Abstract
It has long been appreciated that highly autoreactive BCRs are actively removed from the developing B cell repertoire by antigen-dependent receptor editing and deletion. However, there is persistent debate about whether mild autoreactivity is simply tolerated or positively selected into the mature B cell repertoire, as well as at what stage, to what extent, under what conditions, and into which compartments this occurs. In this study, we describe two minor, trackable populations of B cells in B1–8i Ig-transgenic mice that express the VH186.2 H chain and recognize a common foreign antigen (the hapten NP), but differ in L chain expression. We use the Nur77-eGFP reporter of BCR signaling to define their reactivity toward endogenous antigens. The less autoreactive of these two populations is strongly counter-selected during development of mature B1a, follicular, and marginal zone B cells. By genetically manipulating strength of BCR signal transduction via titration of surface CD45 expression, we demonstrate that this B cell population is not negatively selected, but instead displays characteristics of impaired positive selection. We demonstrate that mild self-reactivity improves the developmental fitness of B cell clones in the context of a diverse population of B cells, and positive selection by endogenous antigens shapes the mature B cell repertoire.
Keywords: B cell, BCR, positive selection, autoreactivity, Nur77, Nr4a1, MZ B cell, B1a cell
Introduction
Due to the random nature of Ig gene rearrangement during early stages of B cell development, both non-functional and highly self-reactive BCRs are generated (1). A large fraction of newly rearranged BCRs bind to self-antigens, including nucleic acids, but these specificities are efficiently removed from the repertoire by receptor editing and deletion, and tolerance mechanisms such as anergy control residual self-reactivity in the periphery (2–4). Conversely, productive rearrangement of the BCR H chain is enforced by an early signaling checkpoint that drives both survival and differentiation (1); if a rearranged Ig H chain successfully pairs with surrogate light chain, the resulting pre-BCR is thought to signal autonomously to mimic an “autoreactive” BCR and promote L chain rearrangement (5). Successful L chain rearrangement is then enforced because the BCR cannot be expressed on the surface of B cells without a functional L chain, and BCR signaling itself is essential for normal B cell development (6). However, although mutations that disrupt BCR signaling machinery perturb B cell development, these genetic lesions affect both tonic and antigen-dependent signaling and do not distinguish between the two (6). Consequently, it is not clear whether mere expression of the BCR on the surface and the tonic signals it provides (which are critical for mature B cell survival) are sufficient for optimal B cell development, or whether recognition of bona fide endogenous antigens is important during this process (7–9). Further, the teleological rationale for antigen-driven B cell development is unclear; unlike antigen-dependent positive selection of T cells during thymic development, which enforces MHC restriction and ensures that mature T cells can recognize peptides in the context of self-MHC, it is not immediately apparent how positive selection of self-reactivity into the B cell repertoire might facilitate host defense. For these reasons, the existence of a bona-fide antigen-dependent positive selection checkpoint during B cell development has been debated.
Nevertheless, there are several lines of evidence supporting a role for antigen-dependent positive selection of specific BCRs into the mature B cell repertoire (9). Most compelling among these, the “innate-like” B1a compartment displays remarkable BCR restriction relative to other B cell subsets; more than a third of the B1a repertoire is comprised of a handful of BCRs with a shared CDR3 sequence that recognizes phosphatidylcholine, an epitope exposed on the surface of dying cells (10, 11). Moreover, Hayakawa and colleagues showed that Thy-1-specific (ATA) B cells require Thy-1 antigen expression in order to enter the B1a compartment and fail to do so in Thy-1-deficient mice (12, 13). Indeed, B1a development is especially vulnerable to any impairment in BCR signal transduction, and this high threshold for BCR signaling is thought to enforce antigen-dependence of this population and favor selection of self-reactivity (14, 15). By contrast, marginal zone (MZ) B cell development in the ATA model is disfavored both at high levels of antigen and in the complete absence of antigen, but proceeds optimally at low levels of antigen, suggesting a much lower signaling threshold for selection into this compartment (16). Consistent with this, MZ B cell development exhibits a much less stringent requirement for robust BCR signal transduction than B1a B cells (14, 17, 18).
Analogous but indirect evidence for positive selection into the follicular (Fo) B2 cell compartment has also been reported; specific BCR sequences are enriched in mature Fo B cells relative to immature B cells, suggesting active selection of at least some clones by antigen, although whether this occurs during or after development is uncertain (19–21). Furthermore, BCR crosslinking can restore development in an Ig transgenic (Ig-Tg) model that does not normally support Fo B cell maturation (22). However, hen egg lysozyme (HEL)-specific B cells with high surface BCR expression and intact BCR signaling machinery populate the B2 cell compartments even in the absence of cognate antigen, calling into question whether antigen recognition is in fact necessary for Fo or MZ B cell development (23, 24). Indeed, ATA B cells (which require low and high Thy-1 antigen expression respectively in order to enter the MZ and B1a compartments) populate the Fo compartment even in Thy-1-deficient animals (12, 13, 16). Further, genetic models harboring truncated BCR constructs have suggested that antigen recognition is dispensable for Fo B cell development and ‘tonic’ BCR signals can suffice (25–27). In addition, existing evidence in favor of antigen-dependent positive selection of B2 cells has several limitations. Bona fide physiologic endogenous antigens may differ significantly in terms of abundance, affinity, and valency from model self-antigens. Most prior studies focus on analysis of Ig-Tg B cells in monoclonal rather than diverse repertoires, but inter-clonal competition can alter B cell development; Freitas and colleagues showed that Ig-Tg B cells from several different Tg mouse lines develop normally in isolation, but exhibit a competitive disadvantage and an increased turnover rate in chimeras harboring competing polyclonal B cells (27, 28). However, the developmental stage and B cell lineage affected were not defined, leaving open the question of whether antigen regulates true positive selection of B2 cells or merely their survival in the mature repertoire after development has already occurred. Most importantly, the actual self-reactivity of most of the Ig-Tg lines examined in these and other studies is unknown, making it challenging to interpret and compare them.
The question that bedevils the B cell field is not whether antigen can promote B cell development – clearly it can – but whether it does so in the context of a diverse BCR repertoire and physiologic endogenous antigens. Here we take advantage of a reporter of BCR signaling, Nur77-eGFP, which serves as a sensitive marker of bona fide endogenous antigen reactivity, in order to define the self-reactivity of individual B cell populations in the context of a polyclonal repertoire (18). We describe two B cell populations in B1–8i H chain Tg mice that each recognize 4-hydroxy-3-nitrophenylacetyl (NP) hapten but have different levels of reactivity towards endogenous antigens (29). These two populations express a common transgenic H chain (VH186.2) and differ only in expression of two different lambda L chains. Both arise at relatively low precursor frequency in the context of a polyclonal repertoire, and we rigorously assessed their competitive fitness at different stages of development. The population with less self-reactivity, NP+ Igλ1+, displays profoundly impaired entry into the peritoneal B1a compartment and counter-selection during development into mature B2 B cell compartments in the spleen. Through genetic modulation of BCR signal strength via titration of CD45 expression, we identify positive and negative selection thresholds for entry of these B cell populations into mature B1 and B2 cell compartments. While the self-reactivity threshold for selection into the B1a compartment is especially high, mere tonic signals are not sufficient for efficient entry into any mature B cell compartment. Rather, we show that endogenous antigen recognition promotes optimal B cell development in the context of a complex peripheral repertoire.
Materials and Methods
Mice
C57BL/6, BoyJ, and B1–8i mice were obtained from Jackson Laboratory (29). Nur77-eGFP BAC Tg (18), IgHEL Tg (MD4) (23), CD45.L/L (lightning) (30), and CD45.H/H (31) mice were previously described. CD45.L/+ mice used in this study have one WT allele and one lightning allele of the Ptprc gene encoding CD45. The lightning allele harbors a previously described point mutation in the first extracellular fibronectin repeat of CD45, resulting in reduced surface expression, but normal splicing, of CD45. CD45.H/+ mice have two copies of endogenous WT CD45 and a single copy of the previously described H Tg, resulting in 50% overexpression of normally spliced CD45. All strains were fully backcrossed to the C57BL/6 genetic background. Mice were housed in a specific pathogen-free facility at the University of California, San Francisco according to university and NIH guidelines. Mice of mixed sex were used unless otherwise noted. In this study, “wild-type” (WT) mice have no BCR transgenes, express allotype [b] BCRs, and express normal levels of CD45.
Antibodies and Reagents
Streptavidin and antibodies to B220, CD5, CD19, CD21, CD23, CD45.1, CD45.2 CD93, IgD, Igλ1, Igλ1,2,3, IgM, IgM[a], and IgM[b], were conjugated to biotin, APC/A647, APC-e780, FITC, PE, PE-Cy7, PerCP-Cy5.5, or Pacific Blue (Tonbo Biosciences, Biolegend, BD Biosciences, eBioscience). NP hapten conjugated to PE was from Biosearch Technologies. pErk Ab for intracellular staining (clone 194g2) was from Cell Signaling Technologies. Donkey anti-rabbit secondary Ab conjugated to APC was from Jackson Immunoresearch. Goat anti-mouse IgM F(ab’)2 stimulatory antibody was from Jackson Immunoresearch.
