Abstract
Background
The epidemiology of hospital adverse reactions, particularly allergic reactions, or hypersensitivity reactions (HSRs), is poorly defined. To determine priorities for allergy safety in healthcare, we identified and described safety reports of allergic reactions.
Methods
We searched the safety report database of a large academic medical center (AMC) from April 2006 through March 2016 using 101 complete, truncated, and/or misspelled keywords related to allergic symptoms, treatments, and culprits (e.g., medications, foods). Patient and event data were summarized for adverse reactions (AR) and two types of ARs, HSRs and side effects/toxicities.
Results
Among 9,111 keyword search-identified events, 876 (10%) were ARs, of which 436 (5%) were HSRs and the remaining 440 (5%) were side effects, reactions or toxicities. While the most common HSRs were simple cutaneous reactions (83%), severe immediate HSRs were also identified: Shortness of breath (16%), anaphylaxis (14%), and angioedema (12%). Most HSRs were caused by drugs (81%), with antibiotics (26%), particularly beta-lactams (11%) and vancomycin (8%), commonly implicated. Other causes of drug HSRs included contrast agents (24%), chemotherapeutics (7%), and opioids (6%). Non-drug HSRs were from blood products (8%), latex (3%), and devices (3%). Food reactions were rarely identified (1%).
Conclusions
We identified ARs, HSRs and side effects/toxicities, contained in a decade of safety reports at an AMC. Allergy safety in the healthcare setting should target approaches to common and severe reactions, with a focus on the safe administration of beta-lactams, vancomycin, contrast agents, chemotherapeutics, and opioids. Priority non-drug HSR culprits include blood products, latex, and devices.
INTRODUCTION
Prevention of adverse reactions (ARs) to medications and other products is important to patient safety.1 In the inpatient setting, reactions are most often due to drugs (i.e., adverse drug reactions, ADRs). ADRs are generally reported by over one-third of patients2 and comprise 11% of malpractice cases in the United States.3 While patients both with and without prior allergies may suffer an AR as a complication of their treatment, hospitalized patients and medically complex patients often have multiple drug allergies and are at particularly high risk for ordering errors and ARs.4–6
Allergic reactions are hypersensitivity reactions (HSRs), immunologically-mediated reactions to specific drug or non-drug culprits. HSRs can occur in the healthcare setting from drugs as well as foods, cleaning solutions, blood products, and/or medical devices. Drug HSRs were previously estimated to occur in approximately 2 per 1,000 hospitalizations.7 Food allergies, documented in the electronic health records (EHRs) of 4% of patients,8 may be an emerging HSR culprit in the healthcare setting. Protecting food-allergic inpatients requires that allergy information is conveyed to nutrition services in addition to the pharmacy where drug allergies are communicated.9,10
Prior epidemiological studies of hospital ARs focused on drug reactions/ADRs, identifying reactions through voluntary reporting,11 stimulated reporting followed by chart review,4 ADR surveillance systems,12 exclusive chart review,13 keyword searches,14 and billing codes.15,16 While studies have previously classified hospital ADRs as Type A (dose-related) and Type B (non-dose related), few studies have estimated the burden of hospital drug-induced HSRs, which are a subset of the Type B ADRs. To date, therefore, little is known about healthcare system HSRs, specifically those that result in safety event reports. The objective of this study was to identify and describe HSRs in a decade of voluntary safety reporting to enhance hospitals’ and healthcare systems’ ability to create informed policy guidance for allergy safety.
METHODS
Study Design and Data Source
We searched safety reports from Massachusetts General Hospital (MGH), a large AMC in Boston, in rL Solutions (rL, Cambridge, MA) from April 2006 through March 2016. At MGH, any employee with a valid EHR login can file safety reports, and there are approximately 20,000 safety reports filed annually. MGH safety events are classified into 22 event types in rL Solutions. While initial software versions relied heavily on free-text event descriptions entered by the reporter, a 2014 upgrade included more coded fields, including inpatient/outpatient, injury yes/no, patient affected details (e.g., name, medical record number, sex, date of birth, location), and general event details (e.g., event date, event time, location of event, reporter role). ADR event types encode fields for medications administered during the event (which may include anti-allergy medications such as antihistamines), event severity, and contributing factors (which may specify that a patient’s allergy was “unknown” or “not noted”). For other event types, and events prior to September 2014, allergy data reside exclusively in the free text field, “brief factual description.”
Safety Report Identification
To identify allergy-related safety events, we applied keyword search on free-text fields for all event types and searched coded fields for ADR event types. Keywords included 101 allergist and pharmacist-devised complete, truncated, and/or misspelled terms related to allergic reactions, culprit agents, and anti-allergy treatment (Supplemental Table 1). If a case matched one or more keywords, at least one co-investigator with advanced training in adverse and allergic reactions (KGB, ARW, YL) reviewed the event file to determine if the event was an AR or HSR. ARs were defined as any undesired responses to either a drug or non-drug culprit. HSRs were defined as ARs with allergic signs or symptoms that may have an immunologically-mediated mechanism to a drug or non-drug culprit. Immunologically-mediated mechanisms that were considered included all HSR types defined by Gell and Coombs classifications,17 but most commonly were a type I (or immunoglobulin-E)-mediated HSR, with symptoms such as itching, hives, swelling, wheezing, and a type IV HSR, which includes morbilliform drug eruptions, immunologic organ reactions (e.g., acute interstitial nephritis), and severe cutaneous adverse reactions (e.g., Stevens-Johnson syndrome). Non-IgE-mediated reactions, direct mast cell reactions clinically indistinguishable from Type I HSRs, were also considered HSRs. AR and HSR events were included in subsequent descriptive analyses while events not describing an AR or HSR were excluded from further review. Any unclear case was discussed by at least two reviewers until a consensus was reached.
Data Extraction and Analysis
Event-specific data were extracted from rL Solutions and included event severity and reporter department. Event severity was determined at the time of safety report triage by five masters/doctorate-prepared nurses at the Edward P. Lawrence Center for Quality and Safety who serve as staff specialists using a standardized scale modified from the Medical Expense Reimbursement Plan (MERP).18 Patient-specific data (e.g., patient demographics, history of adverse or allergy reactions documented in the EHR, comorbidities, primary diagnoses, level of care, hospitalization length of stay [LOS]) were extracted from “Data 4 Quality” (D4Q) repository, an internally managed structured query language (SQL) database. Comorbidities and primary admission diagnosis were defined by ICD9/10 codes matching the medical encounter dates, with diagnosis categorization.19 LOS was calculated as the integer difference between the discharge date and admission date.
