2015a;10:e0117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collazos J, Asensi V, Martin G, Montes AH, Suárez‐Zarracina T, Valle‐Garay E. The effect of gender and genetic polymorphisms on matrix metalloprotease (MMP) and tissue inhibitor (TIMP) plasma levels in different infectious and noninfectious conditions. Clin Exp Immunol. 2015;182:213‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brilha S, Sathyamoorthy T, Stuttaford LH, et al. Early secretory antigenic target‐6 drives matrix metalloproteinase‐10 gene expression and secretion in tuberculosis. Am J Resp Cell Mol Biol. 2017;56:223‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lou J, Wang Y, Zhang Z, et al. Activation of MMPs in Macrophages by Mycobacterium tuberculosis via the miR‐223‐BMAL1 Signaling Pathway. J Cell Biochem. 2017;118:4804‐4812. [DOI] [PubMed] [Google Scholar]
- 45. Azikin W, Amiruddin L, Husein A, et al. Matrix Metalloproteinase‐9 (MMP‐9) Level in Tuberculosis Exposed and Infected Children. Am J Health Res. 2017;1:7‐10. [Google Scholar]
- 46. Chongwe G, Michelo C, Sinkala E, et al. Mycobacterium avium lysate induces matrix metalloproteinase‐1 in intestinal tissue and peripheral blood: Observations from selected hospital based Zambian adults. Inter J Infec Dis. 2018;71:73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumar NP, Moideen K, Viswanathan V, et al. Elevated levels of matrix metalloproteinases reflect severity and extent of disease in tuberculosis‐diabetes co‐morbidity and are predominantly reversed following standard antituberculosis or metformin treatment. BMC Infec Dis. 2018;18:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao D, Feng C, Zhuang H. The effect of minimally invasive operation on the serum interleukins and matrix metalloproteinases of old patients with spinal tuberculosis and perioperative nursing. Biomed Res. 2017;28:9097‐9102. [Google Scholar]
- 49. Chodobski A, Zink BJ, Szmydynger‐Chodobska J. Blood‐brain barrier pathophysiology in traumatic brain injury. Translat Stroke Res. 2011;2:492‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. 2016;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roine I, Pelkonen T, Lauhio A, et al. Changes in MMP‐9 and TIMP‐1 concentrations in cerebrospinal fluid after 1 week of treatment of childhood bacterial meningitis. J Clin Microbiol. 2015;53:2340‐2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ong C, Pabisiak PJ, Brilha S, et al. Complex regulation of neutrophil‐derived MMP‐9 secretion in central nervous system tuberculosis. J Neuroinflam. 2017;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li XP, Ding GL, Chen JL, et al. Expression of matrix metalloproteinase‐9 in mice with tuberculous meningitis and its significance. Int J Clin Exp Pathol. 2016;9(8):8132‐8141. [Google Scholar]
- 54. Clark I, Swingler T, Sampieri C, Edwards D. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362‐1378. [DOI] [PubMed] [Google Scholar]
- 55. Mancini A, di Battista JA. Transcriptional regulation of matrix metalloprotease gene expression in health and disease. Front Biosci. 2006;11:423‐446. [DOI] [PubMed] [Google Scholar]
- 56. Reunanen N, Li SP, Ahonen M, et al. Activation of p38 MAPK enhances collagenase‐1 (matrix metalloproteinase (MMP)‐1) and stromelysin‐1 (MMP‐3) expression by mRNA stabilization. J Biol Chem. 2002;277:32360‐32368. [DOI] [PubMed] [Google Scholar]
- 57. Chernov AV, Sounni NE, Remacle AG, Strongin AY. Epigenetic control of the invasion‐promoting MT1‐MMP/MMP‐2/TIMP‐2 axis in cancer cells. J Biol Chem. 2009;284:12727‐12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tocchi A, Parks WC. Functional interactions between matrix metalloproteinases and glycosaminoglycans. FEBS J. 2013;280:2332‐2341. [DOI] [PubMed] [Google Scholar]
- 59. Rosenberg GA. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist. 2002;8:586‐595. [DOI] [PubMed] [Google Scholar]
- 60. Price NM, Farrar J, Chau T, Mai N, Hien TT, Friedland JS. Identification of a matrix‐degrading phenotype in human tuberculosis in vitro and in vivo . J Immunol. 2001;166:4223‐4230. [DOI] [PubMed] [Google Scholar]