Flow cytometry
Cells were stained with antibodies, Fc block (2.4G2), and NP-PE diluted in PBS with 2% fetal calf serum, 2 mM EDTA, and penicillin/streptomycin/glutamine. Samples were collected on a BD LSRFortessa (BD Biosciences, Franklin Lakes, NJ) and analyzed with FlowJo (v9.9.4; FlowJo, LLC, Ashland, OR).
Vital dye loading
Cells were loaded with CellTrace Violet (CTV; Invitrogen) per the manufacturer’s instructions except at 5 ×106 cells/ml rather than 1 × 106 cells/ml.
Adoptive transfer and immunization
Splenocytes from B1–8i mice were harvested into single cell suspensions, subjected to red blood cell lysis with ACK buffer, and loaded with vital dye as described above. 4×106 cells in 200 μL total volume were injected into each CD45.1 host via the tail vein. Hosts were either immunized IP with 10μg NP-KLH / alum (1:1), or held as controls. After 3 days, hosts splenocytes were harvested and analyzed by flow staining.
Intracellular staining
Following stimulation, cells were fixed in 2% paraformaldehyde for 10 minutes, permeabilized on ice with 100% methanol for 30 minutes, and stained with anti-pErk followed by lineage markers and secondary antibody (40 minutes incubation for primary and secondary staining). Antibody concentrations were previously described (32).
Bone marrow chimeras
Male BoyJ hosts were lethally irradiated twice at 330 mC, 4 hours apart, and reconstituted with 5 × 106 1:1 mixed B1–8i CD45.1/2 Nur77-eGFP and WT CD45.2/2 Nur77-eGFP bone marrow cells. Chimeric mice were sacrificed and analyzed 7 weeks after reconstitution.
Light chain sequencing and alignment
Splenocytes were stained and sorted using a FACSAria II (Becton Dickinson), dividing B220+/CD21lo/CD23+/NP+ cells into Igλ1+ and Igλ1− populations. RNA was isolated via Trizol (Life technologies)/chloroform phase separation. cDNA was generated using a Onestep RT PCR kit (Qiagen), with nested PCR primers to amplify light chain sequences (Primers: VL1/2: ctgctaccggttcctgggcccaggctgttgtgactcag; VL3: ctgctaccggttcctgggcccaacttgtgctcactcag; JL1: ttgggctggccaaggacagtcagtttggttcc; JL2: ttgggctggccaaggacagtgaccttggttcc; JL3: ttgggctggccaaggacagtcaatctggttcc). cDNA was gel-purified and inserted into the PCR-2.1 Topo vector (Topo-TA kit, Invitrogen), and transformed into Top10 competent E. coli (Invitrogen) via heat shock. Colonies were picked and PCR amplified using M13 primers from the Topo-TA kit. Amplified DNA was treated with recombinant Shrimp Alkaline Phosphatase and Exonuclease 1 (New England Biolabs), and sent to ELIM Biopharm for sequencing. The returned sequences were uploaded to IMGT_V-QUEST (http://www.imgt.org/IMGT_vquest/vquest) for VJ alignment and light chain identification (see Supplemental Table 1).
Statistical analysis
Statistics were calculated using GraphPad Prism (v.7.0b; GraphPad Software La Jolla, CA). Welch’s t test was used to compare two groups unless otherwise noted. One-way ANOVA was used to compare three or more groups, and Tukey’s and Dunnett’s multiple comparisons tests were used when comparing groups to a single reference group or to every other group, respectively. Error bars correspond to mean ± SEM.
Results
B1–8i mice have two largely monoclonal NP-binding B cell populations with different self-reactivity
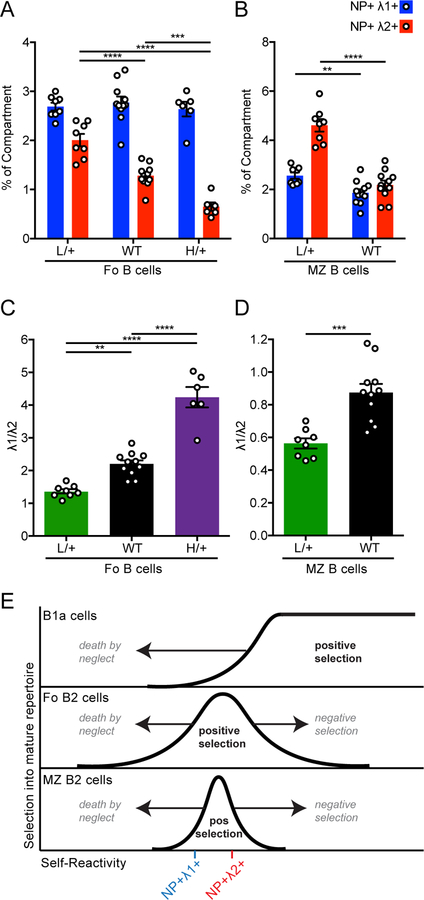
B1–8i mice harbor a transgenic Ig H chain knocked-in to the endogenous locus, generating a BCR repertoire in which a substantial proportion of B cells (approximately 5%) recognize NP hapten (29). During our analysis of B1–8i mice, we noticed two distinct NP-binding populations that each express the VH186.2 H chain Tg (marked by IgH[a] allotype) and Igλ L chains, but can be distinguished on the basis of Igλ1 expression (Fig. 1A, Supplemental Figs. 1A, B). To further characterize these two populations, we sorted them in order to sequence their L chains. As expected, all of the sequences from the Igλ1+ population encode VL1, with the vast majority also encoding the J1 region (Fig. 1B, Supplemental Table 1). The NP-binding (NP+) Igλ1− population expresses exclusively VL2 and mostly J2 segments (Fig. 1B, Supplemental Table 1). Indeed, genomic structure of the murine lambda light chain locus makes J2 the main viable recombination target downstream of VL2 (Supplemental Fig. 1C). To simplify our nomenclature, we will henceforth refer to the NP+ Igλ1− population as NP+ Igλ2+, given this population’s exclusive usage of VL2. We observed little junctional diversity in the CDR3 region of the cloned sequences (which spans the V-J junction) among each of these two populations, implying that each of the two NP-binding populations are largely monoclonal (Fig. 1C). Alignment of VL1J1 and VL2J2 consensus sequences reveals that the two are highly similar, as previously described (Fig. 1D) (33). This degree of similarity spanning their antigen-binding domains may help explain why the small hapten NP is bound by both populations. However, several residues differ between VL1J1 and VL2J2 specifically in the CDR2 and CDR3 regions, and there is also considerable evolutionary divergence between the adjoining C1 and C2 regions, albeit outside conventional antigen-binding domains (Fig. 1D) (33). This raises the possibility that endogenous antigen interactions may differ between these L chains. Importantly, both NP-binding populations are responsive to immunization with NP-haptenated protein after adoptive transfer, implying that these BCRs are both functional (Supplemental Fig. 1D–F).
Figure 1. B1–8i mice have two NP-binding B cell populations that express different light chains.
(A) Representative gating of NP-binding lymph node (LN) B cells in B1–8i mice (left) and corresponding gates overlaid on one another (right) to facilitate tracking these populations throughout subsequent figures on basis of color. Representative of n = 8 mice.
(B) NP+ Igλ1+ and NP+ Igλ1- populations as gated in (A) were sorted, RNA was prepared, and individual light chains (VL through JL segments) were cloned and sequenced. Graphs depict light chain V-region and J-region identity in NP+ Igλ1+ and NP+ Igλ1- populations assigned by IMGT (see Supplemental Table 1 for individual alignments).
(C) Individual cloned sequences aligned to consensus CDR3 (spanning V-J junction) of VL1J1 and VL2J2, the two most prevalent sequences isolated from NP+ Igλ1+ and NP+ Igλ1- populations respectively in (B).
(D) Amino acid similarity between consensus VL1 / J1C1 and VL2 / J2C2 light chain sequences. VL and JL were sequenced for this manuscript, and CL sequences were previously described (48).