The offending drugs were classified into a hierarchy of drug categories generally following commercial knowledge bases (e.g., First Databank) including parent categories (e.g., antibiotics) and intermediate categories (e.g., penicillins); non-drug culprits were classified based on type of culprit agent. We determined the route that triggered the reaction and documented reaction symptoms and findings (e.g., itching, flushing, rash, shortness of breath); reactions were grouped into reaction categories considering organ of involvement and mechanism (i.e., HSR or side effect/toxicity). We defined severe immediate HSRs as those with respiratory symptoms, cardiac symptoms, angioedema, or anaphylaxis.
We report descriptive characteristics, such as numbers with frequencies for binary variables and medians with interquartile ranges for continuous data. We compared binary frequencies with Fisher’s exact test and continuous variables with Wilcoxan rank sum test, with a two-sided p<0.05 considered statistically significant.
RESULTS
Number of Adverse Reactions and Hypersensitivity Reactions Identified
Of 9,111 reports identified, 876 (10%) were ARs. ARs were HSRs (n=436, 50%) and side effect reactions or toxicities (n=440, 50%, Figure 1). ARs were from drugs (i.e., ADRs) in 686 (78%) cases. Non-drug culprits (n=190), included foods in 7 (<1.0%) cases. ARs and HSRs detected remained generally consistent over the study period, from 62–103 and 30–57 events per year, respectively (Figure 2).
Figure 1. Events and reactions identified through voluntary safety reporting.
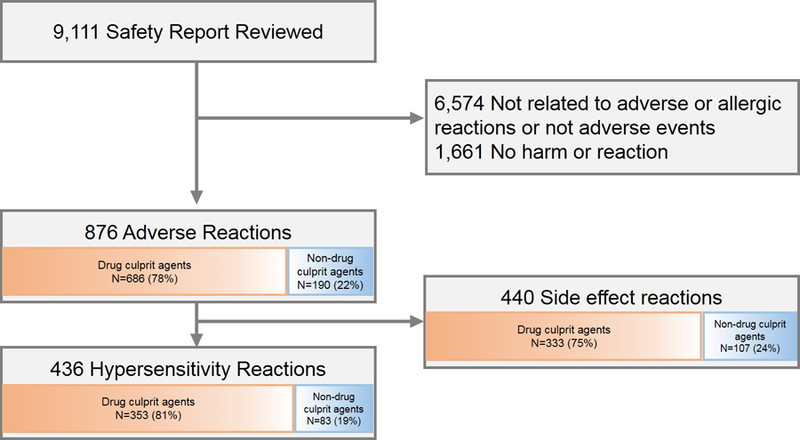
Of 9,111 reviewed event reports, 876 (10%) represented adverse reactions. Adverse reactions were either hypersensitivity reactions (n=436, 50%) or side effect/toxicity reactions (n=440, 50%).
Figure 2. Safety reports indicating adverse reactions and hypersensitivity reactions identified per year.
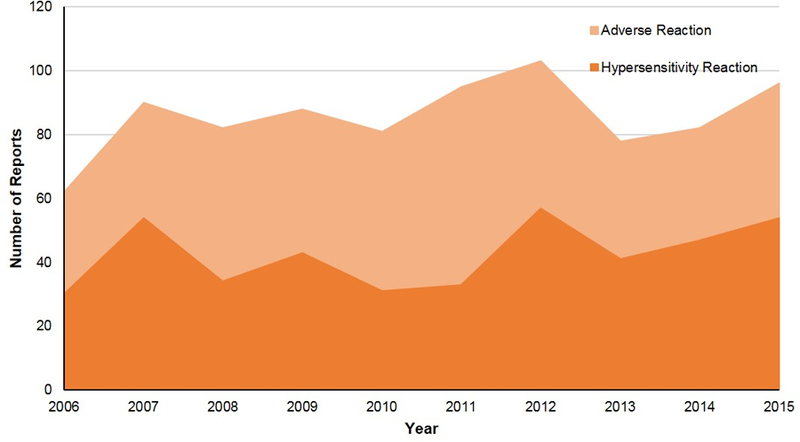
Adverse reactions and hypersensitivity reactions identified from allergy safety report searching remained consistent over the study period (2006 through 2015); for adverse reactions from 62–103 events per year, and for hypersensitivity reactions from 30–57 events per year.
Event Details
Most ARs (78%) were near misses (Table 1). HSRs resulted in lower patient harm than side effect/toxicity reactions, including lower serious injury or high risk for serious injury events (2% vs 5%), lower minor or temporary harm (10% vs 18%), and higher near misses (83% vs 72%, p<0.001).
Table 1.
Safety report details of patients who experienced adverse reactions, including both hypersensitivity reactions and side effect reactions
| HSR reports (n=436) |
Side effect reports (n=440) |
Total AR Reports (n=876) |
p-value* | |
|---|---|---|---|---|
| Event severity † | <0.001 | |||
| Near misses | 363 (83) | 316 (72) | 679 (78) | |
| Minor or temporary harm | 45 (10) | 78 (18) | 123 (14) | |
| Serious injury or high risk for serious injury | 8 (2) | 23 (5) | 31 (4) | |
| Reporter | ||||
| Nurses | 89 (20) | 151 (34) | 240 (27) | <0.001 |
| Pharmacists | 149 (34) | 91 (21) | 240 (27) | <0.001 |
| Radiology provider | 77 (18) | 29 (7) | 106 (12) | <0.001 |
| Medicine provider | 20 (5) | 51 (12) | 71 (8) | <0.001 |
| Surgery provider | 18 (4) | 21 (5) | 39 (5) | 0.74 |
| Anesthesia provider | 16 (4) | 26 (6) | 42 (5) | 0.15 |
| Emergency service provider | 13 (3) | 16 (4) | 29 (3) | 0.71 |
| Other‡ | 54 (12) | 55 (13) | 109 (12) | >0.99 |
Data presented as number (%)
Fisher’s exact test.
43 AR reports had event severity missing
Other includes pediatric services, blood transfusion services, IV therapy, neurology, nutrition and food services, obstetrics/gynecology, oncology, orthopedic surgery, pathology, physical/occupational therapy, psychiatry, respiratory care services, transplant services, and urology
Overall, nurses (27%) and pharmacists (27%) were the most common groups voluntarily reporting ARs. Providers in radiology (12%), medicine (8%), and surgery (5%) also filed AR reports. Providers in many other departments, including orthopedics, pathology, physical/occupational therapy, psychiatry, respiratory care, transplant, and urology, rarely filed AR, HSR, or side effect reports.
