Abstract
BACKGROUND
Spontaneous peritonitis is an infection of ascitic fluid without a known intra-abdominal source of infection. spontaneous fungal peritonitis (SFP) is a potentially fatal complication of decompensated cirrhosis, defined as fungal infection of ascitic fluid in the presence of ascitic neutrophil count of greater than 250 cells/mL.
AIM
To determine the prevalence of fungal pathogens, management and outcomes (mortality) of SFP in critically ill cirrhotic patients.
METHODS
Studies were identified using PubMed, EMBASE, Cochrane Central Register of Controlled Trials and Scopus databases until February 2019. Inclusion criteria included intervention trials and observation studies describing the association between SFP and cirrhosis. The primary outcome was in-hospital, 1-mo, and 6-mo mortality rates of SFP in cirrhotic patients. Secondary outcomes were fungal microorganisms identified and in hospital management by anti-fungal medications. The National Heart, Lung and Blood Institute quality assessment tools were used to assess internal validity and risk of bias for each included study.
RESULTS
Six observational studies were included in this systematic review. The overall quality of included studies was good. A meta-analysis of results could not be performed because of differences in reporting of outcomes and heterogeneity of the included studies. There were 82 patients with SFP described across all the included studies. Candida species, predominantly Candida albicans was the fungal pathogen in majority of the cases (48%-81.8%) followed by Candida krusei (15%-25%) and Candida glabrata (6.66%-20%). Cryptococcus neoformans (53.3%) was the other major fungal pathogen. Antifungal therapy in SFP patients was utilized in 33.3% to 81.8% cases. The prevalence of in hospital mortality ranged from 33.3% to 100%, whereas 1-mo mortality ranged between 50% to 73.3%.
CONCLUSION
This systematic review suggests that SFP in end stage liver disease patient is associated with high mortality both in the hospital and at 1-mo, and that antifungal therapy is currently underutilized.
Keywords: Spontaneous fungal peritonitis, Bacterial peritonitis, Liver, Cirrhosis, Critical
Core tip: Spontaneous fungal peritonitis (SFP) in patients with cirrhosis is associated with high in-hospital mortality rate of 33.3% to 100% and 1-mo mortality rate of 50% to 73.3%. In our systematic review of the literature, despite such high mortality rates, the condition is under diagnosed and antifungal therapy is underutilized; 33.3% to 81.8% SFP patients received anti-fungal therapy. High clinical suspicion, new methods of early diagnosis and empiric treatment in critically ill patients with peritonitis may improve outcomes.
INTRODUCTION
Spontaneous peritonitis (SP), defined as an infection of the ascitic fluid without any apparent intra-abdominal source of infection, is a potentially fatal complication of decompensated cirrhosis and occurs in approximately 12% of patients with end stage liver disease (ESLD) with mortality rates up to 40%[1,2]. It has a culture positive and a culture negative variant, also known as culture negative neutrocytic ascites (CNNA)[3]. SP is further classified into spontaneous bacterial peritonitis (SBP) and spontaneous fungal peritonitis (SFP) on the basis of microbiological cultures performed on ascitic fluid[4]. Another classification of SP includes nosocomial SP which is defined as SP which is diagnosed 48-72 h after hospital admission and community acquired (CA) SP if it is diagnosed on admission or within 2 d of presentation to the hospital[5].
SFP, a catastrophic and underestimated complication of ESLD is defined as fungal infection of the ascitic fluid and the presence of ascitic neutrophil count of > 250 cells/mL[6]. It is distinct from fungi ascites which has a neutrophil count of < 250 cells/mL in the ascitic fluid[4]. Cirrhosis with concomitant critical illness is a relevant combination that causes acquired immunodeficiency leading to increased risk of developing SFP[2]. Scarce data exists regarding clinical course, risk factors, management and outcomes of SFP particularly in critically ill patients. The aim of this systematic review was to determine the prevalence of fungal micro-organisms, management and mortality rates of critically ill cirrhotic patients with SFP.
