ABSTRACT
Background
There may be differences in hematological parameters between meat-eaters and vegetarians.
Objective
The aim of this study was to perform cross-sectional analyses of hematological parameters by diet group in a large cohort in the United Kingdom.
Methods
A complete blood count was carried out in all UK Biobank participants at recruitment (2006–2010). We examined hemoglobin, red and white blood cell counts, and platelet counts and volume in regular meat eaters (>3 times/wk of red/processed meat consumption, n = 212,831), low meat eaters (n = 213,092), poultry eaters (n = 4815), fish eaters (n = 10,042), vegetarians (n = 6548), and vegans (n = 398) of white ethnicity and meat eaters (n = 3875) and vegetarians (n = 1362) of British Indian ethnicity.
Results
In both white and British Indian populations, compared with regular meat eaters (or meat eaters in Indians), the other diet groups had up to 3.7% lower age-adjusted hemoglobin concentrations (difference not significant in white vegan women) and were generally more likely to have anemia (e.g., 8.7% of regular meat eaters compared with 12.8% of vegetarians in white premenopausal women; P < 0.05 after Bonferroni correction). In the white population, compared with regular meat eaters, all other diet groups had lower age- and sex-adjusted total white cells, neutrophils, lymphocytes, monocytes, and eosinophils (P-heterogeneity < 0.001 for all), but basophil counts were similar across diet groups; in British Indians, there was no significant difference in any of the white blood cell counts by diet group. Compared with white regular meat eaters, the low meat eaters, poultry eaters, fish eaters, and vegans had significantly lower platelet counts and higher platelet volume, whereas vegetarians had higher counts and lower volume. Compared with British Indian meat eaters, vegetarians had higher platelet count and lower volume.
Conclusions
In the UK Biobank, people with low or no red meat intake generally had lower hemoglobin concentrations and were slightly more likely to be anemic. The lower white blood cell counts observed in low and non-meat eaters, and differences in mean platelet counts and volume between diet groups, warrant further investigation. This observational study was registered at http://www.isrctn.com/ as ISRCTN10125697.
Keywords: UK Biobank, vegetarian, vegan, hematology, blood count, anemia, ethnicity
Introduction
Vegetarian and vegan diets are associated with lower BMI, blood pressure, and blood cholesterol concentrations (1–3). At the same time, the long-term exclusion of animal foods may lead to inadequate intakes of some essential nutrients that are not easily obtained from plant sources, especially without fortification (4, 5), and this may in turn affect hematological parameters. For example, red meat is a good source of heme iron, and poultry and fish also contain heme iron, which is more bioavailable than plant sources of iron; therefore, lack of meat or fish consumption may increase risk of iron-deficiency anemia (6). Indeed, several small studies reported that vegetarians and vegans had higher rates of anemia and lower hemoglobin or red blood cell counts compared with nonvegetarians (7–11). Vitamin B-12 is only present in animal foods; vegetarians, or vegans who exclude all animal foods from their diet, generally have a higher prevalence of vitamin B-12 deficiency (4, 12). Vitamin B-12 has a crucial role in various cellular processes, including the maturation of red blood cells (13), and may play a role in the platelet life cycle (14–16). Intake of dietary protein, or specific amino acids, which may be low in vegetarian diets (17), may also have a role in supporting the immune system, including production or activation of white blood cells (18, 19). Other nutrients, such as zinc, vitamin A, and riboflavin, which are less bioavailable in plant-based diets (5, 20), have also been linked to blood cell or hemoglobin production (21–25).
There is limited robust evidence on the influence of vegetarian diets on hematological parameters, which might be reflective of anemia or immune status. Here, we examine the associations between varying degrees of animal source food exclusion and hematological indices in a large population-based cohort of ∼500,000 participants in the United Kingdom who self-identified as white British or British Indians.
Methods
Study design and participants
The UK Biobank is a prospective cohort of >500,000 people aged 40–69 y, who were recruited in 2006–2010 across the United Kingdom (26). The scientific rationale and design of the UK Biobank study have been described in detail elsewhere (27). In brief, people who lived within traveling distance (∼25 km) of 1 of the 22 assessment centers across England, Wales, and Scotland were identified from National Health Service registers and invited to participate in the study. Permission for access to patient records for recruitment was approved by the Patient Information Advisory Group (now the National Information Governance Board for Health and Social Care) in England and Wales and by the Community Health Index Advisory Group in Scotland. Overall, ∼5.5% of the invitees attended a baseline visit (28) during which they gave informed consent to participate in UK Biobank using a signature capture device and completed a touch-screen questionnaire that asked about sociodemographic characteristics, lifestyle exposures [including diet, supplement use (e.g., formulations containing B vitamins, folic acid, multiple micronutrients, or iron), and smoking status], and general health and medical history. All participants also completed a computer-assisted personal interview and had physical measurements and blood samples taken. In addition to a self-administered questionnaire, additional dietary information was collected using a Web-based 24-h dietary assessment tool (29), which was administered ≤5 times in a large subsample of participants (∼210,000). This study was registered at http://www.isrctn.com/ as ISRCTN10125697.
Ethnicity classification
On the touch-screen questionnaire, participants were asked to identify their ethnicity from options of “White,” “Mixed,” “Asian or Asian British,” “Black or Black British,” “Chinese,” “Other ethnic group,” “Do not know,” or “Prefer not to answer.” Participants were included for analyses if they self-identified as “white” or as “Asian or Asian British” and subsequently as “Indian.” For consistency, participants are subsequently referred to as “white” or “British Indian.” The white population was included because it made up the majority of the UK Biobank population (∼94%), and the British Indian population was included due to the large proportion of vegetarians in this population group (24.6% compared with 1.7% in the overall cohort). The number of vegetarians in the other ethnic groups was small; therefore, other ethnic groups were excluded from these analyses.
Diet group classification
To determine the classification of diet groups, we used methods previously described (1, 30). Briefly, participants were asked their frequency of consumption of processed meat, beef, lamb or mutton, pork, poultry (e.g., chicken or turkey), oily fish, other types of fish, eggs or foods containing eggs, and dairy products, in 6 categories of frequency ranging from “Never” to “Once or more daily.” Based on these questions, 6 diet groups were defined for the white British population: regular meat eaters (red and processed meat consumption >3 times/wk), low meat eaters (red and processed meat consumption ≤3 times/wk), poultry eaters (participants who ate poultry but no red or processed meat, regardless of whether they ate fish, dairy products, or eggs), fish eaters (participants who ate fish but no red or processed meat or poultry), vegetarians (participants who did not eat meat, poultry, or fish), and vegans (participants who further excluded dairy products and eggs). Two diet groups were defined for the British Indian population: meat eaters (ate any combination of red or processed meat or poultry) and vegetarians (excluding vegans). Information collected from the Web-based 24-h dietary assessment tool was used to estimate food and nutrient intakes (e.g., iron and vitamin B-12) in each diet group, based on McCance and Widdowson's The Composition of Foods and its supplements (29, 31).
Blood measurements and hematological assays
Blood sampling was performed by either a phlebotomist or a nurse in all participants except for a small proportion (0.3%) who declined, were deemed unable to undergo sampling, or where the attempt was abandoned for either technical or health reasons. Nonfasting blood samples were taken from a vein in the inner elbow using an 18-G vacutainer needle and barrel or, if that appeared unsuitable, from a vein on the back of the hand using a 21-G Safety Lok butterfly needle (BD) connected to a vacutainer barrel (27). For hematological assays, blood was collected into a 4-mL EDTA vacutainer and dispatched to the central processing laboratory in temperature-controlled shipping boxes (at 4°C) (32). Complete blood cell counts were conducted using a Coulter Counter (Beckman Coulter), typically within 24 h of blood collection (32, 33). Because the hematological assays were performed throughout a long recruitment period (∼5 y), variation in blood count caused by laboratory drift cannot be ruled out despite efforts in quality control (further details are provided in the Supplementary Methods) (32), but any possible variation should be independent of diet group.
