ABSTRACT
Background
Child stunting is a major public health problem, afflicting 155 million people worldwide. Lack of animal-source protein has been identified as a risk, but effects of animal protein supplementation are not well established.
Objective
The aim of this study was to investigate effects of animal protein supplementation in mothers, preterm infants, and term infants/children on birth and growth outcomes.
Methods
PubMed, EMBASE, Cochrane library, Web of Science, Cumulative Index of Nursing and Allied Health Literature, and Latin American and Caribbean Health Sciences Literature were searched for randomized controlled trials of animal protein supplementation in mothers or infants and children (≤age 5 y), evaluating measures of anthropometry (≤age 18 y). Main outcomes included birth weight, low birth weight, small for gestational age at birth; height, height-for-age, weight, weight-for-age, weight-for-length, stunting, and wasting ≤18 y of age. Data were extracted independently in duplicate, and findings pooled using inverse variance meta-analysis. Heterogeneity was explored using I2, stratified analysis, and meta-regression, and publication bias by funnel plots, Egger's test, and fill/trim methods.
Results
Of 6808 unique abstracts and 357 full-text articles, 62 trials were included. The 62 trials comprised over 30,000 participants across 5 continents, including formula-based supplementation in infants and food-based supplementation in pregnancy and childhood. Maternal supplementation increased birth weight by 0.06 kg, and both formula and food-based supplementation in term infants/young children increased weight by ≤0.14 kg. Neither formula nor food-based supplementation for term infants/young children increased height, whereas the height-for-age z-score was increased in the food-based (+0.06 z-score) but not formula-based (−0.11 z-score) trials reporting this outcome. In term infants, the weight-for-length z-score was increased in trials of formula (+0.24 z-score) and food supplementation (+0.06 z-score), whereas food supplementation was also associated with reduced odds of stunting (−13%).
Conclusions
Supplementation of protein from animal-source foods generally increased weight and weight-for-length in children, but with more limited effects on other growth outcomes such as attained height.
Keywords: dietary protein, child, maternal, weight, height, anthropometric, birth weight, meta-analysis
Introduction
Suboptimal growth in young children is among the most common forms of undernutrition worldwide, with manifestations including low birth weight (LBW), low childhood height and weight, stunting, and wasting (1). With serious ramifications for physical and cognitive development, improved child growth is a major global target in the context of the United Nation's Sustainable Development Goals. For normal growth, sufficient dietary protein during pregnancy and early childhood is critical, in particular from animal-source foods due to their complete amino acid profile, contents and bioavailability of lysine, sulfur amino acids, and threonine, and associated insulin-like growth factors, iron, zinc, and vitamin B12 (2–6). Extreme protein deficiency leads to hypoalbuminemic malnutrition, metabolic abnormalities, and delayed development; and animal protein-rich foods and supplements have shown beneficial effects in severely undernourished children (7) and in specially formulated food supplements to treat acute malnutrition and wasting (8). However, the role of animal protein in situations of less extreme protein inadequacy (quantity or quality) is less well established.
In animal models, linear growth is sensitive to total dietary protein, for instance acting through stimulation of insulin-like growth factor-1 and its binding proteins (9–11). In humans, observational studies have found that stunting and other sub-optimal growth outcomes are often associated with diets high in staple starches and low in animal sources of protein (12–14). Yet, whereas some studies suggest that higher dietary animal protein is associated with higher growth rates in young children (5, 6), other studies suggest that increased consumption of animal protein or animal foods could result in excessive (obesogenic) growth, for example mediated by insulin-like growth factor-1 (5, 15, 16). Thus, uncertainty remains about the expected impacts of supplemental animal protein and foods in supporting optimal growth in children (13, 17–20), including effects on birth outcomes, linear growth, and prevention of stunting and wasting. In addition, such effects could vary by the period of supplementation, i.e., during pregnancy/lactation, in preterm infants, or during infancy/early childhood.
To address these important gaps in knowledge, we performed a systematic review and meta-analysis of randomized controlled trials to determine the effects of maternal, preterm infant, and term infant/child supplementation of animal protein on child birth and growth outcomes. Elucidating these relations, as well as the remaining evidence gaps, is essential to inform strategies and policymaking to reduce undernutrition globally.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines during all stages of implementation, analysis, and reporting of this meta-analysis.
Primary exposures and outcomes
The primary exposure of interest was the consumption of protein from animal sources, including meat, seafood (including fish), dairy products (including milk), and eggs, as well as animal milk-based infant formulas, by children aged 5 y or younger and pregnant women or postpartum lactating women. The primary outcomes included birth weight and risk of LBW (<2500 g), intrauterine growth retardation, or premature birth (gestational age <37 wk); and height, height-for-age z-score (HAZ), weight, weight-for-age z-score (WAZ), weight-for-length z-score (WFL), and risk of stunting, assessed ≤18 y of age.
Search strategy
Multiple electronic databases were searched for relevant articles including PubMed, EMBASE, The Cochrane Library Web of Science, Cumulative Index of Nursing and Allied Health Literature, and Latin American and Caribbean Health Sciences Literature (LILACS). This was supplemented with hand searching of citation lists and electronic searching of the first 20 “related articles” on PubMed for all included full-text publications; searches of the international standard randomized control trial number register (http://www.isrctn.com/); and contacts with experts. Searches were performed without restrictions on years or language through 25 February 2015 (and updated through to June 2018), with examples of search terms including: (dietary protein OR meat OR dietary supplements OR fortified food OR protein supplement) AND (body height OR growth child development OR pregnancy outcome OR birth weight OR stunting OR height) AND (child OR infant OR pregnant women) AND (clinical trials OR randomized controlled trial). Complete search strategies for each database are presented in the supplementary materials. Title and abstracts of all identified references were screened by 1 investigator (LP). For any potentially relevant article, the full text was retrieved and independently assessed in duplicate by 4 investigators (LP, SK, DK, VM) to determine eligibility, with discrepancies resolved by consensus.
Study selection
Inclusion criteria
We included all randomized control trials that evaluated the effect of animal-source food intake in pregnancy, lactation, or children ≤age 5 y, including premature infants, low-birth-weight infants, and stunted or otherwise malnourished children, on growth outcomes as described above, including an effect measure and information to compute its standard error.
Exclusion criteria
We excluded studies with duration <3 mo; or where the interventions included multiple dietary or other elements that did not allow the isolation of an effect of animal protein or animal food consumption across groups. We also excluded observational studies, cross-sectional ecological studies, commentaries, general reviews, case reports, or trials conducted in populations with major chronic disease (e.g., sickle-cell disease, cystic fibrosis, HIV infection, and phenylketonuria). When duplicate publications from the same study were identified, we included the publication reporting the largest number of participants for each outcome of interest.
Data extraction
Data from included studies were independently extracted by 4 investigators in duplicate (LP, SK, DK, VM) using a standardized electronic form, with any differences resolved by consensus. Information was extracted on the study (first author, years, design, location), participants (sample size, age, sex, race, baseline nutritional status, birth status [term, preterm], socio-economic status, baseline proportion of stunting, primary method of feeding [breastfed, formula fed]), intervention (quantity and source of animal protein, assessment methods, energy adjustment), outcome types, follow-up (duration of follow-up, dropout rate), and growth outcomes (effect size, associated measure of uncertainty). The dose of each intervention was standardized to grams of animal protein per 1000 kcal regular diet. If the precise protein content of the supplement was not reported, it was estimated using the American Diabetes Association and Academy for Nutrition and Dietetics Diabetic Exchange Lists (when not specified, dairy products were assumed to be whole milk, and meat to be medium fat) (21). When volume of formula was not reported, American Academy of Pediatrics (22) recommendations were used to estimate the amount consumed by infants at the mean age of the intervention group. If the total number of calories was not reported, this was estimated using NHANES data based on the age group of the study (23). Direct author contacts were attempted for all missing data.
Quality assessment
Study quality was assessed using the Cochrane Collaborations risk-of-bias tool, evaluating potential for selection bias, performance bias, detection bias, attrition bias, and reporting bias through a 6-question quality control checklist (24). Each question was answered as low risk of bias (score = 1), high risk of bias (score = −1), or unclear (score = 0); and values were summed (potential range: −6 to +6) (Supplemental Table 1). Scores were grouped in approximate tertiles with values of −6 to 0 considered as low quality, 1–3 as medium quality, and 4–6 as high quality.
Statistical analysis
Analyses were stratified by period of supplementation: maternal, preterm, and term/early childhood. For continuous outcomes (e.g., height, HAZ, weight, WAZ, WFL), the primary effect measure was the mean difference in changes from baseline to follow-up in the intervention compared with the control group. If mean changes from baseline were not reported, the difference in follow-up measures between treatment groups was used. For binary outcomes (e.g., risk of stunting, wasting), we extracted the reported OR across treatment groups. The SE for each effect measure was extracted or directly calculated from other reported uncertainty measures (SD, 95% CI, P value). We utilized the values from intent-to-treat analysis as the default. For trials reporting effects by stratum (e.g., by sex or randomized factorial design), we calculated the study-specific effect of animal protein by inverse-weighted meta-analysis across subgroups within that trial. Findings across trials were pooled using inverse variance-weighted meta-analyses (25); random effects weights were also evaluated in sensitivity analyses.
Heterogeneity was assessed using the I2 statistic, with thresholds of <25%, 50%, and >75% considered to represent low, moderate, and high heterogeneity, respectively. We evaluated prespecified sources of potential heterogeneity including country income (high, low/middle), baseline nutritional status (average/unspecified, >30% malnourished), dose of protein supplementation (> or <median), energy supplementation (isocaloric-protein, energy-protein), intervention duration (> or <median), and study quality score (low, medium/high), using stratified analyses and meta-regression to test statistical significance of potential differences. We hypothesized that benefits of supplementation would be greater in low-/middle-income countries than in high-income countries, in malnourished than in average/unspecified nutritional status, in higher than in lower dose of protein, in energy-protein supplementation than in isocaloric-protein supplementation, and in studies with longer intervention duration, and higher study quality score. In post hoc exploratory analyses, we also assessed heterogeneity by child age at baseline and at follow-up. Potential for small-study effects was evaluated by visual inspection of funnel plots and Egger's and Begg's tests (26). For both stratified analyses and evaluation of small-study effects, we focused on outcomes with at least 10 estimates to facilitate statistical power. All analyses were performed with STATA 14 (StataCorp) (2-tailed α = 0.05).
Results
Study characteristics
Of 6808 articles, 62 randomized controlled trials met eligibility criteria (Supplemental Figure 1, Supplemental Information 1), totaling 30,349 unique participants. The trials were conducted across 5 continents including 16 trials in the North America/Caribbean, 16 in Europe, 9 in Asia, 6 in Central and South America, 13 in Africa, and 2 across multiple continents (Table 1). Thirteen trials were conducted with pregnant women, 6 in preterm infants, and 43 in term infants/early childhood. Twenty-eight trials evaluated supplements or foods based on animal protein; 34 trials evaluated a mix of animal and plant protein. Trials of formula-based supplementation in infancy were generally isocaloric, whereas trials of food-based supplementation in pregnancy and childhood generally provided both animal protein and calories. The mean age at randomization was 31.4 y, and gestational age was on average 19.3 wk for pregnant mothers, 1 wk for preterm infants, and 9.3 mo for term infants/children. The mean intervention duration was 23.3 wk for trials during pregnancy and 26 wk for trials in infants/children; with a mean difference in protein between intervention arms of 9.85 g/1000 kcal in pregnant women, 5.6 in preterm infants, and 7.15 in term infants/children.
