Abstract
Background:
Tamoxifen-inducible Cre/lox site-specific recombination technology has been widely used to generate conditional transgenic mice. As an estrogen receptor ligand, tamoxifen itself potentially affects energy metabolism, which may confound interpretation of data especially in metabolic studies. Considering sexual dimorphism, in this study, the effects of low-dose tamoxifen administration on energy metabolism, and browning of adipose tissues in female and male mice were investigated.
Methods:
Female and male C57/BL6 mice were injected with tamoxifen oil solution (i.p.) and then housed at both room temperature (23 ± 2 °C) and cold environment (6 ± 1 °C). Serum, brown and white adipose tissues were obtained, and the effects of tamoxifen administration on energy metabolism and the browning of adipose tissues were evaluated.
Results:
At 25 mg/kg body weight (BDW), tamoxifen administration for 3 alternative days decreased the percentage of inguinal and gonadal white adipose tissue weights in female mice accompanied by the up-regulation of thermogenesis in adipose tissues. In contrast, this dosage of tamoxifen did not induce noticeable changes in the energy metabolism and thermogenesis of adipose tissue in male mice under room temperature. Consistently, under cold stimulus, substantial browning of adipose tissues was observed in female mice injected with tamoxifen (50 mg/kg BDW, single injection) but not in male mice. Two-way ANOVA tests also demonstrated significant interactions between tamoxifen treatment and gender on the expression of thermogenic markers in adipose tissues.
Conclusion:
Tamoxifen, even at a low dose, remarkably increases thermogenesis in adipose tissues of female mice; meanwhile, such a low dose could be used in male mice for inducing gene recombination without confounding the interpretation of data related to metabolism and thermogenesis of adipose tissues.
Keywords: Tamoxifen, Adipose Tissue, Sex Dimorphism, Energy Metabolism, Mice
1. Introduction
Compared to germline gene knockout, the Cre/lox site-specific recombination system exhibits tremendous advantages due to its capability to control gene recombination temporally and spatially (1). In this system, a conditional knockout is achieved through the controlled expression of Cre recombinase. The most common approach is to fuse the Cre recombinase with a mutated ligand-binding domain of the estrogen receptor (ER) (2). These fusion proteins prevent the binding of estrogen under physiological conditions, but they are responsive to external estrogen receptor ligand, 4-hydroxy-tamoxifen, an active tamoxifen metabolite (3). The binding of 4-hydroxy-tamoxifen induces conformational changes of the Cre-ER fused proteins, which leads to their nuclear translocation to elicit gene recombination. While this system achieves great success in generating various transgenic mouse mutants, its function as an estrogen receptor ligand may generate side effects.
In addition to its expression in reproductive tissues, the estrogen receptor is also widely distributed in the brain and nonneural peripheral tissues such as adipose tissue (4). In the central nerve system, when estrogen binds to its receptor, the feeding behavior of rat is altered along with energy metabolism (5). Similarly, estradiol affects energy expenditure in adipose tissue, which includes suppressed lipoprotein lipase activity, reduced lipogenesis and promoted lipolysis and the release of free fatty acids (6, 7). While both two estrogen receptors, ERα and ERβ, regulate adiposity and fat distribution, ERα has the dominant effects (5). Tamoxifen could mimic the effects of estrogen, enhancing fatty acid utilization and reducing body weight gain (8). When used in mice for inducing Cre expression, these off-target effects of tamoxifen may confound data interpretation, especially in studies related to energy metabolism and adipose biology.
The dosage and duration of tamoxifen administration, for the purpose of Cre recombinase activation, varies in different studies. Typically, administration of tamoxifen with a daily dosage of 40–100 mg/kg body weight (BDW) for 5 consecutive days, is used to induce target gene knockout (2, 9–13). However, these tamoxifen dosages have been reported to be unnecessarily high for efficient gene knockout and can induce serious side-effects in a wide range of tissues (14–17). To optimize the dosage of tamoxifen for efficient gene recombination, several studies have been carried out aimed to decrease tamoxifen-dose requirements. In a bone study, a dosage of tamoxifen at 10 mg/kg BDW for 4 days yielded similar gene recombination efficiency with those at 100 mg/kg BDW for 4 days (14). In a heart study, a dosage of tamoxifen at 40 mg/kg BDW for 2 days successfully knocked-out over 94% target genes (15). In our previous study, a lower dosage of tamoxifen (25 mg/kg BDW, for 3 alternative days) reduced side effects and achieved sufficient knockout efficiency in adipose tissues (18). Although these low dosages are expected to reduce side-effects of tamoxifen (14, 19), tamoxifen’s effects on adipose tissues have not yet been directly examined. Furthermore, considering the sex differences of female and male mice in terms of adipose tissue deposition, hormonal receptor distribution, and neural responses to signals regulating adipose tissue metabolism (5, 20, 21), the responsiveness of adipose tissues to tamoxifen administration in both genders is expected to be different. The objective of this study was to explore whether tamoxifen, even at low doses, affects adipose tissue metabolism in both genders of mice.
