Abstract
Purpose:
To characterize differences in resource utilization and cost of managing enrollees with exfoliation glaucoma (XFG) compared to primary open-angle glaucoma (POAG).
Design:
Retrospective utilization and cost comparison using Medicare claims data.
Methods:
We identified Medicare beneficiaries with XFG or POAG and ≥5 years of continuous enrollment from January 2008 to December 2014. We distinguished newly-diagnosed cases from those with pre-existing disease. We compared ophthalmic resource utilization and costs over 2 years of follow-up for persons with newly-diagnosed and pre-existing XFG versus those with POAG.
Main Outcome Measures:
Number of clinic visits, diagnostic procedures, medication fills, laser and incisional surgery, and mean eyecare costs per beneficiary.
Results:
Among 192 eligible enrollees (median age 77.6 years) with newly-diagnosed XFG and 7339 enrollees (median age 77.3 years) with newly-diagnosed POAG, those with XFG had more office visits (mean, 9.1 vs. 7.9; p=0.001), cataract surgery (34.9% vs. 19.0%; p<0.0001) and glaucoma surgery (28.7% vs. 19.7%, p=0.002). They also experienced 27% higher mean total eyecare costs ($3260 vs. $2562, p=0.0001) over 2 years of follow-up. Among 2745 enrollees (median age 80.5 years) with pre-existing XFG and 89036 persons (median age 79.5) with pre-existing POAG, persons with XFG had more office visits (mean 9.3 vs. 7.3; p<0.0001), perimetry (85.3% vs. 79.8%; p<0.0001), cataract surgery (23.4% vs. 12.3%; p<0.0001), laser trabeculoplasty (18.6% vs. 9.6%; p<0.0001), trabeculectomy (8.1 vs. 1.8%; p<0.0001) and experienced 37% higher total mean eyecare costs ($3764 vs. $2739; p<0.0001).
Conclusions:
Healthcare resource utilization and costs are substantially higher for managing patients with XFG compared to POAG.
Introduction
With the passage of the Affordable Care Act and more recently the Medicare Access and CHIP Reauthorization Act (MACRA) legislation, health policymakers have been looking for alternative models for provider reimbursement such as those that involve bundling of payments for health care services into episodes of care instead of the traditional fee-for-service (FFS) payment approach. Unlike traditional FFS reimbursement which incentivizes increased resource utilization, bundled payment designs provide clinicians a single payment to cover all services related to a specific condition or treatment over a specified time period. The acceptability of these alternative payment models to patients and health care providers requires that such models are capable of accounting for disease severity and complexity when determining proper payment for a given bundle or episode of care. For example, if insurers were to create a bundled payment for glaucoma care such that providers are allocated the same payment amount for patients with all types, severities, and complexities of glaucoma, this could incentivize eye care providers to perform additional and potentially unnecessary testing and treatment to some patients with less severe disease while, at the same time, could also incentivize them to undertreat other patients with more complex or severe disease since the payment is the same for all patients. By identifying differences in resource utilization and costs for patients with different types or level of disease, insurers can factor this into the amount of payment for an episode or bundle of care such that it promotes high value, cost-effective care.
Resource use and costs, and how they vary by disease severity, have been characterized for patients with primary open-angle glaucoma (POAG).1–5 For example, Lee and colleagues demonstrated that the mean direct annual medical costs of caring for patients with mild stage glaucoma were nearly fourfold lower than other patients with very advanced disease.1 Likewise, Pasquale and coworkers found that patients with ocular hypertension accrued much lower annual charges relative to others with POAG.2 Yet little is known the extent resource utilization and costs vary for patients with one type of glaucoma versus another and for those with newly-diagnosed (incident) disease versus patients with pre-existing (prevalent) disease.
Exfoliation syndrome glaucoma is the most common secondary open-angle glaucoma. Similar to POAG, this condition often affects both eyes but can be very asymmetric. Patients with XFG are known to experience higher peak intraocular pressures (IOP)6–10, more rapid visual field deterioration6–8, higher rates of failure of medical therapies, and more frequent need for surgical interventions compared to patients with POAG. 6,11,12 Therefore, we hypothesize that patients with XFG experience greater resource utilization and costs compared to persons with POAG, though the extent by which differences in resource use and costs exist is unknown. Thus, the purpose of this study is to compare resource utilization and costs of managing Medicare beneficiaries with XFG to those with POAG and whether differences in ophthalmic resource utilization and total eyecare costs are different for those with newly-diagnosed disease versus others who have had the conditions for a while.
Methods
Data Source
We used a Medicare claims dataset consisting of a nationally-representative 20% sample of all persons with Medicare Parts A, B and D health insurance coverage during January 1, 2008 to December 31, 2014. The database contains detailed records of all ocular and nonocular conditions based on International Classification of Disease, 9th Revision - Clinical Modification13 (ICD-9-CM) billing codes and all visits, diagnostic, and therapeutic procedures based on Current Procedural Terminology14 (CPT-4) codes. Since all eligible patients had Part D coverage, we were able to capture all outpatient medications filled during the study period as well. The database also contains information on demographics, type of healthcare provider for each encounter, and amount paid for all services rendered. Researchers have used this database in the past to study patients with glaucoma and other ocular conditions4,15 and the study was approved by the Institutional Review Board at the University of Michigan.
Study Participants/Inclusion and Exclusion Criteria
We identified all eligible enrollees with records of XFG (ICD-9-CM: 365.52) or POAG (ICD-9-CM: 365.10–365.15) during January 1, 2011 to December 31, 2012. To help ensure that the patient was not miscoded as one of these conditions, we required eligible enrollees to have ≥ 1 confirmatory diagnosis of the condition on a separate date. If an enrollee received diagnoses of both POAG and XFG, they were classified as XFG. To be more certain these persons had bona fide XFG or POAG and were not glaucoma suspects or “rule out” cases, we excluded those who had been diagnosed with either of these conditions but had no record of any medical, laser, or surgical interventions for glaucoma. Other inclusion criteria for this analysis were age at plan enrollment of ≥ 65 years, continuous enrollment in FFS Medicare for at least 5 years, Part D coverage for outpatient medication prescriptions for all 5 years, and ≥ 2 to visits to an eye care provider (ophthalmologist or optometrist). We excluded beneficiaries residing outside the United States, persons with missing demographic information, and persons in Medicare Advantage plans. Individuals in Medicare Advantage plans were excluded since the database does not contain complete data of all healthcare services rendered for these enrollees.
