Abstract
Background
We investigated the individual and additive effects of three modifiable maternal metabolic factors, including pre-pregnancy overweight/obesity, gestational weight gain (GWG), and gestational diabetes mellitus (GDM), on early childhood growth trajectories and obesity risk.
Methods
A total of 1 425 mother-offspring dyads (953 black and 472 white) from a longitudinal birth cohort were included in this study. Latent class growth modeling was performed to identify the trajectories of body mass index (BMI) from birth to four years in children. Poisson regression models were used to examine the associations between the maternal metabolic risk factors and child BMI trajectories and obesity risk at four years.
Results
We identified three discrete BMI trajectory groups, characterized as rising-high-BMI (12.6%), moderate-BMI (61.0%), or low-BMI (26.4%) growth. Both maternal pre-pregnancy obesity (adjusted relative risk [adjRR] = 1.96; 95% CI: 1.36–2.83) and excessive GWG (adjRR = 1.71, 95% CI: 1.13–2.58) were significantly associated with the rising-high-BMI trajectory, as manifested by rapid weight gain during infancy and a stable but high BMI until four years. All three maternal metabolic indices were significantly associated with childhood obesity at age four years (adjRR for pre-pregnancy obesity = 2.24, 95% CI: 1.62–3.10; adjRR for excessive GWG = 1.46, 95% CI: 1.01–2.09; and adjRR for GDM = 2.14, 95% = 1.47–3.12). In addition, risk of rising-high BMI trajectory or obesity at age four was stronger among mothers with more than one metabolic risk factor. We did not observe any difference in these associations by race.
Conclusion
Maternal pre-pregnancy obesity, excessive GWG, and GDM individually and jointly predict rapid growth and obesity at age four in offspring, regardless of race. Interventions targeting maternal obesity and metabolism may prevent or slow the rate of development of childhood obesity.
Keywords: Childhood obesity, gestational diabetes, gestational weight gain, growth trajectory metabolic factors, pre-pregnancy obesity, racial disparity
Introduction
Childhood obesity has become one of the most serious public health challenges. In the US, one-third of children and adolescents aged 2–19 years were overweight (16.2%) or obese (17.2%) in 2013–2014.1 Childhood obesity can cause a wide range of serious consequences later in the life course, such as adult obesity,2–4 type 2 diabetes,5,6 cardiovascular disease,7,8 and may also have psychological or behavioral consequences.9 It also has imposed a significant economic impact on the health care system.10–12 It is critically important to identify risk factors for the development of childhood obesity and improve its early prevention and long-term health outcomes.
Accumulating evidence has strongly supported intrauterine events in the origins of childhood obesity. In particular, it has been suggested that maternal metabolic conditions during pregnancy, such as obesity and insulin resistance, may influence the fetal environment to ultimately affect long-term obesity risk in children.13 Prior studies have suggested that prenatal metabolic-related factors, including maternal pre-pregnancy overweight/obesity, excess gestational weight gain (GWG), and gestational diabetes mellitus (GDM), may increase the risk for childhood obesity.14–17 However, there are still gaps in our knowledge regarding the associations of these prenatal metabolic factors with childhood growth and overweight/obesity risk in offspring.
First, although childhood growth trajectories have been shown to be more predictive for obesity risk in later life than a single growth measurement,18 very few studies have examined the associations between these prenatal metabolic factors and childhood growth trajectories.19,20 The prevalence of obesity is higher in African American children than white children.21,22 However, compared with white populations, fewer studies have included blacks and many fewer have examined the potential racial-/ethnic-specific effects of these prenatal metabolic factors on child’s growth and obesity risk within diverse samples.23–26 Finally, previous studies mostly focused on one or two maternal factors, and few have investigated the joint effects of these three major prenatal metabolic factors on children’s growth and obesity risk in children. Therefore, the aim of this study was to systematically examine the individual and additive effects of the three modifiable maternal metabolic factors (pre-pregnancy overweight/obesity, GWG, and GDM) on childhood growth trajectories and obesity risk at age four years and to investigate the potential racial disparity in these effects using a longitudinal birth cohort of black and white mother-child dyads in the US.
Subjects and Methods
Study subjects
All the study mothers and children were from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. The CANDLE study is a prospective birth cohort of mother-child dyads in Shelby County, Tennessee. The study has been described previously.27–29 Briefly, the CANDLE study enrolled 1 503 healthy women aged 16–40 years during their second trimester of a singleton pregnancy between 2006 and 2011. Exclusion criteria included existing chronic disease requiring medication (e.g., hypertension, diabetes, and sickle cell disease), known pregnancy complications (e.g., complete placenta previa and oligohydramnios), and plans to deliver at a nonparticipating hospital.28 Of 1 455 live births, 1 425 mother-child pairs were included in this study, including 953 black (66.9%) and 472 white (33.1%) mother-child pairs. The CANDLE study was conducted in accordance with the Helsinki Declaration and approved by the Institutional Review Board of The University of Tennessee Health Science Center. Informed consent was given by participants 18 years or older, and assent was given by those 16–17.9 years, with consent provided by their legally authorized representative prior to enrollment.
