Abstract
High-risk myelodysplastic syndrome/acute myeloid leukemia patients have a very poor survival after azacitidine failure. Guadecitabine (SGI-110) is a novel subcutaneous hypomethylating agent which results in extended decitabine exposure. This multicenter phase II study evaluated the efficacy and safety of guadecitabine in high-risk myelodysplastic syndrome and low blast count acute myeloid leukemia patients refractory or relapsing after azacitidine. We included 56 patients with a median age of 75 years [Interquartile Range (IQR) 69-76]. Fifty-five patients received at least one cycle of guadecitabine (60 mg/m2/d subcutaneously days 1-5 per 28-day treatment cycles), with a median of 3 cycles (range, 0-27). Eight (14.3%) patients responded, including two complete responses; median response duration was 11.5 months. Having no or few identified somatic mutations was the only factor predicting response (P=0.035). None of the 11 patients with TP53 mutation responded. Median overall survival was 7.1 months, and 17.9 months in responders (3 of whom had overall survival >2 years). In multivariate analysis, IPSS-R (revised International Prognostic Scoring System) score other than very high (P=0.03) primary versus secondary azacitidine failure (P=0.01) and a high rate of demethylation in blood during the first cycle of treatment (P=0.03) were associated with longer survival. Thus, guadecitabine can be effective, sometimes yielding relatively prolonged survival, in a small proportion of high-risk myelodysplastic syndrome/low blast count acute myeloid leukemia patients who failed azacitidine. (Trial registered at clinicaltrials.gov identifier: 02197676)
Introduction
The first generation hypomethylating agents (HMA) azacitidine (AZA) or decitabine are considered to be the reference treatment for high-risk myelodysplastic syndromes (MDS) and low blast count acute myeloid leukemia (AML) (<30% marrow blasts) in elderly patients who are not candidates for allogeneic stem cell transplantation (allo-SCT). However, responses are seen in only 50-60% of patients, and the median overall survival (OS) of 18-24 months obtained with azacitidine remains modest.1 Moreover, median survival after HMA failure is only approximately six months.2
The hypomethylating activity of AZA and decitabine depends on their incorporation during the S phase of the cell cycle into RNA or DNA, respectively.3 This suggests a relationship between duration of drug exposure and effectiveness of the HMA. Half-life of first generation HMA is approximately 30 minutes, which might limit their activity in slowly dividing MDS cells.4 Furthermore, a recent study found a high response rate of 67% in unfavorable risk MDS/AML (specifically TP53 mutated patients) after serial 10-day cycles of decitabine, a regimen with a longer exposure to decitabine than the classical 5-day schedule.5
Guadecitabine (SGI-110) is a novel, second-generation hypomethylating drug. It is a dinucleotide of decitabine (the active metabolite) and deoxyguanosine, resistant to cytidine deaminase, the main enzyme responsible for decitabine degradation. Cleavage of the phosphodiester bond between the two parts of the dinucleotide results in a slow release of decitabine, prolonging the HMA exposure in cells.6 A phase I/II study found a 52% (55 of 107) response rate to guadecitabine in treatment naïve AML with tolerable toxicity.7 Guadecitabine was also studied in 19 patients with relapsed or refractory MDS after HMA, with a 32% response rate.8
These results prompted the GFM group to propose guadecitabine as a salvage treatment in a larger series of higher-risk MDS and low blast count AML patients after AZA failure, not candidates for intensive chemotherapy or allo-SCT.
