Abstract
Background:
Despite increasing emphasis on reducing radiation exposure from myocardial perfusion imaging (MPI), the use of radiation-sparing practices (RSP) at nuclear laboratories remains limited. Defining real-world impact of RSPs on effective radiation dose (E) can potentially further motivate their adoption.
Methods:
MPI studies performed between 1/2010 and 12/2016 within a single health system were included. Mean E was compared between sites with ‘basic’ RSP (defined as elimination of thallium-based protocols and use of stress-only (SO) imaging on conventional single photon emission computed tomography (SPECT) cameras) and those with ‘advanced’ capabilities (sites that additionally used solid-state detector (SSD) SPECT cameras, advanced post-processing software (APPS) or positron emission tomography (PET) imaging), after matching patients by age, gender and weight. Contributions of individual RSP to E reduction were determined using multiple linear regression after adjusting for factors affecting tracer dose.
Results:
Among 55,930 MPI studies performed, use of advanced RSP was associated with significantly lower mean E compared to basic RSP (7 ± 5.6 mSv and 16 ± 5.4 mSv, respectively; P<0.001), with a greater likelihood of achieving E < 9 mSv (65.7% vs. 10.8%, respectively; OR=15.8 [95%CI 14–17.8]; P<0.0001). Main driver of E reduction was SO-SSD SPECT (mean reduction= 11.5 mSv), followed by use of SO-SPECT+APPS (mean reduction=10.1 mSv), PET (mean reduction= 9.7 mSv) and elimination of thallium protocols (mean reduction= 9.1 mSv); P<0.0001 for all comparisons.
Conclusion:
In a natural experiment with implementation of radiation-saving practices at a large health care system, stress-only protocols use in conjunction with modern SPECT technologies, use of PET and elimination of thallium-based protocols were associated with greatest reductions in radiation dose. Availability of several approaches to dose reduction within a health system can facilitate achievement of targeted radiation benchmarks in a greater number of performed studies.
Keywords: myocardial perfusion imaging, radiation exposure, effective dose
Introduction
Minimizing radiation exposure to patients undergoing radionuclide perfusion imaging has garnered increasing attention in recent years, driven by concerns about the potential long-term hazards of ionizing radiation and the desire to improve patient safety.1–3 As a result, major medical societies have issued recommendations for greater adoption of radiation-sparing interventions with myocardial perfusion imaging (MPI), in accordance with the central principles of radiation safety.4–6
Several radiation-sparing practices (RSP) are currently available for use with single photon emission computed tomography (SPECT) MPI.7 These range from simply avoiding radiopharmaceuticals with higher radioactivity, such as thallium-labelled tracers, to incorporating innovative hardware and software technologies and adoption of patient-centered imaging protocols aimed at lowering administered tracer dosage, and hence lowering radiation dose.8–11 The use of positron emission tomography (PET) offers another opportunity to assess myocardial perfusion with significantly lower radiation as compared with conventional SPECT.12
Radiation exposure from MPI at the majority of nuclear laboratories across the world, however, continues to exceed recommended targets.13–16 Furthermore, a smaller proportion of US-based laboratories achieve the endorsed levels of radiation exposure compared with non-US centers,17 with much of this variation in laboratory-level effective radiation dose (E) likely driven by slow and erratic adoption of RSPs within health care systems in the US. This apparent disconnect between strong societal recommendations and suboptimal real-world RSP implementation may be partly due to limited evidence comparing the efficacy of different radiation-saving interventions, especially when implemented concurrently within the same laboratory.
To further clarify the advantages of RSP adoption, as has been achieved by several groups,18, 19 it is critical to quantify radiation reductions with individual RSPs, so as to better illuminate the potential for improvement in MPI safety. While the intention of the current study was not to describe novel approaches to dose reduction, we sought to determine the feasibility and outcomes of sequential RSP implementation within a single health system and to determine the contribution of each of these interventions to improvement in radiation exposure with MPI.
Methods
Data source and participating sites
We used the electronic database of Saint Luke’s Mid America Heart Institute to identify patients referred for clinically-indicated SPECT or PET MPI studies between 1/1/2010 and 12/31/2016. The database includes information on MPI studies performed at multiple sites throughout the health system, including 4 large, hospital-based laboratories located within the Kansas City metropolitan area, and 5 affiliated, community-based sites located in surrounding rural communities. All studies are electronically transmitted, irrespective of the site of performance, to a central nuclear cardiology laboratory at Saint Luke’s Mid America Heart Institute (Kansas City, MO), where image processing takes place and information on patient demographics, risk factors, stress protocols, radiopharmaceutical used (type and administered activity), imaging protocol and camera system use are entered by trained staff. Exercise or pharmacologic stress (with dipyridamole, adenosine, regadenoson, and dobutamine) was used according to standard protocols and image acquisition was completed in accordance with published guidelines.5, 20 The study was reviewed and approved by the Institutional Review Board and waiver for informed consent was granted based on the retrospective design of the study.