Maternal measures
Self-administered questionnaires were used to collect information on age, race/ethnicity, education, insurance type, marital status, parity, cigarette smoking and alcohol use during pregnancy, and medical history at enrollment. At enrollment, trained research staff administered the Block Food Frequency Questionnaire (FFQ) was used to capture the mothers’ nutritional intake during the previous three months and estimate total energy intake. Self-reported height and weight prior to pregnancy were collected at enrollment and used to calculate pre-pregnancy BMI as weight (in kilograms) divided by the square of height (in meters). Mothers were categorized as normal weight or underweight with BMI < 25 kg/m2, overweight with 25 ≤ BMI<30 kg/m2 and obese with BMI ≥ 30 kg/m2. GWG was calculated by subtracting pre-pregnancy weight from maternal weight at delivery and was categorized based on the recommendations of the Institute of Medicine30: underweight mothers (BMI < 18.5 kg/m2) who gained 12.7–18.1 kg, normal-weight mothers (18.5 ≤ BMI<25 kg/m2) who gained 11.3–15.9 kg, overweight mothers (25 ≤ BMI<30 kg/m2) who gained 6.8–11.3 kg, and obese mothers (BMI ≥ 30.0 kg/m2 ) who gained 5.0–9.1 kg were categorized as adequate GWG; mothers who gained weight above or below this criterion were categorized as excessive or inadequate GWG, respectively. GDM was assessed by self-report at the clinical visits during second and third trimesters and at delivery.
Child measures
Birth weight and length of the CANDLE children were extracted from medical charts by research assistants.31 The body weight and length/height were also measured at each annual visit until four years old using the methods guided by the NHANES protocol.32 Body weight was measured using a digital scale. Recumbent length was obtained at the year-one visit and standing height was obtained for those two years or older. CDC has recommended to use the World Health Organization growth standards for children < 2 years and the Center for Disease Control and Prevention growth charts for children aged 2–19 years to assess children’s growth/obesity risk in the US.33 Since the obesity status at 4 years is a major outcome in this study, we calculated the sex- and age-specific BMI z score and percentile for each child using this recommended method by CDC. According to CDC, for children aged two years and older, childhood overweight was defined as a BMI at or above the 85th percentile and below the 95th percentile, and obesity was defined as a BMI at or above the 95th percentile. The information on breastfeeding was collected using the Infant Feeding Practices Questionnaire at the home visit at four weeks and the clinic visit at one year.
Statistical analysis
The characteristics between black and white mother-child pairs were compared using t tests for continuous variables and χ2 tests for categorical variables. The latent class growth modeling approach was used to identify subgroups that shared a similar underlying trajectory based on children’s BMI z scores using the SAS procedure PROC TRAJ.34,35 Selection of the best-fitting trajectory model was assessed using the Bayesian Information Criterion and the requirement of the number of subjects in each trajectory group no less than 5% of the total subjects. The number and percentage of individuals with available data for each predictor and outcome variable are shown in Supplementary Table S1. About 88.5% CANDLE children had at least two measurements of BMI at the five-time points during the follow-up. Multiple imputations were conducted to account for both missing children’s weight and height/length measurement data and mothers’ predictor and covariate data with ten imputations based on the Markov-chain Monte Carlo method using the SAS procedure PROC IM. Because odds ratios (ORs) are likely to overstate risk of predictors for common outcomes and both the growth trajectory groups and obesity rate at age four were common (over 10%), we calculated relative risks (RRs) for studied maternal metabolic factors instead of ORs. Two approaches, the log binomial regression and Poisson regression with robust variance estimation, have been used in prospective studies with common outcomes.36–38 Sometimes log binomial regression models do not converge. We also experienced the same problem. Thus, we used Poisson regression with robust variance estimated to compute the RRs and 95% confidence intervals implemented through PROC GENMOD in SAS. To examine their independent effects, the three maternal characteristics were included in the Poisson regression models simultaneously. Potential confounding factors, including maternal demographic (age, race, and marital status), socioeconomic (education and insurance type), dietary and lifestyle during pregnancy (energy intake, alcohol drinking, and tobacco use), reproductive history (parity), and child factors (child sex), were included in the regression model. Considering that birth weight, gestational age, and breastfeeding may mediate the effects of prenatal metabolic factors on childhood growth and obesity, these factors were further included in the regression models to examine whether the studied maternal metabolic factors had additional effects on childhood outcomes independent of these potential mediators.20 The interaction terms of these maternal metabolic factors with race were added in the regression models with the adjustment for all the aforementioned covariates to examine the potential differences in the associations between prenatal metabolic factors and childhood outcomes between blacks and whites. We also accessed the additive effects of the three maternal metabolic factors using a cumulative score (any two or all three factors) on children’s growth. Data were analyzed using SAS (Version 9.4; SAS Institute, Cary, North Carolina, USA). All statistical tests were 2-sided, and a P-value <0.05 was considered statistically significant.