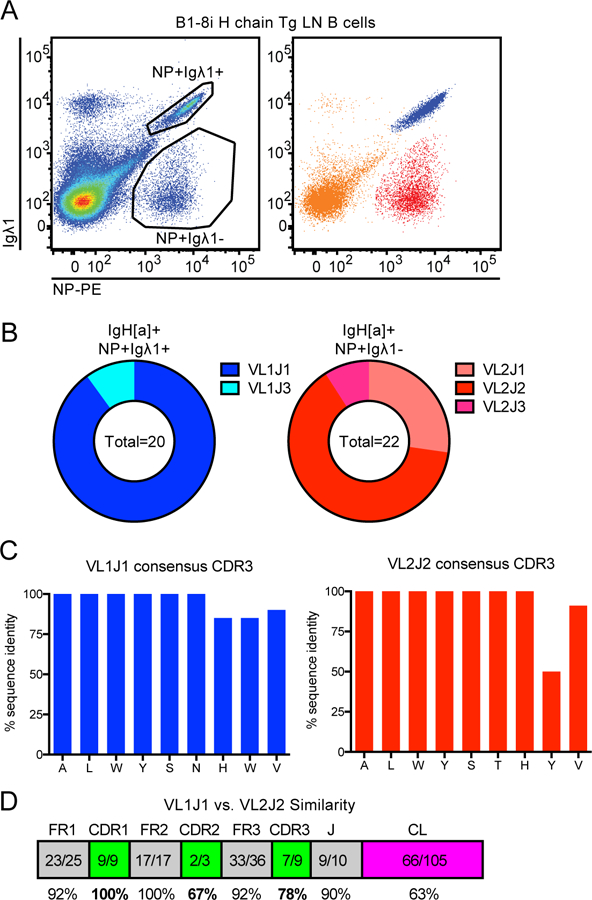
We therefore sought to compare the self-reactivity of these two NP-binding B cell populations. We observed that NP+ Igλ2+ Fo B cells express substantially less surface IgM than the NP+ Igλ1+ population (Fig. 2A), a phenotype characteristic of autoreactivity (18, 34). By contrast, the two populations have comparable levels of surface IgD, expression of which is invariant across the normal mature Fo B cell repertoire, irrespective of self-reactivity (Fig. 2B)(18). To directly measure the recognition of endogenous antigens by each population, we crossed B1–8i mice to the Nur77-eGFP reporter, which is regulated by the regulatory region of the immediate early gene Nr4a1/Nur77. This reporter reads out recent BCR-antigen encounter in vivo and consequently marks reactivity to endogenous antigens (18). Importantly, not only is endogenous antigen recognition by the BCR both necessary and sufficient for reporter expression, but signaling through other immunoreceptors on B cells does not regulate reporter expression in mature B cells under steady state conditions in vivo (32). Despite expressing more surface BCR than the NP+ Igλ2+ population (Fig. 2A–C), the Fo mature NP+ Igλ1+ B cells express significantly less Nur77-eGFP, consistent with reduced self-reactivity (Fig. 2D). These observations were consistent across both lymph node and splenic Fo B cell populations (Supplemental Fig. 2A–C).
Figure 2. Igλ1+ and Igλ2+ NP-binding B cell populations exhibit different cross-reactivity to endogenous antigens.
(A-D) NP+ Igλ1+ and NP+ Igλ2+ LN B cells were analyzed for surface IgM expression (A), surface IgD expression (B), NP binding (C), and Nur77-eGFP expression (D). Values in A-D were obtained from n = 4–5 mice, and p-values for A-D pairwise comparisons were calculated using Welch’s t test.
(E-H) Representative histograms and MFI ± SEM of Nur77-eGFP expression in splenic follicular (Fo) mature (E, F) or marginal zone (MZ) (G, H) B cells from B1–8i, WT, and MD4 mice. Values were obtained from n = 3 mice of each genotype. (Fo mature = CD23+CD93−, MZ = CD21hi CD23lo). One-way ANOVA with Tukey’s multiple comparisons test was used to calculate p-values in F, H.
**p<0.01, ***p<0.001, ****p< 0.0001.
To place the self-reactivity of each population in the context of previously characterized BCR repertoires, we compared Nur77-eGFP expression in the NP-binding populations to that of the bulk B1–8i B cell repertoire, WT polyclonal B cells, and HEL-specific B cells from MD4 mice lacking expression of the cognate HEL antigen (Figs. 2E–H). Bulk VH186.2 H chain Tg B cells in B1–8i mice express lower levels of Nur77-eGFP relative to WT mice, implying that fixing this H chain confers subtly reduced self-reactivity on the entire repertoire. Consistent with this, surface IgM in bulk B1–8i B cells was higher than that of bulk WT B cells (Supplemental Figs. 2D–G). As we previously showed, IgHEL Tg B cells in the absence of cognate antigen express very low levels of Nur77-eGFP, consistent with minimal self-reactivity(18). Notably, NP+ Igλ1+ Fo B cells also express a very low level of Nur77-eGFP comparable to IgHEL B cells, indicative of little-to-no self-antigen recognition (Fig. 2E, F). The marginal zone (MZ) compartment displays a greater dynamic range of Nur77-eGFP expression than Fo B cells; reporter GFP expression in NP+ Igλ1+ MZ B cells is even lower than that of IgHEL MZ B cells (Fig. 2G,H). By contrast, the NP+ Igλ2+ population expresses higher Nur77-eGFP than IgHEL B cells in both B2 compartments, but nevertheless remains lower than that of bulk B1–8i Fo B cells and WT Fo and MZ B cells in the spleen (Fig. 2E–H). In lymph nodes, we observe a similar trend; the B1–8i bulk B cell population spans a range of Nur77-eGFP and surface IgM expression, while NP+ Igλ1+ and NP+ Igλ2+ sit at the lower and upper bounds of self-reactivity in this repertoire respectively (Supplemental Figs. 2D–G). In summary, we have identified two minor, trackable, and largely monoclonal B cell populations with distinct levels of self-reactivity, both of which exist at the low end of the normal spectrum observed in WT mice.
Development of B1a cells expressing the B1–8i H chain transgene is highly disfavored
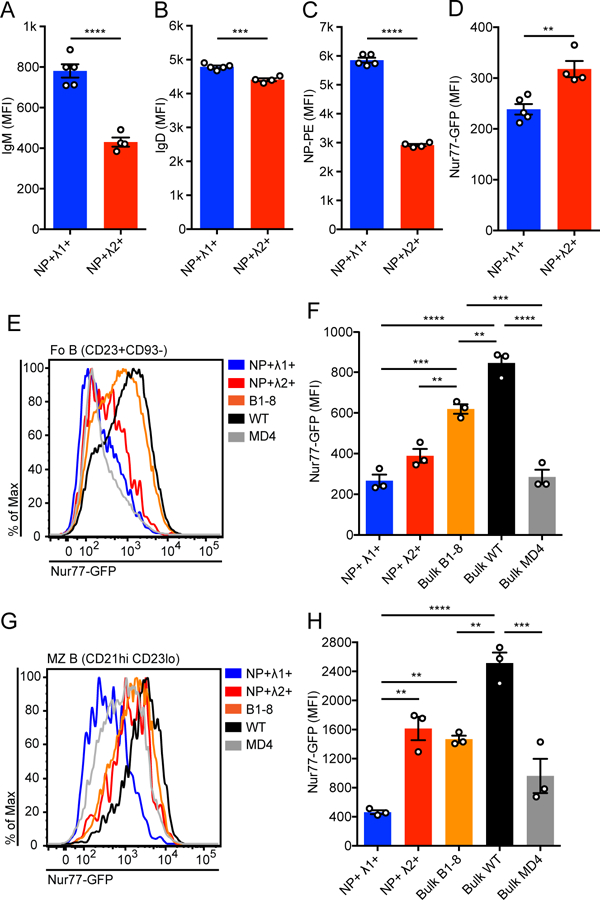
Selection into the innate-like B1a compartment requires recognition of endogenous antigens sufficient to drive strong BCR signaling (12–15). Consistent with the high self-reactivity of these cells, we recently showed that B1a cells express much higher levels of Nur77-eGFP reporter than B2 cells, and this was higher still among phosphatidylcholine-binding B1a cells that mark highly enriched, extremely self-reactive clones in this compartment (35). We first sought to determine whether NP+ cells with low self-reactivity were indeed precluded from entering the B1a compartment in B1–8i mice. Relative to WT mice, the peritoneal cavity (PerC) B1a compartment is substantially smaller in B1–8i mice harboring a single copy of the VH186.2 H chain Tg (Fig. 3A, Supplemental Fig. 2H), perhaps due to reduced or altered self-reactivity of the bulk B1–8i repertoire (Fig. 2E–H). More strikingly, few of the B1a B cells that do develop in these B1–8i mice express the knocked-in heavy chain (marked by IgM[a] allotype), and the vast majority express endogenous heavy chains (IgM[b]), either alone or in combination with the B1–8i heavy chain (Fig. 3B, C). A similar phenomenon has been described in both IgHEL Tg and anti-Thy-1 (ATA) Tg mice lacking Thy-1 antigen (12, 35). This stands in marked contrast to the B2 compartment in B1–8i mice, which is populated almost exclusively by VH186.2 H chain-expressing cells (Fig. 3B, C). NP-binding cells are largely excluded from the B1a compartment in B1–8i mice, and of the few that do develop, most express the Igλ2 L chain rather than the less self-reactive Igλ1 L chain, and these cells co-express an endogenous H chain along with the B1–8i H chain (Fig. 3D, E). By contrast, NP-binding cells readily enter the B2 compartment, and both Igλ1+ and Igλ2+ populations are capable of adopting this fate at comparable rates (Fig. 3D, E). These results suggest that the low self-reactivity of NP+ B1–8i cells, and NP+ Igλ1+ cells in particular, disfavors their entry into the B1a compartment, consistent with prior studies. Moreover, this provides independent evidence to confirm low self-reactivity of the entire B1–8i VH186.2 H chain repertoire, and the Igλ1+ NP-binding population in particular.
