Abstract
Background
The United States (U.S.) Preventive Services Task Force (USPSTF) recommends lung cancer screening among individuals aged 55–80 years with a 30 pack-year cigarette smoking history and, if stopped smoking, quit within 15 years. Two-thirds of newly-diagnosed lung cancer patients do not meet these criteria; 18·8% (1,285 of 6,838 patients) are reported to be either with ≥15 quit-years (long-term quitters [LTQ]) or aged 50–54·9 years (younger age group [YAG]). We assessed survival outcomes of these two subgroups in two prospectively followed cohorts.
Methods
Between January 1, 1997, and December 31, 2017, 8,739 lung cancer patients aged 50–80 years, with ≥30 pack-years smoked, either current or former smokers with ≤30 quit-years, were identified from a hospital (Mayo Clinic) or a community (Olmsted County) cohort in the U.S. with a median follow-up 6·5 (IQR 3·8–10·0) years. Patients were divided into those meeting USPSTF criteria (USPSTF group) versus LTQ or YAG. The risk of death at five-year post diagnosis was analyzed with/without matching age and pack-years smoked for LTQ. The USPSTF group was subdivided into a 55–69 and a 70–80 age subgroup for analysis.
Findings
The median survival time was 16·9 months (95% CI, 16·2–17·5); the proportions of LTQ, YAG, and USPSTF group who survived at 5 years after diagnosis were 23% (323 of 1,404 patients), 18.5% (131 of 708 patients), and 19.2% (1,272 of 6,627 patients), respectively. In both cohorts, LTQ did not have significantly different risk of death as the USPSTF group (hazard ratio [HR]=0·97–1·02, p>0·05); matched analysis showed similar results. The YAG also did not have significantly different risk of death as the USPSTF group (HR=1·16, p>0·05); age-group stratified analysis yielded similar findings, HR of 0·93–1·29 (all p>0·30).
Interpretation
Lung cancer patients with ≥15 quit-years and those up to 5-years younger than the age recommended for screening who otherwise meet the USPSTF criteria, demonstrate a similar risk of death from lung cancer as the USPSTF group. Additionally, screening for YAG may confer the advantage of increased smoking cessation. Individuals in these two subgroups may gain more benefit from screening owing to lung cancer detected at an earlier stage and the evolving treatment paradigm.
Keywords: Lung cancer, Screening, Low-dose CT, smoking cessation, Former smokers, Younger age
Introduction
Lung cancer continues to be the leading cause of deaths from malignancy worldwide.1,2 Survival remains poor given that most patients are diagnosed at an incurable stage.3 This has prompted the wide-spread effort to develop safe and effective screening methods to detect lung cancer at a curable stage. After the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality associated with screening high-risk individuals with low-dose computed tomography (LDCT),4 the United States (U.S.) Preventive Services Task Force (USPSTF) recommends screening for lung cancer in individuals aged 55–80 years, with a smoking history of ≥30 pack-years, and who currently smoke or have quit within the past 15 years.5 A comparative modeling study based on the USPSTF criteria predicted that an estimated 18,000 lives per year could be saved in the U.S. if LDCT can be widely used in the eligible population.6 However, the Surveillance Epidemiology and End Results Program (SEER) database as well as data from two other independent cohorts demonstrated that only one third of lung cancer patients would have met the USPSTF screening criteria,7–9 suggesting that a large number of potentially high-risk individuals are not eligible for LDCT screening. Among patients outside of the USPSTF-defined high-risk population, the three largest subgroups with potentially high risk for lung cancer are patients who had quit smoking 15–30 years prior to diagnosis (long-term quitters [LTQ]), those aged 50–54·9 years at the time of lung cancer diagnosis (younger age group [YAG]), and those who had a smoking history of around 20–30 pack-years,8,9 providing evidence that the risk for developing lung cancer in these individuals was still substantially elevated.10,11 Our previous reports revealed that two-thirds of newly-diagnosed lung cancer patients do not meet the USPSTF criteria and 18·8% (1,285 of 6,838 patients) are either LTQ or YAG.8,9 In contrast to the USPSTF-defined high-risk population, multiple organizations including the International Association for the Study of Lung Cancer (IASLC), the American Association of Thoracic Surgery (AATS), the American College of Chest Physicians (ACCP), and the National Comprehensive Cancer Network (NCCN) have recommended LDCT screening of patients according to NLST smoking history criteria or patients aged about 50–54·9 years along with one additional lung cancer risk factor, which could include occupational exposure or a history of pulmonary disease.12–14 Recently, the NELSON randomized-controlled screening trial has demonstrated a reduced risk of death from lung cancer of 26% in individuals aged 50–74 years, with a smoking history of more than 10 cigarettes daily for over 30 years or more than 15 cigarettes daily for over 25 years, and who currently smoke or have quit within the past 10 years. The NELSON trial thus included individuals with younger age and also those with a lesser smoking history than NLST.15
Now screening has been an essential component of an endeavor to decrease the burden from lung cancer in the high-risk population.16 The ultimate measure of an effective screening tool is mortality reduction, and it remains unclear whether additional expensive randomized trials will be performed to prove adjustments in criteria. We explored whether the potentially high-risk subgroups that are not eligible for USPSTF screening criteria have similar lung cancer mortality as those who are eligible. To our knowledge, no study has directly compared risk of death between lung cancer patients meeting USPSTF screening criteria and those who were ineligible due to 15 years or longer since quitting smoking (LTQ) or age of 50–54·9 years at the time of lung cancer diagnosis (YAG). We conducted the current study to (1) estimate the risk of death at five years post diagnosis, (2) assess the five-year death risk after matching age and pack-years smoked for LTQ, and (3) perform age-group stratified analysis for YAG.
Methods
Study Population
A total of 8,739 lung cancer patients aged 50–80 years, with ≥30 pack-years smoked, either current or former smokers with ≤30 quit-years, were identified from a hospital or a community cohort. 8,031 patients were 55 to 80 years old whom were divided into those meeting USPSTF screening criteria (USPSTF group) versus LTQ in both cohorts. Based on age at the time of diagnosis, 7,335 enrolled patients with <15 quit-years were divided into the 55–80 (USPSTF group) versus the 50–54·9 (YAG) group. The LTQ and YAG group were selected based on our previous studies,8,9 where we evaluated top 7 potential high-risk subgroups by frequency that missed USPSTF criteria for LDCT screening, and where the LTQ and YAG were ranked the highest. All cases were enrolled from two prospectively observed cohorts of patients with pathologically diagnosed primary lung cancer. The community cohort was composed of patients in the Rochester Epidemiology Project database with medical records of all people residing in Olmsted County, whereas the hospital cohort diagnosed at the Mayo Clinic in Minnesota between 1997 and 2015 (excluding Olmsted County residents). The community cohort (N=941) was matched to the same 19 years of diagnosis as the hospital cohort (N=7,798).9 The population consists of approximately 140,000 persons, 83% of whom are non-Hispanic whites, and it is socioeconomically similar to the white population of the U.S. and is representative of the population of the Midwestern U.S.8,9,17 The Institutional Review Boards (IRB) of Mayo Foundation and Olmsted County Medical Center approved this medical record-based study.
Data Collection
Detailed procedures of patient enrollment, data collection, and routine follow-up have been reported in previous studies8,9,17 and the appendix (p2), including the definition of pack-years, and quit-years.8,9,18 Never-smoker was defined as an individual who had smoked fewer than 100 cigarettes during his/her lifetime. Former smoker was defined as an individual with ≥1 quit-years before lung cancer diagnosis. Current smokers were defined as individuals who were actively smoking, and who had stopped smoking within one year before diagnosis.
