ABSTRACT
Background
Total energy expenditure (TEE) data in patients with early-stage cancer are scarce, precluding an understanding of energy requirements.
Objective
The objective was to cross-sectionally characterize TEE in patients with colorectal cancer (CRC) and to compare measured TEE with energy recommendations. It was hypothesized that TEE would differ according to body mass, body composition, and physical activity level (PAL) and current energy recommendations would have poor individual-level accuracy.
Methods
Patients with newly diagnosed CRC had resting energy expenditure (REE) measured by indirect calorimetry and TEE by doubly labeled water. Hypermetabolism was defined as REE > 110% of that predicted from the Mifflin St.-Jeor equation. Body composition was assessed via DXA. Physical activity was determined as the ratio of TEE to REE (TEE:REE) (PAL) and residual activity energy expenditure (RAEE). TEE was compared with energy recommendations of 25–30 kcal/d and Dietary Reference Intakes (DRIs) using Bland–Altman analyses. Patients were stratified according to median BMI, PAL, and sex-specific ratio of fat mass (FM) to fat-free mass (FFM).
Results
Twenty-one patients (M:F 14:7; mean ± SD BMI: 28.3 ± 4.9 kg/m2, age: 57 ± 12 y) were included. Most (n = 20) had stage II–III disease; 1 had stage IV. Approximately half (n = 11) were hypermetabolic; TEE was not different in those with hypermetabolism and REE as a percentage of predicted was not correlated with TEE. Mean ± SD TEE was 2473 ± 499 kcal/d (range: 1562–3622 kcal/d), or 29.7 ± 6.3 kcal/kg body weight (range: 20.4–48.5 kcal/kg body weight). Mean ± SD PAL was 1.43 ± 0.27. The energy recommendation of 25 kcal/kg underestimated TEE (−12.6% ± 16.5%, P = 0.002); all energy recommendations had wide limits of agreement (the smallest was DRI with measured PAL: −21.2% to 29.3%). Patients with higher BMI and FM:FFM had higher bias using kilocalories per kilogram recommendations; bias from several recommendations was frequently lower (i.e. underestimation) in patients with higher PAL and RAEE.
Conclusions
TEE variability was not reflected in energy recommendations and error was related to body weight, body composition, and physical activity. This trial was registered at clinicaltrials.gov as NCT03131921.
Keywords: energy expenditure, energy metabolism, cancer, energy requirements, energy balance, nutritional assessment, dietary intake, body composition, physical activity
Introduction
Energy balance is the long-term relation between energy intake and total energy expenditure (TEE; the sum of energy required for bodily maintenance at rest, movement, and food digestion, absorption, and transport). Characterizing TEE is therefore essential for understanding energy requirements needed to support or modulate energy balance. This concept is especially relevant for individuals with cancer because body weight and body composition changes [i.e., loss of fat-free mass (FFM)] can be detrimental to prognosis (1–3). Conversely, weight gain during cancer treatment may not confer a survival advantage in some circumstances (1), might worsen pre-existing comorbidities, and may increase secondary disease risk in patients with obesity (4, 5).
In oncology, most of our understanding of energy expenditure comes from studies of resting energy expenditure (REE), which is the largest component of TEE in nonathletic populations. However, in patients with cancer, REE might be affected by changes in body composition, systemic inflammation, or tumor burden and predicted REE may not correlate with TEE (6). Because the ratio of TEE to REE is indicative of physical activity level (PAL), absence of a relation between REE and TEE indicates that variable physical activity might affect TEE within this population, rather than REE alone.
To date, only 4 reports have measured TEE in cancer using objective and accurate techniques such as doubly labeled water (DLW) or bicarbonate-urea (6–9), which severely limits the current understanding of energy requirements in oncology settings. The majority of patients in these previous studies had advanced (i.e., stage IV) disease (6) or severe weight loss (i.e., 19% of pre-illness body weight) (7). However, this likely represents a small proportion of patients with certain types of cancer. For example, colorectal cancer (CRC) is the third most commonly diagnosed cancer in the world (10); improvements in screening practices, lower incidence of risk factors, and effective treatment options have led to a higher proportion of cancer cases diagnosed at earlier stages (11), where severe wasting/weight loss [i.e., cachexia (12)] and high systemic inflammation are less common (13). These patients also have a high prevalence of obesity at diagnosis and weight gain during curative-intent treatment (14).
Owing to the paucity of data characterizing TEE in patients with cancer, current oncology energy intake recommendations are based on an estimate of 25–30 kcal/kg body weight with a call for further research (15). However, basing recommendations on body weight alone would likely overestimate energy requirements in individuals with obesity and underestimate it in those with low body weight (16). Furthermore, such recommendations do not consider body composition, physical activity, cancer type, or disease stage, which might affect TEE.
The objectives of the current study were to compare TEE with current energy recommendations and to characterize TEE in relation to body weight, body composition, and physical activity. It was hypothesized that current energy recommendations would have poor individual-level accuracy and TEE would differ according to body mass, body composition, and PAL categories.
Methods
Study and subjects
This analysis is part of a larger cross-sectional study (NCT03131921) measuring energy expenditure, body composition, physical activity, and dietary intake in patients with cancer (17). Patients with stage II–IV CRC were recruited from the Cross Cancer Institute in Edmonton, Alberta, Canada. In line with common practice in gastrointestinal oncology, patients with stage II or III CRC were considered to have “early-stage” disease. In addition, patients with lympho-vascular invasion, T4 tumor size, gastrointestinal obstruction, or high tumor grade were considered to have a high risk of recurrence and were advised to undergo surgical removal of the tumor. Recruitment for the full ongoing trial began in April, 2016; between March, 2017 and January, 2018, patients were offered additional TEE and body composition assessments. This study was approved by the Health Research Ethics Board of Alberta and informed consent was obtained from all patients before study assessments. Inclusion criteria were recent cancer diagnosis, being aged 18–90 y, and able to communicate freely in English. Exclusion criteria included anticancer therapy or surgery within the past 4 wk, confinement to a wheelchair, medications or conditions that might affect body composition or metabolism (steroids, hormone replacement, or unstable thyroid disease), inability to breathe under the calorimetry hood for 30 min, pregnancy, or breastfeeding. All measurements were completed within (before or after) 2 wk of starting anticancer therapy, where applicable.
Patient-reported measures
Individuals in this study were asked to complete several profiling questionnaires. Patients completed the Patient-Generated Subjective Global Assessment (PG-SGA)—short form (18), which consists of 4 sections: weight (score range: 0–5), food intake (score range: 0–4), symptoms (score range: 0–24), and activities and function (score range: 0–3). Lower scores indicate better results in each section. The European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire—C30 (version 3.0) (19) was also completed; only overall quality of life score (range: 1–7) was used in this analysis, with higher scores representing better quality of life. The International Physical Activity Questionnaire (IPAQ)—Long Form (20) was used to measure subjective physical activity; continuous values from the IPAQ were expressed as metabolic equivalency of tasks (MET) minutes per week.
