Caspase-3 and -7 are redundantly required to amplify the extrinsic and intrinsic apoptotic cascade in human leukemia.
Abstract
Apoptosis is a complex multi-step process driven by caspase-dependent proteolytic cleavage cascades. Dysregulation of apoptosis promotes tumorigenesis and limits the efficacy of chemotherapy. To assess the complex interactions among caspases during apoptosis, we disrupted caspase-8, -9, -3, -7, or -6 and combinations thereof, using CRISPR-based genome editing in living human leukemia cells. While loss of apical initiator caspase-8 or -9 partially blocked extrinsic or intrinsic apoptosis, respectively, only combined loss of caspase-3 and -7 fully inhibited both apoptotic pathways, with no discernible effect of caspase-6 deficiency alone or in combination. Caspase-3/7 double knockout cells exhibited almost complete inhibition of caspase-8 or -9 activation. Furthermore, deletion of caspase-3 and -7 decreased mitochondrial depolarization and cytochrome c release upon apoptosis activation. Thus, activation of effector caspase-3 or -7 sets off explosive feedback amplification of upstream apoptotic events, which is a key feature of apoptotic signaling essential for efficient apoptotic cell death.
INTRODUCTION
Programmed cell death is vital to development and maintenance of healthy tissues in multicellular animals, and a lack of apoptotic cell death is thought to be a key driver of oncogenesis (1). Nonetheless, it remains unclear whether apoptosis and its mediators are specifically required for cancer cell killing by many chemotherapeutic drugs. In addition to their clear functional roles in apoptotic cell death, increasing functions for caspases have been identified in biological processes as diverse as cell proliferation, differentiation, tissue homeostasis, and immune regulation (2, 3); thus, a better understanding of the regulation of caspase signaling may yield valuable insight into many broader biological processes.
Originally identified among other family members of Caenorhabditis elegans death proteins (4), it is now known that caspases are key proteases that activate and mediate apoptotic cell death through a cascade of protein cleavage. Apoptotic caspases are hierarchically organized into apical caspases (caspase-2, -8, -9, and -10) and effector caspases (caspase-3, -7, and -6) (5). Apoptosis can be induced by either extrinsic stimulus through death receptors [such as the tumor necrosis factor–α (TNFα) or FAS receptors] that specifically activate caspase-8 or intrinsic stimulus (such as expression of BCL2 family BH3-only proteins BIM or puma) that leads to mitochondrial depolarization and activation of caspase-9 (6, 7).
Most models of caspase activation present a hierarchical and linear proteolytic cascade, wherein upstream caspases directly cleave effector caspases that subsequently mediate destruction of the cell through direct cleavage of a broad range of substrates (8, 9), and activation of downstream death mediators such as caspase-activated deoxyribonuclease (10). In addition to models of hierarchical caspase activation, a number of reports have shown that effector caspases may feedback on caspase-8 to promote extrinsic apoptosis (11–14) and caspase-9, BID, and other BCL2 family members to promote intrinsic apoptosis (8, 15, 16). Despite many studies, the difficulty of specifically inactivating caspases has confounded analyses of the specific roles of individual effector caspases in apoptosis within an intact human cell.
It is generally believed that effector caspase-3 and -7 are functionally redundant in the activation of apoptosis (17), with the contribution of caspase-6 remaining unclear. Caspase-3 and -7 can be individually removed from certain mouse strains with no overt phenotype, but caspase-3/7 double-knockout animals cannot survive because of fatally disorganized heart development, although mouse embryonic fibroblasts derived from mouse embryonic tissue show profound resistance to apoptotic cell death not seen in single knockouts (16). Despite their functional redundancy, a number of publications have indicated some differences in substrate specificity and function between caspase-3 and -7 (18, 19); thus, the individual contribution of effector caspases to apoptotic activation remains unclear. Despite their central role in mediating apoptosis, mutations or deletions of caspase-3 and -7 seem only to be relatively rarely detected in tumor tissue (20), potentially supporting a view that redundancy and genomic segregation of effector caspases may limit the risk of inactivation through mutation in cancer cells.
Here, we have undertaken a systematic analysis of the individual and combined roles of effector caspase-3, -7, and -6 in chemotherapy-induced cell death within human leukemic cell lines. Using CRISPR-Cas9 gene targeting, we have specifically disrupted the effector caspase genes alone or in all possible combinations. We demonstrate that caspase-3 and -7 indeed do show complete redundancy in activating apoptosis in response to both extrinsic and intrinsic apoptosis triggers, with no apparent role for caspase-6. Furthermore, the absence of both caspase-3 and -7 leads to defective activation of upstream caspase-8 and -9 and a decrease of mitochondria membrane depolarization with delayed cytochrome c release, underlining the role of effector caspase feedback in activating the apoptotic cascade at different levels.
RESULTS
Caspase-8 and -9 are both activated by extrinsic and intrinsic apoptotic stimuli
To investigate the activation of initiator caspases during apoptosis, leukemia cells were treated with selective extrinsic or intrinsic apoptotic stimuli. To activate extrinsic apoptosis, we treated NALM6 leukemia cells with a combination of TNF and Birinapant (Bir/TNF), a small-molecule SMAC mimetic that blocks cellular inhibitor of apoptotic proteins. We have previously shown that this combination induces cell death that is strictly dependent on the extrinsic caspase-8 apoptotic pathway in human leukemia cells (21). Following the induction of apoptotic cell death by Bir/TNF, we observed coordinated appearance of cleaved/activated forms of both caspase-8 and -9 via Western blot (Fig. 1A). We also observed progressive loss of the inactive full length and appearance of cleaved forms of effector caspase-3, -7, and -6, between 16 and 30 hours after treatment (Fig. 1A).
Fig. 1. Caspase-8 and -9 are both activated during intrinsic and extrinsic apoptosis.