Results
The characteristics of the studied mother-child pairs are listed in Table 1. Overall, black mothers were younger, completed fewer years of education, and were more likely to be single and have Medicaid/Medicare insurance compared to white mothers. In addition, black mothers had lower rates of smoking and alcohol drinking during pregnancy, but higher pre-pregnancy BMI than white mothers. There were no difference in GWG or GDM among the CANDLE mothers by race. The children born to black mothers had a lower birth weight and shorter length compared to those born to white mothers, and the prevalence of breastfeeding was also lower among black mothers. However, the BMI-z-scores at ages 1–4 and overweight/obesity rates at four-years-old were not significantly different between the children with black and white mothers.
Table 1.
Characteristics of the CANDLE mothers and children according to mothers’ race
| Variables | All (n=1,425) |
Blacks (n = 953) |
Whites (n = 472) |
P value a |
|---|---|---|---|---|
| Maternal | ||||
| Age, years | 26.0 ± 5.4 | 24.8 ± 5.2 | 28.6 ± 4.9 | < 0.001 |
| Education (≤ 12 years), % | 59.3 | 73.5 | 30.5 | < 0.001 |
| Marital status (single), % | 43.4 | 60.2 | 9.3 | < 0.001 |
| Insurance (Medicaid or Medicare), % | 59.7 | 77.3 | 23.9 | < 0.001 |
| Smoking during pregnancy, % | 9.3 | 7.9 | 12.3 | 0.01 |
| Alcohol drinking during pregnancy, % | 8.2 | 5.3 | 14.0 | < 0.001 |
| Total energy intake, kcals | 2726.4 ± 1666.5 | 3120.8 ± 1865.5 | 1986.4 ± 789.0 | < 0.001 |
| Parity (primiparous), % | 31.1 | 28.8 | 35.8 | < 0.001 |
| Pre-pregnancy BMI, kg/m2 | 27.5 ± 7.5 | 28.4 ± 8.0 | 25.8 ± 6.0 | < 0.001 |
| Gestational weight gain, kg | 14.6 ± 7.4 | 14.4 ± 7.8 | 14.9 ± 6.6 | 0.26 |
| Gestational diabetes, % | 5.4 | 4.7 | 6.6 | 0.15 |
| Child | ||||
| Gestational age at birth, weeks | 38.7 ± 1.9 | 38.7 ± 2.1 | 38.9 ± 1.4 | 0.01 |
| Male, % | 50.4 | 51.4 | 48.3 | 0.27 |
| Birth weight, kg | 3.2 ± 0.6 | 3.1 ± 0.6 | 3.4 ± 0.5 | < 0.001 |
| Birth length, cm | 50.1 ± 3.1 | 49.6 ± 3.2 | 51.0 ± 2.6 | < 0.001 |
| Breastfed status, % | 65.0 | 54.8 | 85.9 | < 0.001 |
| BMIz at birth | −0.4 ± 1.1 | −0.5 ± 1.1 | −0.2 ± 1.0 | < 0.001 |
| BMIz at age 1 | 0.8 ± 1.1 | 0.7 ± 1.2 | 0.8 ± 1.0 | 0.56 |
| BMIz at age 2 | 0.3 ± 1.2 | 0.3 ± 1.3 | 0.3 ± 1.1 | 0.70 |
| BMIz at age 3 | 0.3 ± 1.3 | 0.3 ± 1.3 | 0.4 ± 1.1 | 0.11 |
| BMIz at age 4 | 0.6 ± 1.1 | 0.6 ± 1.2 | 0.5 ± 1.0 | 0.54 |
| Overweight at age 4, % | 14.3 | 13.4 | 16.3 | 0.22 |
| Obesity at age 4, % | 16.6 | 17.9 | 13.8 | 0.10 |
BMIz, age- and sex-specific body mass index z score.
For comparisions between blacks and whites.