Anthropometry and body composition
Height and weight were measured using a Health-O-Meter Professional digital scale with height rod (model number: 597KL), with shoes and heavy clothing removed. One-month and 6-mo previous weight change percentages were collected from the PG-SGA. BMI was calculated as kg/m2 and classified according to the WHO's cutoffs (21).
Body composition was assessed by DXA (Lunar iDXA, GE Healthcare; Encore 2001 software version 13.60) within a median 9 (IQR: 7–14) d of energy expenditure assessments. Fat mass (FM) and fat-free mass (FFM) were expressed adjusting for height in m2 [fat mass index (FMI) and fat-free mass index (FFMI)] and as a ratio (FM:FFM), to represent metabolic load and capacity as explained elsewhere (22). Percentage body fat was also reported. Appendicular skeletal muscle index (ASMI) was calculated as the sum of lean soft tissue from limbs divided by height squared (kg/m2), with low ASMI defined as <5.45 for women and <7.26 for men (23). Similarly, FFMI <15 for women and <16 for men were used to define “myopenia” for exploratory purposes (24).
REE
An indirect calorimeter with ventilated hood system (VMax Spectra 29N, Nutritional Assessment Instrument; Sensor-Medics) was used to measure REE. This particular system is considered one of the most accurate metabolic carts (25) and has been used as a gold standard in previous studies (26, 27). Volume and air flow were calibrated before each measurement using a 3-L syringe. Gas analyzers were calibrated before each test with standard gas concentrations of 20.95% oxygen and 0.03% carbon dioxide. The fraction of expired carbon dioxide was kept between 0.75 and 0.80 for as much time as possible. Breath samples were collected for 30 min and only steady-state data (variations in volume of oxygen and carbon dioxide of ≤10% over 5 consecutive minutes) were used. The abbreviated Weir equation (28) was used to calculate REE. Respiratory quotient was calculated as the ratio of carbon dioxide produced to oxygen consumed (CO2:O2). Measured REE was compared with predicted REE to identify high or low REE, or hyper- or hypometabolism, respectively. The Mifflin St.-Jeor equation was used for predicted REE because it predicts REE with the most accuracy (29).
TEE
TEE was the primary outcome of this investigation and was assessed using DLW over 14 d. Stock doses were formulated using 10 atom% oxygen 18 (18O) and 99.9 atom% deuterium (2H) based on 1 g 18O and 0.1 g 2H per kilogram of body weight per patient. A single baseline urine sample was collected before dosing (predose). Patients drank the dose with a straw followed by ∼50 mL tap water to rinse the dose cup; actual dose was therefore assumed to be the same as the dose given. All patients were asked to collect a urine sample 4.5 and 6 h after dosing and 1–2 times/d for the following 13 d. Only isotope enrichments from urine samples from predose, 4.5 and 6 h postdose, and days 3, 7, and 14 were analyzed.
Measurements of 2H and 18O isotope enrichments from stock doses and urine samples were analyzed by using a dual-inlet chromium reduction and continuous flow isotope ratio mass spectrometer at the NIH. Natural logarithms of 2H and 18O enrichments were regressed against time, with slopes of regression lines representing rates of 2H and 18O loss from body water (kH and kO, respectively). 2H and 18O dilution spaces (NH and NO, respectively) were determined by dividing administered isotopes (in moles) by the intercepts. Total body water was then calculated as (30, 31):
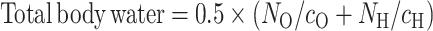 |
(1) |
where cH and cO were the 2H and 18O pool sizes relative to total body water. To account for some isotopes entering organic pools, nonaqueous cH was assumed to be 1.041 and cO was assumed to be 1.007. The isotope fractionation for 2H leaving the body as water vapor is 0.946 times the true rate of water it equilibrates with and the fractionation factor for 18O leaving the body as carbon dioxide is 1.038 times the true rate of carbon dioxide production (32). We assumed breath was saturated with water vapor and nonsweat skin water vapor loss was proportional to exposed skin surface; therefore the simplified equation from the International Atomic Energy Agency (32) was used to calculate carbon dioxide as follows:
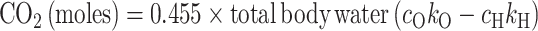 |
(2) |
Carbon dioxide was used in the modified Weir equation to calculate TEE as:
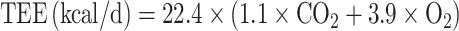 |
(3) |
where oxygen (in liters per day) was calculated by:
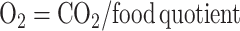 |
(4) |
The food quotient was assumed to be 0.86, representative of a typical diet at the population level (33).
Quality control measures to screen for unacceptable estimates included confirming the following for each patient: 18O enrichment/intercept >0.08, linear fit of 2H and 18O slopes, kO/kH 1.1–1.7, similar residuals of predicted and measured 2H and 18O, and NH/NO 1.0–1.7. One patient provided urine samples for isotope analysis on days 11 and 17 and both were assessed. Another patient underwent unexpected surgery on day 5 and had 4 d of samples; because all the aforementioned quality control measures were met (including kO/kH = 1.315 and NH/NO = 1.050) and our results were similar with and without this patient, the data were kept in the final analyses.
TEE was expressed as kcal/d and kcal/kg body weight measured at the study visit (the same day as isotopic dosing and REE measurement). Predicted TEE was calculated as 25 kcal/kg and 30 kcal/kg body weight based on internationally accepted clinical oncology guidelines from the European Society for Clinical Nutrition and Metabolism (15) and from Dietary Reference Intakes (DRIs) (34), using the overweight and obese–specific equation where appropriate. For exploratory purposes, IPAQ categories were used to determine physical activity categories for the DRI TEE equation as follows: sedentary, IPAQ category 1; low active, IPAQ category 2; active, IPAQ category 3.
Physical activity
PAL was determined as the ratio of TEE to REE. Because PAL is a ratio method and subject to bias because the regression intercept is not zero (35) (or could be indicative of a nonlinear relation), activity was also expressed as residual activity energy expenditure (RAEE) (36). This was calculated as the residual from TEE (dependent) and REE (independent), with positive values being associated with higher-than-average physical activity and negative numbers being associated with lower-than-average physical activity (expressed in kcal/d).
Patients were asked to wear ActiCal accelerometers (Phillips Respironics) during the 14-d collection period on the right hip. A 15-s epoch length was used. Patients were also asked to keep a record of wear times, including time awoken in the morning and time to bed in the evening. A valid day of monitoring was defined as ≥12 h of wear time (37). Only patients with ≥4 valid days of accelerometer monitoring were included (38). TEE calculations from ActiCal were also compared with measured TEE.