2. Materials and methods
Animal and experimental design
Animal studies were performed according to the guidelines of the National Institutes of Health and approved by the Animal Use and Care Committee at Washington State University (Permit No. 04518). Wild-type (WT) C57BL/6 mice were initially purchased from the Jackson Laboratory (Bar Harbor, Maine) and then bred at Washington State University. Male and female mice at 3 to 4 months of age were randomly divided into 4 groups (6 mice for each group), respectively. Experiments were conducted under two conditions, room temperature (23 ± 2 °C) and cold environment (6 ± 1 °C). For experiments conducted under room temperature, one group of mice were intraperitoneally injected with 25 mg/kg BDW tamoxifen dissolved in corn oil for 3 alternative days, while the other group were treated with only corn oil. Mice were housed in a temperature-controlled environment (23 ± 2 °C, alternating 12-h light/dark cycle) with ad libitum access to food and water, and euthanized 3 days after the final tamoxifen injection. Blood samples, brown adipose tissue (BAT), inguinal white adipose tissue (ingWAT), and gonadal white adipose tissue (gonWAT) were rapidly collected. Fat tissues from one side were fixed in 4% paraformaldehyde for sectioning and staining, and tissues from the other side were rapidly frozen in liquid nitrogen and stored at −80°C until further analyses.
For cold stimulus, mice were injected with tamoxifen at 50 mg/kg BDW once and kept in a cold room for 4 days before they were euthanized. Here, a single tamoxifen injection at a higher dose was used in order to avoid repeated injections during the cold exposure, which would increase the stress levels of mice. Samples were collected as described above.
Body temperature
Rectal temperature of mice was measured before transferring into the cold room and recorded twice daily at 9:00 am and 5:00 pm in the following days using a TH-5 Thermalert Monitoring Thermometer (Physitemp Instruments, Inc., Clifton, NJ, USA).
Serum profile analysis
After the mice were euthanized, blood samples were collected and centrifuged for serum separation. The concentration of glucose was measured by a glucometer (Bayer Contour, Tarrytown, NY, USA). The concentration of insulin was determined by a Mouse Ultrasensitive insulin ELISA Kit (ALPCO Diagnostics, Salem, NH, USA). The content of free fatty acids was measured using an EnzyChrom™ Free Acid Assay Kit (BioAssay System, CA, USA).
Tissue processing and histochemical analyses
Paraffin-embedded adipose tissue sections (5-μm thick) were rehydrated through a series of incubation in xylene and ethanol solutions, and then used for H&E staining as previously described (22). Imaging was performed with an EVOS microscope (Advanced Microscopy Group, Bothell, WA, USA). Adipocyte diameters were measured using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Quantitative real-time PCR (qRT-PCR) analyses
As previously described (22), total RNA was isolated using TRIzol reagent (Sigma, Saint Louis, MO, USA), followed by reverse-transcription to cDNA using iScriptTM cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA). qRT-PCR was carried out by the CFX RT-PCR detection system (Bio-Rad). After normalization to 18s rRNA content, relative mRNA expression was determined using the method of 2-ΔΔCt (23). Table S1 shows the primer sequences.
Immunoblotting analyses
Immunoblotting analyses were performed as previously described (22) using the Odyssey Infrared Image System (LI-COR Biosciences, Lincoln, NE, USA). Band densities of target proteins were normalized to β-tubulin content. Antibodies against uncoupling protein 1 (UCP1, no. 14670) and cytochrome C (Cyt C, no. 4280) were purchased from Cell Signaling (Danver, MA, USA) and diluted 1: 1,000 for use. IRDye 800CW goat anti-rabbit and IRDye 680 goat anti-mouse secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE, USA), and diluted 1: 10,000 for use.
Statistical analyses
All data are expressed as mean ± SEM. Two-way ANOVA was performed to assess the interactions between tamoxifen administration and gender using the General Linear Model, followed by least significant difference (LSD) post hoc comparison to evaluate differences among treatments (SAS Institute Inc., Cary, NC, USA). A significant difference was considered as p<0.05.