TABLE 1.
Characteristics of randomized trials examining the effect of animal protein supplementation on growth outcomes in infants and children
| Study author/date | Country (urban or rural) | Age, y for mothers, mo for children (GA in wk for newborns) | No. of subjects | Special population characteristics | Breast- (B) or formula-(F) fed | SES1 | Intervention duration, wk (duration to latest follow-up, wk) | Intervention feeding | Control feeding | Difference in animal protein between groups, g/1000 kcal | Outcomes | Quality Score4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnant mothers | ||||||||||||
| Adams 1978 (27) | USA (urban) | – (–) | 145 | — | — | — | 19 | 40 g/d protein | Usual diet | 18.2 | BW | 1 |
| 6 g/d protein | 3.2 | |||||||||||
| Adu-Afarwuah 2015 (28) | GHA (mixed) | 26.7 (16.2) | 708 | — | — | Low | 24 | Lipid-based nutrient supplement with 18 micronutrients; 2.6 g/d protein | Nutrient supplement with 18 micronutrients | 1.1 | BW, % LBW (<2500 g), % SGA (BW <10th percentile for infants of same GA) | 6 |
| Chan 2006 (29) | USA (mixed) | 16.6 (18) | 49 | — | — | — | 22 | 4 servings/d of dairy (milk, yogurt, cheese); ∼32 g/d protein2 | 4 servings/d of calcium-fortified orange juice; 0 g/d protein2 | 13.9 | BW | −2 |
| Kardjati 1988 (30) | IDN (rural) | 25 (–) | 185 | — | — | Low | 13 | Supplement: high-energy “jamu”; 7.1 g/d protein | Supplement: low-energy “jamu”; 6.2 g/d protein | −0.41 | BW | −1 |
| Mardones- Santander 1988 (31) | CHL (urban) | 22.8 (14.5) | 782 | All underweight (WFL at 14 wk <95% standard) | — | Low | 29 | Powdered milk; 27.9 g/d protein | Powdered milk-based fortified product; 14.5 g/d protein | 6.27 | BW, % LBW (≤2500 g), % SGA | 6 |
| Mridha 2016 (32) | BGD (rural) | 22 (13.1) | 3440 | 31% underweight (BMI <18.5 kg/m2) | — | Low | 27 | Lipid-based nutrient supplement with iron and folic acid; 2.6 g/d protein | Nutrient supplement with iron and folic acid | 1.1 | BW, % LBW (<2500 g), % SGA (BW <10th percentile for infants of same GA) | 6 |
| Rush 1980 (33) | USA (urban) | – (–) | 768 | All underweight | — | — | 15 | Beverage; 10 g/d protein | Usual diet and multivitamins | 2.53 | BW | 1 |
| Beverage; 6 g/d protein | 16.9 | |||||||||||
| Viegas 1982 (34) | UK (urban) | 28 (28) | 44 | — | — | Low | 12 | Chocolate-flavored skim milk powder, multivitamin; 10.6 g/d protein2 | Usual diet, multivitamin, and glucose syrup2 | 4.52 | BW | −1 |
| Wohlleb 1983 (35) | TWN (rural) | 26.5 (–) | 110 | — | — | Low | 40 (92) | Chocolate-flavored, nutrient-rich supplement; 40 g/d protein | Chocolate-flavored, nutrient-rich supplement; 0 g/d protein | 24.1 | BW, weight, height | 2 |
| Pregnant mothers and offspring | ||||||||||||
| Ashorn 2015 (36) | MWI (rural) | Mothers: 25 | 1211 | Neither LBW nor SGA | — | Low | 23 | Lipid-based nutrient supplement; 2.6 g/d protein | Nutrient supplement (same micronutrients as lipid-based nutrient supplement); 0 g/d protein | 1.1 | BW, weight, height, % LBW, % SGA | 6 |
| Children: 6 | 52.1 | 2.54 | ||||||||||
| Elwood 1981 (37) | UK (urban) | Mothers: 25 (–) | — | — | — | Low | 260 | Milk tokens given to pregnant mothers; 19 fl oz milk average consumption; ∼18 g/d protein | Usual diet, no milk tokens | 1.36 | Weight, height | −2 |
| Children: Newborn (0 mo) | 513 | — | — | Milk tokens given to children up to 5 y; 19 fl oz milk average consumption; ∼18 g/d protein | ||||||||
| Mora 1981 (38) | COL (urban) | Mothers: 25.5 (26) | — | — | — | Low | 169 | Skim milk, enriched bread, vegetable oil; 38.4 g/d protein in 3rd trimester | Usual diet | 18.1 | BW, % LBW, weight, height | 1 |
| Children: Newborn (0 mo) | 131 | — | — | Same; 30 g/d protein | 19.3 | |||||||
| Schroeder 1995 (39) | GTM (rural) | – (–) | 399 | — | — | Low | 13 | Protein calorie supplement “Atole” (dry skim milk, sugar, incaparina); 11.5 g/d protein | Low-calorie supplement “Fresco” drink (sugar, flavorings); 0 g/d protein | 4.71 | BW, weight, height | 1 |
| 3 | 514 | 7.80 | ||||||||||
| 12 | 317 | 11.49 | ||||||||||
| 24 | 408 | 15.09 | ||||||||||
| 36 | 491 | 12.47 | ||||||||||
| 48 | 502 | 11.58 | ||||||||||
| 60 | 425 | 11.0 | ||||||||||
| 72 | 416 | 10.52 | ||||||||||
| Preterm infants | ||||||||||||
| Aimone 2009 (40) | CAN (mixed) | 2 | 32 | All LBW (<1800 g) and some SGA3 | Mainly B | — | 12 (44) | Nutrient-enriched human milk; 3.6 g protein/100 mL human milk | Unfortified human milk | 9.04 | Weight, height | 2 |
| Amesz 2010 (41) | NL (urban) | — | 93 | Some LBW (≤1750 g) | F | — | 24 (34) | Nutrient-enriched formula; 1.7 g protein/100 mL2 | Standard term formula; 1.47 g protein/100 mL2 | 3.43 | WAZ, HAZ | −2 |
| Carver 2001 (42) | USA (urban) | 0 | 54 | All LBW (BW ≤1800 g) | F | — | 60 | Postdischarge formula; 2.6 g/100 kcal | Term formula; 2.1 g/100 kcal | 4.01 | Weight, height | 5 |
| Cooke 2010 (43) | UK (mixed) | 0 | 113 | All LBW (≤1500 g) | F | — | 26 (52) | Preterm formula to 6 mo corrected age; 2.2 g protein/100 mL2 | 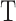 erm formula to 6 mo corrected age; 1.4 g protein/100 mL2 erm formula to 6 mo corrected age; 1.4 g protein/100 mL2
|
8.79 | Weight | −2 |
| Embleton 2005 (44) | UK (urban) | 0.75 | 50 | All LBW (≤1750 g) and SGA (SDS < −2 SD at that gestation) | F | High | 18 | Formula; 2.6 g protein/100 mL2 | Formula; 2.2 g protein/100 mL2 | 4.98 | Weight, height, WFL, HAZ | −2 |
| Formula; 2.4 g protein/100 mL2 | 2.48 | |||||||||||
| Koo 2006 (45) | USA (urban) | 0 | 76 | All LBW (630–1620 g) and SGA3 | F | — | 52 | Nutrient-enriched formula; 2.6 g protein/100 kcal | Term formula; 2.14 g protein/100 kcal | 6.79 | Weight, height, WAZ, HAZ | 6 |
| Term infants (formula intervention) | ||||||||||||
| Borschel 2013 (46) | USA (mixed) | 0.19 | 157 | Neither LBW nor SGA | F | — | 15 | Formula; 2.03 g protein/100 mL2 | Formula; 1.89 g protein/100 mL2 | 2.07 | Weight, height | −2 |
| Fazzolari-Nesci 1992 (47) | SWE (mixed) | 0 | 10 | Neither LBW nor SGA | F | — | 12 | Formula; 0.157 g protein/mL2 | Formula; 0.137 g protein/mL2 | 1.6 | Weight, height | 2 |
| Fleddermann 2014 (48) | SRB | 0.93 | 164 | Neither LBW nor SGA | F | — | 13.1 | Formula; 1.5 g protein/100 mL2 | Formula; 1.3 g protein/100 mL | 2.79 | Weight, height | 6 |
| Fomon 1995 (49) | USA (urban) | 0 | 29 | Neither LBW nor SGA | F | High | 15 | Formula; 1.5 g protein/100 mL2 | Formula; 0.83 g protein/100 mL2 | 8.08 | Weight, height | −1 |
| Graham 1996 (50) | PER (urban) | 9.8 | 30 | All stunted (>3rd percentile NCHS WFL), all SGA3 | F | Low | 13 | Formula: 6.7%E protein, 8.0%E protein | Formula: 5.5%E protein | 5.16, 9.27 | Weight, height | 5 |
| 19.6 | 28 | Formula: 6.4%E protein , 8.0%E protein | Formula: 4.7%E protein | 3.98, 8.93 | ||||||||
| Hanning 1992 (51) | CAN (mixed) | 0 | 95 | Neither LBW nor SGA | F | — | 12 | Formula; 1.59 g protein/100 mL2 | Formula; 1.33 g protein/100 mL2 | 3.89 | Weight, height | 3 |
| Koletzko 2009 (15) | BEL, GER, ITA, POL, ES (urban) | 0.5 | 636 | — | F | Medium | 49.5 (101) | Formula; 2.2 g protein/100 kcal followed by 4.4 g protein/100 kcal | Formula; 1.77 g protein/100 kcal followed by 2.9 g protein/100 kcal | 16.65 | Weight, height, WAZ, HAZ, WFL | 6 |
| Larnkjaer 2009 (52) | DNK (urban) | 9.1 | 86 | Neither LBW nor SGA | Mainly F | High | 13 | Whole milk; ∼10 g/d protein | Formula; ∼1.35 g protein/100 mL | 29.6 | Weight, height, | 6 |
| Lien 2004 (53) | USA (mixed) | 0.25 | 135 | Neither LBW nor SGA | F | — | 12 | Formula; 1.5 g protein/100 mL | Formula; 1.44 g protein/100 mL | 0.57 | Weight, height | 5 |
| Lonnerdal 1990 (54) | TWN (urban) | 0 | — | — | Mainly F | — | 12 | Formula; 1.4 g protein/100 mL2 | Formula; 1.29 g protein/100 mL2 | 1.94 | Weight, height | 6 |
| — | Formula; 1.5 g protein/100 mL2 | 3.88 | ||||||||||
| Lonnerdal 1998 (55) | SWE (mixed) | 0 | 22 | Neither LBW nor SGA | F | — | 19.8 | Formula; 1.5 g protein/100 mL | Formula; 1.3 g protein/100 mL | 1.6 | Weight, height | −3 |
| Oropeza-Ceja 2018 (56) | MEX (urban) | 0.7 | 96 | — | F | Medium | 13.9 | Formula; 1.9 g protein/100 kcal | Formula; 1.43 g protein/100 kcal | 4.7 | Weight, height, WAZ, HAZ, WFL | 6 |
| Formula; 2.18 g protein/100 kcal | 7.5 | |||||||||||
| Raiha 2002 (57) | ITA (urban) | 05 | 58 | Neither LBW nor SGA | F | Medium | 13 | Formula; 2.2 g protein/100 kcal2 | Formula; 1.8 g protein/100 kcal2 | 0.46 | Weight, height, WAZ, HAZ | 1 |
| Rzehak 2009 (58) | GER (urban) | 0 | 235 | Neither LBW nor SGA | F | High | 16 (256) | Formula; 1.6 g protein/100 mL2 | Formula; 1.4 g protein/100 kcal | 2.67 | Weight, height | −1 |
| Formula; 1.9 g protein/100 mL2 | 6.72 | |||||||||||