Medical variables
At the time of assessment, patients were scheduled to begin either radiation, chemotherapy, combined radiation and chemotherapy, or surveillance. Neutrophil:lymphocyte ratio from medical records was used as a measure of systemic inflammation; only the value closest to the study date was assessed in a cross-sectional manner. Prospective weight change over treatment or surveillance was also acquired from medical records and expressed as % weight change/100 d to account for varying follow-up appointment dates.
Statistical analysis
All data were assessed using SPSS software version 24 (IBM Corp.), with the threshold for significance set at P ≤ 0.05. Normality in variables was determined using the Shapiro–Wilk test. Nonnormally distributed variables were reported as median and IQR; otherwise, values are expressed as mean ± SD. Effect size for post hoc sample size analysis was calculated using TEE data (n = 12) at baseline from an ongoing clinical trial in a similar population (39). An effect size of 0.73 and α of 0.05 yielded a power of 0.89 to detect a mean difference of 246 ± 334 kcal/d between measured and predicted TEE from the DRI energy recommendation using a 2-tailed paired-samples t test.
Pearson correlation coefficients or Spearman rank-order correlations (for nonparametric variables) described relations between variables. BMI and PAL were split by the sample median and FM:FFM was split by the sex-specific sample median to explore differences in energy expenditure. Paired t tests assessed differences in parameters within individuals. Independent-samples t tests or the Mann–Whitney U test (when dependent variables were nonnormally distributed for each group of the independent variable) determined differences between patient groups stratified by sex, previous radiotherapy (yes or no), percentage REE from predicted, ASMI, PAL median, RAEE (negative compared with positive residuals), BMI median, sex-specific FM:FFM median, or TEE. Bland–Altman analyses were used to assess the agreement between measured and predicted TEE from current energy intake recommendations and ActiCal-derived TEE. Bias indicates group-level agreement and is the mean difference between predicted minus measured values. Limits of agreement, or bias ± 1.96 SD, indicates agreement for each individual. Bias and limits of agreement were expressed as percentages to account for body size and individual energy expenditure. Proportional bias was quantified by Pearson correlation coefficients between the mean of measured and predicted TEE and bias to determine if there were trends in the magnitude of bias with increasing TEE.
Results
Patients
Between 1 March, 2017 and 31 January, 2018, 143 patients with CRC were approached to participate, with 49 completing REE measurements (39.8% overall accrual). Of those, a total of 21 patients (14 men) completed the optional DLW assessments (42.8% accrual of those who completed basic study measurements), with 20 completing body composition and accelerometer measurements (Supplemental Figure 1). Patient characteristics are presented in Table 1. Only 1 patient had stage IV disease; this patient was not an outlier in terms of energy expenditure or body composition measurements. All other patients had stage II (n = 3, 14.3%) or stage III (n = 17, 80.1%) disease and most individuals presented with overweight (n = 8, 38.1%) or obesity (n = 8, 38.1%). Mean previous 1-mo weight change was −1.5% ± 3.4% (range: −7.9% to 4.9%) and previous 6-mo weight change was −5.3% ± 5.1% (range: −20.0% to 0%), with no differences in weight loss between sexes. Seven patients had weight loss >5% in the past 6 mo. Four patients had undergone neoadjuvant combined radiotherapy and chemotherapy (>1 mo before study inclusion), with 2 having colon cancer and 2 having rectal cancer. There were no differences in anthropometric, demographic, energy expenditure (including PAL), or body composition variables between those who had received or not received radiotherapy. Most (n = 17) patients had undergone surgery for early-stage high-risk disease before (n = 10, median: 49 d; IQR: 45–65 d from study visit) or after (n = 7, median: 102 d; IQR: 95–102 d) the study visit. Because many individuals will experience recurrence after curative treatment (40) owing to the presence of microscopic residual disease after surgery, individuals in this study were still considered as patients with cancer after surgical resection. Most (n = 10, 47.6%) were scheduled to undergo adjuvant chemotherapy with folinic acid, fluorouracil, and oxaliplatin, with the remaining patients scheduled to begin neoadjuvant radiochemotherapy (n = 8, 38.1%), neoadjuvant short-course radiotherapy (n = 2, 9.5%), or surveillance (n = 1, 4.8%).
TABLE 1.
Characteristics of 21 patients with colorectal cancer1
| Characteristic | Total (n = 21)2 | Men (n = 14) | Women (n = 7) | P value3 |
|---|---|---|---|---|
| Age, y | 57 ± 12 (34–73) | 55 ± 13 (34–72) | 59 ± 13 (40–73) | 0.582 |
| Body weight, kg | 85.1 ± 18.4 (54.3–131.1) | 91.5 ± 17.3 (68.6–131.1) | 72.5 ± 14.0 (54.3–92.6) | 0.021 |
| BMI, kg/m2 | 28.3 ± 4.9 (20.9–39.5) | 29.2 ± 4.9 (20.9–39.5) | 26.7 ± 4.9 (22.0–35.0) | 0.294 |
| FM, kg | 28.8 ± 12.3 (9.9–59.8) | 29.5 ± 13.8 (9.9–59.8) | 27.6 ± 9.6 (16.5–41.4) | 0.754 |
| FM index, kg/m2 | 9.6 ± 3.8 (3.1–18.0) | 9.3 ± 13.8 (3.1–18.0) | 10.1 ± 3.4 (6.3–15.1) | 0.651 |
| Percentage fat | 32.9 ± 8.7 (14.7–45.6) | 30.6 ± 9.1 (14.7–45.6) | 37.3 ± 6.3 (27.6–44.4) | 0.101 |
| FFM, kg | 56.3 ± 10.7 (37.6–74.1) | 62.6 ± 6.8 (48.1–74.1) | 44.6 ± 5.1 (37.6–51.8) | <0.001 |
| FFM index, kg/m2 | 18.6 ± 2.4 (14.1–22.2) | 19.8 ± 1.8 (16.5–22.2) | 16.5 ± 1.9 (14.1–19.8) | 0.001 |
| FM:FFM | 0.51 ± 0.19 (0.17–0.84) | 0.46 ± 0.19 (0.17–0.84) | 0.61 ± 0.16 (0.38–0.80) | 0.102 |
| Appendicular skeletal muscle, kg | 23.9 ± 5.2 (16.2–42.6) | 26.8 ± 3.8 (20.3–42.6) | 18.5 ± 2.1 (16.2–21.4) | 0.001 |
| Appendicular skeletal muscle index, kg/m2 | 7.8 ± 1.2 (5.7–12.3) | 8.3 ± 1.1 (6.9–12.3) | 6.9 ± 0.9 (5.7–8.4) | 0.018 |
| REE, kcal/d | 1698 (IQR: 1446–2009) | 1841 (IQR: 1668–2077) | 1423 (IQR: 1388–1500) | <0.001 |
| Respiratory quotient | 0.80 ± 0.05 (0.73–0.93) | 0.81 ± 0.05 (0.73–0.93) | 0.79 ± 0.03 (0.74–0.82) | 0.393 |
| TEE, kcal/d | 2473 ± 499 (1562–3622) | 2646 ± 490 (1929–3622) | 2127 ± 313 (1562–2509) | 0.020 |
| TEE, kcal/kg body weight | 29.7 ± 6.3 (20.4–48.5) | 29.7 ± 7.1 (20.4–48.5) | 29.8 ± 4.8 (25.1–36.1) | 0.952 |
| PAL | 1.43 ± 0.27 (1.04–2.16) | 1.40 ± 0.29 (1.04–2.16) | 1.49 ± 0.22 (1.04–1.76) | 0.463 |
Values are mean ± SD (range) or median (IQR) for nonnormality between groups. PAL is TEE:REE. FFM, fat-free mass; FM, fat mass; PAL, physical activity level; REE, resting energy expenditure; TEE, total energy expenditure.
n = 20 total and n = 13 men with body composition measurements.
