ABSTRACT
Background
Maternal nutrition and genetics are determinants of breast-milk nutrient composition and, as such, are determinants of the nutritional exposure of breastfed infants.
Objectives
The aim of this study was to determine whether common maternal single nucleotide polymorphisms (SNPs) in folate-dependent enzymes are associated with breast-milk folate content in a cohort of mothers enrolled in the Maternal–Infant Research on Environmental Chemicals (MIREC) study.
Methods
The MIREC study is a Canadian prospective pregnancy cohort study that recruited 2001 participants between 2008 and 2011. Five folate-related SNPs—MTHFR 677C>T (rs1801133), MTHFR 1298A>C (rs1801131), MTHFR 1793G>A (rs2274976), MTR 2756A>G (rs1805087), and MTRR 66A>G (rs1801394)—were genotyped. Breast milk was sampled ∼1 mo postpartum, and tetrahydrofolate (THF), 5-methyl-THF, 5-formyl-THF, 5,10-methenyl-THF, and unmetabolized folic acid (UMFA) were measured using liquid chromatography–tandem mass spectrometry in a subset of participants (n = 551). Associations were assessed using Wald's test. Associations were considered significant if P ≤ 0.01 (Bonferroni correction for multiple testing).
Results
None of the SNPs were associated with total breast-milk folate. However, the MTHFR 677C>T SNP was associated with breast-milk UMFA (R2 = 0.01; unadjusted P = 0.004), explaining a small portion of total variance; this association remained significant when adjusted for other covariates, including supplemental folic acid consumption. The MTHFR 1793G>A and MTRR 66A>G SNPs tended to be associated with 5-methyl-THF (R2 = 0.008, P = 0.04) and reduced folates (THF + 5-methyl-THF + 5-formyl-THF + 5,10-methenyl-THF; R2 = 0.01, P = 0.02), respectively.
Conclusions
We found that total breast-milk folate content was not associated with any of the folate-related SNPs examined. The association between the MTHFR 677C>T SNP and breast-milk UMFA, albeit modest, highlights the need to better understand the determinants of breast-milk folate and the impact they might have on milk folate bioavailability.
Keywords: folate, unmetabolized folic acid, breast milk, supplements, methylenetetrahydrofolate reductase, methionine synthase, methionine synthase reductase
Introduction
Exclusively breastfed infants are unique in that in addition to their own genetics, physiology, and metabolism, their nutritional exposure is dependent on maternal factors. Although it is accepted that both maternal nutrition and genetics influence the composition of breast milk, only a limited number of studies have examined these relations (1). Several single nucleotide polymorphisms (SNPs) in the genes of folate-dependent enzymes modify folate requirements and metabolism, but whether or how these SNPs influence breast-milk folate content is unknown. Although genetic variants in folate-dependent enzymes may or may not change the total folate content of breast milk, which is highly regulated, they could alter the relative proportions of specific folate vitamers. Given that not all folate vitamers are equally bioavailable, genetic polymorphisms could modify the bioavailability of folates in breast milk and, by extension, the folate exposure of breastfed infants.
Several SNPs in the MTHFR, MTR, and MTRR genes are associated with altered folate metabolism and status biomarkers. Methylenetetrahydrofolate reductase (MTHFR) irreversibly reduces 5,10-methylenetetrahydrofolate (5,10-methylene-THF) to 5-methyl-THF, the main circulating folate vitamer and a major folate constituent in breast milk (2–5). There are a number of common SNPs in the MTHFR gene, including 677C>T (rs1801133), 1298A>C (rs1801131), and 1793G>A (rs2274976). The MTHFR 677C>T SNP results in a thermolabile protein with 35% and 70% lower enzymatic activity in heterozygotes and homozygotes, respectively (6). The MTHFR 677C>T TT genotype is associated with lower plasma and red blood cell (RBC) folate, higher plasma homocysteine, and altered RBC folate vitamer distribution, such that 5-methyl-THF is lower and nonmethylated (formylated) folates are higher (7–11). Homozygosity for the MTHFR 1298A>C SNP is associated with lower enzymatic activity, although the enzyme is not thermolabile and this SNP is in linkage disequilibrium (LD) with the MTHFR 677C>T SNP (12, 13). The MTHFR 1793G>A SNP results in a missense mutation in the regulatory domain of the protein, suggesting it could affect enzyme activity (14–16).
Methionine synthase (MTR) catalyzes the remethylation of homocysteine to form methionine using 5-methyl-THF as the methyl donor. The MTR 2756A>G SNP (rs1805087) is a missense mutation in the domain required for vitamin B-12 (cobalamin) cofactor methylation; it is associated with lower folate and higher homocysteine concentrations, albeit inconsistently (17, 18). Methionine synthase reductase (MTRR) reduces the inactive cobalamin–MTR complex into the active complex (19). The MTRR 66A>G SNP (rs1801394) results in a less efficient reductive repair of the inactive MTR enzyme, possibly due to a reduced affinity for MTR; the SNP is a modest determinant of elevated homocysteine (17, 20, 21).
We previously reported that supplemental folic acid (FA) intake above the recommended 400 µg FA/d is a determinant of folate vitamer distribution and the presence of unmetabolized folic acid (UMFA) in breast milk in a cohort of mothers enrolled in the Maternal–Infant Research on Environmental Chemicals (MIREC) study (3, 22). Given the effects of common SNPs in folate-dependent enzymes on 1-carbon metabolism, we hypothesized that they could also influence folate vitamer concentrations in breast milk.
Methods
Ethics
The MIREC study was approved by the Research Ethics Boards of Health Canada, Ste-Justine's Hospital in Montreal, and the academic and hospital ethics committees of the 10 study sites throughout Canada (22). All participants provided informed consent upon enrolment.