Side effect events were more commonly filed than HSRs by nurses (34% vs 20%, p<0.001) and by medicine providers (12% vs 5%, p<0.001). HSRs were more commonly filed than side effect events by pharmacists (34% vs 21%, p<0.001) and radiology providers (18% vs 7%, p<0.001).
Patient Details
Patients who experienced ARs had a median age of 56 years [IQR 35 years, 70 years], and 52% of them were female (Table 2). Nine reactions occurred in pediatric patients. Most patients (76%) were white. HSR patients, compared to side effect/toxicity patients, were more frequently female (55% vs 48%, p=0.07) and younger (median age 52 years vs 59 years, p<0.001). Overall, 294 AR patients (34%) had a prior adverse or allergic reaction documented in the EHR. More HSR patients than side effect patients had a prior allergy listed in the EHR (39% vs 28%, p<0.001).
Table 2.
Demographic characteristics of patients who experienced adverse and hypersensitivity reactions
| HSR patients (n=436) |
Side effect patients (n=440) |
Total AR patients (n=876) |
p-value* | |
|---|---|---|---|---|
| Female | 238 (55) | 213 (48) | 451 (52) | 0.07 |
| Age (years), Med [IQR] | 52 [31, 67] | 59 [40, 74] | 56 [35, 70] | <0.001 |
| Race | 0.52 | |||
| White | 328 (75) | 337 (77) | 665 (76) | |
| Black | 22 (5) | 28 (6) | 50 (6) | |
| Asian | 22 (5) | 13 (3) | 35 (4) | |
| Hispanic | 4 (<1) | 4 (<1) | 8 (<1) | |
| American Indian | 2 (<1) | 0 (0) | 2 (<1) | |
| Other | 31 (7) | 30 (7) | 61 (7) | |
| Declined or unknown | 27 (6) | 28 (6) | 55 (6) | |
| Prior ADR | 171 (39) | 123 (28) | 294 (34) | <0.001 |
| Patient comorbidities | ||||
| Malignancy | 114 (26) | 113 (26) | 227 (26) | 0.88 |
| Atopic diseases | 95 (22) | 81 (18) | 176 (20) | 0.24 |
| Diabetes | 73 (17) | 97 (22) | 170 (19) | 0.05 |
| Renal failure | 71 (16) | 86 (20) | 157 (18) | 0.22 |
| Primary diagnosis † | ||||
| Circulatory system diseases | 63 (15) | 102 (23) | 165 (19) | 0.001 |
| Neoplasms | 61 (14) | 41 (9) | 102 (12) | 0.04 |
| Injury and poisoning | 42 (10) | 38 (9) | 80 (9) | 0.64 |
| Gastrointestinal diseases | 36 (8) | 36 (8) | 72 (8) | >0.99 |
| Respiratory diseases | 30 (7) | 35 (8) | 65 (7) | 0.61 |
| Highest level of care | ||||
| Inpatient | 250 (57) | 223 (51) | 473 (54) | 0.05 |
| Intensive care unit | 112 (26) | 152 (35) | 264 (30) | 0.005 |
| Cardiac care unit | 17 (4) | 26 (6) | 43 (5) | 0.21 |
| Emergency department | 36 (8) | 8 (2) | 44 (5) | <0.001 |
| Outpatient | 10 (2) | 9 (2) | 19 (2) | 0.82 |
| Neurologic intensive care unit | 3 (<1) | 13 (3) | 16 (2) | 0.02 |
| Unknown | 8 (2) | 9 (2) | 17 (2) | >0.99 |
| Length of stay (days), Med [IQR] ‡ | 6 [3, 14] | 11 [5, 23] | 9 [4, 19] | <0.001 |
AR patients commonly carried a diagnosis of malignancy (26%), atopic diseases (20%), diabetes (19%), and renal failure (18%). Side effect patients more frequently had diabetes (22% vs 17%, p=0.05) than HSR patients. The most common primary diagnoses of the patients who experienced ARs were circulatory system diseases (19%), neoplasms (12%), injury/poisoning (9%), gastrointestinal diseases (8%), and respiratory diseases (7%). HSR patients more frequently had neoplasms as the primary diagnosis compared to side effect patients (14% vs 9%, p=0.04).
ARs, HSRs, and side effect reactions were all most commonly seen in patients on general inpatient units (54%, 57%, 51% respectively). The intensive care unit (ICU) was also a common setting with 30% of ARs seen in ICUs. Of the ARs seen in ICUs, side effect reactions were more common than HSRs (35% vs 26%, p=0.005). Of inpatients, length of stay was a median of 9 days for ARs; LOS was longer for side effect/toxicity reactions than HSR reactions (median 11 days vs 6 days, p<0.001).
HSR Reactions, Culprit Agents, and Route
For all ARs, the most common reactions reported included itching (14%), shortness of breath (13%), rash (13%), and anaphylaxis (12%, Table 3). HSRs were primarily cutaneous (83%). The most common reactions were itching/pruritus (27%), rash (25%), hives (20%), shortness of breath (16%), anaphylaxis (14%), and angioedema (12%). Common side effect reactions included hematologic side effects (19%), altered mental status (14%), hypotension (11%), shortness of breath (10%), and gastrointestinal symptoms (8%).
Table 3.
Most commonly reported adverse reactions overall and grouped into hypersensitivity reactions and side effect reactions
| Adverse reactions (n=876) | |
|---|---|
| Itching | 119 (14) |
| Shortness of breath* | 111 (13) |
| Rash† | 111 (13) |
| Anaphylaxis | 109 (12) |
| Hives | 90 (10) |
| Blood-related reactions | 85 (10) |
| Altered mental status | 70 (8) |
| Angioedema‡ | 66 (8) |
| Tachycardia or bradycardia | 64 (7) |
| Erythema or flushing | 62 (7) |
|
Hypersensitivity reactions (n=436) | |
| Itching | 116 (27) |
| Rash† | 109 (25) |
| Hives | 89 (20) |
| Shortness of breath* | 69 (16) |
| Anaphylaxis | 60 (14) |
| Angioedema‡ | 54 (12) |
| Erythema or flushing | 47 (11) |
| Tachycardia or bradycardia | 29 (7) |
| Gastrointestinal symptoms§ | 18 (4) |
| Fever or chills | 11 (3) |
|
Side effects reactions (n=440) | |
| Hematologic side effect reactions | 84 (19) |
| Altered mental status | 61 (14) |
| Hypotension | 49 (11) |
| Shortness of breath* | 42 (10) |
| Gastrointestinal symptoms§ | 36 (8) |
| Tachycardia or bradycardia | 35 (8) |
| Electrolyte abnormality | 30 (7) |
| Fever or chills | 29 (7) |
| Hypertension | 28 (6) |
| Skin break | 28 (6) |
Data presented as number (%). Note that percentages do not add up to 100% as patients could have had more than one reaction.
