Abstract
Background.
The evolution of influenza A viruses results in birth cohorts that have different initial influenza virus exposures. Historically, A/H3 predominant seasons have been associated with more severe influenza-associated disease; however, since the 2009 pandemic, there are suggestions that some birth cohorts experience more severe illness in A/H1 predominant seasons.
Methods.
United States influenza virologic, hospitalization, and mortality surveillance data during 2000–2017 were analyzed for cohorts born between 1918 and 1989 that likely had different initial influenza virus exposures based on viruses circulating during early childhood. Relative risk/rate during H3 compared with H1 predominant seasons during prepandemic versus pandemic and later periods were calculated for each cohort.
Results.
During the prepandemic period, all cohorts had more influenza-associated disease during H3 predominant seasons than H1 predominant seasons. During the pandemic and later period, 4 cohorts had higher hospitalization and mortality rates during H1 predominant seasons than H3 predominant seasons.
Conclusions.
Birth cohort differences in risk of influenza-associated disease by influenza A virus subtype can be seen in US influenza surveillance data and differ between prepandemic and pandemic and later periods. As the population ages, the amount of influenza-associated disease may be greater in future H1 predominant seasons than H3 predominant seasons.
Keywords: birth cohort, influenza, influenza hospitalization, influenza morality, influenza surveillance
Influenza viruses are constantly changing and as a result cause significant morbidity and mortality during annual epidemics and periodic pandemics [1–5]. As influenza A viruses have evolved and emerged or re-emerged during the past 100 years, the subtypes and antigenic properties of the viruses circulating in the human population at any given time have changed (Figure 1). Before 1977, a single influenza A subtype circulated; H1 viruses circulated from at least 1918 until 1957, H2 viruses circulated from 1957 until 1968, and H3 viruses circulated from 1968 to 1977. In 1977, H1 viruses re-emerged and began to cocirculate with H3 viruses. Currently, both influenza A subtypes and 2 influenza B lineages cocirculate at different proportions during annual epidemics. Since 1976, the highest rates of severe influenza-related illness and death usually occur in seasons during which H3 viruses are predominant [1–3].
Figure 1.
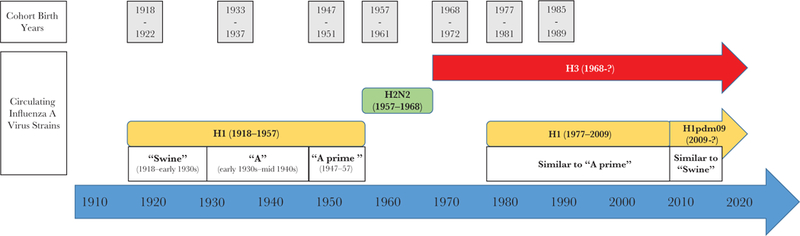
Influenza A virus circulation, 1918 through 2017.
As a result of the frequently changing landscape of influenza A virus circulation, different birth cohorts have different influenza A virus exposure histories. The concept of immunologic imprinting or “original antigenic sin,” first described in the mid-1900s, suggests that the influenza virus with which an individual is first infected has a lasting impact on subsequent immune responses, such that subsequent influenza infections cause a boost to antibodies against the initial virus [6]. More recently, a modified version of this phenomenon, termed antigen imprinting or antigenic seniority, proposes that there is a relative immune response to each subsequent strain one is infected with and the antibody response is highest against viruses one is exposed to in childhood [7, 8]. Some studies suggest that the age distribution of severe illness noted during the 2009 pandemic (greater in children and younger adults than among those ≥65 years) may be at least partially explained by this phenomenon because the pandemic virus was related to the H1 viruses that were the initial influenza infection for persons born in the early to mid-1900s [5, 9–11].
Since the 2009 pandemic, US influenza surveillance data have suggested a distribution of severe influenza illness different from what was seen in prepandemic seasons. For example, surveillance for laboratory-confirmed, influenza-associated hospitalizations found that older adults continued to have higher mean hospitalization rates during H3 compared with H1 predominant seasons, but younger adults had higher mean hospitalization rates during H1 predominant seasons [12]. To explore this apparent shift in disease burden of influenza A subtypes since the emergence of the H1 virus that caused the 2009 pandemic (H1pdm09), and to determine whether adults were affected differentially based on their original influenza A virus exposure, we compared US influenza laboratory, hospitalization, and mortality surveillance data for 7 adult birth cohorts during the 2000–2001 through 2016–2017 influenza seasons.
METHODS
Birth Cohorts
Seven 5-year birth cohorts with birth years from 1918 to 1989 were assessed. Birth cohort was used as a proxy for initial influenza A virus exposure. Cohort start years were selected to coincide with the emergence of a new influenza A subtype or significant antigenic changes to the circulating H1 virus [9, 10, 13–15]. Changes within H1 viruses were selected for birth cohort determination because, during the past 100 years, these viruses circulated for a longer period of time than H3 viruses, and because they emerged and re-emerged at 3 separate time points (Figure 1). In addition, since the development of influenza vaccine, H1 viruses exhibited fewer substantial antigenic changes, necessitating fewer vaccine strain updates than H3 viruses [16]. The birth cohorts analyzed had a likely initial influenza virus exposure to H1 viruses (1918, 1933, and 1947 cohorts), H2 viruses (1957 cohort), H3 viruses (1968 cohort), or a mix of H1 and H3 viruses (1977 and 1985 cohorts) (Table 1). The mixed virus cohorts were included in part to assess whether mixed exposure might, at a population level, have an advantage during this current period of H1 and H3 virus cocirculation. Five-year cohorts were used to maximize the likelihood that cohort members’ initial influenza virus infection was caused by a similar virus; almost all children have been infected with an influenza A virus by the time they are 6 years old [17], and the potential for mixing during transition from one virus group to another is minimized with a 5-year cohort.