Distinguishing Beneficiaries with Newly-Diagnosed versus Pre-existing POAG and XFG
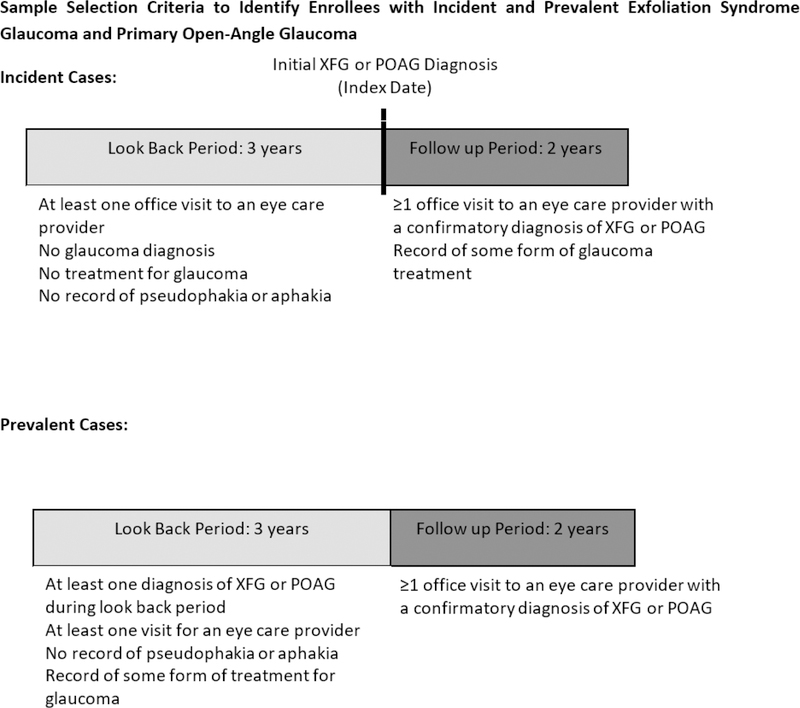
To identify persons with newly-diagnosed (incident) XFG or POAG, we required them to have no record of these conditions during their first 3 years in the health plan and ≥ 1 visit to an eye care provider during that period to give each person an opportunity to get diagnosed with these conditions. Since distinguishing POAG from XFG can be challenging after cataract surgery, we excluded persons who were pseudophakic or aphakic during their first 3 years in the plan. Persons who were diagnosed with other forms of glaucoma besides XFG or POAG during their first 3 years in the plan were also excluded, though enrollees who were classified as glaucoma suspects (ICD9: 365.00, 365.01, 365.04) or XFG suspect (ICD9–366.11) during this time period were not excluded and were thus eligible to be classified as incident cases during the follow-up period. Incident cases of XFG or POAG required one or more confirmatory diagnosis on a separate date during the follow up period for inclusion. Enrollees with ≥ 1 record of XFG or POAG during their first 3 years in the plan were categorized with pre-existing disease (prevalent cases) and included if they also had 1 or more confirmatory diagnosis of the condition during the follow up period. (Figure 1)
Figure 1.
Sample Selection Criteria to Identify Enrollees with Incident and Prevalent Exfoliation Syndrome Glaucoma and Primary Open-Angle Glaucoma
POAG = primary-open angle glaucoma; XFG = exfoliation syndrome glaucoma
Resource Use and Costs
The outcome of interest was ophthalmic resource use and total eyecare costs during a 24-month period. This 24-month period included the date of initial diagnosis of XFG or POAG. Resource use was identified by CPT billing codes and grouped into the following categories: visits to eye care providers; diagnostic procedures to monitor glaucoma (perimetry, fundus photography, other ocular imaging (i.e. optical coherence tomography), gonioscopy, other glaucoma diagnostic tests); laser and incisional glaucoma surgery; cataract surgery; outpatient prescription fills for the following classes of glaucoma medications: topical beta blockers, oral and topical carbonic anhydrase inhibitors, prostaglandin analogues, parasympathomimetics, and alpha agonists. Online Table 1, available at http://www.ajo.com lists the specific CPT codes included in the analysis. We also looked at the mean costs of each of these services and how they varied for enrollees with XFG versus POAG over the 24-month follow-up period. Mean costs for each category were calculated by dividing the total cost for each of the resource categories by the number of beneficiaries in the XFG or POAG groups. Dollar value paid by Medicare rather than physician charges were used to calculate cost.
Analysis
Statistical analyses were performed using SAS software version 9.2 (SAS Institute, Cary, North Carolina, USA). Participant characteristics were summarized for the entire sample using means and standard deviations (SD) for continuous variables and frequencies and percentages for categorical variables.
We determined the mean ± SD number of eye clinic visits, glaucoma diagnostic testing, glaucoma surgeries, and glaucoma medication fills over 24 months of follow-up for those with newly-diagnosed XFG and POAG. The mean number of each of these services were compared using an unpaired t-test. We also assessed the proportion of enrollees undergoing these services at least once during the 24 months and used a chi-square test to compare the groups. We performed similar comparisons for those with pre-existing XFG versus those with pre-existing POAG. Total eyecare costs were calculated as the sum of all costs for eye clinic visits, glaucoma diagnostic testing, glaucoma and cataract surgery, and outpatient glaucoma medication costs over the 24-month period. Comparisons of the mean costs for those with newly-diagnosed and pre-existing XFG versus POAG were performed using an unpaired t-test. For all analyses, p≤0.05 was considered statistically significant.