Results
Tamoxifen administration affected body composition and metabolic parameters in a gender-specific manner
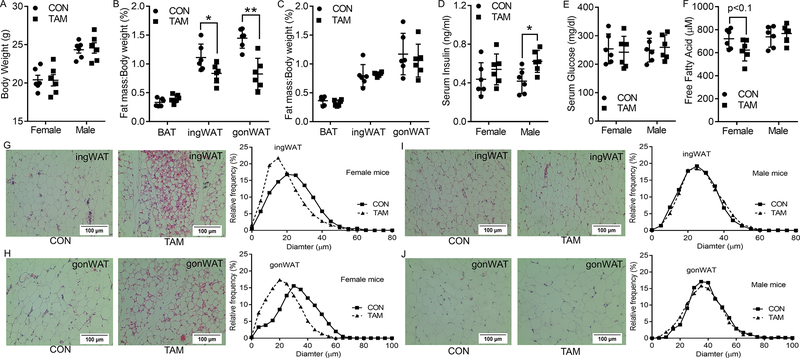
When mice were housed at room temperature and euthanized 3 days after the last injection of tamoxifen, no significant difference (p>0.05) was observed in the body weights of the control and tamoxifen-treated groups, for both genders (Fig. 1A). However, a total dosage of 75 mg/kg BDW tamoxifen administration used in this study altered the body fat composition of female mice, with a 24.3% reduction in ingWAT and 42.8% reduction in gonWAT, though no difference in BAT ratio was found (p=0.105, Fig. 1B). In contrast, the same dosage of tamoxifen administrated to male mice did not induce significant changes in their body fat composition including BAT, ingWAT and gonWAT (Fig. 1C). Two-way ANOVA tests also demonstrated significant interaction between tamoxifen treatment and gender on the ratio of ingWAT (p<0.05) and gonWAT (p<0.05) while a tendency of interaction on the ratio of BAT (p<0.1) was also detected.
Figure 1.
Effects of tamoxifen administration on body composition, serum metabolic parameters, and histological structure of fat tissues in both genders of mice under room temperature. A, body weight. B-C, fat index (ratio of fat tissues weight to the whole-body weight) of BAT, ingWAT and gonWAT of female (B) and male mice (C). D-F, serum insulin (D), glucose (E), and free fatty acid (F) levels. G-H, representative H&E staining and percentage distribution of adipocyte diameters for ingWAT (G) and gonWAT (H) sections of female mice. I-J, representative H&E staining and percentage distribution of adipocyte diameters for ingWAT (I) and gonWAT (J) sections of male mice. *p<0.05, ** p<0.01. Data are expressed as mean ± SEM (n=6).
Tamoxifen induced a 24.8% increase in the serum insulin level of female mice but did not reach statistical significance (Fig. 1D). In addition, no change in glucose levels were found in female mice after tamoxifen administration (Fig. 1E). In male mice, though tamoxifen injection increased serum insulin level (Fig. 1D), no change in glucose concentration was found (Fig. 1E). In addition, tamoxifen treatment showed a decreased tendency of serum free fatty acid level in female mice (p<0.1, Fig. 1F) while no change was observed in male mice (Fig. 1F). A tendency of interaction between tamoxifen administration and gender on the concentration of free fatty acids (p<0.1) was detected while no interactions on insulin and glucose levels were found.
Tamoxifen administration induced thermogenesis of adipose tissues in female mice but not in male mice at room temperature
To understand the sex-specific effects of tamoxifen on adipose tissue metabolism, histological structures and thermogenesis of BAT, ingWAT, and gonBAT were analyzed.
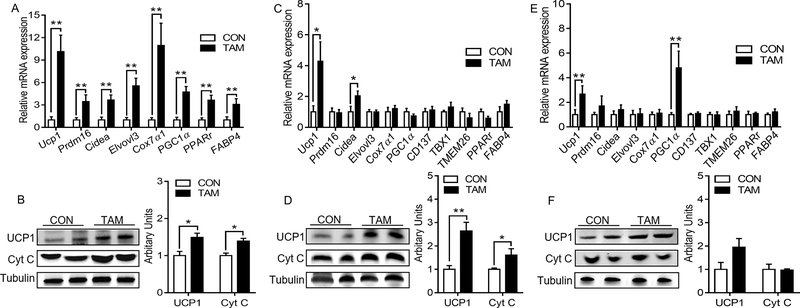
Compared with the control group, tamoxifen administration induced substantial changes in the histological structures and thermogenesis of adipose tissues in female mice at the room temperature. HE staining showed more multilocular and mitochondria-rich adipocytes in ingWAT (Fig. 1G) and gonWAT (Fig. 1H) of tamoxifen-treated female mice. There were also noticeable shifts of adipocyte diameters to smaller sizes for both ingWAT and gonWAT. Though the differences between the control and tamoxifen-treated groups in BAT structures were not ostensible (Fig. S1A), mRNA expression of thermogenic genes including uncoupling protein 1 (Ucp1), PR domain-containing 16 (Prdm16), cell death-inducing DFFA-like effector A (Cidea), elongation of very long-chain fatty acids protein 3 (Elvovl3), Cytochrome c oxidase subunit Vlla polypeptide 1 (Cox7α1) and peroxisomal proliferator-activated receptor γ coactivator-1 α (Pgc1α) were significantly up-regulated in the tamoxifen-treated female mice at room temperature (Fig. 2A). In addition, administration of tamoxifen also increased mRNA expression of peroxisomal proliferator-activated receptor γ (Pparγ) and fatty-acid binding protein 4 (Fabp4). Consistently, the tamoxifen group showed higher protein levels of cytochrome c (Cyt C) which is an essential component of the mitochondrial respiratory chain, and UCP1 which mediates adaptive non-shivering thermogenesis (Fig. 2B).