Figure 3. Development of B1–8i NP+ Igλ1+ B1a cells is highly disfavored.
(A) Peritoneal compartment sizes in B1–8i and WT mice. Values were obtained from n = 12 B1–8i and n = 7 WT mice. Welch’s t test was used to calculate p-values. (B1a = CD19+CD5+CD23−, B1b = CD19+CD5-CD23−, B2 = CD19+CD5-CD23+; see gating in Supplemental Fig. 1D).
(B, C) Representative gating and quantification of heavy chain usage in B1–8i peritoneal B cell subsets. A concatenated sample from n = 4 mice is displayed for clarity, and gating is representative of n = 7 mice. The knocked-in B1–8i heavy chain is allotype [a], and the endogenous heavy chain is allotype [b].
(D, E) Representative gating and quantification of light chain usage in NP-binding peritoneal populations from B1–8i mice. A concatenated sample from n = 3 mice is displayed for clarity, and gating is representative of n = 12 mice. Values were obtained from n = 12 mice, and a ratio paired t test was used to calculate p values.
*p<0.05, ***p<0.001, ****p< 0.0001.
NP+ Igλ1+ cells are strongly counter-selected during B2 cell development
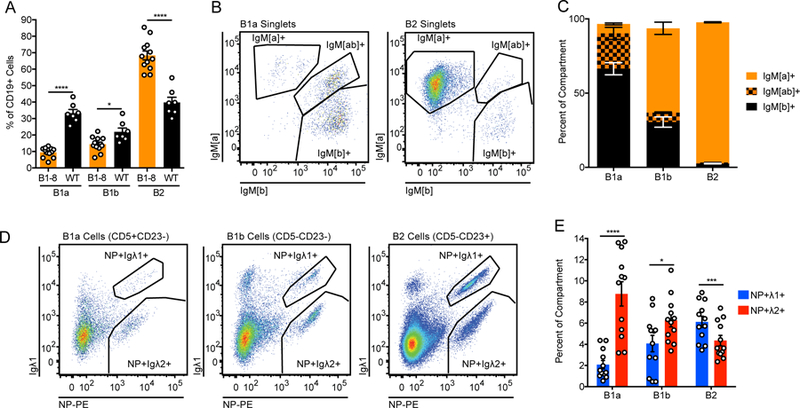
The poor development of NP+ B1a cells in B1–8i mice is consistent with an extensive body of literature demonstrating dependence of the B1a population on antigen recognition, enforced by a stringent requirement for strong BCR signaling (12–15). However, whether self-antigen recognition is required for the optimal development of B2 (Fo and MZ) subsets is much less clear. Since NP+ Igλ1+ and NP+ Igλ2+ B cells express BCRs with different (but low) reactivity towards self-antigens, comparing their developmental progression could provide evidence for or against selection of self-reactivity into polyclonal B2 cell compartments. Strikingly, NP+ Igλ1+ and NP+ Igλ2+ B cells have vastly different propensities to progress through sequential splenic stages of development (Fig. 4A, B). By contrast to the NP+ Igλ2+ population, a much smaller proportion of NP+ Igλ1+ B cells are found in the mature Fo and MZ compartments relative to the precursor immature T1 compartment (Fig. 4A, B). Disproportionate accumulation of NP+ Igλ1+ cells in the immature compartment implies a defect in development of NP+ Igλ1+ relative to NP+ Igλ2+ mature B cells. Viewed alternatively, NP+ Igλ1+ cells start off as a substantial fraction of the entire T1 compartment (8%) and are counter-selected relative to the rest of the B cell repertoire as development proceeds to the mature Fo and MZ stages (Fig. 4C). On the other hand, the proportion of NP+ Igλ2+ cells in each developmental stage and mature B cell compartment does not change substantially as development proceeds (Fig. 4D). These data reveal dramatic counter-selection during B2 development of NP+ Igλ1+ cells with the lowest self-reactivity relative to modestly self-reactive NP+ Igλ2+ and bulk repertoire VH186.2 H chain B cells (Fig. 4E). As a result, the ratio of Igλ1+ to Igλ2+ cells within the NP+ population falls dramatically between the T1 and mature stages of development (Fig. 4F). Importantly, we observe analogous counter-selection of NP+ Igλ1+ but not NP+ Igλ2+ cells between immature and mature B2 compartments across both bone marrow and peripheral lymphoid organs (Supplemental Figs. 2I, J).
Figure 4. Development of B1–8i NP+ Igλ1+ follicular and marginal zone B cells is highly disfavored.
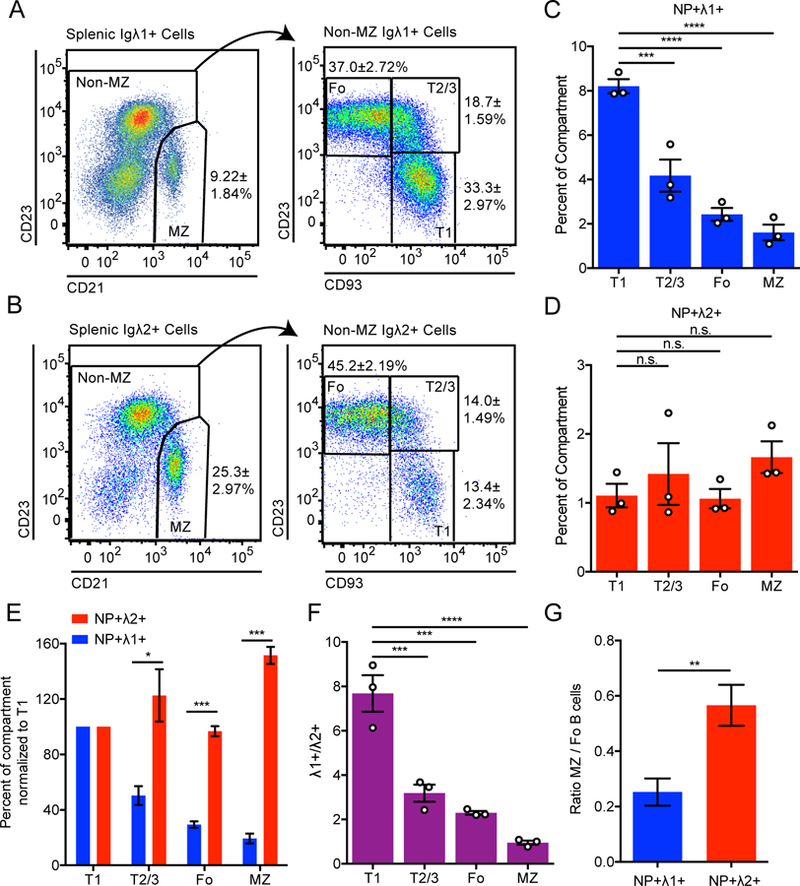
(A-B) Representative subset gating of NP+ Igλ1+ (A) and NP+ Igλ2+ (B) splenic B cells. Values represent the percentage of splenic B cells of a given genotype belonging to that compartment. A concatenated sample from n = 4 mice is displayed for gating, and statistics were calculated using each mouse individually. Mean ± SEM is displayed for each subset. (T1 = CD23−CD93+, T2/3 = CD23+CD93+, Fo = CD23+CD93−, MZ = CD21hi CD23lo).
(C-D) Percentage of NP+ Igλ1+ (C) and NP+ Igλ2+ (D) cells relative to total B220+ cells in each compartment in B1–8i H chain Tg mice. Values represent mean ± SEM of 3 mice.
(E) Graph represents data from (C, D) normalized to percentage in the T1 compartment. Welch’s t test was used to calculate p-values.
(F) Ratio of NP+ Igλ1+ to NP+ Igλ2+ cells in each compartment extracted from data plotted in (C, D).
(G) Graph depicts ratio of MZ to Fo B cells from data in (A, B) among NP+ Igλ1+ and NP+ Igλ2+ B cells. Ratio paired t-test was used to calculate p value.
One-way ANOVA with Dunnett’s multiple comparisons test was used to compare T2/3, Fo, and MZ populations to the T1 population and calculate p-values in C, D, F.
*p<0.05, **p<0.01, ***p<0.001, ****p< 0.0001.
IgHEL Tg B cells enter both the Fo and MZ B cell compartments when they develop in the absence of cognate antigen (23, 24). By contrast, Hayakawa and Hardy previously showed that ATA B cells similarly mature into Fo B cells in the absence of Thy-1 antigen, but MZ B cells do not (16). We therefore examined the relative propensity of NP+ populations in B1–8i mice to enter the MZ and Fo B cell compartments. Interestingly, NP+ Igλ1+ cells with minimal self-reactivity are even more disfavored in the mature MZ compartment than in the mature Fo compartment (Figs. 4A, G). By contrast, modestly self-reactive NP+ Igλ2+ cells enter the MZ compartment much more readily (Figs. 4B, E-G). Our data not only reveals a lower bound of self-reactivity below which optimal maturation of B2 cells is impaired, but also suggests that this threshold is lower for Fo than for MZ B cells, as proposed by Hayakawa and Hardy (16, 36).