Methods
Trial design
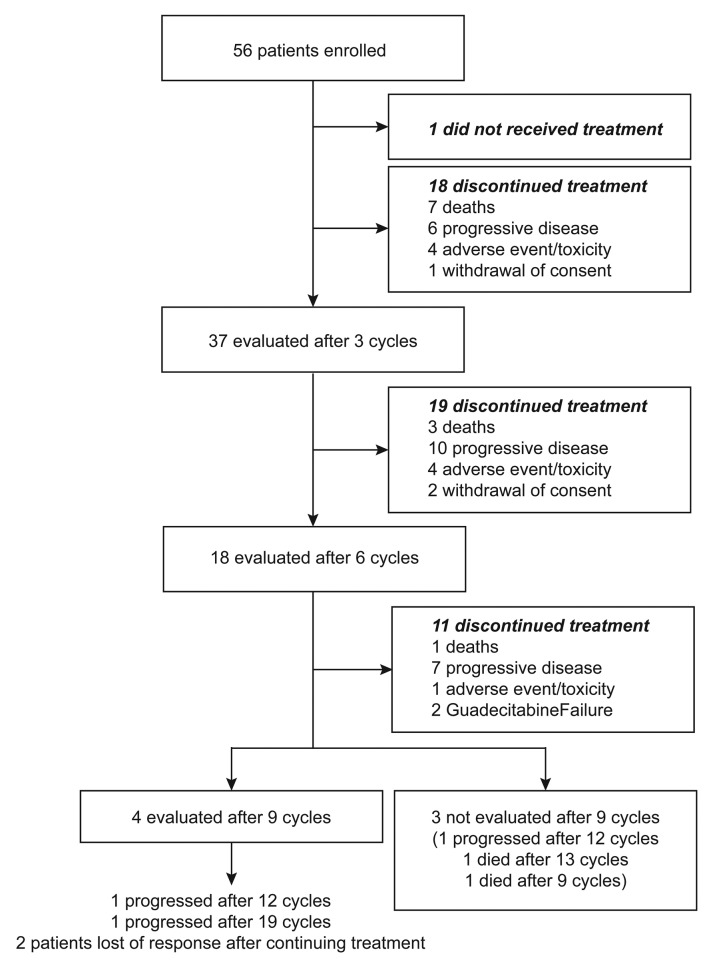
This was a national, GFM-sponsored multicenter phase II clinical trial (clinicaltrials.gov identifier: 02197676) evaluating the efficacy of guadecitabine in higher-risk MDS and low-blast count AML patients, refractory or relapsing after AZA treatment. A first cohort of 21 patients was planned with the objective to stop the study if four patients or less would respond after six cycles of guadecitabine or experience a high toxicity. After review by an independent Data Safety Monitoring Board (DSMB), toxicity was considered acceptable and five patients were responders. The extended cohort included 36 patients. Because late responders had been reported in previous studies (Issa et al., 2019, personal communication), response was also evaluated after nine cycles of guadecitabine in the extended cohort (Online Supplementary Figure S1).
Patients
Inclusion criteria were: i) age >18 years; i) diagnosis of MDS or chronic myelomonocytic leukemia (CMML), with white blood cell (WBC) count <13×109/L according to the World Health Organization (WHO) 2008 criteria9 with international prognostic scoring system (IPSS) intermediate-2 or high-risk MDS10 or AML with 20-30% marrow basts [AML/refractory anemia with excess blasts in transformation (RAEB-t) according to the French-American-British (FAB) classification11]; iii) refractoriness to azacitidine, i.e. at least six cycles without response [complete response (CR), partial response (PR), marrow CR or stable disease with hematologic improvement (HI), according to International Working Group (IWG) 2006 criteria]12 or relapse after a response. Non-responders were eligible only in the absence of overt progression, i.e. at least doubling of marrow blast percentage between start of HMA and protocol screening. Patients eligible for intensive chemotherapy or allo-SCT were excluded.
Other eligibility criteria included an Eastern Co-operative Oncology Group (ECOG) performance status of 0-2, and adequate liver and hepato-renal functions (creatinine <1.5 times the upper limit of normal, and creatinine clearance ≥+50 mL/min, total bilirubin and transaminase <1.5 times the upper normal limit). The protocol was approved by the Comité de Protection des Personnes Paris-Ile de France, the ethical committee whose approval is valid for all participating French institutions. All patients provided written informed consent.
Treatment
Patients received 60 mg/m2 subcutaneous guadecitabine on days 1-5 of 28-day treatment cycles (the recommended drug regimen in previous studies).7,8 Treatment was continued until progression, death, unacceptable toxicity, or no response after six cycles (extended to 9 cycles after the first 20 patients). Dose reductions to 45 and even 30 mg/m2/d were allowed to manage toxicity.