Results
There were 4541378 Medicare enrollees who met our inclusion criteria. Of these individuals, 346626 patients had a diagnosis of XFG or POAG between 1/½011 and 12/3½012 and among these, 137941 had three years of lookback data, two years of follow up data and had at least two visits to eye care providers. After excluding persons who were aphakic, pseudophakic and patients without any record of treatment, 99312 patients were remaining in our study sample. There were 192 eligible Medicare enrollees who had been diagnosed with incident XFG and 7339 persons with incident POAG. There were also 2745 eligible enrollees with prevalent XFG and 89036 enrollees with prevalent POAG identified. The median age of those with incident XFG (77.6 years) and POAG (77.3 years) were relatively similar (p=0.32). The median age of persons with pre-existing XFG was 80.5 years and those with pre-existing POAG was 79.5 years (p<0.0001). There were slightly greater proportions of females with XFG compared to POAG (68.8% versus 64.5% for incident cases, p=0.22 and 70.9% versus 67.9%, p=0.001 for prevalent cases). Among those with XFG, a greater proportion were white race compared to those with POAG (97.4% versus 78.2% for incident cases, p<0.0001 and 93.0% versus 77.6% for prevalent cases, p<0.0001). (Table 1)
Table 1:
Characteristics of Study Sample
| Incident Cases | Prevalent Cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| POAG | XFG | POAG | XFG | ||||||
| N | % | N | % | N | % | N | % | ||
| Total | 7339 | 192 | 89036 | 2745 | |||||
| Race/ethnicity | White | 5736 | 78.2% | 187 | 97.4% | 69114 | 77.6% | 2552 | 93.0% |
| Black | 809 | 11.0% | 0 | 0.0% | 11812 | 13.3% | 32 | 1.2% | |
| Hispanic | 272 | 3.7% | 0 | 0.0% | 2824 | 3.2% | 54 | 2.0% | |
| Asian | 319 | 4.3% | 2 | 1.0% | 3286 | 3.7% | 41 | 1.5% | |
| Native American | 28 | 0.4% | 1 | 0.5% | 344 | 0.4% | 12 | 0.4% | |
| Other | 173 | 2.4% | 2 | 1.0% | 1627 | 1.8% | 54 | 2.0% | |
| Sex | Male | 2603 | 35.5% | 60 | 31.3% | 28538 | 32.1% | 799 | 29.1% |
| Female | 4734 | 64.5% | 132 | 68.8% | 60469 | 67.9% | 1946 | 70.9% | |
| Median Age (years) | 77.3 | 77.6 | 79.5 | 80.5 | |||||
| Comorbid Ocular Disease | PDR | 81 | 1.1% | 0 | 0.0% | 1244 | 1.4% | 6 | 0.2% |
| NPDR | 281 | 3.8% | 1 | 0.5% | 3642 | 4.1% | 49 | 1.8% | |
| AMD | 1356 | 18.5% | 32 | 16.7% | 14681 | 16.5% | 531 | 19.3% | |
| DME | 125 | 1.7% | 1 | 0.5% | 2218 | 2.5% | 47 | 1.7% | |
| RD | 109 | 1.5% | 2 | 1.0% | 1219 | 1.4% | 39 | 1.4% | |
Primary open-angle glaucoma (POAG); Exfoliation syndrome glaucoma (XFG); Proliferative Diabetic Retinopathy (PDR); Non-Proliferative Diabetic Retinopathy (NPDR); Age-Related Macular Degeneration (AMD); Diabetic Macular Edema (DME); Retinal Detachment (RD).
Incident cases were patients with no record of any glaucoma during their first 3 years in the plan and then received a diagnosis of XFG or POAG. Prevalent cases were patients with ≥1 diagnosis of XFG or POAG during their first 3 years in the plan.
Comparison of Ophthalmic Resource Use and Total Eyecare Costs for Enrollees with Newly-Diagnosed Exfoliation Syndrome Glaucoma Versus Primary Open-Angle Glaucoma
Eye Clinic Visits / Diagnostic Testing
The mean number of eye clinic visits over the 24-month follow-up period was higher for enrollees with newly-diagnosed XFG compared to those with POAG (XFG: 9.1 eye visits per patient over the two years; POAG: 7.9 eye visits per patient over the two years) p=0.001. Nearly all enrollees in both groups underwent some form of glaucoma diagnostic testing over the 24 months (98.4% for those with XFG versus 97.0% for enrollees with POAG, p=0.25) though enrollees with XFG underwent a greater number of glaucoma diagnostic tests (mean: 5.0 for those with XFG versus 4.0 for those with POAG, p<0.0001) over the 24-month follow-up period. In terms of specific glaucoma diagnostic tests, a greater proportion of persons with newly-diagnosed XFG underwent gonioscopy (54.7% versus 37.0%; p<0.0001) and fundus photography (47.9% versus 40.4%, p=0.04) compared to those with POAG while there were no statistically significant differences in proportions receiving perimetry (86.5% versus 84.7%; p=0.50) or other ocular imaging (OCT) (75.5% versus 76.0%; p=0.88) between the 2 groups. (Table 2)
Table 2.