Classification of anemia, low platelet count, and elevated platelet volume
Anemia was defined as hemoglobin concentrations of <130 g/L and <120 g/L for men and women, respectively, based on WHO criteria (34). Anemia severity was classified as mild (hemoglobin 110–129 g/L in men and 110–119 g/L in women), moderate (80–109 g/L in both sexes), and severe (<80 g/L in both sexes). To adjust for the effect of smoking on hemoglobin, when using hemoglobin levels to define anemia, 3 g/L was subtracted for all participants who indicated they were smokers (35), but original values of hemoglobin were used in all other analyses. If anemia was present, it was further defined as microcytic or macrocytic, based on a mean corpuscular volume of <80 fL or >100 fL, respectively (36). Secondarily, we also tested for any differences in the classification by correcting for hemoglobin levels using more detailed categorization of smoking status (<10 cigarettes smoked per day, no adjustment; ≥10 and <20 cigarettes smoked per day, −3 g/L; ≥20 and <40 cigarettes smoked per day, −5 g/L; ≥40 cigarettes smoked per day, −7 g/L; and unknown amount, −3 g/L).
For platelet count and volume, low platelet count was defined as <169.06 × 109 cells/L, and elevated platelet volume was defined as >11.24 fL, both as specified by the manufacturer's reference range (33).
Statistical analyses
Baseline characteristics of the cohort were tabulated by 6 diet groups in white British participants and by 2 diet groups in British Indian participants. The primary outcomes of this research were blood counts and prevalence of anemia, and the secondary outcomes were prevalence of subtypes of anemia and prevalence of low platelet counts and elevated platelet volume. Linear regressions were modeled to estimate the adjusted mean levels (95% CIs) of each hematological parameter of interest, including hemoglobin, red blood cell count, reticulocyte percentage, immature reticulocyte fraction, total white blood cell count, neutrophils, lymphocytes, monocytes, eosinophils, basophils, platelet count, and platelet volume.
To estimate mean levels of hemoglobin, red blood cell count, reticulocyte percentage, and immature reticulocyte fraction, the regression model was stratified by sex and menopausal status (men, premenopausal women, and postmenopausal women) and adjusted for age of recruitment (5-y age groups from <45, 45–49, 50–54, 55–59, 60–64, and ≥65 y). We additionally adjusted for smoking status (never, previous, current <15 cigarettes per day, current ≥15 cigarettes per day, and unknown) in a second model. There was little difference in reticulocyte percentage and immature reticulocyte fraction by sex and menopausal status; thus, we combined the 3 groups and adjusted for sex in the regression model for our main results and presented the stratified results secondarily.
To estimate mean levels of white cells (total white blood cell count, neutrophils, lymphocytes, monocytes, eosinophils, and basophils) and platelets (platelet count and platelet volume), the regression model was adjusted for age and sex and subsequently for smoking status. In addition, as a third model, we restricted the analysis to participants who answered “No” to the question “Do you have any long-standing illness, disability, or infirmity?” to remove the possible confounding effect of chronic illnesses on white cell or platelet count.
For classification of anemia, low platelet count, and elevated platelet volume, numbers and percentages of people in each diet group were reported as observed, based on criteria as previously described. For anemia, we additionally restricted the analyses to people who reported no iron or B vitamins supplement use.
For each baseline characteristic and each hematological parameter of interest, post hoc pairwise comparisons based on linear regression models were used to test for significant differences between diet groups. For the white population, Bonferroni correction for multiple comparisons between the 6 diet groups was applied, and results reported here represent significant differences after Bonferroni correction, and by using regular meat eaters as the reference group, unless otherwise stated. All statistical analyses were performed using Stata release 15.1 (StataCorp), and 2-sided P values <0.05 were considered significant.
Results
After excluding participants who had no hematological data (n = 24,336), reported other or unknown ethnicities (n = 21,851), or did not answer a sufficient number of questions to be classified into a diet group (n = 3393), 447,726 white British and 5237 British Indian participants were included in this analysis. Of the white British participants, 212,831 were classified as regular meat eaters, 213,092 as low meat eaters, 4815 as poultry eaters, 10,042 as fish eaters, 6548 as vegetarians, and 398 as vegans. Of the British Indian participants, 3875 were classified as meat eaters and 1362 as vegetarians. A participant flowchart of the inclusion and exclusion criteria of this study is shown in Supplemental Figure 1.
Participant characteristics
Characteristics of UK Biobank participants are shown in Tables 1 and 2. In the white population, low or non-meat eaters (fish eaters, vegetarians, and vegans) were more likely to be women, and non-meat eaters were younger. Except for vegans, low and non-meat eaters were less likely to report current smoking and the presence of long-standing illness. Non-meat eaters had a higher reported use of some specified supplements (formulations containing B vitamins, folic acid, multiple micronutrients, or iron), and low and non-meat eaters had on average higher total iron intake from foods (but lower or no iron intake from red or processed meat) and lower vitamin B-12 intake from foods (especially in vegetarians and vegans). Regarding the British Indian participants, vegetarians were slightly older and more likely to be women. They were less likely to report current smoking, more likely to report specified supplement use [formulations containing B vitamins, folic acid, multiple micronutrients, or iron (significant in women only)], and had lower mean vitamin B-12 intake from foods.
TABLE 1.