Includes bronchospasm, wheezing, and chest tightness
Type of rash not further specified
Includes swelling and throat tightness
Includes abdominal pain, gastrointestinal upset, nausea, vomiting, and/or diarrhea
Drug HSR culprits (81%) included antibiotics (n=115, 26%), often beta-lactams (11%) and vancomycin (8%, Table 4). Contrast agents (n=106, 24%), chemotherapeutics (n=29, 7%), and opioids (n=25, 6%) were also identified as HSR causes. Antibiotics (26% vs 6%, p<0.001), contrast agents (24% vs 5%, p<0.001), and chemotherapeutics (7% vs 2%, p=0.002) more frequently caused HSRs than side effect reactions. Opioids (15% vs 6%, p<0.001), anticoagulants (17% vs 1%, p=<0.001), cardiovascular agents (7% vs 3%, p=0.002), and insulin (3% vs. 0%, p<0.001) more commonly caused side effect reactions than HSRs.
Table 4.
Adverse and hypersensitivity reactions identified through voluntary reporting classified by culprit agents and route
| HSR reports (n=436) |
Side effect reaction reports (n=440) |
Total AR reports (n=876) |
p-value* | |
|---|---|---|---|---|
| Drug Culprit Agents | 352 (81) | 332 (75) | 684 (78) | |
| Antibiotics | 115 (26) | 25 (6) | 140 (16) | <0.001 |
| Penicillins | 16 (4) | 5 (1) | 21 (2) | 0.02 |
| Cephalosporins | 30 (7) | 3 (<1) | 33 (4) | <0.001 |
| Vancomycin | 36 (8) | 11 (3) | 47 (5) | <0.001 |
| Fluoroquinolones | 12 (3) | 2 (0.5) | 14 (2) | 0.007 |
| Sulfonamides | 8 (2) | 3 (0.7) | 11 (1) | 0.14 |
| Macrolides | 1 (<1) | 1 (0.2) | 2 (<1) | >0.99 |
| Other antibiotics | 21 (5) | 6 (1) | 27 (3) | 0.003 |
| Contrast agents | 106 (24) | 20 (5) | 126 (14) | <0.001 |
| Opioids | 25 (6) | 64 (15) | 89 (10) | <0.001 |
| Anticoagulants | 5 (1) | 75 (17) | 80 (9) | <0.001 |
| Cardiovascular agents † | 11 (3) | 31 (7) | 42 (5) | 0.002 |
| Chemotherapeutics ‡ | 29 (7) | 10 (2) | 39 (5) | 0.002 |
| General anesthetics | 12 (3) | 20 (5) | 32 (4) | 0.21 |
| Vasopressors § | 2 (<1) | 15 (3) | 17 (2) | 0.002 |
| Insulins | 0 (0) | 13 (3) | 13 (2) | <0.001 |
| Antipsychotics | 5 (1) | 8 (2) | 13 (2) | 0.58 |
| Immunosuppressants | 8 (2) | 4 (<1) | 12 (1) | 0.26 |
| Anxiolytics | 2 (<1) | 9 (2) | 11 (1) | 0.06 |
| Local anesthetics | 3 (<1) | 7 (2) | 10 (1) | 0.34 |
| Anticonvulsants | 1 (<1) | 9 (2) | 10 (1) | 0.02 |
| Antidotes or reversal agents ǁ | 2 (<1) | 7 (2) | 9 (1) | 0.18 |
| Hormones | 4 (1) | 4 (1) | 8 (1) | 0.99 |
| Antiemetics | 3 (<1) | 4 (1) | 7 (1) | >0.99 |
| Electrolyte supplements | 2 (<1) | 4 (1) | 6 (1) | 0.69 |
| Topical agents | 5 (1) | 1 (<1) | 6 (1) | 0.12 |
| Diuretics | 2 (<1) | 4 (1) | 6 (1) | 0.69 |
| Respiratory agents | 1 (<1) | 4 (1) | 5 (1) | 0.37 |
| Acetaminophen | 3 (1) | 2 (<1) | 5 (1) | 0.69 |
| Aspirin | 1 (<1) | 3 (1) | 4 (<1) | 0.62 |
| Non-steroidal anti-inflammatory drugs | 1 (<1) | 3 (1) | 4 (<1) | 0.62 |
| Vaccines | 1 (<1) | 3 (1) | 4 (<1) | 0.62 |
| Antihistamines | 0 (0) | 3 (1) | 3 (<1) | 0.25 |
| Antidepressants | 2 (<1) | 1 (<1) | 3 (<1) | 0.62 |
| Radioactive isotopes | 2 (<1) | 1 (<1) | 3 (<1) | 0.62 |
| Vitamins | 3 (1) | 0 (0) | 3 (<1) | 0.12 |
| Antivirals | 0 (0) | 2 (<1) | 2 (<1) | 0.50 |
| Corticosteroids | 0 (0) | 2 (<1) | 2 (<1) | 0.50 |
| Other anti-inflammatory drugs | 1 (<1) | 1 (<1) | 2 (<1) | 0.99 |
| Gastrointestinal agents | 2 (<1) | 0 (0) | 2 (<1) | 0.25 |
| Other drug agents ¶ | 8 (2) | 10 (2) | 18 (2) | 0.81 |
| Non-Drug Culprit Agents | 84 (19) | 108 (25) | 192 (22) | |
| Blood products | 35 (8) | 29 (7) | 64 (7) | 0.44 |
| Adhesives | 8 (2) | 20 (5) | 28 (3) | 0.03 |
| Devices | 11 (3) | 15 (3) | 26 (3) | 0.55 |
| Tubing, needles, or catheters | 2 (<1) | 17 (4) | 19 (2) | <0.001 |
| Fluid and electrolyte solutions | 4 (1) | 11 (3) | 15 (2) | 0.12 |
| Latex | 12 (3) | 3 (1) | 15 (2) | 0.02 |
| Other non-drug agents** | 4 (1) | 7 (2) | 11 (1) | 0.55 |
| Nutritional supplements | 3 (1) | 6 (1) | 9 (1) | 0.51 |
| Food | 5 (1) | 2 (<1) | 7 (1) | 0.29 |
| Route †† | ||||
| Intravenous | 326 (75) | 247 (56) | 573 (65) | <0.001 |
| Oral/nasogastric | 43 (10) | 58 (13) | 101 (12) | 0.14 |
| Intradermal | 1 (<1) | 28 (6) | 29 (3) | <0.001 |
| Epidural | 3 (1) | 5 (1) | 8 (1) | 0.73 |
| Intramuscular | 2 (<1) | 5 (1) | 7 (1) | 0.45 |
| Transdermal | 0 (0) | 3 (1) | 3 (<1) | 0.25 |
| Other | 5 (1) | 6 (1) | 11 (1) | >0.99 |
| Unknown | 4 (1) | 6 (1) | 10 (1) | 0.75 |
| Non-medication | 52 (12) | 82 (19) | 134 (15) | 0.006 |
Fisher’s exact test.