All differences tested using independent-samples t test except in the case of nonnormality wherein Mann–Whitney U test was used.
Patient-reported measures
Most patients had low scores for all PG-SGA boxes, indicating good nutritional status and physical function. Most (n = 11, 52.4%) scored 0 for weight change. All patients scored 0 (n = 9, 42.9%) or 1 (n = 12, 57.1%) for food intake. Symptom score was variable (range: 0–6), with most (n = 13, 61.9%) indicating no symptoms. Within activities and function, most patients indicated they were “normal with no limitations” (n = 10, 47.6%) or “not my normal self, but able to be up and about with fairly normal activities” (n = 9, 42.9%), with 2 (9.5%) selecting “able to do little activity and spend most of the day in bed or chair.” Median global quality of life score was 75 (IQR: 58.3–83.3), corresponding to median 5.5 (IQR: 4.5–6.0) on a scale of 1–7. Self-reported physical activity from IPAQ was highly variable: median (IQR) walking was 693 (396–2871) MET-min/wk and median (IQR) moderate activity was 900 (300–1875) MET-min/wk. Most (n = 17, 81.0%) did not report vigorous activity. Median (IQR) total reported MET-minutes per week was 1955 (1265–5724) MET-min/wk.
Anthropometrics and body composition
Anthropometric and body composition variables are presented in Table 1. As expected, FFM and FFMI were lower in women; however, there were no differences in FM or FMI between the sexes. Median BMI was 28.7; median FM:FFM was 0.44 in men and 0.63 in women.
Energy expenditure description
All measures of TEE from DLW met quality control estimates. Mean tracer elimination rate (kO/kH) from DLW was normal (1.281 ± 0.050) and 2H:18O distribution volume (NH/NO) was 1.036 ± 0.018. Men had higher REE and TEE, but not PAL (Table 1). Group median (IQR) REE was 1698 (1446–2009) kcal/d (mean ± SD: 1764 ± 415 kcal/d), which was higher than the Mifflin St.-Jeor prediction (median: 1545; IQR: 1411–1817 kcal/d, P = 0.001). Approximately half (n = 11, 52.4%) of patients had hypermetabolism and none had measured REE < 90% of predicted (suggestive of hypometabolism). Patients with hypermetabolism had lower mean PAL (1.31 ± 0.22 compared with 1.56 ± 0.26, P = 0.024) and RAEE (−179 ± 318 compared with 196 ± 373 kcal/d from the regression line, P = 0.022). However, percentage REE bias was not correlated with TEE in kilocalories per day or kilocalories per kilogram per day and there were no differences in TEE, or percentage previous 1-mo or 6-mo weight change, between groups; in other words, higher than “expected” REE was associated with lower physical activity but did not relate to total energy requirements or weight change.
Characteristics of TEE and PAL are presented in Table 1. A wide variability in TEE expressed as kcal/d (range: 1562–3622 kcal/d) and kcal · kg body weight–1 · d–1 (range: 20.4–48.5 kcal · kg body weight–1 · d–1) was observed. Men had higher absolute TEE than women, although TEE in kilocalories per kilogram body weight and PAL were not different between sexes. Approximately half (n = 12, 57.1%) of patients fell within the range 25–30 kcal/kg body weight (Figure 1). Mean PAL was 1.43 ± 0.27 (median: 1.49) and was also variable, ranging from 1.04 to 2.16.
FIGURE 1.
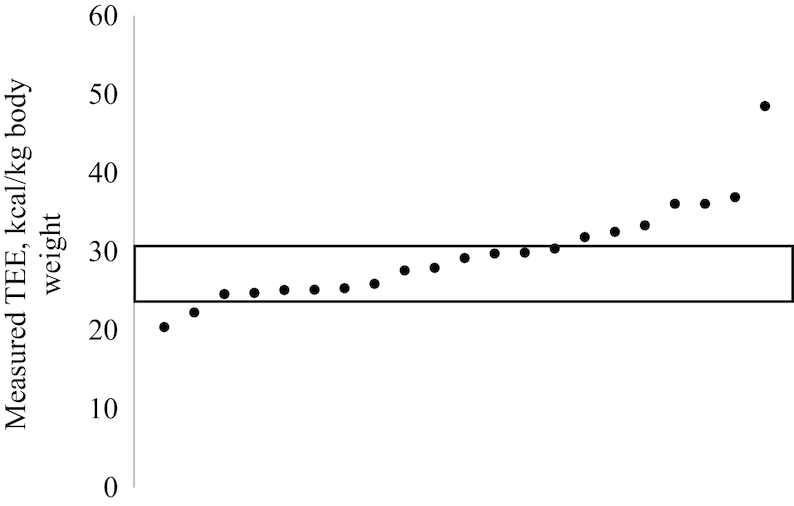
Range of measured TEE in kilocalories per kilogram of body weight in 21 patients with colorectal cancer. Each point is a patient. The box represents current recommendations of 25–30 kcal/kg body weight from the European Society for Clinical Nutrition and Metabolism (15). TEE, total energy expenditure.
Relations between energy expenditure variables and age, body weight, FM, and FFM are shown in Table 2. REE and TEE were positively correlated with body weight and FFM, with higher correlations observed with FFM than with body weight. PAL and RAEE were not related to any variable. Four patients had low ASMI (all men) and 2 of these had weight loss >2% in the previous 6 mo (i.e., cachectic). There were no differences in any anthropometric, energy expenditure, or physical activity variables between individuals with low and normal ASMI; these results were the same when only men were assessed. Similarly, only 1 patient had FFMI below predefined cutoff values, precluding any further comparison.
TABLE 2.