Baseline characteristics of white British participants by diet group in the UK Biobank1
| Meat eaters | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Regular consumption (>3 times/wk) (max n = 212,831)2 | Low consumption (≤ 3 times/wk) (max n = 213,092)2 | Poultry eaters (max n = 4815) | Fish eaters (max n = 10,042) | Vegetarians (max n = 6548) | Vegans (max n = 398) | P-het3 |
| Age, y | 56.8 ± 8.1a | 56.9 ± 7.9b | 56.7 ± 8.0a,b | 54.2 ± 8.0c | 52.8 ± 7.9d | 54.3 ± 7.9c | <0.001 |
| Women, n (%) | 91,398 (42.9)a | 135,635 (63.7)b | 3741 (77.7)c | 7253 (72.2)d | 4418 (67.5)e | 232 (58.3)b | <0.001 |
| Premenopausal | 20,791 (22.7) | 29,570 (21.8) | 810 (21.7) | 2287 (31.5) | 1636 (37.0) | 76 (32.8) | |
| Postmenopausal | 67,145 (73.5)a | 101,184 (74.6)b | 2797 (74.8)a,b | 4643 (64.0)c | 2583 (58.5)d | 149 (64.2)a,b,c,d | <0.001 |
| Smoking status, n (%) | |||||||
| Previous | 76,402 (36.0) | 74,826 (35.2) | 1675 (34.9) | 3705 (37.0) | 2213 (33.9) | 157 (39.5) | |
| Current | 25,980 (12.2)a | 18,852 (8.9)b | 360 (7.5)c | 719 (7.2)b,c | 512 (7.8)c | 31 (7.8)a,b,c | <0.001 |
| Has a long-standing illness, n (%) | 71,900 (34.6)ha | 63,876 (30.7)b | 1498 (31.8)b | 2703 (27.5)c | 1835 (28.7)c,d | 140 (35.8)a,b,d | <0.001 |
| Regular supplement user, n (%) | |||||||
| B vitamins | 7541 (3.5)a | 9369 (4.4)b | 449 (9.3)c | 739 (7.4)d | 541 (8.3)c,d | 76 (19.1)e | <0.001 |
| Folic acid | 3985 (1.9)a | 4883 (2.3)b | 214 (4.4)c | 335 (3.3)d | 199 (3.0)d | 19 (4.8)c,d | <0.001 |
| Multiple micronutrients | 40,911 (19.2)a | 48,649 (22.8)b | 1561 (32.4)c,d | 3122 (31.1)c | 2248 (34.3)d,e | 155 (38.9)e | <0.001 |
| Iron, in men | 2333 (1.9)a | 1615 (2.1)a | 50 (4.7)b | 160 (5.7)b,c | 134 (6.3)c | 15 (9.0)c | <0.001 |
| Iron, in premenopausal women | 1226 (5.9)a | 1888 (6.4)a | 76 (9.4)b | 300 (13.1)c | 236 (14.4)c | 7 (9.2)a,b,c | <0.001 |
| Iron, in postmenopausal women | 1667 (2.5)e | 2749 (2.7)e | 152 (5.4)d | 327 (7.0)c | 215 (8.3)b | 23 (15.4)a | <0.001 |
| Mean dietary iron intake, mg/d (95% CI)4 | 13.7 (13.7, 13.8)a | 13.5 (13.5, 13.5)b | 13.9 (13.7, 14.1)b | 14.6 (14.5, 14.7)c | 14.6 (14.4, 14.7)c | 16.9 (16.4, 17.4)d | <0.001 |
| From red or processed meat4 | 1.42 (1.41, 1.43)a | 1.02 (1.01, 1.03)b | — | — | — | — | <0.001 |
| From poultry and fish4 | 0.56 (0.55, 0.56)a | 0.60 (0.59, 0.60)b | 0.74 (0.71, 0.76)c | 0.48 (0.46, 0.49)d | — | — | <0.001 |
| Mean dietary vitamin B-12 intake, µg/d (95% CI)4 | 6.74 (6.71, 6.77)a | 6.48 (6.45, 6.51)b | 6.42 (6.23, 6.60)b | 6.03 (5.91, 6.14)c | 2.96 (2.82, 3.10)d | 0.88 (0.31, 1.45)e | <0.001 |
1Values are means ± SDs unless otherwise indicated; n = 447,726. Groups that do not share a superscript letter were significantly different at the 5% level from post hoc pairwise comparisons based on linear regression models and after Bonferroni correction for multiple comparisons. max, maximum.
Includes participants who consume any red or processed meat (beef, lamb, pork, and processed meat), regardless of whether they consume poultry, fish, dairy, or eggs. Cutoffs of regular and low consumption were determined based on consumption of red and processed meat as reported on the touch-screen questionnaire.
Represents P for heterogeneity across the six diet groups, estimated by regressing each baseline characteristic against diet group.
Estimated based on a subsample who completed ≥1 Web-based 24-h dietary assessment and based on intakes from food sources only. Assessments were averaged for participants who completed >1 assessment. The numbers of white British participants who completed ≥1 dietary assessments were as follows: 87,525 regular meat eaters, 93,513 low meat eaters, 2165 poultry eaters, 5486 fish eaters, 3745 vegetarians, and 232 vegans. Estimates were adjusted for age at recruitment (<45, 45–49, 50–54, 55–59, 60–64, and ≥65 y).
TABLE 2.
Baseline characteristics of British Indian participants by diet group in the UK Biobank1
| Characteristics | Meat eaters (max n = 3875) | Vegetarians (max n = 1362) | P-het2 |
|---|---|---|---|
| Age, y | 53.7 ± 8.4 | 55.0 ± 7.9 | <0.001 |
| Women, n (%) | 1621 (41.8) | 877 (64.4) | <0.001 |
| Premenopausal | 565 (34.9) | 229 (26.1) | |
| Postmenopausal | 980 (60.5) | 615 (70.1) | <0.001 |
| Smoking status, n (%) | |||
| Previous | 530 (13.8) | 87 (6.4) | |
| Current | 350 (9.1) | 30 (2.2) | <0.001 |
| Has a long-standing illness, n (%) | 1170 (31.5) | 385 (29.5) | 0.18 |
| Regular supplement user, n (%) | |||
| Vitamin B | 198 (5.1) | 89 (6.5) | 0.047 |
| Folic acid or folate | 133 (3.4) | 67 (4.9) | 0.014 |
| Multivitamins ± minerals | 971 (25.1) | 387 (28.4) | 0.015 |
| Iron, in men | 82 (3.6) | 23 (4.7) | 0.25 |
| Iron, in premenopausal women | 79 (14.0) | 46 (20.1) | 0.032 |
| Iron, in postmenopausal women | 77 (13.6) | 38 (16.6) | 0.033 |
| Mean dietary iron intake, mg/d (95% CI)3 | 11.8 (11.6, 12.1) | 11.6 (11.1, 12.1) | 0.34 |
| From red or processed meat3 | 0.72 (0.65, 0.78) | — | — |
| From poultry and fish3 | 0.60 (0.56, 0.63) | — | — |
| Mean dietary vitamin B-12 intake, µg/d (95% CI)3 | 5.20 (4.95, 5.44) | 2.55 (2.10, 3.00) | <0.001 |
Values are means ± SDs unless otherwise indicated; n = 5237. max, maximum.
Represents P for heterogeneity across the 2 diet groups, estimated by regressing each baseline characteristic against diet group.
Estimated based on a subsample who completed ≥1 Web-based 24-h dietary assessment and based on intakes from food sources only. Assessments were averaged for participants who completed >1 assessment. The numbers of British Indian participants who completed ≥1 dietary assessments were as follows: 1333 meat eaters, 397 vegetarians, and 248 vegans. Estimates were adjusted for age at recruitment (<45, 45–49, 50–54, 55–59, 60–64, and ≥65 y).