- 61. Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP–7, MMP–9 and the inhibitor TIMP–1 in pulmonary tuberculosis. Clin Immunol. 2009;133:126‐131. [DOI] [PubMed] [Google Scholar]
- 62. Hwang K‐E, Shon Y‐J, Cha B‐K, et al. Tissue inhibitor of metalloproteinase–1 is responsible for residual pleural thickening in pleural tuberculosis. Tohoku J Exp Med. 2015;235:327‐333. [DOI] [PubMed] [Google Scholar]
- 63. Ugarte‐Gil CA, Elkington P, Gilman RH, et al. Induced sputum MMP–1, –3 & –8 concentrations during treatment of tuberculosis. PLoS ONE. 2013;8:e61333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walker NF, Clark SO, Oni T, et al. Doxycycline and HIV infection suppress tuberculosis‐induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185(9):989‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen Y, Wang J, Ge P, et al. Tissue inhibitor of metalloproteinases 1, a novel biomarker of tuberculosis. Mol Med Reports. 2017;15:483‐487. [DOI] [PubMed] [Google Scholar]
- 66. Rand L, Green JA, Saraiva L, Friedland JS, Elkington P. Matrix metalloproteinase–1 is regulated in tuberculosis by a p38 MAPK–dependent, p–aminosalicylic acid–sensitive signaling cascade. J Immunol. 2009;182:5865‐5872. [DOI] [PubMed] [Google Scholar]
- 67. Pawluczyk I, Patel SR, Kevin PG. Pharmacological enhancement of the kallikrein‐kinin system promotes anti‐fibrotic responses in human mesangial cells. Cell Physiol Biochem. 2006;18:327‐336. [DOI] [PubMed] [Google Scholar]
- 68. Saunders WB, Bayless KJ, Davis GE. MMP‐1 activation by serine proteases and MMP‐10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325‐2340. [DOI] [PubMed] [Google Scholar]
- 69. Marceau F. Kinin B1 receptors: a review. Int Immunopharmacol. 1995;30:1‐26. [DOI] [PubMed] [Google Scholar]
- 70. Wang G, Ye Y, Zhang X, Song J. Bradykinin stimulates IL‐6 production and cell invasion in colorectal cancer cells. Oncol Rep. 2014;32:1709‐1714. [DOI] [PubMed] [Google Scholar]
- 71. Eric J, Gabra BH, Sirois P. Implication of the bradykinin receptors in antigen‐induced pulmonary inflammation in mice. Br J Pharmacol. 2003;138:1589‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kimura A, Takahiro K, Ryuichi O, et al. Localization of Bradykinin B2 Receptor in the Follicles of Porcine Ovary and Increased Expression of Matrix Metalloproteinase‐3 and ‐20 in Cultured Granulosa Cells by Bradykinin Treatment. Biol Reprod. 2001;65:1462‐1470. [DOI] [PubMed] [Google Scholar]
- 73. Schanstra JP, Neau E, Drogoz P, et al. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest. 2002;110:371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zaczynska E, Gabra BH, Sirois P. Bradykinin stimulates MMP‐2 production in guinea pig tracheal smooth muscle cells. Inflammation. 2003;27:5. [DOI] [PubMed] [Google Scholar]
- 75. Ehrenfeld P, Conejeros I, Pavicic MF, et al. Activation of kinin B1 receptor increases the release of metalloproteases‐2 and ‐9 from both estrogen‐sensitive and ‐insensitive breast cancer cells. Cancer Lett. 2011;301:106‐118. [DOI] [PubMed] [Google Scholar]
- 76. Webba JG, Yanga X, Craig EC. Bradykinin activation of extracellular signal‐regulated kinases in human trabecular meshwork cells. Exp Eye Res. 2011;92(6):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yoon H, Blaber SI, Li WU, Scarisbrick IA, Blaber M. Activation profiles of human kallikrein‐related peptidases by matrix metalloproteinases. Biol Chem. 2013;394(1):137‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sundararajan S, Babu S, Das SD. Comparison of localized versus systemic levels of Matrix metalloproteinases (MMPs), its tissue inhibitors (TIMPs) and cytokines in tuberculous and non–tuberculous pleuritis patients. Hum Immunol. 2012;73:985‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22(10):571‐579. [DOI] [PubMed] [Google Scholar]
- 80. Elkington PT, Green JA, Emerson JE, et al. Synergistic up‐regulation of epithelial cell matrix metalloproteinase‐9 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2007;37:431‐437. [DOI] [PubMed] [Google Scholar]
- 81. O'Kane CM, Boyle JJ, Horncastle DE, Elkington PT, Friedland JS. Monocyte‐dependent fibroblast CXCL8 secretion occurs in tuberculosis and limits survival of mycobacteria within macrophages. J Immunol. 2007;178:3767‐3776. [DOI] [PubMed] [Google Scholar]