Correlations between energy expenditure, demographic, and body composition variables1
| Age | Weight | FM | FFM | FM:FFM | |
|---|---|---|---|---|---|
| REE2 | −0.353 | 0.729* | 0.388 | 0.873* | −0.029 |
| TEE | −0.382 | 0.558* | 0.350 | 0.658* | 0.025 |
| PAL | 0.163 | −0.366 | −0.396 | −0.255 | −0.273 |
| RAEE | 0.083 | 0.050 | −0.093 | 0.213 | −0.197 |
Values are r values. n = 21. *P < 0.05, correlation. FFM, fat-free mass; FM, fat mass; PAL, physical activity level; RAEE, residual activity energy expenditure (residual from TEE and REE); REE, resting energy expenditure; TEE, total energy expenditure.
Spearman's rank-order correlation; all other values derived from Pearson correlation.
Agreement with energy recommendation estimations
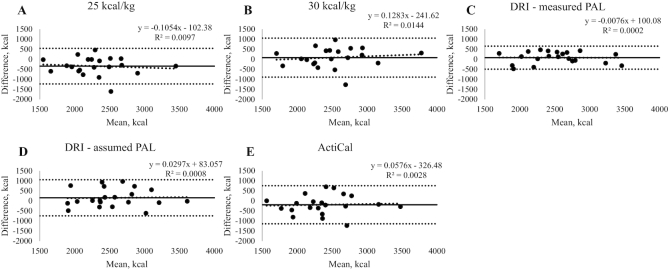
Energy recommendations were correlated with measured TEE in all equations (r: 0.548–0.826, P: 0.010 to <0.001). Predicted energy recommendation with 25 kcal/kg was lower than measured TEE (2128 ± 459 compared with 2473 ± 499 kcal/d, P = 0.002), but all other estimations were not different at a group level (Table 3). However, less than one-half of patients had TEEs within 10% of all recommendations. Wide limits of agreement were also observed between TEE and all energy recommendations; for example, even the recommendation with the smallest limits of agreement (DRI with measured PAL) under-predicted by ≤22.5% below (484 kcal/d) to 22.7% above (468 kcal/d) measured TEE (Figure 2). Using assumed PAL from IPAQ categories did not improve the prediction ability and produced the widest limits of agreement (−33.5%, 50.2%, or −742, 1060 kcal/d). No proportional bias was apparent in any recommendation.
TABLE 3.
Agreement between measured and estimated TEE1
| Proportional bias2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| kcal/d | Percentage bias | r | P | LOA | Absolute LOA | Minimum difference | Maximum difference | Within 10% of measured TEE | |
| Measured TEE | 2473 ± 499 | ||||||||
| 25 kcal/kg | 2128 ± 459* | −12.6 ± 16.5 | −0.099 | 0.670 | −45.1, 19.8 | 64.9 | −48.5 | 22.4 | 7 (33.3) |
| 30 kcal/kg | 2554 ± 551 | 4.8 ± 19.9 | 0.120 | 0.604 | −34.1, 43.8 | 77.8 | −38.2 | 46.9 | 8 (38.1) |
| DRI − measured PAL | 2554 ± 495 | 4.1 ± 12.9 | −0.012 | 0.958 | −21.2, 29.3 | 50.5 | −22.5 | 22.7 | 10 (47.6) |
| DRI − assumed PAL | 2632 ± 510 | 8.3 ± 21.4 | 0.029 | 0.901 | −33.5, 50.2 | 83.8 | −22.5 | 48.9 | 10 (47.6) |
| ActiCal | 2359 ± 549 | −4.6 ± 19.5 | 0.125 | 0.600 | −42.7, 33.6 | 76.3 | −35.1 | 43.3 | 9 (42.9) |
Values are means ± SDs, percentages, or n (%) unless otherwise indicated. n = 21. *P ≤ 0.05, difference between measured TEE and energy intake recommendations via paired-samples t test. DRI, Dietary Reference Intake; LOA, limits of agreement; PAL, physical activity level; TEE, total energy expenditure.
Proportional bias determined as Pearson correlation between bias and mean of measured and predicted TEE.
FIGURE 2.
Bland–Altman plots of measured compared with predicted TEE from 25 kcal/kg (A), 30 kcal/kg (B), DRI with measured PAL (C), DRI with assumed PAL (D), and ActiCal accelerometer (E) in 21 patients with colorectal cancer. The middle solid line represents bias (mean difference between measured and predicted TEE) and the 2 parallel dotted lines represent the 95% limits of agreement (bias ± 1.96 SD). Proportional bias was determined as the Pearson correlation coefficient between the mean of measured and predicted TEE and bias; no proportional bias was apparent in any recommendation. PAL was measured as TEE:resting energy expenditure. DRI was calculated using measured PAL and estimated from a subjective questionnaire. DRI, Dietary Reference Intake; PAL, physical activity level; TEE, total energy expenditure.
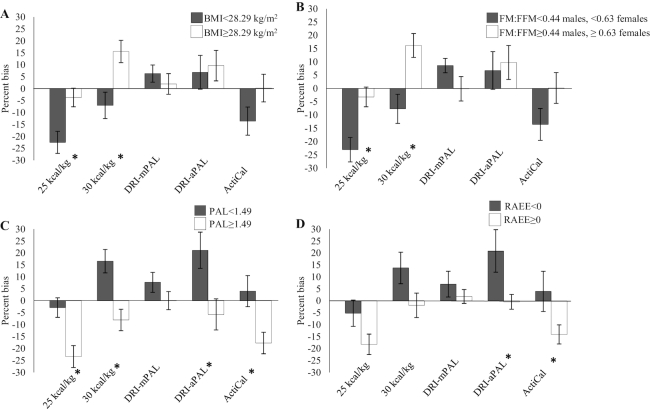
Body weight, FM, and FM:FFM were positively correlated with percentage bias using 25 kcal/kg and 30 kcal/kg (Table 4). PAL and RAEE were negatively correlated with percentage bias from 25 kcal/kg, 30 kcal/kg, DRI with assumed PAL, and ActiCal TEE. Mean percentage bias using 25 kcal/kg and 30 kcal/kg was lower (i.e., underestimation) in those with BMI and FM:FFM below the medians (median BMI: 28.29; median FM:FFM: men, 0.44; women, 0.63) (Figure 3). Bias was frequently lower in those with higher PAL and RAEE (Figure 3). Patients with TEE > 30 kcal/kg (n = 7) had lower BMI (24.1 ± 3.3 compared with 30.4 ± 4.2, P < 0.001), higher PAL (1.67 ± 0.23 compared with 1.31 ± 0.20, P = 0.001), and higher RAEE (309 ± 387 compared with −154 ± 291 kcal/d, P = 0.006). The REE bias from Mifflin St.-Jeor equations was not related to the bias from TEE equations.
TABLE 4.
