Abstract
Twice-weekly carfilzomib is approved at 27 and 56 mg/m2 to treat relapsed multiple myeloma patients. In the phase III study ARROW, once-weekly 70 mg/m 2 carfilzomib prolonged the median progression-free survival of relapsed multiple myeloma patients in comparison with twice-weekly 27 mg/m2 carfilzomib, without adding significant toxicity. Data were pooled from two phase I/II studies of newly diagnosed multiple myeloma patients who received nine induction cycles of carfilzomib (either 70 mg/m2 once-weekly or 36 mg/m2 twice-weekly), cyclophosphamide and dexamethasone, followed by carfilzomib maintenance. Overall, 121 transplant-ineligible patients with newly diagnosed multiple myeloma were analyzed (once-weekly, n=63; twice-weekly, n=58). We found no significant difference in median progression-free survival [35.7 months (95%CI: 23.7-not reached, NR) vs. 35.5 months (95%CI: 24.3-NR); HR: 1.39; P=0.26] and 3-year overall survival [70% [95%CI: 59%-84%) vs. 72% (95%CI: 60%-85%); HR: 1.27; P=0.5] between once-weekly and twice-weekly carfilzomib. From the start of maintenance, 3-year progression-free survival [47% (95%CI: 33%-68%) vs. 51% (95%CI: 38%-70%); HR: 1.04; P=0.92] and overall survival [72% (95%CI: 58%-89%) vs. 73% (95%CI: 59%-90%); HR: 0.82; P=0.71] were similar in the once- versus twice-weekly carfilzomib. The rate of grade 3-5 hematologic (24% vs. 30%; P=0.82) and non-hematologic (38% vs. 41%; P=0.83) adverse events was similar in the two groups. Once-weekly 70 mg/m2 carfilzomib as induction and maintenance therapy for newly diagnosed multiple myeloma patients was as safe and effective as twice-weekly 36 mg/m2 carfilzomib and provided a more convenient schedule. The trials are registered at clinicaltrials.gov identifiers: 01857115 (IST-CAR-561) and 01346787 (IST-CAR-506).
Introduction
In the last two decades, several novel agents of various classes have been developed and approved to treat multiple myeloma (MM), resulting in improved overall survival (OS) for both transplant-eligible and -ineligible patients.1 Among new agents, the immunomodulatory drugs (IMiD) thalidomide and lenalidomide, and the proteasome inhibitor (PI) bortezomib, have been included in the initial treatment for newly diagnosed (ND) MM patients. Bortezomib, a first-generation PI, proved to be a very effective anti-MM agent. It was initially approved for the relapse setting and then approved for upfront therapy. Despite the efficacy of bortezomib, its long-term administration is limited by the emergence of peripheral neuropathy, which was reported in 4-13% of patients (grade 3-4).2,3
Carfilzomib, a second-generation PI, showed significant activity among patients with relapsed and/or refractory (RR) MM and was approved by US Food and Drug Administration and the European Medicines Agency in combination with dexamethasone or lenalidomide-dexamethasone (Rd) for the treatment of RRMM patients. Given the efficacy displayed by carfilzomib in the relapse setting, several trials tested carfilzomib as part of upfront therapy for NDMM patients, either with Rd (KRd) or with alkylating agents, such as melphalan-prednisone (KMP) or cyclophosphamide-dexamethasone (KCyd).4–7
Carfilzomib is currently approved with the twice-weekly schedule at a dose of 27 mg/m2 over a 2-10-minute (min) infusion period when administered alone or in combination with lenalidomide and dexamethasone, or at a dose of 56 mg/m2 over a 30-min infusion period when given in combination with dexamethasone (Kd). Nonetheless, other doses (up to 70 mg/m2) and schedules (once weekly) have been shown to be promising.