Outcomes
The primary outcome was to assess the overall survival, defined as the time from the date of diagnosis to death from any cause in the hospital and community cohort; those alive or lost to follow-up were defined as censored. The risk of death at five-year post diagnosis was analyzed for LTQ, YAG, and USPSTF group.
Statistical Analysis
The patient characteristics are presented as the mean (standard deviation, SD) and median (interquartile range, IQR) for continuous variables and frequency (percentage) for categorical variables. Demographic and clinical characteristics were assessed using Wilcoxon rank-sum test and chi-square test for continuous and categorical variables, respectively. Overall survival was evaluated using the Kaplan-Meier method and log-rank test. Models were developed from the hospital cohort and validated in the community cohort. Univariate Cox proportional hazard (Cox) models were performed for evaluating the association of known prognostic factors (i.e., age at diagnosis, sex, cigarette smoking history, tumor histology, stage, and treatment modalities) with estimated five-year survival. Multivariate Cox models were performed using the significant variables (p<0·10) in the univariate analysis, and hazard ratios (HR) with 95% confidence intervals (CI) were calculated. Cox model assumption was verified by rejecting non-proportional hazards using Kaplan-Meier curves on key variables that show neither crossing nor levelling off or sharply dropping to zero and rejecting collinearity or lack of independence after checking pairwise correlations among key covariates.
A matching study was used to balance the distributions of age at diagnosis and pack-years smoked for LTQ and those meeting USPSTF screening criteria, using pair matching without replacement.19 The maximum allowable difference of 3 years in age and 10 pack-years smoked was calculated to produce well-balanced matched groups on baseline characteristics while including optimal case numbers. The kernel density plots were used for visual comparisons of the matching results. Additionally, bootstrapping test was used as an internal cross-validation, where 1000 replicate samples were drawn with replacements from the original sample, each with the same sample size.
Considering age and competing causes of death, age-group stratified analysis was performed, and the five-year risk of death in the subgroups were compared with that in YAG. All statistical tests were done as 2-tailed and P levels of <0·05 were considered statistically significant. Analyses were performed using SAS, v.9·4 (SAS Institute Inc.) without missing data.
Role of the funding source
The study funders had no role in the design of this study, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study, and the final responsibility for the decision to submit for publication.
Results
The recruitment of 8,739 lung cancer patients started from January 1, 1997 to date of last follow-up until December 31, 2017 or the time of death from any cause with a median follow-up 6·5 (IQR 3·8–10·0) years. Of 8,739 patients, 7,846 (89.8%) were U.S. Whites. The median survival time was 16·9 months (95% CI, 16·2–17·5); the proportions of LTQ, YAG, and USPSTF group who survived at 5 years after diagnosis were 23% (323 of 1,404 patients), 18.5% (131 of 708 patients), and 19.2% (1,272 of 6,627 patients), respectively. Among total patients in the hospital cohort, 30 (2·3%) of the 1,299 LTQ, 104 (1·8%) of 5,869 in USPSTF group, and 14 (2·2%) of 630 in YAG were unknown for stage. 57 (4·4%) of 1,299 LTQ, 246 (4·2%) of 5,869 in USPSTF group, and 19 (3%) of 630 in YAG were unknown for treatment. The characteristics and comparisons of 8,031 patients aged 55 to 80 years with ≤30 quit-years from hospital and community lung cancer cohorts, stratified by USPSTF criteria, are shown in Table 1. LTQ were significantly associated with older age, male gender, fewer pack-years smoked, and adenocarcinoma histology compared with those meeting USPSTF screening criteria.
Table 1.
Characteristics of 8,031 patients aged 55–80 years in the hospital and community cohort with long-term quitters, diagnosed 1997–2015
| Hospital Cohort (n=7168) | Community Cohort (n=863) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Long-term quitters* | USPSTF criteria† | Total | P value | Long-term quitters* | USPSTF criteria† | Total | P value |
| (N=1299) | (N=5869) | (N=7168) | (N=105) | (N=758) | (N=863) | |||
| Age at diagnosis, mean (SD), year | 71·7 (5·7) | 66·7 (6·5) | 67·6 (6·6) | <0·0001 | 72·7 (5·6) | 67·8 (6·7) | 68·4 (6·8) | <0 0001 |
| Sex, No- (%) | ||||||||
| Female | 394 (30·3%) | 2477 (42·2%) | 2871 (40·1%) | <0· 0001 | 27 (25·7%) | 317 (41·8%) | 344 (39·9%) | 0·002 |
| Male | 905 (69·7%) | 3392 (57·8%) | 4297 (59·9%) | 78 (74·3%) | 441 (58·2%) | 519 (60·1%) | ||
| Race/ethnicity, No. (%) | ||||||||
| Caucasian | 1192 (91·8%) | 5210 (88·8%) | 6402 (89·3%) | 0·002 | 103 (98·1%) | 731 (96·4%) | 834 (96·6%) | 0·38 |
| Others | 107 (8·2%) | 659 (11·2%) | 766 (10·7%) | 2 (1·9%) | 27 (3·6%) | 29 (3·4%) | ||
| Cigarette smoking status at diagnosis, No. (%) | ||||||||
| Former | 1299 (100·0%) | 2262 (38·5%) | 3561 (49·7%) | NA | 105 (100·0%) | 296 (39·1%) | 401 (46·5%) | NA |
| Current | NA | 3607 (61·5%) | 3607 (50·3%) | NA | 462 (60·9%) | 462 (53·5%) | ||