Hemoglobin levels, red blood cell counts, and prevalence of anemia
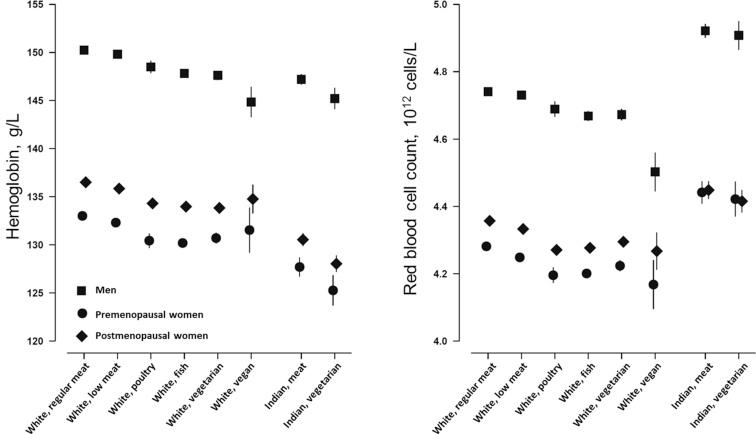
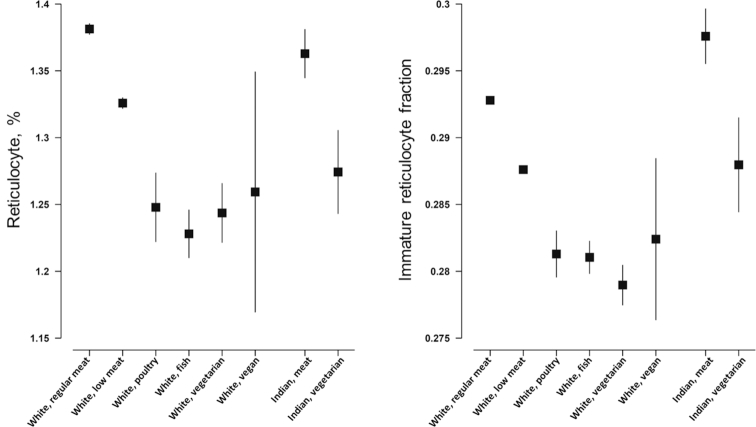
Mean hemoglobin concentration, red blood cell count, reticulocyte percentage, and immature reticulocyte fraction are shown in Figures 1 and 2 and Supplemental Tables 1–6. In white British participants, compared with regular meat eaters, all other diet groups had lower hemoglobin concentrations [e.g., 150.3 g/L (95% CI: 150.2, 150.3 g/L) in regular meat-eating men compared with 144.8 g/L (95% CI: 143.2, 146.3 g/L) in vegan men], but the difference was not statistically significant in vegan women (Figure 1 and Supplemental Tables 1–6). All other diet groups also had lower red blood cell counts, lower reticulocyte percentages (not significant in vegans), and lower immature reticulocyte fraction (not significant in vegan men and premenopausal women who were low meat eaters, poultry eaters, or vegans). In British Indians, vegetarians had lower mean hemoglobin [e.g., 147.2 g/L (95% CI: 146.7, 147.7 g/L) in meat-eating men compared with 145.2 g/L (95% CI: 144.1, 146.2 g/L) in vegetarian men], reticulocyte percentage (not significant in premenopausal women), and immature reticulocyte fraction (not significant in premenopausal women), but similar red blood cell counts. In all populations, results were similar with or without adjustment for smoking.
FIGURE 1.
Hemoglobin concentration and red blood cell count by diet group and ethnicity in the UK Biobank. Point estimates represent adjusted mean levels (95% CIs), estimated based on linear regression models. All estimates were adjusted for age at recruitment (<45, 45–49, 50–54, 55–59, 60–64, and ≥65 y) and smoking (never, previous, current <15 cigarettes/d, current ≥15 cigarettes/d, and unknown). Total numbers of men, premenopausal women, and postmenopausal women, respectively, in the diet groups were as follows: white regular meat eaters: 121,433, 20,791, 67,145; white low meat eaters: 77,457, 29,570, 101,184; white poultry eaters: 1074, 810, 2797; white fish eaters: 2789, 2287, 4643; white vegetarians: 2130, 1636, 2583; white vegans: 166, 76, 149; Indian meat eaters: 2254, 565, 980; Indian vegetarians: 485, 229, 615. P for heterogeneity across the diet groups (stratified by ethnicity and estimated by regressing each variable against diet group) was 0.01 for hemoglobin in British Indian premenopausal women, >0.5 for red blood cell count in all British Indians, and <0.001 for all other variables.
FIGURE 2.
Reticulocyte percentage and immature reticulocyte fraction by diet group and ethnicity in the UK Biobank. Point estimates represent adjusted mean levels (95% CIs), estimated based on linear regression models. All estimates were adjusted for age at recruitment (<45, 45–49, 50–54, 55–59, 60–64, and ≥65 y), sex, and smoking (never, previous, current <15 cigarettes/d, current ≥15 cigarettes/d, and unknown). Total numbers of participants in the diet groups were as follows: white regular meat eaters, 212,831; white low meat eaters, 213,092; white poultry eaters, 4815; white fish eaters, 10,042; white vegetarians, 6548; white vegans, 398; Indian meat eaters, 3875; and Indian vegetarians, 1362. P for heterogeneity across the diet groups (stratified by ethnicity and estimated by regressing each variable against diet group) was >0.2 for reticulocyte percentage and immature reticulocyte fraction in British Indian premenopausal women and <0.001 for all other variables.
Numbers and proportions of people with anemia are reported in Tables 3 and 4. In white men, the proportion of people with anemia was significantly higher in fish eaters compared with regular meat eaters (3.9% compared with 2.9%, respectively), and although proportions with anemia were also higher in poultry eaters, vegetarians, and vegans, the difference was not statistically significant (Table 3). In both white premenopausal and postmenopausal women, compared with regular meat eaters, low meat eaters, poultry eaters, fish eaters, and vegetarians (12.8% of vegetarians compared with 8.7% of regular meat eaters in premenopausal women and 5.8% of vegetarians compared with 3.4% of regular meat eaters in postmenopausal women) were more likely to have anemia. In British Indians, vegetarian men and postmenopausal women were more likely to have anemia compared with meat eaters (12.6% of vegetarian men compared with 7.6% of meat eaters and 19.2% of vegetarian postmenopausal women compared with 13.3% of meat eaters), but there was no statistically significant difference in premenopausal women (26.6% of vegetarian premenopausal women compared with 20.5% of meat eaters) (Table 4). Results were similar when we restricted the analyses to participants who did not report taking iron or B vitamin supplements and also when we applied more detailed correction for smoking; proportions of participants with microcytic or macrocytic anemia were small in all subgroups (Supplemental Tables 7 and 8).
TABLE 3.
Anemia prevalence in white British participants by diet group in the UK Biobank1
| Meat eaters | |||||||
|---|---|---|---|---|---|---|---|
| Classification2 | Regular consumption (>3 times/wk) (total n = 212,831)3 | Low consumption (≤3 times/wk) (total n = 213,092)3 | Poultry eaters (total n = 4815) | Fish eaters (total n = 10,042) | Vegetarians (total n = 6548) | Vegans (total n = 398) | P-het4 |
| Anemia in men, n (%) | 3517 (2.9)a | 2200 (2.8)a | 43 (4.0)a,b | 108 (3.9)b | 83 (3.9)a,b | 11 (6.6)a,b | <0.001 |
| Mild | 3239 (2.7) | 2049 (2.6) | 38 (3.5) | 104 (3.7) | 78 (3.7) | 9 (5.4) | |
| Moderate | 261 (0.2) | 142 (0.2) | 5 (0.5) | 4 (0.1) | 5 (0.2) | 1 (0.6) | |
| Severe | 17 (0.0)a | 9 (0.0)a | 0 (0.0)a,b | 0 (0.0)a | 0 (0.0)a,b | 1 (0.6)b | <0.001 |
| Anemia in premenopausal women, n (%) | 1804 (8.7)a | 2888 (9.8)b | 105 (13.0)c | 309 (13.5)c | 209 (12.8)c | 6 (7.9)a,b,c | <0.001 |
| Mild | 1346 (6.5) | 2097 (7.1) | 81 (10.0) | 230 (10.1) | 146 (8.9) | 4 (5.3) | |
| Moderate | 442 (2.1) | 766 (2.6) | 23 (2.8) | 75 (3.3) | 63 (3.9) | 0 (0.0) | |
| Severe | 16 (0.1)a | 25 (0.1)b | 1 (0.1)b,c | 4 (0.2)c | 0 (0.0)c | 2 (2.6)a,b,c | <0.001 |
| Anemia in postmenopausal women, n (%) | 2300 (3.4)a | 3838 (3.8)b | 154 (5.5)c | 248 (5.3)c | 149 (5.8)c | 6 (4.0)a,b,c | <0.001 |
| Mild | 1974 (2.9) | 3331 (3.3) | 140 (5.0) | 218 (4.7) | 129 (5.0) | 5 (3.4) | |
| Moderate | 315 (0.5) | 494 (0.5) | 13 (0.5) | 30 (0.6) | 20 (0.8) | 0 (0.0) | |
| Severe | 11 (0.0)a | 13 (0.0)b | 1 (0.0)c | 0 (0.0)c | 0 (0.0)c | 1 (0.7)a,b,c | <0.001 |
n = 447,726. Groups that do not share a superscript letter were significantly different at the 5% level from post hoc pairwise comparisons based on linear regression models and after Bonferroni correction for multiple comparisons.