The current twice-weekly schedule may not be very convenient for patients (particularly for elderly patients with limited access to hospital facilities), affecting their quality of life and treatment compliance. In order to improve the convenience of the carfilzomib schedule, preliminary studies tested higher doses of carfilzomib administered in a once-weekly schedule. The phase Ib/II CHAMPION-1 study tested different doses of once-weekly carfilzomib in RRMM patients to define its maximum tolerated dose (MTD) combined with dexamethasone.8 The MTD of once-weekly carfilzomib proved to be 70 mg/m2 over a 30-min infusion period, displaying good efficacy and tolerability. Based on these results, a phase III study (ARROW) was initiated to compare twice-weekly carfilzomib at the dose of 27 mg/m2 with once-weekly carfilzomib at the dose of 70 mg/m2.9 Among 578 RRMM patients, once-weekly carfilzomib improved the overall response rate (ORR; 62.9% vs. 40.8%) and prolonged median progression-free survival (PFS) as compared to twice-weekly carfilzomib (median PFS, 11.2 vs. 7.6 months), with a similar rate of grade 3-4 adverse events (68% vs. 62%). A major limitation of the ARROW study was the low dose (27 mg/m2) of carfilzomib in the twice-weekly arm as compared to the 70 mg/m2 dose adopted in the once-weekly arm. This low dose was determined according to the carfilzomib approval at the time of study design.
We previously published data from two phase I/II (IST-CAR-561) and phase II (IST-CAR-506) studies investigating once-weekly (70 mg/m2) and twice-weekly (36 mg/m2) carfilzomib combined with cyclophosphamide and dexamethasone (KCyd) as initial treatment for transplant-ineligible NDMM patients.6,7 In both trials, KCyd was shown to be a safe and effective option for NDMM patients. Here we report the results of a pooled analysis of these two studies.
Methods
Study design and participants
For this analysis, we pooled together data from two phase I/II (IST-CAR-561; clinicaltrials.gov identifier: 01857115) and phase II (IST-CAR 506; clinicaltrials.gov identifier: 01346787) studies; these studies were led by the same co-operative groups. Patients were recruited from 14 sites across Italy (hospitals, clinics, oncology or medical centers). Both trials enrolled NDMM patients older than 65 years of age or younger but not eligible for autologous stem-cell transplantation. Inclusion and exclusion criteria are similar between the two source studies and have been previously published.6,7 Ethics committees or institutional review boards at the study sites approved both studies, which were carried out in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Procedures
In both studies, patients received nine 4-week induction cycles with carfilzomib, cyclophosphamide (orally, 300 mg on days 1, 8 and 15) and dexamethasone (40 mg on days 1, 8, 15 and 22). In the IST-CAR 561 study, patients received once-weekly carfilzomib at the dose of 70 mg/m2 (on days 1, 8 and 15), while in the IST-CAR 506 study patients received twice-weekly carfilzomib at the dose of 36 mg/m2 (on days 1, 2, 8, 9, 15 and 16). After the induction phase, patients received maintenance treatment with carfilzomib as single agent, which was administered at the same dose and schedule of the induction phase and until progressive disease or intolerable toxicity. Details of study procedures have been previously published.6,7
Outcomes
Focusing on patients who received once- versus twice-weekly carfilzomib, the primary goals of this analysis were: (1) to compare PFS, PFS-2 and OS from the date of entry onto the trial in the intention-to-treat (ITT) population; (2) to compare PFS from start of maintenance therapy (PFS_m), PFS 2 from start of maintenance therapy (PFS-2_m) and overall survival from start of maintenance therapy (OS_m) in a population who completed the induction phase and started maintenance treatment. (Note that PFS-2 was calculated from the date of enrollment to the date of second relapse/progression or death or the date the patient was last known to be in remission.)
Secondary end points were responses, time to response, and safety of once- versus twice-weekly carfilzomib.
Responses were recorded at the beginning of every cycle, according to the International Myeloma Working Group (IMWG) criteria. All adverse events (AE) were assessed during each cycle and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).10 Fluorescence in situ hybridization (FISH) was centrally assessed with a 10% cut-off for numerical aberrations and a 15% cut-off for IgH translocations; high-risk FISH was defined by the presence of at least one of the following chromosomal abnormalities: del(17p), t(4;14) or t(14;16).11 Frailty status was evaluated according to the IMWG Frailty Score.12
The intention-to-treat population consisted of all the enrolled patients and was the basis for the analysis of efficacy end points. Patients were analyzed according to initial treatment assignment. The safety population was defined as all the enrolled patients who received at least one dose of carfilzomib, cyclophosphamide or dexamethasone, and was the basis for the analysis of the safety end points.