Figure 2.
Effects of tamoxifen administration on browning of adipose tissues in female mice under room temperature. A, C, E, mRNA expression of thermogenic and adipogenic genes in BAT (A), ingWAT (C), and gonWAT (E). B, D, F, representative images of immunoblotting and arbitrary units for UCP1 and Cytochrome C (Cyt C) in BAT (B), ingWAT (D), and gonWAT (F). *p<0.05, ** p<0.01. Data are expressed as mean ± SEM (n=6).
In ingWAT, the expression of Ucp1 and Cidea was increased in female mice housed at room temperature; no significant changes in other thermogenic genes and beige adipocyte-selective markers including cluster differentiation 137 (CD137), T-box1 (Tbx 1), and transmembrane protein 26 (Tmem26) were found (Fig. 2C). Also, no changes in Pparγ and Fabp4 expression were observed. At the protein level, UCP1 and Cyt C were up-regulated in the tamoxifen-treated females (Fig. 2D). Similarly, in gonWAT, Ucp1 and Pgc1α were up-regulated at the mRNA levels while no changes of other thermogenic genes and beige adipocyte-selective markers were observed (Fig. 2E). At the protein level, no changes were found for UCP1 and Cyt C expression (Fig. 2F).
In contrast, the same dose of tamoxifen administrated to male mice did not induce noticeable change in the histological structures of BAT (Fig. S2A), ingWAT (Fig. 1I), and gonWAT (Fig. 1J). The adipocyte diameter distribution was not altered in both ingWAT and gonWAT of male mice. In agreement, the expression of thermogenic markers was not altered in the adipose tissues of male mice (Fig. S2, B-G).
Two-way ANOVA analysis showed that there were functional interactions between tamoxifen administration and gender on the expression of Ucp1 (p<0.05), Prdm16 (p<0.05), Cidea (p<0.01), Elvovl3 (p<0.01), Cox7α1 (p<0.01), Pgc1α (p<0.05), Pparγ (p<0.05), Fabp4 (p<0.01) in BAT, Ucp1 (p<0.05) and Cidea (p<0.05) in ingWAT, and Ucp1 (p = 0.057) and Pgc1α (p<0.05) in gonWAT. At the protein level, significant interaction between tamoxifen treatment and gender was observed for the expression of UCP1 (p<0.05) in BAT, and expression of UCP1 (p<0.01) and Cyt C (p<0.05) in ingWAT. No significant interaction on other thermogenic markers was detected in BAT, ingWAT and gonWAT.
Tamoxifen administration affected adipose tissue mass, body temperature homeostasis and serum metabolic parameters under cold stimulus in a gender specific manner
Cold exposure enhances BAT activity and stimulates beige adipocyte activation in white adipose tissue (24–26). Following tamoxifen injection, mice were housed in a cold room and euthanized 4 days after. For female mice, the ratio of gonWAT decreased significantly after tamoxifen administration. The ratio of ingWAT showed a decreased tendency (p<0.1) in tamoxifen-treated group while no changes were noted in the ratio of BAT (Fig. 3A and 3B). However, cold stimulus did not change the body weight and fat mass ratios (BAT, ingWAT and gonWAT) of male mice (Fig. 3A and 3C). A tendency of interaction between treatment and gender on the ratio of gonWAT (p<0.1) was detected while no interactions (p>0.05) on the ratios of BAT and ingWAT were found.
Figure 3.
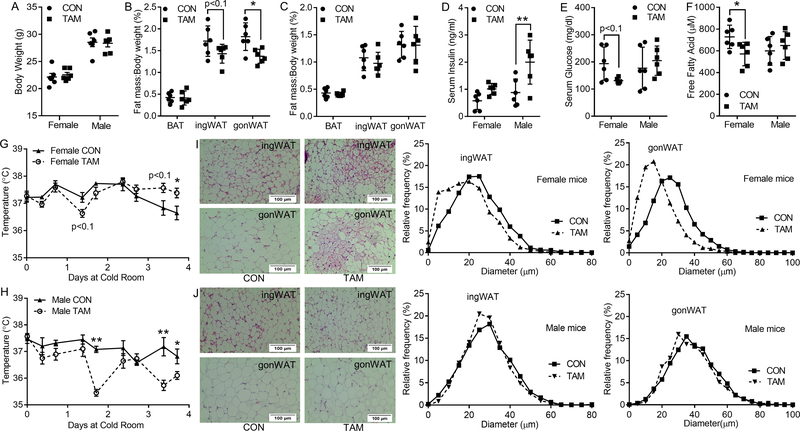
Effects of tamoxifen administration on body composition, serum metabolic parameters, body temperature, and histological structure of fat tissues in both genders of mice under cold environment. A, body weight. B-C, fat index (ratio of fat tissues weight to the whole-body weight) of BAT, ingWAT, and gonWAT of female (B) and male mice (C). D-F, serum insulin (D), glucose (E), and free fatty acid (F) levels. G-H, body temperature changes of male (G) and female (H) mice after exposed at cold environment. I-J, representative H&E staining and percentage distribution of adipocyte diameters for ingWAT and gonWAT sections of male (I) and female (J) mice. *p<0.05, ** p<0.01. Data are expressed as mean ± SEM (n=6).