Anemia is defined as hemoglobin <130 g/L for men and <120 g/L for women. Mild anemia, hemoglobin 110–129 g/L in men and 110–119 g/L in women; moderate anemia, hemoglobin 80–109 g/L (both sexes); and severe anemia, hemoglobin <80 g/L (both sexes). For defining anemia and all subtypes of anemia, hemoglobin is adjusted by −3 g/L in current smokers.
Includes participants who consume any red or processed meat (beef, lamb, pork, and processed meat), regardless of whether they consume poultry, fish, dairy, or eggs. Cutoffs of regular and low consumption were determined based on consumption of red and processed meat as reported on the touch-screen questionnaire.
Represents P for heterogeneity across the 6 diet groups, estimated by regressing each row variable against diet group.
TABLE 4.
Anemia prevalence in British Indian participants by diet group in the UK Biobank1
| Classification | Meat eaters (total n = 3875) | Vegetarians (total n = 1362) | P-het2 |
|---|---|---|---|
| Anemia in men, n (%) | 172 (7.6) | 61 (12.6) | <0.001 |
| Mild | 157 (7.0) | 59 (12.2) | |
| Moderate | 15 (0.7) | 2 (0.4) | |
| Severe | 0 (0.0) | 0 (0.0) | 0.002 |
| Anemia in premenopausal women, n (%) | 116 (20.5) | 61 (26.6) | 0.061 |
| Mild | 80 (14.2) | 38 (16.6) | |
| Moderate | 35 (6.2) | 23 (10.0) | |
| Severe | 1 (0.2) | 0 (0.0) | 0.042 |
| Anemia in postmenopausal women, n (%) | 130 (13.3) | 118 (19.2) | 0.002 |
| Mild | 106 (10.8) | 92 (15.0) | |
| Moderate | 24 (2.4) | 24 (3.9) | |
| Severe | 0 (0.0) | 2 (0.3) | <0.001 |
n = 5237. Anemia is defined as hemoglobin <130 g/L for men and <120 g/L for women. Mild anemia, hemoglobin 110–129 g/L in men and 110–119 g/L in women; moderate anemia, hemoglobin 80–109 g/L (both sexes); and severe anemia, hemoglobin <80 g/L (both sexes). For defining anemia and all subtypes of anemia, hemoglobin is adjusted by −3 g/L in current smokers.
Represents P for heterogeneity across the 2 diet groups, estimated by regressing each row variable against diet group.
White blood cell count
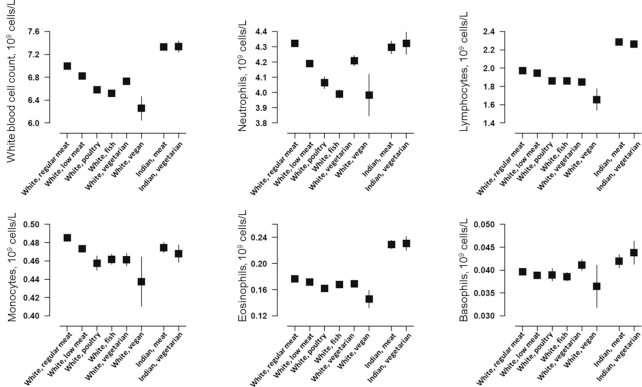
Total and specific (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) white blood cell counts are shown by diet group in Figure 3 and Supplemental Tables 9 and 10. In the white population, compared with regular meat eaters, all other diet groups had lower mean counts of total white cells [e.g., 7.02 × 109 cells/L (95% CI: 7.01, 7.03 × 109 cells/L) in regular meat eaters compared with 6.22 × 109 cells/L (95% CI: 6.01, 6.43 × 109 cells/L) in vegans], neutrophils, lymphocytes, monocytes, and eosinophils; basophil counts appeared similar across diet groups, although counts were low overall. In the Indian population, there was no significant difference in any of the white blood cell counts between meat eaters and vegetarians. Results were similar with or without adjustment for smoking and when including only people who reported having no long-standing illness (n = 295,590 in white British participants; n = 3465 in British Indian participants).
FIGURE 3.
White blood cell counts by diet group and ethnicity in the UK Biobank. Point estimates represent adjusted mean levels (95% CIs), estimated based on linear regression models. All estimates were adjusted for age at recruitment (<45, 45–49, 50–54, 55–59, 60–64, and ≥65 y), sex, and smoking (never, previous, current <15 cigarettes/d, current ≥15 cigarettes/d, and unknown). Total numbers of participants in the diet groups were as follows: white regular meat eaters, 212,831; white low meat eaters, 213,092; white poultry eaters, 4815; white fish eaters, 10,042; white vegetarians, 6548; white vegans, 398; Indian meat eaters, 3875; and Indian vegetarians, 1362. P for heterogeneity across the diet groups (stratified by ethnicity and estimated by regressing each variable against diet group) was >0.1 for all variables in British Indians and <0.001 for all variables in white British participants.
Platelet count and volume
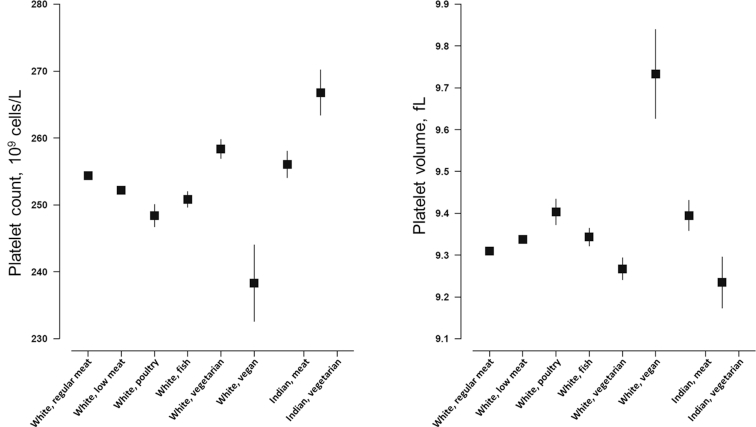
Mean levels of platelet count and platelet volume are plotted in Figure 4 and shown in Supplemental Tables 9 and 10. Compared with white regular meat eaters (mean: 254.5 × 109 cells/L; 95% CI: 254.2, 254.7 × 109 cells/L), the low meat eaters, poultry eaters, fish eaters, and vegans (mean: 238.2 × 109 cells/L; 95% CI: 232.5, 243.9 × 109 cells/L) had lower mean platelet counts, whereas vegetarians had higher counts (mean: 258.2 × 109 cells/L; 95% CI: 256.8, 259.7 × 109 cells/L). On the other hand, vegetarians had lower mean platelet volume (mean: 9.27 fL; 95% CI: 9.24, 9.29 fL), whereas the other diet groups, especially vegans (mean: 9.73 fL; 95% CI: 9.63, 9.84 fL), had higher platelet volumes compared with regular meat eaters (mean: 9.31 fL; 95% CI: 9.31, 9.32 fL). In British Indians, vegetarians had higher mean platelet count (mean: 266.7 × 109 cells/L; 95% CI: 263.3, 270.0 × 109 cells/L) but lower mean platelet volume (mean: 9.24 fL; 95% CI: 9.18, 9.30 fL) compared with meat eaters (mean: 256.1 × 109 cells/L; 95% CI: 254.1, 258.1 × 109 cells/L; mean: 9.39 fL; 95% CI: 9.36, 9.43 fL). Results were consistent when participants were classified as having low platelet count or elevated platelet volume (Supplemental Tables 7 and 8).