| Schmelzle 2003 (59) | GER (mixed) | 0.23 | 101 | Neither LBW nor SGA | F | — | 11 | Formula; 1.7g protein/100 mL | Formula; 1.5g protein/100 mL | 4.02 | Weight, height | 4 |
| Timby 2014 (60) | SWE (urban) | 1.5 | 148 | Neither LBW nor SGA | F | High | 26 (52) | Formula; 1.27 g protein/100 mL | Formula; 1.2 g protein/100 mL | −0.46 | WAZ, HAZ | −1 |
| Turck 2006 (61) | FRA (urban) | 0.12 | 74 | Neither LBW nor SGA | F | High | 17 | Formula; 2.6 g protein/100 kcal2 | Formula; 1.8 g protein/100 kcal2 | 9.45 | Weight, height | −1 |
| Weber 2014 (62) | BEL, GER, ITA, POL, ES (mixed) | 0.46 | 448 | Neither LBW nor SGA | F | Medium | 52 (312) | Formula; ∼2.6 g protein/100 mL2 | Formula; ∼1.4 g protein/100 mL2 | 22.8 | Weight, height, WAZ, HAZ | −1 |
| Ziegler 2003 (63) | USA (urban) | 0.25 | 33 | Neither LBW nor SGA | F | High | 15 | Formula; 2.39 g protein/100 mL2 | Formula; 1.92 g protein/100 mL2 | 5.35 | Weight, height | 1 |
| Ziegler 2015 (64) | USA (urban) | 3 | 174 | — | F | High | 38.7 | Formula; 1.39 g protein/100 mL | Formula; 1.08 g protein/100 mL | 5.4 | WAZ, HAZ | 5 |
| Term infants/children (whole-food intervention) | ||||||||||||
| Ackatia-Armah 2015 (65) | MLI (rural) | 14.6 | 623 | All stunted (WFL < −2 SD) | — | Low | 12 | Corn–soy blend “plus plus”: refined cereal–legume–milk blend; 18.4 g/d protein2 | Less refined cereal–legume flour mix: millet, beans, sugar, and oil; 15.5 g/d protein2 | 2.19 | Weight, length, WFL | 3 |
| Alarcon 2003 (66) | TWN, PHL (mixed) | 48.5 | 91 | All stunted (<25th percentile WFL) | Mainly B | — | 12 | Pediasure, 40 mL/kg/d; 12%E as protein | Usual diet | 38.2 | Weight, height, WAZ, HAZ | −2 |
| Bauserman 2015 (67) | DRC (rural) | 6 | 222 | 34.7% stunted (HAZ < −2 SD) | B | Low | 52 | Caterpillar cereal; 6.9 g/d protein for 6–12 mo of age and 10.3 g/d protein for 12–18 mo of age | Usual diet | 8.5 | WAZ, HAZ, WFL, stunting, wasting | 6 |
| Christian 2015 (68) | BGD (rural) | 6.2 | 2962 | −24.7% stunted (HAZ < −2 SD) | — | Low | 52 | Chickpea-based ready-to-eat food containing sugar, soybean oil, and whole-milk powder; 15 g protein/100 g | Usual diet | 4 | Weight, height, HAZ, HAZ%, WFL% | 0 |
| Lentil-based ready-to-eat food containing sugar, soybean oil, and whole-milk powder; 11 g protein/100 g | 5.47 | |||||||||||
| Fabiansen 2017 (69) | BFA (rural) | 11.5 | 1609 | All with moderate acute malnutrition (MUAC ≥ 115 mm and <125 mm and/or WFL ≥±3 and <±2) | — | Low | 12 | Corn–soy blend or lipid-based supplement with dehulled or isolate soy (factorial trial design); 20% of total protein from skimmed milk | Corn–soy blend or lipid-based supplement with dehulled or isolate soy (factorial trial design); no skimmed milk | 2.79 | Weight, height, WFL | 5 |
| Corn–soy blend or lipid-based supplement with dehulled or isolate soy (factorial trial design); 50% of total protein from skimmed milk | 6.99 | |||||||||||
| He 2005 (70) | CHN (urban) | 50.6 | 402 | All stunted3 | — | Medium | 39 | 1 cup yogurt/d (125 g); 8 g/d protein | Usual diet | 4.35 | Weight, height, WAZ, HAZ | 4 |
| Heikens 1993 (71) | JAM (urban) | 14.6 | 75 | All underweight (<80% NCHS WAZ) and malnourished3 | — | Low | 13 (26) | High-energy supplement: full-milk cream powder, sugar, oil mixed with water; 20.6 g/d protein | Usual diet | 15.62 | Weight, height | 0 |
| Heikens 1989 (72) | JAM (urban) | 14.4 | 82 | All underweight (<80% NCHS WAZ) and malnourished3 | — | Low | 13 (26) | High-energy supplement: full-milk cream powder, sugar, oil mixed with water; 20.6 g/d protein | Usual diet | 15.62 | Weight, height | 0 |
| Iannotti 2017 (73) | ECU (rural) | 7.6 | 163 | 38% stunted (HAZ < −2 SD) | — | Low | 25.8 | 1 egg; 6.46 g/d protein | Usual diet | 6.46 | WAZ, HAZ, WFL, stunting | 4 |
| Krebs 2012 (74) | GTM, DRC, ZMB, PAK (mixed) | 6 | 1062 | 33% stunted (HAZ < −2 SD), some SGA3 | Mixed | Low | 52 | Lyophilized beef; ∼8 g/d protein2 | Precooked rice and soy flour with micronutrients; ∼2 g/d protein2 | 6.26 | WAZ, HAZ, WFL, HAZ%, height | 6 |
| Lin 2008 (75) | MWI (rural) | 6 | 240 | — | B | Low | 52 | Corn porridge fortified with fish powder; 11.2 g/d protein2 | Peanut and soy-based fortified spread; 5.8 g/d protein2 | 4.69 | Weight, height | 1 |
| Long 2012 (76) | KEN (rural) | 25.5 | 193 | 27% stunted3 | — | Low | 22 | Meat porridge; 13 g/d protein2 | Plain millet-based porridge (sugar, margarine); 3.4 g/d protein2 | 5.77 | Weight, height, WAZ, WFL, HAZ | 6 |
| Whole-milk porridge; 5.9 g/d protein2 | 1.41 | |||||||||||
| Maleta 2015 (77) | MWI (rural) | 5.9 | 1291 | 29.3% moderately to severely stunted (HAZ < −2.0) | Mainly F | Low | 52 | Milk containing maize-based porridge; 2.5 g/d protein | Nonmilk containing maize-based porridge; 1.0 g/d protein | 1.5 | Weight, height, WAZ, HAZ, WFL, stunting, wasting | 4 |
| Milk containing maize-based porridge; 5.0 g/d protein | Milk containing maize-based porridge; 2.0 g/d protein | 3.0 | ||||||||||
| Mangani 2015 (78) | MWI (rural) | 6 | 376 | 8.5% stunted (HAZ < −3 SD) | — | Low | 52 | Milk-protein–based fortified soy–corn flour; 8.2 g/d protein | Usual diet | 6.49 | Weight, height, WAZ, WFL, HAZ | 6 |
| Nikiema 2014 (79) | BFA (rural) | 13.2 | 1369 | All stunted (WFL < −2 SD) | — | Low | 12 | Corn–soy blend with added micronutrients: maize, soya, sugar, dried skim milk, and soybean oil; 10.4 g/d protein2 | Ready-to-use supplementary food: peanut butter, vegetable oil, sugar, soy flour, shea butter, added micronutrients; 8.7 g/d protein2 | 1.47 | Height (length gain), WFL, HAZ | 1 |
| Schlossman 2017 (80) | GNB (rural) | 15.7 | 327 | All with mild to moderate acute malnutrition (WAZ <1.0, or HAZ < 2.0, or WFL < 2.0) | — | Low | 12.9 | Soy- and dairy-protein paste; 0.6 g/d protein | Usual diet | 0.773 | WAZ, HAZ, WFL | 3 |
| 38.8 | 159 | Soy- and dairy-protein paste; 1.2 g/d protein | 1.2 | |||||||||
| Simondon 1996 (81) | COG, SEN, BOL, NCL (mixed) | 4 | 447 | Neither LBW nor SGA | Mixed | Medium | 12 | Precooked wheat, maize, soybean flour, milk powder, soybean oil, palm oil, sugar; 6.74 g/d protein | Usual diet | 6.47 | Weight, height | 3 |
| Skau 2015 (82) | KHM (rural) | 5.9 | 180 | 18% stunted (HAZ < −2 SD) | — | Low | 38.7 | Vegetable oil, maize, soya, skimmed milk powder; 16.8 g/d protein | Vegetable oil, sugar, maize, soya; 14.6 g/d protein | 1.76 | Weight, height, WAZ, WFL, HAZ | 4 |
| Stobaugh 2016 (7) | MWI, MOZ (rural) | 16.4 | 2230 | All with moderate acute malnutrition (MUAC ≥ 115 mm and <125 mm without bipedal edema) | Mainly B | Low | 12 | Dairy-based ready-to-use supplementary food | Soy-based ready-to-use supplementary food | Weight, height, WFL | 6 | |
| Tang 2014 (83) | USA (urban) | 5 | 42 | Neither LBW nor SGA | B | — | 17 | Pureed meat and gravy; ∼8 g/d protein | Cereal; ∼2–3 g/d protein | 30.3 | WAZ, WFL, HAZ | −1 |
| Tang 2014 (84) | CHN (rural) | 7 | 1318 | 30% stunted3, neither LBW nor SGA | Mixed | Low | 52 | Boiled pork, every other day; ∼14.8 g/d protein2 | Commercially available packaged pressed rice cereal; ∼3.9 g/d protein2 | 0.88 | Weight, height, WAZ, WFL, HAZ | −1 |
| Tavill 1969 (85) | MAR (urban) | 6 | 88 | Neither LBW nor SGA | — | Low | 26 | Fish-protein concentrate; ∼13 g/d protein | Usual diet | 12.97 | Weight, height | −1 |
| Walker 1996 (86) | JAM (urban) | 18.7 | 63 | All stunted (HAZ < −2 SD) | — | — | 52 (103.2) | Milk-based formula, skim milk powder, and cornmeal; 14 g protein/100 mL | Usual diet | 15.2 | Weight, height, WFL, HAZ | −1 |
If not directly reported, estimated from the study descriptorsby 2 investigators independently and in duplicate. BEL, Belgium; BFA, Burkina Faso; BGD, Bangladesh; BOL, Bolivia; BW, birth weight; CAN, Canada; CHL, Chile; CHN, China; COG, Congo; COL, Colombia; DNK, Denmark; DRC, Democratic Republic of Congo; ECU, Ecuador; ES, Spain; FRA, France; GA, Gestational age; GER, Germany; GHA, Ghana; GNB, Guinea-Bissau; GTM, Guatemala; HAZ, height-for-age z-score; HAZ%, percentage height-for-age z-score(stunting); IDN, Indonesia; ITA, Italy; JAM, Jamaica; KEN, Kenya; KHM, Cambodia; LBW, low birth weight; MAR, Morocco; MEX, Mexico; MLI, Mali; MOZ, Mozambique; MUAC, midupper arm circumference; MWI, Malawi; NCHS, National Center for Health Statistics; NCL, New Caledonia; NL, Netherlands; PAK, Pakistan; PER, Peru; PHL, Philippines; POL, Poland; SEN, Senegal; SES, socio-economic status; SGA, small for gestational age; SRB, Serbia; SWE, Sweden; TWN, Taiwan; UK, United Kingdom; USA, United States of America; WAZ, weight-for-age z-score; WFL, weight-for-length z-score; WFL%, percentage weight-for-length z-score; ZMB, Zambia; –, information not applicable or not available.