Includes adenosine, amiodarone, captopril, digoxin, diltiazem, doxazosin, hydralazine, labetalol, lisinopril, metoprolol, milrinone, nicardipine, nifedipine, nitroglycerin, verapamil, and valsartan. Three events were from an ACE-inhibitor.
Includes anastrazole, carboplatin, cetuximab, doxorubicin, erlotinib, etoposide, ifosfamide, mitomycin, ofatumumab, oxaliplatin, paclitaxel, panitumumab, rituximab, taxotere.
Includes dopamine, epinephrine, phenylephrine, norepinephrine, neosynephrine, vasopressin.
Includes acetylcysteine, aminocaproic acid, deferasirox.
Includes acarbose, anti-thymocyte globulin, carbidopa-levodopa, citrate, dexmedetomidine, dextran, edrophonium, EMD experimental medication, glyburide, mivacurium, pramipexole, probenecid, sildenafil, simvastatin, sitagliptin, and tizanidine.
Includes burn from electrophysiology lab, dressing materials, support bandage, support stockings, oxygen equipment, tourniquet, adhesive remover, warming system materials, and chlorine bleach.
Excludes food ARs, all of which were oral/nasogastric.
Non-drug HSRs (n=84, 19%) included HSRs from blood products (n=35, 8%), latex (n=12, 3%), devices (n=11, 3%), and adhesive (n=8, 2%). Latex more frequently caused HSRs than side effect reactions (3% vs <1%, p=0.02); adhesive more frequently caused side effect reactions than HSRs (5% vs 2%, p=0.03).
There were seven food reactions identified, including 5 HSRs (71%); with culprit foods including pine nuts, unspecified nuts, wheat, mushroom, and artificial sweetener. Two HSRs occurred in patients without previously documented food allergies: One HSR was due to food brought into the hospital by a patient’s family member, and one food reaction was due to an incorrect diet order.
Routes by which culprit agents resulted in ARs included intravenous (65% for ARs, 75% for HSRs, and 56% for side effect reactions) and oral/nasogastric (12% for ARs, 10% for HSRs, and 13% for side effect reactions).
Reactions commonly occurred to contrast agents, blood products, vancomycin, chemotherapeutics, cephalosporins, opioids, penicillins, cardiovascular agents, and adhesives (Figure 3). HSRs from contrast agents included itching (n=45), erythema (N=7), and rash (n=7); severe immediate HSR symptoms to contrast media (n=47) included respiratory symptoms (n=29), cardiac symptoms (n=3), angioedema (n=13), and anaphylaxis (n=3). Other culprit agents for severe immediate HSRs (n=174) included blood products (n=26), chemotherapeutics (n=16), opioids (n=14), cephalosporins (n=13), cardiovascular agents (n=10), vancomycin (n=7), penicillins (n=3), and adhesives (n=2).
Figure 3. Common agents for hypersensitivity reactions captures by voluntary reporting.
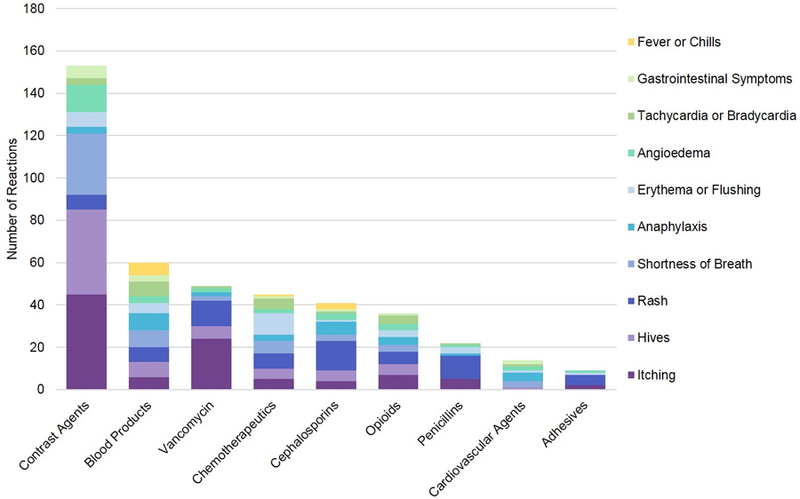
This figure displays the most common causative agents for hypersensitivity reactions identified by safety reports, and the indicated reactions. Cardiac symptoms included tachycardia, tachypnea, palpitations, bradycardia, cardiac arrest, reduced cardiac function, chest pain, chest tightness or pressure, and abnormal electrocardiogram. Mental status changes include agitation, anxiety, hallucination, behavioral and movement change, fatigue, psychosis, sedation, somnolence, unconsciousness, confusion, delirium, depression. Gastrointestinal symptoms included nausea, vomiting, diarrhea, stomach distress, and small bowel obstruction. Respiratory symptoms included airway compromise, shortness of breath, wheezing, bronchospasm, decreased oxygen saturation, dyspnea, acute respiratory failure, stridor, lung crackles, pulmonary edema, cyanosis, sneezing, coughing, and congestion.
Of 60 anaphylaxis reports, culprit agents were blood products (n=8), cephalosporins (n=6), opiates (n=4), cardiovascular agents (n=4), contrast agents (n=3), chemotherapeutics (n=3), and vancomycin (n=2). Of 54 angioedema reports, culprits were contrast agents (n=13), cephalosporins (n=3), blood products (n=3), opiates (n=3), vancomycin (n=2), and chemotherapeutics (n=2). Seven severe cutaneous adverse reactions were identified from antibiotics (penicillin, cephalosporin, ciprofloxacin, sulfamethoxazole/trimethoprim), a cardiovascular agent (diltiazem), an immunosuppressive agent (leflunomide), and a blood product (fresh frozen plasma). There were no hemolytic blood reactions identified in the sample.