FIGURE 4.
Platelet count and volume by diet group and ethnicity in the UK Biobank. Point estimates represent adjusted mean levels (95% CIs), estimated based on linear regression models. All estimates were adjusted for age at recruitment (<45, 45–49, 50–54, 55–59, 60–64, and ≥65 y), sex, and smoking (never, previous, current <15 cigarettes/d, current ≥15 cigarettes/d, and unknown). Total numbers of participants in the diet groups were as follows: white regular meat eaters, 212,831; white low meat eaters, 213,092; white poultry eaters, 4815; white fish eaters, 10,042; white vegetarians, 6548; white vegans, 398; Indian meat eaters, 3875; and Indian vegetarians, 1362. P for heterogeneity across the diet groups (stratified by ethnicity and estimated by regressing each variable against diet group) was <0.001 for all variables.
Discussion
The current study, with >450,000 participants, is the largest study ever conducted to examine hematological parameters by degrees of animal food consumption in white British and British Indian individuals. In the white British population, compared with regular meat eaters, low or non-meat eaters generally had lower hemoglobin concentrations and were more likely to have anemia, and they had lower white blood cell counts. Vegans had lower mean platelet count and higher mean platelet volume, whereas the reverse was true for vegetarians. In British Indians, vegetarians had lower mean hemoglobin, were more likely to be anemic, and had higher platelet count but lower platelet volume compared with meat eaters. In addition to hematological conditions such as anemia or low platelet count, differences in blood count could be related to future risk of diseases (e.g., cardiovascular disease) or mortality (37–39).
Only a few small existing studies (n vegetarians <100) have reported on blood cell counts in different diet groups, but our findings corroborate their results. Studies have reported that compared with nonvegetarians, vegetarians or vegans had lower hemoglobin levels (7, 9, 10) or lower red blood cell count (8, 11), but the sample sizes of these studies were too small to accurately assess differences in anemia. Other studies have also reported lower white blood cell counts (including lymphocytes and neutrophils) (7, 14, 40, 41) or platelet counts (41) in vegetarians or vegans compared with nonvegetarians. The only study that included British Indian vegetarians (n = 23) found that they had lower hemoglobin but higher platelet count compared with “Caucasian” vegetarians (n = 22) (11), which appeared consistent with our results. On the other hand, a few studies reported that compared with nonvegetarians, vegetarians or vegans had similar levels of hemoglobin (42) or white blood cell counts (8), or higher red blood cell counts (43). Overall, because these previous studies were small, they may lack sufficient statistical power to detect potential differences.
Low or non-meat eaters in the UK Biobank would have little or no intake of heme iron (estimated to be ∼40% of iron from meat sources) (44), which is more easily absorbed than non-heme iron (present in plant foods such as cereals, vegetables, pulses, and fruits) (45, 46). Although vegetarian and vegan diets are usually high in vitamin C (47), which enhances iron absorption, plant-based diets also contain significant amounts of phytates and tannins, which inhibit non-heme iron absorption (45). Previous studies showed that vegetarians had lower non-heme and total iron absorption, as well as lower ferritin concentrations, compared with nonvegetarians, despite similar or higher total dietary iron intake (11, 41, 48–50). Given the crucial role of iron in hemoglobin synthesis and red blood cell production (34), it would be expected that compared with regular meat eaters, low or non-meat eaters may have lower levels of hemoglobin and red blood cells and a higher risk of anemia (51). However, as shown in Tables 1 and 2, low or non-meat eaters were more likely to take iron supplements, as well as multivitamins that may contain iron, which could help prevent or correct anemia. For example, anemia in vegans in the UK Biobank seemed to occur mostly in those who were not taking iron supplements, although numbers were too small to make valid conclusions. In addition, zinc is a catalyst in iron metabolism (21) and is less bioavailable in vegetarian diets (5), and low serum zinc levels have been associated with anemia (21, 22). Vegetarians or vegans also tend to have lower intakes of other micronutrients, such as vitamin A or riboflavin (20), which might also have roles in blood cell or hemoglobin production (24, 25).
Another explanation for differences in red blood cell count is that given the crucial role of vitamin B-12 in erythropoiesis, deficiency in this nutrient in vegetarians or vegans may result in red blood cells that do not develop normally and are too large to leave the bone marrow (13, 52, 53), which subsequently manifests as lower counts of reticulocytes and mature red blood cells in these diet groups. For subtypes of anemia, macrocytic anemia can be induced by vitamin B-12 deficiency but can be masked by the simultaneous presence of microcytic anemia in cases of severe iron deficiency or high folate intake (11, 14, 54); previous studies have shown that all 3 exposures are likely in vegetarians (11, 12, 41). Numbers for anemia subtypes were small in this study, however, and serum levels of relevant nutrients and other parameters that would be used in a clinical setting to classify anemia were not available. Hence, it was not possible to determine how these factors affected the distribution of anemia subtypes in different diet groups.
Although anemia might also be caused by β-thalassemia (52), there is no reason to suspect this genetic condition should vary by diet group, but ethnic differences are possible (53). The prevalence of anemia in British Indians in our cohort was much higher than in the corresponding white British groups; iron intake, especially iron from red or processed meat in the meat eaters, and dietary vitamin B-12 intake were also lower in British Indians. However, detailed comparisons of hematological characteristics across different ethnicities are beyond the scope of the current study, which aimed to assess the differences across diet groups.
A high white blood cell count has been proposed as an early marker of inflammation, and in prospective studies it has been associated with a higher risk of chronic diseases or death (37–39). Evidence linking vegetarian diets to risk of chronic inflammation is limited, but some studies suggest that long-term vegetarianism is associated with lower concentrations of other inflammatory biomarkers, such as C-reactive protein (55, 56). On the other hand, a low white blood cell count may also be an indication of an impaired immune system or abnormal bone marrow pathology (57). Vitamin B-12 deficiency is a reversible cause of bone marrow failure (58), and some case reports have suggested a link between vitamin B-12 deficiency (more likely in vegetarians and vegans) and pancytopenia (low counts of red blood cells, white blood cells, and platelets) (59, 60). Differences in intake of other nutrients between the diet groups might also explain the differences in white blood cell count. Some evidence, largely from in vitro or animal studies, suggests that dietary proteins or specific amino acids might be integral to proper functioning of the immune system, including the production of blood cells (18, 19, 61, 62). Parallel evidence from large-scale human studies is lacking, but previous studies do show differences in dietary and serum amino acids between different diet groups (17). Alternatively, zinc deficiency has also been linked to impaired growth and functioning of immune cells (23). Note that although there were differences between the diet groups, all groups had mean white blood cell counts within the normal range (38, 57).
In addition to their role in blood clotting, platelets are also believed to be involved in chronic inflammation, via interactions with endothelial cells and white blood cells (63, 64); hence, it might be reasonable that diet groups with a low white blood cell count should also have a low platelet count. Alternatively, due to the relatively high vitamin B-12 content in platelets compared to red blood cells (15), it has been suggested that vitamin B-12 has a prominent role in the platelet cell life cycle, and low platelet count and large platelet volume can be a symptom of vitamin B-12 deficiency (14). Studies have documented that low platelet counts in vitamin B-12-deficient patients have responded to vitamin B-12 therapy (16, 65). This fits with the observations of low platelet count and large platelet volume in the vegans in this study, but it does not explain the reverse pattern in vegetarians, who also have relatively low vitamin B-12 intakes.