The intervention and control supplements were isocaloric.
Not defined in the report.
The Cochrane Collaboration's tool for assessing risk of bias was used to assess potential for selection bias, performance bias, detection bias, attrition bias, and reporting bias through a 6-question quality control checklist (24). Each question was answered as low risk of bias (score = 1), high risk of bias (score = −1), or unclear (score = 0); and values were summed (potential range: −6 to +6). Scores were grouped in approximate tertiles with values of −6 to 0 considered as low quality, 1–3 as medium quality, and 4–6 as high quality.
Population is <25% or >75% males.
Maternal supplementation
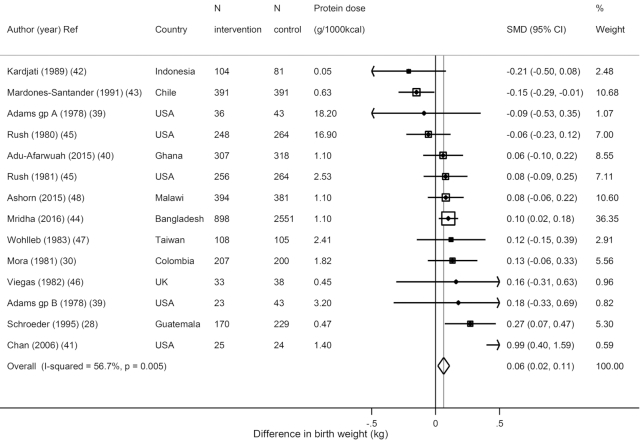
Among trials during pregnancy, protein supplementation significantly increased birth weight (N = 14 estimates from 12 trials, n = 8132 total participants; weighted mean difference [WMD] = 0.06 kg; 95% CI: 0.02, 0.11 kg; I2 = 56.7%) (Figure 1). Maternal supplementation did not significantly reduce the risk of LBW (N = 5, n = 6121; OR: 0.89; 95% CI: 0.78, 1.02; I2 = 0.8%) or small for gestational age (SGA) (N = 4, n = 5674; OR: 0.98; 95% CI: 0.87, 1.10; I2 = 80.3%) (Supplemental Figure 2), or increase height (N = 3, n = 1490; WMD = 0.01 cm; 95% CI: −0.09, 0.11 cm; I2 = 59.7%) or weight (N = 2, n = 636; WMD = −0.08 kg; 95% CI: −0.23, 0.08 kg; I2 = 0.0%) during later childhood (Supplemental Figure 3).
FIGURE 1.
Effects of protein supplementation on birth weight in kilograms from 14 estimates in 12 trials including 8132 subjects. SMD, standardized (weighted) mean difference.
Three trials supplemented both mothers (during pregnancy and/or breastfeeding) and their children after birth, all with combined energy–animal protein supplementation: increases were seen in both child height (N = 2, n = 3276; WMD = 0.09 cm; 95% CI: 0.02, 0.15 cm; I2 = 61.8%) and weight (N = 3, n = 4227; WMD = 0.10 kg; 95% CI: 0.04, 0.16 kg; I2 = 71.6%) (Supplemental Figure 4).
Preterm infant supplementation
Supplementation of preterm infants (comparing formula with higher compared with lower animal protein) did not significantly affect child height (N = 5, n = 262; WMD = 0.06 cm; 95% CI: −0.22, 0.34 cm; I2 = 96.9%) and reduced HAZ (N = 4, n = 269; WMD = −1.31 z-score; 95% CI: −1.60, −1.01; I2 = 96.8%) (Supplemental Figure 5), although these trials were quite small. Preterm supplementation did not significantly affect child weight (N = 6, n = 373; WMD = 0.19 kg; 95% CI: −0.03, 0.42 kg; I2 = 96.2%) (Supplemental Figure 6). Only 2 trials reported weight for age, which decreased (N = 2, n = 169; WMD = −0.81 z-score; 95% CI:−1.16, −0.46; I2 = 98.4%), and 1 trial provided 2 estimates for weight for length, which also decreased (N = 2, n = 100; WMD = −1.57 z-score; 95% CI: −2.02, −1.12; I2 = 0.0%) (Supplemental Figure 7).
Term infant/child supplementation
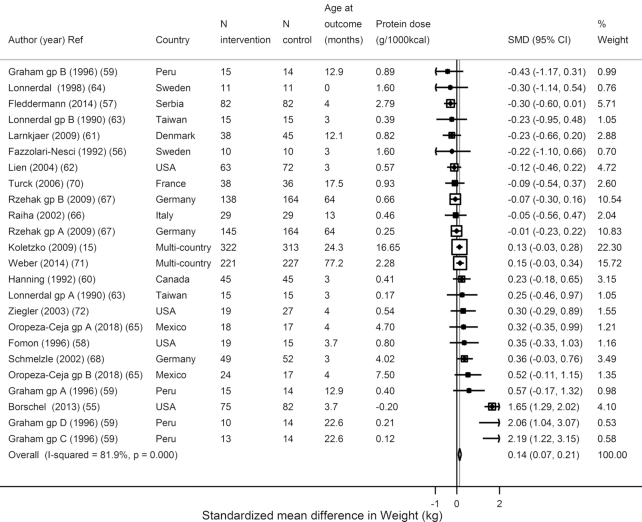
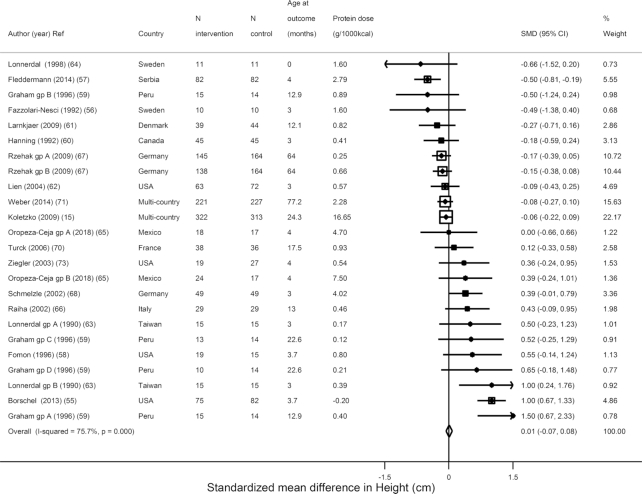
In trials of formula supplementation (higher compared with lower protein content) among term infants/children, supplementation significantly increased weight (N = 24, n = 2923; WMD = 0.14 kg; 95% CI: 0.07, 0.21 kg; I2 = 81.9%) (Figure 2) but not height (N = 24, n = 2920; WMD = 0.01 cm; 95% CI: −0.07, 0.08 cm; I2 = 75.7%) (Figure 3). Only 7 estimates from 6 trials reported weight- and height-for-age z-scores: WAZ was not reduced (N = 7, n = 1532; WMD = −0.01 z-score; 95% CI: −0.11, 0.09; I2 = 95.1%), but HAZ (N = 7, n = 1532, WMD = −0.11 z-score; 95% CI: −0.22, −0.0.01; I2 = 90.3%) was reduced (Supplemental Figure 8). Only 3 studies reported WFL: WFL was increased (N = 3, n = 711; WMD = 0.24 z-score; 95% CI: 0.09, 0.38; I2 = 0.0%) (Supplemental Figure 9).
FIGURE 2.
Effects of term child protein formula supplementation on weight in kilograms from 24 estimates from 18 trials including 2923 subjects. SMD, standardized (weighted) mean difference.
FIGURE 3.
Effects of term child protein formula supplementation on height in centimeters from 24 estimates in 18 trials including 2920 subjects. SMD, standardized (weighted) mean difference.
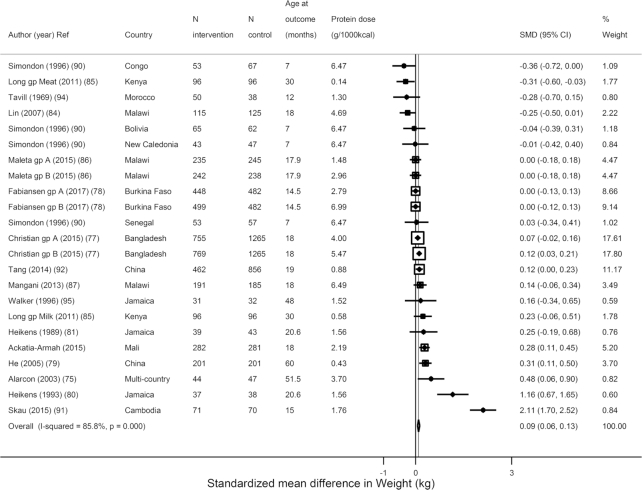
Similar to formula trials, the trials testing food-based animal protein showed that supplementation significantly increased weight (N = 23 estimates, n = 11,195; WMD: 0.09 kg; 95% CI: 0.06, 0.13 kg; I2 = 85.8%) (Figure 4) but not height (N = 25, n = 13,626; WMD = −0.02 cm; 95% CI: −0.06, 0.01 cm; I2 = 97.8%) (Figure 5). However, in 19 trials assessing z-scores, HAZ increased (N = 19, n = 11,098; WMD = 0.06 z-score; 95% CI: 0.02, 0.10; I2 = 57.9%) (Supplemental Figure 10). In these food-based trials, the source was most often milk; yogurt, fish, and red meat were also used. Consistent with the overall weight effects, a tendency toward an increase was seen in weight for age (N = 15, n = 5611; WMD = 0.05 z-score; 95% CI: 0.00, 0.10; I2 = 80.6%) and an increase in weight for length (N = 19, n = 11,251; WMD = 0.06 z-score; 95% CI: 0.02, 0.10; I2 = 87.2%) (Supplemental Figure 11). Only 3 trials evaluated stunting, finding a significant reduced risk of stunting (N = 6 estimates, n = 5138; OR: 0.87; 95% CI: 0.77, 0.97; I2 = 0), but no reduced risk of wasting (N = 5 estimates, n = 5267; OR: 0.99; 95% CI: 0.84, 1.16; I2 = 0.0%) (Supplemental Figure 12).
FIGURE 4.
Effects of term child protein food-based supplementation on weight in kilograms from 23 estimates from 16 trials including 11,195 subjects. SMD, standardized (weighted) mean difference.
FIGURE 5.
Effects of term child protein food-based supplementation on height in centimeters from 25 estimates from 18 trials including 13,626 subjects. SMD, standardized (weighted) mean difference.