| Pack-years, median (IQR)‡ | 47 (36·0, 65·0) | 56 (44·0, 80·0) | 54 (41·0, 78·0) | <0·0001 | 52 (40·0, 75·0) | 60 (46·0, 80·0) | 60 (45·0, 80·0) | 0·004 |
| Tumor stage, No. (%)§ | ||||||||
| NSCLC, No. (%) | 1143 (88·0%) | 4864 (82·9%) | 6007 (83·8%) | 95 (90·5%) | 625 (82·5%) | 720 (83·4%) | ||
| I | 357 (31·2%) | 1300 (26·7%) | 1657 (27·6%) | 32 (33·7%) | 199 (31·8%) | 231 (32·1%) | ||
| II | 139 (12·2%) | 538 (11·1%) | 677 (11·3%) | 0·001 | 9 (95%) | 48 (7·7%) | 57 (7·9%) | 0·89 |
| III | 271 (23·7%) | 1388 (28·5%) | 1659 (27·6%) | 25 (26·3%) | 170 (27·2%) | 195 (27·1%) | ||
| IV | 376 (32·9%) | 1638 (33·7%) | 2014 (33·5%) | 29 (30·5%) | 208 (33·3%) | 237 (32·9%) | ||
| SCLC, No. (%) | 126 (9·7%) | 901 (15·4%) | 1027 (14·3%) | 10 (9·5%) | 133 (17·5%) | 143 (16·6%) | ||
| Limited | 53 (42·1%) | 399 (44·3%) | 452 (44·0%) | 0·64 | 3 (30·0%) | 51 (38·3%) | 54 (37·8%) | 0·60 |
| Extensive | 73 (57·9%) | 502 (55·7%) | 575 (56·0%) | 7 (70·0%) | 82 (61·7%) | 89 (62·2%) | ||
| Histology, No. (%) | ||||||||
| Adenocarcinoma | 647 (49·8%) | 2367 (40·3%) | 3014 (42·0%) | 57 (54·3%) | 266 (35·1%) | 323 (37·4%) | ||
| Squamous cell carcinoma | 341 (26·3%) | 1612 (27·5%) | 1953 (27·2%) | 27 (25·7%) | 239 (31·5%) | 266 (30·8%) | ||
| Small cell carcinoma | 128 (9·9%) | 915 (15·6%) | 1043 (14·6%) | <0·0001 | 10 (9·5%) | 135 (17·8%) | 145 (16·8%) | 0·003 |
| Other NSCLC|| | 34 (2·6%) | 178 (3·0%) | 212 (3·0%) | 3 (2·9%) | 48 (6·3%) | 51 (5·9%) | ||
| Unspecified NSCLC | 149 (11·5%) | 797 (13·6%) | 946 (13·2%) | 8 (7·6%) | 70 (9·2%) | 78 (9%) | ||
| Treatment, No. (%)¶ | ||||||||
| Surgery only | 389 (29·9%) | 1359 (23·2%) | 1748 (24·4%) | 34 (32·4%) | 193 (25·5%) | 227 (26·3%) | ||
| Chemotherapy only | 216 (166%) | 975 (16·6%) | 1191 (166%) | <0·0001 | 18 (171%) | 116 (153%) | 134 (155%) | 0·60 |
| Chemotherapy & Radiation | 189 (14·5%) | 1150 (19·6%) | 1339 (18·7%) | 17 (16·2%) | 170 (22·4%) | 187 (21·7%) | ||
| Radiation only | 94 (7·2%) | 385 (6·6%) | 479 (6·7%) | 13 (12·4%) | 80 (10·6%) | 93 (10·8%) | ||
| Surgery & Chemotherapy | 83 (6·4%) | 300 (5·1%) | 383 (5·3%) | 2 (1·9%) | 28 (3·7%) | 30 (3·5%) | ||
| Surgery & Radiation | 28 (2·2%) | 128 (2·2%) | 156 (2·2%) | 4 (3·8%) | 21 (2·8%) | 25 (2·9%) | ||
| Surgery & Chemotherapy & Radiation | 59 (4·5%) | 355 (6·0%) | 414 (5·8%) | 3 (2·9%) | 31 (4·1%) | 34 (3·9%) | ||
| Other treatment | 39 (3·0%) | 230 (3·9%) | 269 (3·8%) | 4 (3·8%) | 22 (2·9%) | 26 (3·0%) | ||
| No treatment | 145 (11·2%) | 741 (12·6%) | 886 (12·4%) | 10 (9·5%) | 97 (12·8%) | 107 (12·4%) | ||
Abbreviations: USPSTF, the U.S. Preventive Services Task Force; SD, standard deviation; IQR, interquartile range; NA, not applicable; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Long-term quitters: individual aged 55 to 80, who have a smoking history of 30 or more pack-years and had quitted smoking for 15–30 years.
USPSTF criteria: individual aged 55 to 80, who have a current or past smoking history of 30 or more pack-years, if former smokers, who had quitted within the past 15 years.
Pack-years: pack smoked daily × years.
Unknown stage: 30 (2·3%) of the 1299 long-term quitters and 104 (1·8%) of 5869 patients among the USPSTF group in the hospital cohort.
Large neuroendocrine and adenosquamous carcinoma.
Unknown treatment: 57 (4·4%) of 1299 long-term quitters and 246 (4·2%) of 5869 patients among the USPSTF group in the hospital cohort.
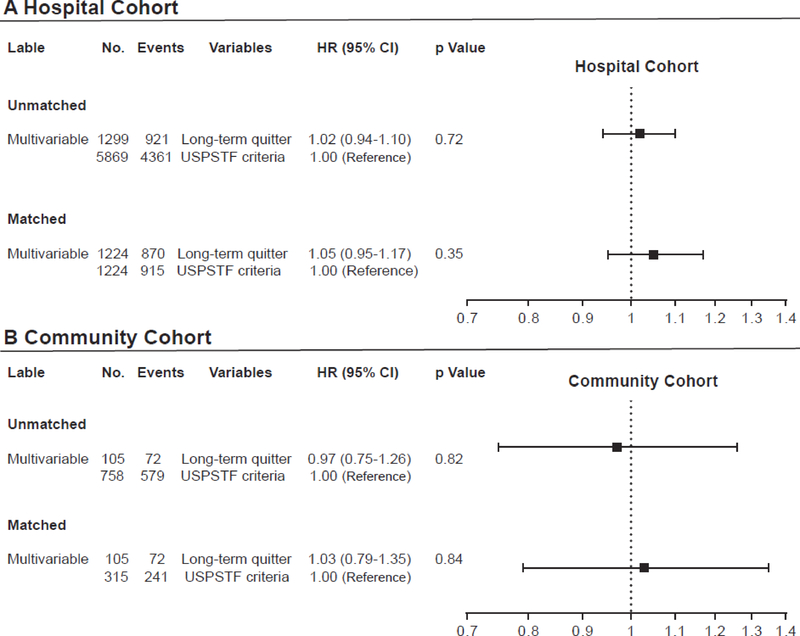
In univariate Cox models, known prognostic factors including age, sex, race, smoking status, pack-years smoked, tumor histology, stage, and treatment modality were significant variables, which were then analyzed in multivariate Cox models. In multivariate analyses, when compared with those meeting USPSTF criteria, LTQ in the hospital cohort were at the same risk of death at 5 years (HR =1·02; 95% CI, 0·94–1·10; p=0·72) after adjustment of the significant variables in univariate analysis (Figure 1A). In the community cohort, LTQ were also at virtually the same risk of death as those meeting the USPSTF criteria (HR=0·97; 95% CI, 0·75–1·26; p=0·82) at 5 years (Figure 1B). A bootstrap validation did not alter the results in the fitness of both models (data not shown).
Figure 1.
Multivariate Cox proportional hazard models of patients meeting USPSTF screening criteria vs. long-term quitter. Results were presented in (A) the hospital cohort and (B) the community cohort, including unmatched and matched analyses for age at diagnosis and pack-years. Multivariate Cox proportional hazard models were used to adjust for age, sex, race, smoking status, pack-years smoked, tumor histology and stage, and treatment modalities.
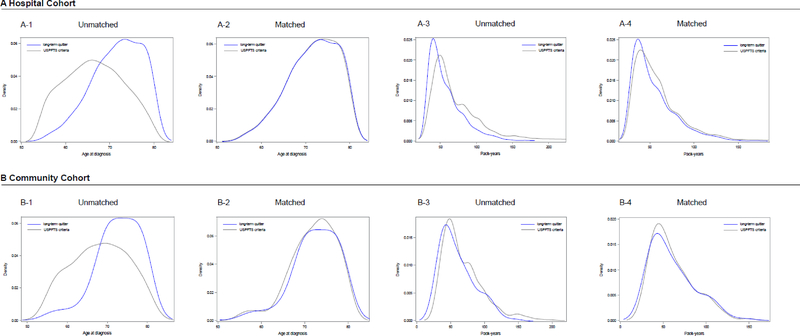
Matching based on age at diagnosis and pack-years smoked yielded 1224 patient pairs in the hospital cohort and 315:105 patient sets in the community cohort, between those meeting USPSTF criteria and the LTQ (Table 2). Figure 2 illustrates the balanced comparison groups, and Table 2 presents well-balanced characteristics except for gender. In multivariate analysis, virtually identical risk of death between LTQ and patients meeting USPSTF criteria were observed in the hospital cohort (HR =1·05; 95% CI, 0·95–1·17; p=0·35) (Figure 1A) and in the community cohort (HR =1·03; 95% CI, 0·79–1·35; p=0·84) (Figure 1B).
Table 2.