Ophthalmic Resource Utilization During 2 Years of Follow-Up for Enrollees with Incident and Prevalent Primary Open-Angle Glaucoma and Exfoliation Syndrome Glaucoma
| Incident Cases | Prevalent Cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| POAG | XFG | p-value | POAG | XFG | p-value | ||||||
| N | % | N | % | N | % | N | % | ||||
| Total enrollees | 7339 | 192 | 89036 | 2745 | |||||||
| Diagnostic Procedures (%) | Any glaucoma diagnostic test* | 7121 | 97.0% | 189 | 98.4% | 0.25 | 83833 | 94.2% | 2657 | 96.8% | <0.0001 |
| Perimetry | 6216 | 84.7% | 166 | 86.5% | 0.50 | 71079 | 79.8% | 2341 | 85.3% | <0.0001 | |
| Gonioscopy | 2716 | 37.0% | 105 | 54.7% | <0.0001 | 23736 | 26.7% | 1082 | 39.4% | <0.0001 | |
| Fundus Photography | 2964 | 40.4% | 92 | 47.9% | 0.036 | 31115 | 35.0% | 974 | 35.5% | 0.56 | |
| Other ocular imaging | 5577 | 76.0% | 145 | 75.5% | 0.88 | 62333 | 70.0% | 1964 | 71.6% | 0.083 | |
| Any other diagnostic test± | 4156 | 56.6% | 135 | 70.3% | 0.0002 | 46188 | 51.9% | 1673 | 61.0% | <0.0001 | |
| Therapeutic Procedures (%) | Any Ocular Surgery** | 3282 | 44.7% | 118 | 61.5% | <0.0001 | 32277 | 36.3% | 1495 | 54.5% | <0.0001 |
| Any Glaucoma Surgery** | 1443 | 19.7% | 55 | 28.7% | 0.002 | 12591 | 14.1% | 860 | 31.3% | <0.0001 | |
| Trabeculectomy | 68 | 0.9% | 4 | 2.1% | 0.10 | 1611 | 1.8% | 233 | 8.1% | <0.0001 | |
| GDI | 26 | 0.4% | 2 | 1.0% | 0.12 | 889 | 1.0% | 103 | 3.8% | <0.0001 | |
| LTP | 1120 | 15.3% | 38 | 19.8% | 0.086 | 8585 | 9.6% | 511 | 18.6% | <0.0001 | |
| Medication | 6839 | 93.2% | 175 | 91.2% | 0.27 | 87629 | 98.4% | 2680 | 97.6% | 0.0012 | |
| Cataract Surgery | 1391 | 19.0% | 67 | 34.9% | <0.0001 | 10964 | 12.3% | 643 | 23.4% | <0.0001 | |
Primary open angle glaucoma (POAG); Exfoliation Glaucoma (XFG); Glaucoma Drainage Implant (GDI); Laser Trabeculoplasty (LTP).
Proportions capture enrollees who underwent 1 or more of a given service during the 2 years of follow-up.
Any glaucoma diagnostic test includes gonioscopy, fundus photography, perimetry, optical coherence tomography.
Any other diagnostic test – see Online Table 1 available at http://www.ajo.com for included CPT codes.
Any Glaucoma Surgery includes laser iridotomy, trabeculoplasty, trabeculectomy and glaucoma drainage implant insertion.
Any Ocular Surgery includes all laser and incisional ophthalmic surgeries, including but not limited to glaucoma surgeries.
Group comparisons were performed using Pearson chi-square test
Therapeutic Interventions
Proportionately, slightly more patients with POAG filled at least 1 prescription for glaucoma medications during the 2-year follow-up period (93.2%) compared to those with XFG (91.2%) (p=0.27). However, a considerably greater proportion of patients with newly-diagnosed XFG (61.5%) underwent some form of laser or incisional intraocular surgery in the first 24 months after initial diagnosis compared to those with newly-diagnosed POAG (44.7%), (p<0.0001). The rate of any ocular surgery in the XFG group was 2.0 procedures per patient over the two years compared to 1.3 procedures per patient over the two years in the group with POAG (p=0.01). The most common ocular surgical procedure for persons in both groups was cataract surgery. During the 24 months after initial diagnosis, 34.9% of persons with XFG underwent cataract surgery compared to 19.0% of those with POAG. (p<0.0001) When considering all laser and incisional glaucoma surgeries together, a greater proportion of patients with XFG underwent at least 1 of these procedures during the 24 months of follow-up (28.7% versus 19.7%, p=0.002), however when we compare proportions undergoing specific surgeries, there were no statistically significant differences in the proportion undergoing LTP (19.8% for XFG versus 15.3% for POAG, p=0.09), trabeculectomy (2.1% for XFG versus 0.9% for POAG, p=0.10), or GDI (1.0% for XFG versus 0.4% for POAG, p=0.12). (Table 2)
Costs
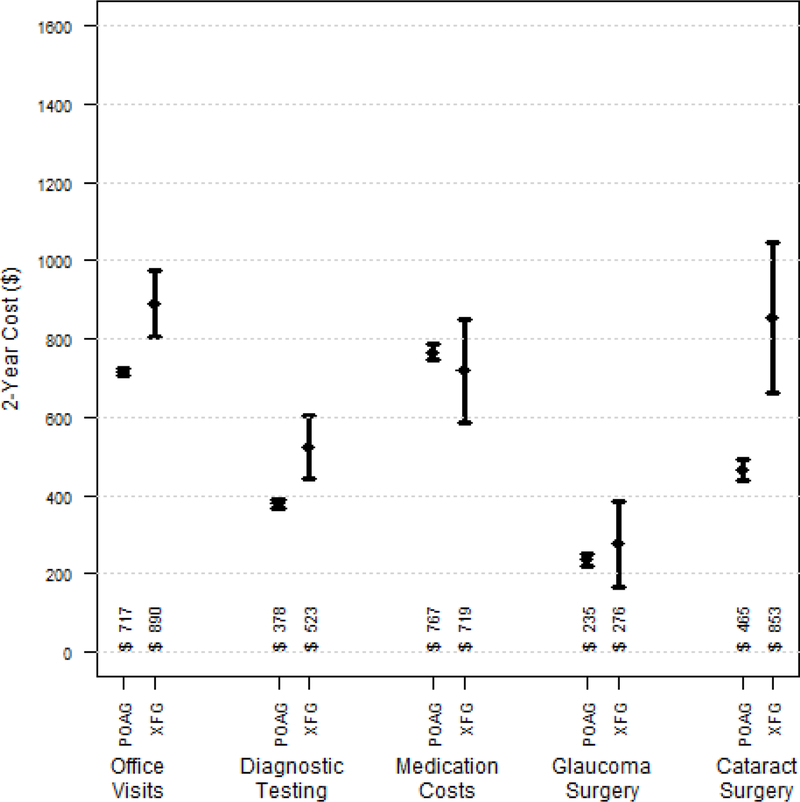
The mean total eyecare costs over the 24 months after initial diagnosis for enrollees with newly-diagnosed XFG were 27% higher than persons with newly-diagnosed POAG ($3260 versus $2562, respectively), p=0.0001. We found the group with XFG had statistically significantly greater costs for eye clinic visits ($890 versus $717, p=0.0001), glaucoma diagnostic testing ($523 versus $378, p=0.0006), and cataract surgery ($853 versus $465, p=0.0001). There were no significant differences in mean costs of glaucoma surgery ($276 versus $235, p=0.47) or glaucoma medications ($719 versus $767, p=0.48) among the 2 groups. (Figure 2)
Figure 2.