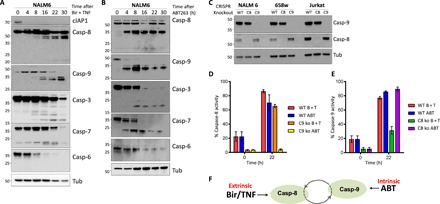
(A and B). Western blot of NALM6 WT cells treated for the indicated time with (A) Birinapant (50 nM) and hTNF (10 ng/ml) or (B) with ABT263 (5 μM). (C) Western blot of control and CRISPR-knockout caspase-8– or -9–deficient leukemia cells; tubulin (Tub) is shown for loading control. (D and E) Activation of caspase-8 and -9, respectively, using caspase-8– or -9–specific FAM-FLICA stains and flow cytometry at indicated time points after treatment with (D) Birinapant (50 nM) and hTNF (10 ng/ml) (B + T) or (E) ABT263 (5 μM) (ABT) in WT or caspase-8 (C8 ko)– and caspase-9 (C9 ko)–deficient cells. (F) Model of apoptotic response activation of caspase-8 and -9 following extrinsic or intrinsic stimuli. Graphs show the mean ± SEM for three repeated experiments performed in duplicate.
To investigate whether we see similar coordinated activation of caspase-8 and -9 following stimulation of intrinsic apoptosis, we treated these cells with a small-molecule inhibitor of the antiapoptotic BCL2 family, ABT263. Similar to our observations with the extrinsic apoptotic trigger Bir/TNF, ABT263 treatment resulted in activation of both caspase-8 and -9 (Fig. 1B). To confirm the specificity of extrinsic (Bir/TNF) or intrinsic (ABT263) apoptotic stimuli, we next used fluorescent protein-tagged lentiviral vectors to deliver CRISPR-Cas9 and targeted single-guide RNAs (sgRNAs) [LentiCRISPR (LC)] to generate specific caspase-8 (casp-8−/−) or caspase-9 (casp-9−/−)–deficient cell lines derived from three different human leukemia (NALM6, 658w, or Jurkat; Fig. 1C). Using these cells, we show that caspase-9–deficient cells show activation of caspase-8 in response to extrinsic apoptotic stimulation with Bir/TNF but not to the intrinsic apoptotic stimulator ABT263 (Fig. 1D). In contrast, caspase-8–deficient cells showed normal caspase-9 activation in response to intrinsic but not to extrinsic apoptotic stimulation (Fig. 1E). These results suggest a model wherein either caspase-8 or -9 is required to initiate an apoptotic response following extrinsic of intrinsic-specific stimulation, but both pathways become activated following stimulation (Fig. 1F).
To examine the downstream effects of loss of caspase-8 or -9 expression, we stained NALM6 wild-type (WT) or caspase-knockout cells with the mitochondrial polarity–dependent dye tetra-methyl rhodamine ester (TMRE). We found that casp-9−/− cells retained their mitochondrial potential while WT or casp-8−/− cells show complete mitochondrial depolarization by 24 hours after ABT263 treatment (Fig. 2, A and B).
Fig. 2. Extrinsic or intrinsic apoptotic inducers differentially trigger mitochondrial depolarization.
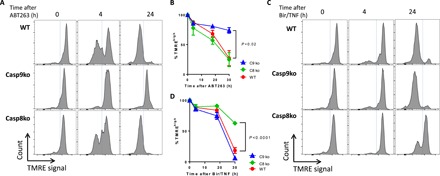
(A to D) Analysis of mitochondria membrane depolarization using TMRE measurements by flow cytometry in WT, caspase-9, and caspase-8 knockout NALM6 cells, upon intrinsic [(A) 5 μM ABT263, quantitation in (B)] or extrinsic trigger [(C) Birinapant (50 nM), TNFa (10 ng/ml), quantitation in (D)]. Graphs show the mean ± SEM for three repeated experiments performed in duplicate. Student’s t test was used for comparison between single time point values as indicated.
In contrast, loss of mitochondrial membrane potential following Bir/TNF was attenuated in caspase-8–deficient but not WT or casp-9−/− cells (Fig. 2, C and D). Agreeing with our TMRE observations, cell viability measurements using mitochondrial activity dye (CCK-8) demonstrate that caspase-9 deficiency partially protected cells from ABT263 but not from Bir/TNF (fig. S1A), whereas caspase-8 deficiency protected cells against Bir/TNF but not ABT263 (fig. S1B). Overall, these results are consistent with the canonical understanding of the apoptotic pathways, wherein caspase-8 and -9 are specifically required to activate extrinsic and intrinsic apoptosis, respectively. Independent of the specific pathway of initiation, however, apoptotic leukemia cells show coordinated activation of both caspase-8 and -9, loss of mitochondrial membrane potential, and cell death. Unexpectedly, caspase-9 deficiency also blocked mitochondria membrane depolarization after ABT263 treatment, a process that is generally considered to be upstream of caspase-9 activation, supporting a previously proposed model of functional feedback regulation at the level of the mitochondrial membrane (22–24).
Caspase-3 and -7 redundantly activate apoptotic cell death with no apparent role for caspase-6 in leukemic cells
To dissect the overlapping roles of the downstream effector caspase proteins in executing apoptotic cell death, we generated effector caspase-deficient leukemic cell lines using our multicolor LC approach as we have previously described (21, 25). We first transduced the acute lymphoblastic leukemia cell lines NALM6, 658w, and Jurkat cells with LC–enhanced green fluorescent protein targeting caspase-7, LC-mCherry targeting caspase-3, and LC-RFP657 targeting caspase-6 (fig. S2A). Using single-cell sorting and screening via Western blotting, we were able to generate clonal cell lines deficient for caspase-3, -7, and -6, or any combination of these proteins derived from three parental leukemia cell lines (Fig. 3, A to C).
Fig. 3. Caspase-3 and -7 are redundantly required for intrinsic or extrinsic apoptosis.
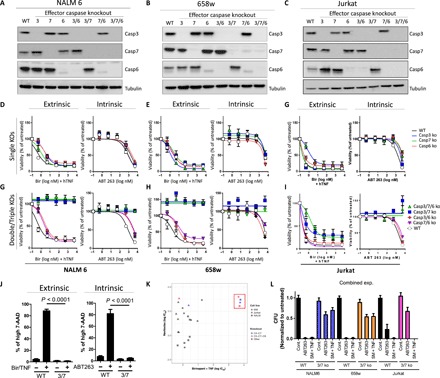
(A to C) Western blot of NALM 6 (A), 658w (B), and Jurkat cells (C) with CRISPR-based deletion of indicated effector caspases to produce single, double, or triple knockouts. (D to F) Cell viability (in % viability compared to control) analyses using CCK-8 of single effector caspase knockouts (KOs) in NALM6 (D), 658w (E), and Jurkat (F) upon incubation with Birinapant (50 nM) and hTNF (10 ng/ml) or ABT263 (5 μM). (G to I) Cell viability analyses (in % viability compared to control) using CCK-8 of indicated double or triple effector caspase knockout in NALM6 (G), 658w (H), and Jurkat (I) upon incubation with Birinapant (50 nM) and hTNF (10 ng/ml) or ABT263 (5 μM). (J) Cell viability using 7-AAD of WT or caspase-3/7 double-knockout NALM6 cells in controls and upon treatment with Birinapant and hTNF or ABT263 for 24 hours. (K) Dotplot indicating IC50 values for indicated WT and knockout cell lines with values for ABT263 given on the y axis and those for Birinapant and hTNF on the x axis. (L) Colony formation assays in Methocult were performed to examine the number of viable cells remaining at 24 to 48 hours after treatment with ABT263 or TNF/SM. Cell viability graphs [shown in (D) to (J)] show the mean ± SEM for three repeated experiments performed in duplicate. Colony-forming units (CFUs) are the average from three experiments each performed in duplicate.