MATERIALS AND METHODS
Data selection
The study was conducted in accordance with PRISMA guidelines[7]. PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and Scopus were searched up to February 5, 2019. The search strategy for PubMed, EMBASE, Cochrane and Scopus included search terms for all databases along with Medical Subject Headings (MeSH) terms for PubMed/Medline, and Emtree terms for EMBASE. No language restrictions were applied. The search strategy was the following for the various databases: (1) MEDLINE (PubMed); (“SFP”) AND (“cirrhosis”[Mesh] OR “cirrhotic”[Mesh] OR "Liver Cirrhosis"[Mesh]); (2) EMBASE; (“Spontaneous” NEAR/2 "fungal peritonitis" OR ”fungal peritonitis”/exp) AND (“cirrhosis” OR “cirrhotic” OR “liver cirrhosis”/exp); (3) CENTRAL; (“SFP” OR “fungal peritonitis”) AND (“"liver cirrhosis"); (4) Scopus; “SFP”.
Intervention trials and observational studies (cross-sectional, case-control and cohort study-designs) describing the association between SFP and cirrhosis in adults (> 18 years) were included. In addition, studies with bacterial and other fungal infections in the presence of concomitant SFP were included. References of review articles and included studies were hand searched to identify any additional studies.
Exclusion criteria were studies involving children (age < 18 years) or those lacking data on outcomes listed below. In addition, review articles, case reports, letter to the editor, comments, perspectives, and animal studies were excluded. The primary outcome was in-hospital, 1-mo, and 6-momortality rates of cirrhotic patients with SFP (in percentage). The secondary outcomes were fungal micro-organisms implicated in SFP cirrhotic patients and in-hospital management by anti-fungal medications (in percentage).
Risk of bias assessment
The National Heart, Lung, and Blood Institute (NHLBI) quality assessment tools were used to assess internal validity and risk of bias for each included study[8]. The following data elements were extracted from included studies: First author, publication year, journal, study design and setting, study population, controls, definition of SFP and its method of diagnosis. Quantitative estimates extracted included: In-hospital, 1-mo and 6-mo mortality rates of cirrhotic patients with SFP; fungal pathogens isolated; and in-hospital management by anti-fungal medications.
Statistical analysis
Two authors (Irfan FB and Farishta M) independently assessed the eligible studies for inclusion, and quality, and performed data extraction. In cases of discrepancy between the two authors, a third author (Tariq T) was consulted to reach consensus. The statistical methods of this study were reviewed by Patrick Karabon, William Beaumont School of Medicine, Oakland University.
RESULTS
Characteristics of included studies
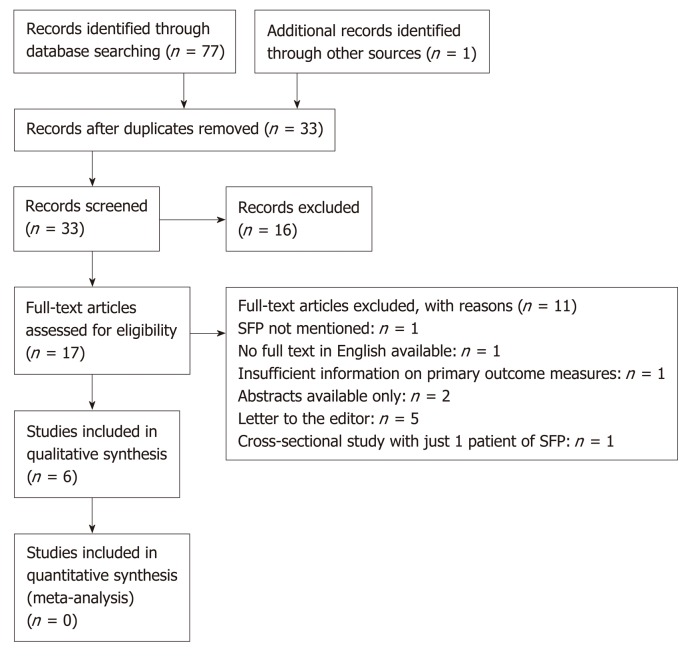
The PRISMA flowchart of included studies selection is shown in Figure 1. There were 6 studies included that evaluated mortality rates of cirrhotic patients with SFP. Of the included 6 studies, 5 studies determined the secondary outcome measure of in-hospital management of SFP patients by anti-fungal medications (Table 1). There was 1 study from South Korea; 1 study from Egypt; 2 studies from Portugal and Germany; 1 study from the United States; and 1 multi-center database study from 28 health centers in United States, Canada and Saudi Arabia. There were 3 cross-sectional studies, 1 case-control study, 1 prospective cohort study, and 1 nested-cohort study. The differences in reporting of outcomes (mortality, micro-organisms and management) and heterogeneity of included studies did not allow a pooled analysis of results.