Statistical analysis
Data from the two studies were pooled together and analyzed. Comparisons between different patient groups were investigated using Fisher exact test. Time to response was calculated from the start of treatment to the date of the first response [complete remission (CR), partial remission (PR)]. PFS was calculated from the date of enrollment to the date of progression or death or the date the patient was last known to be in remission. PFS-2 was calculated from the date of enrollment to the date of second relapse/progression or death or the date the patient was last known to be in remission. OS was calculated from the date of enrollment to the date of death or the date the patient was last known to be alive. PFS_m, PFS-2_m and OS_m were calculated from the date of the start of maintenance therapy. In order to account for potential con-founders, the comparison once- versus twice-weekly carfilzomib was adjusted for age, International Staging System (ISS), FISH, Frailty Score, and, in relation to the maintenance analysis, for response to induction therapy.
Time-to-event data were analyzed using the Kaplan-Meier method; survival curves were compared with the log-rank test. Results are presented as hazard ratios (HRs), 95% confidence intervals (95% CIs), and two-sided P-values. Data were censored on September 30th 2015 for the IST-CAR-506 study and on April 30th 2018 for the IST-CAR-561 study. Data were analyzed using R software (version 3.5.1).
Results
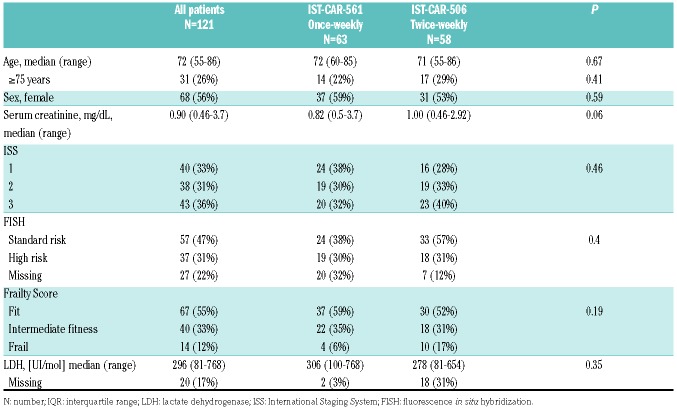
One hundred and twenty-one transplant-ineligible NDMM patients were analyzed: 63 from the IST-CAR- 561 study (once-weekly carfilzomib) and 58 from the IST-CAR-506 study (twice-weekly carfilzomib). Patients’ characteristics are listed in Table 1. The median age at diagnosis in the entire population was 72 years (range, 55-86 years). Cytogenetic data were available in 94 patients: 37 (31%) had high-risk chromosomal abnormalities by FISH, including 10% of patients with t(4;14), 3% with t(14;16), and 18% with del(17p), while 57 patients (47%) were classified as standard-risk. No significant differences were observed in the two groups between the percentage of patients with ISS 3 disease (32% vs. 40%; P=0.45), high-risk FISH (30% vs. 31%; P=0.40) or Frailty Score (6% vs. 17%; P=0.09). The median follow up of the entire cohort was 39 months [interquartile range (IQR): 31-47], without any difference between the two groups.
Table 1.
Patients’ characteristics.
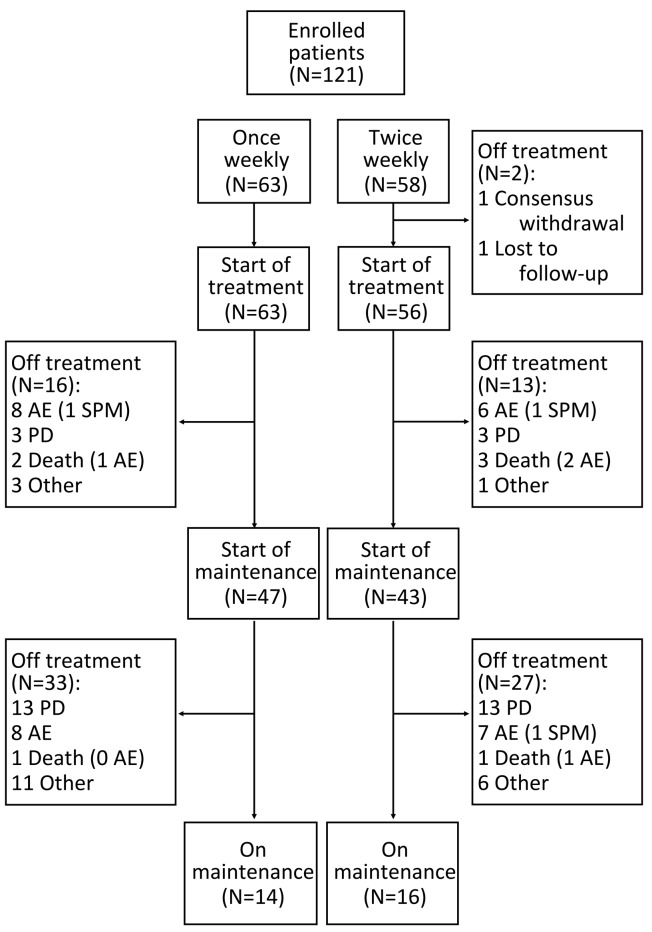
Overall, 119 of 121 patients enrolled in the studies started induction therapy (Figure 1): 63 in the once-weekly group and 56 in the twice-weekly group. Two patients did not start therapy in the twice-weekly group: one withdrew consent and one was lost to follow up. Ninety patients entered the maintenance phase: 47 (75%) and 43 (74%) in the once- and twice-weekly groups, respectively (Figure 1).