Characteristics of matched sets in the hospital and community cohort with long-term quitters, diagnosed 1997–2015
| Hospital Cohort (n=2448) | Community Cohort (n=420) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Long-term quitters* | USPSTF criteria1† | Total | P value | Long-term quitters* | USPSTF criteria1† | Total | P value |
| (N=1224) | (N=1224) | (N=2448) | (N=105) | (N=315) | (N=420) | |||
| Age at diagnosis, mean (SD), year | 71·6 (5·7) | 71·4 (5 ·6) | 71·5 (5· 6) | 0·35 | 72·7 (5·6) | 72·0 (5·4) | 72·2 (5·4) | 0·17 |
| Pack·years, mean (SD), | 54 ·1 (23 ·5) | 54·8 (23· 0) | 54·5 (23·2) | 0·17 | 59· 9 (25 ·8) | 60·8 (24·8) | 60 ·6 (25· 0) | 0·55 |
| Pack·years, median (IQR)‡ | 47(36· 0–65 ·0) | 50(38·0–65·0) | 49(37· 0–65· 0) | 0·17 | 52(40·0,75·0) | 53(40··0,75·0) | 52··8(40·0,75·0) | 0·55 |
| Sex, No. (%) | ||||||||
| Female | 37·2 (30·4%) | 47·6 (38· 9%) | 84·8· (34 ·6%) | <0·0001 | 27 (25·7%) | 15·1 (47· 9%) | 17·8 (42· 4%) | <0 ·0001 |
| Male | 852 (69 ·6%) | 748 (61 ·1%) | 1600 (65 ·4%) | 78 (74·3%) | 164 (52· 1%) | 242 (57·6%) | ||
| Race/ethnicity, No. (%) | ||||||||
| Caucasian | 1133 (92 ·6%) | 1116 (91·2%) | 2249 (91 ·9%) | 0·21 | 103 (98 ·1%) | 308 (97 ·8%) | 411 (97 ·9%) | 0·85 |
| Others | 91 (7 ·4%) | 108 (8 ·8%) | 199 (8·1%) | 2 (1·9%) | 7 (2·2%) | 9 (2 ·1%) | ||
| Cigarette smoking status at diagnosis, No. (%) | ||||||||
| Former | 1224 (100· 0%) | 640 (52·3%) | 1864 (76 ·1%) | NA | 105 (100 ·0%) | 159 (50· 5%) | 264 (62 ·9%) | NA |
| Current | NA | 584 (47·7%) | 584 (23 ·9%) | NA | 156 (49· 5%) | 156 (37 ·1%) | ||
| Tumor stage, No. (%) | ||||||||
| NSCLC, No. (%) | 1100 (89 ·9%) | 1102 (90 ·0%) | 2202 (90 ·0%) | 96 (91·4%) | 270 (85·7%) | 366 (87 ·1%) | ||
| I | 354 (32·2%) | 351 (31·9%) | 705 (32·0%) | 32 (33·3%) | 90 (33·3%) | 122 (33·3%) | ||
| II | 136 (12· 4%) | 135 (12 ·3%) | 271 (12·3%) | 0·20 | 9 (9 ·4%) | 20 (7·4%) | 29 (7·9%) | 0·94 |
| III | 258 (23·5%) | 298 (27·0%) | 556 (25·2%) | 25 (26·0%) | 74 (27·4%) | 99 (27 ·0%) | ||
| IV | 352 (32·0%) | 318 (28 ·9%) | 670 (30·4%) | 30 (31·3%) | 86 (319%) | 116 (317%) | ||
| SCLC, No. (%) | 124 (10·1%) | 122 (10·0%) | 246 (10· 0%) | 9 (8·6%) | 45 (14·3%) | 54 (12·9%) | ||
| Limited | 51 (41·1%) | 48 (39·3%) | 99 (40·2%) | 0·78 | 3 (33·3%) | 21 (46· 7%) | 24 (44·4%) | 0·46 |
| Extensive | 73 (58·9%) | 74 (60·7%) | 147 (59· 8%) | 6 (66· 7%) | 24 (53·3%) | 30 (55·6%) | ||
| Histology, No. (%) | ||||||||
| Adenocarcinoma | 613 (50·1%) | 548 (44 ·8%) | 1161 (47 ·4%) | 57 (54·3%) | 118 (37· 5%) | 175 (41·7%) | ||
| Squamous cell carcinoma | 321 (26·2%) | 389 (31· 8%) | 710 (29 ·0%) | 27 (25·7%) | 100 (31·7%) | 127 (30·2%) | ||
| Small cell carcinoma | 125 (10·2%) | 125 (10 2%) | 250 (10·2%) | 0·03 | 10 (9·5%) | 45 (14·3%) | 55 (13·1%) | 0·04 |
| Other NSCLC§ | 33 (2·7%) | 28 (2·3%) | 61 (2 ·5%) | 3 (2·9%) | 19 (6· 0%) | 22 (5·2%) | ||
| Unspecified NSCLC | 132 (10· 8%) | 134 (10·9%) | 266 (10 ·9%) | 8 (7·6%) | 33 (10·5%) | 41 (9 ·8%) | ||
| Treatment, No. (%) | ||||||||
| Surgery only | 388 (31·7%) | 370 (30·2%) | 758 (31·0%) | 0·39 | 34 (32·4%) | 87 (27·6%) | 121 (28 ·8%) | 0·76 |
| Chemotherapy only | 213 (17·4%) | 204 (16 ·7%) | 417 (17·0%) | 18 (17·1%) | 40 (12· 7%) | 58 (13· 8%) | ||
| Chemotherapy & Radiation | 184 (15 ·0%) | 203 (16 ·6%) | 387 (15 ·8%) | 17 (16·2%) | 67 (21·3%) | 84 (20·0%) | ||
| Radiation only | 91 (7 ·4%) | 100 (8·2%) | 191 (7 ·8%) | 13 (12 ·4%) | 39 (12 ·4%) | 52 (12 ·4%) | ||
| Surgery & Chemotherapy | 82 (6·7%) | 58 (4·7%) | 140 (5 ·7%) | 2 (1·9%) | 11 (3 ·5%) | 13 (3·1%) | ||
| Surgery & Radiation | 26 (2 ·1%) | 37 (3·0%) | 63 (2·6%) | 4 (3·8%) | 10 (3·2%) | 14 (3·3%) | ||
| Surgery & Chemotherapy & Radiation | 61 (5· 0%) | 60 (4 ·9%) | 121 (4 ·9%) | 3 (2·9%) | 7 (2·2%) | 10 (2·4%) | ||
| Other treatment | 39 (3·2%) | 43 (3·5%) | 82 (3·3%) | 4 (3·8%) | 10 (3·2%) | 14 (3·3%) | ||
| No treatment | 140 (11·4%) | 149 (12 ·2%) | 289 (11 ·8%) | 10 (9· 5%) | 44 (14· 0%) | 54 (12 ·9%) | ||
Abbreviations: USPSTF, the U.S. Preventive Services Task Force; SD, standard deviation; IQR, interquartile range; NA, not applicable; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Long-term quitters: individual aged 55 to 80, who have a smoking history of 30 or more pack-years and had quitted smoking for 15–30 years.
USPSTF criteria : individual aged 55 to 80, who have a current or past smoking history of 30 or more pack-years, if former smokers, who had quitted within the past 15 years.
Pack-years: pack smoked daily × years.
Large neuroendocrine and adenosquamous carcinoma
Figure 2.
Kernel density plots illustrated the balance improvement for age at diagnosis and pack-years between patients meeting USPSTF screening criteria and long-term quitters. Results are presented in (A) the hospital cohort and (B) the community cohort, including unmatched and matched analyses.