To test the effect of specific deficiency in effector caspases on apoptotic cell death, we treated WT or caspase-knockout cells with either extrinsic (Bir/TNF) or intrinsic (ABT263) apoptotic stimuli. Both extrinsic and intrinsic apoptotic stimuli lead to potent killing of all single caspase–deficient cell lines, with no apparent dosage effects of single loss of any effector caspases (Fig. 3, D to F). In contrast to single-knockout lines, NALM6 and 658w cells lacking either caspase-3 and -7 (casp3/7−/−) or caspase-3, -7, and -6 (casp3,7,6−/−) showed a profound resistance to extrinsic and intrinsic apoptosis (Fig. 3, G to I). We confirmed similar cell death results using 7-amino-actinomycin D (7-AAD) and flow cytometry to examine the loss of cell membrane integrity following apoptosis induction in NALM6 WT and casp3/7-deficient cells (Fig. 3J).
In the case of extrinsic apoptosis, we note an over 4-log increase in median inhibitory concentration (IC50) response to Bir/TNF treatment in NALM6 and 658w cells lacking caspase-3 and -7 (Fig. 3K). In contrast to this, Jurkat cells lacking caspase-3 and -7 were resistant to ABT but remained sensitive to Bir/TNF induced (Fig. 3K), likely because of the fact that Jurkat cells undergo necroptotic death upon TNF stimulation and inhibition of the extrinsic apoptotic pathway (21). We also observed similar induction of cell death following treatment of WT, caspase-3/7, or caspase-3/7/6 knockout cells with a Fas ligand, which is also known to induce apoptotic and necroptotic cell death (fig. S2B) (26). In line with this, necrostatin rescues Jurkat cells from Birinapant-induced cell death (fig. S2C). This observation supports a model of the necroptosis pathway as a specific alternative to extrinsic cell death with no substantial role during intrinsic apoptotic death.
We next confirmed the loss of effector caspase activity in double-knockout cells using a fluorogenic substrate assay. As expected, fluorogenic caspase-3/7 substrate activity was completely absent in casp3/7−/− cells (fig. S2D). Although fluorogenic substrates have been observed to be somewhat promiscuously targeted by various caspases, the complete absence of signal in casp3/7−/− cells supports a conclusion that effector caspases are functionally redundant in these cells, and caspase-6 does not sustain any residual DEVD-cleavage activity in the absence of caspase-3 and -7. A scatter plot summarizing calculated IC50 responses for all knockout cell lines tested demonstrates a clear segregation of cell lines lacking both caspase-3 and -7, with no obvious effect for additional loss of caspase-6, with the exception of the necroptotic death pathway in Jurkat cells (Fig. 3K).
To confirm whether caspase-3 and -7–deficient cells display long-term viability after apoptotic stimulation, we performed washout experiments to remove Bir/TNF or ABT263 from cultures. After 24 hours, we observed sustained viability of casp3/7−/− cells, as they were able to reach similar cell density compared to untreated WT cells after 1 to 4 days (fig. S2E). Furthermore, we also performed a colony-forming unit assay in methylcellulose to determine the number of viable colonies within WT or caspase-3/7−/− cells after 24 to 48 hours of treatment with intrinsic or extrinsic apoptotic stimulus. In this case, we observed that the vast majority of cells remain viable in caspase-3/7 knockout cells after induction of apoptosis (Fig. 3L and fig. S2, F to H). Overall, these results show that expression of either caspase-3 or -7 is adequate for potent activation of apoptosis in response to specific extrinsic or intrinsic apoptotic stimulus, and their combined loss prevents the vast majority of apoptotic cell death.
Caspase-3 or -7 is required for full activation of apical caspases
We hypothesized that, in the absence of effector caspases, cells stimulated to undergo apoptosis might accumulate high levels of activated apical caspases. Thus, we performed a time-course Western blot analysis to investigate the relative amount of pro- and cleaved caspases at various time points after apoptosis induction. In the absence of single caspase-3, -7, or -6, we observed no significant change in the appearance of any cleaved caspases following treatment with a specific extrinsic apoptotic stimulus (Fig. 4A). These results agree with our observations in cell viability assays, wherein there were no apparent dosage effects associated with single loss of caspase-3 or -7 in terms of caspase activation or apoptotic cell death.
Fig. 4. Simultaneous absence of caspase-3 and -7 results in a significant decrease in caspase-8 and -9 activation in extrinsic apoptosis.