DISCUSSION
By searching a decade of voluntary reporting safety data from a large AMC, we identified a variety of allergy-related incidents, including 436 HSRs. Patients with HSRs were more commonly female, younger, and had a prior documented allergy of some type compared to patients with side effect or toxicity reactions. Most hypersensitivity reactions (81%) were from drug culprits, including antibiotics (26%), often beta-lactams (11%) and vancomycin (8%), contrast agents (24%), chemotherapeutics (7%), and opioids (6%). Non-drug HSRs (19%) included reactions to blood products (8%), latex (3%) and devices (3%). Allergy safety event epidemiology can facilitate targeted programs for safe allergy practices in healthcare.
Approximately one in five documented ADRs is an HSR.20,21 While we found that approximately half of ARs were HSRs, this increased HSR proportion was by design, given our aim to identify HSRs using keywords that targeted allergic-type reactions and treatments. Evidence-based methodologies to prevent ADRs include choosing the lowest effective dose, prescribing oral over parenteral medications, and performing drug-specific monitoring.22,23 EHRs enforce this safe prescribing and monitoring with computerized physician order entry and clinical decision support.4,24 HSR prevention often includes similar principles,25,26 but also requires assessing prior allergies before ordering medications, foods, or other treatments, and ensuring adherence to drug administration protocols, including appropriate premedications, such as those required for chemotherapeutics, or for patients with prior contrast reactions to receive radiocontrast media.27,28
In our study, HSRs were less morbid and resource-intensive than side effects and toxicities, resulting in 3% less serious injury or high risk for serious injury events and 7% less minor or temporary harm. Further, hospitalized patients with HSRs required a lower level of inpatient care and had a five-day shorter median LOS than those with side effect reactions. Although most true immunologic HSRs are not preventable (i.e., they are idiosyncratic),25,26 rapid recognition and treatment of these reactions can lead to decreased morbidity and resource utilization. Most HSRs have been shown to be simple, cutaneous reactions,29,30 and even severe immediate HSRs are often reversible with appropriate medical treatments (e.g., epinephrine for anaphylaxis).31 However, prior studies support that anaphylaxis is poorly recognized and undertreated in healthcare settings (i.e., not treated with epinephrine, despite a clear indication), suggesting that additional attention to devising and implementing HSR/anaphylactic protocols should still be prioritized. 32,33
Compared to patients who experienced HSRs, those who experienced side effects more commonly had a diagnosis of circulatory disease, which is consistent with cardiovascular medications tending to cause more side effect reactions than HSRs. The most common side effect reactions identified in this study were hematologic side effects, altered mental status, and hypotension, and the most common culprits for these types of reactions were anticoagulants, opioids, and blood products; other drugs such as insulin and non-drug culprits such as tubing, needles, and catheters were more likely to cause side effect reactions than HSRs.
Similar to prior safety report data, we found that registered nurses were the most common healthcare team members filing safety reports, possibly due to their role in medication administration and their familiarity with safety reporting systems.34–36 While prior studies have described pharmacists as reporting 5–8% of ADEs,37 we found pharmacists to be more frequent AR/HSR reporters, consistent with drugs being the most common culprits and pharmacists’ crucial role in medication safety and allergy checking.38 Finally, we found that locations with frequent use of highly allergenic agents (e.g., radiology and contrast agents, medicine and antibiotics) commonly reported HSR events. Although general anesthetics were attributed to anaphylaxis events in our sample, and anesthesia is a setting with exposure to antibiotics, latex, and other allergenic drugs and non-drugs, Anesthesia as a department was an uncommon area from which AR safety reports were generated.
Antibiotics, the most common culprit drug class for HSRs, comprised over one quarter of the HSRs captured. This is largely consistent with findings from other sites and employing different methods: Spontaneous reporting found that antibiotics were the second most common class of drugs to cause ADRs (17%), and active surveillance found that antibiotics were the most common culprit (37%).39 Beta-lactams and vancomycin were particularly significant. Although most general prescribers are not comfortable prescribing beta-lactams to patients with prior penicillin allergy,40,41 10–15% of patients report a penicillin allergy.2 With guideline-based approaches proven useful, safe, and effective,42 hospitals should standardize antibiotic management for patients with beta-lactam allergy histories. Vancomycin can cause rate-dependent infusion reactions (“red man syndrome”), non-IgE-mediated anaphylaxis, and delayed cutaneous eruptions ranging from mild to severe.43,44 This study identified that vancomycin administration warrants increased attention to recommended infusion practices (e.g., rates <16.7 mg/min) and evidence-based approaches to reduce symptoms in patients with prior vancomycin infusion reactions.26,45
While one prior study found contrast agents responsible for 1 in 10 reactions,39 we found that they comprised almost one quarter of HSRs. Hospitals often have standardized and effective (<5% have breakthrough reactions) contrast allergy premedication protocols,46–49 but few have standardized responses to acute contrast reactions. Pilot educational programs using simulation and algorithms have been used in radiology settings,50 but given the frequency and severity of these reactions, standardized, algorithmic, hospital-wide management approaches should be adopted.
While opioids represented just 6% of HSRs in this study, prior studies found that opioids were more common ADR culprits: 30% with a spontaneous reporting, 21% with an active surveillance, and 7% using claims data (ICD and E-Codes).16,39 In addition to causing deleterious side effects (e.g., hypotension, respiratory depression), opioids frequently cause itching, hives, and rash through direct mast cell activation, a non-immunologic reaction that can be confused for an immunologically-mediated HSR.26,51 Although antihistamines can minimize these symptoms,26 to date, opioid HSR guidelines or algorithms have not been broadly implemented in the healthcare setting. Effective protocols might advise when patients may safely continue opioids despite symptoms and detail premedications that may be used (e.g., nonsedating antihistamines such as fexofenadine).26
HSRs from blood products, latex, devices, and adhesives cumulatively caused 15% of ARs, 12% of HSRs, and 19% of side effect reactions. While hospitals have clear guidance for suspected blood transfusion reactions,52 the policies that exist for latex exposure often contain outdated information from the latex allergy epidemic of the 1990s.53 With the prevalence of latex allergy decreasing, many patients who are labeled latex-allergic are actually latex tolerant.54,55 Revised latex policies should include a protocol for withdrawing medications or vaccines through latex stoppers (e.g., the “one-stick” method); the evaluation for latex allergy in patients who experience anaphylaxis without a clear, discernable trigger (e.g., perioperative anaphylaxis); and follow up care for patients labeled as latex-allergic to receive confirmatory testing.26
While these data come from a large AMC over a 10-year time frame, important study limitations exist. The data come from a voluntary (spontaneous) reporting database, and prior studies suggest that only 1 in 10 ADRs are spontaneously reported.56,57 More events would have been identified with active reporting methods with chart reviews,58,59 computer-triggered monitoring,60 or active surveillance programs.39 Because the voluntary reporting database did not have an established method for identification of allergy-related safety reports, we used a novel, yet untested, group of keywords to identify allergy events. While we could not determine the sensitivity of this method, specificity was adequate for ARs (approximately 10% yield), but almost infeasible for HSRs (approximately 5% yield). Improved methods for identifying allergy events are needed to facilitate regular identification and tracking of HSRs with the goal of promoting allergy safety. Event review was retrospective, therefore subject to potential misclassification or misattribution of causative agents; however, reviewers were experienced, and prior data demonstrate that most reported cases have at least probable causality between reaction and culprit agent.39 Finally, we report on one AMC in the greater Boston area; among other resources, MGH has specialty access to Allergy/Immunology, hospital protocols for many common HSR drug culprits, and a unified approach to anaphylaxis.42,61 Therefore, we may describe events with different outcomes than may be expected in other environments with different patients, resources, or programs.