Biological studies
Somatic mutations were screened on bone marrow cells by a next-generation sequencing (NGS) assay for a selected panel of 36 genes (Online Supplementary Appendix and Online Supplementary Table S1) at inclusion for all patients and on sequential bone marrow samples in some responders.
Global DNA methylation was measured in 53 patients by the long interspersed nuclear element (LINE1) methylation assay, and changes in methylation from baseline were assessed as described in the Online Supplementary Appendix.
End points
The primary end point was hematologic response (CR, PR, marrow CR or stable disease with HI according to IWG 2006 criteria)12 Secondary end points were duration of response, rate of progression to AML, overall survival, toxicity profile of guadecitabine. All patients who achieved a CR, PR, marrow CR or HI after 3, 6 or 9 (for the 36 patients of the extended cohort) cycles of guadecitabine were considered responders and were allowed to continue treatment until loss of response, progression, or death.
Statistical analysis
Based on the results of phase III trials of decitabine for the treatment of MDS13 and AML,14 it was expected that guadecitabine would achieve at least 30% hematologic responses. A Bryant and Day 2-stage phase II design was used. Assuming a 20% response rate (CR+PR+ marrow CR + HI according to IWG 2006 criteria) under the null, controlling for type I and II error rates at α=0.05 and β=0.2 respectively, 19 patients had to be accrued for stage 1 of the trial to demonstrate a benefit of 20% (i.e. a response rate of >40%). At the end of Stage 1, the study would stop if there were four responders or less. If there were at least five responders, 35 additional patients had to be included in the study. We assumed that approximately 10% of the population may not be evaluable for response and that 56 patients should, therefore, be included.