Potential sources of heterogeneity
Using stratified analyses and meta-regression, we evaluated prespecified potential sources of heterogeneity (Table 2). Among trials of pregnant women evaluating birth weight, no significant heterogeneity was identified by country income, baseline nutritional status, dose of protein supplementation, intervention duration, or study quality score. Compared with isocaloric-protein supplementation (one trial only), energy-protein supplementation to pregnant women was more effective in increasing birth weight (P heterogeneity = 0.002; birth weight WMD = 0.089 kg; 95% CI: 0.040, 0.137 kg).
TABLE 2.
Sources of potential heterogeneity among randomized trials examining the effect of animal protein supplementation on growth outcomes in infants and children1
| Pregnant mothers | Term children, formula-based | Term children, food-based | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth weight, kg | Height, cm | Weight, kg | Height, cm | Weight, kg | Height-for-age, z-score | Weight-for-age, z-score | Weight-for-length, z-score | |||||||||
| Potential sources of heterogeneity | N | Pooled estimate (95% CI) | N | Pooled estimate (95% CI) | N | Pooled estimate (95% CI) | N | Pooled estimate (95% CI) | N | Pooled estimate (95% CI) | N | Pooled estimate (95% CI) | N | Pooled estimate (95% CI) | N | Pooled estimate (95% CI) |
| Country income | ||||||||||||||||
| High | 7 | 0.064 (−0.037, 0.166) | 18 | 0.111 (0.036, 0.187) | 18 | −0.016 (−0.092, 0.059) | 0 | 0 | 1 | −0.075 (−0.717, 0.566) | 1 | 0.097 (−0.545, 0.739) | 1 | 0.185 (−0.458, 0.828) | ||
| Low/middle | 7 | 0.063 (0.012, 0.115) | 6 | 0.632 (0.323, 0.942) | 6 | 0.361 (0.063, 0.660) | 25 | −0.024 (−0.059, 0.010) | 23 | 0.095 (0.057, 0.133) | 18 | 0.059 (0.021, 0.097) | 15 | 0.050 (−0.004, 0.103) | 18 | 0.060 (0.023, 0.098) |
| P heterogeneity, univariate (multivariate)2 | 0.986 | 0.001* (0.621) | 0.016* (0.680) | NA | NA | 0.682 | 0.886 | 0.705 | ||||||||
| Baseline nutritional status3 | ||||||||||||||||
| Average/unspecified | 10 | 0.107 (0.033, 0.180) | 20 | 0.120 (0.045, 0.194) | 20 | −0.010 (−0.085, 0.064) | 14 | 0.082 (0.033, 0.132) | 14 | 0.071 (0.022, 0.120) | 9 | 0.056 (0.004, 0.108) | 7 | −0.031 (−0.121, 0.058) | 11 | 0.161 (0.071, 0.252) |
| >30% malnourished | 4 | 0.036 (−0.023, 0.094) | 4 | 0.805 (0.386, 1.225) | 4 | 0.480 (0.084, 0.875) | 11 | −0.130 (−0.179, −0.081) | 9 | 0.131 (0.071, 0.190) | 10 | 0.061 (0.006, 0.117) | 8 | 0.094 (0.028, 0.160) | 12 | 0.040 (−0.001, 0.081) |
| P heterogeneity, univariate (multivariate)2 | 0.140 | 0.002* (0.341) | 0.017* (0.539) | <0.001* (0.578) | 0.128 | 0.891 | 0.026* (0.671) | 0.017* (0.595) | ||||||||
| Dose of protein supplementation4 | ||||||||||||||||
| >Median | 7 | 0.058 (−0.030, 0.147) | 8 | 0.108 (0.006, 0.211) | 8 | −0.088 (−0.191, 0.015) | 16 | 0.102 (0.059, 0.146) | 16 | 0.097 (0.053, 0.140) | 9 | 0.100 (0.046, 0.154) | 8 | 0.082 (−0.007, 0.171) | 10 | 0.131 (0.081, 0.182) |
| <Median | 7 | 0.065 (0.012, 0.119) | 16 | 0.175 (0.069, 0.280) | 16 | 0.104 (−0.000, 0.209) | 9 | −0.245 (−0.302, −0.187) | 7 | 0.089 (0.012, 0.166) | 10 | 0.019 (−0.034, 0.072) | 7 | 0.032 (−0.034, 0.098) | 9 | −0.024 (−0.079, 0.032) |
| P heterogeneity, univariate (multivariate)2 | 0.894 | 0.376 | 0.010* (0.224) | 0.001* (0.189) | 0.864 | 0.037* (0.275) | 0.382 | 0.001* (0.163) | ||||||||
| Energy supplementation5 | ||||||||||||||||
| Isocaloric-protein | 1 | −0.150 (−0.290, −0.009) | 24 | 0.141 (0.067, 0.214) | 24 | 0.007 (−0.067, 0.080) | 4 | 0.058 (−0.018, 0.133) | 4 | 0.038 (−0.038, 0.113) | 2 | 0.128 (−0.050, 0.306) | 2 | 0.127 (−0.051, 0.306) | 5 | 0.054 (−0.018, 0.126) |
| Energy-protein | 13 | 0.089 (0.040, 0.137) | 0 | 0 | 21 | −0.047 (−0.086, −0.007) | 19 | 0.114 (0.071, 0.158) | 17 | 0.055 (0.017, 0.094) | 13 | 0.042 (−0.013, 0.098) | 14 | 0.063 (0.020, 0.107) | ||
| P heterogeneity, univariate (multivariate)2 | 0.002* | NA | NA | 0.016* (0.772) | 0.084 | 0.435 | 0.373 | 0.836 | ||||||||
| Intervention duration4 | ||||||||||||||||
| >Median | 5 | 0.056 (−0.001, 0.114) | 6 | 0.064 −(0.029, 0.157) | 6 | −0.101 (−0.194, −0.008) | 14 | −0.081 (−0.125, −0.038) | 13 | 0.101 (0.056, 0.146) | 15 | 0.066 (0.024, 0.108) | 15 | 0.023 (−0.033, 0.079) | 12 | 0.057 (−0.001, 0.116) |
| <Median | 9 | 0.076 (−0.173, 0.284) | 18 | 0.270 (0.150, 0.391) | 18 | 0.184 (0.064, 0.303) | 11 | 0.080 (0.021, 0.138) | 10 | 0.080 (0.011, 0.150) | 4 | 0.025 (−0.064, 0.114) | 3 | 0.285 (0.119, 0.451 | 7 | 0.063 (0.015, 0.112) |
| P heterogeneity, univariate (multivariate)2 | 0.694 | 0.008* (0.507) | 0.001* (0.954) | <0.001* (0.483) | 0.627 | 0.417 | 0.003* (0.205) | 0.882 | ||||||||
| Study quality score6 | ||||||||||||||||
| Low (−6 to 0) | 3 | 0.055 (0.017, 0.109) | 7 | 0.182 (0.073, 0.290) | 7 | 0.013 (−0.095, 0.121) | 8 | 0.105 (0.051, 0.159) | 8 | 0.114 (0.060, 0.167) | 6 | 0.107 (0.052, 0.161) | 3 | 0.198 (0.090, 0.306) | 3 | 0.040 (−0.069, 0.148) |
| Medium/high (1 to 6) | 11 | 0.064 (0.017, 0.111) | 17 | 0.106 (0.006, 0.206) | 17 | 0.001 (−0.099, 0.101) | 17 | −0.119 (−0.165, −0.073) | 15 | 0.076 (0.022, 0.130) | 13 | 0.013 (−0.040, 0.066) | 12 | 0.003 (−0.058, 0.064) | 16 | 0.064 (0.024, 0.103) |
| P heterogeneity, univariate (multivariate)2 | 0.944 | 0.316 | 0.867 | <0.001* (0.685) | 0.327 | 0.015* (0.419) | 0.002* (0.103) | 0.687 | ||||||||
| Age at supplementation4, | ||||||||||||||||
| >Median | 5 | 0.043 (−0.029, 0.115) | 5 | 0.304 (0.003, 0.605) | 5 | 0.138 (−0.154, 0.430) | 18 | −0.050 (−0.087, −0.013) | 16 | 0.095 (0.054, 0.135) | 15 | 0.058 (0.018, 0.098) | 11 | 0.061 (0.001, 0.121) | 15 | 0.068 (0.029, 0.108) |
| <Median | 9 | 0.068 (−0.031, 0.167) | 19 | 0.130 (0.055, 0.206) | 19 | −0.002 (−0.078, 0.073) | 7 | 0.177 (0.074, 0.280) | 10 | 0.098 (−0.004, 0.199) | 7 | 0.064 (−0.050, 0.179) | 4 | 0.009 (−0.106, 0.123) | 4 | −0.004 (−0.118, 0.111) |
| P heterogeneity, univariate (multivariate)2 | 0.976 | 0.273 | 0.363 | 0.001* (0.395) | 0.958 | 0.920 | 0.424 | 0.243 | ||||||||
| Age at follow-up4, | ||||||||||||||||
| >Median | 7 | 0.061 (−0.027, 0.150) | 10 | 0.098 (0.008, 0.189) | 10 | −0.087 (−0.177, 0.004) | 12 | 0.092 (0.037, 0.146) | 12 | 0.077 (0.023, 0.132) | 12 | 0.105 (0.042, 0.168) | 14 | 0.086 (0.023, 0.150) | 16 | 0.031 (−0.014, 0.076) |
| <Median | 7 | 0.051 (−0.002, 0.105) | 14 | 0.224 (0.097, 0.351) | 14 | 0.187 (0.062, 0.313) | 13 | −0.103 (−0.148, −0.058) | 11 | 0.111 (0.059, 0.164) | 7 | 0.032 (−0.015, 0.079) | 4 | −0.034 (−0.130, 0.063) | 6 | 0.124 (0.058, 0.190) |
| P heterogeneity, univariate (multivariate)2 | 0.852 | 0.113 | 0.001* (0.282) | <0.001* (0.605) | 0.378 | 0.068 | 0.042* (0.720) | 0.023* (0.125) | ||||||||
Only pooled analyses with 10 or more estimates were evaluated in these stratified analyses and tests for heterogeneity. For each stratum, the pooled estimate (95% CI) was calculated using inverse variance-weighted meta-analyses. *Values indicate P < 0.05. HAZ, height-for-age z-score.
P value for heterogeneity calculated using univariate meta-regression analysis. For each outcome, multivariate meta-regression was subsequently performed including all factors with univariate P < 0.05 together in the same model.
Defined according to the study definition (e.g., HAZ < 2SD).
Median value defined within each target population separately (e.g., maternal supplementation, term infants).
When energy intakes were not specified, an intervention compared with a “usual diet” control was considered to be an energy-protein (not isocaloric-protein) intervention, whereas a control that used a specific control supplement was considered to be isocaloric-protein.
Study quality was assessed using the Cochrane Collaboration risk-of-bias tool, evaluating potential for selection bias, performance bias, detection bias, attrition bias, and reporting bias through a 6-question quality control checklist (24). Each question was answered as a low risk of bias (score = 1), high risk of bias (score = −1), or unclear (score = 0), and values were summed (potential range: −6 to +6). Scores were grouped in approximate tertiles with values of −6 to 0 considered as low quality, 1–3 as medium quality, and 4–6 as high quality.
For trials of formula-based supplementation in term children, univariate meta-regression suggested that studies from low- and middle-income countries (N = 6) showed larger increases in both weight (P heterogeneity = 0.001) and height (P heterogeneity = 0.02) than studies from high-income countries (N = 18); similar stratification was identified by baseline nutritional status and intervention duration. Doses of animal protein supplementation below median showed larger effects. No significant heterogeneity was identified by study quality score or in multivariate meta-regression.