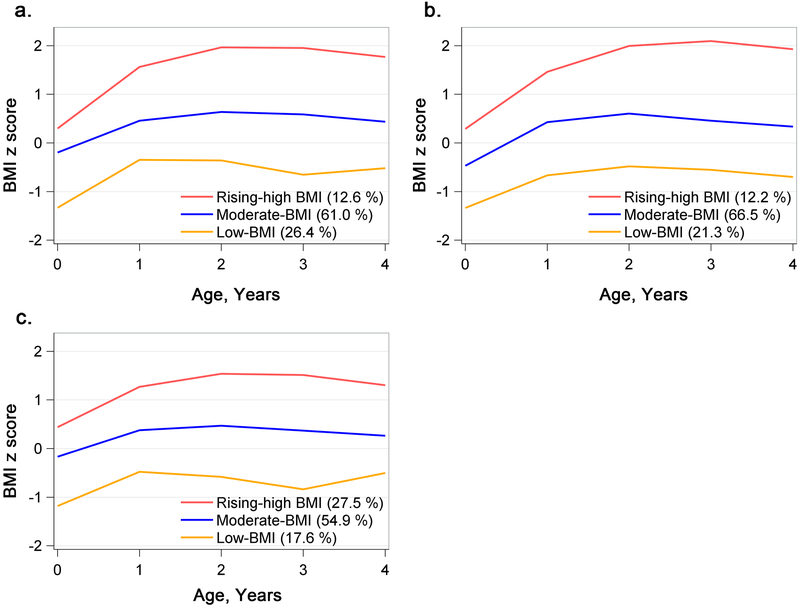
A three-class model of postnatal growth was the best fitting model in the trajectory analysis of the CANDLE children (Figure 1). A large majority of the children (61.0% of the cohort) were classified as a moderate-BMI trajectory characterized by an average size at birth (the average BMI-z-score close to zero) followed by a moderate BMI-z-score gain rate and an average z-score ranging from 0 to 1. The second trajectory group (24.4% of the cohort) exhibited relatively lower birth size and rapid BMI gain during the first year and stayed at a relatively lower but normal BMI level (the average BMI-z-score ranging from −1 to 0, the low-BMI group). The third and final trajectory group (12.6% of the cohort) started from an average birth size followed by a rapid BMI gain during the first year and stable high BMI until four-years-old (the rising-high-BMI group). The BMI growth trajectories were similar between the children with black and those with white mothers.
Figure 1.
BMI-z-score trajectories among the CANDLE participants. Panels a, b, and c show trajectories among the overall sample, blacks, and whites, respectively. BMI, body mass index.
The three maternal metabolic factors significantly varied among the growth trajectory groups (all P’s < 0.05) (Table 2). Mothers with overweight/obesity, excessive GWG, and GDM were more likely to have children within the rising-high-BMI trajectory group. Both rapid weight gain and high BMI during early childhood may be associated with increased risk for obesity in later life. 39–42 Therefore, we computed the RR for each maternal metabolic factor associated with the rising-high BMI trajectory. After adjusting for multiple confounding factors, pre-pregnancy obesity and excessive GWG were significantly associated with the rising-high-BMI trajectory and an increased risk for obesity at age four among the children (Table 3). GDM was not independently associated with the rising-high-BMI trajectory but was significantly associated with childhood obesity at age four. Additional adjustment for the potential mediators, including birth weight, gestational age, and breastfeeding, slightly attenuated the associations, but the RRs still remained significant (Table 3). We also calculated the RRs of these maternal metabolic factors associated with the low-BMI trajectory. The association directions between these maternal factors and the low-BMI trajectory were opposite of those associated with rising-high BMI trajectory. For example, pre-pregnancy obesity was negatively associated with the low-BMI trajectory (Supplementary Table S2 and Table S3).
Table 2.
Maternal metabolic risk factors overall and according to the BMI-z-score trajectory classes
| Maternal metabolic risk factors | All | Low-BMI | Moderate-BMI | Rising- High-BMI |
P value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Pre-pregnancy BMI status, kg/m2 (n =1,420) | |||||
| Normal or underweight, < 25 | 637 (44.9) | 200 (31.4) | 391 (61.4) | 46 (7.2) | <0.001 |
| Overweight, 25-29.9 | 351 (24.7) | 90 (25.6) | 217 (61.8) | 44 (12.5) | |
| Obesity, ≥ 30 | 432 (30.4) | 86 (19.9) | 258 (59.7) | 88 (20.4) | |
| Gestational weight gain (n =1,313) | |||||
| Adequate | 321 (24.4) | 82 (25.5) | 212 (66) | 27 (8.4) | <0.001 |
| Inadequate | 244 (18.6) | 90 (36.9) | 133 (54.5) | 21 (8.6) | |
| Excessive | 748 (57.0) | 166 (22.2) | 463 (61.9) | 119 (15.9) | |
| Gestational diabetes (n =1,420) | |||||
| No | 1,344 (94.6) | 363 (27) | 819 (60.9) | 162 (12.1) | 0.005 |
| Yes | 76 (5.4) | 11 (14.5) | 48 (63.2) | 17 (22.4) | |
BMI, body mass index
Table 3.
