Erdheim Chester Disease (ECD) is a rare histiocytic neoplasm that is classified as a macrophage-dendritic cell neoplasm in the 2016 WHO classification of hematopoietic and lymphoid malignancies.1 Histiocytic neoplasms have been historically categorized into: 1) Langerhans cell histiocytosis (LCH), and 2) non-Langerhans histiocytosis (non-LCH), including Erdheim–Chester disease (ECD), juvenile xanthogranuloma (JXG), and Rosai– Dorfman disease (RDD). ECD is a malignant disorder characterized by accumulation of clonally derived foamy histiocytes, fibrosis and inflammation affecting multiple organs including bones, lungs, heart, kidneys, and brain, presenting as fatigue, bone pain, diabetes insipidus, panhypopituitarism, cerebral and cerebellar disease, cardiac and pulmonary disease, renal failure and retroperitoneal and mediastinal fibrosis.3 Approximately 50% of patients with ECD have a BRAFV600E mutation, and those with wild-type BRAF generally have other activating mutations in the MAPK pathway.2,4
As an early step in differentiation, hematopoietic stem cells differentiate to lymphoid and myeloid lineages. Monocytes are derived from myeloid progenitors. Blood monocytes can differentiate to macrophages and dendritic cells. Macrophages are large cells residing in tissues and are mainly involved in clearing pathogens and debris. Dendritic cells are antigen presenting cells residing in the skin, mucosa, and lymphoid tissues and their main job is antigen presentation and activation of T cells. Dendritic cells residing in the skin are called Langerhans Cells. Histiocyte is a general term referring to tissue-resident macrophages and dendritic cells.4–6 The exact cell of origin of ECD is not known, but three lines of evidence support that it arises from myeloid progenitors: 1) similar expression signatures and BRAFV600E mutations have been found in histiocytic neoplasms and the blood monocyte and hematopoietic stem cell/progenitors,7–11 2) histiocytosis like lesions were generated by xenotransplantation of CD34+ cells from a patient with ECD11 and 3) approximately 10% of patients with non-Langerhans Histiocytosis have concurrent myeloid neoplasms.12 Here we report a patient with BRAFV600E mutated ECD who developed BRAFV600E mutated acute monocytic leukemia (AML-M5). Whole exome sequencing confirmed that the ECD and AML had multiple shared mutations and arose from the same cell of origin. This case adds to the current body of evidence suggesting that ECD should be categorized as a myeloid/myeloproliferative neoplasm with a chance of progression to acute myeloid leukemia.
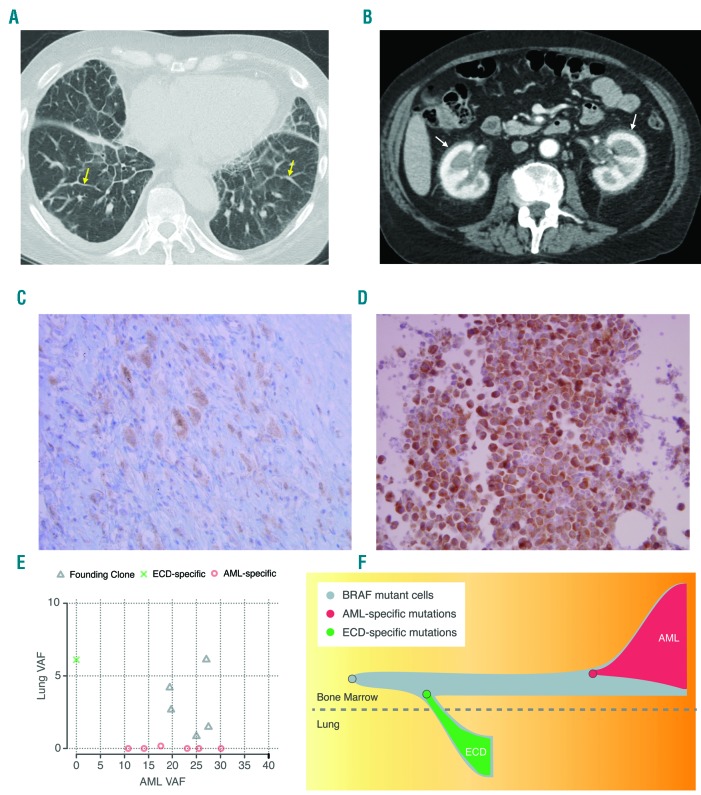
An 80 year old man presented with dyspnea on exertion. A computed tomography (CT) scan showed ground glass opacities with septal wall thickening in the lungs and retroperitoneal fibrosis (Figure 1A and 1B). Whole body positron emission tomography (PET) showed Fludeoxyglucose (FDG) avid sclerotic changes in the tibial midshafts bilaterally, retroperitoneal, perirenal, and mediastinal fibrotic changes with mild FDG uptake, and septal line thickening with increased FDG uptake in the lungs. Brain magnetic resonance imaging (MRI) showed scattered white matter T2 hyperintensities. Cardiac MRI revealed circumferential wall thickening of the right atrium with normal left and right ventricular systolic function. He underwent video-assisted thoracoscopic surgery and wedge resection of the right lower lobe that confirmed ECD. Sections of the lung biopsy showed dense bands of mixed inflammatory infiltrate, consisting of small lymphocytes, eosinophils, and clusters of atypical histiocytic cells. Atypical histiocytic cells stained strongly positive for CD68 and Factor XIII, weakly positive for S100, and negative for CD1a. Mutation analysis of lung tissue was performed using both immunohistochemistry