Mean Total 2-Year Costs Per Beneficiary for Various Diagnostic and Therapeutic Services for Enrollees with Newly-Diagnosed Primary Open-Angle Glaucoma (n=7339) and Exfoliation Syndrome Glaucoma (n=192).
p<0.001 for clinic visit costs; p<0.001 for glaucoma diagnostic testing costs; p<0.001 for cataract surgery costs; p=0.46 for glaucoma surgery costs; p=0.48 for glaucoma medication costs. Error bars in figure reflect 95% confidence intervals.
POAG = primary-open angle glaucoma; XFG = exfoliation syndrome glaucoma
Comparison of Ophthalmic Resource Use and Total Eyecare Costs for Enrollees with Prevalent Exfoliation Syndrome Glaucoma Versus Primary Open-Angle Glaucoma
Eye Clinic Visits / Diagnostic Testing
The mean number of eye clinic visits over the 24-month follow-up period was higher for enrollees with pre-existing XFG compared to patients with POAG (XFG: 9.3 eye care visits per patient over the two years; POAG: 7.3 eye care visits per patient over the two years, p<0.0001). The majority of the enrollees in both groups underwent some form of glaucoma diagnostic testing over the 24 months (96.8% for those with XFG versus 94.2% for enrollees with POAG, p<0.0001). Similar to incident cases, enrollees with pre-existing XFG underwent a greater number of glaucoma diagnostic tests over the 24-month period (mean, 4.3 tests) compared to those with POAG (mean, 3.7 tests), p<0.0001. In terms of specific glaucoma diagnostic tests, a greater proportion of persons with prevalent XFG compared to those with POAG underwent gonioscopy (39.4% versus 26.7%; p<0.0001) and perimetry (85.3% versus 79.8%, p<0.0001) while there were no statistically significant differences in proportions undergoing fundus photography (35.5% versus 35.0%; p=0.56) or other ocular imaging (OCT) (71.6% versus 70.0%; p=0.08) between the 2 groups.
Therapeutic Interventions
Nearly all the patients in both groups had records of at least 1 glaucoma medication fill during the 2-year follow-up period (97.6% for those with XFG versus 98.4% for those with POAG), p=0.001. A substantially greater proportion of patients with prevalent XFG (54.5%) underwent some form of laser or incisional intraocular surgery during the 24-month follow-up period compared to those with prevalent POAG (36.3%), p<0.0001. The rate of any ocular surgery in the XFG group was 1.5 procedures per patient over the two years of follow-up compared to 1.0 procedure per patient over the two years of follow-up for the group with POAG (p<0.0001). Similar to those with incident disease, the most common surgical procedure for persons in both groups was cataract surgery. During the 24 months of follow-up, 23.4% of persons with XFG underwent cataract surgery compared to 12.3% of those with POAG. (p<0.0001) When considering all laser and incisional glaucoma surgeries together, a greater proportion of patients with XFG underwent at least 1 of these procedures during the 24 months (31.3% versus 14.1%, p<0.0001) and when we assess specific glaucoma surgeries, we observed greater proportions of persons with XFG undergoing LTP (18.6% versus 9.6%, p<0.0001), trabeculectomy (8.1% versus 1.8%, p<0.0001) and GDI insertion (3.8% versus 1.0%, p<0.0001). (Table 2)
Costs
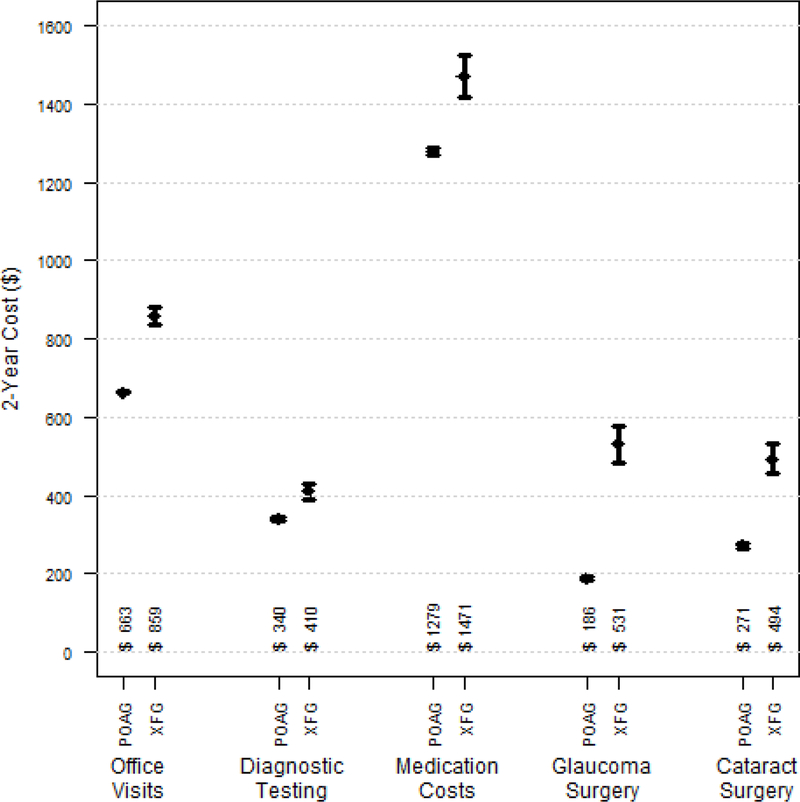
The mean overall eye care costs over the 24 months for enrollees with prevalent XFG were 37% higher than persons with prevalent POAG ($3764 vs. $2739, respectively); p<0.0001), which amounts to more than $1000 over the 24 months. We found the group with XFG had statistically significantly greater costs of eye clinic visits ($859 versus $663, p<0.0001), glaucoma diagnostic testing ($410 versus $340, p<0.0001), cataract surgery ($494 versus $271, p<0.0001), glaucoma medications ($1471 versus $1279, p<0.0001), and glaucoma surgery ($531 versus $186, p<0.0001) over the 2 years of follow-up. (Figure 3)
Figure 3.