Though tamoxifen induced only a 0.8-times increment of insulin level in female mice (p>0.05), it was accompanied by about 32.5% reduction in glucose level (p<0.1), suggesting enhanced utilization of glucose (Fig. 3D and 3E). Tamoxifen increased the insulin levels in male mice by nearly 1.3 times (p<0.01), with no change in the glucose levels (Fig. 3D and 3E). Besides, serum free fatty acids were decreased in tamoxifen-treated female mice while no changes were found in male mice (Fig. 3F). Two-way ANOVA tests demonstrated a significant interaction between tamoxifen treatment and gender on the serum concentration of free fatty acids (p<0.05), while there was a tendency of interaction on glucose concentration (p<0.1) and no interaction on insulin concentration.
Tamoxifen-treated female mice also showed a relatively lower body temperature during the first two days, although the body temperature increased and became significantly higher than control group at day 4 of cold exposure; this shows that tamoxifen induced thermogenesis in female mice (Fig. 4G). On the other hand, male mice treated with tamoxifen tended to have a lower temperature compared to the control group (Fig. 4H). Significant interactions between tamoxifen treatment and gender on the body temperature of mice at day 4 of cold exposure were observed by the two-way ANOVA analysis.
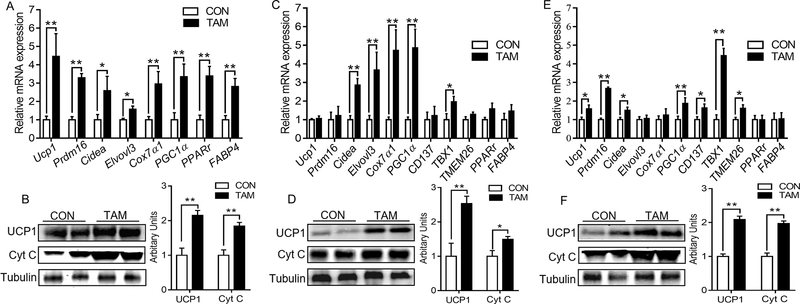
Figure 4.
Effects of tamoxifen administration on browning of adipose tissues in female mice under cold environment. A, C, E, mRNA expression of thermogenic and adipogenic genes in BAT (A), ingWAT (C), and gonWAT (E). B, D, F, Representative images of immunoblotting and arbitrary units for UCP1 and Cytochrome C (Cyt C) in BAT (B), ingWAT (D), and gonWAT (F). *p<0.05, **p<0.01. Data are expressed as mean ± SEM (n=6).
Tamoxifen administration induced browning of white adipose tissue in female mice under a cold stimulus
For females, cold stimulation increased thermogenesis in adipose tissues of tamoxifen-treated mice. Though no apparent differences were noted in the histological structures of BAT (Fig. S1B), mRNA expression of Ucp1, Prdm16, Cidea, Elvovl3, Cox7α1, Pgc1α, Pparγ and Fabp4 were increased (Fig. 4A). The UCP1 and Cyt C contents at the protein level were also up-regulated (Fig. 4B). For ingWAT and gonWAT, more multilocular and mitochondria-rich adipocytes were found after tamoxifen administration (Fig. 3I). Analysis on adipocyte diameter distributions showed more abundant small adipocytes in both ingWAT and gonWAT after tamoxifen treatment. Correspondingly, Cidea, Elvovl3, Cox7α1, Pgc1α, and Tbx1 mRNA expression were significantly increased in ingWAT of female mice treated with tamoxifen (Fig. 4C). At the protein level, UCP1 and Cyt C expression were also upregulated (Fig. 4D). For gonWAT, tamoxifen administration up-regulated the expression of Ucp1, Prdm16, Cidea, Pgc1α, CD137, Tbx1, and Tmem26 while no difference in Pparγ and Fabp4 was found (Fig. 4E). Meantime, UCP1 and Cyt C contents were increased at the protein level (Fig. 4F). These data showed that cold exposure amplified the browning of adipose tissue induced by tamoxifen in female mice.