The characteristics and comparisons of 7,335 patients aged 50–80 years with <15 quit-years from hospital and community lung cancer cohorts, stratified by USPSTF criteria are shown in Table 3. 5,869 patients in the hospital cohort and 758 patients in the community cohort met the USPSTF screening criteria. In both cohorts with predominantly male gender, there were statistically significant differences in the age variable between the USPSTF group and YAG, and most of the patients were Caucasian. A significant majority of patients were current smokers. Most patients were stage III/IV and the most prevalent histology was adenocarcinoma. Comparing to the USPSTF group, the YAG was associated with current smoker, female sex, stage III/IV lung cancer, and adenocarcinoma when compared with those meeting USPSTF criteria.
Table 3.
Characteristics of 7,335 patients aged 50–80 years in the hospital and community cohort with younger age group, diagnosed 1997–2015
| Hospital Cohort (n=6499) |
Community Cohort (n=836) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Younger age group* | USPSTF criteria† | Total | P value | Younger age group* | USPSTF criteria† | Total | P value |
| (N=630) | (N=5869) | (N=6499) | (N=78) | (N=758) | (N=836) | |||
| Age at diagnosis, mean (SD), year | 52·1 (1·4) | 66·7 (6 ·5) | 65 ·3 (7·6) | <0 ·0001 | 52·3 (1·4) | 67·8 (6·7) | 66·3 (7·8) | <0 ·0001 |
| Sex, No. (%) | ||||||||
| Female | 285 (45·2%) | 2477 (42·2%) | 2762 (42·5%) | <0 0001 | 39 (50· 0%) | 317 (41·8%) | 356 (42 ·6%) | 0·16 |
| Male | 345 (54 ·8%) | 3392 (57·8%) | 3737 (57·5%) | 39 (50· 0%) | 441 (58 ·2%) | 480 (57 ·4%) | ||
| Race/ethnicity, No. (%) | ||||||||
| Caucasian | 534 (84·8%) | 5210 (88 ·8%) | 5744 (88 ·4%) | 0 ·003 | 76 (97· 4%) | 731 (96 ·4%) | 807 (96 ·5%) | 0·65 |
| Others | 96 (15 ·2%) | 659 (11· 2%) | 755 (11 ·6%) | 2 (2·6%) | 27 (3·6%) | 29 (3·5%) | ||
| Cigarette smoking status at diagnosis, No. (%) | ||||||||
| Former | 114 (18·1%) | 2262 (38·5%) | 2376 (36 ·6%) | <0 ·0001 | 17 (21·8%) | 296 (39 ·1%) | 313 (37·4%) | 0· 003 |
| Current | 516 (819%) | 3607 (61 5%) | 4123 (63 4%) | 61 (78· 2%) | 46·2 (60 ·9%) | 523 (62·6%) | ||
| Pack-years, median (IQR)‡ | 48 (35 0,65 0) | 56 (44 0,80 0) | 55 (43 0,80 0) | <0 0001 | 45 (36 ·0,64 ·0) | 60 (46· 0,80 ·0) | 60 (45 ·0,80 ·0) | <0· 0001 |
| Tumor stage, No. (%)§ | ||||||||
| NSCLC, No. (%) | 527 (83·7%) | 4864 (82 ·9%) | 5391 (83· 0%) | 66 (84 ·6%) | 625 (82·5%) | 691 (82 ·7%) | ||
| I | 69 (13·1%) | 1300 (26· 7%) | 1369 (25· 4%) | 16 (24 ·2%) | 199 (31·8%) | 215 (31·1%) | ||
| II | 61 (11·6%) | 538 (11 ·1%) | 599 (11· 1%) | <0· 0001 | 4 (6 ·1%) | 48 (7·7%) | 52 (7·5%) | 0·50 |
| III | 172 (32·6%) | 1388 (28 ·5%) | 1560 (28 9%) | 19 (28 ·8%) | 170 (27·2%) | 189 (27· 4%) | ||
| IV | 225 (42··7%) | 1638 (33· ·7%) | 1863 (34 ·6%) | 27 (40 ·9%) | 208 (33·3%) | 235 (34·0%) | ||
| SCLC, No. (%) | 89 (14·1%) | 901 (154%) | 990 (15 ·2%) | 12 (15·4%) | 133 (17·5%) | 145 (17·3%) | ||
| Limited | 38 (42··7%) | 399 (44 ·3%) | 437 (44 ·1%) | 0·77 | 3 (25·0%) | 51 (38·3%) | 54 (37·2%) | 0·36 |
| Extensive | 51 (57·3%) | 502 (55·7%) | 553 (55·9%) | 9 (75 ·0%) | 82 (61·7%) | 91 (62·8%) | ||
| Histology, No. (%) | ||||||||
| Adenocarcinoma | 298 (47·3%) | 2367 (40·3%) | 2665 (41· 0%) | 41 (52·6%) | 266 (35 ·1%) | 307 (36 ·7%) | ||
| Squamous cell carcinoma | 104 (16·5%) | 1612 (27·5%) | 1716 (26 ·4%) | 15 (19·2%) | 239 (31·5%) | 254 (30· 4%) | ||
| Small cell carcinoma | 91 (14·4%) | 915 (15·6%) | 1006 (15 ·5%) | <0 ·0001 | 12 (15·4%) | 135 (17·8%) | 147 (17·6%) | 0·02 |
| Other NSCLC|| | 23 (3–7%) | 178 (3 0%) | 201 (3 1%) | 2 (2–6%) | 48 (63%) | 50 (6 ·0%) | ||
| Unspecified NSCLC | 114 (18·1%) | 797 (13· 6%) | 911 (14·0%) | 8 (10·3%) | 70 (9 ·2%) | 78 (9·3%) | ||
| Treatment, No. (%)¶ | ||||||||
| Surgery only | 91 (14·4%) | 1359 (23·2%) | 1450 (22 ·3%) | 19 (24 ·4%) | 193 (25·5%) | 212 (25·4%) | ||
| Chemotherapy only | 131 (20 ·8%) | 975 (16 ·6%) | 1106 (17 ·0%) | <0 0001 | 12 (15·4%) | 116 (15·3%) | 128 (15·3%) | 0·75 |
| Chemotherapy & Radiation | 163 (25 ·9%) | 1150 (19·6%) | 1313 (20 ·2%) | 24 (30·8%) | 170 (22 ·4%) | 194 (23 ·2%) | ||
| Radiation only | 32 (5·1%) | 385 (6 ·6%) | 417 (6· 4%) | 5 (6· 4%) | 80 (10·6%) | 85 (10·2%) | ||
| Surgery & Chemotherapy | 35 (5·6%) | 300 (5 ·1%) | 335 (5·2%) | 4 (5 ·1%) | 28 (3·7%) | 32 (3·8%) | ||
| Surgery & Radiation | 12 (1·9%) | 128 (2·2%) | 140 (2· 2%) | 1 (1·3%) | 21 (2· 8%) | 22 (2·6%) | ||
| Surgery & Chemotherapy & Radiation | 57 (9· 0%) | 355 (6·0%) | 412 (6·3%) | 3 (3·8%) | 31 (4·1%) | 34 (4 ·1%) | ||
| Other treatment | 33 (5·2%) | 230 (3·9%) | 263 (4 ·0%) | 3 (3·9%) | 22 (2·9%) | 25 (3·0%) | ||
| No treatment | 57 (9· 0%) | 741 (12·6%) | 798 (12 ·3%) | 7 (9· 0%) | 97 (12·8%) | 104 (12·4%) | ||
Abbreviations: USPSTF, the U.S. Preventive Services Task Force; SD, standard deviation; IQR, interquartile range; NA, not applicable; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Younger age group: individuals aged 50–54·9, who have a 30 or more pack-year smoking history, and currently smoke or have quitted within the past 15 years.