Figure 1.
Analysis profile. N: number; AE: adverse events; PD: progressive disease; SPM: second primary malignancy.
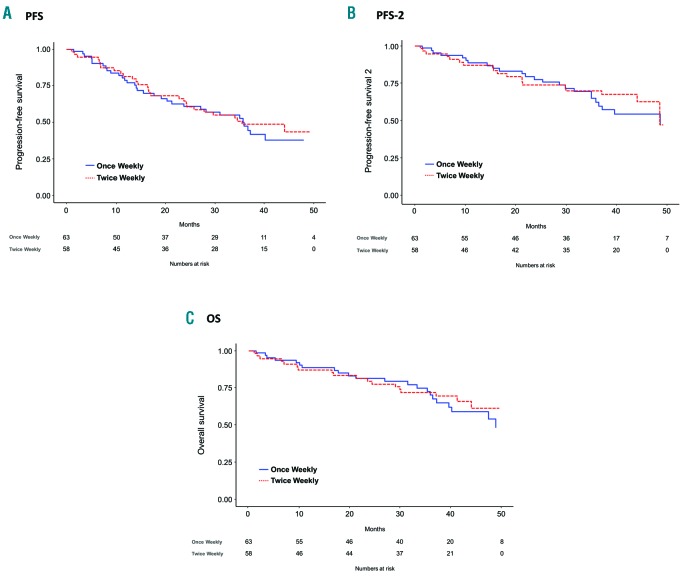
In the ITT population, the median PFS from enrollment was 35.7 months (95%CI: 23.7-NR) in the once-weekly group and 35.5 months (95%CI: 24.3-NR) in the twice- weekly group, with, respectively, 47% and 49% of patients alive and free from progression at three years (Figure 2A). When adjusting for age, ISS, FISH, and Frailty Score, no significant differences in the risk of progression or death were observed between the once-weekly and the twice-weekly carfilzomib groups (HR: 1.39; P=0.26). Median PFS-2 was similar in patients receiving once-weekly (48.6 months; 95%CI: 36.5-NR) and twice-weekly (48.5 months; 95%CI: 44.1-NR) carfilzomib (HR: 1.25; P=0.51) (Figure 2B). At three years, median OS was not reached in either group, with 70% and 72% of patients alive in the two groups, respectively (Figure 2C). No difference in the risk of death was observed between the once-weekly and the twice-weekly carfilzomib groups when adjusting for age, ISS, FISH and Frailty Score (HR: 1.27; P=0.50). We also assessed PFS and OS according to cytogenetic risk. No significant difference in 3-year PFS (52% vs. 43%; HR: 0.76; P=0.38) and 3-year OS (78% vs. 73%; HR: 0.71; P=0.36) was reported between standard-and high-risk FISH patients, with a greater reduction in the risk of progression or death in the once-weekly (HR: 1.17; P=0.72) than in the twice-weekly carfilzomib group (HR: 0.52; P=0.12; interaction P=0.19).
Figure 2.