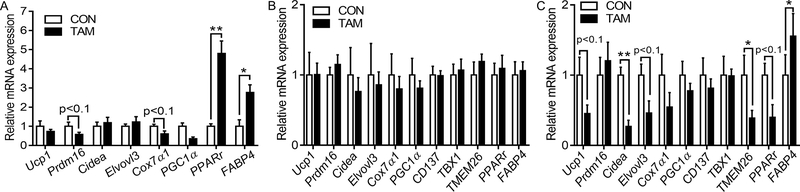
For male mice under cold exposure, tamoxifen did not induce noticeable changes in the histological structures of BAT (Fig. S3A). Surprisingly, at molecular levels, tamoxifen administration induced a tendency of down-regulation for Prdm16 and Cox7α1 (P<0.1, Fig. 5A). However, the expression of Pparγ and Fabp4 was up-regulated. No difference in Ucp1, Cidea, Elvol3 and Pgc1α mRNA expression was found for male mice, neither for UCP1 and Cyt C protein contents (Fig. S3B). In agreement, for ingWAT and gonWAT of male mice, no noticeable multilocular and mitochondria-rich adipocytes were visible after tamoxifen treatment (Fig. 3J). No obvious shift of adipocyte diameter distribution was found in both ingWAT and gonWAT. Correspondingly, tamoxifen treatment did not induce significant difference in the expression of thermogenic markers and adiposity related genes in ingWAT, nor for UCP1 and Cyt C protein contents (Fig. S3C). Similarly, in gonWAT of male mice, no difference of UCP1 and Cyt C at the protein level was observed (Fig. S3D), but tamoxifen treatment decreased Cidea and Tmem26 expression while there was also a tendency of decrease (p<0.1) for Ucp1, Elvovl3, and Pparγ (Fig. 5C). In addition, mRNA expression of Fabp4 was up-regulated after tamoxifen administration. Thus, tamoxifen only had minor effects on the browning of adipose tissue in male mice exposed to cold.
Figure 5.
Effects of tamoxifen administration on mRNA expression of thermogenic and adipogenic genes in BAT (A), ingWAT (B), and gonWAT (C) of male mice. *p<0.05, ** p<0.01. Data are expressed as mean ± SEM (n=6).
In addition, we compared UCP1 and Cyt C expression at the protein level in BAT and WAT of both genders of mice at room temperature and cold environment (Fig. S4). After 4 days’ cold exposure, the expression of UCP1 in BAT was significantly enhanced in both genders of mice. Cold stimuli also induced a 0.5-fold up-regulation of UCP1 in ingWAT of male mice (p=0.16) and 2.0-fold up-regulation (p<0.01) in female mice. In gonWAT, there was a tendency of increased expression of UCP1 (p<0.1) in female mice but not in male mice. However, no changes of Cyt C were found in BAT and WAT of both genders of mice after the cold stimuli. We also compared the expression levels of UCP1 in tamoxifen-treated BAT and WAT (Fig. S5). At room temperature, the UCP1 contents (relative to the contents of tubulin) in ingWAT and gonWAT, respectively was about 13% and 10% of its expression level in BAT. After cold stimuli, the ratio increased to 50% for ingWAT and 31% for gonWAT.
Two-way ANOVA tests showed that there were significant interactions between tamoxifen treatment and gender on the mRNA expression of Ucp1, Prdm16, Cox7α1, and Pgc1α, and on UCP1 and Cyt C expression at the protein levels in BAT. In ingWAT, interactions between tamoxifen treatment and gender were observed for the mRNA expression of Cidea, Elvovl3, Cox7α1, and Pgc1α. There was also a significant interaction on the expression of UCP1 at the protein level. In gonWAT, significant interactions on mRNA expression of Ucp1, Cidea, Pgc1α, CD137, Tbx1 and Tmem26 and protein expression of UCP1 and Cyt C were also demonstrated. No interaction was found for other thermogenic markers at both mRNA and protein levels. These data confirmed that tamoxifen administration influenced the expression of thermogenic markers in BAT and WAT with a gender-specific manner.
Discussion
There are two types of thermogenic adipocytes in mammals, brown and beige adipocytes (27), which play crucial roles in maintaining the body temperature through non-shivering thermogenesis (28). Beige adipocytes reside inside WAT and are inducible in response to external cues such as cold stimulus and β3-adrenergic receptor agonist (27). The differentiation and thermogenesis of brown/beige adipocytes are accompanied with the expression of numerous marker genes including Prdm16, Ucp1, Cidea, Elvov3, Cox7α1, Pgc1α, and Cyt C (29, 30). TBX1, TMEM26, and CD137 are selective markers for beige adipocytes, while PPARγ and FABP4 controls both white and brown/beige adipogenic differentiation (31–33). These genetic markers of adipogenesis provide valuable information about molecular changes and energy expenditure in adipose tissues.