Among trials of food-based supplementation in term children, effects on height were smaller, in studies of malnourished compared with average/unspecified nutritional status (P heterogeneity < 0.001), whereas effects on WAZ and WFL were greater in studies of average/unspecified nutritional status (P heterogeneity < 0.026 for both). In univariate meta-regression, potential heterogeneity was also identified by dose of protein supplementation, presence of energy supplementation, intervention duration, and study quality score for studies of the effect of height. Heterogeneity was identified by dose and study quality score in studies of HAZ meta-regression, and for baseline nutritional status, intervention duration and study quality, for studies of the effect on WAZ. Only baseline nutritional status and animal protein supplementation dose shower heterogeneity effects in studies of WFL. None of these interactions remained significant in multivariate meta-regression. No variables were found to drive heterogeneity in studies of the effect on weight.
In post hoc analyses of interaction by age at follow-up, children below the median age at follow-up showed stronger effects in the association of formula-based supplementation and weight and the association of food-based supplementation and WFL. Above the median age at follow-up was associated with stronger effects of term children food-based supplementation on height and WAZ.
Evaluation of small-study effects
Visual inspection of funnel plots and Egger's and Begg's tests did not provide any evidence for meaningful small-study effects (Supplemental Figure 13).
Discussion
This systematic review and meta-analysis of 62 controlled trials comprising over 30,000 participants across 5 continents found that supplementation of protein from animal-source foods generally increased weight in children; yet, it had limited effects on other outcomes such as attained height or stunting. For instance, maternal supplementation increased birth weight by 0.06 kg (with no significant effect on LBW or SGA), and both formula and food-based supplementation in term infants/young children increased weight by 0.14 and 0.09 kg, respectively. The strongest effects were seen in trials where both mothers and children were supplemented, with 100-g increases in weight and a mean 0.1-cm increase in height. However, neither formula nor food-based supplementation for term infants/young children increased height, whereas HAZ was increased in food-based (+0.06 z-score) but not formula-based trials (−0.11 z-score) reporting this outcome. Whether this latter difference is due to chance or benefits of nonprotein components of animal-source foods remains unclear, and the limited number of studies reporting on HAZ outcomes after term formula supplementation (N = 7) adds uncertainty about this finding. We conducted a post hoc meta-analysis of height and weight in studies of food-based supplementation in term children in the subgroup of studies that reported HAZ and WAZ, respectively, as an outcome. However, we still did not find a positive effect of food-based supplementation on height, and the significant effect on weight was maintained in this subgroup of studies. Supplementation in preterm infants did not significantly improve growth outcomes; in contrast, lower height for age, weight for age, and weight for length (although based on only 6 trials totaling <400 participants) were shown. In sum, these findings do not provide strong evidence for the benefits of animal-protein supplementation in mothers and preterm infants, and during infancy/early childhood on growth outcomes other than weight and WFL, and stunting for food supplementation in term children; the potential effects of food-based supplementation on HAZ require further study.
Formula-based trials generally provided isocaloric animal protein supplementation, whereas trials in pregnant mothers and food-based trials in children generally provided both animal protein and additional calories from foods (“balanced energy–animal protein supplementation”). Increases in birth weight with the latter approach in pregnant mothers support recommendations from a 2013 narrative review (87), although the effects of the increased calories compared with animal protein per se cannot be distinguished in these interventions. The increased height in term children given balanced energy–animal protein supplementation was based on very few trials with particular characteristics: all were conducted in the 1970s, including in rural Guatemala, urban slums in Columbia, and small industrial towns in South Wales (39, 37, 38). Although our meta-analysis supports increased birth weight with balanced energy–animal protein supplementation from foods to mothers, our findings also highlight the relatively few studies testing this approach in children after birth, indicating a need for additional trials in this area.
During exclusive breastfeeding, protein accounts for ∼5% of energy intake, which generally increases to ∼15% energy when complementary foods are introduced (88). In general, protein requirements for infants and young children have been determined by the Adequate Intake method, where recommended intakes are set at the mean protein intake of healthy breastfeeding children, or ∼1.5 g/kg/d for infants 0–6 mo of age (89). These levels of intake are several-fold higher than physiologic requirements to prevent clinical amino acid deficiencies.
Yet, it remains unclear whether the amino acid requirements for body maintenance are the same as those for new tissue deposition (recovery from undernutrition) (90), and the appropriate dietary protein intake for optimal growth in children has remained uncertain. WHO has argued for inclusion of animal protein in supplementary foods in the management of moderate acute malnutrition (wasting), suggesting that “animal-source foods are more likely to meet the amino acid and other nutrient needs of recovering children” (91). However, few prior studies have systematically reviewed whether animal protein promotes optimal growth. In a recent meta-analysis, balanced energy and protein supplementation during pregnancy reduced stillbirth by 40% (95% CI: 6, 61%) and SGA by 21% (95% CI: 10, 31%), and increased birth weight by 0.04 kg (95% CI: 0.005, 0.08 kg) (92). Conversely, based on 2 trials of isocaloric-protein supplementation during pregnancy, no benefits on birth outcomes were identified; effects of either of these approaches on linear growth after birth were not evaluated (92). Another meta-analysis focused on balanced protein-energy supplementation and birth outcomes, but without differentiating plant compared with animal sources (93). Supplementation significantly increased birth weight, but not birth length or birth head circumference; again, effects on linear growth after birth were not evaluated. Consistent with our quantitative results, a recent narrative review of lipid compared with grain-based supplemental foods in the management of moderate wasting concluded that “benefits of dairy in Ready-to-Use-Food require further investigation” (94). Our finding significantly extends and expands these results by investigating animal protein supplementation during pregnancy, in preterm infants, and in term infants/children; evaluating linear growth after birth; and formally considering heterogeneity by a range of underlying characteristics.
Our systematic review also highlights the variation in the populations studied, and the nature and doses of supplementation strategies. Continuing knowledge gaps are identified because trials have used varying kinds of foods, types and levels of protein, and forms of intervention, all of which continue to make a clear articulation of the role of animal-source proteins on nutrition outcomes challenging. The differences between food-based and formula-based interventions that we identified could indicate an effect of the overall food, although chance or other design and population difference could also explain these findings. Our results highlight the need for further studies of this question, including trials concurrently testing isocaloric animal protein, animal protein including its calories, and animal foods. The heterogeneity identified in our meta-analysis as well as prior reviews indicates a need for more standardized approaches to evaluate specific forms and doses of animal protein, repeated episodes of growth failure (wasting as well as linear growth retardation), and, of course, the role of aggravating factors beyond diet. Several recently completed or ongoing trials are testing various forms and levels of animal-source foods in products specifically designed to treat acute or severe wasting in children in low-income countries. As demonstrated by our current investigation, there can be wide variability in prevalence of wasting or stunting among such populations at baseline. Among term children, we found that formula-based supplementation had larger effects on weight and height in populations with lower baseline nutritional status, the at-risk group that would drive such interventions. These same groups consistently experience greater benefits on growth in studies of pregnant women or food-based supplementation in children. These interactions by baseline nutritional status are biologically plausible and policy-relevant. Of note, however, is that few studies document the background quantity or quality (completeness) of usual total protein intake at baseline. Relatively modest differences in baseline characteristics of undernutrition, background diets, and corresponding doses and durations of supplementation could have meaningful impacts on effectiveness.
Our investigation has several strengths. Extensive searches of multiple databases, hand searching of citations, and searches of electronically linked studies reduce the likelihood that major studies corresponding to our inclusion criteria were missed. Strict inclusion criteria and duplicate data extraction reduced the possibilities of error and bias. Plausible sources of heterogeneity and the potential for small-study effects were quantitatively evaluated. Studies were identified across a range of countries, increasing generalizability. We evaluated supplementation in mothers, preterm infants, and term infants/young children, providing a more complete picture of effects across the early life course. We focused on randomized controlled trials, increasing inference for a cause-and-effect relation. Strengths of our investigation include the evaluation of different related outcomes (e.g., weight, WAZ, height, HAZ), allowing assessment for concordance of findings and elucidating robustness of the results, as well as evaluation of prespecified sources of heterogeneity to identify potential reasons for differences across studies. The observed unexplained discrepancies (e.g., for weight compared with WAZ for formula supplementation) suggest that further research is needed to confirm the effects of animal protein and animal-source foods on these outcomes.
Potential limitations should be considered. As with any meta-analysis, results are limited by the availability of studies focusing on specific outcomes of interest; for instance, relatively few studies evaluated age-specific growth outcomes, wasting, or stunting assessed using different metrics. Although we evaluated several potential sources of heterogeneity, other unknown sources, including the possibility of chance, remained. In terms of quality, many published papers evaluated using Cochrane criteria for risks of bias demonstrated elements that were either “unclear” or of relatively low rigor. This argues for much more attention to study quality going forward. For some outcomes, the small numbers and sizes of identified trials limited statistical power. On the other hand, this is the largest meta-analysis on this topic to date, serving to highlight the specific gaps in information for certain outcomes of concern.
In summary, this meta-analysis summarizes available evidence for the role of animal protein supplementation on birth, weight, and linear growth outcomes early in life. Supplementation during pregnancy and infancy/childhood increased child weight and food-based supplementation in childhood increased WAZ and reduced the risk of stunting. Only supplementation of animal-source foods (but not formula) during infancy/childhood increased height for age. Benefits on growth outcomes in preterm infants were not identified. Overall, too few high-quality studies were identified to allow for definitive conclusions on linear growth. This does not negate the benefits of balanced (protein plus energy) supplementation for recovery from wasting in children of undernourished mothers or the consideration of other potential benefits, such as on cognitive outcomes, which require separate assessment. WHO continues to support its 2012 call for use of animal-source protein in food supplements intended to manage existing malnutrition. As such, many argue that protein quality (including defined as deriving from animal-source foods) “is important to child health and not a fallacy” (95). We find that convincing evidence from multiple sources pointing to significant benefits beyond weight remains elusive and is therefore a priority for future policy-relevant research.
Supplementary Material
Acknowledgments
We thank Fumiaki Imamura (Medical Research Council Epidemiology, University of Cambridge, UK) for useful comments and suggestions on statistical analysis.
The authors’ responsibilities were as follows—LP, WF, CD, and DM: designed the research; MS, LP, SK, DK, VM, and PW: conducted the research; LP: performed the statistical analysis; LP, PW, and DM: wrote the paper; LP and DM: had primary responsibility for the final content. DM reports research funding from the Bill & Melinda Gates Foundation and the NIH. DM reports personal fees from GOED, DSM, Nutrition Impact, Pollock Communications, Bunge, Indigo Agriculture, Amarin, Acasti Pharma, and America's Test Kitchen; scientific advisory board, Omada Health, Elysium Health, and DayTwo; and chapter royalties from UpToDate; all outside the submitted work. The other authors report no conflicts of interest.
Notes
This work was supported by a grant from the Bill & Melinda Gates Foundation (PI-Mozaffarian). CD was supported in part by the NIH K24 DK104676; and PW, in part by USAID award AID-OAA-L-10-00006 through the Feed the Future Innovation Lab for Nutrition. The funders had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of this manuscript.