Definition of RSP levels
The following practices were considered RSPs: elimination of thallium (Tl)-based protocols (including studies performed with Tl alone and dual-isotope studies), use of solid-state detector (SSD) cameras, use of stress-only (SO) protocols, utilization of advanced post-processing software (+APPS) with conventional SPECT cameras and use of PET.
Imaging sites within the network were grouped according to the level of RSP implementation. Sites with ‘basic RSP’ included those that implemented changes in radiotracer usage (namely elimination of Tl-based protocols) or adopted SO protocols on conventional SPECT cameras without adjunctive use of APPS. We chose this definition since such interventions can be adopted by any site performing cardiac radionuclide imaging without the need for major updates to existing imaging systems and are therefore reflective of what could be considered the most basic interventions to reduce E in “real-world” practices. Sites with ‘advanced RSP’, on the other hand, included those that additionally implemented hardware- or software-based interventions, such as incorporation of SSD cameras, PET scanners or installation of APPS onto standard Anger cameras. Using these definitions, community-based, non-Metro imaging sites within the system (n=5) were deemed to have ‘basic RSP’ capabilities while hospital-based sites (n=4) were considered to have ‘advanced RSP’ capabilities. Sites with basic RSP were used as a comparator group in this analysis to provide a frame of reference for the changes in radiation exposure seen at sites with advanced capabilities. Notably, radiation reduction capabilities were comparable across sites within each group (Supplemental Table S1). Imaging equipment and processing software integration at participating sites are described below and summarized in Figure 1.
Figure 1:
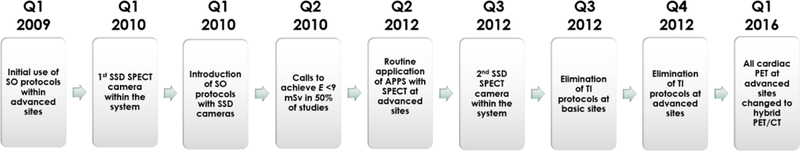
Timeline for the introduction of different radiation-sparing practices at participating sites.
Selection and dosing of radiopharmaceuticals
During the study period, a combination of 99mTc-based, 201Tl-based or dual isotope imaging protocols were used at sites with basic and advanced capabilities. However, the use of Tl-based SPECT imaging gradually phased out, and these studies were no longer performed after the 4th quarter of 2012 at the main sites and after the 3rd quarter of 2012 at outlying sites. The use of weight-adjusted radioisotope dose with SPECT was introduced at all sites early during the study period.
Camera systems and processing software
At the 4 metro locations, SPECT imaging was performed using a variety of conventional Anger SPECT cameras from a variety of vintages, with line source for attenuation correction, including 2 small field-of-view (CardioMD, Philips Healthcare, Milpitas, CA) and 2 large field-of-view cameras (ADAC, Philips Healthcare, Milpitas, CA). In the 1st quarter of 2010, an SSD SPECT camera (D-SPECT, Spectrum Dynamics, Sarasota, FL) was introduced at one of the main nuclear laboratories, and subsequently at one other major site in the 3rd quarter of 2012. The use of SSD cameras enables the use of significantly lower radiotracer doses without compromising image quality and these cameras were generally utilized in protocols to maximize radiation sparing rather that to shorten acquisition time. Participating non-metro sites used conventional large-field-of-view Anger SPECT cameras (Symbia Intevo, Siemens, Munich, Germany; ECAM, Siemens, Munich, Germany) with or without attenuation correction. These sites did not have access to SSD camera technology or implementation of the APPS on their conventional camera systems.
Consistent implementation of APPS started in the 2nd quarter of 2012 with use of ASTONISH-128 (Philips Healthcare, Milpitas, CA); a processing software that involves iterative reconstruction and depth-dependent resolution recovery, in conjunction with an existing conventional small-field-of-view SPECT camera (CardioMD, Philips Healthcare, Milpitas, CA). APPS implementation contributes to dose reduction either through allowing the use of lower tracer dosage in conjunction with full time imaging, or through enhancing image quality via the software specifications when used with full-dose tracer injection and shortened imaging duration.21
Imaging protocols
The use of SO protocols in appropriately selected patients was introduced at the 4 main imaging sites prior to the beginning of the study period.22 Imaging was performed with conventional SPECT systems (large field-of-view with line source attenuation correction), using low-dose (10–19.9 mCi) or high-dose (≥20 mCi) 99mTc injections. The availability of SSD cameras allowed the concomitant use of SO protocols on these camera systems, thereby enabling the use of ultra-low doses of 99mTc (less than 10 mCi), which was incorporated into the routine workflow of sites with SSD SPECT cameras. In addition, when APPS became available with small field-of-view Anger cameras at the main laboratories (the 2nd quarter of 2012), default protocols on those cameras were changed from high-dose stress-first to low dose stress-first protocols (or ultra-low dose) became routine at these sites. Representative examples of SO protocol applications with various cameras are shown in Figure 2.