The response rate was estimated in the intention-to-treat sample, with 95% exact confidence interval. (95%CI:) The Kaplan and Meier method was used for analysis of progression-free survival and overall survival. Median and 25% and 75% quartiles, were estimated.
Analysis was performed on SAS (SAS Cary, NC, USA) and R v.3.3.2 (https://www.R-project.org/) softwares. Two-sided P<0.05 was considered statistically significant.
Results
Patients’ baseline characteristics
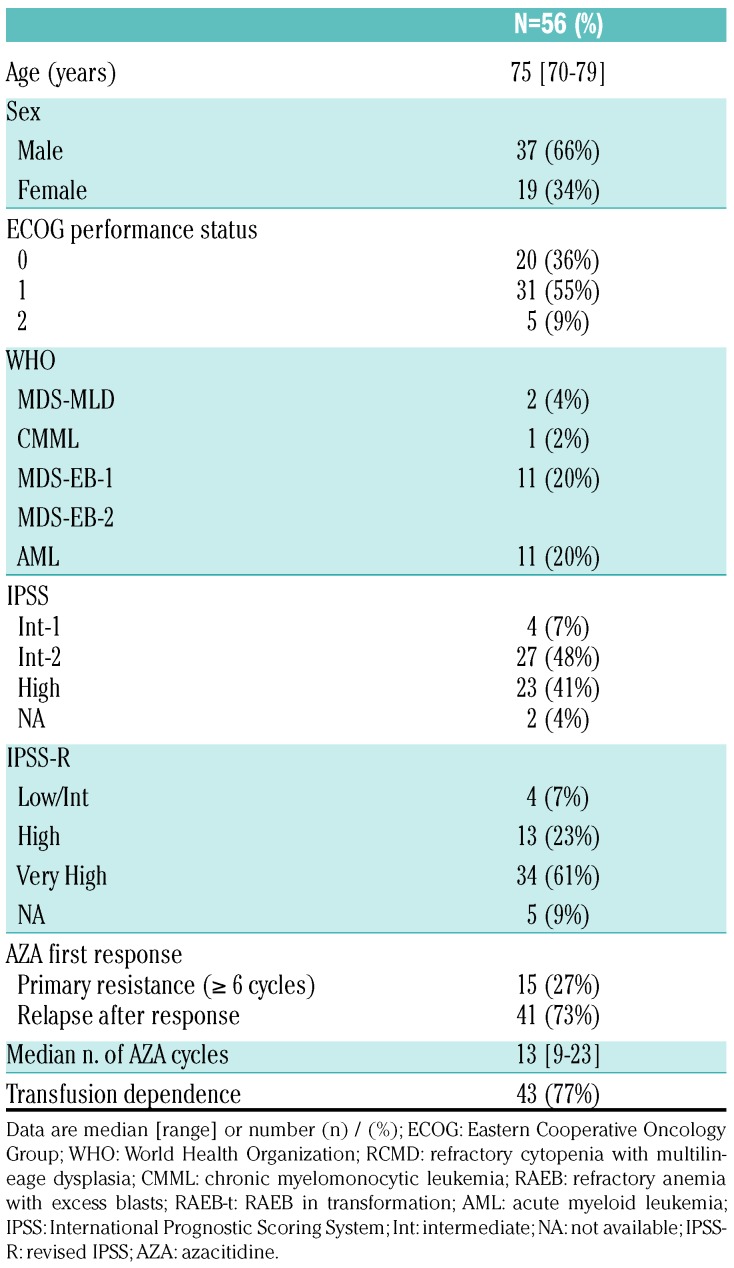
Between August 2014 and January 2016, we enrolled 56 patients in 13 French centers; one patient died from infection before receiving guadecitabine (Table 1 and Figure 1). Of the 56 patients (intent-to-treat population), 66% were males, with a median age of 75 years (range, 70-79). At inclusion, according to WHO classification, 11 (20%) patients had RAEB-1, 31 (55%) had RAEB-2, and 11 (20%) had AML. Thirty-four (61%) patients had very high-risk IPSS-R and 43 (77%) patients were red blood cell transfusion-dependent (TD), while ECOG status was >1 in five (9%) patients. The median number of prior AZA cycles was 13 (range 6-23). Forty-one (73%) patients had relapsed after a response to AZA, while 15 (27%) had primary resistance. The median IQR time interval between the HMA failure and study initiation was 50 days (33; 76) (range, 0-251 days).
Table 1.
Patients’ baseline characteristics.
Figure 1.
Flow chart of the study.
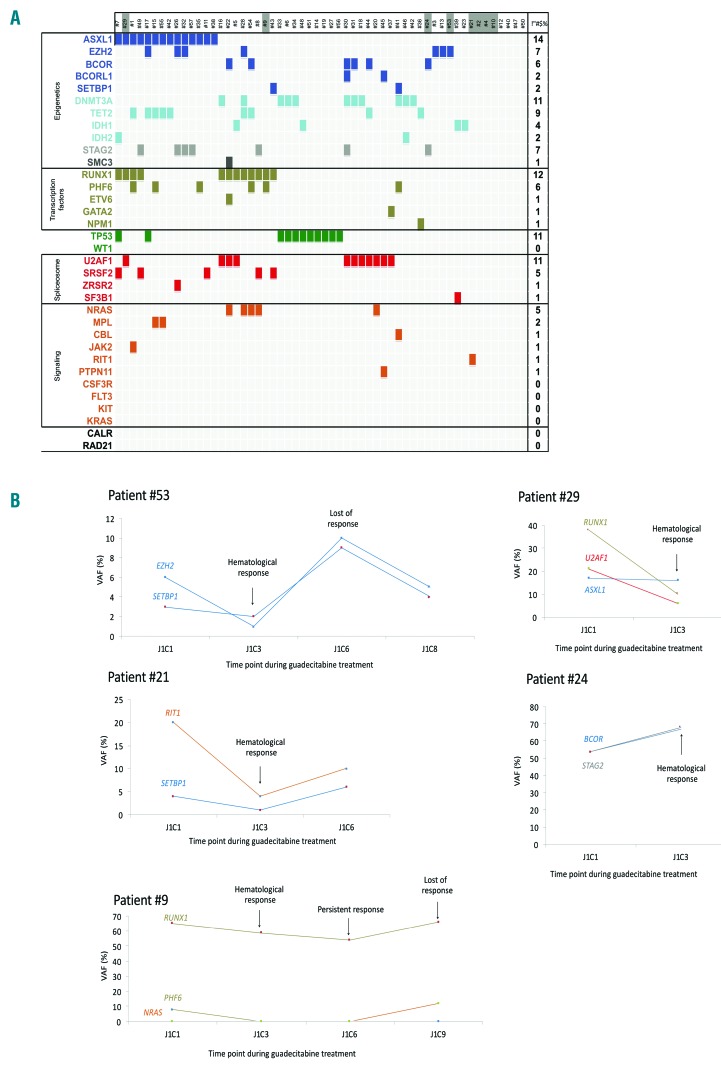
Forty-nine (87.5%) of the 56 patients had at least one somatic mutation, with a median number of two mutations (range 0-7), the most common being ASXL1 (n=14, 25%), RUNX1 (n=12, 21%), TP53 (n=11, 20%), U2AF1 (n=11, 20%), and DNMT3A (n=11, 20%) (Figure 2A). Median variant allele frequency (VAF) of those mutations was 29.5% (Online Supplementary Figure S2). Baseline methylation levels of LINE-1 were similar in blood and bone marrow samples with an average of 73% and 71%, respectively.