Once-weekly versus twice-weekly carfilzomib in patients with newly diagnosed multiple myeloma. (A) Intention-to-treat progression-free survival (ITT PFS). (B) Intention-to-treat progression-free survival 2 (ITT PFS-2). (C) Intention-to-treat overall survival (ITT OS). (Note that PFS-2 was calculated from the date of enrollment to the date of second relapse/progression or death or the date the patient was last known to be in remission.)
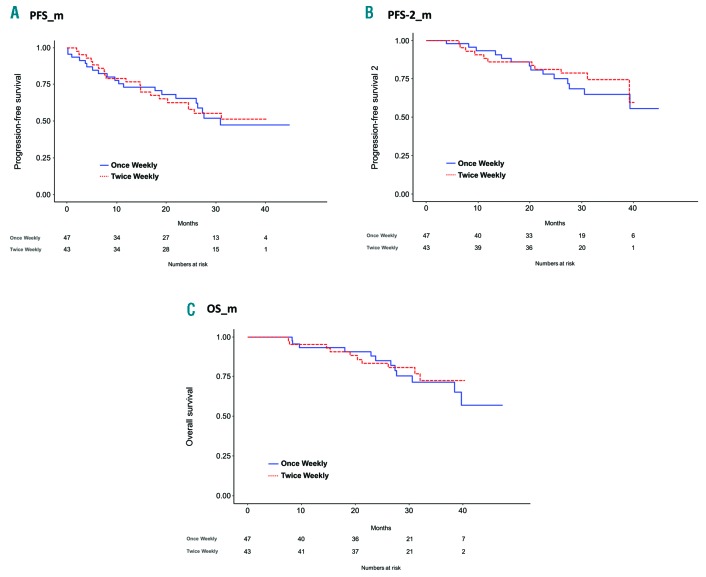
The median duration of maintenance was 17 months (IQR: 4-28) in the once-weekly and 20 months (IQR 7-32) in the twice-weekly group (P=0.17). At three years, PFS_m was 47% (95%CI: 33%-68%) and 51% (95%CI: 38%-70%) in the once-weekly group and in the twice-weekly group, respectively (Figure 3A), with no significant difference in the risk of progression (HR: 1.04; P=0.92) within the two groups when adjusting for age, ISS, FISH, Frailty Score and response to induction. No differences in 3-year PFS-2_m (65% vs. 74%; HR: 0.85; P=0.74) and OS_m (72% vs. 73%; HR: 0.82; P=0.71) were observed between the two groups (Figure 3B and C).
Figure 3.
Analysis from start of maintenance therapy. (A) Progression-free survival from start of maintenance therapy (PFS_m). (B) Progression-free survival 2 from start of maintenance therapy (PFS-2_m). (C) Overall survival from start of maintenance therapy (OS_m). (Note that PFS-2 was calculated from the date of enrollment to the date of second relapse/progression or death or the date the patient was last known to be in remission.)
Overall, the proportion of patients achieving a PR or better was 92% in the once-weekly versus 90% in the twice-weekly group (P=0.76), including 22% and 29% of patients obtaining a CR or better (P=0.41). Responses were rapid: median time to PR or better was 1.9 months in the once-weekly group and 1.2 months in the twice-weekly group.
Carfilzomib dose reduction was necessary in 18 (29%) patients receiving the once-weekly schedule and in 17 (30%) patients receiving the twice-weekly schedule. The median relative dose intensity of carfilzomib [once weekly 97.6% (IQR 88.3-100%); twice weekly 97.2% (IQR 90.4-100%)] was similar in the two groups (P=0.75). Dexamethasone dose reduction was necessary in 13 (21%) patients receiving the once-weekly schedule and in 18 (32%) patients receiving the twice-weekly schedule. The median relative dose intensity of dexamethasone [once weekly 100% (IQR 82.6-100%); twice weekly 100% (IQR 88.5-100%)] was similar in the two groups (P=0.85). Cyclophosphamide dose reduction was necessary in 7 (11%) patients receiving the once-weekly schedule and in 15 (27%) patients receiving the twice-weekly schedule. Nevertheless, the median relative dose intensity of cyclophosphamide [once weekly 96.85% (IQR 90.8-100%); twice weekly 96.75% (IQR 88.6-100%)] was similar in the two groups (P=0.97). The most common AE leading to carfilzomib dose reduction were acute kidney injury (1 patient in the once-weekly group and 2 patients in the twice-weekly group), infections (2 patients in each group), and hypertension (4 patients in the once-weekly group and none in the twice-weekly group). Treatment-related AE leading to the discontinuation of carfilzomib occurred in 17 (27%) patients in the once-weekly group and 17 (30%) patients in the twice-weekly group. The most common AE leading to carfilzomib discontinuation were cardiac injury (6 patients in the once-weekly group and 6 patients in the twice-weekly group), infections (3 patients in the once-weekly group and 3 patients in the twice-weekly group), and thromboembolism (2 patients in the once-weekly group and 1 in the twice-weekly group). Cardiac events leading to drug discontinuation during induction (3 and 2) and maintenance (3 and 4) occurred at similar rates in patients receiving once- versus twice-weekly carfilzomib, respectively. Similarly, the rates of infections leading to drug discontinuation were similar during induction (2 and 3) and maintenance (1 and 0) between the once- versus twice-weekly groups.