Figure 2:
Change in E with the implementation of each radiation-sparing practice at sites with advanced RSP capabilities
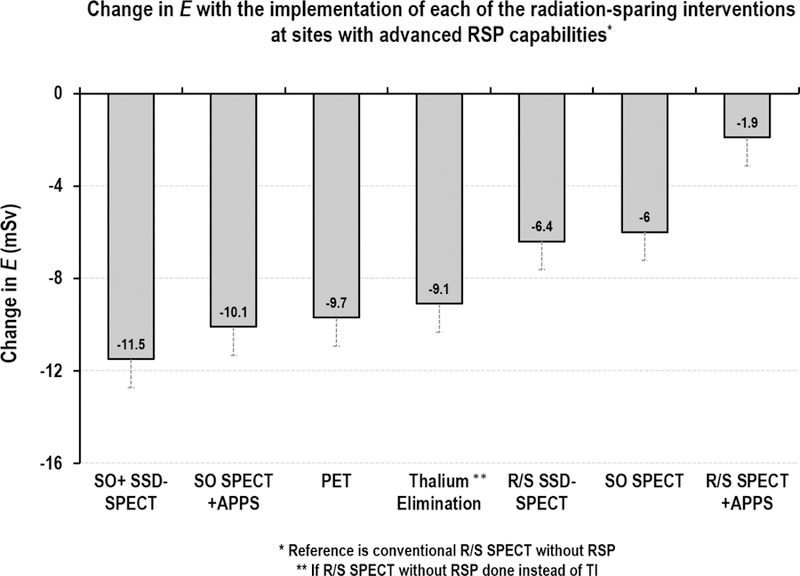
Graph depicts change in E with use of each of the radiation-sparing interventions (adjusted for age, gender, BMI and other factors that may influence selection of RSP modality), if that particular intervention is used instead of R/S conventional SPECT without RSP.
Imaging protocols at the outlying imaging facilities, on the other hand, were limited to rest/stress or, occasionally high-dose SO protocols on conventional SPECT cameras (large field-of-view with or without attenuation correction).
Utilization of PET
PET MPI was consistently offered at the 4 main sites throughout the study duration. It was initially offered using a combination of PET/CT cameras and dedicated cardiac PET systems with line source attenuation correction. Furthermore, image acquisition was performed in both 2-D and 3-D modes. Later, all PET imaging took place on a variety of later generation PET/CT systems in 3-D mode. PET capabilities were not available at any of the outlying sites throughout the study duration.
Radiation dose estimation
The administered dose of radiotracer (in mCi) for rest and stress acquisitions was recorded at the time of study performance and prospectively entered into the database. E was estimated from the administered radiotracer dose of 201Tl and 99mTc-sestamibi using published conversion factors,23, 24 and similarly for 82Rb and 18F-FDG.25 99mTc-tetrofosmin, which is associated with a lower radiation dose, was used in <1% of the studies during the study period. Therefore, the same conversion formula for 99mTc-sestamibi was used to estimate E for these studies. Studies with missing data and those performed for purposes other than assessment of ischemia or viability using other radiopharmaceuticals (such as assessment of cardiac sympathetic nerve activity or assessment of possible cardiac amyloidosis) were excluded from the calculation of effective dose.
Contributions to E from 153Gd line source transmission (for attenuation correction with SPECT MPI, estimated at 0.05 mSv), 68Ge transmission (for attenuation correction with PET MPI, estimated at 0.08 mSv), or from low-dose CT (for attenuation correction on PET/CT camera systems, estimated at 0.5 mSv) were not included in final E estimates given the small magnitude of radiation contributed as compared with that associated with radiotracer injection.2, 7
Statistical analyses
Demographics of patients who underwent MPI at sites with basic or advanced RSP capabilities were compared using t-tests for continuous variables and x2 for categorical variables. Mean achieved E was compared between sites with basic and advanced RSP, in the overall population and in an age-, gender- and weight-matched (5:1) population, using paired sample t-test. Matching was performed to account for differences in patient characteristics that may affect radiation dose. The adequacy of the match was verified by reporting P values and standardized differences between the distributions. The proportion of studies performed with E <9 mSv in each group was also compared using x2 test.
Afterwards, absolute reduction in E achieved with individual radiation saving interventions was determined, using R/S conventional SPECT without implementation of any additional RSP as a reference. This was accomplished using multiple linear regression after adjusting for covariates potentially affecting radiation dose (weight) or test selection (age, gender, presence of CAD, prior revascularization, prior stroke, peripheral arterial disease, left bundle branch block and chronic obstructive pulmonary disease), and with E as the outcome variable. For the purpose of the last aim, studies performed at sites with advanced and basic capabilities were analyzed separately. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Comparison of effective dose by RSP level
During the study period, a total of 55,930 MPI studies were performed; of them 52,735 (94.3%) were performed at sites with advanced RSP and the rest at sites with basic capabilities. There were differences in patient characteristics between sites, such that patients who underwent MPI at advanced RSP sites were older and more often males with lower BMI compared with those who underwent testing at sites with basic capabilities. Therefore, patients were matched (5:1) by age, gender, BMI and year of testing. The matched cohort comprised 17,976 patients, of whom 14,980 imaged at sites with advanced RSP and 2996 patients at sites with basic RSP. The mean age of the matched cohort was 63.8 ± 12.1 years and BMI of 33.1 ± 7.4 with predominance of female patients (53.2%). Characteristics of patients by imaging sites before and after matching are shown in Table 1.