Strengths of this study include the large sample size of ∼450,000 white and 5000 British Indians in the United Kingdom with complete blood counts; thus, this is the largest study ever conducted on the hematological and associated parameters by degrees of animal food consumption. In the white population, 6 distinct diet groups were included, which allowed the comparison of characteristics across varying degrees of consumption of animal source foods, and we were also able to explore hematological indices separately in British Indians. As with all observational studies, some self-selection bias may be present, and only a modest proportion (5.5%) of invited participants agreed to take part, but a representative cohort is not necessary for making valid assessments of exposure–outcome associations (28). Because the study is cross-sectional, it was not possible to determine causality, and generalizability to other populations, especially ethnic groups other than white British or British Indians, might be limited. The findings would have been enhanced by the inclusion of various relevant indices, including serum ferritin, serum vitamin B-12, and other related markers, which were not available in this study. In addition, we did not determine the prevalence of genetic hemoglobin disorders, which can affect hemoglobin concentrations.
In conclusion, in this large population study in the United Kingdom, people with low or no red meat intake generally had lower hemoglobin concentrations and were slightly more likely to be anemic. In the white population, but not the British Indians, low or non-meat eaters also had lower white blood cell counts. Both white and British Indian vegetarians had higher mean platelet counts and lower mean platelet volumes compared with meat eaters, whereas white vegans had lower mean platelet counts but higher platelet volumes. Future studies should investigate the mechanisms that explain these differences because they might be related to chronic disease risk.
Supplementary Material
Acknowledgments
This research was conducted using the UK Biobank Resource under application 3037. The authors’ responsibilities were as follows—TYNT, TJK, and KEB: conceived and designed the research question; TYNT and KEB: analyzed the data; TYNT, TJK, and KEB: wrote the first draft of the manuscript; KG, TJG, WG, and TAS: provided input on data analysis and interpretation of results and reviewed subsequent drafts; and all authors: revised the manuscript critically for important intellectual content and read and approved the final manuscript. The authors report no conflicts of interest related to the study.
Notes
This work was supported by the UK Medical Research Council (MR/M012190/1), Wellcome Trust Our Planet Our Health (Livestock, Environment and People, LEAP 205212/Z/16/Z), and Cancer Research UK (C8221/A19170). KEB is supported by the Girdlers’ New Zealand Health Research Council Fellowship. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
The UK Biobank is an open-access resource. Bona fide researchers can apply to use the UK Biobank data set by registering and applying at http://www.ukbiobank.ac.uk/register-apply.
Supplemental Methods, Supplemental Figure 1, and Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
References
- 1. Tong TY, Key TJ, Sobiecki JG, Bradbury KE. Anthropometric and physiologic characteristics in white and British Indian vegetarians and nonvegetarians in the UK Biobank. Am J Clin Nutr. 2018;107:909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appleby PN, Davey GK, Key TJ. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public Health Nutr. 2002;5:645–54. [DOI] [PubMed] [Google Scholar]
- 3. Bradbury KE, Crowe FL, Appleby PN, Schmidt JA, Travis RC, Key TJ. Serum concentrations of cholesterol, apolipoprotein A-I and apolipoprotein B in a total of 1694 meat-eaters, fish-eaters, vegetarians and vegans. Eur J Clin Nutr. 2014;68:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pawlak R, Lester SE, Babatunde T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr. 2014;68:541–8. [DOI] [PubMed] [Google Scholar]
- 5. Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. 2003;78:633S–9S. [DOI] [PubMed] [Google Scholar]
- 6. Gibson S, Ashwell M.. The association between red and processed meat consumption and iron intakes and status among British adults. Public Health Nutr. 2003;6:341–50. [DOI] [PubMed] [Google Scholar]
- 7. Pongstaporn W, Bunyaratavej A. Hematological parameters, ferritin and vitamin B12 in vegetarians. J Med Assoc Thai. 1999;82:304–11. [PubMed] [Google Scholar]
- 8. Elorinne AL, Alfthan G, Erlund I, Kivimäki H, Paju A, Salminen I, Turpeinen U, Voutilainen S, Laakso J. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians. PLoS One. 2016;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gear JS, Mann JI, Thorogood M, Carter R, Jelfs R. Biochemical and haematological variables in vegetarians. Br Med J. 1980;280:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li D, Sinclair AJ, Mann NJ, Turner A, Ball MJ. Selected micronutrient intake and status in men with differing meat intakes, vegetarians and vegans. Asia Pac J Clin Nutr. 2000;9:18–23. [DOI] [PubMed] [Google Scholar]
- 11. Reddy S, Sanders T.. Haematological studies on pre-menopausal Indian and Caucasian vegetarians compared with Caucasian omnivores. Br J Nutr. 1990;64:331–8. [DOI] [PubMed] [Google Scholar]
- 12. Gilsing AMJ, Crowe FL, Lloyd-Wright Z, Sanders TAB, Appleby PN, Allen NE, Key TJ. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr. 2010;64:933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105–31. [DOI] [PubMed] [Google Scholar]
- 14. Obeid R, Geisel J, Schorr H, Hübner U, Herrmann W. The impact of vegetarianism on some haematological parameters. Eur J Haematol. 2002;69:275–9. [DOI] [PubMed] [Google Scholar]
- 15. Weiss HJ, Kelly A, Herbert V. Vitamin B12 and folate activity in normal human platelets. Blood. 1968;31:258–62. [PubMed] [Google Scholar]
- 16. Andrès E, Affenberger S, Zimmer J, Vinzio S, Grosu D, Pistol G, Maloisel F, Weitten T, Kaltenbach G, Blicklé JF. Current hematological findings in cobalamin deficiency: a study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28:50–6. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ, Appleby PN, Key TJ, Travis RC. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr. 2016;70:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daly JM, Reynolds J, Sigal RK, Shou J, Liberman MD. Effect of dietary protein and amino acids on immune function. Crit Care Med. 1990;18:S86–93. [PubMed] [Google Scholar]
- 19. Li P, Yin Y-L, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–52. [DOI] [PubMed] [Google Scholar]
- 20. Kristensen NB, Madsen ML, Hansen TH, Allin KH, Hoppe C, Fagt S, Lausten MS, Gøbel RJ, Vestergaard H, Hansen T et al.. Intake of macro- and micronutrients in Danish vegans. Nutr J. 2015;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelkitli E, Ozturk N, Aslan NA, Kilic-Baygutalp N, Bayraktutan Z, Kurt N, Bakan N, Bakan E. Serum zinc levels in patients with iron deficiency anemia and its association with symptoms of iron deficiency anemia. Ann Hematol. 2016;95:751–6. [DOI] [PubMed] [Google Scholar]
- 22. Houghton LA, Parnell WR, Thomson CD, Green TJ, Gibson RS. Serum zinc is a major predictor of anemia and mediates the effect of selenium on hemoglobin in school-aged children in a nationally representative survey in New Zealand. J Nutr. 2016;146:1670–6. [DOI] [PubMed] [Google Scholar]
- 23. Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powers HJ, Hill MH, Mushtaq S, Dainty JR, Majsak-Newman G, Williams EA. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am J Clin Nutr. 2011;93:1274–84. [DOI] [PubMed] [Google Scholar]