Tamoxifen-inducible Cre/lox recombination system is widely used due to its advantages in avoiding embryonic lethality and compensatory changes present in mice with constitutive gene deficiency, as well as providing the flexibility of tissue and temporal specific knockout (34). However, in this system, a high level of tamoxifen (40–100 mg/kg BDW for 5 consecutive days) was commonly used, which may have side effects on bone phenotype (14), renal fibrosis (16), hepatic metabolism (35), cardiac function (15), and reproduction system (17), etc. Recent studies suggested that this level of dosage might be unnecessarily high for efficient gene recombination (14, 15, 19). Consistently, in our previous study, 25 mg/kg BDW tamoxifen injection for 3 alternative days was sufficient to induce transgene recombination (18). In the current study, we analyzed whether this low dosage of tamoxifen can reduce side effects on adipose tissue metabolism in mice.
The low dosage of tamoxifen administration used in our study induced substantial changes in female mice including decreased ingWAT and gonWAT ratios, decreased serum free fatty acid level, elevated beige adipocyte formation in WATs, and up-regulated thermogenesis of fat tissues especially in BAT. Compared with female mice, this low level of tamoxifen injection did not change the fat tissue ratio, serum glucose and free fatty acid levels, and thermogenesis of adipose tissues in male mice, though it increased serum insulin level. Thus, this low dose of tamoxifen only had negligible side effects on male mice, which is in contrast to a previous report where a high dosage of 50 or 100 mg/kg BDW I.P. injection of tamoxifen to male mice for 5 consecutive days caused fat mass reduction and browning of adipose tissues (36, 37).
Adipose tissues predominantly store excessive energy in the form of triacylglycerols and hydrolyze them to release free fatty acids for use by other organs in response to systematic energy demand (38). Decreased serum free fatty acid level may reflect elevated systematic energy deprivation. Heat production in BAT and beige adipocytes in WAT is mainly controlled by neurotransmitter, norepinephrine, released from sympathetic nervous system (28). Norepinephrine binds with β-adrenergic receptors (βARs) on brown/beige adipocytes and induces signaling cascades to up-regulate thermogenic gene expression and lipolysis. BAT is more densely innervated by peripheral sympathetic nerves than WAT, which may explain the higher sensitivity of BAT to tamoxifen-induced thermogenesis than WAT. Furthermore, the level of sympathetic innervation is substantially higher in gonWAT of female mice versus male mice (21). Estradiol supplementation enhanced the sympathetic activity of female mice while ovariectomic mice had decreased sympathetic activity and attenuated induction of UCP1 by β3-adrenergic receptor agonist (21). These findings provide an explanation for the higher sensitivity of adipose tissues in female mice to tamoxifen administration.
Cold exposure is sensed by the central nervous system which transduce signals by peripheral sympathetic system to BAT and WAT (24). It not only activates thermogenesis in brown and beige adipocytes, but also stimulates the differentiation of resident precursor cells to form new brown and beige adipocytes. As a result, the difference in thermogenesis between male and female mice in response to tamoxifen treatment was magnified under cold stimulus. Compared with the control group, tamoxifen-treated female mice consumed more free fatty acids and glucose for heat production. This is consistent with smaller adipocytes present in ingWAT and gonWAT accompanied by enhanced expression of thermogenic genes and proteins after tamoxifen injection in female mice. In contrast, a low dose of tamoxifen administration to male mice did not induce significant thermogenesis. In fact, several thermogenic genes in BAT and gonWAT were decreased at transcriptional levels after tamoxifen stimuli. The body temperature was also slightly lower in tamoxifen-treated male mice compared with the control group. Consistently, a recent study reported the adverse effects of tamoxifen to the testis, and tamoxifen as an endocrine disruptor may induce systemic physiological and endocrinological changes in male mice (17).
In summary, tamoxifen injection, even at a low dose (25 mg/kg BDW tamoxifen administration for 3 alternative days), could induce substantial changes in thermogenesis of female mice, though only minor effects were observed for male mice. Cold stimulus further aggravated the sensitivity of adipose tissues in female mice to tamoxifen administration. Therefore, extra attention should be paid for the interpretation of data obtained from female mice subjected to tamoxifen-induced gene knockout, especially for metabolic studies.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01-HD067449 and R21-AG049976) to M.D.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Supplementary information is available at International Journal of Obesity’s website.