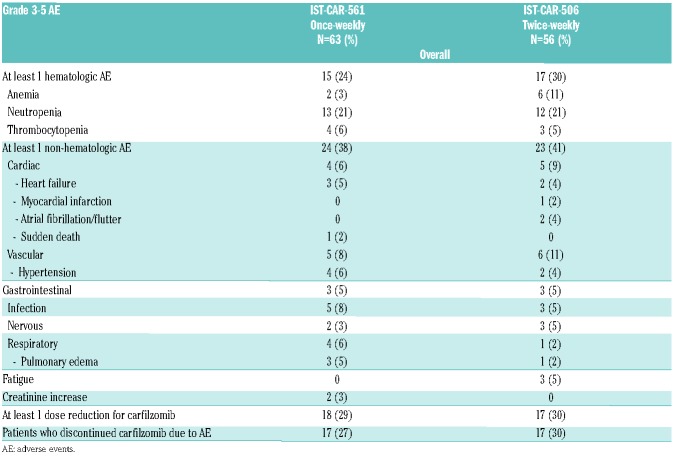
Overall, the incidence of treatment-related grade 3-5 AE was similar in the once-weekly and the twice-weekly carfilzomib groups, both in terms of hematologic (24% vs. 30%; P=0.82) and non-hematologic (38% vs. 41%; P= 0.83) AE. The most frequent non-hematologic grade ≥3 AE were infections [5 (8%) in the once-weekly group vs. 3 (5%) in the twice-weekly group], respiratory [4 (6%) vs. 1 (2%)], cardiac [4 (6%) vs. 5 (9%)] and hypertension [4 (6%) vs. 2 (4%)]. The incidence of treatment-related grade 3-5 AE during carfilzomib maintenance was low, with comparable rates of hematologic (0% vs. 5%) and non-hematologic (21% vs. 23%) AE in the once-weekly and the twice-weekly groups. The most frequent ≥3 AE was hypertension [4 (9%) vs. none]. All AE are reported in Table 2.
Table 2.
Grade 3-5 treatment-related adverse events during induction and maintenance therapy.
Discussion
In this pooled analysis of two phase I/II studies comparing two alternative schedules of carfilzomib, transplant-ineligible NDMM patients who received once-weekly carfilzomib at the dose of 70 mg/m2 showed similar response rates as compared to patients treated with twice-weekly carfilzomib at the dose of 36 mg/m2. Moreover, the analysis did not report differences in terms of PFS, PFS-2, and OS. Administering high-dose carfilzomib (70 mg/m2) in a once-weekly schedule did not impair the safety profile of the KCyd combination in comparison with a lower (36 mg/m2) twice-weekly schedule.
