Myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) are clonal hematopoietic stem/progenitor cell (HSPC) disorders mainly affecting the elderly population.1 Hypomethylating agents (HMA) like azacitidine and decitabine have become the standard of care in elderly patients with high-risk (HR) MDS or AML unfit for intensive treatment approaches. Until today, responses to HMA have occured in less than 50% of patients and are not durable, with only a few patients achieving long-lasting remissions.2,3 Prognostic clinical markers, such as presence of peripheral blasts, high transfusion burden, and poor performance status, have been identified as indicators of a worse outcome of HMA-based therapy.1,4 Moreover, responses to HMA are especially short-lived in patients with adverse risk cytogenetic abnormalities compared to those with normal karyotype.1
Craddock et al. evaluated the impact of mutational profile on clinical response to azacitidine by analyzing 250 patients with newly diagnosed, relapsed, or refractory AML or HR-MDS. Lower complete response (CR) rates occurred in patients with an IDH2 and STAG2 mutation, higher CR rates in patients with NPM1 mutation. Mutations in CDKN2A, IDH1, TP53, NPM1, and FLT3-ITD were associated with a worse overall survival (OS) in univariate analysis, while multivariate analysis showed a decrease in OS in patients with CDKN2A, IDH1, or TP53 mutations. Moreover, ASXL1 and ETV6 were associated with short response duration after azacitidine treatment.5
Despite all efforts to try to select patients based on their cytogenetic and molecular characteristics, failing HMA therapy is still associated with a dismal prognosis reflected by a median survival of six months.6 Until now, almost nothing has been known about the mechanisms underlying HMA-resistance. Thus, as frequently as possible, patients experiencing HMA failure should be evaluated for clinical trial options, given the current absence of any available standard treatment in that setting. In clinically fit patients with HR-MDS or secondary AML (sAML) and normal karyotype, intensive chemotherapy with a subsequent allogeneic stem cell transplantation may also be considered.
In this issue of the Journal, Sébert et al. report results of a phase II study of the Groupe Francophone des Myélodysplasies (GFM) investigating the novel HMA guadecitabine (SGI-110) as a salvage treatment in HR-MDS and low blast count AML (<30% bone marrow blasts) patients after azacitidine failure.7 Guadecitabine is a dinucleotide of decitabine and deoxyguanosine with similar potency but longer half life due to resistance to cytidine deaminase degradation. This results in an extended exposure of blasts to its active metabolite decitabine. The study included fifty-six patients (median age 75 years) who failed or relapsed after at least six previous azacitidine cycles. Patients’ characteristics indicated a study population with advanced disease, including 87.5% of patients carrying high-risk somatic mutations such as ASXL1 (25%), RUNX1 (21%), TP53 (20%) and U2AF1 (20%).7 Patients achieving hematologic response after 3, 6 or 9 cycles of guadecitabine (60 mg/m2/day subcutaneously days 1-5 of 28-day treatment cycles) were considered to be responders and were allowed to continue treatment until loss of response. Sébert et al. identified eight (14.3%) responding patients, including two CR, one partial response (PR), three hematologic improvements (HI), and two marrow CR (mCR). Median response duration was 11.5 months and median OS 7.1 months; responders had a prolonged median OS of 17.9 months.7
Therefore, even after failure to an HMA, HMA-based treatment with guadecitabine can be an effective alternative treatment option prolonging survival in a small proportion of HR-MDS and AML patients, with a toxicity profile similar to that of standard HMA. The authors present an analysis of prognostic factors for response and prolonged OS after guadecitabine treatment. Especially patients with primary azacitidine failure, absence or limited number of somatic mutations and lower methylation level in blood during the first cycle of treatment benefited from guadecitibaine treatment.7 A phase III trial comparing guadecitabine with treatment of choice in MDS patients after HMA failure is currently ongoing (clinicaltrials.gov identifier: 02907359).