Supplemental Table 1, Supplemental Figures 1–13 and Supplemental Information 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: HAZ, height-for-age z-score; LBW, low birth weight; SGA, small for gestational age; WAZ, weight-for-age z-score; WFL, weight-for-length z-score; WMD, weighted mean difference.
References
- 1. United Nations Children's Fund, the World Health Organization and World Bank Group. Joint Child Malnutrition Estimates. (2016 Edition)2016; May 31, 2017. Available from: http://www.who.int/nutgrowthdb/estimates/en/. [Google Scholar]
- 2. Prendergast AJ, Humphrey JH.. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34(4):250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michaelsen KF, Hoppe C, Roos N, Kaestel P, Stougaard M, Lauritzen L et al.. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr Bull. 2009;30(3 Suppl):S343–404. [DOI] [PubMed] [Google Scholar]
- 4. Allen LH, Dror DK.. Effects of animal source foods, with emphasis on milk, in the diet of children in low-income countries. Néstle Nutr Workshop Ser Pediatr Program. 2011;67:113–30. [DOI] [PubMed] [Google Scholar]
- 5. Wiley AS. Cow milk consumption, insulin-like growth factor-I, and human biology: a life history approach. Am J Hum Biol. 2012;24(2):130–8. [DOI] [PubMed] [Google Scholar]
- 6. Molgaard C, Larnkjaer A, Arnberg K, Michaelsen KF. Milk and growth in children: effects of whey and casein. Nestle Nutr Workshop Ser Pediatr Program. 2011;67:67–78. [DOI] [PubMed] [Google Scholar]
- 7. Stobaugh HC, Ryan KN, Kennedy JA, Grise JB, Crocker AH, Thakwalakwa C et al.. Including whey protein and whey permeate in ready-to-use supplementary food improves recovery rates in children with moderate acute malnutrition: a randomized, double-blind clinical trial. Am J Clin Nutr. 2016;103(3):926–33. [DOI] [PubMed] [Google Scholar]
- 8. Webb P. How strong is our evidence for effective management of wasting? A review of systematic and other reviews. Food Nutr Bull. 2015;36(1 Suppl):S65–71. [DOI] [PubMed] [Google Scholar]
- 9. Elango R, Ball RO. Protein and Amino Acid Requirements during Pregnancy. Adv Nutr. 2016;7(4):839S–44S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rush EC, Katre P, Yajnik CS. Vitamin B12: one carbon metabolism, fetal growth and programming for chronic disease. Eur J Clin Nutr. 2014;68(1):2–7. [DOI] [PubMed] [Google Scholar]
- 11. Dror DK, Allen LH.. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev. 2014;72(2):68–81. [DOI] [PubMed] [Google Scholar]
- 12. Manary M. Inadequate dietary protein intake: when does it occur and what are the consequences?. Food Nutr Bull. 2013;34(2):247–8. [DOI] [PubMed] [Google Scholar]
- 13. Briend A, Akomo P, Bahwere P, De Pee S, Dibari F, Golden MH et al.. Developing food supplements for moderately malnourished children: lessons learned from ready-to-use therapeutic foods. Food Nutr Bull. 2015;36(1 Suppl):S53–8. [DOI] [PubMed] [Google Scholar]
- 14. Millward DJ. Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr Res Rev. 2017;30(1):50–72. [DOI] [PubMed] [Google Scholar]
- 15. Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M et al.. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89(6):1836–45. [DOI] [PubMed] [Google Scholar]
- 16. Patro-Golab B, Zalewski BM, Kouwenhoven SM, Karas J, Koletzko B, Bernard van Goudoever J et al.. Protein Concentration in Milk Formula, Growth, and Later Risk of Obesity: A Systematic Review. J Nutr. 2016;146(3):551–64. [DOI] [PubMed] [Google Scholar]
- 17. Harding JE, Cormack BE, Alexander T, Alsweiler JM, Bloomfield FH. Advances in nutrition of the newborn infant. Lancet. 2017;389(10079):1660–8. [DOI] [PubMed] [Google Scholar]
- 18. Abrams SA, Hawthorne KM, Pammi M. A systematic review of controlled trials of lower-protein or energy-containing infant formulas for use by healthy full-term infants. Adv Nutr. 2015;6(2):178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fenton TR, Premji SS, Al-Wassia H, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev. 2014(4):CD003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hay WW, Thureen P.. Protein for preterm infants: how much is needed? How much is enough? How much is too much?. Pediatr Neonatol. 2010;51(4):198–207. [DOI] [PubMed] [Google Scholar]
- 21. Choose Your Foods: Exchange Lists for Diabetes. American Diabetes Association and the Academy of Nutrition and Dietetics Chicago/Alexandria, VA, 2007; [Google Scholar]
- 22. Amount and Schedule of Formula Feedings: American Academy of Pediatrics; 2009; [updated 2015]. Available from: https://www.healthychildren.org/English/ages-stages/baby/feeding-nutrition/Pages/Amount-and-Schedule-of-Formula-Feedings.aspx. [Google Scholar]
- 23. National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention; 2018. Available from: https://www.cdc.gov/nchs/nhanes/index.htm. [Google Scholar]
- 24. Higgins JPT, Green S(eds), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0[updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org. [Google Scholar]
- 25. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 26. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S et al.. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet. 2013;382(9890):452–77. [DOI] [PubMed] [Google Scholar]
- 28. Schroeder DG, Martorell R, Rivera JA, Ruel MT, Habicht JP. Age differences in the impact of nutritional supplementation on growth. J Nutr. 1995;125(4 Suppl):1051s–9s. [DOI] [PubMed] [Google Scholar]
- 29. Elwood PC, Haley TJ, Hughes SJ, Sweetnam PM, Gray OP, Davies DP. Child growth (0-5 years), and the effect of entitlement to a milk supplement. Arch Dis Child. 1981;56(11):831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mora JO, Herrera MG, Suescun J, de Navarro L, Wagner M. The effects of nutritional supplementation on physical growth of children at risk of malnutrition. Am J Clin Nutr. 1981;34(9):1885–92. [DOI] [PubMed] [Google Scholar]
- 31. Michaelsen KF, Greer FR.. Protein needs early in life and long-term health. Am J Clin Nutr. 2014;99(3):718S–22S. [DOI] [PubMed] [Google Scholar]
- 32. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes - Food and Nutrition Board - Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Washington, DC: National Academy Press; 2002. [Google Scholar]
- 33. Briend A, Khara T, Dolan C. Wasting and stunting–similarities and differences: policy and programmatic implications. Food Nutr Bull. 2015;36(1 Suppl):S15–23. [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization. Technical note: supplementary foods for the management of moderate acute malnutrition in infants and children 6–59 months of age. Geneva, Switzerland 2012. [Google Scholar]
- 35. Ota E, Hori H, Mori R, Tobe-Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2015(6):CD000032. [DOI] [PubMed] [Google Scholar]
- 36. Stevens B, Buettner P, Watt K, Clough A, Brimblecombe J, Judd J. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: a systematic review and meta-analysis. Matern Child Nutr. 2015;11(4):415–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suri DJ, Moorthy D, Rosenberg IH. The Role of Dairy in Effectiveness and Cost of Treatment of Children With Moderate Acute Malnutrition: A Narrative Review. Food Nutr Bull. 2016;37(2):176–85. [DOI] [PubMed] [Google Scholar]
- 38. Semba RD. The Rise and Fall of Protein Malnutrition in Global Health. Ann Nutr Metab. 2016;69(2):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adams SO, Barr GD, Huenemann RL. Effect of nutritional supplementation in pregnancy. I. Outcome of pregnancy. J Am Diet Assoc. 1978;72(2):144–7. [PubMed] [Google Scholar]
- 40. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, Arimond M, Vosti S, Dewey KG. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am J Clin Nutr. 2015;101(4):835–46. [DOI] [PubMed] [Google Scholar]
- 41. Chan GM, McElligott K, McNaught T, Gill G. Effects of dietary calcium intervention on adolescent mothers and newborns: A randomized controlled trial. Obstet Gynecol. 2006;108(3 Pt 1):565–71. [DOI] [PubMed] [Google Scholar]
- 42. Kardjati S, Kusin JA, De With C. Energy supplementation in the last trimester of pregnancy in East Java: I. Effect on birthweight. Br J Obstet Gynaecol. 1988;95(8):783–94. [DOI] [PubMed] [Google Scholar]
- 43. Mardones-Santander F, Rosso P, Stekel A, Ahumada E, Llaguno S, Pizarro F et al.. Effect of a milk-based food supplement on maternal nutritional status and fetal growth in underweight Chilean women. Am J Clin Nutr. 1988;47(3):413–9. [DOI] [PubMed] [Google Scholar]
- 44. Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA et al.. Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am J Clin Nutr. 2016;103(1):236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rush D, Stein Z, Susser M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics. 1980;65(4):683–97. [PubMed] [Google Scholar]
- 46. Viegas OA, Scott PH, Cole TJ, Eaton P, Needham PG, Wharton BA. Dietary protein energy supplementation of pregnant Asian mothers at Sorrento, Birmingham. II: Selective during third trimester only. Br Med J (Clin Res Ed). 1982;285(6342):592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wohlleb JC, Pollitt E, Mueller WH, Bigelow R. The Bacon Chow study: maternal supplementation and infant growth. Early Hum Dev. 1983;9(1):79–91. [DOI] [PubMed] [Google Scholar]
- 48. Ashorn P, Alho L, Ashorn U. Supplementation of Maternal Diets during Pregnancy and for 6 Months Postpartum and Infant Diets Thereafter with Small-Quantity Lipid-Based Nutrient Supplements Does Not Promote Child Growth by 18 Months of Age in Rural Malawi: A randomized controlled trial, J Nutr. 2015;145(6):1345–53. [DOI] [PubMed] [Google Scholar]
- 49. Aimone A, Rovet J, Ward W, Jefferies A, Campbell DM, Asztalos E et al.. Growth and body composition of human milk-fed premature infants provided with extra energy and nutrients early after hospital discharge: 1-year follow-up. J Pediatr Gastroenterol Nutr. 2009;49(4):456–66. [DOI] [PubMed] [Google Scholar]
- 50. Amesz EM, Schaafsma A, Cranendonk A, Lafeber HN. Optimal growth and lower fat mass in preterm infants fed a protein-enriched postdischarge formula. J Pediatr Gastroenterol Nutr. 2010;50(2):200–7. [DOI] [PubMed] [Google Scholar]
- 51. Carver JD, Wu PY, Hall RT, Ziegler EE, Sosa R, Jacobs J et al.. Growth of preterm infants fed nutrient-enriched or term formula after hospital discharge. Pediatrics. 2001;107(4):683–9. [DOI] [PubMed] [Google Scholar]
- 52. Cooke RJ, Griffin IJ, McCormick K. Adiposity is not altered in preterm infants fed with a nutrient-enriched formula after hospital discharge. Pediatr Res. 2010;67(6):660–4. [DOI] [PubMed] [Google Scholar]
- 53. Embleton ND, Cooke RJ.. Protein requirements in preterm infants: effect of different levels of protein intake on growth and body composition. Pediatr Res. 2005;58(5):855–60. [DOI] [PubMed] [Google Scholar]