To date, two doses of twice-weekly carfilzomib, 27 mg/m2 and 56 mg/m2, have been approved for the treatment of RRMM patients, based on the results of the phase III ASPIRE and ENDEAVOR trials. In the ASPIRE study, carfilzomib was tested at the dose of 27 mg/m2.13 However, a higher dose of carfilzomib (36 mg/m2) had been investigated in combination with lenalidomide and dexamethasone, and was shown to be safe and effective for NDMM patients.4,14–16 In the ENDEAVOR trial, which compared Kd versus bortezomib-dexamethasone (Vd), carfilzomib was administered at the dose of 56 mg/m2.17,18
Despite the great results yielded by the introduction of carfilzomib, treatment compliance and quality of life of young active patients, as well as those of elderly patients with reduced mobility, are compromised by the need for frequent visits to the outpatient clinic for carfilzomib dosing. From this point of view, a shift from the current twice-weekly to a once-weekly dosing schedule would decrease by 50% patient visits to health care facilities, with a subsequent improvement in quality of life and a reduction in drug and health care costs. For these purposes, higher doses of carfilzomib, administered once-weekly, were tested in the relapse setting in a phase Ib/II study and in a subsequent phase III study.6–9 Once-weekly carfilzomib yielded a higher ORR as compared to twice-weekly carfilzomib, resulting in prolonged median PFS (11.2 vs. 7.6 months) without significantly increasing the rate of AE or the risk of treatment discontinuation due to AE. However, the major limitation of the ARROW study was the low dose adopted for the twice-weekly arm, which was chosen by the investigators because it was the approved dose at the time of trial design. Indeed, higher doses of carfilzomib (up to 36 mg/m2 when given in combination and 70 mg/m2 alone) have been safely delivered both in upfront and relapse settings.5,8,14–20
To our knowledge, this is the first analysis to compare two different schedules and doses of carfilzomib (70 mg/m2 once-weekly vs. 36 mg/m2 twice-weekly) as induction and maintenance therapies for elderly, transplant-ineligible NDMM patients.
In the ITT analysis, we observed no significant differences in 3-year PFS (47% vs. 49%), PFS-2 (62% vs. 70%) and OS (70% vs. 72%) in patients receiving once- versus twice-weekly carfilzomib. The risks of dose reduction or treatment discontinuation were equal between the two groups. Of note, delivering 70 mg/m2 of carfilzomib in a single dose did not increase the risk of grade 3-5 hematologic (24% vs. 30%; P=0.82) and non-hematologic (38% vs. 41%; P=0.83) AE, as compared to a twice-weekly administration of 36 mg/m2 of carfilzomib. Importantly, no new cardiovascular safety risks were identified with once-weekly carfilzomib treatment at the 70 mg/m2 dose.
The aim of continuous treatment is to prolong PFS and OS among NDMM patients without negatively affecting their quality of life. For this purpose, we compared once-versus twice-weekly maintenance with carfilzomib. Among patients who received carfilzomib maintenance, we did not observe any significant differences in terms of 3-year PFS_m (47% vs. 51%), PFS-2_m (65% vs. 74%), and OS_m (72% vs. 73%) between the once-weekly and twice-weekly schedules. Continuous treatment with single-agent carfilzomib was well tolerated and grade 3-5 AE were infrequent in both groups, although patients in the once-weekly arm were at higher risk of developing grade 3-5 hypertension (9% vs. 0%) as compared to patients in the twice-weekly group.
As previously reported, carfilzomib is able to at least partially abrogate the unfavorable prognostic significance of high-risk FISH cytogenetic abnormalities.21 In this trial, we observed no difference between standard and high-risk FISH patients in terms of 3-year PFS (52% vs. 43%) and OS (78% vs. 73%), with a greater reduction in the risk of progression or death for high-risk FISH patients (as compared to standard-risk FISH patients) in the once-weekly (HR: 1.17) than in the twice-weekly (HR: 0.52) carfilzomib schedule. These results compared favorably with those observed in the ARROW trial.9 However, due to the high frequency of patients with unknown cytogenetic risk and the small number of patients, these results should be interpreted with caution and this evidence must be confirmed by further studies.
The major limitation of our analysis was the non-randomized design of the two phase I/II studies. Indeed, the study populations were slightly different (e.g. frailty status, FISH data availability), but the most important inclusion and exclusion criteria, as well as the treatment schema, were identical. Furthermore, the reproducibility of the results in the community setting was limited both by the lower percentage of older patients (≥75 years) as compared to other studies (such as the FIRST and the ALCYONE), and by the fact that patients included in this analysis were treated in the context of clinical trials in a limited number of selected, experienced centers. With this limitation, our results should be interpreted with caution, even though they should be considered as the basis of future randomized trials.
In conclusion, a once-weekly 70 mg/m2 infusion of carfilzomib was shown to be as safe and effective as a twice weekly 36 mg/m2 infusion for the initial treatment of elderly transplant-ineligible NDMM patients, both as induction therapy in combination with cyclophosphamide and dexamethasone and as single-agent maintenance. This analysis supports the use of high-dose once-weekly carfilzomib and provides the rationale for the investigation of once- versus twice-weekly carfilzomib as initial treatment for MM patients.