Table 1.
Most Likely Initial Influenza A Virus Subtype Exposure by Birth Cohort
| Cohort Name | Birth Years | Cohort Type | Influenza A Subtype(s) Circulating During Birth Years |
|---|---|---|---|
| 1918 | 1918-1922 | H1 cohort | A/H1 |
| 1933 | 1933-1937 | H1 cohort | A/H1 (significant drift compared with 1918 H1) |
| 1947 | 1947-1951 | H1 cohort | A/H1 (further significant drift compared with 1918 and 1933 H1) |
| 1957 | 1957-1961 | H2 cohort | A/H2 |
| 1968 | 1968-1972 | H3 cohort | A/H3 |
| 1977 | 1977-1981 | Mixed cohort | A/H1 (similar to 1947 H1), A/H3 |
| 1985 | 1985-1989 | Mixed cohort | A/H1 (slightly less similar to 1947 H1), A/H3 |
Study Period
Data from US influenza seasons from October 2000 through September 2017 were analyzed. Most seasons began in early October and continued through the following September. Due to the emergence of the H1pdm09 virus and start of the pandemic in April 2009, the 2008–2009 season ended in late April 2009, and the 2009–2010 season encompassed influenza activity occurring from late April 2009 through September 2010.
Influenza Disease Data Sources
We looked at data from 3 US influenza surveillance systems that capture clinically significant influenza-associated illness: the US World Health Organization (WHO) Collaborating Laboratories System, the US Influenza Hospitalization Surveillance Network (FluSurv-NET), and the National Center for Health Statistics (NCHS) Mortality Surveillance System. Specimen level results from influenza testing performed on specimens collected during routine patient care at outpatient, emergency department, and inpatient settings and reported to the Centers for Disease Control and Prevention by US WHO Collaborating Laboratories [18] were analyzed. Between 72 and 113 laboratories participated each season with between 28% (prepandemic period) and 53% (pandemic and later period) of laboratories reporting specimen-level data. Specimen-level data with complete information for date of specimen collection or receipt, assay result, and patient birthdate or age were analyzed. Assay method was not available at the specimen level; however, the most commonly used test methods at public health laboratories before the 2009 pandemic were viral culture and serology-based assays, and since the pandemic the most commonly used test methods have been molecular-based assays.
Laboratory-confirmed, influenza-associated hospitalization data from FluSurv-NET [18, 19] for the 2005–2006 season (first season adults were included) through the 2016–2017 season were analyzed. Population-based surveillance occurred in selected counties of between 10 and 16 states per season and allowed the estimation of crude birth cohort-specific hospitalization rates. Date of hospitalization and influenza test, test result, test type (viral culture, direct/indirect fluorescent antibody assay, rapid influenza diagnostic test [RIDT], molecular assay), and patient birthdate/age were analyzed. Persons positive for influenza B, positive using a test that did not distinguish between influenza A or B, or likely had a hospital-acquired influenza infection (first influenza positive more than 3 days after hospital admission) were excluded. For patients with discordant results between an RIDT and a molecular assay on specimens collected on the same day, the RIDT results were excluded. If specimens were collected on different days, the positive result, regardless of test type, was accepted because it was not possible to determine whether timing of exposure, antiviral treatment, or other issues were responsible for the discordant results. The most recent census data available at the start of each season were used for cohort denominators. Census data for catchment area counties participating in FluSurv-NET were available in 1-year increments for persons aged 1 to 84 years and were estimated for those ≥85 years by applying single-year proportions in countrywide census data (available in 1-year increments up to 99 years of age) to the aggregate catchment area population denominator for persons ≥85 years.
Mortality data from the NCHS National Vital Statistics System [20] were analyzed. An influenza death was defined as one with an influenza code (ICD-10 J10-J11) listed anywhere on the death certificate. Final mortality data from 2000 to 2016 and preliminary 2017 mortality data (as of October 10, 2018) were used. Cohort denominators were determined using the US census estimates in one year age increments available in July preceding each season.
Comparing H1 and H3 Predominant Seasons
Each influenza season was categorized as H1 or H3 predominant based on the influenza A subtype reported most frequently by US WHO Collaborating Laboratories. The predominant influenza A subtype accounted for between 41% and 99% of circulating viruses during the prepandemic seasons and between 46% and 95% of the circulating viruses during pandemic and later seasons (Table 2). To control for the difference in circulating H1 viruses and changes in awareness, testing, and reporting of influenza that occurred as a result of the pandemic, 2 time periods were defined: prepandemic (2000–2001 through 2008–2009 seasons) and pandemic and later (2009–2010 through 2016–2017 seasons). The 3 outcomes tested across seasons during each time period for each birth cohort are (1) percentage of specimens testing positive for influenza A (percentage positivity), (2) rate of influenza A-associated hospitalizations, and (3) rate of influenza-coded deaths. Relative risks (percentage positivity) and relative rates (influenza hospitalizations and deaths) of H3 compared with H1 predominant seasons by birth cohort were calculated using generalized estimating equations with influenza subtype, pandemic period, and their interaction as main effects and season as a random effect. To account for the small number of seasons, confidence intervals and statistical significance were assessed using a wild bootstrap method [21] with 1000 replications and a 6-point distribution for the weights [22]. All analyses were performed with SAS version 9.4 (SAS Institute).
Table 2.