- 25. García-Casal MN, Layrisse M, Solano L, Barón MA, Arguello F, Llovera D, Ramírez J, Leets I, Tropper E. Vitamin A and β-carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J Nutr. 1998;128:646–50. [DOI] [PubMed] [Google Scholar]
- 26. Collins R. What makes UK Biobank special? Lancet. 2012;379:1173–4. [DOI] [PubMed] [Google Scholar]
- 27. UK Biobank Coordinating Centre. UK Biobank: Protocol for a Large-scale Prospective Epidemiological Resource [Internet]. 2007. Available from: http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf. [Google Scholar]
- 28. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu B, Young H, Crowe FL, Benson VS, Spencer EA, Key TJ, Appleby PN, Beral V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011;14:1998–2005. [DOI] [PubMed] [Google Scholar]
- 30. Bradbury KE, Tong TYN, Key TJ. Dietary intake of high-protein foods and other major foods in meat-eaters, poultry-eaters, fish-eaters, vegetarians, and vegans in UK Biobank. Nutrients. 2017;9:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holland B, Welch A, Unwin I, Buss D, Paul A, Southgate DAT. McCance and Widdowson's the Composition of Foods. 5th ed Cambridge (UK): Royal Society of Chemistry; 1991. [Google Scholar]
- 32. Elliott P, Peakman TC.. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–44. [DOI] [PubMed] [Google Scholar]
- 33. Sheard SM, Froggatt J.. UK Biobank Haematology Data Companion Document [Internet]. 2017. Available from: https://biobank.ctsu.ox.ac.uk/crystal/docs/haematology.pdf. [Google Scholar]
- 34. WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity: Vitamin and Mineral Nutrition Information System Background [Internet]. 2011. Available from: https://www.who.int/vmnis/indicators/haemoglobin/en/. [Google Scholar]
- 35. Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Haemoglobin adjustments to define anaemia. Trop Med Int Heal. 2008;13:1267–71. [DOI] [PubMed] [Google Scholar]
- 36. Buttarello M. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how?. Int J Lab Hematol. 2016;38:123–32. [DOI] [PubMed] [Google Scholar]
- 37. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, or leukocyte count with coronary heart disease. J Am Med Assoc. 1998;279:1477–82. [DOI] [PubMed] [Google Scholar]
- 38. Shah AD, Thornley S, Chung S-C, Denaxas S, Jackson R, Hemingway H. White cell count in the normal range and short-term and long-term mortality: international comparisons of electronic health record cohorts in England and New Zealand. BMJ Open. 2017;7:e013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kabat GC, Kim MY, Manson JE, Lessin L, Lin J, Wassertheil-Smoller S, Rohan TE. White blood cell count and total and cause-specific mortality in the Women's Health Initiative. Am J Epidemiol. 2017;186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nazarewicz R. [The effect of vegetarian diet on selected biochemical and blood morphology parameters]. Rocz Panstw Zakl Hig. 2007;58:23–7. [PubMed] [Google Scholar]
- 41. Haddad EH, Berk LS, Kettering JD, Hubbard RW, Peters WR. Dietary intake and biochemical, hematologic, and immune status of vegans compared with nonvegetarians. Am J Clin Nutr. 1999;70:586–93. [DOI] [PubMed] [Google Scholar]
- 42. Harvey LJ, Armah CN, Dainty JR, Foxall RJ, Lewis DJ, Langford NJ, Fairweather-Tait SJ. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br J Nutr. 2005;94:557. [DOI] [PubMed] [Google Scholar]
- 43. Sambol SZ, Stimac D, Orlić ZC, Guina T. Haematological, biochemical and bone density parameters in vegetarians and non-vegetarians. West Indian Med J. 2009;58:512–7. [PubMed] [Google Scholar]
- 44. Cross AJ, Harnly JM, Ferrucci LM, Risch A, Mayne ST, Sinha R. Developing a heme iron database for meats according to meat type, cooking method and doneness level. Food Nutr Sci. 2012;3:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hallberg L, Hulthén L.. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability. Am J Clin Nutr. 2000;71:1147–60. [DOI] [PubMed] [Google Scholar]
- 46. Wallace DF. The regulation of iron absorption and homeostasis. Clin Biochem Rev. 2016;37:51–62. [PMC free article] [PubMed] [Google Scholar]
- 47. Sobiecki JG, Appleby PN, Bradbury KE, Key TJ. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr Res. 2016;36:464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hunt JR, Roughead ZK. Nonheme-iron absorption, fecal ferritin excretion, and blood indexes of iron status in women consuming controlled lactoovovegetarian diets for 8 wk. Am J Clin Nutr. 1999;69:944–52. [DOI] [PubMed] [Google Scholar]
- 49. Haider LM, Schwingshackl L, Hoffmann G, Ekmekcioglu C. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58:1359–74. [DOI] [PubMed] [Google Scholar]
- 50. Pawlak R, Bell K.. Iron status of vegetarian children: a review of literature. Ann Nutr Metab. 2017;70:88–99. [DOI] [PubMed] [Google Scholar]
- 51. WHO. Blood Donor Counselling Implementation Guidelines [Internet]. 2014. Available from: http://www.who.int/bloodsafety/voluntary_donation/Blooddonorcounselling.pdf. [PubMed] [Google Scholar]
- 52. Karimi M, Cohan N, De Sanctis V, Mallat NS, Taher A. Guidelines for diagnosis and management of β-thalassemia intermedia. Pediatr Hematol Oncol. 2014;31:583–96. [DOI] [PubMed] [Google Scholar]
- 53. Galanello R, Origa R. β-Thalassemia. Orphanet J Rare Dis. 2010;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kwok T, Cheng G, Woo J, Lai WK, Pang CP. Independent effect of vitamin B12 deficiency on hematological status in older Chinese vegetarian women. Am J Hematol. 2002;70:186–90. [DOI] [PubMed] [Google Scholar]
- 55. Jaceldo-Siegl K, Haddad E, Knutsen S, Fan J, Lloren J, Bellinger D, Fraser GE. Lower C-reactive protein and IL-6 associated with vegetarian diets are mediated by BMI. Nutr Metab Cardiovasc Dis. 2018;28:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haghighatdoost F, Bellissimo N, Totosy De Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2017;20:2713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Osei-Bimpong A, Mclean R, Bhonda E, Lewis SM. The use of the white cell count and haemoglobin in combination as an effective screen to predict the normality of the full blood count. Int J Lab Hematol. 2012;34:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stabler SP. Vitamin B deficiency. N Engl J Med. 2013;368:149–60. [DOI] [PubMed] [Google Scholar]
- 59. Randhawa J, Ondrejka SL, Setrakian S, Taylor H. What should I know before ordering a bone marrow aspiration/biopsy in patients with vitamin B12 deficiency?. BMJ Case Rep. 2013;2013:12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Braza JM, Bhargava P.. Medical mystery: a 71-year-old man with pancytopenia—the answer. N Engl J Med. 2008;359:1941. [DOI] [PubMed] [Google Scholar]
- 61. Cunha MCR, Lima F da S, Vinolo MAR, Hastreiter A, Curi R, Borelli P, Fock RA. Protein malnutrition induces bone marrow mesenchymal stem cells commitment to adipogenic differentiation leading to hematopoietic failure. PLoS One. 2013;8:e58872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fock RA, Blatt SL, Beutler B, Pereira J, Tsujita M, de Barros FE, Borelli P. Study of lymphocyte subpopulations in bone marrow in a model of protein-energy malnutrition. Nutrition. 2010;26:1021–8. [DOI] [PubMed] [Google Scholar]
- 63. Stokes KY, Granger DN.. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McNicol A, Israels SJ. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008;8:99–117. [DOI] [PubMed] [Google Scholar]
- 65. Bolaman Z, Kadikoylu G, Yukselen V, Yavasoglu I, Barutca S, Senturk T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clin Ther. 2003;25:3124–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.