USPSTF criteria: individuals aged 55–80, who has a 30 or more pack-year smoking history, and currently smoke or have quitted within the past 15 years.
Pack-years: packs smoked daily × years for former smoker.
Unknown stage: 14 (2·2%) of 630 patients among the younger age group and 104 (1·8%) of 5869 patients among the USPSTF group in the hospital cohort
Large neuroendocrine and adenosquamous carcinoma
Unknown treatment: 19 (3%) of 630 patients among the younger age group and 246 (4·2%) of 5869 patients among the USPSTF group in the hospital cohort
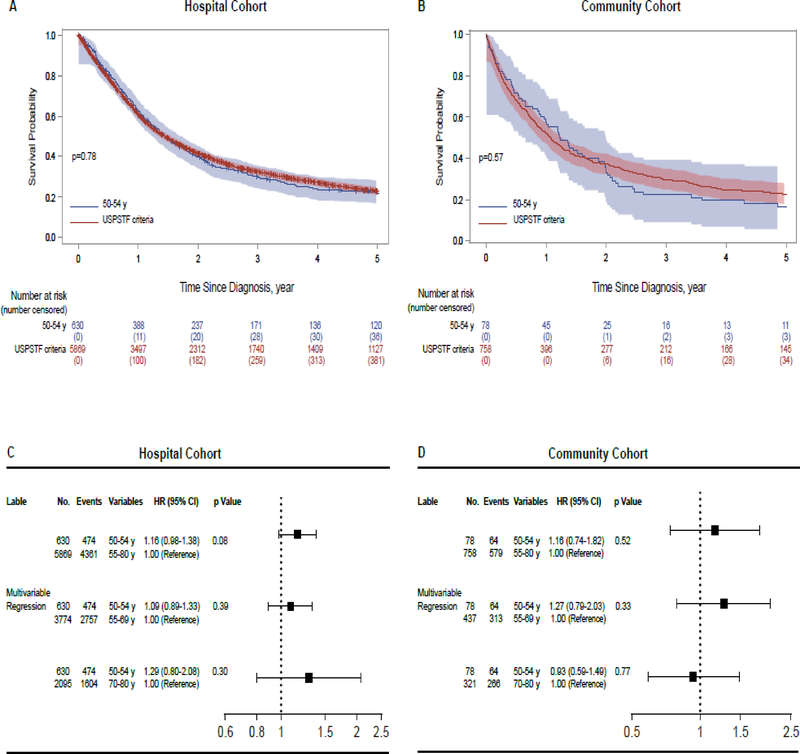
For patients in the hospital cohort, median survival time (MST) in the YAG and USPSTF group were 17·4 (95% CI, 15·5–19·7) and 17·2 (95% CI, 16·5–18·2) months, respectively. As shown in Figure 3A, the 5-year survival rates in the YAG and USPSTF group were 22% (95% CI, 17–27) and 23% (95% CI, 21–25), respectively (p=0·78). In the community cohort, MST for the YAG and USPSTF group were 14·7 (95% CI, 10·7–22·4) and 12·8 (95% CI, 11·1–14·4) months, respectively. The 5-year survival rates in the YAG and USPSTF group were 16% (95% CI, 5–33) and 23% (95% CI, 18–28), respectively (p=0·57) (Figure 3B). In both cohorts, 5-year survival rate in YAG was no better than in the USPSTF group. In univariate Cox models, sex, cigarette smoking status, pack-years smoked, quit-years, tumor histology, stage, and treatment modality were significant variables, which were then analyzed in multiple Cox models. In multivariate analyses, the YAG in the hospital cohort demonstrated the same risk of death as those meeting the USPSTF criteria at 5 years (HR =1·16; 95% CI, 0·98–1·38; p=0·08) (Figure 3C). In the community cohort, the YAG showed the similar risk of death (HR =1·16; 95% CI, 0·74–1·82; p=0·52) compared with those meeting USPSTF criteria (Figure 3D).
Figure 3.
(A) In the hospital cohort, the 5-year survival rates of the younger age group (YAG) and USPSTF group were 22% (95% CI, 17–27) vs. 23% (95% CI, 21–25), respectively. The 5-year survival rate in the YAG was not better than that in the USPSTF group (p=0·78). (B) In the community cohort, the 5-year survival rates of the YAG and USPSTF group were 16% (95% CI, 5–33) vs. 23% (95% CI, 18–28), respectively. The 5-year survival rate in the YAG was not better than that in the USPSTF group (p=0·57). The results of multivariable Cox proportional hazard models of patients meeting USPSTF screening criteria vs. YAG were presented in (C) the hospital cohort and (D) the community cohort.
With regards to age and competing causes of death, the USPSTF group (patients aged 55–80 years) was subdivided into five USPSTF subgroups according to consecutive 5-year age groups. The data of age-group stratified analysis are described in the appendix (pp3–4). Accordingly, the following three USPSTF subgroups, 55–59, 60–64, and 65–69, with similar risks were combined into the 55–69 years USPSTF subgroup (designated as the younger USPSTF subgroup), and the 70–74 and 75–80 age groups were combined into the 70–80 years USPSTF subgroup (designated as the older USPSTF subgroup).
In multivariate risk-adjusted analysis, the YAG did not have significantly different risk of death as two subgroups including the younger USPSTF subgroup (HR = 1·09; 95% CI, 0·89–1·33; p=0·39), and the older USPSTF subgroup (HR = 1·29; 95% CI, 0·8–2·08; p=0·3), in the hospital cohort (Figure 3C). Similarly, the YAG in the community cohort did not show different risk of death as the younger USPSTF subgroup (HR = 1·27; 95% CI, 0·79–2·03; p=0·33) (Figure 3D). The YAG demonstrated the same risk of death in comparison with the older USPSTF subgroup (HR = 0·93; 95% CI, 0·59–1·49; p=0·77) at 5 years.
Discussion
This study tested the hypothesis that LTQ or YAG had the same risk of death from lung cancer as those meeting USPSTF screening criteria in the prospective hospital and community cohorts, suggesting that lung cancer in these subgroups have a similar lethality when compared to those meeting USPSTF criteria. Most patients (7,846 [89.8%] of 8,739) were U.S. Whites; the effect of racial/ethnic background was analyzed but was not significant as an independent covariate, which may reflect the small sample size of non-Whites. Our previous study demonstrated that expanding the USPSTF screening criteria to include LTQ may save more lives without significantly increasing the number of cases of over-diagnosis.9 The current study indicated that LTQ were at the same risk of death as patients meeting USPSTF criteria. Additionally, our results showed that there was more early-stage lung cancer among LTQ than the USPSTF group in the hospital and community cohorts (Table 1) (p=0·001 and 0·89, respectively). Therefore, in terms of tumor stage, LTQ may gain more survival benefit from screening due to the detection of more early-stage lung cancer.
Lung cancer patients aged 50–54·9 years (YAG) who otherwise meet the USPSTF screening criteria are one of the three largest subgroups with potentially high risk for lung cancer outside of the USPSTF-defined high-risk population.8,9 Our data indicated that YAG had the same risk of death as the USPSTF group, suggesting that the risk in YAG might be underestimated by the USPSTF criteria. Thus, using 55 years of age as the lower limit in the USPSTF criteria may exclude younger patients who might benefit from having their lung cancer detected at a potentially curable stage. Furthermore, the incidence and mortality of most malignancies, including lung cancer, usually increase with age until about 80 years old.3,20 However, the older age group in this study did not have higher risk of death compared to YAG, consistent with previous reports suggesting that lung cancer occurring in younger individuals may represent a biologically distinct subgroup with higher mortality,21 so it is essential to identify and screen high-risk younger individuals. A recent study showed that 50 years of age was considered as a useful cut point regarding the prognosis and genomic alterations in non-small cell lung cancer,22 which is supportive by the present study where patients aged 50–54·9 years had the same risk of death from lung cancer as the older age group. Collectively, lung cancer patients aged 50 years or older represent a subgroup with the similar disease entity, and therefore, patients within YAG should also be included in the screening criteria.