Mean Total 2-Year Cost Per Beneficiary for Various Diagnostic and Therapeutic Services for Enrollees with Pre-existing Primary Open-Angle Glaucoma (n=89036) and Exfoliation Syndrome Glaucoma (n=2745).
p<0.0001 for all cost comparisons. Error bars in figure reflect 95% confidence intervals.
POAG = primary-open angle glaucoma; XFG = exfoliation syndrome glaucoma
Discussion
Using data from a nationally-representative sample of Medicare beneficiaries, we compared resource utilization and total eyecare costs for patients with XFG to others with POAG. We learned that for both incident cases as well as those with preexisting disease, the group with XFG received more visits to eye care professionals, underwent more diagnostic testing, and a greater proportion received cataract surgery and laser and incisional glaucoma surgery during the 2 years of follow-up compared to those with POAG. This resulted in nearly $700 in more total eyecare costs for those with incident disease and more than $1000 in total eyecare costs for those with preexisting disease during the follow-up period for those with XFG. While it is well established that patients with XFG tend to be more challenging to manage relative to those with POAG, these results help quantify the extent of the differences in resource utilization and costs between the groups.
When comparing resource utilization, our findings demonstrate that clinicians similarly manage most patients with newly-diagnosed XFG and POAG using perimetry and OCT. However, one notable difference in diagnostic testing utilization between the 2 groups was observed for gonioscopy. This may be attributable to clinicians evaluating the drainage angle to help look for evidence to substantiate the diagnosis of XFG (i.e. Sampaolesi’s line), to assess whether their patients with XFG are candidates for LTP, or to check for narrow angles which can be associated with XFG.16 Moreover, a greater proportion of patients with XFG are monitored by glaucoma subspecialists, who may be more apt to perform gonioscopy compared to other eye care providers. Among patients with preexisting disease, we observed a greater preponderance of patients with XFG who underwent perimetry compared to those with pre-existing POAG. Patients with XFG may have more severe disease8 and thus require more intensive monitoring with perimetry to check for disease progression compared to their counterparts with POAG.
In terms of therapeutic procedures, in our analyses, more than one quarter of the patients with newly-diagnosed XFG underwent laser or incisional glaucoma surgery in at least one eye and more than one third of them underwent cataract surgery during their first two years following XFG diagnosis. These proportions were much higher for both glaucoma and cataract surgery relative to enrollees with newly-diagnosed POAG. Patients with XFG may present with denser cataracts 17,18 or clinicians may recommend surgery earlier in the course of the disease for those with XFG 19 to try to reduce the risk of complications associated with delaying surgery until the cataract is more mature. Among those with pre-existing disease, the proportions who underwent LTP and cataract surgery were approximately double and the proportions who underwent trabeculectomy or GDI were four-fold higher in the XFG cohort. These differences in utilization translated into much greater costs to manage those with XFG.
When comparing costs of glaucoma care for the 2 groups, we identified several salient findings. In the first two years following initial diagnosis, patients with XFG experienced more costs associated with eye clinic visits, glaucoma diagnostic testing, and cataract surgery but no significant difference in costs for glaucoma medications or glaucoma surgery. For those with pre-existing disease, the group with XFG not only had greater costs for eye clinic visits and glaucoma diagnostic testing, but also glaucoma medications and surgery. This suggests that it becomes more challenging and costly to manage patients with XFG over time. If the disease trajectory is more rapid for patients with XFG8, and a greater proportion progress from mild to severe disease, this would in turn affect costs.20 Patients with XFG may also have more difficult to control intraocular pressure6,7,10 or larger fluctuations in intraocular pressure6,7, necessitating more aggressive medical or surgical glaucoma care over time12. Finally, a greater proportion of patients with XFG may get referred to glaucoma subspecialists to care for them, and these providers may be more aggressive at managing them medically and surgically relative to other eye care providers.
There has been a growing interest among health policymakers to look for alternatives to FFS Medicare to try to curtail rising healthcare costs. One alternative to reimbursing providers based on FFS that has been receiving a lot of attention is the creation of episodes of care. All healthcare services that constitute a given episode are lumped into a single bundled payment that is given to a healthcare provider to manage all aspects of care for the condition of interest over a set period of time. Examples of Centers for Medicare & Medicaid Services demonstration projects involving episodes of care include knee and hip replacement surgery21. While we are unaware of any episodes of care that have been developed specifically for glaucoma, in the event that policymakers or insurers look to develop episodes of care or bundling of payments for this condition, it will be imperative for them to consider the type of glaucoma, and its severity and complexity, and the length of time one has the condition when determining the appropriate payment for such a bundle. Our findings that patients with newly-diagnosed XFG cost, on average, approximately $350 more per year to manage, and those with established disease cost, on average, more than $500 more per year to manage highlights the importance of considering the type of glaucoma in such payment algorithms. If policymakers reimburse eye care providers the same amount of money to manage patients with XFG as they do for POAG, providers may be forced to have to skimp on care because of insufficient resources to manage these patients properly. Furthermore, some eye care providers may opt not to care for patients with XFG, knowing they may be unable to offer these patients the same quality of care as others with POAG. Given that patients with XFG tend to have higher intraocular pressures6,7,10 and wider fluctuations in IOP6,7 compared to persons with POAG, these patients need greater, not less, monitoring and surveillance to prevent disease progression and risk of blindness.