Subjects
The MIREC study is a national-level, multiyear cohort study that recruited women between February 2008 and March 2011 from 10 sites located in Vancouver, Edmonton, Winnipeg, Sudbury, Ottawa, Kingston, Hamilton, Toronto, Montreal, and Halifax (22). Eligibility criteria included ability to consent and to communicate in English or French, age ≥18 y, <14 wk gestation, and willing to provide a sample of cord blood and planning on delivering at a local hospital. Women with a specific medical history, described in Arbuckle et al. (22), were excluded from the study. Of the 2001 women who consented, of whom 18 withdrew, 1385 completed visit 6 (2–10 wk postpartum), and 1017 provided a milk sample at that time (Figure 1). Of those milk samples, 561 were allocated proportionally for folate analysis based on maternal age (<30 and ≥30 y), parity (primiparous and multiparous), and region (Maritimes, Quebec, Ontario, Prairies, and British Columbia) (3). Of the 561 women with milk folate analysis, 551 had genotyping data for at least 1 SNP of interest.
FIGURE 1.
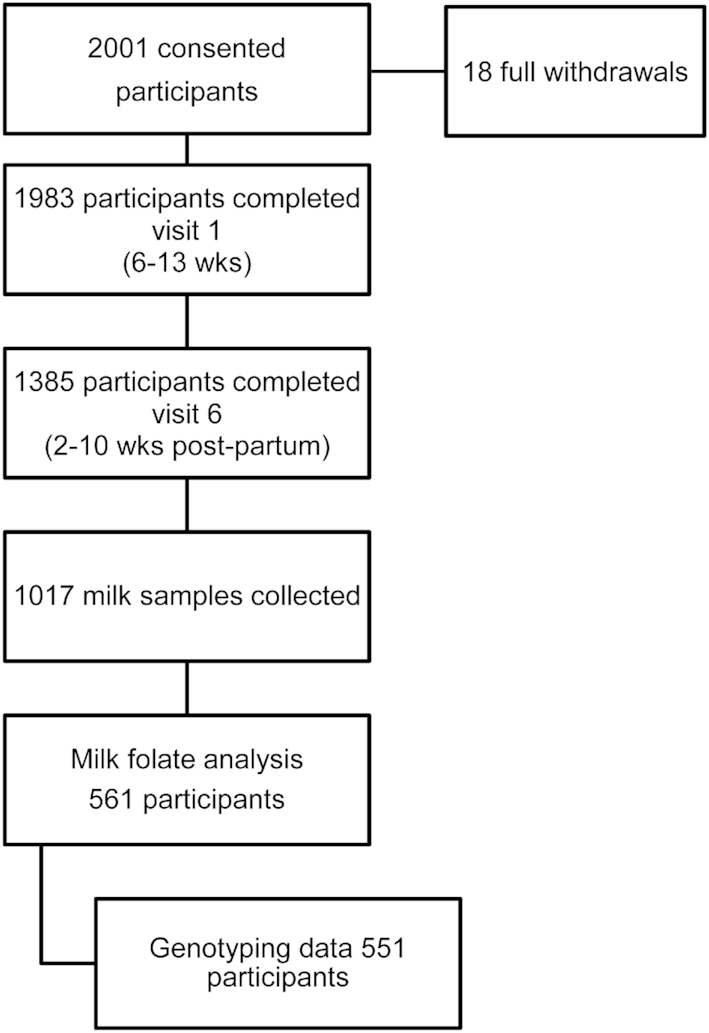
Study flowchart.
Maternal characteristics of interest were age, time from parturition to breast-milk sampling, education, household income, FA-supplement use and dose, milk type (foremilk, hindmilk, or both), time of day of collection (morning, afternoon, evening, overnight, or combined), duration of sample storage at −20°C to −38°C, duration of sample storage at −80°C, and breast-milk folates. Maternal education was defined as less than a postsecondary education; college/trade school diploma, or undergraduate degree; and a graduate degree. Annual household income was defined as lower income (<$60,000), which comprised the bottom quartile, and middle-high income (≥$60,000), which comprised the upper 3 quartiles. Milk type was defined as foremilk, hindmilk, or both when the mother combined foremilk and hindmilk. The time of day the milk was collected was defined as morning, afternoon, evening, overnight, or combined when more than 1 period was indicated by the mother.
Identification of FA-supplement users and determination of total daily FA dose
FA-supplement users were defined as previously described (3). At the time of milk sampling, participants were queried about supplement and medication intake in the past 30 d. Participants were asked to provide the name and description of the product, the drug identification number on the bottle (drug identification number or natural product number), the amount taken each time (number of pills, tablets, capsules, teaspoons, etc.), and the frequency. A supplement user was defined as someone who consumed FA in the form of a multi- or single-vitamin supplement. A total of 78 unique vitamin supplements containing FA were identified by the participants. The FA content and recommended daily intake for each product were obtained from the Health Canada Licensed Natural Health Products Database (23). For products that were not present in the database, the FA content and recommended daily intake were identified on the manufacturer's website. If the participant indicated a brand but not the specific product, the mean FA content for all prenatal supplements from that manufacturer was used (n = 8 participants). For vitamin supplements that were not found in the Licensed Natural Health Products Database, or if the manufacturer's website could not be found, the mean FA content for all supplement products identified in the sample was used to estimate the total daily FA intake (786 µg FA; n = 8 participants). When the participant did not indicate the frequency or number of pills consumed, the daily intake recommended by the manufacturer was assumed (n = 9 participants). The FA content from all vitamin supplements was summed to calculate total daily FA intake from supplements for each participant.