Table 1:
Comparison of patient characteristics and effective dose between sites with and without advanced RSP capabilities, before and after matching for patient characteristics
| Before match | After 5:1 match | |||||
|---|---|---|---|---|---|---|
| Advanced RSP | Basic RSP | P-value | Advanced RSP | Basic RSP | P-value | |
| N | 52735 | 3195 | 14980 | 2996 | ||
| Age (years) | 65.5 ± 12.2 | 62.9 ± 12.9 | <0.001 | 63.8 ±12.1 | 63.8 ±12.2 | 0.94 |
| Women (%) | 23333 (44.2) | 1741 (54.5) | <0.001 | 7970 (53.2) | 1594 (53.2) | 1.0 |
| BMI (kg/m2) | 30.5 ± 7.2 | 33.7 ± 9.1 | <0.001 | 33 ± 7.3 | 33.1 ± 7.4 | 0.58 |
| E (mSv) | 6.3 ± 7.6 | 17.7 ± 9.5 | <0.001 | 7 ± 5.6 | 16 ± 5.4 | <0.001 |
| E< 9 mSv (%) | 37107 (70.4) | 334 (10.5) | <0.001 | 9840 (65.7) | 325 (10.8) | <0.001 |
Continuous variables presented as mean ± standard deviation; categorical variables as percentages.
BMI: body mass index; E: effective dose; RSP: radiation-sparing practices; SD: standard deviation
Mean E was lower at sites with advanced capabilities compared with those having only basic capabilities (6.3 ± 7.6 vs. 17.7 ± 9.5 mSv, mean between-group difference of 10.8 ± 8.2 mSv, P<0.001). This difference persisted after matching for patient characteristics (7 ± 5.6 vs. 16 ± 5.4 mSv, mean between-group difference of 9.5 ± 11.2 mSv, P<0.001). Additionally, a greater proportion of studies performed at sites with advanced capabilities were done with an effective dose <9 mSv per study compared with those done at sites with only basic RSP capabilities: 70.4% and 8.9%, respectively, in the unmatched cohort (OR of 24.5; [95%CI 22.1–271]; P<0.001), and 65.7% vs. 10.8%, respectively, in the matched cohort (OR 15.8; [95%CI 14–17.8]; P<0.001).
Reduction in effective dose with individual RSPs
Mean E and patient-dependent determinants of testing modality were then compared across the RSP spectrum within sites with advanced RSP capabilities (Table 2). Mean E was significantly different between various RSP’s; Tl-based protocols were associated with the highest mean E (22.3 ± 8.7 mSv) whereas SO-SSD SPECT was associated with the lowest mean E (1.9 ± 1.8 mSv, P value for between-group comparison <0.001). In addition, there were significant differences in age, gender, BMI and prevalence of CAD, prior revascularization, prior stroke, peripheral arterial disease, left bundle branch block and chronic obstructive pulmonary disease across the groups (P value for all between-modality comparisons <0.001). In addition, dose of radiopharmaceutical varied significantly between interventions, with the highest total 99mTc dose used for patients who underwent R/S SPECT without RSP (48.7 ± 13.8 mSv in advanced RSP sites and 57.8 ± 12.1 mSv in basic RSP sites) and the lowest in patients who underwent SO-SSD SPECT (6.5 ± 6.2 mSv), followed by SO-SPECT+APPS (9.3 ± 0.7 mSv), both only available at advanced sites (Supplemental Table S2).