Our study has several limitations. First, we lacked data about the status of each enrollee’s glaucoma prior to plan entry. Second, while we used a 3-year lookback period to try to properly categorize and distinguish those with incident disease from those with pre-existing disease, it is possible that some patients with pre-existing disease may have simply not sought eye care for the initial 3 years in the plan and thus, in the dataset appear as incident cases. Prior work from our group has demonstrated that a 3-year lookback period is often sufficient to distinguish persons with incident from non-incident glaucoma22. Third, some patients in each group may have been misdiagnosed or miscoded with the conditions of interest. To try to limit the potential for misdiagnosis, we restricted our study sample to persons who were phakic as it is often easier to distinguish XFG from POAG prior to cataract removal. In addition, to limit the potential for miscoding to affect our findings we required a confirmatory diagnosis of the condition of interest on a separate date. In the event that some patients with XFG were misclassified as POAG or vice versa, this would bias our findings to the null. Fourth, claims data lacks information about clinical variables of interest such as the level of IOP, results from the diagnostic testing to adequately capture the complexity of each patient’s disease, and details about the optic nerve or nerve fiber layer. Glaucoma severity at the time of incident diagnosis was coded for very few patients (only 7%) so unfortunately, this could not be considered in our analyses. Finally, since our study sample were enrollees in FFS Medicare, it is uncertain whether the findings generalize to patients with other types of health insurance, those who are uninsured, or those residing outside of the US.
In conclusion, healthcare resource utilization and cost associated with caring for patients with XFG are significantly higher compared to others with POAG on almost every metric we evaluated. The findings from these analyses highlight the importance of considering the type of glaucoma, its severity, and the length of time with the condition, when insurers and policy-makers develop algorithms for alternative payment models to ensure patients are able to receive high quality, cost-effective care.
Supplementary Material
Table 3.
Ophthalmic Resource Utilization During 2 Years of Follow-Up for Enrollees with Incident and Prevalent Primary Open-Angle Glaucoma and Exfoliation Syndrome Glaucoma
| Incident Cases | Prevalent Cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| POAG | XFG | p-value | POAG | XFG | p-value | ||||||
| N | % | N | % | N | % | N | % | ||||
| Total enrollees | 7339 | 192 | 89036 | 2745 | |||||||
| Diagnostic Procedures (%) | Any glaucoma diagnostic test* | 7121 | 97.0% | 189 | 98.4% | 0.25 | 83833 | 94.2% | 2657 | 96.8% | <0.0001 |
| Perimetry | 6216 | 84.7% | 166 | 86.5% | 0.50 | 71079 | 79.8% | 2341 | 85.3% | <0.0001 | |
| Gonioscopy | 2716 | 37.0% | 105 | 54.7% | <0.0001 | 23736 | 26.7% | 1082 | 39.4% | <0.0001 | |
| Fundus Photography | 2964 | 40.4% | 92 | 47.9% | 0.036 | 31115 | 35.0% | 974 | 35.5% | 0.56 | |
| Other ocular imaging | 5577 | 76.0% | 145 | 75.5% | 0.88 | 62333 | 70.0% | 1964 | 71.6% | 0.083 | |
| Any other diagnostic test± | 4156 | 56.6% | 135 | 70.3% | 0.0002 | 46188 | 51.9% | 1673 | 61.0% | <0.0001 | |
| Therapeutic Procedures (%) | Any Ocular Surgery** | 3282 | 44.7% | 118 | 61.5% | <0.0001 | 32277 | 36.3% | 1495 | 54.5% | <0.0001 |
| Any Glaucoma Surgery** | 1443 | 19.7% | 55 | 28.7% | 0.002 | 12591 | 14.1% | 860 | 31.3% | <0.0001 | |
| Trabeculectomy | 68 | 0.9% | 4 | 2.1% | 0.10 | 1611 | 1.8% | 233 | 8.1% | <0.0001 | |
| GDI | 26 | 0.4% | 2 | 1.0% | 0.12 | 889 | 1.0% | 103 | 3.8% | <0.0001 | |
| LTP | 1120 | 15.3% | 38 | 19.8% | 0.086 | 8585 | 9.6% | 511 | 18.6% | <0.0001 | |
| Medication | 6839 | 93.2% | 175 | 91.2% | 0.27 | 87629 | 98.4% | 2680 | 97.6% | 0.0012 | |
| Cataract Surgery | 1391 | 19.0% | 67 | 34.9% | <0.0001 | 10964 | 12.3% | 643 | 23.4% | <0.0001 | |
Primary open angle glaucoma (POAG); Exfoliation Glaucoma (XFG); Glaucoma Drainage Implant (GDI); Laser Trabeculoplasty (LTP).
Proportions capture enrollees who underwent 1 or more of a given service during the 2 years of follow-up.
Any glaucoma diagnostic test includes gonioscopy, fundus photography, perimetry, optical coherence tomography.
Any other diagnostic test – see Online Table 1 available at http://www.ajo.com for included CPT codes.
Any Glaucoma Surgery includes laser iridotomy, trabeculoplasty, trabeculectomy and glaucoma drainage implant insertion.
Any Ocular Surgery includes all laser and incisional ophthalmic surgeries, including but not limited to glaucoma surgeries.