- 82. Elkington P, Emerson JE, Lopez‐Pascua L, et al. Mycobacterium tuberculosis Up‐Regulates Matrix Metalloproteinase‐1 Secretion from Human Airway Epithelial Cells via a p38 MAPK Switch. J Immunol. 2005;175(8):5333‐5340. [DOI] [PubMed] [Google Scholar]
- 83. Yun P, Decarlo AA, Collyer C, Hunter N. Hydrolysis of interleukin‐12 by porphyromonas gingivalis major cysteine proteinases may affect local gamma interferon accumulation and the Th1 or Th2 T‐cell phenotype in periodontitis. Infect Immun. 2001;69:5650‐5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Queiroz‐Junior CM, Silva M, Corrêa JD, et al. A controversial role for IL‐12 in immune response and bone resorption at apical periodontal sites. Clin Dev Immunol. 2010;327417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Murphy KM, Ouyang W, Farrar JD, et al. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451‐494. [DOI] [PubMed] [Google Scholar]
- 86. Miao L, Zhan S, Liu J. Interleukin‐12‐mediated expression of matrix metalloproteinases in human periodontal ligament fibroblasts involves in NF‐κB activation. Biosci Reports. 2017;37:BSR20170973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roupakia E, Markopoulos GS, Kolettas E. IL‐12‐mediated transcriptional regulation of matrix Metalloproteinases. Biosci Reports. 2018;38:BSR20171420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Torrado E, Cooper AM. IL‐17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trajkovic V, Stosic‐Grujicic S, Samardzic T, et al. Interleukin‐17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol. 2001;119:183‐191. [DOI] [PubMed] [Google Scholar]
- 90. Gideon HP, Phuah JiaYao, Myers AJ, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro‐ and anti‐inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015;11:e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Aguilo N, Alvarez‐Arguedas S, Uranga S, et al. Pulmonary but not subcutaneous delivery of BCG vaccine confers protection to tuberculosis‐susceptible mice by an interleukin 17‐dependent mechanism. J Infect Dis. 2016;213:831‐839. [DOI] [PubMed] [Google Scholar]
- 92. Wang M, Xu G, Lü L, et al. Genetic polymorphisms of IL‐17A, IL‐17F, TLR4 and miR‐146a in association with the risk of pulmonary tuberculosis. Sci Rep. 2016;6:28586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gopal R, Monin L, Slight S, et al. Unexpected role for IL‐17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10:e1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Freches D, Korf H, Denis O, et al. Mice genetically inactivated in interleukin‐17A receptor are defective in long‐term control of Mycobacterium tuberculosis infection. Immunology. 2013;140:220‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Okamoto Yoshida Y, Umemura M, Yahagi A, et al. Essential role of IL‐17A in the formation of a mycobacterial infection‐induced granuloma in the lung. J Immunol. 2010;184:4414‐4422. [DOI] [PubMed] [Google Scholar]
- 96. Singh S, Maniakis‐Grivas G, Singh UK, et al. Interleukin‐17 regulates matrix metalloproteinase activity in human pulmonary tuberculosis. J Pathol. 2018;244:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Murayama R, Kobayashi M, Takeshita A, et al. MAPKs, activator protein‐1 and nuclear factor‐κB mediate production of interleukin‐1β‐stimulated cytokines, prostaglandin E2 and MMP‐1 in human periodontal ligament cells. J Periodontal Res. 2011;46:568‐575. [DOI] [PubMed] [Google Scholar]
- 98. O’Kane CM, Elkington PT, Jones MD, et al. STAT3, p38 MAPK, and NF‐kappaB drive unopposed monocyte‐dependent fibroblast MMP‐1 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2010;43:465‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wu Y, Zhu L, Liu L, Zhang J, Peng B. Interleukin‐17A stimulates migration of periodontal ligament fibroblasts via p38 MAPK/NF‐κB‐dependent MMP‐1 expression. J Cell Physiol. 2014;229:292‐299. [DOI] [PubMed] [Google Scholar]
- 100. Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460:98‐102. [DOI] [PubMed] [Google Scholar]