Figure 2.
Molecular characteristics of the patients after azacitidine (AZA) failure and during guadecitabine treatment. (A) Spectrum of mutations in the 56 high-risk myelodypslastic syndrome (MDS) patients included (refractory to or relapsing after AZA therapy) in 36 selected genes. Each column represents an individual patient sample, and each colored cell represents a mutation of the gene or gene group listed to the left of that row. The number of mutations in each row is indicated in the column on the right. Darker cells of patient numbers indicate responders to guadecitabine. (B) Evolution of different clones, according to variant allele frequency (VAF), in five patients responding to guadecitabine after AZA failure.
Treatment outcomes
Fifty-five patients received at least one cycle of guadecitabine. The median number of treatment cycles received was three (range, 0-27), eight patients having received only one cycle. Most patients received the planned dose in all treatment cycles, but 18 patients had a dose reduction.
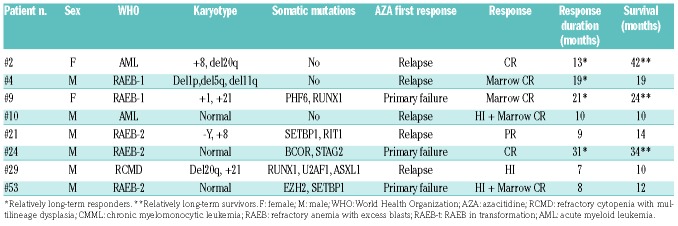
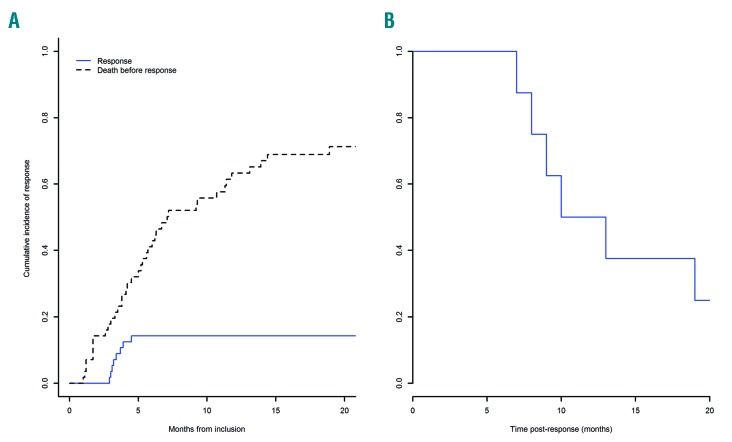
Eight of 56 (14.3%) patients responded, including two CR, one PR, three hematologic improvements, and two marrow CR (Table 2). Seven patients had responded by three cycles and one additional patient by six cycles, but we observed no later response (after 9 cycles) in this study. The median duration of response was 11.5 months (95%CI: 9; not available) (Figure 3B), and response was longer than 12 months in four patients (13, 19, 21, 31 months, respectively).
Table 2.
Baseline characteristics and outcome of the responders.
Figure 3.
Response to guadecitabine of patients treated after azacitidine failure. (A) Cumulative incidence of response. (B) Duration of response.