Milk collection, handling, and storage
Mothers were asked to collect breast milk between the third and eighth week after delivery. Mothers were advised to follow their normal routine, feeding their infants or pumping milk for their infants first and to collect the last amount of milk for the study. Mothers expressed both left and right breasts, if possible, by hand or sterilized breast pump into provided sterile glass jars. Milk collection was repeated, if necessary, until 200 mL was collected. Milk was stored in a refrigerator during collection for up to 3 d or until 200 mL was achieved, at which point the jar was placed in the freezer. The sample was frozen at the end of the third day, even if the milk had not reached 200 mL. Mothers were asked to record the date; time of day (morning, 0600–1159; afternoon, 1200–1759; evening, 1800–2359; or overnight, 24:00–0559); and whether the milk was foremilk, hindmilk, or both. A research nurse collected the frozen milk sample from the participant's home. Samples were stored in a domestic freezer until shipped frozen on dry ice to the Food Research Division of Health Canada in Ottawa, Ontario.
All sample handling was performed under yellow light. Samples were stored at −38°C until aliquotting, at which point milk samples were thawed at room temperature in the dark for ∼1 h. Jars were heated to 38°C with shaking for 30 min and aliquotted into amber glass jars and stored at −80°C. Aliquots for folate analysis were shipped overnight frozen on dry ice to the Health Canada Quebec Regional Laboratory in Longueuil, Quebec, and stored at −80°C until folate analysis.
Measurement of breast-milk folate via liquid chromatography–tandem mass spectrometry
Breast-milk folates were measured with the use of liquid chromatography–tandem mass spectrometry, as previously described (3). A detailed protocol can be found in the Supplemental Methods of Page et al. (3), including limit of detection (LOD)/limit of quantification and method performance data. In brief, folates were extracted from breast milk with an acidic buffer, which was followed by a multistep enzymatic digestion using amylase, protease, and folate deconjugase from rat serum. Proteins were precipitated and the extracts purified via solid-phase extraction. Quantitation was achieved with the use of internal standards and an external standard calibration curve. Given the differences in stability for each folate, matching internal standards were included to correct for losses during sample workup as well as interconversion between folate forms. Samples were analyzed by liquid chromatography–tandem mass spectrometry in positive electrospray mode, and FA, THF, 5-methyl-THF, 5-formyl-THF, and 5,10-methenyl-THF were quantified. The sum of these folates was reported as total folate. FA and 5-methyl-THF are reported separately. Reduced folates represent the sum of THF, 5-formyl-THF, 5,10-methenyl-THF, and 5-methyl-THF. Interconversions of THF, 5-formyl-THF, and 5,10-methenyl-THF preclude them from being reported individually with certainty.
SNP genotyping
A panel of 33 SNPs related to various MIREC endpoints were selected for genotyping a priori to recruitment of women to the study. Of these, 5 common SNPs in 3 folate-related genes—MTHFR 677C>T (rs1801133), MTHFR 1298A>C (rs1801131), MTHFR 1793G>A (rs2274976), MTR 2756A>G (rs1805087), and MTRR 66A>G (rs1801394)—were genotyped. DNA was extracted from 200 μL whole-blood aliquots in 96-well format using the DNeasy blood and tissue kit (Qiagen) following the manufacturer's protocol. Double-stranded DNA concentration was assessed using the Quant-it PicoGreen assay (Invitrogen). Genotyping was performed using the Sequenom MassARRAY system (Sequenom).
Statistics
For calculating total breast-milk folate and prevalence of detectable UMFA, undetectable UMFA was treated as 0. For calculating the mean UMFA concentration of a group, UMFA samples less than the LOD (<0.9 nmol/L; n = 21) were assigned a value of one-half the LOD (0.45 nmol/L). Breast-milk folate data were skewed; therefore, data were log-transformed for statistical analysis. Descriptive data are presented as means ± SEMs or percentages. A chi-square test was used to determine differences in frequencies. Missing data were excluded from chi-square analyses. Statistical analyses for descriptive data were performed with the use of Sigmaplot 13 (Systat Software).
For the genetic association analysis, the Wald's test for quantitative traits was performed using PLINK (24). With Bonferroni correction for multiple testing, P values ≤ 0.01 were considered statistically significant. Because our previous analysis identified a number of significant covariates of breast-milk folate vitamers, we also included them in the analysis presented here. These covariates included maternal age, maternal education, time from parturition, duration of sample storage at −20°C to −38°C, duration of sample storage at −80°C, and total daily FA intake from supplements (3). We performed a 1-way ANCOVA with Holm–Sidak pairwise post hoc analysis adjusting for these variables to assess their impact on significant genotype–folate vitamer associations and those approaching significance (P < 0.05). Maternal age was not included in the ANCOVA analyses because it demonstrated a collinear relation with total daily FA intake from supplements. Models were run with all covariates included (model 1) or with only those variables found to be significant for the model for each genotype–folate vitamer to limit overspecification (model 2). In addition to total daily FA intake from supplements, model 2 for each association included duration of sample storage at −20°C to −38°C for the MTHFR 677C>T × UMFA analysis, time from parturition for the MTHFR 1793G>A × 5-methyl-THF and MTRR 66A>G × reduced folates analyses, and time from parturition and maternal education for the MTRR 66A>G × 5-methyl-THF analysis.
Results
The mean maternal age was 32.6 y (Table 1). A majority of the women had income >$60,000 (78.5%) and had completed a college diploma or higher level of education (91.1%). Milk sampling was completed within ∼1 month of delivery, with the majority of samples representing a combination of foremilk and hindmilk and collection times. A majority of women (71.7%) self-identified as FA-supplement users, with a median total daily supplemental FA intake of 1000 µg. The mean total folate, reduced folates, 5-methyl-THF, and UMFA in breast milk were 116.1 ± 1.9, 68.9 ± 1.3, 47.5 ± 1.0, and 47.2 ± 1.6 nmol/L, respectively. UMFA was detectable in the breast milk of 96.2% of participants. The genotype frequencies among the women included in the milk folate analysis did not differ from those observed in the full MIREC cohort (Supplemental Table 1) and were similar to those observed in a previously reported nationally representative sample of the Canadian population (8).