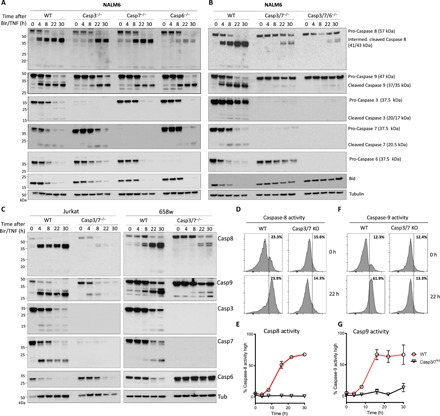
(A to C) Western blot of NALM6 wild-type (WT) and caspase-3, -7, and -6−/− cells as well as caspase-3/7−/− or caspase-3/7/6−/− cells untreated or treated with Birinapant (50 nM) and hTNF (10 ng/ml) for the indicated times. Cells were lysed and processed for Western blot. (A) Disrupting a single effector caspase-3, -7, or -6 results in no difference in caspase-8 or -9 activation. Incubation with caspase-8 antibody shows appearance of intermediate cleaved caspase-8 (41/43 kDa) and active caspase-8 (18 kDa) in WT cells and in caspase-3, -7, or -6−/−. Incubation with caspase-9 antibody shows appearance of cleaved caspase-9 (37/35 kDa) in WT cells and in caspase-3, -7, or -6−/−. Line graphs show the mean ± SEM for two repeated experiments performed in duplicate. (B) Disruption of caspase-3 and -7 simultaneously results in significant delay of the appearance of intermediate cleaved caspase-8 and no appearance of active caspase-8 as well as significant reduction and delay of cleaved caspase-9. Similar results in caspase-3/7/6−/−. (C) Jurkat WT and caspase-3/7−/− cells as well as 658w WT and caspase-3/7−/− cells show analog results. (D to G) NALM6 WT or caspase-3/7−/− cells were left untreated or treated for 22 hours with Birinapant (50 nM) and hTNF (10 ng/ml). Treated WT NALM6 cells show increased cleavage of caspase-8 (E) and caspase-9 (G) substrates over indicated time, whereas caspase-3/7−/− show no increased activity for caspase-8 and -9 substrates. Quantitation of caspase-8 and -9 activity is given (E and G), respectively. Histograms show representative data from at least two experiments performed in duplicate, with the mean from these ± SEM presented in the line graphs.
In contrast to single knockouts, we observed highly delayed and decreased activation of caspase-8 and almost no apparent activation of caspase-9 in cells lacking both caspase-3 and -7 (Fig. 4B). Similarly, decreased apical caspase activation could be detected in the 658w and Jurkat cell lines following treatment with Bir/TNF (Fig. 4C). We also observed that caspase-3/7 knockout cells show no apparent cleavage of Bid, a well-known direct substrate of caspase-8 (Fig. 4, A and B) (27). Similarly, fluorogenic caspase-8 and -9 substrates revealed a lack of apical caspase activity in caspase-3/7–deficient cells following treatment with Bir/TNF (Fig. 4, D to G). Overall, these results point to the unexpected conclusion that full activation of both the initiator and effector caspases is dependent on the presence of effector caspases within leukemia cell lines.
To pursue this conclusion, we next performed similar experiments to examine the activation of activator caspases following stimulation with the intrinsic apoptotic inducer ABT263 in caspase-3/7–deficicent cells. In this case, treatment of casp3/7−/− NALM6 cells also resulted in lower and delayed cleavage of caspase-8 and -9 (Fig. 5A) and markedly decreased caspase cleavage of fluorogenic caspase substrates (Fig. 5, B to E).
Fig. 5. Simultaneous absence of caspase-3 and -7 is required for significant decrease of caspase-8 and -9 activation in intrinsic apoptosis.
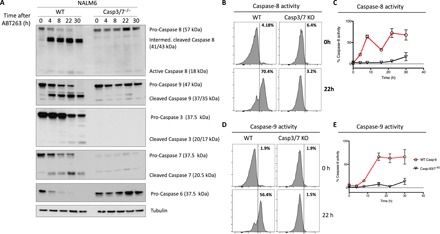
(A) NALM6 WT or caspase-3/7−/− cells were left untreated or treated for the indicated time with ABT263 5 μM. Cells were lysed and processed for Western blot. (B to E) NALM6 WT or caspase-3/7−/− cells were left untreated or treated 22 hours with ABT263 at a concentration of 5 μM. WT NALM6 cells treated with ABT263 show metabolic activity for caspase-8 (B and C) and caspase-9 (D and E) substrates. Caspase-3/7−/− cells show no increased metabolic activity for caspase-8 (B and C) and caspase-9 (D and E) compared to untreated cells. Quantitation of caspase-8 and -9 activity, respectively. Histograms show representative data from at least two independent experiments performed in duplicate, with the mean from these ± SEM presented in the line graphs.
Together, these results support a model wherein either caspase-3 or -7 can provide essential feedback signaling upon apical caspases, driving cells to full apoptotic cell death.
Caspase-3 or -7 activity is required for mitochondrial depolarization and cytochrome c release
We lastly wished to investigate a canonical upstream marker of apoptosis within caspase-3/7–deficient cells, mitochondrial outer membrane permeabilization. As measured by TMRE staining and flow cytometry, treatment of WT NALM6 cells with ABT263 results in a complete loss of mitochondrial membrane potential within 24 hours, whereas caspase-3/7 knockout cells remained mostly TMREhigh (Fig. 6, A and B). Casp-3/7−/− cells did exhibit a consistent partial loss of TMRE signal at 24 hours after treatment with ABT263.
Fig. 6. Caspase-3– and -7–deficient cells maintain mitochondrial membrane polarity following intrinsic or extrinsic apoptotic stimuli.
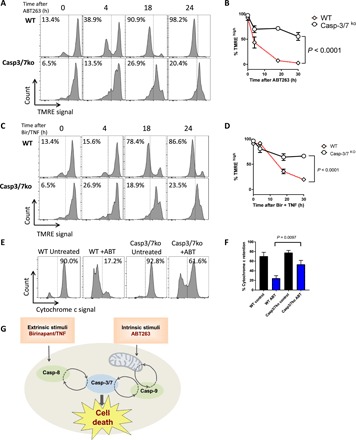
(A) TMRE staining and (B) quantification of mean TMRE signal in NALM6 WT and caspase-3/7 knockout cells treated with ABT263 (5 μM) for the indicated time points. TMRE flow cytometry measurements (A and C) and quantification plot (B and D) of NALM6 WT and caspase-3/7 knockout cells treated with Birinapant (50 nM) and TNF (10 ng/ml) for the indicated time points. Cytochrome c release measurement by flow cytometry (E) and its corresponding quantification graph (F) of NALM6 WT and caspase-3/7 knockout cells untreated (control) or treated with ABT263 (5 μM). Flow cytometry graphs are representative images of three independent experiments. Quantification graphs show the mean from three independent experiments ± SEM. (G) Graphical model of feedback activation by caspase-3 and -7 upon apoptotic stimulation. Histograms show representative data from at least three independent experiments performed in duplicate, with the mean from these ± SEM presented in the line or bar graphs.
Similar to our earlier observations of the lock-step activation of both intrinsic and extrinsic apoptotic caspases, mitochondrial depolarization occurred in WT NALM6 cells following treatment with the extrinsic apoptotic stimulus Bir/TNF (Fig. 6, C and D). Similar to intrinsic stimulation with ABT263, we only observed a partially depolarized phenotype in caspase-3/7–deficient cells following treatment with Bir/TNF (Fig. 6, C and D).