Associations of the maternal metabolic factors with the rising-high-BMI trajectory and obesity risk at age four in the children
| Maternal metabolic factors | Rising-high-BMI trajectory |
Obesity at age four | ||
|---|---|---|---|---|
| RR (95% CI) |
P value |
RR (95% CI) |
P value |
|
| Before adjusting for potential mediatorsa | ||||
| Pre-pregnancy overweight/obesity | ||||
| Normal weight | 1 | 1 | ||
| Overweight | 1.32 (0.87-2.01) | 0.19 | 1.49 (1.03-2.15) | 0.04 |
| Obesity | 2.31 (1.61-3.31) | <0.001 | 2.42 (1.76-3.32) | <0.001 |
| Gestational weight gain | ||||
| Adequate | 1 | 1 | ||
| Inadequate | 1.27 (0.72-2.22) | 0.41 | 1.02 (0.61-1.73) | 0.93 |
| Excessive | 1.86 (1.22-2.82) | 0.004 | 1.53 (1.07-2.20) | 0.02 |
| Gestational diabetes | ||||
| No | 1 | 1 | ||
| Yes | 1.26 (0.75-2.13) | 0.39 | 2.19 (1.52-3.14) | <0.001 |
| After adjusting for potential mediatorsb | ||||
| Pre-pregnancy overweight/obesity | ||||
| Normal weight | 1 | 1 | ||
| Overweight | 1.21 (0.80-1.84) | 0.37 | 1.42 (0.98-2.05) | 0.07 |
| Obesity | 1.96 (1.36-2.83) | <0.001 | 2.24 (1.62-3.10) | <0.001 |
| Gestational weight gain | ||||
| Adequate | 1 | 1 | ||
| Inadequate | 1.32 (0.76-2.30) | 0.33 | 1.06 (0.63-1.79) | 0.83 |
| Excessive | 1.71 (1.13-2.58) | 0.01 | 1.46 (1.01-2.09) | 0.04 |
| Gestational diabetes | ||||
| No | 1 | 1 | ||
| Yes | 1.17 (0.71-1.92) | 0.54 | 2.14 (1.47-3.12) | <0.001 |
BMI, body mass index; CI, confidence interval; RR, relative risk.
Adjusted for maternal age, maternal race, education, insurance type, marital status, total energy intake, alcohol intake and tobacco use during pregnancy, parity, and child sex.
Further adjusting for the potential mediators including birth weight, child gestational age, and breastfed status.
We also examined the additive effects of the three maternal metabolic factors (1, 2 or 3 factors present) on childhood growth trajectory and obesity risk. The children’s risk of being classified into the rising-high-BMI group and being obese at age four increased with the increasing number of maternal adverse metabolic factors (Table 4). The black and white subsamples did not differ in individual or additive effects of these three maternal metabolic factors on growth trajectories and childhood obesity risk (Supplementary Table S4).
Table 4.
Associations of the number of maternal metabolic factors with the rising-high-BMI trajectory and obesity risk in the children at four years
| Number of maternal metabolic factors |
Rising-high-BMI trajectory | Obesity at age four | ||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Before adjusting for potential mediatorsa | ||||
| 0 | 1 | 1 | ||
| 1 | 1.78 (1.04-3.05) | 0.04 | 1.64 (0.97-2.78) | 0.06 |
| 2 | 3.09 (1.87-5.10) | <0.001 | 3.03 (1.95-4.72) | <0.001 |
| 3 | 3.34 (1.40-7.98) | 0.007 | 5.86 (3.04-11.29) | <0.001 |
| After adjusting for potential mediatorsb | ||||
| 0 | 1 | 1 | ||
| 1 | 1.56 (0.91-2.68) | 0.11 | 1.53 (0.91-2.59) | 0.11 |
| 2 | 2.38 (1.42-4.00) | 0.001 | 2.63 (1.68-4.11) | <0.001 |
| 3 | 2.57 (1.09-6.04) | 0.03 | 5.06 (2.52-10.14) | <0.001 |
BMI, body mass index; CI, confidence interval; RR, relative risk.
Adjusted for maternal age, maternal race, education, insurance type, marital status, total energy intake, alcohol intake and tobacco use during pregnancy, parity, and child sex.
Further adjusting for the potential mediators including birth weight, child gestational age, and breastfed status.
Discussion
In this prospective birth cohort of black and white Americans, we identified three distinct BMI growth trajectories during early childhood. Two maternal metabolic risk factors, pre-pregnancy obesity and excessive GWG, were significantly associated with childhood growth trajectory, predicting increased risk for the trajectory characterized by rapid weight gain during the first year of life and stable high BMI until age four (the rising-high-BMI group). We also found that three modifiable maternal metabolic factors (pre-pregnancy obesity, excessive GWG, and GDM) independently predicted increased risk for childhood obesity. Children of the mothers with a greater number of these metabolic risk factors had greater risk for having the rising-high-BMI trajectory and being obese during early childhood.
Recent progress in statistical methods makes it possible to study the potential heterogeneity of BMI changes during early childhood. Individual children may belong to distinct BMI trajectories, which may confer different risks towards the subsequent development of obesity or other health outcomes. Although the numbers and patterns of the BMI trajectories are varied among populations due to different study designs (e.g., duration and frequency of follow-up assessments), the trajectories observed in our study, especially the rising-high-BMI trajectory, are consistent with those reported in previous studies.18,20,39,43,44 The trajectories exhibiting either rapid BMI gain or stable high BMI have been linked with increased risk for obesity in later life,39–42 and even showed a better predictive value than a single time obesity assessment.18 Aris et al. found that the area under the receiver operating characteristics curve for predicting childhood obesity at five years was significantly higher in models that included BMI trajectories in the first two years compared to those including BMI at age two years only.18 All these findings support the potential application of early childhood BMI trajectories in the prediction of health outcomes in later childhood, or even adulthood, and highlight the need to investigate risk factors for those BMI trajectories that are associated with adverse health outcomes in children.