and polymerase chain reaction (PCR). BRAFV600E immunostaining was positive in atypical histiocytic cells (Figure 1C) and PCR testing for the BRAFV600E mutation in lung tissue confirmed the presence of the mutation. A CBC was notable for: white blood cell count (WBC) 19,000/μL, absolute neutrophil count (ANC) 15,500/μL, absolute monocyte count 1760/μL, absolute lymphocyte count 1750/μL, hemoglobin 10.3 g/dl, and platelet count 213,000μL. The patient was offered enrollment in a phase 1 clinical trial using Vemurafenib - a selective BRAFV600E inhibitor - or Interferon α as standard of care. He chose not to get treatment, and was observed. During an observation period of over 2 years, he had stable mild shortness of breath as the main symptom of his ECD. His labs during this period showed normal electrolytes, normal renal and liver function tests, WBCs of 16000-29000/μL, ANC of 14000-27000/μL, absolute monocyte counts of 1200-3400/μL, hemoglobin of 11-12 g/dl, and normal platelet counts. He declined to undergo bone marrow aspiration and biopsy for further evaluation of his monocytosis. Analysis of peripheral blood by morphology and flow cytometry showed neutrophilic leukocytosis with no dysplasia or blasts.
Figure 1.
Imaging, immunohistochemistry staining for BRAFV600E, clonal inference plot and evolutionary trajectory of the ECD and AML. (A) CT chest showing basilar predominant septal line thickening (yellow arrows). (B) CT abdomen showing bilateral perinephric soft tissue thickening (“hairy kidney”) indicating retroperitoneal fibrosis. (C) Immunohistochemistry staining for BRAFV600E in the Lung tissue (ECD specimen at 40× magnification). (D) Immunohistochemistry staining for BRAFV600E in bone marrow (AML specimen at 40× magnification). (E) Clonal inference plot of single-nucleotide variants discovered from exome sequencing. (F) Inferred evolutionary trajectory, starting from a shared BRAFV600E mutation and developing into ECD and AML.
Twenty six months after diagnosis of ECD, he presented with worsening fatigue and shortness of breath. CBC showed WBC of 57,400/μL, hemoglobin of 10.6 g/dL, and platelet count of 37,000/μL with 11% circulating blasts. Bone marrow aspiration and biopsy revealed AML-M5, with 80% cellularity and 30% blasts. Blasts were positive for myeloperoxidase, CD43, CD68, lysozyme, CD163 (very small subset), and negative for CD34, CD117, CD3, CD20, CD79a, CD61, Factor XIIIa, CD15, CD138, CD1a, S100, cytokeratin, and CD30. BRAFV600E immunostaining of the bone marrow core showed diffuse positivity of the blasts for BRAFV600E mutation (Figure 1D) and PCR testing for BRAFV600E mutation in bone marrow confirmed the mutation. The patient was offered treatment with hypomethylating agents or Vemurafenib but declined and proceeded with palliative care. He died of progressive leukemia a few weeks later.
The patient was consented and enrolled in an institutional tissue banking protocol for myeloid malignancies. DNA was extracted from the ECD specimen (lung tissue biopsy), the bone marrow specimen (at the time of progression to AML-M5), and normal skin as a proxy for germline DNA. Enhanced exome sequencing was performed, utilizing a standard exome reagent with additional probes for recurrent mutations in AML spiked in for greater coverage. Further details on sequencing, somatic variant calling, and clonal inference are described in the Online Supplementary Methods.
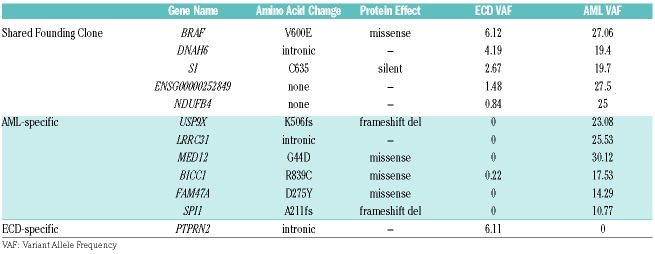
We sequenced the normal skin and AML specimens to mean coverages of 88× and 142×, respectively. The very low tumor cellularity of the ECD sample (~10%) necessitated deeper sequencing (1349× coverage). Fourteen somatic variants were identified, including known cancer-driving mutations in BRAFV600E and IDH2R140Q (Figure 1E and 1F, Table 1, and Online Supplementary Table 1).