To functionally demonstrate the feedback control effect of caspase-3 and -7 on mitochondrial membrane integrity, we also analyzed cytochrome c release upon intrinsic apoptosis activation in WT and casp-3/7−/− cells. As anticipated on the basis of on our TMRE analyses, leukemic cells deficient for caspase-3 and -7 showed a marked decrease in cytochrome c release compared to WT controls upon treatment with ABT263 (Fig. 6, E and F). These results indicate that, in the absence of caspase-3 and -7, intrinsic or extrinsic apoptotic stimuli do indeed result in a shift in mitochondrial polarization and limited release of cytochrome c, but a new stable mitochondrial membrane state can be reached in the absence of feedback signaling provided by effector caspases.
DISCUSSION
As a process, apoptosis integrates a multitude of pro– and anti–cell death signals to a single decision for life or death. This type of “all-or-none” response to quantitative inputs is common in biological phenomena such as cell proliferation and differentiation and is often associated with a network topology containing positive feedback loops (28). While the ability of caspases to self-cleave provides one level of such feedback activity (17), we find that this feedback extends to cross activation between both the extrinsic and intrinsic pathways, with specific activation of either extrinsic or intrinsic apoptosis leading to near simultaneous activation of both caspase-8 and -9 in all apoptotic cells tested in this experiment. This supports a model wherein, although initiation of caspases is segregated by distinct pathways, an apoptotic signal, once having reached a threshold level, leads to a simultaneous activation across both arms of the apoptotic network. Several signaling mechanisms connecting intrinsic and extrinsic apoptotic pathways have been previously identified such as Bid cleavage by caspase-8, which can lead to intrinsic mitochondrial apoptotic signaling (29), and the secondary mitochondrial activator of caspases that blocks cIAP function to greatly increase extrinsic apoptotic death receptor signaling (30, 31). Nonetheless, we find it curious that in those cells tested here, caspase-8 and -9 activation occurs consistently in complete lock step, and we would question whether detectable caspase-8 or -9 activation within cells is more often a function of feedback activation rather than primary induction by specific intrinsic or extrinsic mechanisms.
The timing of caspase-8 and -9 activation was also coordinated with progressive conversion of effector caspase-3, -7, and -6 from their pro to active forms. The specific role of these individual effector caspases in apoptosis has thus far remained unclear; in particular, the individual functional contribution of different effector caspases has not been investigated within the context of living human cells. Although previous evidence has suggested redundant function between caspase-3 and -7 in knockout healthy mice (16), we still questioned whether these enzymes would function in an additive and/or redundant capacity and what the dosage effects of single loss of effector caspases would be in a human and disease context. CRISPR-mediated gene editing, which enables precision inactivation of single or multiple genes simultaneously, allows us to address this point in human cancerous cells. We find here that complete removal of either caspase-3, -7, or -6 resulted in no significant difference in response to specific inducers of extrinsic and intrinsic apoptosis. These results indicate that the dose of effector caspase expression may be at least somewhat irrelevant to the apoptotic decision, and the presence of either caspase-3 or -7 is adequate to fulfill their role in apoptotic cell death within human leukemic cell lines. Our protein expression analysis also did not indicate compensatory increases in caspase expression when a single-effector caspase was genetically deleted.
In contrast to our observations with single-knockout cell lines, leukemic cells deficient in both caspase-3 and -7 showed profound resistance to both extrinsic and intrinsic apoptotic stimuli. While differences in the substrate targets of caspase-3 and -7 could hypothetically result in differential apoptotic activation under some contexts, we find that, in response to acute apoptotic stimulation of human leukemic cells, either caspase-3 or -7 is adequate for robust apoptotic cell death. Consistent with previous observations (32), loss of caspase-6 expression seemed to be irrelevant to apoptosis in this context. Caspase-3 and -7 are found on separate chromosomes in the human genome, whereas caspase-6 is found on the same arm of chromosome 4 as caspase-3; this could perhaps point to evolutionary pressure to genomically segregate redundant and essential caspase genes and may further explain why complete loss of effector caspases is rarely seen in cancer cells.
Upon apoptotic stimulation of our caspase-3/7 double-knockout cell lines, we also observed a strong decrease in apical caspase-8 and -9 activation. This adds to a growing body of evidence to indicate that effector caspase feedback increases apical caspase activation. Cell-free systems have shown significantly reduced caspase-9 cleavage in the absence of caspase-3 (18, 33), although caspase-8 seems to be less sensitive to a caspase-3 activity in a similar system (13). Direct cleavage of upstream caspases by caspase-3 or 7 does not only necessarily result in activation, particularly in the context of caspase-9 but also involves general disinhibition of caspases through effector caspase-mediated cleavage of the endogenous caspase inhibitor XIAP (12, 34, 35). Whether through direct cleavage or indirectly through deactivation of inhibitory molecules, the data presented here clearly demonstrate that caspase-3 and -7 act as essential and redundant signal amplifiers of both apoptotic programs within intact leukemia cells. This corroborates earlier data in normal hematopoiesis obtained in mice (36), where deletion of caspase-3 and -7 results in some defects in the hematopoietic system (16, 37) and where absence of caspase-9 leads to severely affected hematopoietic stem cell function (38, 39) and confers resistance to infection by RNA and DNA viruses (40).
Mitochondrial outer membrane permeabilization is a microcosm of the larger apoptotic program, in that it directly disrupts the cellular process of mitochondrial energy production while also providing a signal to increase apoptotic signaling through release of cytochrome c (41). In the absence of caspase-9 or -3/7 expression, we observed that cells were able to maintain mitochondrial membrane potential and show limited cytochrome c release after treatment with direct inhibitors of BCL2. Similar results were previously shown using caspase-3/7 double-knockout mouse embryonic fibroblasts (16). Similar to previous observations (42), we also saw a consistent decrease in mitochondrial polarization following apoptotic induction in caspase-3/7–deficient cells, suggesting that in the absence of effector caspase feedback, caspase-9 and BCL2 family proteins will reach a new equilibrium state without triggering full depolarization. Similarly, partial cytoplasmic cytochrome c following ABT263 treatment has also been observed in caspase-9–deficient cells (22). Such feedback control of mitochondrial activity will have to be taken into account when interpreting BH3 profiling results and apoptotic dependencies (43). These data support a model wherein feedback regulation lies at the heart of the apoptotic program, wherein caspase-9 and casp-3/7–dependent cleavage of antiapoptotic BCL2 family members enhances mitochondrial depolarization and release of cytochrome c (15, 23, 24).