In our study, both pre-pregnancy obesity and excessive GWG were significantly associated with the rising-high-BMI trajectory, which is in line with previous studies.18,20,43 Most of the previous studies did not adjust for birth weight or gestational age at birth in their analyses; these factors might mediate the effects of the maternal metabolic factors on child growth and are therefore important to include.18,20 However, we found that pre-pregnancy obesity and excessive GWG were still significantly associated with increased risk for the rising-high BMI trajectory even after adjusting for the birth weight and gestational age, although their effects were attenuated. Additionally, we did not observe a significant association between GDM and the rising-high BMI trajectory, which is consistent with the recent study finding of a Spanish birth cohort.20 CANDLE excluded existing diabetes patients at recruitment. Therefore, the relatively low GDM rate among the CANDLE mothers (4.7% in black and 6.6% in white mothers) might partially explain the negative findings due to a limited statistical power. It has been reported that the national prevalence of GDM was as high as 9.2% in an analysis in 2014 according to CDC.45
Although maternal pre-pregnancy obesity, excessive GWG, and GDM have been widely studied for their associations with birth and metabolic outcomes in children, very few studies have evaluated their independent effects (e.g., through adjustment for each other in a single model) or additive effects on childhood obesity risk.46,47 Our findings provide further evidence for their independent effects on childhood obesity which are only partially mediated by birth weight. In particular, the finding that children with mothers having a greater number of metabolic risk factors had a higher risk for childhood obesity will strongly support and guide the early intervention of each of these modifiable maternal factors for preventing obesity in offspring.
The possible mechanisms explaining the increased risk for obesity among the children with obese mothers include the inherited genetic susceptibility to obesity, the effects of maternal obesity on the intrauterine environment, and the maternal role in shaping the child’s postnatal diet and physical activity.26,48 Excessive GWG indicates more maternal fat deposition and possibly a state of maternal abnormal metabolism.49–51 This altered maternal environment may interplay with placental factors that lead to an increased supply of fuels to the fetus.52 Another possible explanation for the association of gestational weight gain with offspring overweight/obesity is that mothers with greater GWG may have children who gain more weight through shared genetic susceptibility, dietary preferences, and physical activity patterns.53 Several mechanisms linking GDM and the risk of offspring obesity have been suggested.54 For example, exposure to maternal diabetes can lead to excess fetal growth in utero, possibly mainly due to an increase in fetal fat mass and alterations in fetal hormone profile. GDM can also result in hyperglycemia, hyperinsulinemia, and elevated leptin synthesis in offspring. In addition, GDM may cause epigenetic changes and influence the expression of genes that direct the accumulation of body fat or related metabolic pathways.55
It has been reported that African American children have a greater prevalence of obesity than white children in the US.21,22 We also observed a higher rate of obesity among black children at age four years compared to white children (17.9% vs. 13.8%), although the difference was not statistically significant, and the growth trajectories from birth through age four were very similar between the black and white children. We also did not identify any difference in the associations of the studied maternal metabolic risk factors with growth trajectories and obesity risk at age four in the children, further suggesting parallel impact of maternal metabolic indices on growth across black and white children. The lack of differences by race may reflect increasing equalization of obesity risk for all race/ethnicities, or the relatively high rates of obesity for members of both groups in the South.56 However, it is most likely due to our study’s rigorous adjustment for obesity-related factors that often differ by race in research samples, such as income, education, and diet quality.22
Our study has several strengths. First, the longitudinal birth cohort with repeated anthropometric measures in the children enabled us to prospectively examine the effects of maternal prenatal factors with childhood growth and classify children into different growth trajectories, reflecting the heterogeneity of BMI changes in early childhood. Our expanded data collection protocols allowed for the assessment of prenatal factors and the association with childhood growth and obesity risk while accounting for important potential confounders. Exclusion of women with existing diabetes at enrollment enabled the examination of diabetes newly developed during pregnancy which predict children’s growth and risk for obesity. Finally, the inclusion of a large number of black and white women enabled generalization of findings to understudied populations as well as the examination of potential racial disparities in the associations of interest.
Limitations include reliance on self-reported pre-pregnancy weight and height, which could lead to misclassification of pre-pregnancy obesity and metabolic indices. However, studies have shown that recall of these factors was reproducible and valid57 and that underestimation,53 which tends to be more frequent than overestimation, most likely would bias estimates toward the null.58 Self-reported GDM during pregnancy may also limit the study power to detect the association between GDM and children’s growth trajectories. Residual confounding may exist because we could not adjust for paternal factors, maternal family history of diabetes or other metabolic disorders, children’s dietary intake, and physical activity which may also have potential effects on childhood obesity. Given the CANDLE mothers were from the South which has a relatively higher prevalence of obesity, the generalization of study findings may be limited to populations with greater risk for obesity. Another limitation is that the variability in the BMI trajectory groups may have not been fully captured due to the restricted sample size. Finally, the smaller numbers of white compared to black women in our sample might undermine the study power to detect racial disparities in these BMI trajectories and associations with maternal factors.