TABLE 1.
Study participant characteristics1
| Characteristic | Value |
|---|---|
| n | 551 |
| Age, y | 32.6 ± 0.2 |
| Time from parturition to milk sampling, d2 | 33.7 ± 0.6 |
| Annual family income, % | |
| Lower income (≤$60,000) | 16.9 |
| Middle-high income (>$60,000) | 78.5 |
| Missing | 4.5 |
| Maternal education, % | |
| < Postsecondary | 8.7 |
| College/trade school diploma or undergraduate degree | 63.9 |
| Graduate degree | 27.2 |
| Missing | 0.2 |
| Time of collection, % | |
| Morning | 16.0 |
| Afternoon | 8.0 |
| Evening | 8.3 |
| Overnight | 2.2 |
| Combined | 57.5 |
| Missing | 8.0 |
| Foremilk or hindmilk, % | |
| Foremilk | 13.1 |
| Hindmilk | 8.9 |
| Combined | 68.6 |
| Missing | 9.4 |
| FA-supplement use, %3 | |
| Nonuser | 28.3 |
| User | 71.7 |
| Total daily supplemental FA intake among supplement users (µg), median (range)3 | 1000 (63, 10,000) |
| Breast milk folates (nmol/L) | |
| Total folate4 | 116.1 ± 1.9 |
| Reduced folates5 | 68.9 ± 1.3 |
| 5-methyl-THF | 47.5 ± 1.0 |
| UMFA6 | 47.2 ± 1.6 |
| Prevalence of detectable UMFA in breast milk, %6 | 96.2 |
Data are presented as mean ± SE, percentage, or median (range). FA, folic acid; THF, tetrahydrofolate; UMFA, unmetabolized folic acid.
n = 522.
Based on self-reported consumption of an FA-containing supplement. Total daily FA from supplements was based on the sum of FA content from all vitamin supplements.
Total folate is the sum of UMFA, THF, 5-methyl-THF, 5,10-methenyl-THF, and 5-formyl-THF.
Reduced folates represents the sum of THF, 5-methyl-THF, 5,10-methenyl-THF, and 5-formyl-THF.
The limit of detection for UMFA was <0.9 nmol/L.
Associations between breast-milk folate vitamers and MTHFR SNPs
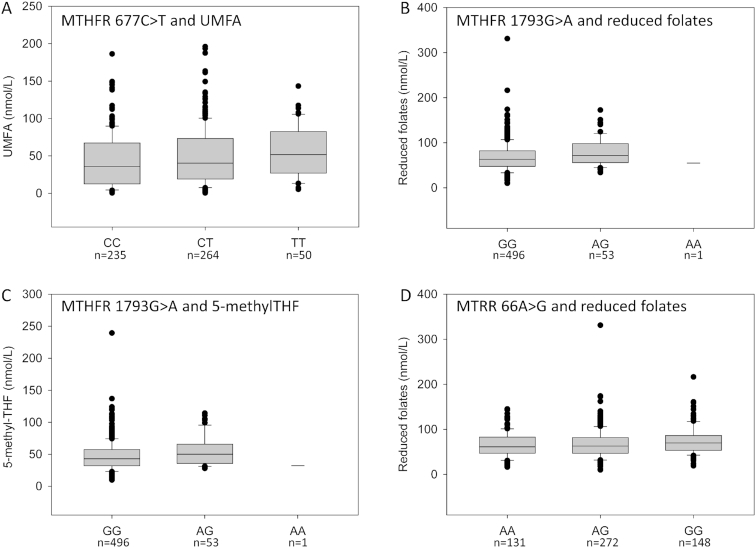
The prevalence of the MTHFR 677C>T genotypes CC, CT, and TT was 42.8%, 48.1%, and 9.1%, respectively (Table 2). The MTHFR 677C>T SNP was not associated with breast-milk total folate, reduced folates, or 5-methyl-THF. However, the T allele was associated with higher breast-milk UMFA concentrations, although its contribution to total variance was small (R2 = 0.01, unadjusted P = 0.004) (Table 2, Figure 2A). The association remained after adjustment for other covariates (model 1: adjusted R2 = 0.10, adjusted P = 0.013; model 2: adjusted R2 = 0.10, adjusted P = 0.006). Proportional to total folate, UMFA was higher among TT individuals than CC individuals (P = 0.009; Supplemental Figure 1). The prevalence of detectable UMFA in breast milk tended to be higher with each additional T allele, increasing from 94% to 97.3% and 100% of participants, respectively (P = 0.05).
TABLE 2.