Table 1.
Mutations identified via exome sequencing. Mutations in up- or down-stream regions are annotated with the nearest gene.
Clonal inference revealed that the BRAFV600E mutation and 4 other mutations were present in a shared founding clone, indicating a common cell of origin (Figure 1E and 1F). Five variants were AML-specific, while only one was specific to the ECD (Table 1 and Online Supplementary Table 1). The IDH2 mutation was present in a distinct population of cells from the tumors, as confirmed by sequencing of a non-malignant biopsy that contained IDH2 but not the founding BRAFV600E mutation, marking a concurrent hematopoietic clone that was not part of either the ECD or AML.
There were five mutations conclusively shared between the ECD (lung) and AML (bone marrow) specimens (BRAF, DNAH6, NDUFB4, SI, and ENSG00000252849) (Table 1 and Online Supplementary Table 1). The presence of all 5 mutations in both the AML and ECD samples provides exceptionally strong evidence that these two malignancies were derived from the same cell of origin.
Though the cells of origin for ECD are still considered unknown, previous studies have suggested that hematopoietic/myeloid progenitor cells might be the precursors of histiocytic neoplastic cells in both ECD and LCH.7–11 Additionally, a recent study observed that 10% of adult patients with non-Langerhans histiocytosis had a concomitant myeloid neoplasm.12 One study reported an ECD/LCH mixed histiocytic neoplasm with an NRAS mutation in ECD/LCH tissue as well as in bone marrow after diagnosis of chronic myelomonocytic leukemia (CMML).12 Another study reported on a patient with mixed histiocytosis and BRAFV600E, TET2, and SRSF2 mutations in LCH cells from skin, and concurrent AML with TET2 and SRSF2 mutations, suggesting a clonal relationship between the two malignancies.11
Our patient had fluctuating monocytosis concurrent with his ECD. We were unable to further analyze his monocytosis with a bone marrow examination prior to presentation with AML, and there was insufficient banked peripheral blood material to investigate BRAF and other mutations in monocytes circulating in blood. It is therefore possible that the patient had CMML concurrent with ECD that progressed to AML 26 months later. In addition, our patient declined treatment with vemurafenib for his ECD. The effect of targeted therapies like Vemurafenib in preventing evolution of ECD to myeloid malignancies has not been studied.
Though our data provides a catalog of single nucleotide variants (SNVs) and small indel mutations, it is difficult to identify with confidence the mutations that cooperated with BRAF to drive malignant transformation. USP9X that is a known tumor suppressor gene in pancreatic ade-nocarcinoma,14 and SPI1 (PU.1) that acts as a tumor suppressor gene in a fraction of patients with AML15 may provide the “second hit” needed for leukemic transformation. The remainder of the mutations in the ECD were not protein-altering, though additional cooperating events may have existed in classes we were not powered to detect (translocations, copy number alterations, etc.), or transcriptional changes that were not assessed.
This case confirms a common cell of origin for BRAFV600E mutated ECD and BRAFV600E mutated AML in one patient, as evidenced by shared BRAFV600E driver mutation and several shared presumed passenger mutations. This adds to the current body of evidence support ing the proposal for categorizing ECD as a myeloid/myeloproliferative malignancy that can progress to AML, and potentially, to other myeloid malignancies.
Footnotes
Funding: AG was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2 TR002346 (principle investigator: Victoria J. Fraser).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond EL, Durham BH, Haroche J, et al. Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov. 2016;6(2):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cline MJ. Histiocytes and Histiocytosis. Blood. 1994;84(9):2840–2853. [PubMed] [Google Scholar]
- 6.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011; 11(11):788–798. [DOI] [PubMed] [Google Scholar]
- 7.Berres ML, Lim KPH, Peters T, et al. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. J Exp Med. 2014;211(4):669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haroche J, Cohen-Aubart F, Charlotte F, et al. The histiocytosis Erdheim-Chester disease is an inflammatory myeloid neoplasm. Expert Rev Clin Immunol. 2015;11(9):1033–1042. [DOI] [PubMed] [Google Scholar]
- 9.Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010; 184(8):4557–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milne P, Bigley V, Bacon CM, et al. Hematopoietic origin of Langerhans cell histiocytosis and Erdheim-Chester disease in adults. Blood. 2017;130(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durham BH, Roos-Weil D, Baillou C, et al. Functional evidence for derivation of systemic histiocytic neoplasms from hematopoietic stem/progenitor cells. Blood. 2017;130(2):176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papo M, Diamond EL, Cohen-Aubart F, et al. High prevalence of myeloid neoplasms in adults with non-Langerhans cell histiocytosis. Blood. 2017;130(8):1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Mancera PA, Rust AG, van der Weyden L, et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486(7402):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller BU, Pabst T, Osato M, et al. Heterozygous PU.1 mutations are associated with acute myeloid leukemia. Blood. 2002;100(3):998–1007. [DOI] [PubMed] [Google Scholar]