In conclusion, our study provides further evidence that, regardless of race, maternal prenatal metabolic factors are associated with childhood growth trajectories and early childhood obesity risk. Clustering of maternal adverse metabolic factors was associated with a substantial increase in the risk of childhood obesity. These findings strongly suggest that early interventions targeting modifiable metabolic risk factors during pregnancy may prevent or delay the development of childhood obesity.
Supplementary Material
Acknowledgements
The CANDLE study was supported by the Urban Child Institute, the University of Tennessee Heath Science Center, and NIH (1R01HL109977) grants.
Footnotes
Conflict of Interest
None.
Supplementary information
Supplementary information is available at the IJO’s website.
References
- 1.Fryar C, Carroll M, Ogden C. Prevalence of Overweight and Obesity Among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 Through 2013–2014. 2016. [Google Scholar]
- 2.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9:474–88. [DOI] [PubMed] [Google Scholar]
- 3.Brisbois TD, Farmer AP, McCargar LJ. Early markers of adult obesity: a review. Obes Rev 2012;13:347–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakshman R, Elks CE, Ong KK. Childhood obesity. Circulation. 2012;126:1770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabelea D, Harrod CS. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev 2013;71 Suppl 1:S62–7. [DOI] [PubMed] [Google Scholar]
- 6.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365:1876–85. [DOI] [PubMed] [Google Scholar]
- 7.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007;357:2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umer A, Kelley GA, Cottrell LE, Giacobbi P Jr., Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr 2011;158:941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes, metabolic syndrome and obesity : targets and therapy. 2010;3:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawley J The economics of childhood obesity. Health affairs (Project Hope). 2010;29:364–71. [DOI] [PubMed] [Google Scholar]
- 12.Trasande L, Chatterjee S. The impact of obesity on health service utilization and costs in childhood. Obesity (Silver Spring). 2009;17:1749–54. [DOI] [PubMed] [Google Scholar]
- 13.Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond). 2016;40:229–38. [DOI] [PubMed] [Google Scholar]
- 14.Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol 2014;211:259 e251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed SR, Ellah MA, Mohamed OA, Eid HM. Prepregnancy obesity and pregnancy outcome. Int J Health Sci (Qassim). 2009;3:203–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Kaseva N, Vaarasmaki M, Matinolli HM, Sipola-Leppanen M, Tikanmaki M, Heinonen K, et al. Pre-pregnancy overweight or obesity and gestational diabetes as predictors of body composition in offspring twenty years later: evidence from two birth cohort studies. Int J Obes (Lond). 2018;42:872–9. [DOI] [PubMed] [Google Scholar]
- 17.Lau EY, Liu J, Archer E, McDonald SM, Liu J. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes 2014;2014:524939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aris IM, Chen LW, Tint MT. Body mass index trajectories in the first two years and subsequent childhood cardio-metabolic outcomes: a prospective multi-ethnic Asian cohort study. Scientific reports. 2017;7:8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH. Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health. 2014;68:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montazeri P, Vrijheid M, Martinez D, Basterrechea M, Fernandez-Somoano A, Guxens M, et al. Maternal Metabolic Health Parameters During Pregnancy in Relation to Early Childhood BMI Trajectories. Obesity (Silver Spring). 2018;26:588–96. [DOI] [PubMed] [Google Scholar]
- 21.Naja F, Hwalla N, Itani L, Karam S, Sibai AM, Nasreddine L. A Western dietary pattern is associated with overweight and obesity in a national sample of Lebanese adolescents (13–19 years): a cross-sectional study. Br J Nutr 2015;114:1909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isong IA, Rao SR, Bind MA, Avendano M, Kawachi I, Richmond TK. Racial and Ethnic Disparities in Early Childhood Obesity. Pediatrics. 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widen EM, Whyatt RM, Hoepner LA, Mueller NT, Ramirez-Carvey J, Oberfield SE, et al. Gestational weight gain and obesity, adiposity and body size in African-American and Dominican children in the Bronx and Northern Manhattan. Matern Child Nutr 2016;12:918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heerman WJ, Bian A, Shintani A, Barkin SL. Interaction between maternal prepregnancy body mass index and gestational weight gain shapes infant growth. Acad Pediatr 2014;14:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta SH, Kruger M, Sokol RJ. Is maternal diabetes a risk factor for childhood obesity? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2012;25:41–4. [DOI] [PubMed] [Google Scholar]
- 26.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–36. [DOI] [PubMed] [Google Scholar]
- 27.Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y, Volgyi E, et al. Early adversity, socioemotional development, and stress in urban 1-year-old children. J Pediatr 2013;163:1733–9.. [DOI] [PubMed] [Google Scholar]