Previous studies demonstrated that the risk of lung cancer exponentially decreased within 15 quit-years;10,11 however, our results indicated that lung cancer patients aged 55–80 years, with ≥30 pack-years smoked and 15–30 quit-years (LTQ) were at the same risk of death as patients with <15 quit-years. Additionally, this study showed that YAG had more current smokers in the community (516 [81·9%] of 630 patients) and hospital (61 [78·2%] of 78 patients) cohorts than the USPSTF group. These findings were compatible with the data in the NLST and the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.4,23 Previous research reported that screen-detected abnormalities were significantly associated with subsequent smoking behavior and may be utilized as a teachable moment for smoking cessation interventions.24 Accordingly, lung cancer screening may discover abnormalities and help to increase the smoking cessation rate in YAG relative to the USPSTF group. From the viewpoint of smoking cessation, the YAG may benefit more from screening which could also impact on other smoking-related diseases, such as stroke, cardiovascular and pulmonary disease. Hence, the morbidity and mortality might be substantially reduced, and life expectancy extended.
Significant progress in lung cancer screening, diagnosis, and treatment has led to remarkably improved survival over the past decades. It has been reported that older patients are more likely to be ineligible for novel therapies due to medical conditions;25,26 consequently, considering the factor of age, the YAG with the same risk of death as the USPSTF group may benefit more than the USPSTF group from continuous advances in lung cancer care.
Cost-effectiveness is especially important for lung cancer screening, and the selection of age groups for screening had a tremendous impact on cost-effectiveness.6 A previous investigation demonstrated that screening individuals aged 50 years or older was the most cost-effective, while screening those aged 60 years or older was the least cost-effective.27 Additionally, screening for LTQ may identify 16% more of screen-detected lung cancers with an acceptable cost and minimal harm.9 Collectively, these studies implied that patients within the criteria of LTQ and YAG should be included in screening criteria; future studies regarding the overall cost-effectiveness of expanding efficacious screening to LTQ and YAG are warranted.
Although this study enrolled the next largest subgroups at high risk for lung cancer (i.e. LTQ; YAG) outside of the USPSTF-defined high-risk population, there may still be potentially high-risk individuals who are ineligible for screening. Hence, there is a need to improve the current screening criteria and the conventional smoking-age-based risk assessment model. Additional large randomized trials that only focus on differing cut-offs of age and smoking history may not be cost-effective; instead, highly promising biological and physiological markers should be tested timely. The molecular biomarkers in the biological fluid, such as blood, urine, saliva or sputum, have been intensively investigated and demonstrated potential ability to enhance the benefit from screening, and to assess the likelihood of malignancy in screen-detected lung nodules.28 Combination of LDCT and biomarkers with optimal discriminating power in pre- or post-screened high-risk individuals has the potential to optimize the screening efficacy.29
A limitation of this study is that all patients were diagnosed with lung cancer, which makes it difficult to accurately examine tradeoffs between benefits and potential harms of screening. Another concern is that approximately one-hundredth of total patients were initially identified via LDCT screening with relatively earlier stage;30 however, the effect of staging has been carefully adjusted in our analyses. Additionally, several unmeasured confounders may affect the survival outcome, such as other comorbidities, personal cancer history, and family history of lung cancer. Furthermore, missing data on stage and treatment were not included in our analyses, although the magnitude of missing data (as specified in Table 1 and 3) is minimal, and the proportions of missing data were similar between those in the USPSTF group and those outside. Despite the limitations, our study has several strengths. First, at the study design phase, we carefully chose two independent cohorts, one being the hospital cohort that represents the natural setting of an observational patient population at a tertiary medical center, the other being the community cohort that represents the Midwest region of the U.S. Data were collected from these two prospectively observed cohorts with long-term follow-up; therefore, longitudinal and comprehensive data can be obtained, which enabled us to adjust for major known prognostic factors. Second, survival models were developed in the hospital cohort and validated in the community cohort, and all patients were diagnosed or treated in the same medical institution. Our results were considered robust only when the data from both cohorts are remarkably consistent. Third, smoking history were obtained from medical records and further confirmed with a follow-up questionnaire or an interview.
In conclusion, our findings suggested that patients with lung cancer who have quit smoking longer than 15-years or of an age up to 5-years younger and who otherwise met the USPSTF screening criteria were not only at substantially high risk for developing the disease, but also manifested a similar risk of death as those meeting the criteria. Additionally, screening for younger age group may confer the advantage of an increased smoking cessation. Finally, individuals in these two subgroups may gain more benefit from screening owing to lung cancer detected at an earlier stage and the evolving treatment paradigm.
Supplementary Material
Research in context.
Evidence before this study
To discover previous clinical investigations of low-dose computed tomography (LDCT) and lung cancer survival, we searched PubMed for articles published up to October 31, 2018, using the terms “Lung cancer”, “screening”, “LDCT”, “smoking cessation”, “former smoker”, “younger age”, without language restrictions. This search revealed that a 20% reduction in lung cancer mortality associated with screening high-risk individuals with LDCT in the National Lung Screening Trial (NLST), the United States (U.S.) Preventive Services Task Force (USPSTF) recommends screening for lung cancer in individuals aged 55–80 years, with a smoking history of 30 or more pack-years, and who currently smoke or have quit within the past 15 years. Recently, the NELSON randomized-controlled screening trial has demonstrated a reduced risk of death from lung cancer of 26% in individuals aged 50–74 years, with a smoking history of more than 10 cigarettes daily for over 30 years or more than 15 cigarettes daily for over 25 years, and who currently smoke or have quit within the past 10 years. However, the Surveillance Epidemiology and End Results Program (SEER) database as well as data from two other independent cohorts demonstrated that only one third of lung cancer patients would have met the USPSTF screening criteria, suggesting that a large number of potentially high-risk individuals are not eligible for LDCT screening. Among patients outside of the USPSTF-defined high-risk population, the three largest subgroups with potentially high risk for lung cancer are patients who had quit smoking 15–30 years prior to diagnosis (long-term quitters [LTQ]), those aged 50–54·9 years at the time of lung cancer diagnosis (younger age group [YAG]), and those who had a smoking history of around 20–30 pack-years, providing evidence that the risk for developing lung cancer in these individuals was still substantially elevated.
Added value of this study
We used two prospectively observed cohorts in the hospital and community to explore whether the potentially high-risk subgroups that are not eligible for USPSTF screening criteria have a similar risk of death from lung cancer as those who are eligible. To the best of our knowledge, no previous study has directly compared risk of death between lung cancer patients who met USPSTF screening criteria and those who were ineligible due to 15 years or longer since quitting smoking (LTQ) or age of 50–54·9 years at the time of lung cancer diagnosis (YAG). Subsequently, we conducted the current study to estimate the risk of death at five years post diagnosis and age-group stratified analysis. In both cohorts, LTQ were at the same risk of death at 5 years as the USPSTF group; matched analysis showed similar results. The YAG group had the same risk of death at five years as the USPSTF group; age-group stratified analysis yielded similar findings. Our study provides further evidence that the LTQ and YAG group are high-risk individuals ineligible for LDCT screening.