Associations between the MTHFR 677C>T, MTHFR 1793G>A, and MTRR 66A>G SNPs and breast-milk folate vitamer concentrations1
| SNP | Genotype | % (n) | Total folate2 (nmol/L) | Reduced folates3 (nmol/L) | 5-methyl-THF (nmol/L) | UMFA (nmol/L) | UMFA prevalence (%)4 |
|---|---|---|---|---|---|---|---|
| MTHFR 677C>T | C|C | 42.8 (235) | 115.2 ± 2.7 | 72.7 ± 2.0 | 50.4 ± 1.5 | 42.5 ± 2.3 | 94.0 |
| C|T | 48.1 (264) | 121.1 ± 2.8 | 71.6 ± 2.1 | 49.5 ± 1.6 | 49.5 ± 2.3 | 97.3 | |
| T|T | 9.1 (50) | 123.5 ± 6.6 | 67.0 ± 4.1 | 45.5 ± 2.6 | 56.4 ± 4.9 | 100.0 | |
| R 2 | — | 0.005 | 0.002 | 0.002 | 0.01 | — | |
| P | — | 0.10 | 0.32 | 0.24 | 0.004 | 0.05 | |
| MTHFR 1793G>A | G|G | 90.2 (496) | 116.0 ± 0.9 | 69.4 ± 0.6 | 47.9 ± 0.5 | 46.6 ± 0.7 | 96.6 |
| A|G | 9.6 (53) | 124.5 ± 2.3 | 79.7 ± 1.9 | 55.9 ± 1.4 | 44.8 ± 2.3 | 92.5 | |
| A|A | 0.2 (1) | 117.3 ± 0.0 | 56.1 ± 0.0 | 32.9 ± 0.0 | 61.2 ± 0.0 | 100 | |
| R 2 | — | 0.003 | 0.008 | 0.008 | <0.001 | — | |
| P | — | 0.19 | 0.04 | 0.04 | 0.82 | 0.32 | |
| MTRR 66A>G | A|A | 23.8 (131) | 112.0 ± 1.5 | 67.2 ± 1.1 | 47.8 ± 0.9 | 44.8 ± 1.3 | 93.9 |
| A|G | 49.4 (272) | 117.2 ± 2.8 | 68.7 ± 2.0 | 47.1 ± 1.5 | 48.5 ± 2.3 | 97.4 | |
| G|G | 26.9 (148) | 120.2 ± 3.4 | 76.2 ± 2.6 | 52.1 ± 1.8 | 44.0 ± 2.9 | 95.9 | |
| R 2 | — | 0.004 | 0.01 | 0.005 | <0.001 | — | |
| P | — | 0.12 | 0.02 | 0.12 | 0.81 | 0.22 |
Genotyping data were available for 549 (MTHFR 677C>T), 550 (MTHFR 1793G>A), and 551 (MTRR 66A>G) individuals. Data are presented as mean ± SE or percentage. Associations were assessed using the Wald's test using PLINK. Associations were considered significant if P ≤ 0.01 based on Bonferroni correction for multiple testing. THF, tetrahydrofolate; UMFA, unmetabolized folic acid.
Total folate represents the sum of folic acid, THF, 5-methyl-THF, 5,10-methenyl-THF, and 5-formyl-THF.
Reduced folates represents the sum of THF, 5-methyl-THF, 5,10-methenyl-THF, and 5-formyl-THF.
The limit of detection for UMFA was <0.9 nmol/L. Differences in frequencies were assessed by chi-square test and considered significant if P ≤ 0.01 based on Bonferroni correction for multiple testing.
FIGURE 2.
Associations between breast-milk folate vitamers and various folate-related SNPs. (A) Association between the MTHFR 677C>T SNP and breast-milk UMFA (P = 0.004). (B and C) Association between the MTHFR 1793G>A SNP and breast milk reduced folates (P = 0.04) and 5-methyl-THF (P = 0.04). (D) Association between the MTRR 66A>G SNP and breast milk reduced folates (P = 0.02). Reduced folates represent the sum of THF, 5-methyl-THF, 5,10-methenyl-THF, and 5-formyl-THF. Associations were assessed using the Wald's test using PLINK. Associations were considered significant if P ≤ 0.01 based on Bonferroni correction for multiple testing. The LOD for UMFA was <0.9 nmol/L; values that fell below the LOD were assigned a value of one-half the LOD for analysis. LOD, limit of detection; SNP, single nucleotide polymorphism; THF, tetrahydrofolate; UMFA, unmetabolized folic acid.
The prevalence of the MTHFR 1298A>C genotypes AA, AC, and CC was 45.4%, 45.9%, and 8.7%, respectively (Supplemental Table 2). The MTHFR 1298A>C SNP was not associated with total folate, reduced folates, 5-methyl-THF, or UMFA. Furthermore, the prevalence of detectable UMFA did not differ among the genotypes.
Because the MTHFR 677C>T and 1298A>C SNPs were in LD, we assessed the association of the 677C>T/1298A>C haplotype with breast-milk folate concentrations (Supplemental Table 3). No significant associations were observed between the haplotypes and breast-milk total folate, reduced folates, or 5-methyl-THF. The MTHFR 677C>T/1298A>C TT/AA haplotype was significantly associated with higher UMFA (P = 0.01).
The prevalence of the MTHFR 1793G>A genotypes GG, GA, and AA was 90.2%, 9.6%, and 0.2%, respectively (Table 2). Because our sample included only 1 participant who was homozygous for the A allele, she was not included in the statistical analysis. The MTHFR 1793G>A SNP was not associated with breast-milk total folate, UMFA, or the prevalence of detectable UMFA. However, the A allele tended to be associated with higher concentrations of reduced folates (R2 = 0.008, P = 0.04) and 5-methyl-THF (R2 = 0.008, P = 0.04) (Table 2, Figure 2B and C). The difference in reduced folates—representing the combination of THF, 5-methyl-THF, 5,10-methenyl-THF, and 5-formyl-THF—was driven primarily by a change in 5-methyl-THF. Adjustment for FA-supplement intake and time from parturition (model 2: adjusted R2 = 0.11, adjusted P = 0.03) or all covariates (model 1: adjusted R2 = 0.12, adjusted P = 0.06) did not strengthen the association between the A allele and 5-methyl-THF.
Associations between breast-milk folate vitamers and MTR 2756A>G
The prevalence of the MTR 2756A>G genotypes AA, AG, and GG was 62.8%, 33.6%, and 3.6%, respectively (Supplemental Table 2). The MTR 2756A>G SNP was not associated with breast-milk total folate, reduced folates, 5-methyl-THF, UMFA, or the prevalence of detectable UMFA.