Table 2:
Basic baseline characteristics and injected radiopharmaceutical dose grouped by radiation-saving practices and level of RSP capabilities
| Sites with advanced RSP | Sites with basic RSP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No RSP (R/S SPECT) |
R/S SSD SPECT | R/S SPECT +APPS | SO- SPECT | SO- SSD SPECT | SO- SPECT + APPS | R/S PET | Tl-based SPECT | p-value | No RSP (R/S SPECT) |
SO- SPECT | Tl-based SPECT | p-value | |
| n | 3144 | 953 | 1033 | 1197 | 1079 | 560 | 6884 | 130 | 2509 | 392 | 95 | ||
| E (± SD) | 14.5 ± 3.8 | 7.6 ± 3.7 | 11.9 ± 3.1 | 8 ± 2.9 | 1.9 ± 1.8 | 2.8 ± 0.2 | 3.5 ± 1.4 | 22.3 ± 8.7 | <0.0001 | 17.1 ± 3.6 | 6.6 ± 2.7 | 24.6 ± 9.2 | <0.001 |
| Age (± SD) | 61.2 ± 11.4 | 62.8 ± 10.8 | 62.6 ± 11 | 57.8 ± 10.7 | 60.1 ± 11.5 | 60.7 ± 10.9 | 67.2 ± 12.2 | 60.3 ± 13.2 | <0.0001 | 63.9 ± 12 | 63.2 ± 13 | 63.8 ± 12.8 | 0.56 |
| BMI (± SD) | 36.8 ± 8.3 | 33.2 ± 7.1 | 33.1 ± 6 | 35.9 ± 8.3 | 30.8 ± 6.5 | 28.4 ± 4.5 | 31.5 ± 6.1 | 29.7 ± 5.2 | <0.0001 | 33.6 ± 7.4 | 31.2 ± 6.6 | 27.6 ± 4.3 | < 0.001 |
| Women (%) | 1654 (52.6%) | 420 (44.1%) | 460 (44.5%) | 670 (56%) | 595 (55.1%) | 301 (53.8%) | 3802 (55.2%) | 68 (52.3%) | <0.0001 | 1332 (53.1) | 220 (56.1) | 42 (44.2) | 0.11 |
| DM (%) | 1276 (40.6%) | 327 (34.3%) | 334 (32.3%) | 337 (28.2%) | 253 (23.4%) | 89 (15.9%) | 2430 (35.3%) | 32 (24.6%) | <0.0001 | - | - | - | - |
| Prior revascul arization (%) | 1010 (32.1%) | 346 (36.3%) | 389 (37.7%) | 86 (7.2%) | 66 (6.1%) | 35 (6.3%) | 2683 (39%) | 41 (31.5%) | <0.0001 | - | - | - | - |
| Known CAD (%) | 1475 (46.9%) | 515 (54%) | 599 (58%) | 315 (26.3%) | 343 (31.8%) | 223 (39.8%) | 3881 (56.4%) | 57 (43.8%) | <0.0001 | - | - | - | - |
| LBBB (%) | 100 (3.2%) | 35 (3.7%) | 35 (3.4%) | 13 (1.1%) | 15 (1.4%) | 6 (1.1%) | 414 (6%) | 3 (2.3%) | <0.0001 | - | - | - | - |
| PAD (%) | 346 (11%) | 88 (9.2%) | 96 (9.3%) | 44 (3.7%) | 25 (4.5%) | 67 (6.2%) | 1158 (16.8%) | 21 (16.2%) | <0.0001 | - | - | - | - |
| COPD (%) | 352 (11.2%) | 112 (11.8%) | 91 (8.8%) | 72 (6%) | 30 (5.4%) | 73 (6.8%) | 965 (14%) | 13 (10%) | <0.0001 | - | - | - | - |
| Stroke (%) | 260 (8.3%) | 62 (6.5%) | 70 (6.8%) | 46 (3.8%) | 28 (5%) | 52 (4.8%) | 809 (11.8%) | 6 (4.6%) | <0.0001 | - | - | - | - |
P value for across-modality comparison within groups
Continuous variables presented as mean ± standard deviation; categorical variables as percentages.
APPS: advanced post-processing software; BMI: body mass index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; E: effective dose; LBBB: left bundle branch block; PAD: peripheral arterial disease; R/S: rest/ stress; SD: standard deviation; SO: stress-only; SSD: solid state detector
In a model adjusted for the covariates above, mean reduction in E relative to R/S SPECT without use of RSP, was greatest with SO-SPECT on SSD camera (mean reduction of 11.5 mSv, 95%CI [11.3 −11.7], p<0.001), followed by SO-SPECT on conventional camera with APPS (mean reduction= 10.1 mSv, 95%CI [9.8 −10.4], p<0.001); PET MPI (mean reduction= 9.7 mSv, 95%CI [9.6 −9.9], p<0.001); elimination of Tl-based protocols (mean reduction= 9.1 mSv, 95%CI [8.5 −9.7], p<0.001); R/S SPECT on SSD camera (mean reduction= 6.4, 95%CI [6.2 −6.7], p<0.001); SO-SPECT with conventional cameras (mean reduction= 6 mSv, 95%CI [5.7 −6.2], p<0.001), and least with R/S SPECT +APPS (mean reduction= 1.9 mSv, 95%CI [1.6 −2.1], p<0.001) (Figure 2).
Discussion
In this study, we examined the association between the implementation of RSPs and radiation exposure at a group of nuclear laboratories within a single health care system. Our data show that a significantly lower E can be achieved at sites incorporating advanced RSP options into workflow, relative to other sites where RSP implementation was more basic. Furthermore, the current detailed comparison among RSPs provides knowledge about the relative efficacy of various dose-reducing strategies. Use of SO protocols in conjunction with innovative SPECT technologies (including SSD cameras or APPS) resulted in the most marked reductions in E, followed by PET MPI and avoidance of Tl-based protocols, whereas use of SO protocol with conventional cameras without APPS, or use of R/S protocol with SSD cameras were associated with more moderate, but still significant, degrees of E reduction.