We identified allergy-related safety reports at an AMC over the last decade; most reactions were from antibiotics, contrast agents, chemotherapeutics, and opioids. Although Allergy/Immunology specialists are trained to identify and treat allergic reactions, access to allergy specialists is limited, even at US AMCs. As such, hospitals have increasingly relied on adoption of protocols for allergy mitigation and treatment. These data suggest that healthcare systems should develop and implement standardized approaches to drug HSRs from beta-lactams, vancomycin, contrast agents, chemotherapeutics, and opioids. Non-drug policies, particularly for blood products, latex, and devices, should also be considered. Finally, a uniform approach to severe immediate reactions, including anaphylaxis is needed.
Supplementary Material
Acknowledgments
The authors thank Xiaoqing Fu, MS and Tyler Harkness for their manuscript preparation assistance.
Funding
This work was supported by NIH K01AI125631, the American Academy of Allergy Asthma and Immunology Foundation, and the MGH Claflin Distinguished Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Institute of Medicine. To Err is Human: Building a Safer Health System Washington, DC: National Academy Press; 2000. [Google Scholar]
- 2.Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy 2016;71(9):1305–13. [DOI] [PubMed] [Google Scholar]
- 3.CRICO. Medication-related malpractice risks. CRICO 2016 CBS Benchmarking Report Available at: https://www.rmf.harvard.edu/Malpractice-Data/Annual-Benchmark-Reports/Risks-in-Medication. Accessed July 10, 2018.
- 4.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274(1):29–34. [PubMed] [Google Scholar]
- 5.Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? Learning from patient-reported incidents. J Gen Intern Med 2005;20(9):830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal KG, Kuhlen JL, Weill AA, et al. Adverse Drug Reactions Associated with Ceftaroline Use: A 2-Center Retrospective Cohort. J Allergy Clin Immunol In Prac 2016;4(4):740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thong BY, Leong KP, Tang CY, et al. Drug allergy in a general hospital: Results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol 2003;90(3):342–7. [DOI] [PubMed] [Google Scholar]
- 8.Acker WW, Plasek JM, Blumenthal KG, et al. Prevalence of food allergies and intolerances documented in electronic health records. J Allergy Clin Immunol 2017; 140 (6):1587–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sergeant P, Kanny G, Morisset M, et al. Food safety of allergic patients in hospitals: implementation of a quality strategy to ensure correct management. Eur Ann Allergy Clin Immunol 2003;35(4):120–3. [PubMed] [Google Scholar]
- 10.Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014;134(5):1016–25 e43. [DOI] [PubMed] [Google Scholar]
- 11.Pathak AK, Kumar M, Dokania S, et al. A retrospective analysis of reporting of adverse drug reactions in a tertiary care teaching hospital: one year survey. J Clin Diagn Res 2016;10(8):FC01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budnitz DS, Pollock DA, Mendelsohn AB, et al. Emergency department visits for outpatient adverse drug events: demonstration for a national surveillance system. Ann Emerg Med 2005;45(2):197–206. [DOI] [PubMed] [Google Scholar]
- 13.Valente S, Murray LP. Creative strategies to improve patient safety: allergies and adverse drug reactions. J Nurses Staff Dev 2011;27(1):E1–5; quiz E6–7. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto T, Gandhi TK, Seger AC, et al. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care 2004;13(4):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Holman CD, Price SD, et al. Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: retrospective cohort study. BMJ 2009;338:a2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane-Gill SL, Van Den Bos J, Handler SM. Adverse drug reactions in hospital and ambulatory care settings identified using a large administrative database. Ann Pharmacother 2010;44(6):983–93. [DOI] [PubMed] [Google Scholar]
- 17.Gell PGH, Coombs RA. The classification of allergic reactions underlying disease First ed. Oxford, England: Blackwell; 1963. [Google Scholar]
- 18.“NCC MERP Index for Categorizing Medication Errors.” National Coordinating Council for Medication Error Reporting and Prevention 2001. [DOI] [PubMed]
- 19.Rassekh SR, Lorenzi M, Lee L, et al. Reclassification of ICD-9 codes into meaningful categories for oncology survivorship research. J Cancer Epidemiol 2010;2010:569517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenthal KG, Lai KH, Huang M, et al. Adverse and hypersensitivity reactions to prescription nonsteroidal anti-inflammatory agents in a large health care system. J Allergy Clin Immunol Pract 2017;5(3):737–43 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly WN. Potential risks and prevention, Part 1: Fatal adverse drug events. Am J Health Syst Pharm 2001;58(14):1317–24. [DOI] [PubMed] [Google Scholar]
- 22.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004;329(7456):15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SD Jr., Landry FJ. Recognizing, reporting, and reducing adverse drug reactions. South Med J 2001;94(4):370–3. [PubMed] [Google Scholar]
- 24.Fortescue EB, Kaushal R, Landrigan CP, et al. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics 2003;111(4 Pt 1):722–9. [DOI] [PubMed] [Google Scholar]
- 25.Solensky R Drug hypersensitivity. Med Clin North Am 2006;90(1):233–60. [DOI] [PubMed] [Google Scholar]
- 26.Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105(4):259–73. [DOI] [PubMed] [Google Scholar]
- 27.Kodzwa R Updates to the ACR Manual on Contrast Media. Radiol Technol 2017;89(2):186–9. [PubMed] [Google Scholar]
- 28.Neuss MN, Gilmore TR, Belderson KM, et al. 2016 Updated American Society of Clinical Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards, Including Standards for Pediatric Oncology. Oncol Nurs Forum 2017;44(1):31–43. [DOI] [PubMed] [Google Scholar]