NP-binding B cells in B1–8i mice develop in the context of a polyclonal repertoire that is less self-reactive than that of WT mice. We therefore sought to determine whether competition with more self-reactive WT B cells might further increase counter-selection of both Igλ1+ and Igλ2+ NP-binding B cells. To do so, we generated competitive radiation chimeras with mixed bone marrow from allotype-marked WT and B1–8i mice harboring the Nur77-eGFP reporter Tg (Figs. 5A–E). Relative expression of Nur77-eGFP in NP+, VH186.2 H chain, and WT B cell populations was virtually identical to earlier observations in intact reporter mice, and supports this readout of self-reactivity as a cell-intrinsic property of these B cell populations (Figs. 2E–H, Figs. 5F, G). We again observed counter-selection of NP+ Igλ1+ during splenic B2 cell development (Fig. 5B). However, neither modestly self-reactive NP+ Igλ2+ nor bulk B1–8i B cells exhibit any competitive advantage or disadvantage during development relative to WT B cells (Figs. 5C–E). This suggests that there is a relatively low threshold above which additional self-reactivity does not confer further competitive advantage for B2 cell development. Collectively, these results suggest that very low self-reactivity, rather than facilitating entry of NP+ Igλ1+ B cells into the mature B2 repertoire, strongly disfavors their development. This forms a contrast to IgHEL Tg and ATA Fo B cell development in the absence of their respective cognate antigens (16, 23).
Figure 5. Development of B1–8i NP+ Igλ1+ but not NP+ Igλ2+ B-2 cells is disfavored in competition with an unrestricted BCR repertoire.
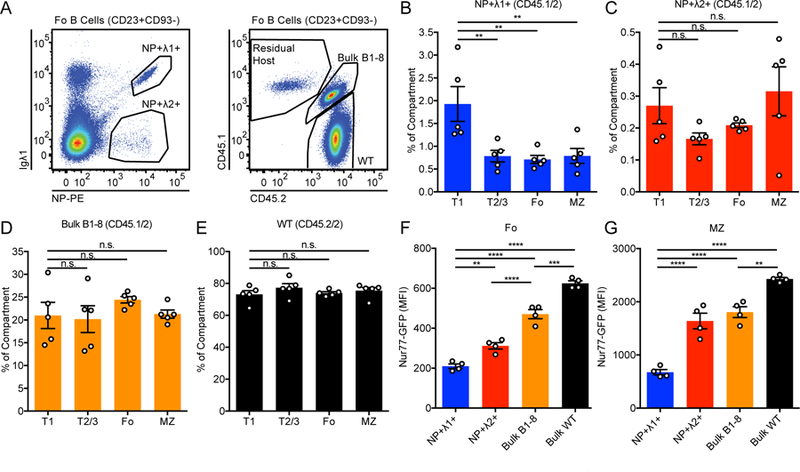
(A) Representative gating of NP+ Igλ1+, NP+ Igλ2+, bulk B1–8, WT, and residual host Fo B cells (CD23+CD93−) in bone marrow chimeras with CD45.1/2 B1–8i and CD45.2/2 WT donors in lethally-irradiated CD45.1/1 hosts.
(B-E) Percentage of NP+ Igλ1+ (B), NP+ Igλ2+ (C), bulk B1–8 (D), and WT (E) cells, normalized to B220+ cells, in splenic compartment. (T1 = CD23−CD93+, T2/3 = CD23+CD93+, Fo = CD23+CD93−, MZ = CD21hi CD23lo). Statistics were calculated for n = 5 chimeric mice, and one-way ANOVA with Dunnett’s multiple comparisons test was used to compare T2/3, Fo, and MZ populations to the T1 reference population and calculate p-values.
(F-G) Nur77-eGFP expression in Fo (G) and MZ (H) B cells from B1–8:WT chimeras described in (A). n = 4 mice were used to calculate Nur77-eGFP MFI and one-way ANOVA with Tukey’s multiple comparisons test was used to calculate p-values.
**p<0.01, ***p<0.001, ****p< 0.0001.
Enhancing BCR signal strength promotes the development of NP+ B1a cells
While low self-reactivity of NP+ Igλ1+ cells (Fig. 2) suggests that their counter-selection during B1a and B2 cell development (Figs. 3, 4) is due to impaired positive selection, we wanted to rule out the possibility that these cells are negatively selected. To distinguish between these two scenarios, we sought to modulate BCR signal strength genetically, independent of BCR specificity (see model, Figs. 6A, B). We crossed the B1–8i H chain Tg onto an allelic series of mice in which expression of the receptor-like tyrosine phosphatase CD45 is varied genetically (30, 37). The primary substrate of CD45 is the c-terminal inhibitory tyrosine of the Src family kinases, which in turn mediate signal transduction downstream of the BCR (38). As a result, increasing CD45 expression in B cells promotes both tonic and inducible antigen receptor signaling (37). By introducing such a genetic perturbation in BCR signal strength and tracking individual BCRs of known self-reactivity, we can isolate the contribution of antigen-dependent (rather than merely tonic) BCR signaling to B cell development. We examined B1–8i mice with low (CD45.L/+) and high (CD45.H/+) CD45 expression, corresponding respectively to a 50% decrease and increase of surface CD45RABC (B220) on B cells relative to physiologic levels (Fig. 6A). Increasing expression of CD45 across this range results in enhanced Erk phosphorylation in response to BCR ligation (Fig. 6C). As we previously reported in allelic series mice harboring an unrestricted BCR repertoire, B1a cell development was enhanced relative to B2 cell development with increasing CD45 expression, consistent with a signal strength-dependent model of B1a cell development (Fig. 6D)(37).
Figure 6. Enhancing BCR signal strength via genetic titration of CD45 surface expression promotes development of NP-binding B1a cells.
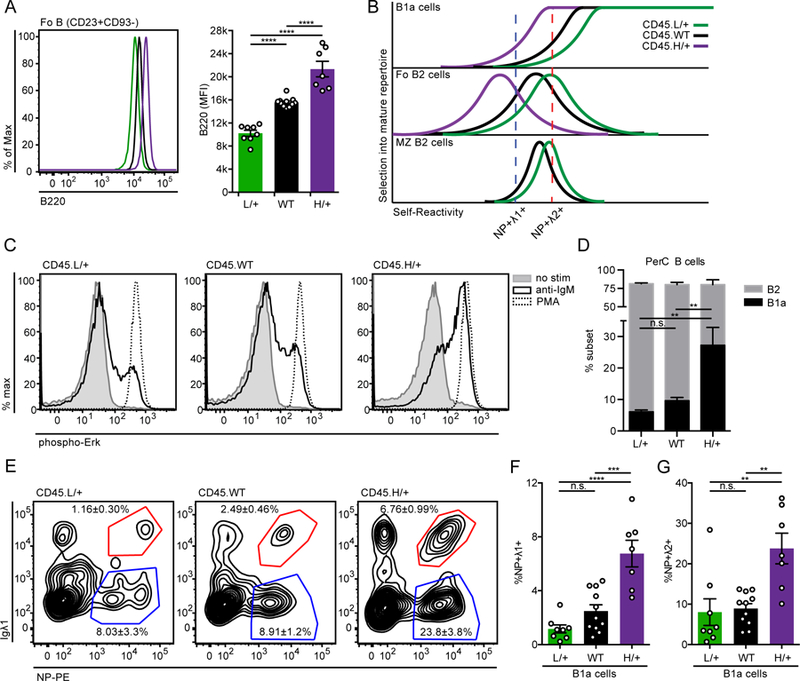
(A) Representative histogram (left) and quantification (right) of B220 expression on Fo B cells (CD23+CD93−) from CD45.L/+, CD45.WT, and CD45.H/+ mice with the B1–8i transgene. Statistics were calculated from n = 7–11 mice of each genotype.
(B) Model depicts efficiency (y-axis) of selecting individual BCRs of varying self-reactivity (x-axis) into the mature repertoire of B1a, Fo, and MZ compartments. Colored graphs depict predicted shifts in the degree of self-reactivity selected into these compartments with altered strength of BCR signal transduction conferred by CD45 titration. L/+ (green) curve shows effect of reducing CD45 expression and signal strength, while H/+ (purple) curve shows effect of increasing CD45 expression and signal strength. With increased signal strength, mature B cell repertoires are rendered less self-reactive because excess signaling compensates for impaired antigen recognition in vivo. The opposite is true for reduced signal strength. Relative self-reactivity of NP-binding Igλ1+ (blue) and Igλ2+ (red) cells is plotted to show how altering signal transduction would be predicted to alter selection of these BCRs into mature B cell compartments.
(C) LN cells were stimulated for 3 minutes with media alone, 10ug/ml anti-IgM, or PMA, then fixed, permeabilized, and stained to detect intra-cellular pErk and the B cell marker CD23. Histograms depict CD23+ B cells and data are representative of at least 3 independent experiments.