In the current era, nuclear laboratories face the challenge of accommodating patients with varying degrees of complexity and cardiovascular risk, and hence there has been growing emphasis on providing a patient-centered approach to imaging.26 As a result, a nuclear laboratory of the modern time needs to be equipped with a wide range of imaging capabilities that can be tailored to meet patients’ needs. Our data provide a frame of reference for physicians ordering MPI to help inform selection of a modality that offers an optimal risk/benefit balance for any given patient. Furthermore, determining the relative efficacy of different RSP’s helps guide laboratories exploring the addition of RSP into their workflow. The current findings should be considered in the context of a guide approximating expected improvements in radiation exposure if a given RSP is implemented—after establishing technical, financial and logistical feasibility of incorporating that intervention—rather than an endorsement of one intervention over others.
This study also demonstrates that SO protocols can have variable impact on radiation dose, depending on the camera system used (Figure 3). For example, combining SO protocol with conventional small field-of-view SPECT cameras equipped with APPS or with SSD cameras allows the use of low or ultra-low 99mTc doses, and hence achieving the largest reduction in E. On the other hand, when SO is used in conjunction with conventional large field-of-view SPECT cameras without APPS, larger 99mTc doses are needed to achieve diagnostic-level studies, and the observed reduction in E would be therefore more modest. Understanding the strengths and limitations of each of the camera systems is critical when balancing the benefits of dose-reducing interventions with the potential risks of adversely affecting diagnostic quality of radionuclide perfusion imaging studies.
Figure 3:
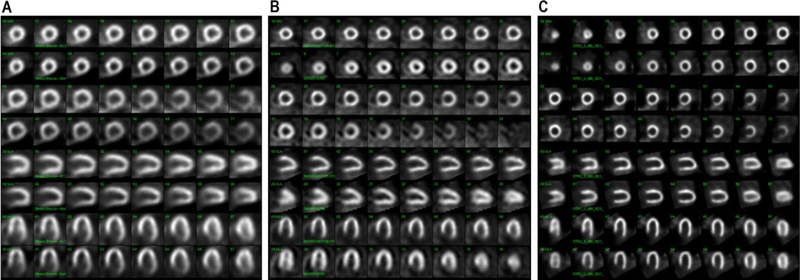
Examples of stress-only application with various SPECT imaging systems.
This figure shows different applications of stress-only (SO) protocols with SPECT MPI and effective radiation dose associated with each of these studies. Panel (A) shows a normal exercise SO-SPECT study performed on a large field-of-view Anger camera with (upper row) and without attenuation correction (lower row) after injection of 29.5 mCi of 99mTc (effective dose 8.7 mSv). In panel (B), a normal exercise SO-SPECT performed on a small field-of-view Anger camera with image processing done using Astonish 128®, an advanced post processing software that involves iterative reconstruction and depth-dependent resolution recovery (upper row). The same study was also processed using traditional filtered back projection (lower row) with clear difference in the quality of the images. This patient was injected with 9.6 mCi of 99mTc (effective dose 2.8 mSv). Panel (C) shows a normal exercise SO-SPECT performed on an SSD SPECT camera in the supine (upper row) and upright positions (lower row) after injection of 5.5 mCi (effective dose 1.6 mSv).
Reducing effective dose in association with RSP adoption also has significant public safety implications, considering the number of MPI studies performed annually.1 Adopting a simple RSP such as eliminating thallium would translate into substantial population-level radiation savings and, in turn, possibly into a potentially significant number of prevented cancers,27 underscoring the relevance of lowering radiation burden with MPI. Such benefits could be potentially amplified if multiple RSP’s are used synergistically within a single testing site.
In a survey of nuclear laboratories worldwide, adherence to laboratory ‘best practices’, including some of the practices considered in the RSP definition used in our study, was associated with reduction in laboratory-level radiation dose.13 The current findings extend those by the INCAPS investigators by providing a more exhaustive evaluation of the efficacy of other radiation- saving interventions—including PET and use of SSD cameras—and by quantifying the benefits of RSP adoption on radiation exposure.
Study limitations
The use of E as a marker of per-study radiation exposure, while it was originally described to assess population effective dose, may not be perfectly accurate, and this issue has been described before.2 Secondly, this study reflects the experience of a single center committed to improving RSP adoption over time. It is conceivable that other sites with access to the same capabilities may have different utilization rates of individual RSP compared to our center. As a result, such sites may achieve a different overall reduction in E, however, efficacy of individual interventions should remain similar, provided patients’ characteristics are similar to those included in the current study. Lastly, the current study design does not allow the examination of other recommended practices to reduce radiation dose, such as screening for the appropriateness of ordered studies or avoiding imaging altogether.
Conclusion
Adoption of advanced radiation-sparing practices is associated with marked reduction in MPI radiation burden, and implementation of such practices significantly increases the likelihood of achieving recommended radiation targets, whereas adoption of more basic radiation-saving interventions results in more modest radiation reduction. The efficacy of interventions across the radiation-sparing spectrum varied significantly, with use of stress-only protocols in conjunction with solid state detector SPECT cameras or conventional SPECT cameras equipped with advanced post-processing software, use of PET MPI and elimination of thallium-based protocols being the practices associated with the greatest reductions in radiation effective dose. Realizing such differences among radiation-saving interventions can allow nuclear laboratories to design radiation-reduction strategies that help them meet radiation targets.