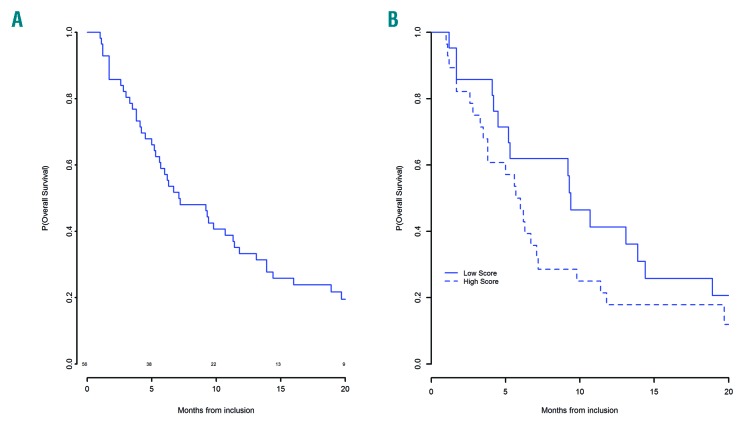
Median overall survival was 7.1 months [95%CI: (5.6;11.8)] with a one-year survival of 33% (95%CI: 22.9;48.4) (Figure 4A). Responders to guadecitabine had a median OS of 17.9 months, and three patients had >2-year OS (24, 34, and 42 months, respectively). Forty-nine patients had died, because of progressing disease in 28 (57.1%), infection in 13 (26.5%), bleeding in two, heart failure in one, and from unknown cause in five. None of the patients had received allogeneic SCT.
Figure 4.
Overall survival of patients treated with guadecitabine after azacitidine failure. (A) Overall survival. (B) Survival according to Nazha score.
Prognostic factors of response and overall survival
Response was seen in four (26%) of the 15 patients with primary failure, and four (9%) of the 41 relapsing patients (P=0.12). The median number of mutations was one [range, 0-3 in responders, compared with 2 (range, 0-6) mutations in non-responders (P=0.035)], and the response rate was significantly higher in patients with no detectable somatic mutations compared to patients with at least one somatic mutation (P=0.036). None of the 11 patients with TP53 mutation achieved response. Clonal architecture was followed during treatment in five responders with mutations at baseline. There was no significant decrease in VAF of the mutated clone(s) at hematologic response (Figure 2B). Treatment with guadecitabine resulted in a maximum LINE-1 demethylation relative to baseline (D0) of 12.3% on day 8 of cycle 1 in peripheral blood samples and of 3.3% on day 28 of cycle 1 in bone marrow samples (Online Supplementary Figure S3).
Except for somatic mutations, no other baseline parameter had significant prognostic value for response, including age, sex, ECOG status, transfusion dependency, baseline hemoglobin, platelet, absolute neutrophil count, bone marrow blast percentage, cytogenetics, IPSS, IPSS-R, type of AZA failure (primary or secondary), LINE1 baseline methylation, or demethylation rate with treatment.
Overall survival was significantly shorter in patients with high IPSS (HR=1.81, 95%CI: 1.1-2.97; P=0.02), with very high IPSS-R (HR=1.5, 95%CI: 1.2-1.87; P=0.0004), and TP53 mutation (HR=2.23, 95%CI: 1.09-4.57; P=0.028), and longer in patients with high demethylation rate in blood on day 8 of the first cycle (P=0.02) (Online Supplementary Figure S4). There was a trend towards shorter survival in patients with a higher number of somatic mutations (HR=1.18, 95%CI: 0.97-1.44; P=0.099) and prolonged OS in patients with primary AZA failure (HR=0.51, 95%CI: 0.25-1.01; P=0.054), and low baseline level of methylation in blood on day 8 of the first cycle (P=0.066) and in bone marrow on day 28 of the first cycle (P=0.083). In multivariate analysis, IPSS-R (P=0.03), demethylation rate in blood (P=0.03) and the type of AZA failure (primary vs. secondary; P=0.01) remained predictive of OS.
Using the recent prognostic model for MDS patients having failed hypomethylating agents15 that includes ECOG >1, very poor cytogenetics, age, bone marrow blasts >20%, transfusion dependency, platelets <30 ×109/L, 21 of 49 patients were classified as low-risk and 28 as high-risk, with a median OS of 9.2 vs. 5.7 months, respectively (HR=1.7, 95%CI: 0.8-3.8; P=0.16) (Figure 4B). This compared with 11 and 4.5 months, respectively, for the patients included in the prognostic model of Nazha et al. who had received various treatments after HMA failure.