References
- 1.Nagy A Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000; 26(2):99–109. [PubMed] [Google Scholar]
- 2.Hayashi S, McMahon AP. Efficient Recombination in Diverse Tissues by a Tamoxifen-Inducible Form of Cre: A Tool for Temporally Regulated Gene Activation/Inactivation in the Mouse. Developmental Biology. 2002; 244(2):305–318. [DOI] [PubMed] [Google Scholar]
- 3.Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic acids research. 1995; 23(10):1686–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J. The many faces of estrogen signaling. Biochemia Medica. 2014; 24(3):329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. The Journal of Steroid Biochemistry and Molecular Biology. 2010; 122(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tara M, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. Journal of Biological Chemistry. 2005; 280(43):35983–35991. [DOI] [PubMed] [Google Scholar]
- 7.Homma H, Kurachi H, Nishio Y, Takeda T, Yamamoto T, Adachi K, et al. Estrogen Suppresses Transcription of Lipoprotein Lipase Gene existence of a unique estrogen response element on the lipoprotein lipase promoter. Journal of Biological Chemistry. 2000; 275(15):11404–11411. [DOI] [PubMed] [Google Scholar]
- 8.Wade GN, Heller HW. Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1993; 264(6):R1219–R1223. [DOI] [PubMed] [Google Scholar]
- 9.Sassmann A, Offermanns S, Wettschureck N. Tamoxifen‐inducible Cre‐mediated recombination in adipocytes. Genesis. 2010; 48(10):618–625. [DOI] [PubMed] [Google Scholar]
- 10.Feil S, Valtcheva N, Feil R. Inducible cre mice. Gene Knockout Protocols: Springer; 2009. p. 343–363. [DOI] [PubMed] [Google Scholar]
- 11.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nature cell biology. 2015; 17(4):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye R, Wang QA, Tao C, Vishvanath L, Shao M, McDonald JG, et al. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Molecular metabolism. 2015; 4(11):771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor α mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proceedings of the National Academy of Sciences. 2001; 98(1):224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong ZA, Sun W, Chen H, Zhang H, Yu-an EL, Lane NE, et al. Optimizing tamoxifen-inducible Cre/loxp system to reduce tamoxifen effect on bone turnover in long bones of young mice. Bone. 2015; 81:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson KB, Winer LH, Mørk HK, Molkentin JD, Jaisser F. Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res. 2010; 19(4):715–725. [DOI] [PubMed] [Google Scholar]
- 16.Falke LL, Broekhuizen R, Huitema A, Maarseveen E, Nguyen TQ, Goldschmeding R. Tamoxifen for induction of Cre-recombination may confound fibrosis studies in female mice. Journal of cell communication and signaling. 2017; 11(2):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SH, O’hara L, Atanassova N, Smith SE, Curley MK, Rebourcet D, et al. Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: implications for inducible transgenics. Scientific Reports. 2017; 7(1):8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Fu X, Liang X, Deavila JM, Wang Z, Zhao L, et al. Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRα+ adipose progenitors. Cell discovery. 2017; 3:17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jardí F, Laurent MR, Dubois V, Khalil R, Deboel L, Schollaert D, et al. A shortened tamoxifen induction scheme to induce CreER recombinase without side effects on the male mouse skeleton. Molecular and cellular endocrinology. 2017; 452:57–63. [DOI] [PubMed] [Google Scholar]
- 20.Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Peña R, et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell metabolism. 2016; 24(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S-N, Jung Y-S, Kwon H-J, Seong JK, Granneman JG, Lee Y-H. Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol Sex Differ. 2016; 7(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Zou T, Gomez NA, Wang B, Zhu M-J, Du M. Raspberry alleviates obesity-induced inflammation and insulin resistance in skeletal muscle through activation of AMP-activated protein kinase (AMPK) α1. Nutr Diabetes. 2018; 8(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001; 25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 24.Kissig M, Shapira SN, Seale P. SnapShot: brown and beige adipose thermogenesis. Cell. 2016; 166(1):258–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young JB, Saville E, Rothwell NJ, Stock MJ, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. The Journal of clinical investigation. 1982; 69(5):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia R, Luo XQ, Wang G, Lin CX, Qiao H, Wang N, et al. Characterization of cold‐induced remodelling reveals depot‐specific differences across and within brown and white adipose tissues in mice. Acta Physiologica. 2016; 217(4):311–324. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K, Maretich P, Kajimura S. The common and distinct features of brown and beige adipocytes. Trends in Endocrinology & Metabolism. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 29.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of clinical investigation. 2011; 121(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Gene Dev. 2013; 27(3):234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012; 150(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013; 19(10):1252. [DOI] [PubMed] [Google Scholar]
- 33.Shan T, Liu W, Kuang S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. The FASEB Journal. 2013; 27(1):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen‐inducible action. Genesis. 2002; 32(1):8–18. [DOI] [PubMed] [Google Scholar]
- 35.Hammad S, Othman A, Meyer C, Telfah A, Lambert J, Dewidar B, et al. Confounding influence of tamoxifen in mouse models of Cre recombinase-induced gene activity or modulation. Arch Toxicol. 2018:1–13. [DOI] [PubMed] [Google Scholar]
- 36.Hesselbarth N, Pettinelli C, Gericke M, Berger C, Kunath A, Stumvoll M, et al. Tamoxifen affects glucose and lipid metabolism parameters, causes browning of subcutaneous adipose tissue and transient body composition changes in C57BL/6NTac mice. Biochemical and biophysical research communications. 2015; 464(3):724–729. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Zou P, Zheng L, Linarelli LE, Amarell S, Passaro A, et al. Tamoxifen reduces fat mass by boosting reactive oxygen species. Cell death & disease. 2015; 6(1):e1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sook Sul H. Triacylglycerol metabolism in adipose tissue. Future lipidology. 2007; 2(2):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.