- 101. Ong C, Elkington PT, Brilha S, et al. Neutrophil‐Derived MMP‐8 Drives AMPK‐Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLoS Pathog. 2015;11(5):e1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Moores RC, Brilha S, Schutgens F, et al. Epigenetic regulation of matrix metalloproteinase‐1 and ‐3 expression in mycobacterium tuberculosis infection. Front Immunol. 2017;8:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schwander S, Dheda K. Human lung immunity against mycobacterium tuberculosis: insights into pathogenesis and protection. Am J Respir Crit Care Med. 2011;183:696‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Via LE, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76(6):2333‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cronan MR, Beerman RW, Rosenberg AF, et al. Macrophage epithelial reprogramming underlies mycobacterial granuloma formation and promotes infection. Immunity. 2016;45:861‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Parasa VR, Rahman MJ, Ngyuen‐Hoang AT, et al. Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue. Dis Model Mech. 2014;7:281‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dartois V. The path of anti‐tuberculosis drugs: From blood to lesions to mycobacterial cells. Nat Rev Microbiol. 2014;12(3):159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dartois V, Barry CE. A medicinal chemists’ guide to the unique difficulties of lead optimization for tuberculosis. Bioorg Med Chem Lett. 2013;23(17):4741‐4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shin SS, Keshavjee S, Gelmanova IY, et al. Development of extensively drug‐resistant tuberculosis during multidrug‐resistant tuberculosis treatment. Am J Respir Crit Care Med. 2010;182:426‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug‐ and extensively drug‐resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80‐86. [DOI] [PubMed] [Google Scholar]
- 111. Yew WW, Sotgiu G, Migliori GB. Update in tuberculosis and non tuberculous mycobacterial disease 2010. Am J Respir Crit Care Med. 2011;184:180‐185. [DOI] [PubMed] [Google Scholar]
- 112. Manjelievskaia J, Erck D, Piracha S, et al. Drug‐resistant TB: deadly, costly and in need of a vaccine. Trans R Soc Trop Med Hyg. 2016;110(3):186‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dye C, Glaziou P, Floyd K, et al. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271‐286. [DOI] [PubMed] [Google Scholar]
- 114. Quiding‐Jarbrink M, Smith DA, Bancroft GJ. Production of matrix metalloproteinases in response to mycobacterial infection. Infect Immun. 2001;69(9):5661‐5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Parks WC, Wilson CL, Lopez‐Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617‐629. [DOI] [PubMed] [Google Scholar]
- 116. Hernandez‐pando R, Orozco H, Arriaga K, et al. Treatment with BB‐94, a broad spectrum inhibitor of zinc‐dependent metalloproteinases, causes deviation of the cytokine profile towards Type‐2 in experimental pulmonary tuberculosis in Balb/c mice. Int J Exp Path. 2000;81:199‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Izzo AA, Izzo LS, Kasimos J, et al. A matrix metalloproteinase inhibitor promotes granuloma formation during the early phase of Mycobacterium tuberculosis pulmonary infection. Tuberculosis. 2004;84(6):387‐396. [DOI] [PubMed] [Google Scholar]
- 118. Volkman HE, Pozos TC, Zheng J, et al. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327(5964):466‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kaplan G, Post FA, Moreira AL, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71:7099‐7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Anandaiah A, Dheda K, Keane J, et al. Novel developments in the epidemic of human immunodeficiency virus and tuberculosis coinfection. Am J Respir Crit Care Med. 2011;183:987‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Singh AK, Gupta UD. Animal models of tuberculosis: lesson learnt. Indian J Med Res. 2018;147:456‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dharmadhikari AS, Nardell EA. What animal models teach humans about tuberculosis. Am J Respir Cell Mol Biol. 2008;39:503‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Beamer GL, Turner J. Murine models of susceptibility to tuberculosis. Arch Immunol Ther Exp. 2005;53(6):469‐483. [PubMed] [Google Scholar]