Figure 1.
PRISMA flow diagram[27].
Table 1.
Management, prognosis and mortality in cirrhotic patients with spontaneous fungal peritonitis
| Study | Country, setting | Study design | Study population |
Management |
Mortality |
| n (%) | n (%) | ||||
| Hwang et al[10], 2014 | South Korea, University Hospital | Retrospective, Cross-sectional | n = 416 patients | 3rd generation cephalosporin (n = 15) | In-hospital: - |
| SBP (n = 401) | Antifungal: n = 5 (33.3%) | 1-mo: 11/15 (73.3%) | |||
| SFP (n = 4) | Amphotericin B: n = 2 | 6-mo: 3/15 (20%) | |||
| Polymicrobial SFP (n = 11) | Liposomal Amphotericin B: n = 1 | ||||
| Fluconazole: n = 2 | |||||
| Hassan et al[9], 2014 | Egypt, University Hospital | Prospective cohort study | n = 46 patients | Not described | In-hospital: 1/3 (33.33%) |
| Control patients with no infection (n = 18) | |||||
| SFP (n = 4; only 3 patients described with ascitic fluid polymorphs > 250 cells/mm3) | |||||
| Karvellas et al[11], 2015 | (CATSS Database) from 28 medical centers in United States, Canada, Saudi Arabia | Retrospective cohort study | n = 126 patients | Anti-fungal: n = 9 (81.8%) | In-hospital: 11/11 (100%) |
| SBP (n = 126) | |||||
| SFP and SBP (n = 11) | |||||
| Bremmer et al[1], 20151 | University of Pittsburgh, United States | Retrospective, cross-sectional study | n = 25 | Antifungal: n = 15 (60%) | In hospital: 15/25 (60%) |
| SFP (n = 25) | One mo: 14/25 (56%) | ||||
| Lahmer, et al[2], 2016 | University Hospital, Germany | Retrospective, cross-sectional study | n = 208 SFP (n = 20) | Antibiotic pretreatment: SFP n = 17 Antifungal: n = 6 (30% of SFP) | In-hospital: 18/20 (90%) |
| SBP (n = 28) | |||||
| Gravito-Soares et al[6], 2017 | University of Coimbra, Coimbra, Portugal | Retrospective, case–control study | n = 231 | Cefotaxime n = 231 | 1 -mo: 4/8 |
| SFP (n = 3) | Antifungal: n = 5/8 (62.5%) | (50%) | |||
| Polymicrobial SFP (n = 5) | Fluconazole: n = 3 | ||||
| SBP (n = 119) | Caspofungin: n = 1 | ||||
| Amphotericin B: n = 1 |
The study was described as a retrospective cohort study design but in the absence of a comparative non-exposure/control group we decided it more appropriately fitted the description of a cross—sectional study. SBP: Spontaneous bacterial peritonitis; SFP: Spontaneous fungal peritonitis.