Distribution of Circulating Influenza Viruses and Predominant Influenza A Subtype, by Season, United States, 2000–2001 to 2016–2017
| Season | Period | Predominant Influenza A Virus Subtype |
Percentage of Circulating Viruses |
||
|---|---|---|---|---|---|
| H1 | H3 | B | |||
| Prepandemic | |||||
| 2000–2001 | A/H1 | 52% | 2% | 47% | |
| 2001–2002 | A/H3 | 2% | 84% | 14% | |
| 2002–2003 | A/H1 | 41% | 17% | 42% | |
| 2003–2004 | A/H3 | 0% | 99% | 1% | |
| 2004–2005 | A/H3 | 0% | 75% | 25% | |
| 2005–2006 | A/H3 | 8% | 72% | 21% | |
| 2006–2007 | A/H1 | 49% | 30% | 21% | |
| 2007–2008 | A/H3 | 18% | 53% | 29% | |
| 2008–2009 | A/H1 | 58% | 8% | 34% | |
| Pandemic and Later | |||||
| 2009–2010 | A/H1pdm09 | 95% | 4% | 1% | |
| 2010–2011 | A/H3 | 28% | 46% | 26% | |
| 2011–2012 | A/H3 | 21% | 60% | 18% | |
| 2012–2013 | A/H3 | 4% | 66% | 30% | |
| 2013–2014 | A/H1pdm09 | 74% | 11% | 15% | |
| 2014–2015 | A/H3 | 0% | 83% | 17% | |
| 2015–2016 | A/H1pdm09 | 56% | 14% | 30% | |
| 2016–2017 | A/H3 | 2% | 75% | 23% | |
RESULTS
Virologic Surveillance
Laboratory testing for influenza increased during the pandemic and remained elevated in subsequent seasons compared with prepandemic testing levels (Table 3). During the prepandemic period, the overall mean number of specimens tested per cohort per season was 305 with the cohort-specific mean number of specimens tested ranging from 125 (1933 cohort) to 632 (1985 cohort). During the pandemic and later period, the overall mean number of specimens tested per cohort per season increased approximately 10-fold to 2941, and the cohort-specific mean number of specimens tested ranged from 1280 (1918 cohort) to 3791 (1985 cohort).
Table 3.
Comparison of Influenza Virologic Surveillance by Birth Cohort, 2000–2001 Through 2016–2017 Seasons - Number of Specimens Tested and Percentage Positive for Influenza A
| Birth Cohort | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1918–1922 | 1933–1937 | 1947–1951 | 1957–1961 | 1968–1972 | 1977–1981 | 1985–1989 | |||||||||
| Season | Predominant Virus |
Percent A Positive |
Number of Specimens Tested |
Percent A Positive |
Number of Specimens Tested |
Percent A Positive |
Number of Specimens Tested |
Percent A Positive |
Number of Specimens Tested |
Percent A Positive |
Number of Specimens Tested |
Percent A Positive |
Number of Specimens Tested |
Percent A Positive |
Number of Specimens Tested |
| Prepandemic | |||||||||||||||
| 2000–2001 | H1 | 1.6 | 123 | 3.5 | 86 | 8.5 | 165 | 24.5 | 241 | 19.1 | 267 | 29.0 | 487 | 28.8 | 313 |
| 2001–2002 | H3 | 30.8 | 221 | 34.4 | 122 | 30.0 | 207 | 32.5 | 268 | 34.1 | 279 | 45.0 | 518 | 37.2 | 462 |
| 2002–2003 | H1 | 14.9 | 101 | 17.6 | 85 | 19.3 | 150 | 16.0 | 237 | 36.1 | 166 | 13.8 | 689 | 23.2 | 745 |
| 2003–2004 | H3 | 31.9 | 238 | 41.9 | 179 | 31.6 | 250 | 32.2 | 311 | 42.7 | 457 | 44.1 | 726 | 43.9 | 1027 |
| 2004–2005 | H3 | 38.1 | 307 | 43.3 | 215 | 41.7 | 345 | 41.3 | 380 | 47.0 | 406 | 35.0 | 488 | 31.4 | 608 |
| 2005–2006 | H3 | 30.0 | 140 | 34.8 | 135 | 33.0 | 206 | 37.4 | 238 | 45.0 | 211 | 34.6 | 292 | 47.7 | 549 |
| 2006–2007 | H1 | 18.1 | 127 | 19.6 | 102 | 24.8 | 165 | 30.0 | 230 | 40.0 | 260 | 35.1 | 288 | 28.8 | 781 |
| 2007–2008 | H3 | 22.5 | 120 | 26.4 | 106 | 24.9 | 221 | 36.1 | 319 | 31.2 | 298 | 44.6 | 359 | 55.8 | 611 |
| 2008–2009 | H1 | 4.7 | 86 | 16.2 | 99 | 22.6 | 146 | 41.0 | 212 | 41.8 | 220 | 44.1 | 286 | 35.3 | 595 |
| Pandemic and Later | |||||||||||||||
| 2009–2010 | H1pdm09 | 4.0 | 1463 | 10.5 | 2621 | 21.2 | 4259 | 32.0 | 5566 | 31.1 | 4332 | 36.3 | 4854 | 46.3 | 6648 |
| 2010–2011 | H3 | 37.4 | 1141 | 34.0 | 1505 | 29.4 | 1894 | 40.6 | 2239 | 39.4 | 1841 | 44.8 | 2241 | 44.6 | 3608 |
| 2011–2012 | H3 | 31.8 | 912 | 27.6 | 1270 | 25.3 | 1555 | 27.3 | 1742 | 30.4 | 1616 | 36.5 | 1721 | 33.3 | 2233 |
| 2012–2013 | H3 | 50.8 | 1963 | 43.1 | 3050 | 36.7 | 3170 | 33.8 | 3155 | 39.3 | 2650 | 43.1 | 2939 | 40.6 | 3368 |
| 2013–2014 | H1pdm09 | 13.5 | 960 | 21.2 | 2563 | 31.1 | 3902 | 38.7 | 4517 | 41.7 | 3655 | 43.5 | 3796 | 41.5 | 4182 |
| 2014–2015 | H3 | 56.2 | 2215 | 43.7 | 4794 | 34.2 | 4752 | 30.7 | 4276 | 29.6 | 3221 | 32.7 | 3487 | 34.0 | 4072 |
| 2015–2016 | H1pdm09 | 7.6 | 631 | 14.0 | 2480 | 21.5 | 3421 | 26.9 | 3830 | 32.3 | 2765 | 30.2 | 2829 | 27.8 | 3257 |
| 2016–2017 | H3 | 48.8 | 957 | 45.3 | 3450 | 41.0 | 3720 | 35.7 | 3542 | 33.5 | 2416 | 32.3 | 2476 | 34.5 | 2956 |
Percentage positivity was significantly higher during prepandemic H3 predominant seasons than H1 predominant seasons for the H1 cohorts (1918, 1933, 1947) with the highest risk in the 1918 cohort (Table 4). The increased positivity in H3 seasons was seen for the other 4 cohorts, but the results were not statistically significant. During the pandemic and later period, the relative risk of having an influenza A-positive specimen remained higher during H3 compared with H1 predominant seasons for the 1918 and 1933 cohorts (relative risk 6.17 and 2.73, respectively). For the 1947 cohort, the relative risk was greater, but not statistically significant, in the postpandemic H3 seasons compared with H1 seasons. For the remaining cohorts (1957, 1968, 1977, and 1985), the relative risk was close to 1.