Side effects
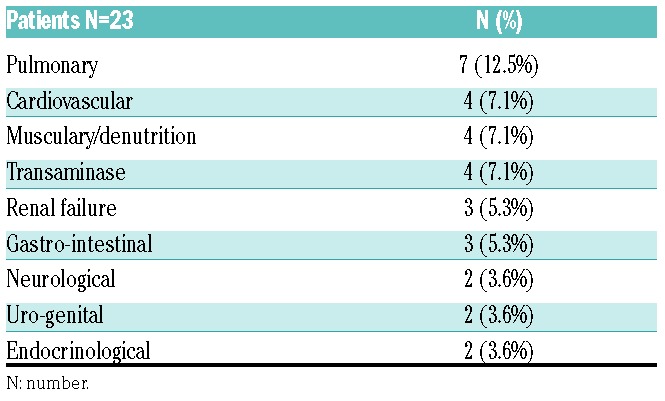
Ninety-nine serious adverse events (SAE) occurred in 44 patients, and they were mostly hematologic, with myelosuppression in 88 of 99 (88%) of events. Thirteen patients were hospitalized for febrile neutropenia with a median duration of hospitalization of 14 days. Grade III-IV non-hematologic toxicities occurring in at least 3% of patients are shown in Table 3. Regarding toxicity at injection points, patients reported less pain and less secondary lesions with subcutaneous guadecitabine injections than with previous AZA injections.
Table 3.
Grade III-IV non-hematologic toxicities during first nine cycles of guadecitabine treatment.
Discussion
In this phase II study, treatment with guadecitabine after AZA failure was generally safe in this elderly population, with limited dose reductions. It yielded a modest ORR of 14.3% and median OS of 7.1 months, but a few longer-term responders were seen, and some biological prognostic factors of response could be identified.
Responders to guadecitabine in our study had a median OS of 17.9 months, compared with six months in non-responders, the reported median survival of high-risk MDS patients after AZA failure in the literature.2 In previous smaller series of decitabine salvage after AZA failure, median OS ranged between 5.9 and 11.8 months.2,15,16 Compared with those series, and also our experience using decitabine in high-risk MDS/CMML patients after AZA failure (that reported no CR and a median response duration of only 3 months),17 the current two CR and median duration of response of 11.5 months achieved with guadecitabine, with 4 of 8 responses exceeding one year, may appear slightly better. A recent study comparing a 5-day regimen of guadecitabine (60-90 mg/m2/d) to a 10-day regimen (60 mg/m2/d) in relapsed or refractory AML reported a response rate of 16% and 30.2% (P=0.1), and an OS of 5 and 7.1 months, respectively, with no significant difference between the two regimens.18 However, only four patients in this study had received first-line treatment with HMA (more than 1 cycle), and predictive factors of response and survival were not analyzed.
In the present series, we observed significant demethylation in blood and bone marrow samples after one cycle of treatment, even if there was no significant correlation between the demethylation rate and the hematologic response, possibly because of the small number of responders. Similar results were reported using decitabine after AZA failure.16 However, a higher demethylation rate in blood on day 8 of cycle 1 was significantly associated with longer OS and remained a significant prognostic factor in multivariate analysis, suggesting that demethylation achieved with guadecitabine after AZA failure is a mechanism implicated in response. This could possibly also explain the relatively good response rate of patients with primary AZA failure, who might have experienced AZA resistance due to low level and duration of HMA exposure, but subsequently responded to guadecitabine, which induced greater demethylation.
To our knowledge, this is the first study analyzing the prognostic factors of response and OS in high-risk MDS/AML receiving a HMA after failure of a first HMA. Previous studies reported that TET2 mutations,19–21 particularly at a VAF >10% and in the absence of co-occurring ASXL1 mutations,20 were associated with higher response rates, whereas a higher number of detectable mutations predicted for lower likelihood of response and complete response, as well as shorter response duration to HMA; however, those studies involved HMA naïve patients.21 In the present study, the only predictive factor of response to guadecitabine was the absence or small number of somatic mutations, associated with a better response to treatment, while no response was observed in 11 TP53 mutated patients. Thus, TP53 mutated MDS/AML patients may have high response rates (although of short duration) with early use of HMA (especially a 10-day regimen of decitabine),5 but after HMA failure, those patients may be particularly resistant to further HMA therapy, even with a different agent.