Here, we have provided a systematic deconstruction of the caspase network in human leukemic cell lines. We show how caspase-3 and -7 specifically function as a redundant central node in this network, without which cells fail to fully activate upstream elements of the apoptotic process. Ultimately, it is these interconnected feedback loops that integrate pro- and antiapoptotic signals into the all-or-none apoptotic switch and regulate the cellular response to perturbations of cellular homeostasis (Fig. 6G). Thus, effector caspase-3 and -7 are crucial and redundant amplifiers of the apoptotic signal, where the presence of either one is sufficient for full activation of both upstream and downstream mechanisms of the apoptotic program. Overall, these results extend a significant body of work identifying feedback mechanisms within the apoptotic program, primarily conducted in mouse cells, frequently also using nonspecific chemical inhibitors, and/or in acellular systems. Bringing this into living human cells, our data place caspase-3 and -7 at the center of a fundamentally recursive apoptotic program, where these enzymes act as redundant signal amplifiers, essential for activation of both upstream and downstream apoptotic processes and efficient cell death.
MATERIALS AND METHODS
LC knockout of caspases
Single and multigene knockouts were generated using the multicolor LC system, which we have previously described (21). Briefly, sgRNA sequences were cloned into multicolor LC vectors via one-pot restriction-ligation reaction using Esp3I restriction enzyme (catalog no. ER0451; Thermo Fisher Scientific). Various sgRNA sequences were tested to identify specific sequences that produced a consistent knockout as examined via Western blot. The following sgRNA sequences, including PAM (protospacer adjacent motif), were chosen for CRISPR targeting: caspase-3, GGAAGCGAATCAATGGACTCTGG; caspase-7, GAAGCACTTGAAGAGCGCCTCGG; caspase-6, AAGATTGTCTCTATCTGCGCAGG; caspase-8, TGATCGACCCTCCGCCAGAAAGG; caspase-9, GTTCAGGCCCCATATGATCGAGG. Target cells were then transduced with specific LC vectors at a multiplicity of infection of ~0.1 and single cell–sorted to produce single- or multi-knockout cell lines as desired. Single-cell clones were screened via Western blotting for desired gene knockouts.
Cell culture
Cell lines used in this study include standard human T cell leukemia (Jurkat), human B cell leukemia (NALM6) cell lines, and a patient-derived B cell leukemia cell line that we have previously reported [658w, (21)]. All cell lines were cultured in RPMI 1640 medium (catalog no. R0883; Sigma-Aldrich) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 0.5% l-glutamine (BioConcept), and 0.5% penicillin/streptomycin (Life Technologies) and incubated at 37° until colonies reached a population size of about 5 × 105 cells/ml. Cells were passaged every 3 days by dilution of the cells to a concentration of around 1 × 105 cells/ml.
Western blot
Cells were harvested and lysed using SDS lysis buffer [62.5 nM tris (pH 6.8), 1% SDS, 0.005% bromophenol blue, 4% glycerol, and 1% (v/v) ß-mercaptoethanol]. Samples were then vortexed and incubated at 95° for 5 min. Lysates were normalized to cell number and subjected to SDS polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and blocked with buffer consisting of TBS (tris-buffered saline)–T with 5% fat-free milk powder. The membranes were then incubated with primary antibodies against caspase-3, -7, -6, -8, or -9 (catalog nos. 9662S, 9492S, 9762S, 9746S, or 9502S, respectively; Cell Signaling), cIAP (catalog no. 3130; Cell Signaling), Bid (catalog no. 2002S; Cell Signaling), or tubulin (catalog no. 081 M4861, 1:1000; Sigma-Aldrich). After washing the membranes with TBS-T at room temperature, we then added the secondary antibodies (1:5000) EasyBlot anti-rabbit or anti-mouse (catalog nos. GTX221666-01 and GTX221667-01, respectively; GeneTex). The reactive bands were visualized by chemiluminescence and captured using Imager Lab.
Cell viability
To quantify the number of living cells, we used Cell Counting Kit-8 (catalog no. CK04-11, 1:10; Dojindo Molecular Technologies), containing WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt]. WST-8 was bioreduced by cellular dehydrogenases to an orange-colored dye that is soluble in tissue culture medium and is directly proportional to the number of living cells. Suspension was incubated for 1 hour at 37°. Viability was quantified by measuring the absorbance of color (450 nm) at the spectrophotometer and normalizing the data to the untreated cells. We also measured cell viability and membrane integrity with 7-AAD viability staining solution (catalog no. 559925; BD Pharmingen). Briefly, cell pellet was resuspended in 0.5 ml of cell staining buffer, and then 5 μl of 7-AAD per million cells was added and incubated for 5 to 10 min in the dark before flow cytometry analysis.
Fluorogenic substrates
Caspase-3/7 as well as caspase-8 and -9 activity was measured using flow cytometry. Briefly, cells were cultured and plated in a concentration of 200,000 cells/ml. We then treated the cells either with ABT263 (5 μM) (intrinsic apoptotic stimulus) or Birinapant (50 nM) and hTNF (10 ng/ml) (B + T) (extrinsic apoptotic stimulus) for 1 to 30 hours. The cell suspension was centrifugated and resuspended in RPMI 1640 to stop the reaction. Using FAM-FLICA Caspase Assay Kit for caspase-8 (catalog no. 910; ImmunoChemistry Technologies), for caspase-9 (catalog no. 912; ImmunoChemistry Technologies), and for caspase-3/7 (catalog no. 93; ImmunoChemistry Technologies), we incubated the cells in 1:30 of 1:5-diluted FLICA staining buffer for 30 min protected from light. Fluorescence was detected by flow cytometry (FAM-FLICA excites at 492 nm and emits at 520 nm).