(D) PerC cells from CD45.L/+, CD45.WT, and CD45.H/+ mice with the B1–8i transgene were stained to detect B cell subsets as gated in Supplemental Fig 1D. Graph depicts % B1a and B2 PerC cells ± SEM in each genotype.
(E-G) Representative gating (E) and quantification of (F) NP+ Igλ1+ and (G) NP+ Igλ2+ B1a cells (CD5+CD23−) isolated from PerC of mice described in (D). In (E), concatenated samples from n = 7–11 mice is displayed for gating, and statistics were calculated using each mouse individually. Mean ± SEM is displayed associated with each subset. WT data in (E-G) contains values from Figure 3E for reference.
One-way ANOVA with Tukey’s multiple comparisons test was used to calculate p-values when analyzing a phenotype (e.g. %NP+ Igλ1+ or B220 MFI) across the full allelic series (A, D, F, and G).
**p<0.01, ***p<0.001, ****p< 0.0001.
If NP+ Igλ1+ B cells are negatively selected in mice expressing physiologic levels of CD45, we would expect that the increased BCR signal strength in CD45.H/+ mice would further prune these cells from the mature B cell repertoire. However, if these cells are counter-selected because of impaired positive selection as we postulate, we would expect that enhancing BCR signal strength would not disfavor their development and might even enhance it (see model, Fig. 6B). Indeed, increasing CD45 expression and BCR signal strength markedly improves the selection of NP+ B1a cells in B1–8i mice, consistent with prior studies showing that self-reactivity and strong BCR signaling are required and tolerated in this compartment (Figs. 6E–G, Supplemental Fig. 3A)(15, 36). This is particularly marked within the NP+ Igλ1+ population, which displays a six-fold relative expansion in the B1a compartment across the CD45 allelic series, confirming that these cells are normally restrained from populating this compartment by impaired positive selection rather than enhanced negative selection (Figs. 6B, F). Nevertheless, NP+ Igλ2+ cells retain a marked advantage over NP+ Igλ1+ cells, consistent with their greater degree of self-reactivity (Figs. 6B, E–G).
Enhancing BCR signal strength favors the selection of NP+ Igλ1+ cells relative to NP+ Igλ2+ cells into the B2 compartment
By contrast to the B1a compartment, the entry of NP+ Igλ1+ cells into the other peritoneal B cell compartments (B1b and B2) appears to be less sensitive to changes in CD45 at the level of perturbation we applied (Supplemental Figs. 3A–C). More strikingly, modestly self-reactive NP+ Igλ2+ cells are progressively disfavored in these compartments with higher expression of CD45 and increasing BCR signal strength, suggesting that they are being negatively selected (Fig. 6B). This supports an extensive body of literature that has identified a profound difference between the thresholds for positive and negative selection in B1a and B2 cell compartments (14, 15).
We next sought to determine the relative contribution of positive and negative selection to the development of NP+ Igλ1+ and NP+ Igλ2+ B2 cells in the spleen. Increasing BCR signal strength leads to a stepwise and progressive loss of NP+ Igλ2+ cells from the follicular repertoire, indicating that the self-reactivity of these cells exists near the threshold of negative selection (Fig. 7A, Model Fig 6B, Supplemental Fig. 3D). This stands in stark contrast to NP+ Igλ1+ cells, which do not change as a proportion of the follicular compartment at the level of CD45 perturbation we probed, indicating that negative selection pressures do not constrain this population (Fig. 7A). It has been previously noted by several independent studies that increasing BCR signal strength preferentially reduces or even eliminates the MZ compartment, implying particular sensitivity of this population to negative selection (14, 17). We previously noted a similar trend with increasing expression of CD45 across the allelic series (18). As MZ development is virtually absent in CD45.H/+ mice, we were only able to probe the effect of lowering signal strength on development of NP+ MZ populations. Similar to the Fo compartment, lowering BCR signal strength does not have a profound effect on the NP+ Igλ1+ population, but it markedly favors the development of additional NP+ Igλ2+ MZ B cells (Fig. 7B). Thus, the relative fitness of NP+ Igλ1+ cells compared to NP+ Igλ2+ cells in the B2 compartments improves with increasing BCR signal strength (Figs. 7C, D). Collectively, these data show that NP+ Igλ1+ B2 cells are not counter-selected by excessive BCR signaling (enhanced negative selection) in the CD45+/+ genetic background, but rather by insufficient BCR signaling (impaired positive selection). By contrast, modestly self-reactive NP+ Igλ2+ cells are negatively selected as BCR signal strength is increased from CD45 L/+ to CD45+/+ mice. Moreover, this is more pronounced in the MZ than the Fo compartment, suggesting that the threshold for negative selection of MZ B cells is in fact lower relative to Fo B cells.
Figure 7. Enhancing BCR signal strength promotes negative selection of NP+ Igλ2+ but not NP+ Igλ1+ cells.
(A-B) Quantification of splenic NP+ Igλ1+ and NP+ Igλ2+ Fo (A) and MZ (CD21hi CD23lo) (B) B cells in mice described in Fig 6. Statistics were calculated from n = 7–11 mice of each genotype.
(C-D) The ratio of Igλ1+ to Igλ2+ cells within the NP+ population was calculated for Fo (C) and MZ (D) populations in mice described above. Statistics were calculated from n = 7–11 mice of each genotype.
(E) Model (analogous to that depicted in Figure 6B) depicts efficiency (y-axis) of selecting individual BCRs of varying self-reactivity (x-axis) into the mature repertoire of B1a, Fo, and MZ compartments. Area to the left of the curve corresponds to BCRs that are counter-selected into a given mature compartment because of insufficient endogenous antigen recognition (failed positive selection or “death by neglect”), while to the right of the curve lie BCRs that are counter-selected because of excess self-reactivity (negative selection). The graphs also depict efficiency of selection of Igλ1+ and Igλ2+ NP-binding populations into mature B cell compartments.
One-way ANOVA with Tukey’s multiple comparisons test was used to calculate p-values when analyzing a phenotype across the full allelic series (A, C). Welch’s t test was used to calculate p-values in (B) and (D). Non-significant findings are omitted for clarity.
**p<0.01, ***p<0.001, ****p< 0.0001.
Discussion
It is well-appreciated that the mature B cell repertoire harbors some degree of self-reactivity despite layered tolerance mechanisms (2, 18), but whether this is merely tolerated or actively selected for during B cell development remains uncertain. Although abundant evidence has established that positive selection by self-antigens is essential for B1a cell development, there has been a long-standing debate about whether tonic BCR signaling is sufficient for B2 cell development, or whether self-antigen recognition also promotes positive selection of B2 cells. A major limitation of prior studies has been the inability to determine the extent of cross-reactivity between BCR Tg cells and endogenous antigens. This has in turn made it challenging to draw definitive conclusions about the minimal amount of self-reactivity and bona fide antigen-dependent signaling required for optimal entry into mature B cell compartments (as opposed to tonic BCR signaling), especially the follicular mature B2 cell compartment. Our study takes a novel approach to study positive and negative selection pressures in mature B cell compartments by capitalizing on the Nur77-eGFP reporter of endogenous antigen recognition, and studying two competing relatively monoclonal B cell populations in the context of a diverse B cell repertoire. This enables us to identify minimal thresholds of antigen-dependent positive selection that are required for optimal entry into all mature B cell compartments (see model Fig. 7E).
We identified two minor B cell populations in B1–8i H chain Tg mice that differ only in their L chain use and consequently in their degree of self-reactivity. We showed that the less self-reactive of these populations, NP+ Igλ1+, experiences counter-selection pressure during development into all mature B cell compartments, including B1a, MZ, and Fo. By contrast, NP+ Igλ2+ B cells with modest self-reactivity, while still counter-selected in the B1a compartment, enter both MZ and Fo B cell compartments with no disadvantage relative to the rest of the B cell repertoire. Since NP+ Igλ1+ B cells express high levels of a functional BCR on their surface (as evidenced by their ready participation in NP-dependent immune responses(39)), these data demonstrate that truly tonic BCR signaling is not sufficient for optimal development into any B2 cell compartment, and the antigen-dependent signaling threshold for optimal entry into these compartments lies somewhere between these two B cell populations (see model, Fig. 7E).
Although Hardy and Hayakawa previously showed that entry of anti-Thy-1 (ATA) B cells into the MZ compartment requires low level Thy-1 antigen expression, ATA cells assumed a mature Fo B cell phenotype even in Thy-1−/− hosts [13]. Interestingly, we find that NP+ Igλ1+ B cells, while disfavored in both Fo and MZ compartments, were significantly more disadvantaged in the MZ compartment relative to more self-reactive NP+ Igλ2+ cells (Figs. 4F). This suggests that the threshold of BCR signaling required for optimal entry into the Fo compartment is lower than that for the MZ compartment, consistent with studies of the ATA model (see model, Fig. 7E)(16). In contrast to the ATA model, IgHEL BCR Tg B cells can enter both the MZ and Fo compartments in the absence of cognate antigen expression, albeit in a monoclonal repertoire with no competition (23, 24). Indeed, we compared the cross-reactivity of IgHEL BCR Tg cells with endogenous antigen and determined that the self-reactivity of these cells indeed lies between that of NP+ Igλ1+ and NP+ Igλ2+ cells. We would propose that ATA cells likely exhibit a low degree of cross-reactivity with endogenous antigens that is above the minimal threshold for Fo entry, but below that of MZ entry (and may correspond to somewhere below that of NP+ Igλ2+ cells and IgHEL BCR Tg cells, but perhaps above that of NP+ Igλ1+).