Correlation of percentage bias between TEE and estimations with patient characteristics1
| Age | Weight | FM | FFM | FM:FFM | PAL | RAEE | |
|---|---|---|---|---|---|---|---|
| 25 kcal/kg | 0.133 | 0.509* | 0.586* | 0.285 | 0.507* | −0.767* | −0.722* |
| 30 kcal/kg | 0.133 | 0.509* | 0.586* | 0.285 | 0.507* | −0.767* | −0.722* |
| DRI − measured PAL | −0.240 | −0.008 | −0.225 | 0.245 | −0.410 | −0.344 | −0.384 |
| DRI − assumed PAL | −0.194 | 0.187 | 0.084 | 0.290 | −0.085 | −0.791* | −0.760* |
| ActiCal | −0.107 | 0.478* | 0.429 | 0.380 | 0.297 | −0.631* | −0.587* |
n = 21. Percentage bias calculated as (energy intake recommendation minus TEE / TEE) × 100. *P < 0.05, Pearson correlation. DRI, Dietary Reference Intake; FFM, fat-free mass; FM, fat mass; PAL, physical activity level; RAEE, residual activity energy expenditure (residual from TEE and resting energy expenditure); TEE, total energy expenditure.
FIGURE 3.
Percentage bias of predicted minus measured total energy expenditure according to the median of BMI (A), FM:FFM (B), PAL (C), and RAEE (D). n = 21. Data are mean ± SEM. *P ≤ 0.05, independent-samples t test. aPAL, assumed PAL from subjective questionnaire; FFM, fat-free mass; FM, fat mass; mPAL, measured PAL; PAL, physical activity level; RAEE, residual activity energy expenditure.
Activity patterns
Mean wear time of the ActiCal devices was 12 ± 3 d, with 20 patients having ≥4 d of wear time and ≥1 weekend (2 d) available. Total IPAQ score was not correlated with any measure of energy expenditure and no other correlations between activity and body composition, physical function, or quality of life were observed.
Clinical parameters
Mean weight change during treatment was −2.4 ± 5.2%/100 d and was not associated with any energy expenditure, body composition, or physical activity variables. Mean neutrophil:lymphocyte ratio was 3.4 ± 2.2 with a range of 1.29–9.33 and was also not associated with any other variable.
Discussion
This study is the first to measure TEE in free-living conditions in patients with primarily early-stage CRC. TEE and PAL were higher than previously reported and were greatly variable. Current energy intake recommendations (15, 34) did not reflect TEE in this cohort. Such discrepancies were due to highly variable body composition and PAL, the latter of which cannot accurately be estimated by patient recall.
As screening and treatment methods continue to improve, it is expected that more patients will be diagnosed at earlier stages of cancer with longer survival; therefore, understanding differences in energy requirements in different cohorts of patients (i.e., early compared with late stages or by cancer type) is important for optimal nutritional care. However, our current knowledge relies primarily on patients with cachexia and/or advanced disease, which might be unrepresentative of many patients with CRC. The largest study to date that objectively measured TEE using DLW included 24 cachectic patients with advanced pancreatic cancer who had a mean BMI of 20 and 19% pre-illness weight loss (7). Mean REE was higher and TEE was lower than predicted; mean ± SD PAL was 1.24 ± 0.20 at baseline. Others have reported overall low PAL (8) and TEE (6) and that structured exercise can increase TEE (9) in sample sizes ranging from 4 to 8 patients with various cancer types. Mean PAL of our sample was 1.43 ± 0.27, which is higher than previously reported in oncology (7, 8); this value corresponds to a “low active” lifestyle (34) and is slightly lower than reported in healthy individuals (PAL: 1.6) (41). Compared with previous research (6, 7), patients in the current sample had generally earlier-stage disease, less weight loss, and lower incidence of low ASMI and low FFMI. Notably, CRC is associated with lower incidence of weight/loss cachexia than are other cancer types (e.g., pancreatic, lung, or gastric cancer) (42). Most individuals in this study also had adequate physical function and PAL was highly variable. In advanced cachectic patients, higher REE and lower TEE may indicate an adaptive response to narrow the gap between TEE and reduced energy intake or a reflection of low physical activity secondary to the disease and its associated side effects (7), which may not occur in earlier-stage CRC. Our findings are novel and suggest that energy metabolism—and therefore energy requirements—differs according to cancer site and stage. Further exploration of the determinants of TEE and PAL according to cancer site and stage is warranted.
We found that energy intake recommendations based on body weight alone were poor assessments of actual energy requirements (assumed to be equal to TEE), with individual differences ranging from −1613 kcal/d (or 48.5%) under-prediction with 25 kcal · kg body weight–1 · d–1 to 968 kcal/d (or 46.9%) over-prediction with 30 kcal · kg body weight–1 · d–1. In addition, a small proportion of energy requirement predictions fell within 10% of measured TEE, ranging from 33.3% using 25 kcal · kg–1 · d–1 to 47.6% using DRI with measured PAL and DRI with assumed PAL. This proportion is smaller than previous reports in healthy adults (62.9–85.7%) (43, 44), suggesting that cancer affects TEE in ways not captured by current energy recommendations.
We found that bias using body weight–based equations was positively related to body weight and composition (i.e., higher body weight, FM, and higher FM:FFM related to over-prediction). Because obesity is a risk factor for several cancers (including CRC) (45, 46), a large number of individuals have obesity at diagnosis (47). However, low FFM is apparent at diagnosis independent of body weight and FM and is not a condition exclusive to advanced cancer (2). Energy recommendations might therefore have widespread error within oncology, although further research in other populations is required.
Whereas previous research suggested that TEE might be lower in the presence of high REE (7), this was not apparent in the current study. Assuming an altered TEE based on REE alone or by applying a universal activity and/or energy factor to measured or estimated REE likely introduces substantial bias in energy recommendations. Several previous studies have investigated REE in patients with CRC (48–52) or mixed tumor types (53, 54). However, many of these were limited in their interpretation of REE in relation to body composition because REE was often divided by measures of muscularity (e.g., FFM), which creates a statistical bias wherein smaller individuals will appear to have higher REE per kilogram of FFM (i.e., patients with low body weight or cachexia might have an artificially high REE), as we (55) and others (56–58) have discussed. Nevertheless, previous studies collectively suggest that REE and body composition might differ according to tumor site (53, 59, 60) and relate to cancer stage and systemic inflammation (51, 61). Although neutrophil:lymphocyte ratio was not associated with energy metabolism in the present analyses, more sensitive indexes of systemic inflammation (i.e., C-reactive protein, IL-6, and TNF-α) might relate to TEE and PAL and should be investigated in more depth. The current study builds upon this line of investigation and provides new evidence that body composition and physical activity might also relate to energy requirements to a greater degree than “high” REE. Equations that incorporate body composition and physical activity and that are developed from oncology populations would likely be more accurate, although further research on the feasibility and accuracy of such approaches is needed.