Measuring mitochondrial depolarization
For the detection of mitochondrial membrane potential changes, NALM6 WT and caspase-3/7−/− cells were plated on a 96-well plate, 30,000 cells/100 μl per well. Cells were then treated with 5 μM ABT263 and 50 nM Birinapant with hTNF (10 ng/ml) for 4, 18, and 24 hours. Cells were stained with 50 nM tetramethylrhodamine ethyl-esther (catalog no. 87917; Sigma-Aldrich) for 5 to 20 min, incubated at 37°C and 0.5% CO2, and shielded from light. Then, they were transferred to fluorescence-activated cell sorting tubes and diluted to 300 μl with 1× phosphate-buffered saline (PBS) and analyzed with flow cytometry.
Cytochrome c release
Cytochrome c release was assessed as previously described (44). Cells were incubated in 1:200 mouse anti–cytochrome c antibody (catalog no. 556432; BD Pharmingen) and 1:200 PE Rat anti-mouse IgG1 (immunoglobulin G1) secondary antibody (catalog no. 550083; BD Pharmingen).
Drugs and chemicals
Recombinant human TNFα was purchased from Gibco/Life Technologies (catalog no. PHC3011). TNFα-neutralizing antibody was purchased from Cell Signaling (catalog no. 7321). Birinapant was purchased from Selleckchem (catalog no. S7015). ABT263 (Navitoclax) was purchased from Selleckchem (catalog no. S1001). Z-VAD-FMK was purchased from ApexBio (catalog no. A1902). Necrostatin-1 was purchased from BioVision (catalog no. 2263-1). FasL was purchased from Enzo Life Sciences (catalog no. ALX-522-001-C010).
Colony-forming unit assay
Nalm6, Jurkat, and 658w WT and casp-3/7−/− cells were plated in 3 ml of RPMI 1640 complete medium in six-well plates at a concentration of 2 × 105 cells per well. The cells were then treated for 24 to 48 hours with either Birinapant (50 nM) and TNF (10 ng/ml), ABT263 (5 mM), or vehicle. The cells were then collected, washed with PBS, mixed with Methocult Optimum media (catalog no. 4034; StemCell Technologies), and plated at a concentration of 1000 cells per well in six-well SmartDish plates (catalog no. 27370; StemCell Technologies). Cells were aliquoted in duplicate samples. After 10 to 14 days, colony-forming units were visually counted under a microscope.
Supplementary Material
Acknowledgments
Funding: This work was supported by the “Stiftung Kinderkrebsforschung Schweiz,” the MAM-Fonds of the Children’s Research Centre of the University Children’s Hospital Zurich, the Empiris Foundation, the clinical research focus program “human hemato-lymphatic diseases” of the University of Zurich, the Swiss Cancer League (KFS 3609-02-2015 and KFS-4384-02-2018), the Novartis Foundation for Biomedical Research, the Swiss National Science Foundation (SNF) (310030-133108), the Canadian Institutes of Health Research (CIHR), the Iten-Kohaut Stiftung, and the Fondation Panacée. Author contributions: S.M. and B.C.B. designed the research. S.M., P.K.C., A.G., H.H., S.J., and M.P.D. performed the experiments. J.-P.B. analyzed data. S.M., A.G., and B.C.B. wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The CRISPR plasmids can be provided by B.C.B. pending scientific review and a completed material transfer agreement. Requests for the CRISPR plasmids should be submitted to B.C.B. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/7/eaau9433/DC1
Fig. S1. Caspase-8 and -9 are specifically required to activate extrinsic and intrinsic apoptosis, respectively.
Fig. S2. Caspase-3 and -7 have overlapping roles in executing apoptotic cell death.
REFERENCES AND NOTES
- 1.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Tummers B., Green D. R., Caspase-8; regulating life and death. Immunol. Rev. 277, 76–89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller N., Ozmadenci D., Ichim G., Stupack D., Caspase-8 function, and phosphorylation, in cell migration. Semin. Cell Dev. Biol. 82, 105–117 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R., The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell 75, 641–652 (1993). [DOI] [PubMed] [Google Scholar]
- 5.McIlwain D. R., Berger T., Mak T. W., Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 5, a008656 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait S. W. G., Green D. R., Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Ramirez M. L. G., Salvesen G. S., A primer on caspase mechanisms. Semin. Cell Dev. Biol. 82, 79–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slee E. A., Harte M. T., Kluck R. M., Wolf B. B., Casiano C. A., Newmeyer D. D., Wang H.-G., Reed J. C., Nicholson D. W., Alnemri E. S., Green D. R., Martin S. J., Ordering the Cytochrome c–initiated Caspase Cascade: Hierarchical Activation of Caspases-2, −3, −6, −7, −8, and −10 in a Caspase-9–dependent Manner. J. Cell Biol. 144, 281–292 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer U., Jänicke R. U., Schulze-Osthoff K., Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 10, 76–100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S., A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391, 43–50 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Eissing T., Conzelmann H., Gilles E. D., Allgöwer F., Bullinger E., Scheurich P., Bistability analyses of a caspase activation model for receptor-induced apoptosis. J. Biol. Chem. 279, 36892–36897 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Ferreira K. S., Kreutz C., MacNelly S., Neubert K., Haber A., Bogyo M., Timmer J., Borner C., Caspase-3 feeds back on caspase-8, Bid and XIAP in type I Fas signaling in primary mouse hepatocytes. Apoptosis 17, 503–515 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Yang S., Thor A. D., Edgerton S., Yang X., Caspase-3 mediated feedback activation of apical caspases in doxorubicin and TNF-α induced apoptosis. Apoptosis 11, 1987–1997 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Van de Craen M., Declercq W., Van den brande I., Fiers W., Vandenabeele P., The proteolytic procaspase activation network: An in vitro analysis. Cell Death Differ. 6, 1117–1124 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Kirsch D. G., Doseff A., Chau B. N., Lim D.-S., de Souza-Pinto N. C., Hansford R., Kastan M. B., Lazebnik Y. A., Hardwick J. M., Caspase-3-dependent Cleavage of Bcl-2 Promotes Release of Cytochrome c. J. Biol. Chem. 274, 21155–21161 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Lakhani S. A., Masud A., Kuida K., Porter G. A. Jr., Booth C. J., Mehal W. Z., Inayat I., Flavell R. A., Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang H. Y., Yang X., Proteases for cell suicide: Functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64, 821–846 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh J. G., Cullen S. P., Sheridan C., Lüthi A. U., Gerner C., Martin S. J., Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. U.S.A. 105, 12815–12819 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamkanfi M., Moreira L. O., Makena P., Spierings D. C. J., Boyd K., Murray P. J., Green D. R., Kanneganti T.