Supplementary Material
Acknowledgments
Disclosures:
Drs. Patel and Al Badarin are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Spertus receives research grant support from Abbott Vascular, Novartis and is the PI of an analytic center for the American College of Cardiology. He serves as a consultant to United Healthcare, Bayer, Janssen, AstraZeneca and Novartis. He has an equity interest in Health Outcomes Sciences.
Dr. Bateman receives research grant support from Astellas and GE Healthcare. He serves as a consultant to GE Healthcare. He has ownership interest in Cardiovascular Imaging Technologies. He has intellectual property rights for Imagen Pro/MD/Q/3D software.
The other authors report no conflicts.
Abbreviations:
- CAD
Coronary artery disease
- E
Effective dose
- MPI
Myocardial perfusion imaging
- PET
Positron emission tomography
- RSP
radiation-sparing practices
- SO
Stress-only
- SPECT
Single photon emission computed tomography
- SSD
solid-state detector
Footnotes
New knowledge gained:
The current data describe improvement in radiation dose following sequential implementation of radiation-saving practices within a large health care system and demonstrate that the most effective interventions were use of stress-only protocols in conjunction with solid-state detector cameras or advanced post-processing-equipped conventional SPECT cameras, use of positron emission tomography and elimination of Tl-based protocols.
References
- 1.Chen J, Einstein AJ, Fazel R, Krumholz HM, Wang Y, Ross JS, Ting HH, Shah ND, Nasir K and Nallamothu BK. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population-based analysis. Journal of the American College of Cardiology 2010;56:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD and Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290–305. [DOI] [PubMed] [Google Scholar]
- 3.Einstein AJ, Weiner SD, Bernheim A, Kulon M, Bokhari S, Johnson LL, Moses JW and Balter S. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA 2010;304:2137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerqueira MD, Allman KC, Ficaro EP, Hansen CL, Nichols KJ, Thompson RC, Van Decker WA and Yakovlevitch M. Recommendations for reducing radiation exposure in myocardial perfusion imaging. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2010;17:709–18. [DOI] [PubMed] [Google Scholar]
- 5.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI and Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016;23:606–39. [DOI] [PubMed] [Google Scholar]
- 6.Einstein AJ, Berman DS, Min JK, Hendel RC, Gerber TC, Carr JJ, Cerqueira MD, Cullom SJ, DeKemp R, Dickert NW, Dorbala S, Fazel R, Garcia EV, Gibbons RJ, Halliburton SS, Hausleiter J, Heller GV, Jerome S, Lesser JR, Raff GL, Tilkemeier P, Williams KA and Shaw LJ. Patient-centered imaging: shared decision making for cardiac imaging procedures with exposure to ionizing radiation. J Am Coll Cardiol 2014;63:1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorbala S, Blankstein R, Skali H, Park MA, Fantony J, Mauceri C, Semer J, Moore SC and Di Carli MF. Approaches to reducing radiation dose from radionuclide myocardial perfusion imaging. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2015;56:592–9. [DOI] [PubMed] [Google Scholar]
- 8.Bateman TM, Heller GV, McGhie AI, Courter SA, Golub RA, Case JA and Cullom SJ. Multicenter investigation comparing a highly efficient half-time stress-only attenuation correction approach against standard rest-stress Tc-99m SPECT imaging. J Nucl Cardiol 2009;16:726–35. [DOI] [PubMed] [Google Scholar]
- 9.Gambhir SS, Berman DS, Ziffer J, Nagler M, Sandler M, Patton J, Hutton B, Sharir T, Haim SB and Haim SB. A novel high-sensitivity rapid-acquisition single-photon cardiac imaging camera. J Nucl Med 2009;50:635–43. [DOI] [PubMed] [Google Scholar]
- 10.Lyon MC, Foster C, Ding X, Dorbala S, Spence D, Bhattacharya M, Vija AH, DiCarli MF and Moore SC. Dose reduction in half-time myocardial perfusion SPECT-CT with multifocal collimation. J Nucl Cardiol 2016;23:657–67. [DOI] [PubMed] [Google Scholar]
- 11.Mercuri M, Pascual TN, Mahmarian JJ, Shaw LJ, Dondi M, Paez D, Einstein AJ and Group II. Estimating the Reduction in the Radiation Burden From Nuclear Cardiology Through Use of Stress- Only Imaging in the United States and Worldwide. JAMA Intern Med 2016;176:269–73. [DOI] [PubMed] [Google Scholar]