- 28.Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Volgyi E, et al. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients. 2015;7:9918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volgyi E, Carroll KN, Hare ME, Ringwald-Smith K, Piyathilake C, Yoo W, et al. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients. 2013;5:1511–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 2009;21:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colon-Ramos U, Racette SB, Ganiban J, Nguyen TG, Kocak M, Carroll KN, et al. Association between dietary patterns during pregnancy and birth size measures in a diverse population in Southern US. Nutrients. 2015;7:1318–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Barres S, Romaguera D, Valvi D, Martinez D, Vioque J, Navarrete-Munoz EM, et al. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: the INMA birth cohort study. Pediatr Obes 2016;11:491–9. [DOI] [PubMed] [Google Scholar]
- 33.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2010;59:1–15. [PubMed] [Google Scholar]
- 34.McCutcheon AL. Latent Class Analysis. SAGE Publications; : Newbury Park, CA, USA: 1987. [Google Scholar]
- 35.Jones B, Nagi ND, Roeder K. SAS Proc edure bas ed on mixture models for estimating developmental trajectories. Sociol Methods Res 2001;29:374–93. [Google Scholar]
- 36.Wacholder S Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986;123:174–84. [DOI] [PubMed] [Google Scholar]
- 37.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–3. [DOI] [PubMed] [Google Scholar]
- 38.Moschonis G, Kalliora AC, Costarelli V, Papandreou C, Koutoukidis D, Lionis C, et al. Identification of lifestyle patterns associated with obesity and fat mass in children: the Healthy Growth Study. Public Health Nutr 2014;17:614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giles LC, Whitrow MJ, Davies MJ, Davies CE, Rumbold AR, Moore VM. Growth trajectories in early childhood, their relationship with antenatal and postnatal factors, and development of obesity by age 9 years: results from an Australian birth cohort study. Int J Obes (Lond). 2015;39:1049–56. [DOI] [PubMed] [Google Scholar]
- 40.Liu JX, Liu JH, Frongillo EA, Boghossian NS, Cai B, Hazlett LJ. Body mass index trajectories during infancy and pediatric obesity at 6 years. Annals of epidemiology. 2017;27:708–15. [DOI] [PubMed] [Google Scholar]
- 41.Rzehak P, Oddy WH, Mearin ML, Grote V, Mori TA, Szajewska H, et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am J Clin Nutr 2017;106:568–80. [DOI] [PubMed] [Google Scholar]
- 42.Peneau S, Giudici KV, Gusto G, Goxe D, Lantieri O, Hercberg S, et al. Growth Trajectories of Body Mass Index during Childhood: Associated Factors and Health Outcome at Adulthood. J Pediatr 2017;186:64–71. [DOI] [PubMed] [Google Scholar]
- 43.Pryor LE, Tremblay RE, Boivin M, Touchette E, Dubois L, Genolini C, et al. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med 2011;165:906–12. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Wang H, Wang Y, Xue H, Wang Z, Du W, et al. Dietary patterns and their associations with childhood obesity in China. Br J Nutr 2015;113:1978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bider-Canfield Z, Martinez MP, Wang X, Yu W, Bautista MP, Brookey J, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes 2017;12:171–8. [DOI] [PubMed] [Google Scholar]
- 47.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr 2015;101:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anzman SL, Rollins BY, Birch LL. Parental influence on children’s early eating environments and obesity risk: implications for prevention. Int J Obes (Lond). 2010;34:1116–24. [DOI] [PubMed] [Google Scholar]
- 49.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol 1976;19:489–513. [DOI] [PubMed] [Google Scholar]
- 50.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Human reproduction update. 2010;16:255–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karachaliou M, Georgiou V, Roumeliotaki T, Chalkiadaki G, Daraki V, Koinaki S, et al. Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol 2015;212:502.e501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Frontiers in genetics. 2011;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr 2008;87:1818–24. [DOI] [PubMed] [Google Scholar]
- 54.Dabelea D The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30 Suppl 2:S169–74. [DOI] [PubMed] [Google Scholar]
- 55.Ge ZJ, Zhang CL, Schatten H, Sun QY. Maternal diabetes mellitus and the origin of non-communicable diseases in offspring: the role of epigenetics. Biol Reprod. 2014;90:139. [DOI] [PubMed] [Google Scholar]
- 56.Okubo H, Crozier SR, Harvey NC, Godfrey KM, Inskip HM, Cooper C, et al. Diet quality across early childhood and adiposity at 6 years: the Southampton Women’s Survey. Int J Obes (Lond). 2015;39:1456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright CS, Weiner M, Localio R, Song L, Chen P, Rubin D. Misreport of gestational weight gain (GWG) in birth certificate data. Matern Child Health J. 2012;16:197–202. [DOI] [PubMed] [Google Scholar]
- 58.McClure CK, Bodnar LM, Ness R, Catov JM. Accuracy of maternal recall of gestational weight gain 4 to 12 years after delivery. Obesity (Silver Spring). 2011;19:1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.