Acknowledgments
We thank the patients who participated in these studies and their families; the study co-investigators, nurses, and coordinators at each of the clinical sites.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/8/1640
Funding
The IST-CAR-561 (NCT01857115) study was sponsored by Stichting Hemato-Oncologie voor Volwassenen Nederland (HOVON, the Netherlands), in collaboration with Fondazione Neoplasie Sangue ONLUS (Italy). The IST-CAR-506 (NCT01346787) study was sponsored by the HOVON Foundation and co-sponsored by Fondazione Neoplasie Sangue ONLUS. Both trials were supported by funding from AMGEN (Onyx Pharmaceuticals), which had no role in study design, data collection, data analysis, data interpretation, writing of the report or publication of this article. The corresponding author had full access to all the data in the two studies and had final responsibility for the decision to prepare and submit this manuscript for publication, together with the other authors.
References
- 1.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.San Miguel JF, Schlag R, Khuageva NK, et al. Persistent Overall Survival Benefit and No Increased Risk of Second Malignancies With Bortezomib-Melphalan-Prednisone Versus Melphalan-Prednisone in Patients With Previously Untreated Multiple Myeloma. J Clin Oncol. 2013;31(4):448–455. [DOI] [PubMed] [Google Scholar]
- 3.Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23):4745–4753. [DOI] [PubMed] [Google Scholar]
- 4.Dytfeld D, Jasielec J, Griffith KA, et al. Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99(9):e162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facon T, Lee JH, Moreau P, et al. Phase 3 Study (CLARION) of Carfilzomib, Melphalan, Prednisone (KMP) v Bortezomib, Melphalan, Prednisone (VMP) in Newly Diagnosed Multiple Myeloma (NDMM). Clin Lymphoma Myeloma Leuk. 2017;17(1):e26–e27. [Google Scholar]
- 6.Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63–69. [DOI] [PubMed] [Google Scholar]
- 7.Bringhen S, D’Agostino M, De Paoli L, et al. Phase 1/2 study of weekly carfilzomib, cyclophosphamide, dexamethasone in newly diagnosed transplant-ineligible myeloma. Leukemia. 2018;32(4):979–985. [DOI] [PubMed] [Google Scholar]
- 8.Berenson JR, Cartmell A, Bessudo A, et al. CHAMPION-1: a phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood. 2016;127(26):3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau P, Mateos M-V, Berenson JR, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018; 19(7):953–964. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute [USA]. Common Terminology Criteria for Adverse Events (CTCAE) Version. 4.0. US Department of Health and Human Services; May 28, 2009. [Google Scholar]
- 11.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. [DOI] [PubMed] [Google Scholar]
- 14.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012; 120(9):1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay F, Rota Scalabrini D, Belotti A, et al. Updated efficacy and MRD data according to risk-status in newly diagnosed myeloma patients treated with carfilzomib plus lenalidomide or cyclosphosphamide: Results from the FORTE trial. HemaSphere 2018;2(S1):6 [Abstract #S109, EHA 2018 23rd Congress]. [Google Scholar]
- 16.Gay F, Foà R, Musto P, et al. Updated efficacy data and MRD analysis according to risk status in newly diagnosed myeloma patients treated with carfilzomib + lenalidomide or cyclophosphamide (FORTE trial). J Clin Oncol. 2018;36(15_suppl) [Abstract #8009, ASCO 2018 Annual Meeting]. [Google Scholar]
- 17.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(10):1327–1337. [DOI] [PubMed] [Google Scholar]
- 19.Boccia RV, Bessudo A, Agajanian R, et al. A Multicenter, Open-Label, Phase 1b Study of Carfilzomib, Cyclophosphamide, and Dexamethasone in Newly Diagnosed Multiple Myeloma Patients (CHAMPION-2). Clin Lymphoma Myeloma Leuk. 2017; 17(7):433–437. [DOI] [PubMed] [Google Scholar]
- 20.Sonneveld P, Asselbergs E, Zweegman S, et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood. 2015; 125(3):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chng W-J, Goldschmidt H, Dimopoulos MA, et al. Carfilzomib–dexamethasone vs bortezomib–dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia. 2017;31(6):1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]