-D., Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood 113, 2742–2745 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson M., Zhivotovsky B., Caspases and cancer. Cell Death Differ. 18, 1441–1449 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McComb S., Aguadé-Gorgorió J., Harder L., Marovca B., Cario G., Eckert C., Schrappe M., Stanulla M., von Stackelberg A., Bourquin J. P., Bornhauser B. C., Activation of concurrent apoptosis and necroptosis by SMAC mimetics for the treatment of refractory and relapsed ALL. Sci. Transl. Med. 8, 339ra70 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Samraj A. K., Sohn D., Schulze-Osthoff K., Schmitz I., Loss of Caspase-9 Reveals Its Essential Role for Caspase-2 Activation and Mitochondrial Membrane Depolarization. Mol. Biol. Cell 18, 84–93 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerrero A. D., Schmitz I., Chen M., Wang J., Promotion of Caspase Activation by Caspase-9-mediated Feedback Amplification of Mitochondrial Damage. J. Clin. Cell. Immunol. 3, 1000126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M., Guerrero A. D., Huang L., Shabier Z., Pan M., Tan T.-H., Wang J., Caspase-9-induced Mitochondrial Disruption through Cleavage of Anti-apoptotic BCL-2 Family Members. J. Biol. Chem. 282, 33888–33895 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Behrmann L., McComb S., Aguadé-Gorgorió J., Huang Y., Hermann M., Pelczar P., Aguzzi A., Bourquin J., Bornhauser B. C., Efficient Generation of Multi-gene Knockout Cell Lines and Patient-derived Xenografts Using Multi-colored Lenti-CRISPR-Cas9. Bio Protoc. 7, e2222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercammen D., Brouckaert G., Denecker G., Van de Craen M., Declercq W., Fiers W., Vandenabeele P., Dual signaling of the fas receptor: Initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188, 919–930 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantari C., Walczak H., Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 1813, 558–563 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q., Bhattacharya S., Andersen M. E., Ultrasensitive response motifs: Basic amplifiers in molecular signalling networks. Open Biol. 3, 130031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Zhu H., Xu C.-J., Yuan J., Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Verhagen A. M., Ekert P. G., Pakusch M., Silke J., Connolly L. M., Reid G. E., Moritz R. L., Simpson R. J., Vaux D. L., Identification of DIABLO, a Mammalian Protein that Promotes Apoptosis by Binding to and Antagonizing IAP Proteins. Cell 102, 43–53 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Du C., Fang M., Li Y., Li L., Wang X., Smac, a Mitochondrial Protein that Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell 102, 33–42 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Klaiman G., Champagne N., LeBlanc A. C., Self-activation of Caspase-6 in vitro and in vivo: Caspase-6 activation does not induce cell death in HEK293T cells. Biochim. Biophys. Acta 1793, 592–601 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Zou H., Yang R., Hao J., Wang J., Sun C., Fesik S. W., Wu J. C., Tomaselli K. J., Armstrong R. C., Regulation of the Apaf-1/Caspase-9 Apoptosome by Caspase-3 and XIAP. J. Biol. Chem. 278, 8091–8098 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Denault J.-B., Eckelman B. P., Shin H., Pop C., Salvesen G. S., Caspase 3 attenuates XIAP (X-linked inhibitor of apoptosis protein)–mediated inhibition of caspase 9. Biochem. J. 405, 11–19 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fakler M., Loeder S., Vogler M., Schneider K., Jeremias I., Debatin K. M., Fulda S., Small molecule XIAP inhibitors cooperate with TRAIL to induce apoptosis in childhood acute leukemia cells and overcome Bcl-2-mediated resistance. Blood 113, 1710–1722 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Marsden V. S., O’Connor L., O’Reilly L. A., Silke J., Metcalf D., Ekert P. G., Huang D. C. S., Cecconi F., Kuida K., Tomaselli K. J., Roy S., Nicholson D. W., Vaux D. L., Bouillet P., Adams J. M., Strasser A., Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419, 634–637 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Janzen V., Fleming H. E., Riedt T., Karlsson G., Riese M. J., Celso C. L., Reynolds G., Milne C. D., Paige C. J., Karlsson S., Woo M., Scadden D. T., Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell Stem Cell 2, 584–594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu E. P., McLellan M., Ding L., Fulton R., Mardis E. R., Wilson R. K., Miller C. A., Westervelt P., DiPersio J. F., Link D. C., Walter M. J., Ley T. J., Graubert T. A., Caspase-9 is required for normal hematopoietic development and protection from alkylator-induced DNA damage in mice. Blood 124, 3887–3895 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White M. J., McArthur K., Metcalf D., Lane R. M., Cambier J. C., Herold M. J., van Delft M. F., Bedoui S., Lessene G., Ritchie M. E., Huang D. C. S., Kile B. T., Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rongvaux A., McArthur K., Metcalf D., Lane R. M., Cambier J. C., Herold M. J., van Delft M. F., Bedoui S., Lessene G., Ritchie M. E., Huang D. C. S., Kile B. T., Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chipuk J. E., Bouchier-Hayes L., Green D. R., Mitochondrial outer membrane permeabilization during apoptosis: The innocent bystander scenario. Cell Death Differ. 13, 1396–1402 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Cepero E., King A. M., Coffey L. M., Perez R. G., Boise L. H., Caspase-9 and effector caspases have sequential and distinct effects on mitochondria. Oncogene 24, 6354–6366 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Sarosiek K. A., Fraser C., Muthalagu N., Bhola P. D., Chang W., McBrayer S. K., Cantlon A., Fisch S., Golomb-Mello G., Ryan J. A., Deng J., Jian B., Corbett C., Goldenberg M., Madsen J. R., Liao R., Walsh D., Sedivy J., Murphy D. J., Carrasco D. R., Robinson S., Moslehi J., Letai A., Developmental Regulation of Mitochondrial Apoptosis by c-Myc Governs Age- and Tissue-Specific Sensitivity to Cancer Therapeutics. Cancer Cell 31, 142–156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterhouse N. J., Trapani J. A., A new quantitative assay for cytochrome c release in apoptotic cells. Cell Death Differ. 10, 853–855 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/7/eaau9433/DC1
Fig. S1. Caspase-8 and -9 are specifically required to activate extrinsic and intrinsic apoptosis, respectively.
Fig. S2. Caspase-3 and -7 have overlapping roles in executing apoptotic cell death.