Associations between breast-milk folate vitamers and MTRR 66A>G
The prevalence of the MTRR 66A>G genotypes AA, AG, and GG was 23.8%, 49.4%, and 26.9%, respectively (Table 2). Although the G allele is generally considered the minor allele, it was more prevalent in our study population than the A allele. The MTRR 66A>G SNP was not associated with breast-milk total folate, 5-methyl-THF, UMFA, or the prevalence of detectable UMFA. The MTRR 66A>G SNP tended to be associated with breast-milk reduced folate concentrations (R2 = 0.01, P = 0.02) (Table 2; Figure 2D). Adjustment for other covariates did not strengthen the association (model 1: adjusted R2 = 0.09, adjusted P = 0.02; model 2: adjusted R2 = 0.09, adjusted P = 0.03).
Discussion
The total folate content of breast milk is tightly regulated and is generally insensitive to dietary folate intake, although it can be lower in cases of chronic deficiency and modestly higher when >400 µg of supplemental FA is consumed (3, 25–28). Total folate represents a mix of folate vitamers, not all of which are equally bioavailable to the infant. We previously demonstrated that supplemental FA intake above the recommended 400 µg/d was associated with a shift in the proportion of breast-milk folate vitamers away from reduced folates and toward UMFA (3). Here, we show that the common MTHFR 677C>T SNP is also associated with breast-milk UMFA.
The MTHFR 677C>T SNP is associated with lower folate status and altered folate vitamer distribution in RBCs such that TT homozygotes have lower 5-methyl-THF and higher nonmethylated (formylated) folates in their RBCs (7, 9, 10). The accumulation of the less stable formylated folates in TT homozygotes has been proposed to explain their lower overall cellular folate (29, 30). One could speculate that a consequence of lower 5-methyl-THF in TT homozygotes might be higher UMFA as a proportion of total folate both in cells and in circulation, which could make it more available for uptake into breast milk. However, the associations between the MTHFR 677C>T SNP and UMFA concentration or as a proportion of total folate in serum/plasma are not well understood. One study reported that the prevalence of detectable plasma UMFA tended to be higher in pregnant women with the TT genotype treated with 400 µg/d of supplemental FA for 22 wk in midgestation compared with women with the CT and CC genotypes (P = 0.08) (31). However, others reported no significant association (32). Our data suggest that the MTHFR 677C>T SNP is associated with an altered distribution of folate vitamers in breast milk in the absence of a significant overall change in total folate; UMFA represented a significantly higher proportion of total folate with a concomitant lower, albeit nonsignificant, proportion of reduced folates (Supplemental Figure 1). It remains to be determined whether this is due to altered distribution of folate vitamers in circulation and their differential uptake into the milk or to altered metabolism in the mammary gland. Either way, the association of the MTHFR 677C>T SNP with circulating and breast-milk UMFA warrants closer examination, as does their potential interaction with FA intake from diet and supplements because FA intake is associated with higher circulating UMFA (33, 34).
None of the associations among the other folate-related SNPs and folate vitamers were significant based on a Bonferroni correction threshold of P ≤ 0.01, including the MTHFR 1298A>C SNP. However, because this SNP is in LD with the MTHFR 677C>T SNP, we examined them together as a haplotype. Only the MTHFR 677C>T/1298A>C TT/AA haplotype was associated with a folate vitamer, namely UMFA. We suggest that this association was driven by its LD with the MTHFR 677C>T SNP, as demonstrated in a genome-wide association study in which the association between the MTHFR 1298A>C and RBC folate was eliminated when the analysis adjusted for the MTHFR 677C>T SNP (13).
Our study has strengths and limitations. A strength was the large sample of women (n = 551) who were genetically comparable to a nationally representative sample of Canadians (8), albeit based on a limited suite of SNPs. However, the participants of the MIREC study were not necessarily representative of the Canadian population with respect to other important variables. For example, they were older with higher socioeconomic status and more likely to consume FA supplements compared with women giving birth in the general Canadian population (35, 36). Given that Canadian women are more likely to breastfeed if they are older, have higher education, and have higher income (35), it is not unexpected that a breast-milk study would select for those women. However, other than FA consumption, these characteristics would not necessarily be expected to influence the association between genetic polymorphisms and folate vitamers in breast milk. Indeed, we found that the MTHFR 677C>T SNP association with UMFA remained significant even after adjustment for supplemental FA intake and other variables.
The use of a quantitative liquid chromatography–tandem mass spectrometry method was critical for measuring the breast-milk folate vitamers, and it allowed us to examine SNP associations. A total folate method, such as the microbiological method, would not have been useful for identifying the differences among the folate vitamers, which potentially influence folate bioavailability and exposure in breastfed infants. A limitation is that we did not measure the 5-methyl-THF oxidation product 4α-hydroxy-5-methyl-THF, which was shown to represent ∼5% of total serum folate (2). It is unknown whether 4α-hydroxy-5-methyl-THF is naturally present in breast milk or how breast-milk handling and preparation might contribute to its formation. However, our method separated 4α-hydroxy-5-methyl-THF chromatographically from the other folate analytes, so it would not have contributed to our reported total folate values. We also did not measure milk folate binding protein concentration or maternal folate status, which could improve the interpretation of the data.
Although the MIREC study is a prospective cohort, the milk sampling was cross-sectional taken at ∼30 d postpartum. It would be of interest to assess whether these genetic associations exist throughout lactation given the changes in breast-milk folate content over time and how they might interact with dietary and supplemental FA intake across time. Milk folate content can also change by milk type (foremilk and hindmilk) and time of day of collection; however, we did not observe an association between milk folate content and these factors. This is likely due to the majority of our samples being a combination of milk types collected across multiple times of day. Despite this limitation, we believe our findings remain important because these samples would likely represent an infant's overall breast-milk folate exposure.
We did not include dietary folate intake in our analysis, and folate status was not assessed in the mothers. We previously showed that the frequency of intake of FA-fortified foods (e.g., white breads and ready-to-eat cereal) did not differ between supplement nonusers and users in this cohort (3). Similar to other reports, we found no association between dietary folate intake and breast-milk folate content, even when the analysis was restricted to supplement nonusers (3). FA-supplement use is the main determinant of folate status and nearly doubles total folate intake in lactating women (37, 38), suggesting that it—and not diet—is the more important determinant of breast-milk folate vitamers.