Physical activity is highly variable in healthy individuals and can significantly affect TEE. In the present study, PAL variability was similar to that of sedentary to lightly active healthy adults (34, 62). According to our data, it appears that physical activity also greatly affected energy requirements in these patients and was the most variable component of TEE. However, subjective measures of physical activity (IPAQ) did not improve estimation of energy requirements and were not related to any physical or clinical measure. This is likely because physical activity is often over- or under-reported (63, 64) and is therefore a poor reflection of actual physical activity engagement. Because physical activity is feasible, safe, and beneficial for patients with cancer (65–67) and affects energy requirements, improved techniques for capturing this modality are needed.
Although this is the largest exploratory study of TEE in early-stage cancer and CRC using several accurate techniques, there are inherent limitations. Firstly, DLW measures TEE over a span of only 2 wk. The impact of anticancer therapy (and associated side effects), body composition changes, or disease progression on TEE and physical activity patterns cannot be assumed, but should be investigated in more depth. Although our sample size was sufficient to detect differences in predicted and measured TEE from the DRI equation, the variability in equation error should be confirmed in samples with larger numbers of individuals and with different tumor types (because energy metabolism might presumably vary in this regard).
In conclusion, TEE and physical activity were highly variable in patients with CRC, which was not apparent in current energy recommendations at an individual level. TEE differed according to categories of body weight, body composition, and physical activity; these variables also affected error associated with energy recommendations. Future research should therefore characterize the feasibility and impact of incorporating body composition and physical activity in the estimation of energy requirements for patients with cancer.
Supplementary Material
Acknowledgments
We thank Dr. Vickie E Baracos for review and discussion of the manuscript.
The authors’ responsibilities were as follows—CMP and SAP: conceptualized the study; SAP, SAE, PJW, TP, HC, MBS, and CMP: were responsible for the research design; SAP: conducted the research and analyzed the data; PJW and HC: provided essential materials; SAP and CMP: wrote the paper and had primary responsibility for final content; and all authors: contributed to data interpretation and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Present address for SAE: Alberta Research Centre for Health Evidence, Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada
Supported by a Canadian Institutes of Health Research New Investigator Salary Award (to CMP) and the Campus Alberta Innovates Program (to CMP) and a Canadian Foundation for Innovation John R Evans Leaders Fund (Project # 34115, to CMP).
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ASMI, appendicular skeletal muscle index; cH, deuterium pool size; cO, oxygen 18 pool size; CRC, colorectal cancer; DLW, doubly labeled water; DRI, Dietary Reference Intake; FFM, fat-free mass; FFMI, fat-free mass index; FM, fat mass; FMI, fat mass index; IPAQ, International Physical Activity Questionnaire; kH, deuterium loss from total body water; kO, oxygen 18 loss from total body water; MET, metabolic equivalency of tasks; NH, deuterium dilution space; NO, oxygen 18 dilution space; PAL, physical activity level; PG-SGA, Patient-Generated Subjective Global Assessment; RAEE, residual activity energy expenditure; REE, resting energy expenditure; TEE, total energy expenditure.
References
- 1. Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, Cespedes Feliciano EM, Xiao J, Caan BJ. Association of weight change after colorectal cancer diagnosis and outcomes in the Kaiser Permanente Northern California population. Cancer Epidemiol Biomarkers Prev. 2017;26:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL, Quesenberry CP, Kwan ML et al.. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross SA, Dzida G, Vora J, Khunti K, Kaiser M, Ligthelm RJ. Impact of weight gain on outcomes in type 2 diabetes. Curr Med Res Opin. 2011;27:1431–8. [DOI] [PubMed] [Google Scholar]
- 5. Demark-Wahnefried W, Campbell K, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(8 Suppl):2277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skipworth RJ, Stene GB, Dahele M, Hendry PO, Small AC, Blum D, Kaasa S, Trottenberg P, Radbruch L, Strasser F et al.. Patient-focused endpoints in advanced cancer: criterion-based validation of accelerometer-based activity monitoring. Clin Nutr. 2011;30:812–21. [DOI] [PubMed] [Google Scholar]
- 7. Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibney E, Elia M, Jebb SA, Murgatroyd P, Jennings G. Total energy expenditure in patients with small-cell lung cancer: results of a validated study using the bicarbonate-urea method. Metabolism. 1997;46:1412–17. [DOI] [PubMed] [Google Scholar]
- 9. Hayes S, Davies PS, Parker T, Bashford J. Total energy expenditure and body composition changes following peripheral blood stem cell transplantation and participation in an exercise programme. Bone Marrow Transpl. 2003;31:331–8. [DOI] [PubMed] [Google Scholar]
- 10. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 11. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. [DOI] [PubMed] [Google Scholar]
- 12. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G et al.. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- 13. Fox KM, Brooks JM, Gandra SR, Markus R, Chiou C-F. Estimation of cachexia among cancer patients based on four definitions. J Oncol. 2009:693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winkels RM, Snetselaar T, Adriaans A, van Warmerdam LJC, Vreugdenhil A, Slooter GD, Straathof JW, Kampman E, van Lieshout R, Beijer S. Changes in body weight in patients with colorectal cancer treated with surgery and adjuvant chemotherapy: an observational study. Cancer Treat Res Commun. 2016;9:111–15. [Google Scholar]
- 15. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S et al.. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. [DOI] [PubMed] [Google Scholar]
- 16. Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188–98. [DOI] [PubMed] [Google Scholar]
- 17. Clinicaltrials.gov. Identifier: NCT03131921. Resting energy expendi-ture in cancer - associations with body composition, dietary intake, and exercise habits. [Internet] Bethesda, MD: National Library of Medicine; 2017; [accessed November 10, 2018]. Available from: https://clinicaltrials.gov/ct2/show/nct03131921. [Google Scholar]
- 18. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12:S15–19. [DOI] [PubMed] [Google Scholar]
- 19. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al.. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- 20. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al.. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sport Exerc. 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 21. World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. WHO Technical Report Series Geneva, Switzerland: WHO; 2000. [PubMed] [Google Scholar]
- 22. Prado CMM, Wells JCK, Smith SR, Stephan BCM, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. [DOI] [PubMed] [Google Scholar]
- 23. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. [DOI] [PubMed] [Google Scholar]