Table 4.
Comparison of Influenza Virologic Surveillance by Birth Cohort, 2000–2001 Through 2016–2017 - Relative Risk of Influenza A Positivity During H3 Compared to H1 Predominant Seasons
| Prepandemic (2000–2001 to 2008–2009 Seasons) | Pandemic and Later (2009–2010 to 2016–2017 Seasons) | |||||
|---|---|---|---|---|---|---|
| Percent A Positive (No. Positive/No. Tested) |
H3 vs HI Predominant Seasons |
Percent A Positive (No. Positive/No. Tested) |
H3 vs HI Predominant Seasons |
|||
| Cohort | H1 Predominant Seasons |
H3 Predominant Seasons |
Relative Risk (95% Confidence Interval) |
H1 Predominant Seasons |
H3 Predominant Seasons |
Relative Risk (95% Confidence Interval) |
| 1918–1922 | 10.07 (44/437) | 32.16 (380/1026) | 3.19 (1.65–11.07) | 7.73 (236/3054) | 47.66 (3426/7188) | 6.17 (3.11–14.27) |
| 1933–1937 | 14.52 (54/372) | 37.65 (285/757) | 2.59 (1.58–4.46) | 15.21 (1166/7664) | 41.50 (5838/14 069) | 2.73 (1.73–4.76) |
| 1947–1951 | 18.69 (117/626) | 33.20 (408/1229) | 1.78 (1.08–3.07) | 24.63 (2853/11 582) | 34.86 (5261/15 091) | 1.42 (0.95–2.18) |
| 1957–1961 | 27.50 (253/920) | 36.15 (548/1516) | 1.31 (0.87–2.10) | 32.78 (4561/13 913) | 33.62 (5027/14 954) | 1.03 (0.70–1.49) |
| 1968–1972 | 33.63 (307/913) | 37.55 (620/1651) | 1.12 (0.73–1.82) | 35.00 (3763/10 752) | 34.25 (4022/11 744) | 0.98 (0.67–1.48) |
| 1977–1981 | 26.46 (463/1750) | 41.13 (985/2383) | 1.56 (0.94–2.88) | 37.17 (4267/11 479) | 37.62 (4840/12 864) | 1.01 (0.70–1.54) |
| 1985–1989 | 28.68 (689/2434) | 40.41 (1316/3257) | 1.41 (0.93–2.10) | 40.60 (5719/14 087) | 37.70 (6122/16 237) | 0.93 (0.61–1.40) |
The bold indicates the value is statistically significant.
Hospitalization Surveillance
During the prepandemic period for which FluSurv-NET data were available (2005–2006 through 2008–2009 seasons), the number of influenza A hospitalizations per birth cohort per season ranged from 24 to 200 (Table 5) with the cohort-specific mean ranging from 41 (1985 cohort) to 102 (1918 cohort). Due at least in part to increased laboratory testing for influenza, the overall mean number of influenza-associated hospitalizations during the pandemic and later period was higher than during the prepandemic period (426 and 67, respectively), and the cohort-specific mean during the pandemic and later period ranged from 250 (1985 cohort) to 668 (1933 cohort) (Table 5).
Table 5.