In conclusion, we found that total breast-milk folate content was not associated with any of the folate-related SNPs examined in this study. This is noteworthy given that maternal serum and cord blood serum folate (39) are associated with the MTHFR 677C>T SNP, such that TT homozygotes have lower status, indicating that breast-milk total folate concentration is regulated independently of genotype. In addition, we showed that the MTHFR 677C>T SNP is associated with breast-milk UMFA, albeit with a small effect size. It is important to understand the determinants of breast-milk folate, genetic or otherwise, as well as their possible interactions because changes in the distribution of folate vitamers could possibly impact the bioavailability of breast-milk folate given the higher affinity of the folate binding protein for FA relative to 5-methyl-THF; this could impact folate absorption in the infant gastrointestinal tract (40, 41). However, we caution against overinterpretation of the data because the breast-milk UMFA concentration was approximately one-third that of FA in infant formula—a concentration that is deemed safe.
Supplementary Material
Acknowledgments
We thank the MIREC Study Group and participants for their contribution to our study.
The authors’ responsibilities were as follows—AJM: designed and supervised the study, finalized the manuscript, and had primary responsibility for the final content; RP: performed the data analysis and drafted the manuscript; AW: oversaw the statistical analysis and interpretation; TEA: Health Canada lead for the MIREC study and contributed to study design; and all authors: read and approved the final manuscript. All authors declare no conflict of interest related to this study.
Notes
The MIREC study was supported by the Chemicals Management Plan at Health Canada, the Ontario Ministry of the Environment, and the Canadian Institutes of Health Research (grant MOP-81285).
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: FA, folic acid; LD, linkage disequilibrium; LOD, limit of detection; MIREC, Maternal–Infant Research on Environmental Chemicals; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; RBC, red blood cell; SNP, single nucleotide polymorphism; THF, tetrahydrofolate; UMFA, unmetabolized folic acid.
References
- 1. Bravi F, Wiens F, Decarli A, Dal Pont A, Agostoni C, Ferraroni M. Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr. 2016;104:646–62. [DOI] [PubMed] [Google Scholar]
- 2. Pfeiffer CM, Sternberg MR, Fazili Z, Lacher DA, Zhang M, Johnson CL, Hamner HC, Bailey RL, Rader JI, Yamini S et al.. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2. Br J Nutr. 2015;113:1965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Page R, Robichaud A, Arbuckle TE, Fraser WD, MacFarlane AJ. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am J Clin Nutr. 2017;105:1101–9. [DOI] [PubMed] [Google Scholar]
- 4. O'Connor DL, Tamura T, Picciano MF. Pteroylpolyglutamates in human milk. Am J Clin Nutr. 1991;53:930–4. [DOI] [PubMed] [Google Scholar]
- 5. Selhub J. Determination of tissue folate composition by affinity chromatography followed by high-pressure ion pair liquid chromatography. Anal Biochem. 1989;182:84–93. [DOI] [PubMed] [Google Scholar]
- 6. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP et al.. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. [DOI] [PubMed] [Google Scholar]
- 7. Tsang BL, Devine OJ, Cordero AM, Marchetta CM, Mulinare J, Mersereau P, Guo J, Qi YP, Berry RJ, Rosenthal J et al.. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: a systematic review and meta-analysis of trials and observational studies. Am J Clin Nutr. 2015;101:1286–94. [DOI] [PubMed] [Google Scholar]
- 8. Zinck JW, de Groh M, MacFarlane AJ. Genetic modifiers of folate, vitamin B-12, and homocysteine status in a cross-sectional study of the Canadian population. Am J Clin Nutr. 2015;101:1295–304. [DOI] [PubMed] [Google Scholar]
- 9. Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA. 1998;95:13217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fazili Z, Pfeiffer CM. Measurement of folates in serum and conventionally prepared whole blood lysates: application of an automated 96-well plate isotope-dilution tandem mass spectrometry method. Clin Chem. 2004;50:2378–81. [DOI] [PubMed] [Google Scholar]
- 11. Friedman G, Goldschmidt N, Friedlander Y, Ben-Yehuda A, Selhub J, Babaey S, Mendel M, Kidron M, Bar-On H. A common mutation A1298C in human methylenetetrahydrofolate reductase gene: association with plasma total homocysteine and folate concentrations. J Nutr. 1999;129:1656–61. [DOI] [PubMed] [Google Scholar]
- 12. Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72. [DOI] [PubMed] [Google Scholar]
- 13. Shane B, Pangilinan F, Mills JL, Fan R, Gong T, Cropp CD, Kim Y, Ueland PM, Bailey-Wilson JE, Wilson AF et al.. The 677C→T variant of MTHFR is the major genetic modifier of biomarkers of folate status in a young, healthy Irish population. Am J Clin Nutr. 2018;108:1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leclerc D, Sibani S, Rozen R. Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of Mutations/Polymorphisms. Austin, TX: Landes Bioscience; 2000–2013. [Google Scholar]
- 15. Rady PL, Szucs S, Grady J, Hudnall SD, Kellner LH, Nitowsky H, Tyring SK, Matalon RK. Genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) in ethnic populations in Texas; a report of a novel MTHFR polymorphic site, g1793a. Am J Med Genet. 2002;107:162–8. [DOI] [PubMed] [Google Scholar]