- 29.Blumenthal KG, Li Y, Acker WW, et al. Multiple drug intolerance syndrome and multiple drug allergy syndrome: epidemiology and associations with anxiety and depression. Allergy 2018. 10.1111/all.13440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalabianloo F, Berstad A, Schjott J, et al. Clinical characteristics of patients with drug hypersensitivity in Norway: a single-centre study. Pharmacoepidemiol Drug Saf 2011;20(5):506–13. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Danoff TM, Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol 2014;133(4):1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerji A, Rudders S, Clark S, et al. Retrospective study of drug-induced anaphylaxis treated in the emergency department or hospital: patient characteristics, management, and 1-year follow-up. J Allergy Clin Immunol Pract 2014;2(1):46–51. [DOI] [PubMed] [Google Scholar]
- 33.Banerji A, Rudders SA, Corel B, et al. Repeat epinephrine treatments for food-related allergic reactions that present to the emergency department. Allergy Asthma Proc 2010;31(4):308–16. [DOI] [PubMed] [Google Scholar]
- 34.Taylor JA, Brownstein D, Christakis DA, et al. Use of incident reports by physicians and nurses to document medical errors in pediatric patients. Pediatrics 2004;114(3):729–35. [DOI] [PubMed] [Google Scholar]
- 35.Evans SM, Berry JG, Smith BJ, et al. Attitudes and barriers to incident reporting: a collaborativehospital study. Qual Saf Health Care 2006;15(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobo MG, Pinheiro SM, Castro JG, et al. Adverse drug reaction monitoring: support for pharmacovigilance at a tertiary care hospital in Northern Brazil. BMC Pharmacol Toxicol 2013;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawcutt DB, Mainie P, Riordan A, et al. Reported paediatric adverse drug reactions in the UK 2000–2009. Br J Clin Pharmacol 2012;73(3):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bondesson A, Eriksson T, Kragh A, et al. In-hospital medication reviews reduce unidentified drug-related problems. Eur J Clin Pharmacol 2013;69(3):647–55. [DOI] [PubMed] [Google Scholar]
- 39.Yun IS, Koo MJ, Park EH, et al. A comparison of active surveillance programs including a spontaneous reporting model for phamacovigilance of adverse drug events in a hospital. Korean J Intern Med 2012;27(4):443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puchner TC Jr., Zacharisen MC. A survey of antibiotic prescribing and knowledge of penicillin allergy. Ann Allergy Asthma Immunol 2002;88(1):24–9. [DOI] [PubMed] [Google Scholar]
- 41.Blumenthal KG, Shenoy ES, Hurwitz S, et al. Effect of a drug allergy educational program and antibiotic prescribing guideline on inpatient clinical providers’ antibiotic prescribing knowledge. J Allergy Clin Immunol Pract 2014;2(4):407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: assessing tools for antimicrobial stewardship. J Allergy Clin Immunol 2017;140(1):154–61 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers AL, Gaedigk A, Dai H, et al. Defining risk factors for red man syndrome in children and adults. Pediatr Infect Dis J 2012;31(5):464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivagnanam S, Deleu D. Red man syndrome. Crit Care 2003;7(2):119–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renz CL, Thurn JD, Finn HA, et al. Antihistamine prophylaxis permits rapid vancomycin infusion. Crit Care Med 1999;27(9):1732–7. [DOI] [PubMed] [Google Scholar]
- 46.Brockow K, Christiansen C, Kanny G, et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005;60(2):150–8. [DOI] [PubMed] [Google Scholar]
- 47.Thomsen HS. Guidelines for contrast media from the European Society of Urogenital Radiology. AJR Am J Roentgenol 2003;181(6):1463–71. [DOI] [PubMed] [Google Scholar]
- 48.Jingu A, Fukuda J, Taketomi-Takahashi A, et al. Breakthrough reactions of iodinated and gadolinium contrast media after oral steroid premedication protocol. BMC Med Imaging 2014;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mervak BM, Davenport MS, Ellis JH, et al. Rates of breakthrough reactions in inpatients at high risk receiving premedication before contrast-enhanced CT. AJR Am J Roentgenol 2015;205(1):77–84. [DOI] [PubMed] [Google Scholar]
- 50.Wang CL, Schopp JG, Petscavage JM, et al. Prospective randomized comparison of standard didactic lecture versus high-fidelity simulation for radiology resident contrast reaction management training. AJR Am J Roentgenol 2011;196(6):1288–95. [DOI] [PubMed] [Google Scholar]
- 51.Zhang B, Li Q, Shi C, Zhang X. Drug-induced pseudoallergy: a review of the causes and mechanisms. Pharmacology 2018;101(1–2):104–10. [DOI] [PubMed] [Google Scholar]
- 52.Tinegate H, Birchall J, Gray A, et al. Guideline on the investigation and management of acute transfusion reactions. Prepared by the BCSH Blood Transfusion Task Force. Br J Haematol 2012;159(2):143–53. [DOI] [PubMed] [Google Scholar]
- 53.Hunt LW, Fransway AF, Reed CE, et al. An epidemic of occupational allergy to latex involving health care workers. J Occup Environ Med 1995;37(10):1204–9. [DOI] [PubMed] [Google Scholar]
- 54.Kelly KJ, Sussman G. Latex allergy: Where are we now and how did we get there? J Allergy Clin Immunol Pract 2017;5(5):1212–6. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein DI. Management of natural rubber latex allergy. J Allergy Clin Immunol 2002;110(2 Suppl):S111–6. [DOI] [PubMed] [Google Scholar]
- 56.Classen DC, Resar R, Griffin F, et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood) 2011;30(4):581–9. [DOI] [PubMed] [Google Scholar]
- 57.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA 1998;280(15):1311–6. [DOI] [PubMed] [Google Scholar]
- 58.Miguel A, Bernardo M, Freitas A, et al. Detection of adverse drug reactions using hospital databases-a nationwide study in Portugal. Pharmacoepidemiol Drug Saf 2013;22(8):907–13. [DOI] [PubMed] [Google Scholar]
- 59.Durrieu G, Batz A, Rousseau V, et al. Use of administrative hospital database to identify adverse drug reactions in a Pediatric University Hospital. Eur J Clin Pharmacol 2014;70(12):1519–26. [DOI] [PubMed] [Google Scholar]
- 60.Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc 1998;5(3):305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blumenthal KG, Shenoy ES, Varughese CA, et al. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol 2015;115(4):294–300 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