We show fall-off of NP+ Igλ1+ B cells occurs between the T1 and the T2/3 stages of splenic development, as well as subsequently upon entry into the mature Fo and MZ B cell compartments. Our data implies that counter-selection of these cells with minimal self-reactivity occurs during development and does not simply represent impaired survival of mature B cells. But, what purpose is served by a positive selection checkpoint during splenic B2 cell development when a pre-BCR signal-dependent checkpoint already exists earlier during bone marrow development? It may simply function as a stringent mechanism to ensure that primary rearrangement of L chains and L chain editing generate a functional BCR. However, a mere requirement for tonic BCR signaling ought to be sufficient to ensure that this is the case. Alternatively, selection of mild B cell autoreactivity into the mature naïve B cell repertoire might be advantageous because the capacity to recognize general biological patterns of self-antigens biases the mature B cell repertoire towards efficient recognition of pathogen-associated patterns as well, and may set the stage for better protective responses (9, 40). Indeed, it has been suggested that the extremely high degree of self-reactivity in the immature B cell repertoire may reflect a germline bias towards such self-recognition (2). Moreover, new evidence suggests that the mature B cell repertoire may be directly shaped by commensal flora, further supporting such a model (41). Indeed, although we have previously shown that Nr4a1/Nur77 expression in bulk splenic B cells is unaltered in germ free mice, we cannot exclude the possibility that recognition of commensal flora by individual BCRs may serve, in lieu of reactivity to bona fide “self”, to positively select a subset of clones into the mature repertoire (32). A related hypothesis is that some self-reactivity may not only be tolerated, but may even be favored in order to avoid generating ‘holes’ in the B cell repertoire that could in turn be exploited by pathogens to evade immune recognition; indeed it has been shown that deletion of critical precursor BCRs generates such holes and poses a barrier to generation of anti-HIV broadly-neutralizing antibodies (42). Anergy, which is reversible, rather than deletion, may be the preferred tolerance mechanism for mature B cells in order to maintain some degree of self-reactivity in the pre-immune B cell repertoire. A final possibility is that positive selection for endogenous antigen recognition generates a poly-reactive primary B cell repertoire. Indeed, Wardemann and colleagues have suggested that the relevant feature of peripheral BCRs is their ‘polyreactivity’ rather than their ‘self-reactivity’. A polyreactive naïve BCR repertoire is more likely to generate a highly polyclonal response to any given foreign antigen. Such diversity in the primary immune response may be important for optimal affinity maturation in the germinal center by facilitating both competition and efficient search of ‘mutational space’ by somatic hypermutation of a range of germline sequences.
By contrast to B2 cells, B1a cells have long been appreciated not only to harbor significant self-reactivity, but also to require strong self-antigen recognition for their development (12, 13). They seem to be largely resistant to negative selection pressures, but rather rely on robust inhibitory tone to maintain their quiescence in the face of chronic self-antigen stimulation (15). What advantage does this confer on the host? Since the B1a compartment is largely fetally-derived and thereafter maintained via self-renewal, it contains germline-encoded BCRs due to a lack of TdT expression during fetal hematopoiesis (15). The most highly prevalent of the ‘innate-like’ BCR specificities selected into the B1a compartment not only recognizes phosphatidylcholine exposed on the surface of dying cells (and may be important for clearance of cellular debris), but is also highly cross-reactive with the polysaccharide coat of streptococcal species. A high threshold for positive selection serves to generate an innate-like B cell compartment enriched for protective BCR specificities that are cross-reactive with conserved pathogen-associated molecular patterns. Since the B1a compartment is thought to be the predominant source of natural serum IgM, and plays an important role in host defense against common bacterial and viral pathogens, these BCRs operate in some ways like classic pattern recognition receptors of the innate immune system and may in turn be subject to considerable evolutionary pressure in the germline.
Despite positive selection of modest self-reactivity, mature B2 cell compartments – unlike B1a cells - do exhibit an upper bound of self-reactivity beyond which reversible tolerance mechanisms such as anergy do not seem to extend (see model Fig. 7E). This corresponds to the threshold for ‘negative selection’ (imposed by both receptor editing and deletion). We titrated BCR signal strength via genetic manipulation of surface CD45 expression to unmask such thresholds. Although titration of CD45 expression in B cells modulates both tonic and antigen-dependent BCR signaling, the effects of modulating CD45 during B cell development depend upon the degree of BCR self-reactivity. For example, IgHEL Tg B cells deficient for CD45 exhibit a profound block in development (attributed to low self-reactivity of this BCR) that can be rescued by introducing expression of cognate sHEL Ag (43). Conversely, we previously reported that CD45 over-expression selectively produced deletion of IgHEL B cells in the presence, but not the absence, of cognate sHEL Ag (37). Thus, titrating strength of signal transduction via CD45 titration has distinct effects on B cell clones with different degrees of self-reactivity. It is this phenomenon that we take advantage of in the present study. Here we find that increasing BCR signal strength results in increasing loss of NP+ Igλ2+ B2 cells, but not of NP+ Igλ1+ B2 cells. We interpret this data to show that modestly self-reactive NP+ Igλ2+ cells lie near the negative selection threshold for B2 cell development, while the low level of endogenous self-reactivity exhibited by NP+ Igλ1+ B cells renders them relatively more resistant to this effect. Similarly, we show that exclusion of both the NP+ Igλ1+ and the NP+ Igλ2+ populations from the B1a compartment is indeed due to insufficient positive selection as expected (see model, Fig. 6B). Moreover, we find that the threshold for positive selection into the B1a compartment overlaps with that for negative selection in the B2 compartment, as previously noted in the ATA model (see model, Fig. 7E)(12, 13, 36). Importantly, we show that counter-selection of NP+ Igλ1+ and NP+ Igλ2+ B cells by both positive and negative selection pressures is relative rather than absolute at each end of the mature B cell repertoire.
There is abundant genetic evidence that the MZ compartment is more sensitive to negative selection than the Fo compartment (14, 17, 37). Indeed, we observed that a relatively subtle increase in signal strength (between L/+ and WT CD45 expression) causes a much sharper fall-off in MZ entry of NP+ Igλ2+ cells than Fo entry of these cells (Figs. 7A, B). We speculate that variation in the relative degree of self-reactivity (marked by Nur77-eGFP expression) of bulk B1–8i and NP+ Igλ2+ cells within Fo and MZ compartments might reflect different thresholds for negative selection in these two compartments such that the most autoreactive bulk B1–8i B cells are preferentially excluded from the MZ compartment (Figs. 2E–H, 5F, G). Our data shows that the MZ B cell compartment exhibits both a higher positive selection threshold and a lower negative selection threshold than the Fo B cell compartment (see model Fig. 7E). We suggest that the MZ compartment tolerates a relatively narrow range of modest self-reactivity, while the Fo compartment is fairly permissive across a broad range of self-reactivity (see model Fig. 7E). MZ B cells are thought to be specialized to mount rapid Ab responses to blood-borne T-independent pathogens by differentiating directly into short-lived plasma cells. Indeed, MZ B are uniquely poised to make rapid polyclonal plasma cell responses to pathogen-associated molecular patterns (PAMPs) via TLRs (44). The unique and narrow spectrum of self-reactivity in the MZ compartment may function both to limit generation of self-reactive autoantibodies, and to select for BCRs that are pre-disposed to recognize general biological patterns. By contrast, Fo B cells are preferentially specialized to mount T-dependent immune responses and to undergo affinity maturation in the germinal center. Since somatic hypermutation can both increase foreign-reactivity and redeem or censor self-reactivity, a broader range of both lower and higher self-reactivity may be tolerated in the Fo compartment (45, 46). In some cases, the pressure to lose autoreactivity can even set cells on a faster trajectory to mutate towards high affinity recognition of foreign antigens in the germinal center (47).
Our work demonstrates directly that modest reactivity to endogenous antigens improves the developmental fitness of B cell clones in the context of a diverse peripheral repertoire. Tonic BCR signaling in the absence of endogenous antigen recognition is not sufficient to drive efficient B cell development. Rather, antigen recognition is necessary for optimal development of not only B1a, but also B2 cells, albeit at much lower levels. We conclude that not only negative selection, but also positive selection by endogenous antigen shapes the mature B cell repertoire and propose that variations in these thresholds across mature B cell subsets facilitate their specialized functions.
Supplementary Material
Key Points.