Comparison of Influenza A Hospitalizations by Birth Cohort, 2005–2006 Through 2016–2017 Seasons - Number and Rate (per 100,000 Population) of Influenza A Hospitalizations
| Birth Cohort | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1918–1922 | 1933–1937 | 1947–1951 | 1957–1961 | 1968–1972 | 1977–1981 | 1985–1989 | |||||||||
| Season | Predom Inant Virus | Rate | Number | Rate | Number | Rate | Number | Rate | Number | Rate | Number | Rate | Number | Rate | Number |
| Prepandemic | |||||||||||||||
| 2005–2006 | H3 | 61.5 | 127 | 19.8 | 88 | 5.7 | 63 | 3.7 | 52 | 2.5 | 34 | 2.6 | 33 | 2.0 | 25 |
| 2006–2007 | H1 | 21.0 | 47 | 12.2 | 62 | 3.2 | 40 | 3.7 | 60 | 1.8 | 28 | 1.6 | 24 | 1.8 | 25 |
| 2007–2008 | H3 | 94.1 | 200 | 34.0 | 175 | 9.3 | 120 | 7.8 | 130 | 4.6 | 75 | 4.3 | 68 | 4.5 | 64 |
| 2008–2009 | H1 | 19.1 | 36 | 7.4 | 38 | 3.6 | 47 | 3.9 | 65 | 2.9 | 47 | 2.6 | 42 | 3.3 | 48 |
| Pandemic and Later | |||||||||||||||
| 2009–2010 | H1pdm09 | 23.5 | 48 | 26.9 | 158 | 28.8 | 429 | 34.9 | 673 | 22.7 | 424 | 24.8 | 463 | 28.3 | 507 |
| 2010–2011 | H3 | 143.1 | 270 | 46.6 | 296 | 18.2 | 299 | 17.3 | 371 | 8.8 | 185 | 8.4 | 177 | 8.7 | 180 |
| 2011–2012 | H3 | 65.1 | 99 | 29.2 | 169 | 7.4 | 113 | 6.1 | 124 | 3.1 | 61 | 2.8 | 56 | 4.0 | 79 |
| 2012–2013 | H3 | 499.1 | 636 | 158.7 | 891 | 43.6 | 663 | 24.2 | 491 | 14.3 | 286 | 11.4 | 232 | 10.9 | 225 |
| 2013–2014 | H1pdm09 | 77.4 | 78 | 72.0 | 374 | 46.5 | 670 | 41.9 | 812 | 25.5 | 491 | 19.1 | 374 | 14.5 | 296 |
| 2014–2015 | H3 | 1094.8 | 907 | 327.9 | 1642 | 72.8 | 1032 | 31.4 | 605 | 16.6 | 321 | 12.9 | 253 | 11.9 | 248 |
| 2015–2016 | H1pdm09 | 64.0 | 43 | 70.2 | 337 | 41.2 | 574 | 34.3 | 656 | 19.3 | 373 | 12.3 | 244 | 10.1 | 215 |
| 2016–2017 | H3 | 829.9 | 441 | 322.8 | 1477 | 83.9 | 1143 | 42.5 | 802 | 18.6 | 357 | 11.7 | 230 | 11.4 | 246 |
The influenza A hospitalization rate per 100 000 population (Table 6) was higher during prepandemic H3 predominant seasons than H1 predominant seasons for all cohorts with relative rates ranging from 3.88 (1918 cohort) to 1.31 (1985 cohort) but statistically significant for only the H1 cohorts (1918, 1933, and 1947). During the pandemic and later period, the rates of influenza A hospitalization remained higher during H3 compared with H1 predominant seasons for the all 3 H1 cohorts but only statistically significant for the 1918 cohort (relative rate 8.58). For the remaining cohorts, the inverse was true; the rate of hospitalization for H3 predominant seasons was lower than H1 predominant seasons, statistically significantly for the 1968 and 1977 cohorts (relative rates 0.54 and 0.50, respectively).
Table 6.
Comparison of Influenza A Hospitalizations by Birth Cohort, 2005–2006 Through 2016–2017 Seasons - Relative Rate of Influenza A Hospitalization During H3 Compared to H1 Predominant Seasons
| Prepandemic (2005–2006 to 2008–2009 Seasons) | Pandemic and Later (2009–2010 to 2016–2017 Seasons) | |||||
|---|---|---|---|---|---|---|
| Hospitalization Rate Per 100 000 (No. of Hospitalizations) | H3vsH1 Predominant Seasons | Hospitalization Rate Per 100 000 (No. of Hospitalizations) | H3 vs HI Predominant Seasons | |||
| Cohort | HI Predominant Seasons | H3 Predominant Seasons | Relative Rate (95% Confidence Interval) |
HI Predominant Seasons | H3 Predominant Seasons | Relative Rate (95% Confidence Interval) |
| 1918–1922 | 20.11 (83) | 78.00 (327) | 3.88 (2.13–6.71) | 45.37 (169) | 389.47 (2353) | 8.58 (1.54–27.71) |
| 1933–1937 | 9.80 (100) | 27.41 (263) | 2.80 (1.41–5.67) | 54.74 (869) | 163.64 (4475) | 2.99 (0.86–7.01) |
| 1947–1951 | 3.40 (87) | 7.64 (183) | 2.24 (1.22–3.88) | 38.69 (1673) | 43.47 (3250) | 1.12 (0.40–2.22) |
| 1957–1961 | 3.81 (125) | 5.95 (182) | 1.56 (0.71–2.96) | 37.09 (2141) | 23.9 (2393) | 0.64 (0.32–1.07) |
| 1968–1972 | 2.33 (75) | 3.67 (109) | 1.58 (0.75–3.15) | 22.50 (1288) | 12.17 (1210) | 0.54 (0.30–0.89) |
| 1977–1981 | 2.11 (66) | 3.57 (101) | 1.69 (0.86–3.38) | 18.63 (1081) | 9.4 (948) | 0.50 (0.28–0.89) |
| 1985–1989 | 2.57 (73) | 3.37 (89) | 1.31 (0.53–3.08) | 17.10 (1018) | 9.41 (977) | 0.55 (0.29–1.12) |
The bold indicates the value is statistically significant.
Mortality Surveillance
During the prepandemic period, the number of influenza-coded deaths per cohort per season ranged from 2 to 538 (Table 7) with the cohort-specific mean ranging from 7 (1977 cohort) to 225 (1918 cohort). Likely due to increases in influenza testing and recognition of the contribution of influenza to a person’s death, the number of influenza-coded deaths for a single cohort in a single season during the pandemic and later period was higher, ranging from 10 to 1249 with the cohort-specific mean ranging from 33 (1985 cohort) to 439 (1918 cohort).