Implications of all the available evidence
Our findings suggested that patients with lung cancer who have quit smoking longer than 15-years or of an age up to 5-years younger and who otherwise met the USPSTF screening criteria were not only at substantially high risk for developing the disease, but also manifested a similar risk of death from lung cancer as those who met the criteria. Thoughtful consideration of the optimal screening criteria is mandatory to guide the decision of screening for individuals with high risk for lung cancer. We highlight the need to improve the current screening criteria and the conventional smoking-age-based risk assessment model. In future, more sophisticated screening program combining LDCT and biomarkers should be developed to identify high-risk individuals that would benefit most from screening.
Acknowledgments
This study is supported by grants R03 CA77118, R01 CA80127 and R01 CA84354 from the National Institutes of Health; R01 AG034676 and R01 AG052425 from the National Institute on Aging; and Mayo Clinic Foundation. We thank Barbara A Abbott for assisting access Rochester Epidemiology Project resource that was made available to this study. The authors appreciate Chia-Sui Weng for her technical assistance with the manuscript.
Funding: National Institutes of Health; Mayo Clinic Foundation
Footnotes
Declaration of interests: All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yung-Hung Luo, Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, 200 First Street SW, Rochester, MN, USA; Department of Chest Medicine, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Taipei City, Taiwan; School of Medicine, National Yang-Ming University, No.155, Sec.2, Linong Street, Taipei, Taiwan; Institute of Clinical Medicine, National Yang-Ming University, No.155, Sec.2, Linong Street, Taipei, Taiwan; Division of Medical Oncology, Mayo Clinic, 200 First Street SW, Rochester, MN, USA.
Lei Luo, Department of Science and Education, Guizhou Province People’s Hospital, No. 83, Zhongshan East Road, Guiyang, Guizhou, China.
Jason A. Wampfler, Division of Biomedical Statistics and Informatics, Department of Health Science Research, Mayo Clinic, 200 First Street SW, Rochester, MN, USA.
Yi Wang, School of Public Health and Management, Wenzhou Medical University, University Town, Chashan, Wenzhou, Zhejiang, China.
Dan Liu, Division of Pulmonary & Critical Care Medicine, West China Hospital, Sichuan University, No.37, Guoxue Alley, Chengdu, Sichuan, China.
Yuh-Min Chen, Department of Chest Medicine, Taipei Veterans General Hospital, No. 201, Sec. 2, Shipai Rd., Taipei City, Taiwan; School of Medicine, National Yang-Ming University, No.155, Sec.2, Linong Street, Taipei, Taiwan; Taipei Cancer Center, Taipei Medical University, No. 252, Wuxing Street, Taipei, Taiwan.
Alex A. Adjei, Division of Medical Oncology, Mayo Clinic, 200 First Street SW, Rochester, MN, USA.
David E. Midthun, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN, USA.
Ping Yang, Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, 200 First Street SW, Rochester, MN, USA; Department of Health Sciences Research, Mayo Clinic, 13400 E Shea Blvd, Scottsdale, AZ, USA.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61(2): 69–90. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cancer: fact sheet no. 297. 2014.
- 3.Institute NC. SEER stat fact sheets: lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html (accessed August 21 2018).
- 4.National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5): 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160(5): 330–8. [DOI] [PubMed] [Google Scholar]
- 6.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014; 160(5): 311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen 2012; 19(3): 154–6. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Midthun DE, Wampfler JA, et al. Trends in the proportion of patients with lung cancer meeting screening criteria. Jama 2015; 313(8): 853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang P, Wang Y, Wampfler JA, et al. Trends in Subpopulations at High Risk for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016; 11(2): 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry JS, Lee PN, Forey BA, Coombs KJ. How rapidly does the excess risk of lung cancer decline following quitting smoking? A quantitative review using the negative exponential model. Regul Toxicol Pharmacol 2013; 67(1): 13–26. [DOI] [PubMed] [Google Scholar]
- 11.Pinsky PF, Zhu CS, Kramer BS. Lung cancer risk by years since quitting in 30+ pack year smokers. J Med Screen 2015; 22(3): 151–7. [DOI] [PubMed] [Google Scholar]
- 12.Field JK, Smith RA, Aberle DR, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2012; 7(1): 10–9. [DOI] [PubMed] [Google Scholar]
- 13.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. The Journal of thoracic and cardiovascular surgery 2012; 144(1): 33–8. [DOI] [PubMed] [Google Scholar]
- 14.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(5 Suppl): e78S–e92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Koning H, Van Der Aalst C, Ten Haaf K, Oudkerk M. PL02.05 Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2018; 13(10): S185. [Google Scholar]
- 16.Sharma D, Newman TG, Aronow WS. Lung cancer screening: history, current perspectives, and future directions. Arch Med Sci 2015; 11(5): 1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z, Aubry MC, Deschamps C, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg 2006; 131(5): 1014–20. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Ruan X, Yang P, Liu H. Comparison of Three Information Sources for Smoking Information in Electronic Health Records. Cancer Inform 2016; 15: 237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayawant N Mandrekar SJM. An Introduction to Matching and its Application using SAS®. http://www2.sas.com/proceedings/sugi29/208-29.pdf (accessed August 21 2018).
- 20.Harding C, Pompei F, Wilson R. Peak and decline in cancer incidence, mortality, and prevalence at old ages. Cancer 2012; 118(5): 1371–86. [DOI] [PubMed] [Google Scholar]
- 21.VandenBussche CJ, Illei PB, Lin MT, Ettinger DS, Maleki Z. Molecular alterations in non-small cell lung carcinomas of the young. Hum Pathol 2014; 45(12): 2379–87. [DOI] [PubMed] [Google Scholar]
- 22.Sacher AG, Dahlberg SE, Heng J, Mach S, Janne PA, Oxnard GR. Association Between Younger Age and Targetable Genomic Alterations and Prognosis in Non-Small-Cell Lung Cancer. JAMA Oncol 2016; 2(3): 313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry SA, Tammemagi MC, Penek S, et al. Predictors of adverse smoking outcomes in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst 2012; 104(21): 1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014; 106(6): dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004; 291(22): 2720–6. [DOI] [PubMed] [Google Scholar]
- 26.Lawler M, Selby P, Aapro MS, Duffy S. Ageism in cancer care. BMJ 2014; 348: g1614. [DOI] [PubMed] [Google Scholar]
- 27.Goffin JR, Flanagan WM, Miller AB, et al. Cost-effectiveness of Lung Cancer Screening in Canada. JAMA Oncol 2015; 1(6): 807–13. [DOI] [PubMed] [Google Scholar]
- 28.Mazzone PJ, Sears CR, Arenberg DA, et al. Evaluating Molecular Biomarkers for the Early Detection of Lung Cancer: When Is a Biomarker Ready for Clinical Use? An Official American Thoracic Society Policy Statement. Am J Respir Crit Care Med 2017; 196(7): e15–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanoue LT, Tanner NT, Gould MK, Silvestri GA. Lung cancer screening. Am J Respir Crit Care Med 2015; 191(1): 19–33. [DOI] [PubMed] [Google Scholar]
- 30.Liu D, Midthun DE, Qiuyuan L, et al. Health and Quality of Life Assessment in Four Groups of Lung Cancer Patients: Low Dose Computed Tomography Screened, Chest X-Ray Detected, Incidentally-Found, and Routinely Diagnosed. C102 ADVANCING LUNG CANCER SCREENING PROCESSES AND OUTCOMES. American Thoracic Society International Conference; 2018: A5983–A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.