- 16. Zhou BS, Bu GY, Li M, Chang BG, Zhou YP. Tagging SNPs in the MTHFR gene and risk of ischemic stroke in a Chinese population. Int J Mol Sci. 2014;15:8931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steluti J, Carvalho AM, Carioca AAF, Miranda A, Gattas GJF, Fisberg RM, Marchioni DM. Genetic variants involved in one-carbon metabolism: polymorphism frequencies and differences in homocysteine concentrations in the folic acid fortification era. Nutrients. 2017;9(6):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li WX, Cheng F, Zhang AJ, Dai SX, Li GH, Lv WW, Zhou T, Zhang Q, Zhang H, Zhang T et al.. Folate deficiency and gene polymorphisms of MTHFR, MTR and MTRR elevate the hyperhomocysteinemia risk. Clin Lab. 2017;63:523–33. [DOI] [PubMed] [Google Scholar]
- 19. Olteanu H, Banerjee R. Human methionine synthase reductase, a soluble P-450 reductase-like dual flavoprotein, is sufficient for NADPH-dependent methionine synthase activation. J Biol Chem. 2001;276:35558–63. [DOI] [PubMed] [Google Scholar]
- 20. Gaughan DJ, Kluijtmans LA, Barbaux S, McMaster D, Young IS, Yarnell JW, Evans A, Whitehead AS. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001;157:451–6. [DOI] [PubMed] [Google Scholar]
- 21. Olteanu H, Munson T, Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry. 2002;41:13378–85. [DOI] [PubMed] [Google Scholar]
- 22. Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, von Dadelszen P, Hemmings DG et al.. Cohort profile: The Maternal–Infant Research on Environmental Chemicals research platform. Paediatr Perinat Epidemiol. 2013;27:415–25. [DOI] [PubMed] [Google Scholar]
- 23. Health Canada. Licensed Natural Health Products Database. [Internet] [cited 29 September, 2015]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/applications-submissions/product-licensing/licensed-natural-health-products-database.html. [Google Scholar]
- 24. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ et al.. Plink: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mackey AD, Picciano MF. Maternal folate status during extended lactation and the effect of supplemental folic acid. Am J Clin Nutr. 1999;69:285–92. [DOI] [PubMed] [Google Scholar]
- 26. Tamura T, Yoshimura Y, Arakawa T. Human milk folate and folate status in lactating mothers and their infants. Am J Clin Nutr. 1980;33:193–7. [DOI] [PubMed] [Google Scholar]
- 27. Bruinse HW, van der Berg H, Haspels AA. Maternal serum folacin levels during and after normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1985;20:153–8. [DOI] [PubMed] [Google Scholar]
- 28. Smith AM, Picciano MF, Deering RH. Folate supplementation during lactation: maternal folate status, human milk folate content, and their relationship to infant folate status. J Pediatr Gastroenterol Nutr. 1983;2:622–8. [PubMed] [Google Scholar]
- 29. Misselbeck K, Marchetti L, Field MS, Scotti M, Priami C, Stover PJ. A hybrid stochastic model of folate-mediated one-carbon metabolism: effect of the common C677T MTHFR variant on de novo thymidylate biosynthesis. Sci Rep. 2017;7:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stover PJ, MacFarlane AJ, Field MS. Bringing clarity to the role of MTHFR variants in neural tube defect prevention. Am J Clin Nutr. 2015;101:1111–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pentieva K, Selhub J, Paul L, Molloy AM, McNulty B, Ward M, Marshall B, Dornan J, Reilly R, Parle-McDermott A et al.. Evidence from a randomized trial that exposure to supplemental folic acid at recommended levels during pregnancy does not lead to increased unmetabolized folic acid concentrations in maternal or cord blood. J Nutr. 2016;146:494–500. [DOI] [PubMed] [Google Scholar]
- 32. Obeid R, Kasoha M, Kirsch SH, Munz W, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. Am J Clin Nutr. 2010;92:1416–22. [DOI] [PubMed] [Google Scholar]
- 33. Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, Johnson CL. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. 2015;145:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stamm RA, March KM, Karakochuk CD, Gray AR, Brown RC, Green TJ, Houghton LA. Lactating Canadian women consuming 1000 µg folic acid daily have high circulating serum folic acid above a threshold concentration of serum total folate. J Nutr. 2018;148:1103–8. [DOI] [PubMed] [Google Scholar]
- 35. Public Health Agency of Canada. What mothers say: The Canadian Maternity Experiences Survey. Ottawa, Canada: Public Health Agency of Canada; 2009. [Google Scholar]
- 36. Miller EC, Liu N, Wen SW, Walker M. Why do Canadian women fail to achieve optimal pre-conceptional folic acid supplementation? An observational study. J Obstet Gynaecol Can. 2011;33:1116–23. [DOI] [PubMed] [Google Scholar]
- 37. Colapinto CK, O'Connor DL, Dubois L, Tremblay MS. Folic acid supplement use is the most significant predictor of folate concentrations in Canadian women of childbearing age. Appl Physiol Nutr Metab. 2012;37:284–92. [DOI] [PubMed] [Google Scholar]
- 38. Sherwood KL, Houghton LA, Tarasuk V, O'Connor DL. One-third of pregnant and lactating women may not be meeting their folate requirements from diet alone based on mandated levels of folic acid fortification. J Nutr. 2006;136:2820–6. [DOI] [PubMed] [Google Scholar]
- 39. Plumptre L, Masih SP, Ly A, Aufreiter S, Sohn KJ, Croxford R, Lausman AY, Berger H, O'Connor DL, Kim YI. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr. 2015;102:848–57. [DOI] [PubMed] [Google Scholar]
- 40. Verwei M, Arkbage K, Mocking H, Havenaar R, Groten J. The binding of folic acid and 5-methyltetrahydrofolate to folate-binding proteins during gastric passage differs in a dynamic in vitro gastrointestinal model. J Nutr. 2004;134:31–7. [DOI] [PubMed] [Google Scholar]
- 41. Nygren-Babol L, Jagerstad M. Folate-binding protein in milk: a review of biochemistry, physiology, and analytical methods. Crit Rev Food Sci Nutr. 2012;52:410–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.