- 124. Rivera‐Marrero CA, Schuyler W, Roser S, et al. Induction of MMP‐9 mediated gelatinolytic activity in human monocytic cells by cell wall components of Mycobacterium tuberculosis. Microb Pathog. 2000;29:231‐244. [DOI] [PubMed] [Google Scholar]
- 125. Taylor JL, Hattle JM, Dreitz SA, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006;74:6135‐6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Driver ER, Ryan GJ, Hoff DR, et al. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56:3181‐3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bouté M, Carreras F, Rossignol C, Doz E, Winter N, Epardaud M. The C3HeB/FeJ mouse model recapitulates the hallmark of bovine tuberculosis lung lesions following Mycobacterium bovis aerogenous infection. Vet Res. 2017;48:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Irwin SM, Driver E, Lyon E, et al. Presence of multiple lesion types with vastly different microenvironments in C3HeB/FeJ mice following aerosol infection with Mycobacterium tuberculosis. Dis Models Mechan. 2015;8:591‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Via LE, Weiner DM, Schimel D, et al. Differential virulence and disease progression following Mycobacterium tuberculosis complex infection of the common marmoset (Callithrix jacchus). Infect Immun. 2013;81:2909‐2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Via LE, Schimel D, Weiner DM, et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [18F]2‐fluoro‐deoxy‐D‐glucose positron emission tomography and computed tomography. Antimicrob Agents Chemother. 2012;56:4391‐4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Subbian S, Tsenova L, Yang G, et al. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol. 2011;1:110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gupta UD, Katoch VM. Animal models of tuberculosis for vaccine development. Indian J Med Res. 2009;129:11‐18. [PubMed] [Google Scholar]
- 133. Dannenberg AM. Pathogenesis of human pulmonary tuberculosis. insights from the rabbit model. Washington, DC: ASM Press; 2006. [Google Scholar]
- 134. Kjellsson MC, Via LE, Goh A, et al. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob Agents Chemother. 2012;56:446‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Subbian S, Tsenova L, Holloway J, et al. Adjunctive phosphodiesterase‐4 inhibitor therapy improves antibiotic response to pulmonary tuberculosis in a rabbit model. EBioMedicine. 2016;4:104‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Subbian S, Tsenova L, O’Brien P, et al. Phosphodiesterase‐4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol. 2011;179:289‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. van der Sar AM, Spaink HP, Zakrzewska A, Bitter W, Meijer AH. Specificity of the zebrafish host transcriptome response to acute and chronic mycobacterial infection and the role of innate and adaptive immune components. Mol Immunol. 2009;46:2317‐2332. [DOI] [PubMed] [Google Scholar]
- 139. Walsh GP, Tan EV, Dela Cruz EC, et al. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med. 1996;2:430‐436. [DOI] [PubMed] [Google Scholar]
- 140. Lin PL, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631‐4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang J, Guo M, Rao Y, et al. Mycobacterium tuberculosis Erdman infection of cynomolgus macaques of Chinese origin. J Thorac Dis. 2018;10:3609‐3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mehra S, Pahar B, Dutta NK, et al. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS ONE. 2010;5:e12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ralph AP, Kenangalem E, Waramori G, et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under‐recognised phenomena. PLoS ONE. 2013;8:e80302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pasipanodya JG, McNabb S, Hilsenrath P, et al. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health. 2010;10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. van Zyl Smit RN, Pai M, Yew WW, et al. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J. 2010;35:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. WHO (2018). Global Tuberculosis Report. Available on: http://apps.who.Int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf(Accessed on November 10, 2018). [Google Scholar]