Tonic BCR signaling is not sufficient for optimal development of any B cell lineage
Antigen recognition is required for development not only of B1a, but also of B2 cells
The minimal threshold for antigen recognition is higher for MZ than for Fo B2 cells
Acknowledgements
We are grateful to Arthur Weiss for helpful suggestions and to Al Roque for help with mouse husbandry.
Funding:
NSF Graduate Research Fellowship 1650113 (MN), HHMI Medical Research Fellows program (JH), NIAMS R01AR069520 (JZ), Rheumatology Research Foundation (JZ)
References
- 1.Melchers F 2015. Checkpoints that control B cell development. J Clin Invest 125: 2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, and Nussenzweig MC. 2003. Predominant autoantibody production by early human B cell precursors. Science 301: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 3.Cambier JC, Gauld SB, Merrell KT, and Vilen BJ. 2007. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nature reviews. Immunology 7: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemazee D 2017. Mechanisms of central tolerance for B cells. Nature reviews. Immunology 17: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzog S, and Jumaa H. 2012. Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr Opin Immunol 24: 166–172. [DOI] [PubMed] [Google Scholar]
- 6.Miosge LA, and Goodnow CC. 2005. Genes, pathways and checkpoints in lymphocyte development and homeostasis. Immunol Cell Biol 83: 318–335. [DOI] [PubMed] [Google Scholar]
- 7.Lam KP, Kühn R, and Rajewsky K. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, Depinho RA, Kutok JL, Kearney JF, Otipoby KL, and Rajewsky K. 2009. PI3 kinase signals BCR-dependent mature B cell survival. Cell 139: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancro MP, and Kearney JF. 2004. B cell positive selection: road map to the primary repertoire? Journal of immunology 173: 15–19. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J, and Herzenberg LA. 2015. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife 4: e09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold LW, Pennell CA, McCray SK, and Clarke SH. 1994. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J Exp Med 179: 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, and Hardy RR. 1999. Positive selection of natural autoreactive B cells. Science 285: 113–116. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa K, Asano M, Shinton SA, Gui M, Wen L-J, Dashoff J, and Hardy RR. 2003. Positive Selection of Anti–Thy-1 Autoreactive B-1 Cells and Natural Serum Autoantibody Production Independent from Bone Marrow B Cell Development. The Journal of Experimental Medicine 197: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, and Rajewsky K. 2004. B cell receptor signal strength determines B cell fate. Nat Immunol 5: 317–327. [DOI] [PubMed] [Google Scholar]
- 15.Berland R, and Wortis HH. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol 20: 253–300. [DOI] [PubMed] [Google Scholar]
- 16.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, and Hayakawa K. 2005. Evidence of marginal-zone B cell-positive selection in spleen. Immunity 23: 297–308. [DOI] [PubMed] [Google Scholar]
- 17.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, and Pillai S. 2001. The Follicular versus Marginal Zone B Lymphocyte Cell Fate Decision Is Regulated by Aiolos, Btk, and CD21. Immunity 14: 603–615. [DOI] [PubMed] [Google Scholar]
- 18.Zikherman J, Parameswaran R, and Weiss A. 2012. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 489: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine MH, Haberman AM, Sant’Angelo DB, Hannum LG, Cancro MP, Janeway CA Jr., and Shlomchik MJ. 2000. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci U S A 97: 2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu H, Tarlinton D, Muller W, Rajewsky K, and Forster I. 1991. Most peripheral B cells in mice are ligand selected. J Exp Med 173: 1357–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viale AC, Coutinho A, and Freitas AA. 1992. Differential expression of VH gene families in peripheral B cell repertoires of newborn or adult immunoglobulin H chain congenic mice. J Exp Med 175: 1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, and Clarke SH. 2003. Evidence for a Ligand-Mediated Positive Selection Signal in Differentiation to a Mature B Cell. The Journal of Immunology 171: 6381–6388. [DOI] [PubMed] [Google Scholar]
- 23.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink R, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, Trent RJ, and Basten A. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334: 676–682. [DOI] [PubMed] [Google Scholar]
- 24.Cyster JG, and Goodnow CC. 1995. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity 2: 13–24. [DOI] [PubMed] [Google Scholar]
- 25.Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, and Monroe JG. 2001. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med 194: 1583–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuentes-Panana EM, Bannish G, Karnell FG, Treml JF, and Monroe JG. 2006. Analysis of the individual contributions of Igalpha (CD79a)- and Igbeta (CD79b)-mediated tonic signaling for bone marrow B cell development and peripheral B cell maturation. Journal of immunology 177: 7913–7922. [DOI] [PubMed] [Google Scholar]
- 27.Rosado MM, and Freitas AA. 1998. The role of the B cell receptor V region in peripheral B cell survival. Eur J Immunol 28: 2685–2693. [DOI] [PubMed] [Google Scholar]
- 28.Freitas AA, Rosado MM, Viale A, and Grandien A. 1995. The role of cellular competition in B cell survival and selection of B cell repertoires. Eur J Immunol 25: 1729–1738. [DOI] [PubMed] [Google Scholar]
- 29.Sonoda E, Pewzner-Jung Y, Schwers S, Taki S, Jung S, Eilat D, and Rajewsky K. 1997. B cell development under the condition of allelic inclusion. Immunity 6: 225–233. [DOI] [PubMed] [Google Scholar]
- 30.Zikherman J, Jenne C, Watson S, Doan K, Raschke W, Goodnow CC, and Weiss A. 2010. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity 32: 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virts EL, Diago O, and Raschke W. 2003. A CD45 minigene restores regulated isoform expression and immune function in CD45-deficient mice: therapeutic implications for human CD45-null severe combined immunodeficiency. Blood 101: 849–855. [DOI] [PubMed] [Google Scholar]
- 32.Noviski M, Mueller JL, Satterthwaite A, Garrett-Sinha LA, Brombacher F, and Zikherman J. 2018. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. eLife 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayzer DJ 1990. Immunoglobulin lambda light chain evolution: Igl and Igl-like sequences form three major groups. Immunogenetics 32: 157–174. [DOI] [PubMed] [Google Scholar]
- 34.Quach TD, Manjarrez-Orduno N, Adlowitz DG, Silver L, Yang H, Wei C, Milner EC, and Sanz I. 2011. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. Journal of immunology 186: 4640–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huizar J, Tan C, Noviski M, Mueller JL, and Zikherman J. 2017. Nur77 Is Upregulated in B-1a Cells by Chronic Self-Antigen Stimulation and Limits Generation of Natural IgM Plasma Cells. Immunohorizons 1: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy RR, and Hayakawa K. 2015. Selection of natural autoreactive B cells. Clin Exp Rheumatol 33: S80–86. [PubMed] [Google Scholar]
- 37.Zikherman J, Doan K, Parameswaran R, Raschke W, and Weiss A. 2012. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci U S A 109: E3–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermiston ML, Zikherman J, and Zhu JW. 2009. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev 228: 288–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob J, Kassir R, and Kelsoe G. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med 173: 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo T, Gautam A, and Wesemann DR. 2019. Affinity war: forging immunoglobulin repertoires. Current Opinion in Immunology 57: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Chaudhary N, Yang N, Granato A, Turner JA, Howard SL, Devereaux C, Zuo T, Shrestha A, Goel RR, Neuberg D, and Wesemann DR. 2018. Microbial symbionts regulate the primary Ig repertoire. J Exp Med 215: 1397–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang R, Verkoczy L, Wiehe K, Alam SM, Nicely NI, Santra S, Bradley T, Pemble CW, Zhang J, Gao F, Montefiori DC, Bouton-Verville H, Kelsoe G, Larimore K, Greenberg PD, Parks R, Foulger A, Peel JN, Luo K, Lu X, Trama AM, Vandergrift N, Tomaras GD, Kepler TB, Moody MA, Liao H, and Haynes BF. 2016. Initiation of immune tolerance–controlled HIV gp41 neutralizing B cell lineages. Science Translational Medicine 8: 336ra362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, and Goodnow CC. 1996. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature 381: 325–328. [DOI] [PubMed] [Google Scholar]
- 44.Meyer-Bahlburg A, and Rawlings DJ. 2012. Differential impact of Toll-like receptor signaling on distinct B cell subpopulations. Front Biosci (Landmark Ed) 17: 1499–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, Langley D, Roome B, Vazquez-Lombardi R, Rouet R, Hermes J, Chan TD, Brink R, Dunn-Walters DK, Christ D, and Goodnow CC. 2014. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A 111: E2567–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reed JH, Jackson J, Christ D, and Goodnow CC. 2016. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med 213: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnett DL, Langley DB, Schofield P, Hermes JR, Chan TD, Jackson J, Bourne K, Reed JH, Patterson K, Porebski BT, Brink R, Christ D, and Goodnow CC. 2018. Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science 360: 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giudicelli V, Duroux P, Ginestoux C, Folch G, Jabado-Michaloud J, Chaume D, and Lefranc MP. 2006. IMGT/LIGM-DB, the IMGT comprehensive database of immunoglobulin and T cell receptor nucleotide sequences. Nucleic Acids Res 34: D781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.