Group comparisons were performed using Pearson chi-square test
Acknowledgements
a. Funding/Support: David Greenfield: P30EY014801 University of Miami core grant, Miami, FL (DSG); David Greenfield and Joshua Stein: Research to Prevent Blindness unrestricted grant to the University of Miami and University of Michigan, New York, NY (DSG + JDS); David Greenfield: an unrestricted grant from Mr. and Mrs. Thorne B. Donnelley, Chicago, IL (DSG); David Greenfield: the Maltz Family Endowment for Glaucoma Research, Cleveland, OH (DSG); Joshua Stein: W.K. Kellogg Foundation, East Grand Rapids, MI (JDS); Joshua Stein: National Eye Institute R01 EY026641, Bethesda, MD (JDS); Siddarth Rathi: American Glaucoma Society Mentoring for Physician-Scientists Award, San Francisco, CA (SR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available at AJO.com
b. Financial Disclosure: Dr. Greenfield serves as a consultant to Aerie Pharmaceuticals (Bedminster Township, NJ), Alcon (Fort Worth, TX), Allergan (Madison, NJ), Bausch + Lomb (Bridgewater, NJ), Quark Pharmaceuticals (Fremont, CA), and Glaukos (San Clemente, CA). Dr. Rathi serves as a consultant to Digisight Technologies (San Francisco, CA) and EyeNovia (New York, NY). Dr Stein and Dr Andrews have no financial disclosures.
References
- 1.Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol 2006;124(1):12–19. [DOI] [PubMed] [Google Scholar]
- 2.Pasquale LR, Dolgitser M, Wentzloff JN, et al. Health care charges for patients with ocular hypertension or primary open-angle glaucoma. Ophthalmology 2008;115(4):633–638. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Cassard SD, Gower EW, Ramulu PY, Jampel HD, Friedman DS. The cost of glaucoma care provided to Medicare beneficiaries from 2002 to 2009. Ophthalmology 2013;120(11)2249–57. [DOI] [PubMed] [Google Scholar]
- 4.Stein JD, Ayyagari P, Sloan FA, Lee PP. Rates of glaucoma medication utilization among persons with primary open-angle glaucoma, 1992 to 2002. Ophthalmology 2008;115(8):1315–9. [DOI] [PubMed] [Google Scholar]
- 5.Stein JD, Niziol LM, Musch DM, et al. Longitudinal trends in resource use in an incident cohort of open-angle glaucoma patients. Am J. Ophthalmol 2012;154(3):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstas AG, Hollo G, Astakhov YS, et al. Factors associated with long-term progression or stability in exfoliation glaucoma. Arch Ophthalmol 2004;122(1):29–33. [DOI] [PubMed] [Google Scholar]
- 7.Konstas AG, Mylopoulos N, Karabatsas CH, et al. Diurnal intraocular pressure reduction with latanoprost 0.005% compared to timolol maleate 0.5% as monotherapy in subjects with exfoliation glaucoma. Eye (Lond) 2004;18(9):893–899. [DOI] [PubMed] [Google Scholar]
- 8.Ritch R, Schlotzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol 2001;45(4):265–315. [DOI] [PubMed] [Google Scholar]
- 9.Ritch R, Schlotzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res 2003;22(3):253–275. [DOI] [PubMed] [Google Scholar]
- 10.Vesti E, Kivela T. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res 2000;19(3):345–368. [DOI] [PubMed] [Google Scholar]
- 11.Tornqvist G, Drolsum LK. Trabeculectomies. A long-term study. Acta Ophthalmol (Copenh) 1991;69(4):450–454. [DOI] [PubMed] [Google Scholar]
- 12.Lim SH, Cha SC. Long-term Outcomes of Mitomycin-C Trabeculectomy in Exfoliative Glaucoma Versus Primary Open-Angle Glaucoma. J Glaucoma 2017;26(4):303–310. [DOI] [PubMed] [Google Scholar]
- 13.American Medical Association. International classification of diseases, 9th revision, clinical modification: ICD-9-CM 1996. [Salt Lake City, UT]: American Medical Association; Medicode. [Google Scholar]
- 14.American Medical Association. CPT 2006 Chicago, IL: AMA Press; 2006. [Google Scholar]
- 15.Newman-Casey PA. Woodward MA Niziol LM Lee PP De Lott LB Brand Medications and Medicare Part D: How Eye Care Providers’ Prescribing Patterns Influence Costs. Ophthalmology 2017;S0161-6420(17):30971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross FJ, Tingey D, Epstein DL. Increased prevalence of occludable angles and angle-closure glaucoma in patients with pseudoexfoliation. Am J Ophthalmol 1994;117(3):333–6. [DOI] [PubMed] [Google Scholar]
- 17.Hiller R, Sperduto RD, Krueger DE. Pseudoexfoliation, Intraocular pressure and senile lens changes in a population-based survey. Arch Ophthal 1982;100(7):1080–2. [DOI] [PubMed] [Google Scholar]
- 18.Kanthan GL, Mitchell P, Burlutsky G, Rochtchina E, Wang JJ. Pseudoexfoliation syndrome and the long-term incidence of cataract and cataract surgery: the blue mountains eye study. Am J Ophthalmol 2013; 155(1):83–88. [DOI] [PubMed] [Google Scholar]
- 19.Desai MA, Lee RK. The Medical and Surgical Management of Pseudoexfoliation Glaucoma. Int Ophthalmol Clin 2008;48(4):95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee PP, Kelly SP, Mills RP, et al. Glaucoma in the United States and Europe: predicting costs and surgical rates based upon stage of disease. J Glaucoma 2007;16(5):471–478. [DOI] [PubMed] [Google Scholar]
- 21.Center for Medicare & Medicaid Services. Comprehensive Care for Joint Replacement Model Available at https://innovation.cms.gov/initiatives/cjr; 2018. Accessed March 3, 2018.
- 22.Stein JD, Blachley TS, Musch DC. Identification of Persons with Incident Ocular Diseases Using Health Care Claims Databases. Am J. Ophthalmol 2013;156(6):1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.