- 147. Sonnenberg P, Glynn J, Fielding K, Murray J, Godfrey‐Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150‐158. [DOI] [PubMed] [Google Scholar]
- 148. Schluger NW, Perez D, Liu YM. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122:597‐602. [DOI] [PubMed] [Google Scholar]
- 149. Walker NF, Wilkinson KA, Meintjes G, et al. Matrix degradation in human immunodeficiency virus type 1–associated tuberculosis and tuberculosis immune reconstitution inflammatory syndrome: a prospective observational study. Clin Infect Dis. 2017;65:121‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV‐infected subjects in the HAART era. PLoS ONE. 2009;4:e6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tadokera R, Meintjes GA, Wilkinson KA, et al. Matrix metalloproteinases and tissue damage in HIV‐tuberculosis immune reconstitution inflammatory syndrome. Eur J Immunol. 2014;44:127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ravimohan S, Tamuhla N, Kung S‐J, et al. Matrix metalloproteinases in tuberculosis‐immune reconstitution inflammatory syndrome and impaired lung function among advanced hiv/tb co‐infected patients initiating antiretroviral therapy. EBioMed. 2016;3:100‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ravimohan S, Tamuhla N, Steenhoff AP, et al. Early immunologic failure is associated with early mortality among advanced HIV‐infected adults initiating antiretroviral therapy with active tuberculosis. J Infect Dis. 2013;208:1784‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ravimohan S, Tamuhla N, Steenhoff AP, et al. Immunological profiling of tuberculosis‐associated immune reconstitution inflammatory syndrome and non‐immune reconstitution inflammatory syndrome death in HIV‐infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis. 2015;15:429‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ljubisavljevic S, Stojanovic I, Basic J, et al. The role of matrix metalloproteinase 3 and 9 in the pathogenesis of acute neuroinflammation. implications for disease modifying therapy. J Mol Neurosci. 2015;56(4):840‐847. [DOI] [PubMed] [Google Scholar]
- 157. Liechti FD, Grandgirard D, Leppert D, Leib SL. Matrix metalloproteinase inhibition lowers mortality and brain injury in experimental pneumococcal meningitis. Infect Immun. 2014;82:1710‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ricci S, Grandgirard D, Wenzel M, et al. Inhibition of matrix metalloproteinases attenuates brain damage in experimental meningococcal meningitis. BMC Infect Dis. 2014;14:3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Oehlers SH, Cronan MR, Scott NR, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517(7536):612‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hawn TR, Matheson AI, Maley SN, Vandal O. Host‐directed therapeutics for tuberculosis: can we harness the host? Microbiol Mol Biol Rev. 2013;77:608‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mayer‐Barber KD, Andrade BB, Oland SD, et al. Host directed therapy of tuberculosis based on interleukin‐1 and type I interferon crosstalk. Nature. 2014;511:99‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Albaiceta GM, Gutierrez‐Fernández A, García‐Prieto E, et al. Absence or inhibition of matrix metalloproteinase‐8 decreases ventilator‐induced lung injury. Am J Respir Cell Mol Biol. 2010;43(5):555‐563. [DOI] [PubMed] [Google Scholar]
- 163. Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13(12):904‐927. [DOI] [PubMed] [Google Scholar]
- 164. Ordonez AA, Pokkali S, Sanchez‐Bautista J, et al. Matrix metalloproteinase inhibition in a murine model of cavitary tuberculosis paradoxically worsens pathology. Brief Report, J Infec Dis. 2019;29;219(4):633‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ordonez AA, Pokkali S, Kim S, et al. Adjunct antibody administration with standard treatment reduces relapse rates in a murine tuberculosis model of necrotic granulomas. PLoS ONE. 2018;13:e0197474. [DOI] [PMC free article] [PubMed] [Google Scholar]