- 54. Koo WW, Hockman EM.. Posthospital discharge feeding for preterm infants: effects of standard compared with enriched milk formula on growth, bone mass, and body composition. Am J Clin Nutr. 2006;84(6):1357–64. [DOI] [PubMed] [Google Scholar]
- 55. Borschel MW, Ziegler EE, Wedig RT, Oliver JS. Growth of healthy term infants fed an extensively hydrolyzed casein-based or free amino acid-based infant formula: a randomized, double-blind, controlled trial. Clin Pediatr (Phila). 2013;52(10):910–7. [DOI] [PubMed] [Google Scholar]
- 56. Fazzolari-Nesci A, Domianello D, Sotera V, Raiha NC. Tryptophan fortification of adapted formula increases plasma tryptophan concentrations to levels not different from those found in breast-fed infants. J Pediatr Gastroenterol Nutr. 1992;14(4):456–9. [DOI] [PubMed] [Google Scholar]
- 57. Fleddermann M, Demmelmair H, Grote V, Nikolic T, Trisic B, Koletzko B. Infant formula composition affects energetic efficiency for growth: the BeMIM study, a randomized controlled trial. Clin Nutr. 2014;33(4):588–95. [DOI] [PubMed] [Google Scholar]
- 58. Fomon SJ, Ziegler EE, Nelson SE, Frantz JA. What is the safe protein-energy ratio for infant formulas?. Am J Clin Nutr. 1995;62(2):358–63. [DOI] [PubMed] [Google Scholar]
- 59. Graham GG, MacLean WC Jr., Brown KH, Morales E, Lembcke J, Gastanaduy A. Protein requirements of infants and children: growth during recovery from malnutrition. Pediatrics. 1996;97(4):499–505. [PubMed] [Google Scholar]
- 60. Hanning RM, Paes B, Atkinson SA. Protein metabolism and growth of term infants in response to a reduced-protein, 40:60 whey: casein formula with added tryptophan. Am J Clin Nutr. 1992;56(6):1004–11. [DOI] [PubMed] [Google Scholar]
- 61. Larnkjaer A, Hoppe C, Molgaard C, Michaelsen KF. The effects of whole milk and infant formula on growth and IGF-I in late infancy. Eur J Clin Nutr. 2009;63(8):956–63. [DOI] [PubMed] [Google Scholar]
- 62. Lien EL, Davis AM, Euler AR. Growth and safety in term infants fed reduced-protein formula with added bovine alpha-lactalbumin. J Pediatr Gastroenterol Nutr. 2004;38(2):170–6. [DOI] [PubMed] [Google Scholar]
- 63. Lonnerdal B, Chen CL.. Effects of formula protein level and ratio on infant growth, plasma amino acids and serum trace elements. I. Cow's milk formula. Acta Paediatr Scand. 1990;79(3):257–65. [DOI] [PubMed] [Google Scholar]
- 64. Lonnerdal B, Hernell O.. Effects of feeding ultrahigh-temperature (UHT)-treated infant formula with different protein concentrations or powdered formula, as compared with breast-feeding, on plasma amino acids, hematology, and trace element status. Am J Clin Nutr. 1998;68(2):350–6. [DOI] [PubMed] [Google Scholar]
- 65. Oropeza-Ceja L, Rosado J, Ronquillo D, García O, Caamaño M, García-Ugalde C et al.. Lower Protein Intake Supports Normal Growth of Full-Term Infants Fed Formula: A randomized controlled trial. Nutrients. 2018;10(7):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Raiha NC, Fazzolari-Nesci A, Cajozzo C, Puccio G, Monestier A, Moro G et al.. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr. 2002;35(3):275–81. [DOI] [PubMed] [Google Scholar]
- 67. Rzehak P, Sausenthaler S, Koletzko S, Reinhardt D, von Berg A, Kramer U et al.. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 y of age: results from the German Infant Nutritional Intervention Study. Am J Clin Nutr. 2009;89(6):1846–56. [DOI] [PubMed] [Google Scholar]
- 68. Schmelzle H, Wirth S, Skopnik H, Radke M, Knol J, Bockler HM et al.. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr. 2003;36(3):343–51. [DOI] [PubMed] [Google Scholar]
- 69. Timby N, Domellof E, Hernell O, Lonnerdal B, Domellof M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr. 2014;99(4):860–8. [DOI] [PubMed] [Google Scholar]
- 70. Turck D, Grillon C, Lachambre E, Robiliard P, Beck L, Maurin JL et al.. Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J Pediatr Gastroenterol Nutr. 2006;43(3):364–71. [DOI] [PubMed] [Google Scholar]
- 71. Weber M, Grote V, Closa-Manesterolo R, Escribano J, Langhendries JP, Dain E et al.. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99(5):1041–51. [DOI] [PubMed] [Google Scholar]
- 72. Ziegler EE, Jeter JM, Drulis JM, Nelson SE, Haschke F, Steenhout P et al.. Formula with reduced content of improved, partially hydrolyzed protein and probiotics: Infant growth and health. Monatsschrift fur Kinderheilkunde. 2003;151(SUPPL. 1):S65–71. [Google Scholar]
- 73. Ziegler EE, Fields DA, Chernausek SD, Steenhout P, Grathwohl D, Jeter JM et al.. Adequacy of infant formula with protein content of 1.6 g/100 kcal for infants between 3 and 12 months. J Pediatr Gastroenterol Nutr. 2015;61(5):596–603. [DOI] [PubMed] [Google Scholar]
- 74. Ackatia-Armah RS, McDonald CM, Doumbia S, Erhardt JG, Hamer DH, Brown KH. Malian children with moderate acute malnutrition who are treated with lipid-based dietary supplements have greater weight gains and recovery rates than those treated with locally produced cereal-legume products: a community-based, cluster-randomized trial. Am J Clin Nutr. 2015;101(3):632–45. [DOI] [PubMed] [Google Scholar]
- 75. Alarcon PA, Lin LH, Noche M Jr., Hernandez VC, Cimafranca L, Lam W, Comer GM. Effect of oral supplementation on catch-up growth in picky eaters. Clin Pediatr (Phila). 2003;42(3):209–17. [DOI] [PubMed] [Google Scholar]
- 76. Bauserman M, Lokangaka A, Gado J, Close K, Wallace D, Kodondi K-K et al.. A cluster-randomized trial determining the efficacy of caterpillar cereal as a locally available and sustainable complementary food to prevent stunting and anaemia. Public Health Nutr. 2015;18(10):1785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Christian P, Shaikh S, Shamim AA, Mehra S, Wu L, Mitra M et al.. Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster-randomized trial. Int J Epidemiol. 2015;44(6):1862–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fabiansen C, Yaméogo CW, Iuel-Brockdorf A-S, Cichon B, Rytter MJ, Kurpad A et al.. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2× 2× 3 factorial trial in Burkina Faso. PLoS Med. 2017;14(9):e1002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. He M, Yang YX, Han H, Men JH, Bian LH, Wang GD. Effects of yogurt supplementation on the growth of preschool children in Beijing suburbs. Biomed Environ Sci. 2005;18(3):192–7. [PubMed] [Google Scholar]
- 80. Heikens GT, Schofield WN, Dawson S. The Kingston Project. II. The effects of high energy supplement and metronidazole on malnourished children rehabilitated in the community: anthropometry. Eur J Clin Nutr. 1993;47(3):160–73. [PubMed] [Google Scholar]
- 81. Heikens GT, Schofield WN, Dawson S, Grantham-McGregor S. The Kingston project. I. Growth of malnourished children during rehabilitation in the community, given a high energy supplement. Eur J Clin Nutr. 1989;43(3):145–60. [PubMed] [Google Scholar]
- 82. Iannotti LL, Lutter CK, Stewart CP, Riofrío CAG, Malo C, Reinhart G et al.. Eggs in early complementary feeding and child growth: A randomized controlled trial. Pediatrics. 2017;140, (1):e20163459. [DOI] [PubMed] [Google Scholar]
- 83. Krebs NF, Mazariegos M, Chomba E, Sami N, Pasha O, Tshefu A et al.. Randomized controlled trial of meat compared with multimicronutrient-fortified cereal in infants and toddlers with high stunting rates in diverse settings. Am J Clin Nutr. 2012;96(4):840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin CA, Manary MJ, Maleta K, Briend A, Ashorn P. An energy-dense complementary food is associated with a modest increase in weight gain when compared with a fortified porridge in Malawian children aged 6–18 months. J Nutr. 2008;138(3):593–8. [DOI] [PubMed] [Google Scholar]
- 85. Long JK, Murphy SP, Weiss RE, Nyerere S, Bwibo NO, Neumann CG. Meat and milk intakes and toddler growth: a comparison feeding intervention of animal-source foods in rural Kenya. Public Health Nutr. 2012;15(6):1100–7. [DOI] [PubMed] [Google Scholar]
- 86. Maleta KM, Phuka J, Alho L, Cheung YB, Dewey KG, Ashorn U et al.. Provision of 10–40 g/d Lipid-Based Nutrient Supplements from 6 to 18 Months of Age Does Not Prevent Linear Growth Faltering in Malawi–3. J Nutr. 2015;145(8):1909–15. [DOI] [PubMed] [Google Scholar]
- 87. Mangani C, Maleta K, Phuka J, Cheung YB, Thakwalakwa C, Dewey K et al.. Effect of complementary feeding with lipid-based nutrient supplements and corn-soy blend on the incidence of stunting and linear growth among 6- to 18-month-old infants and children in rural Malawi. Matern Child Nutr. 2015;11:Suppl 4:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nikiema L, Huybregts L, Kolsteren P, Lanou H, Tiendrebeogo S, Bouckaert K et al.. Treating moderate acute malnutrition in first-line health services: an effectiveness cluster-randomized trial in Burkina Faso. Am J Clin Nutr. 2014;100(1):241–9. [DOI] [PubMed] [Google Scholar]
- 89. Schlossman N, Brown C, Batra P, de Sa AB, Balan I, Balan A et al.. A randomized controlled trial of two ready-to-use supplementary foods demonstrates benefit of the higher dairy supplement for reduced wasting in mothers, and differential impact in infants and children associated with maternal supplement response. Food Nutr Bull. 2017;38(3):275–90. [DOI] [PubMed] [Google Scholar]
- 90. Simondon KB, Gartner A, Berger J, Cornu A, Massamba JP, San Miguel JL et al.. Effect of early, short-term supplementation on weight and linear growth of 4-7-mo-old infants in developing countries: a four-country randomized trial. Am J Clin Nutr. 1996;64(4):537–45. [DOI] [PubMed] [Google Scholar]
- 91. Skau JK, Touch B, Chhoun C, Chea M, Unni US, Makurat J et al.. Effects of animal source food and micronutrient fortification in complementary food products on body composition, iron status, and linear growth: a randomized trial in Cambodia. Am J Clin Nutr. 2015;101(4):742–51. [DOI] [PubMed] [Google Scholar]
- 92. Tang MH, Krebs NF.. High protein intake from meat as complementary food increases growth but not adiposity in breastfed infants: A randomized trial. Am J Clin Nutr. 2014;100(5):1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tang M, Sheng XY, Krebs NF, Hambidge KM. Meat as complementary food for older breastfed infants and toddlers: A randomized, controlled trial in rural China. Food Nutr Bull. 2014;35(4 Suppl):S188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tavill F, Gonik A.. Use of fish protein concentrate in the diets of weanling infants. A study of 88 infants from a low socioeconomic population, Casablanca, Morocco. Am J Clin Nutr. 1969;22(12):1571–6. [DOI] [PubMed] [Google Scholar]
- 95. Walker SP, Grantham-McGregor SM, Himes JH, Powell CA, Chang SM. Early childhood supplementation does not benefit the long-term growth of stunted children in Jamaica. J Nutr. 1996;126(12):3017–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.