- 12.Tout D, Davidson G, Hurley C, Bartley M, Arumugam P and Bradley A. Comparison of occupational radiation exposure from myocardial perfusion imaging with Rb-82 PET and Tc-99m SPECT. Nuclear medicine communications 2014;35:1032–7. [DOI] [PubMed] [Google Scholar]
- 13.Einstein AJ, Pascual TN, Mercuri M, Karthikeyan G, Vitola JV, Mahmarian JJ, Better N, Bouyoucef SE, Hee-Seung Bom H, Lele V, Magboo VP, Alexanderson E, Allam AH, Al-Mallah MH, Flotats A, Jerome S, Kaufmann PA, Luxenburg O, Shaw LJ, Underwood SR, Rehani MM, Kashyap R, Paez D, Dondi M and Group II. Current worldwide nuclear cardiology practices and radiation exposure: results from the 65 country IAEA Nuclear Cardiology Protocols Cross-Sectional Study (INCAPS). Eur Heart J 2015;36:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerome SD, Tilkemeier PL, Farrell MB and Shaw LJ. Nationwide Laboratory Adherence to Myocardial Perfusion Imaging Radiation Dose Reduction Practices: A Report From the Intersocietal Accreditation Commission Data Repository. JACC Cardiovasc Imaging 2015;8:1170–6. [DOI] [PubMed] [Google Scholar]
- 15.Lindner O, Pascual TN, Mercuri M, Acampa W, Burchert W, Flotats A, Kaufmann PA, Kitsiou A, Knuuti J, Underwood SR, Vitola JV, Mahmarian JJ, Karthikeyan G, Better N, Rehani MM, Kashyap R, Dondi M, Paez D and Einstein AJ. Nuclear cardiology practice and associated radiation doses in Europe: results of the IAEA Nuclear Cardiology Protocols Study (INCAPS) for the 27 European countries. Eur J Nucl Med Mol Imaging 2016;43:718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka R, Kubo N, Miyazaki Y, Kawahara M, Takaesu J and Fukuchi K. Current status of stress myocardial perfusion imaging pharmaceuticals and radiation exposure in Japan: Results from a nationwide survey. J Nucl Cardiol 2017;24:1850–1855. [DOI] [PubMed] [Google Scholar]
- 17.Mercuri M, Pascual TN, Mahmarian JJ, Shaw LJ, Rehani MM, Paez D, Einstein AJ and Group II. Comparison of Radiation Doses and Best-Practice Use for Myocardial Perfusion Imaging in US and Non-US Laboratories: Findings From the IAEA (International Atomic Energy Agency) Nuclear Cardiology Protocols Study. JAMA Intern Med 2016;176:266–9. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RC, O’Keefe JH, McGhie AI, Bybee KA, Thompson EC and Bateman TM. Reduction of SPECT MPI Radiation Dose Using Contemporary Protocols and Technology. JACC Cardiovasc Imaging 2017. [DOI] [PubMed]
- 19.Songy B, Guernou M, Hivoux D, Attias D, Lussato D, Queneau M, Bonardel G and Bertaux M. Prognostic value of one millisievert exercise myocardial perfusion imaging in patients without known coronary artery disease. J Nucl Cardiol 2018;25:120–130. [DOI] [PubMed] [Google Scholar]
- 20.Dorbala S, Di Carli MF, Delbeke D, Abbara S, DePuey EG, Dilsizian V, Forrester J, Janowitz W, Kaufmann PA, Mahmarian J, Moore SC, Stabin MG and Shreve P. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med 2013;54:1485–507. [DOI] [PubMed] [Google Scholar]
- 21.Venero CV, Heller GV, Bateman TM, McGhie AI, Ahlberg AW, Katten D, Courter SA, Golub RJ, Case JA and Cullom SJ. A multicenter evaluation of a new post-processing method with depth- dependent collimator resolution applied to full-time and half-time acquisitions without and with simultaneously acquired attenuation correction. J Nucl Cardiol 2009;16:714–25. [DOI] [PubMed] [Google Scholar]
- 22.Gowd BM, Heller GV and Parker MW. Stress-only SPECT myocardial perfusion imaging: a review. J Nucl Cardiol 2014;21:1200–12. [DOI] [PubMed] [Google Scholar]
- 23.1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP 1991;21:1–201. [PubMed] [Google Scholar]
- 24.Conversion coefficients for use in radiological protection against external radiation. Adopted by the ICRP and ICRU in September 1995. Ann ICRP 1996;26:1–205. [PubMed] [Google Scholar]
- 25.Case JA, deKemp RA, Slomka PJ, Smith MF, Heller GV and Cerqueira MD. Status of cardiovascular PET radiation exposure and strategies for reduction: An Information Statement from the Cardiovascular PET Task Force. J Nucl Cardiol 2017;24:1427–1439. [DOI] [PubMed] [Google Scholar]
- 26.Depuey EG, Mahmarian JJ, Miller TD, Einstein AJ, Hansen CL, Holly TA, Miller EJ, Polk DM and Samuel Wann L. Patient-centered imaging. J Nucl Cardiol 2012;19:185–215. [DOI] [PubMed] [Google Scholar]
- 27.https://www.fda.gov/radiationemittingproducts/radiationemittingproductsandprocedures/medicalimaging/medicalxrays/ucm115329.htm. US Food and Drug Administration. What are the radiation risks from CT?
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.