- 24. Fearon K, Evans WJ, Anker SD. Myopenia—a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle. 2011;2:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper JA, Watras AC, O'Brien MJ, Luke A, Dobratz JR, Earthman CP, Schoeller DA. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009;109:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woo P, Murthy G, Wong C, Hursh B, Chanoine J-P, Elango R. Assessing resting energy expenditure in overweight and obese adolescents in a clinical setting: validity of a handheld indirect calorimeter. Pediatr Res. 2016;81:51. [DOI] [PubMed] [Google Scholar]
- 27. Reeves MM, Capra S, Bauer J, Davies PS, Battistutta D. Clinical accuracy of the MedGem indirect calorimeter for measuring resting energy expenditure in cancer patients. Eur J Clin Nutr. 2005;59:603–10. [DOI] [PubMed] [Google Scholar]
- 28. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105:775–89. [DOI] [PubMed] [Google Scholar]
- 30. Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267:E585–90. [DOI] [PubMed] [Google Scholar]
- 31. Speakman JR, Nair KS, Goran MI. Revised equations for calculating CO2 production from doubly labeled water in humans. Am J Physiol. 1993;264:E912–17. [DOI] [PubMed] [Google Scholar]
- 32. International Atomic Energy Agency. Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques. IAEA Human Health Series No. 3 Vienna: IAEA; 2009. [Google Scholar]
- 33. International Dietary Energy Consultancy Group. The Doubly-Labelled Water Method for Measuring Energy Expenditure: Technical Recommendations for use in Humans. A Consensus Report by the IDECG Working Group. Prentice AM.editor. Vienna: International Atomic Energy Agency; 1990. [Google Scholar]
- 34. Food and Nutrition Board of the Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington (DC): The National Academies Press; 2004. [DOI] [PubMed] [Google Scholar]
- 35. Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995;19:644–52. [PubMed] [Google Scholar]
- 36. Most J, Vallo PM, Gilmore LA, St Amant M, Hsia DS, Altazan AD, Beyl RA, Ravussin E, Redman LM. Energy expenditure in pregnant women with obesity does not support energy intake recommendations. Obesity (Silver Spring). 2018;26:992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herrmann SD, Barreira TV, Kang M, Ainsworth BE. Impact of accelerometer wear time on physical activity data: a NHANES semisimulation data approach. Br J Sport Med. 2014;48:278–82. [DOI] [PubMed] [Google Scholar]
- 38. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sport Exerc. 2008;40:181–8. [DOI] [PubMed] [Google Scholar]
- 39. Clinicaltrials.gov. Identifier: NCT02788955. Protein Recommen-dations to Increase Muscle (PRIMe). [Internet] Bethesda, MD: National Library of Medicine; 2017[accessed November 10, 2018]. Available from: https://clinicaltrials.gov/ct2/show/nct02788955. [Google Scholar]
- 40. Mejri N, Dridi M, Labidi S, El Benna H, Daoud N, Boussen H. Annual hazard rate of relapse of stage II and III colorectal cancer after primary therapy. Clin Transl Oncol. 2017;19:1524–30. [DOI] [PubMed] [Google Scholar]
- 41. Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 42. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ et al.. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med. 1980;69:491–7. [DOI] [PubMed] [Google Scholar]
- 43. Ndahimana D, Lee SH, Kim YJ, Son HR, Ishikawa-Takata K, Park J, Kim EK. Accuracy of dietary reference intake predictive equation for estimated energy requirements in female tennis athletes and non-athlete college students: comparison with the doubly labeled water method. Nutr Res Pract. 2017;11:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim E-K, Kim J-H, Kim M-H, Ndahimana D, Yean S-E, Yoon J-S, Kim J-H, Park J, Ishikawa-Takata K. Validation of dietary reference intake equations for estimating energy requirements in Korean adults by using the doubly labeled water method. Nutr Res Pract. 2017;11:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- 46. Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8:e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, Xiao J, Caan BJ. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. 2016;2:1137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nixon DW, Kutner M, Heymsfield S, Foltz AT, Carty C, Seitz S, Casper K, Evans WK, Jeejeebhoy KN, Daly JM et al.. Resting energy expenditure in lung and colon cancer. Metabolism. 1988;37:1059–64. [DOI] [PubMed] [Google Scholar]
- 49. Hansell DT, Davies JW, Burns HJ. Effects of hepatic metastases on resting energy expenditure in patients with colorectal cancer. Br J Surg. 1986;73:659–62. [DOI] [PubMed] [Google Scholar]
- 50. Maguire R, McMillan DC, Wallace AM, McArdle C. A longitudinal study of leptin and appetite, resting energy expenditure and body fat mass in weight-stable cancer patients. Cytokine. 2002;20:174–7. [DOI] [PubMed] [Google Scholar]
- 51. Ravasco P, Monteiro-Grillo I, Camilo M. Colorectal cancer: intrinsic characteristics modulate cancer energy expenditure and the risk of cachexia. Cancer Invest. 2007;25:308–14. [DOI] [PubMed] [Google Scholar]
- 52. Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cao D, Wu G, Zhang B, Quan Y, Wei J, Jin H, Jiang Y, Yang Z. Resting energy expenditure and body composition in patients with newly detected cancer. Clin Nutr. 2010;29:72–7. [DOI] [PubMed] [Google Scholar]
- 54. Souza MTP, Singer P, Ozorio GA, Rosa VM, Alves MMF, Mendoza López RV, Waitzberg DL. Resting energy expenditure and body composition in patients with head and neck cancer: an observational study leading to a new predictive equation. Nutrition. 2018;51–52:60–5. [DOI] [PubMed] [Google Scholar]
- 55. Purcell SA, Elliott SA, Baracos VE, Chu QSC, Prado CM. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr. 2016;70(11):1230–8. [DOI] [PubMed] [Google Scholar]
- 56. Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–8. [DOI] [PubMed] [Google Scholar]
- 57. Hill RJ, Cleghorn GJ, Withers GD, Lewindon PJ, Ee LC, Connor F, Davies PS. Resting energy expenditure in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:342–6. [DOI] [PubMed] [Google Scholar]
- 58. Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C et al.. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fredrix EW, Soeters PB, Rouflart MJ, von Meyenfeldt MF, Saris WH. Resting energy expenditure in patients with newly detected gastric and colorectal cancers. Am J Clin Nutr. 1991;53:1318–22. [DOI] [PubMed] [Google Scholar]
- 60. Fredrix EW, Soeters PB, Wouters EF, Deerenberg IM, von Meyenfeldt MF, Saris WH. Effect of different tumor types on resting energy expenditure. Cancer Res. 1991;51:6138–41. [PubMed] [Google Scholar]
- 61. Ravasco P, Monteiro-Grillo I, Camilo M. How relevant are cytokines in colorectal cancer wasting?. Cancer J. 2007;13:392–8. [DOI] [PubMed] [Google Scholar]
- 62. FAO/WHO/UNU. Human Energy Requirements. Report of a joint FAO/WHO/UNU expert consultation. Rome: FAO; 2001. [Google Scholar]
- 63. Lewis LS, Hernon J, Clark A, Saxton JM. Validation of the IPAQ against different accelerometer cut-points in older cancer survivors and adults at risk of cancer. J Aging Phys Act. 2018;26:34–40. [DOI] [PubMed] [Google Scholar]
- 64. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A et al.. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exerc. 2010;42:1409–26. [DOI] [PubMed] [Google Scholar]
- 66. Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, May AM, Galvão DA, Chinapaw MJ et al.. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. [DOI] [PubMed] [Google Scholar]
- 67. Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: a systematic review. Physiother Canada. 2010;62:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.