Table 7.
Comparison of Influenza Mortality by Birth Cohort, 2000–2001 through 2016–2017 Seasons - Number and Rater (Per 100,000) of Influenza Deaths
| Birth Cohort | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1918–1922 | 1933–1937 | 1947–1951 | 1957–1961 | 1968–1972 | 1977–1981 | 1985–1989 | |||||||||
| Season | Predom Inant Virus | Rate | Number | Rate | Number | Rate | Number | Rate | Number | Rate | Number | Rate | Number | Rate | Number |
| Prepandemic | |||||||||||||||
| 2000–2001 | H1 | 0.88 | 53 | 0.24 | 24 | 0.07 | 14 | 0.03 | 6 | 0.02 | 5 | 0.03 | 5 | 0.02 | 5 |
| 2001–2002 | H3 | 2.33 | 132 | 0.40 | 39 | 0.08 | 15 | 0.03 | 6 | 0.01 | 2 | 0.03 | 6 | 0.04 | 8 |
| 2002–2003 | H1 | 0.89 | 47 | 0.17 | 16 | 0.06 | 12 | 0.04 | 8 | 0.02 | 4 | 0.02 | 3 | 0.04 | 8 |
| 2003–2004 | H3 | 10.96 | 538 | 1.39 | 132 | 0.32 | 59 | 0.12 | 27 | 0.08 | 17 | 0.07 | 14 | 0.11 | 23 |
| 2004–2005 | H3 | 10.12 | 457 | 0.93 | 87 | 0.26 | 49 | 0.08 | 19 | 0.06 | 12 | 0.01 | 2 | 0.05 | 11 |
| 2005–2006 | H3 | 5.38 | 222 | 0.74 | 68 | 0.16 | 30 | 0.08 | 19 | 0.05 | 10 | 0.02 | 4 | 0.04 | 8 |
| 2006–2007 | H1 | 2.25 | 84 | 0.40 | 36 | 0.14 | 25 | 0.05 | 12 | 0.01 | 3 | 0.02 | 4 | 0.01 | 2 |
| 2007–2008 | H3 | 13.52 | 454 | 1.23 | 107 | 0.42 | 77 | 0.17 | 38 | 0.07 | 14 | 0.09 | 19 | 0.06 | 13 |
| 2008–2009 | H1 | 1.21 | 36 | 0.24 | 20 | 0.12 | 21 | 0.12 | 27 | 0.04 | 9 | 0.03 | 7 | 0.05 | 11 |
| Pandemic and Later | |||||||||||||||
| 2009–2010 | H1pdm09 | 3.14 | 84 | 1.09 | 91 | 0.84 | 152 | 0.76 | 174 | 0.47 | 97 | 0.32 | 66 | 0.25 | 54 |
| 2010–2011 | H3 | 10.91 | 247 | 1.25 | 100 | 0.58 | 105 | 0.37 | 84 | 0.17 | 36 | 0.20 | 41 | 0.15 | 31 |
| 2011–2012 | H3 | 6.64 | 129 | 0.65 | 50 | 0.15 | 27 | 0.13 | 30 | 0.08 | 17 | 0.05 | 10 | 0.05 | 11 |
| 2012–2013 | H3 | 50.12 | 828 | 4.78 | 358 | 1.16 | 204 | 0.57 | 128 | 0.29 | 60 | 0.18 | 36 | 0.09 | 20 |
| 2013–2014 | H1 pdm09 | 10.32 | 142 | 3.49 | 252 | 1.96 | 342 | 1.83 | 410 | 0.97 | 203 | 0.56 | 115 | 0.28 | 61 |
| 2014–2015 | H3 | 110.35 | 1249 | 12.66 | 877 | 2.26 | 390 | 0.85 | 190 | 0.31 | 66 | 0.23 | 47 | 0.14 | 31 |
| 2015–2016 | H1pdm09 | 12.55 | 115 | 4.69 | 311 | 1.96 | 335 | 1.40 | 311 | 0.85 | 179 | 0.41 | 84 | 0.17 | 37 |
| 2016–2017 | H3 | 99.34 | 720 | 14.05 | 885 | 2.83 | 477 | 1.24 | 272 | 0.46 | 97 | 0.24 | 51 | 0.09 | 20 |
The influenza death rate per 100 000 population (Table 8) during the prepandemic period was higher during H3 predominant seasons than H1 predominant seasons for all birth cohorts (statistically significant for 1918, 1937, 1947, and 1968) with the relative rate ranging from 6.54 (1918 cohort) to 1.67 (1957 and 1968 cohorts). During the pandemic and later period, the relative rate of an influenza death was higher during H3 than H1 predominant seasons for the 1918 and 1933 cohorts (5.64 [statistically significant] and 2.14 times higher, respectively), whereas the remaining cohorts had a higher rate of influenza deaths during H1 seasons (relative rates from 0.90 [1947 cohort] to 0.37 [1968 cohort]; statistically significant for 1957, 1968, 1977, and 1985 cohorts).
Table 8.