One other promising approach to improving efficacy of hypomethylation in HR-MDS and AML patients is the addition of the orally selective B-cell lymphoma 2 (BCL-2) inhibitor venetoclax to HMA. BCL-2 protein, a key regulator of leukemic blast survival, has been reported to play an important role in regulating apoptosis via the intrinsic mitochondrial cell death. Overexpression of the BCL-2 protein has been shown to be associated with poor outcomes, conferring chemotherapeutic resistance in AML.8 Recent data suggest that 400 mg of venetoclax has an optimal benefit-risk profile when used in combination with azacitidine.8 This combination has already demonstrated impressive rates of CR both in the frontline and relapse settings: AML patients treated with first-line venetoclax and HMA showed favorable overall response rates (ORR) (CR: 71% and CRi: 74%).9,10 Median duration of response after achieving CR was 21.2 months and median overall survival was 16.9 months.9 Comparing these results with historically poor outcome data of single agent azacitidine treatment (CR rates approx. 20%; OS not exceeding 12 months in AML patients3), it becomes clear that this novel targeted combined strategy will potentially dominate the future treatment landscape in HR-MDS and AML patients not eligible for intensive induction therapy. Nevertheless, previous clinical trials in AML demonstrated a toxicity profile that represents cause for concern. Over 50% of included patients developed grade ≥3 neutropenia, leading to a high incidence of treatment interruption and subsequent study discontinuation due to progressive disease (PD).9,11 Recruitment is currently underway for a clinical study evaluating the combination of venetoclax with azacitidine in patients with HR-MDS after HMA failure (clinicaltrials.gov identifier: 02966782).
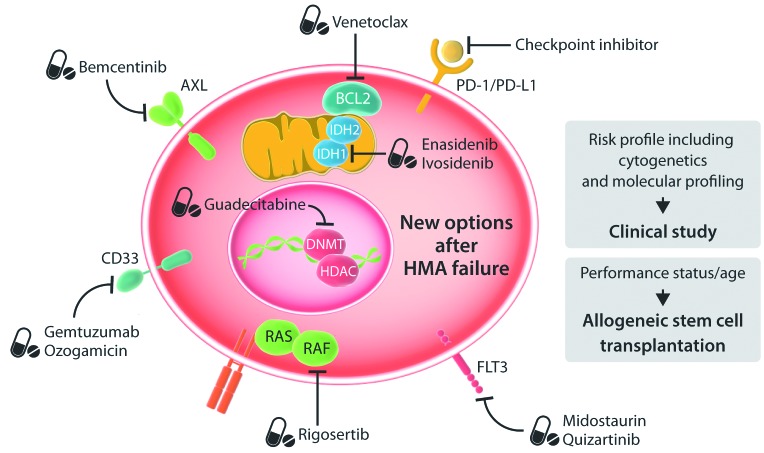
Figure 1.
New options after hypomethylating agent failure.
It is known that HMA can reduce immune response by upregulation of inhibitory immune checkpoint molecule expression. Therefore, preventing resistance to HMA by combining HMA and checkpoint inhibition is another possible new treatment strategy which is currently under investigation in several clinical trials. We reported on a patient with sAML undergoing single agent pembrolizumab (anti-PD-1) treatment.12 After two months of therapy, platelet count increased in line with a response according to International Working Group (IWG) 2018 criteria, together with clearance of IDH1 mutation.12 Recently Daver et al. reported on a phase II study evaluating response to azacitidine and nivolumab in relapsed/refractory AML patients. In HMA pretreated patients, ORR was 22% and median OS for the 70 included patients was 6.3 months.13
Gemtuzumab ozogamicin (GO), a humanized anti-CD33 antibody conjugate, is currently licensed by both the US Food and Drug Administration and the European Medicines Agency in combination with daunorubicin and cytarabine for the treatment of de novo CD33-positive AML patients. Moreover, available data suggest activity of GO in combination with HMA. The maturation of AML blasts increases CD33 expression after HMA therapy, resulting in an enhanced uptake of GO by blast cells.14 A phase II clinical trial in older AML patients evaluated the combination of hydroxyurea followed by azacitidine for seven days and GO on day eight. Results demonstrated CR in 44% of patients in the good risk group (age 60-69 years or performance status 0-1) and 35% (19 of 59 patients) CR rate in the poor risk group (age ≥70 years and performance status 2 or 3).14 In a phase II study in newly diagnosed or relapsed HR-MDS and AML patients, the combination of decitabine with GO achieved CR/CRi in 35% of patients (39 of 110 patients).15
Rigosertib (ON-01910), a multikinase inhibitor, is currently undergoing evaluation in a randomized phase III trial (clinicaltrials.gov identifier: 02562443) in HR-MDS patients after HMA failure. Results of a previous phase III study demonstrated that patients treated with rigosertib had longer (8.6 vs. 5.3 months) median OS compared to patients receiving best supportive care after HMA failure.16 The combination of rigosertib with azacitidine after HMA failure was recently evaluated in a phase II trial, showing an ORR of 54%, including 8% CR in this patient population; the safety profile was similar to those described for azacitidine alone.17
One interesting new therapeutic target in patients failing HMA is the selective inhibition of AXL, a surface membrane protein kinase receptor on blast cells. Signaling through AXL seems to stimulate a number of pro-survival pathways and enables malignant cells to develop resistance to conventional chemotherapies.18 Preclinical studies with bemcentinib, an orally selective small molecule AXL inhibitor, demonstrated in vitro and in mouse models that leukemic proliferation was blocked by interference with AXL signaling.18 Thus, AXL represents a promising target and bemcentinib a possible new treatment option for HR-MDS or AML patients.18 The efficacy and safety of bemcentinib is currently being evaluated in a phase II study (BERGAMO trial; clinicaltrials.gov identifier: 03824080) within the European Myelodysplastic Syndromes Cooperative Group (EMSCO) in patients with HR-MDS or AML after HMA failure. Other potentially available therapeutic approaches after failing HMA include the use of targeted molecular therapies, e.g. with IDH or FLT3-inhibitors. IDH mutations are quite common in MDS (10-15% of patients) and data in relapsed AML have so far proved promising.19 FLT3-inhibitors have already been approved in the US for second-line treatment of patients with AML and may, therefore, offer a therapeutic option in rare FLT3 mutated cases with disease progression.20
In conclusion, patients with HR-MDS or AML failing HMA remain a population with a dismal outcome and limited therapeutic options. In the future, a personalized targeted treatment strategy on the basis of the patient’s molecular profile, cytogenetics, and previous therapies may be the best approach. Until then, translational studies based on a variety of prospective clinical trials are urgently required to overcome the enormous unmet medical need for additional treatment options.