Regarding OS, our multivariate analysis showed that primary AZA failure (vs. secondary failure), low to high R-IPSS and higher demethylation in blood (on day 8 of cycle1) were associated with better OS, factors that could help select patients more likely to benefit from second-line treatment with guadecitabine. The survival impact of guadecitabine did not seem to differ between “low-risk” and “high-risk” patients, based on Nazha et al.’s scoring system for HMA failure patients.
Altogether, our study suggests that some selected patients [primary AZA failure and low to high IPSS-R, patients with no or few somatic mutations (especially no TP53 mutations), patients with higher demethylation rate in blood during the first cycle of treatment] may benefit from guadecitabine treatment after AZA failure. Our results also suggest that primary AZA failures may be better candidates than secondary AZA failures to receive guadecitabine. An international phase III study (clinicaltrials.gov identifier: 02907359) is underway to compare guadecitabine treatment with best investigator’s choice in high-risk MDS patients relapsing or failing after first-line AZA or decitabine.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/8/1565
References
- 1.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009; 10(3):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29(24):3322–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karahoca M, Momparler RL. Pharmacokinetic and pharmacodynamic analysis of 5-aza-2′-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin Epigenetics. 2013;5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Issa J-PJ, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009; 15(12):3938–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch JS, Petti AA, Miller CA, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med. 2016; 375(21):2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang JC, Warner SL, Vollmer D, et al. S110, a 5-Aza-2′-deoxycytidine-containing dinucleotide, is an effective DNA methylation inhibitor in vivo and can reduce tumor growth. Mol Cancer Ther. 2010;9(5):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Roboz GJ, Kropf PL, et al. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol. 2017;18(10):1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Issa J-PJ, Roboz G, Rizzieri D, et al. Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose-escalation phase 1 study. Lancet Oncol. 2015;16(9):1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 11.Bennett JM, Catovsky D, Daniel MT, et al. Criteria for the diagnosis of acute leukemia of megakaryocyte lineage (M7). A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103(3):460–462. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. [DOI] [PubMed] [Google Scholar]
- 13.Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–1996. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazha A, Komrokji RS, Garcia-Manero G, et al. The efficacy of current prognostic models in predicting outcome of patients with myelodysplastic syndromes at the time of hypomethylating agent failure. Haematologica. 2016;101(6):e224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borthakur G, Ahdab SE, Ravandi F, et al. Activity of decitabine in patients with myelodysplastic syndrome previously treated with azacitidine. Leuk Lymphoma. 2008;49(4):690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duong VH, Bhatnagar B, Zandberg DP, et al. Lack of objective response of myelodysplastic syndromes and acute myeloid leukemia to decitabine after failure of azacitidine. Leuk Lymphoma. 2015;56(6):1718–1722. [DOI] [PubMed] [Google Scholar]
- 18.Harel S, Cherait A, Berthon C, et al. Outcome of patients with high risk Myelodysplastic Syndrome (MDS) and advanced Chronic Myelomonocytic Leukemia (CMML) treated with decitabine after azacitidine failure. Leuk Res. 2015;39(5):501–504. [DOI] [PubMed] [Google Scholar]
- 19.Roboz GJ, Kantarjian HM, Yee KWL, et al. Dose, schedule, safety, and efficacy of guadecitabine in relapsed or refractory acute myeloid leukemia. Cancer. 2018; 124(2):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25(7):1147–1152. [DOI] [PubMed] [Google Scholar]
- 21.Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124(17):2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montalban-Bravo G, Takahashi K, Patel K, et al. Impact of the number of mutations in survival and response outcomes to hypomethylating agents in patients with myelodysplastic syndromes or myelodysplastic/myeloproliferative neoplasms. Oncotarget. 2018;9(11):9714–9727. [DOI] [PMC free article] [PubMed] [Google Scholar]