Comparison of Influenza Mortality by Birth Cohort, 2000–2001 through 2016–2017 seasons - Relative Rate of Influenza Mortality During H3 Compared to H1 Predominant Seasons
| Prepandemic (2000–2001 to 2008–2009 Seasons) | Pandemic and Later (2009–2010 to 2016–2017 Seasons) | |||||
|---|---|---|---|---|---|---|
| Death Rate Per 100 000 (No. of Deaths) | H3 vs H1 Predominant Seasons | Death Rate Per 100 000 (No. of Deaths) | H3 vs HI Predominant Seasons | |||
| Cohort | HI Predominant Seasons | H3 Predominant Seasons | Relative Rate (95% Confidence Interval) |
H1 Predominant Seasons | H3 Predominant Seasons | Relative Rate (95% Confidence Interval) |
| 1918–1922 | 1.22 (220) | 7.98 (1803) | 6.54 (2.89–12.98) | 7.29 (362) | 41.12 (3173) | 5.64 (1.08–18.74) |
| 1933–1937 | 0.26 (96) | 0.93 (433) | 3.58 (1.99–6.46) | 2.90 (645) | 6.22 (2270) | 2.14 (0.35–6.33) |
| 1947–1951 | 0.10 (72) | 0.25 (230) | 2.50 (1.26–5.02) | 1.52 (799) | 1.37 (1203) | 0.90 (0.30–1.94) |
| 1957–1961 | 0.06 (53) | 0.10 (109) | 1.67 (0.75–4.26) | 1.28 (860) | 0.63 (704) | 0.49 (0.20–0.95) |
| 1968–1972 | 0.03 (21) | 0.05 (55) | 1.67 (1.05–4.19) | 0.70 (437) | 0.26 (276) | 0.37 (0.17–0.66) |
| 1977–1981 | 0.02 (19) | 0.05 (45) | 2.50 (0.58–3.94) | 0.40 (246) | 0.18 (185) | 0.45 (0.25–0.75) |
| 1985–1989 | 0.03 (26) | 0.06 (63) | 2.00 (0.99–4.47) | 0.26 (169) | 0.10 (113) | 0.38 (0.27–0.74) |
The bold indicates the value is statistically significant.
DISCUSSION
This analysis of US influenza surveillance data by birth cohort rather than traditional age groups (eg, children, adults, elderly) suggests, on a population level, that initial influenza A virus subtype exposure may affect the clinical impact of influenza in subsequent years. Before 2009, more influenza-associated hospitalization and death occurred during H3 predominant seasons than H1 predominant seasons for all birth cohorts studied with the largest and statistically significant effect seen in those birth cohorts that had their first influenza virus exposure with an H1 virus (the 1918, 1933, and 1947 cohorts). However, since the H1pdm09 virus emerged, birth cohorts born after 1957 experienced more severe illness (statistically significant for the 1968 and 1977 cohorts) and deaths (statistically significant for 1957 and later cohorts) during H1 seasons, whereas the 1918 through 1947 H1 cohorts continued to have more severe illness and deaths (statistically significant for hospitalizations and deaths for the 1918 cohort) in H3 seasons. Thus, as time passes and there are fewer persons alive who typically fare better in H1 predominant seasons (the H1 cohorts), and persons that typically fare worse in H1 seasons (cohorts born after 1957) age and become at higher risk for severe influenza complications, seasons with a predominance of H1pdm09 virus infections may be associated with more severe illness than H3 predominant seasons.
Our results were generally consistent across the 3 surveillance systems, with the percentage of specimens positive for influenza A and influenza hospitalization and mortality rates greater during prepandemic H3 than H1 predominant seasons for all cohorts (statistically significant for all H1 cohorts for all indicators and for mortality in the 1968 cohort). In contrast, since the pandemic, the 1957 and later cohorts experienced more hospitalizations and deaths during H1 predominant seasons (statistically significant for mortality in all 4 cohorts and hospitalization in the 1968 and 1977 cohorts). The variability in amount and intensity of influenza activity from season to season, even among seasons with a similar predominant influenza A subtype, resulted in larger confidence intervals, and in some cases confidence intervals that included 1, even for cohorts that experienced a large disparity in rates or percentage positive. Even in the presence of this known variability among influenza seasons, many cohorts saw statistically significant differences in activity based on the predominant virus and saw changes in these differences between the 2 study periods.
Our findings might be explained in part by the evolutionary pattern of H1 viruses since 1918. The H1 viruses circulating during 1918 underwent antigenic drift over time, with notable changes in 1933 and 1947, and multiple related but distinguishable groups of H1 viruses circulated from 1947 to 1957 [9, 10, 13–15]. The H1 viruses that re-emerged in 1977 were most similar to the viruses that circulated in the early 1950s [23]. Studies have shown that the H1pdm09 virus that emerged in 2009 was antigenically more similar to the H1 viruses that caused the 1918 pandemic than to the later prepandemic H1 viruses [9, 10]. Based on these antigenic properties, we hypothesized, and our data supported, that (1) the 1918 cohort would have the greatest protection against currently circulating H1pdm09 viruses followed by the 1933 and 1947 cohorts and (2) all 3 H1 cohorts would fare better during pandemic and later H1 predominant seasons than cohorts with initial exposure to H2, H3, or later prepandemic H1 viruses. Although the H1 viruses that re-emerged in 1977 were similar to those seen between 1947 and 1957, they cocirculated with H3 viruses, thereby reducing the probability that an individual in the 1977 or 1985 cohort had an initial influenza exposure to an H1 virus and potentially resulting in less protection against H1pdm09 viruses for these cohorts compared with the 1947 cohort.
The birth cohort effects related to initial exposure to H1 viruses were more apparent than effects related to initial exposure to H3 viruses, possibly due to the overall slower rate of change among H1 viruses and the emergence in 2009 of the H1pdm09 virus that was antigenically similar to the 1918 H1 viruses. This difference in imprinting effects of H1 compared with H3 viruses could be due to larger antigenic differences between the H3 viruses that circulated during and immediately after the 1968 pandemic and the H3 viruses that circulated during the study period.