References
- 1.Platzbecker U. Treatment of MDS. Blood. 2019;133(10):1096–1107. [DOI] [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403–411. [DOI] [PubMed] [Google Scholar]
- 5.Craddock CF, Houlton AE, Quek LS, et al. Outcome of Azacitidine Therapy in Acute Myeloid Leukemia Is not Improved by Concurrent Vorinostat Therapy but Is Predicted by a Diagnostic Molecular Signature. Clin Cancer Res. 2017;23(21):6430–6440. [DOI] [PubMed] [Google Scholar]
- 6.Komrokji RS. Treatment of Higher-Risk Myelodysplastic Syndromes After Failure of Hypomethylating Agents. Clin Lymphoma Myeloma Leuk. 2015;15 Suppl:S56–59. [DOI] [PubMed] [Google Scholar]
- 7.Sebert M, Renneville A, Bally C, et al. A phase II study of guadecitabine in higher-risk myelodysplastic syndrome and low blast count acute myeloid leukemia after azacitidine failure. Haematologica. 2019, 104(8):1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinardo CD, Pratz KW, Potluri J, et al. Durable response with venetoclax in combination with decitabine or azacitadine in elderly patients with acute myeloid leukemia (AML). J Clin Oncol. 2018;36(15_suppl): 7010–7010. [Google Scholar]
- 9.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollyea DA, Pratz KW, Jonas BA, et al. Venetoclax in Combination with Hypomethylating Agents Induces Rapid, Deep, and Durable Responses in Patients with AML Ineligible for Intensive Therapy. Blood. 2018;132(Suppl 1):285–285. [Google Scholar]
- 11.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–228. [DOI] [PubMed] [Google Scholar]
- 12.Kubasch AS, Wehner R, Bazzurri S, et al. Clinical, molecular, and immunological responses to pembrolizumab treatment of synchronous melanoma and acute myeloid leukemia. Blood Adv. 2018;2(11):1187–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daver N, Garcia-Manero G, Basu S, et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019;9(3):370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nand S, Othus M, Godwin JE, et al. A phase 2 trial of azacitidine and gemtuzumab ozogamicin therapy in older patients with acute myeloid leukemia. Blood, 2013;122(20):3432–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daver N, Kantarjian H, Ravandi F, et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30(2):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Manero G, Fenaux P, Al-Kali A, et al. ONTIME study investigators Rigosertib versus best supportive care for patients with high- risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(4):496–508. [DOI] [PubMed] [Google Scholar]
- 17.Navada SC, Garcia-Manero G, Atallah EL, et al. Phase 2 Expansion Study of Oral Rigosertib Combined with Azacitidine (AZA) in Patients (Pts) with Higher-Risk (HR) Myelodysplastic Syndromes (MDS): Efficacy and Safety Results in HMA Treatment Naïve & Relapsed (Rel)/Refractory (Ref) Patients. Blood. 2018;132(Suppl 1):230. [Google Scholar]
- 18.Medyouf H. The microenvironment in human myeloid malignancies: emerging concepts and therapeutic implications. Blood. 2017;129(12): 1617–1626. [DOI] [PubMed] [Google Scholar]
- 19.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortes J, Perl AE, Döhner H, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018;19(7):889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]