Our analysis of US influenza surveillance data to compare birth cohort impacts required the assumption that all influenza activity in a season was due to the predominant influenza A subtype and all or most members of a birth cohort had a similar initial influenza virus exposure. We controlled for the effect of influenza B virus cocirculation in the laboratory and hospitalization data by analyzing only influenza A virus data (this was not possible for the mortality data), and we structured the birth cohort start years and duration to maximize the likelihood that cohort members had similar initial influenza A virus exposures. Furthermore, because the percentage of circulating viruses accounted for by the predominant influenza A subtype was lower during H1 predominant prepandemic seasons and H3 predominant pandemic and later seasons, any bias potentially caused by attributing all influenza-related illness to the predominant virus would underestimate the true relative risk of H3 viruses during the prepandemic period and H1 viruses during the pandemic and later period. It is possible that a change in virulence of the H1 virus could account for some of the changes identified in this analysis; however, if this were a significant contributing factor, we would have expected to see a uniform impact for each cohort rather than the differential impact across cohorts that was observed.
To control for changes in influenza recognition and testing practices that occurred during the study period, most notably as a result of the 2009 pandemic, and potential age-related differences in recognition of influenza and influenza testing practices, we stratified the analysis by birth cohort (as a proxy for age) and time period (prepandemic versus pandemic and later). We also determined that the different duration of the 2008–2009 and 2009–2010 seasons relative to other seasons in the study period should not significantly affect hospitalization or mortality rates because 2008–2009 activity was below baseline levels when the season ended, and little influenza activity was reported beyond the 52nd consecutive week of the 2009–2010 season. In the analysis of virologic data, we were not able to control for the relative contribution of specimens from outpatient and inpatient settings. The proportion of each may vary from season to season and may have contributed to the slightly different pattern of influenza A positivity compared with hospitalization and mortality rates in the pandemic and later period.
CONCLUSIONS
This analysis of data from 3 separate US influenza surveillance systems provides a clear indication that before the 2009 pandemic, H3 viruses caused more severe illness than H1 viruses in all birth cohorts; however, since the pandemic, there has been a paradigm shift such that more severe disease/death is now occurring in H1 rather than H3 predominant seasons for those in the 1957 and later birth cohorts. With the emergence of the H1pdm09 virus and the changing demographics of individuals with different initial influenza virus exposure histories, H1 predominant seasons may become the “high severity” influenza seasons of the future. As influenza viruses continue to evolve, it will be necessary to revisit these analyses to determine whether the morbidity and mortality patterns described here continue. Analyses of other influenza data by birth cohort, rather than age group, may also contribute to understanding the complex interplay of factors affecting the clinical impact of influenza virus infection.
Acknowledgments.
We thank Dr. David Benkeser (Rollins School of Public Health at Emory University); state, county, city, and territorial health departments and public health laboratories; the US World Health Organization collaborating laboratories; FluSurv-NET; and the National Center for Health Statistics, Centers for Disease Control and Prevention.
Financial support. This work was funded by the Centers for Disease Control and Prevention.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–40. [DOI] [PubMed] [Google Scholar]
- 2.Thompson MG, Shay DK, Zhou H, et al. Estimates of deaths associated with season influenza - United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–62. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. https://www.cdc.gov/flu/about/burden/index.html. Accessed 20 December 2018.
- 4.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 1998; 178:53–60. [DOI] [PubMed] [Google Scholar]
- 5.Shrestha SS, Swerdlow DL, Borse RH, et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009-April 2010). Clin Infect Dis 2011; 52 (Suppl 1):S75–82. [DOI] [PubMed] [Google Scholar]
- 6.Francis T On the doctrine of original antigenic sin. Proc Am Philos Soc 1960; 104:572–78. [Google Scholar]
- 7.Henry C, Palm AE, Krammer F, Wilson PC. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol 2018; 39:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P. Neutralizing antibodies against previously-encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 2013; 5: 198RA107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–52. [DOI] [PubMed] [Google Scholar]
- 11.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–9. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. FluView interactive, hospitalizations. https://gis.cdc.gov/GRASP/Fluview/FluHospRates.html Accessed 20 December 2018.
- 13.Nelson MI, Viboud C, Simonsen L, et al. Multiple reassortment events in the evolutionary history of H1N1 influenza A since 1918. PLoS Pathog 2008; 4:e1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilbourne ED, Smith C, Brett I, Pokorny BA, Johansson B, Cox N. The total influenza vaccine failure of 1947 revisited: major intrasubtypic antigenic change can explain failure of vaccine in a post-World War II epidemic. Proc Natl Acad Sci U S A 2002; 99:10748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci 2001; 356:1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Influenza Research Database. World Health Organization recommendations for composition of influenza vaccines, 1974–2018. https://www.fludb.org/brc/vaccineRecommendspg?decorator=influenza Accessed 20 December 2018.
- 17.Bodewes R, de Mutsert G, van der Klis FR, et al. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol 2011; 18:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Overview of influenza surveillance in the United States. https://www.cdcgov/flu/weekly/overview.htm Accessed 20 December 2018.
- 19.Chaves SS, Lynfield R, Lindegren ML, Bresee J, Finelli L. The US influenza hospitalization surveillance network. Emerg Infect Dis 2015; 21:1543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National vital statistics system - mortality data. https://www.cdc.gov/nchs/nvss/deaths.htm Accessed 20 December 2018.
- 21.Cameron AC, Gelbach JG, and Miller DL. Bootstrap-based improvements for inference with clustered errors. Rev Econ Stat 2008; 90:414–27. [Google Scholar]
- 22.Webb M Reworking wild bootstrap based inference for clustered errors. Queens Economics Department Working Paper, No. 1315 2013. https://econpapers.repec.org/paper/qedwpaper/1315.htm. Accessed 8 May 2019. [Google Scholar]
- 23.Rozo M, Gronvall GK. The reemergent 1977 H1N1 strain and the gain-of-function debate. mBio 2015; 6:e01013–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