There was a total of 82 cirrhotic patients with SFP in all the included 6 studies. Of the total 82 SFP patients, 27 patients had polymicrobial SFP. Candida spp. was the fungal pathogen in the majority of cases: Candida albicans (48%-81.8%); Candida krusei (15%-25%); Candida glabrata (6.66%-20%); Candida parapsilosis (5%-16%); Candida tropicalis (6.66%-12%); Candida kefyr (10%); Candida lusitaniae (12.5%); and Candida zeylanoides (4%). Besides candida spp., other significant fungal pathogens included Cryptococcus neoformans (53.3%). Antifungal therapy utilization ranged from 33.3% to 81.8%. The prevalence of in-hospital mortality ranged from 33.3%-100%, 1-mo mortality had a range of 50%-73.3%. Only 1 study described 6-mo mortality of 20% in their study.
Micro-organisms, management and mortality in cirrhotic patients with SFP
Bremmer et al[1], conducted a retrospective study and identified patients with fungal ascitic fluid cultures through microbiology records. Exclusion criteria included patients without a history of cirrhosis or if an alternative reason for peritonitis was found. There were 25 SFP cirrhotic patients with the following fungal infections: 48% (12/25) patients had Candida albicans; 20% (5/25) patients had C. glabrata; 16% (4/25) patients had C. parapsilosis; 12% (3/25) patients had C. tropicalis; and 4% (1/25) patient had C zeylanoides. Antifungal therapy was given to 60% (15/25) patients. There were 15 patients that were treated with antifungal medications, 3 patients had persistent or recurrent peritonitis. The 1 mo and in-hospital mortality were 56% (14/25) and 60% (15/25), respectively. There was no statistically significant difference in mortality between patients managed with caspofungin (38%), fluconazole (57%) or patients from whom antifungals were withheld electively (25%). The median time to death was 6 d (IQR: 3-7)[1].
Gravito-Soares et al[6], carried out a case-control study, with 8 cirrhotic SFP patients compared with 119 cirrhotic SBP (control) patients. Of 8 cirrhotic SFP patients, 62.5% (5/8) patients had co-infection by bacteria and fungi. Antifungal therapy was utilized in 7 (87.5%) patients. Appropriate antifungal therapy was given to 62.5% (5/8) patients: Candida albicans infection was treated with Fluconazole (2/3); Candida lusitaniae infection treated with Fluconazole (1/1); Candida tropicalis managed with Caspofungin (1/1); and Geotrichum capitatus treated with Amphotericin B (1/1). There were 2 patients (25%) with Candida krusei that had resistance to initial antifungal therapy with Fluconazole; and 1 patient died due to late diagnosis of SFP. The 30-d (1 mo) mortality was 50% (4/8) and overall mortality was 62.5% (5/8) of cirrhotic SFP patients in the study. The mean time duration between SFP diagnosis and death was 17.6 d ± 11.5 d.
Hassan et al[9] carried out a prospective cohort study including 46 ESLD patients; 18 control patients with no infection and 28 patients with invasive fungal infection. Of 28 cases, 4 (16%) patients had SFP. Although 4 patients were described as having SFP, ascitic fluid polymorphs > 250 cells/mm3 were only described in three patients, and only 2 patients had fungal micro-organisms (Aspergillus niger and Candida albicans) isolated from ascitic fluid. Management of SFP patients was not described. Of the three SFP patients with ascitic fluid polymorphs > 250 cells/mm3, in-hospital mortality occurred in only one patient[9].
Hwang et al[10] conducted a retrospective cross-sectional study and compared SFP patients with SBP patients. During the study period of 5 years, 416 patients with SP were included of which 15 (3.6%) had SFP and 410 (96.4%) had SBP. Eleven out of 15 SFP patients had concomitant bacterial infection. The fungal isolates identified in SFP patients were the following: Candida albicans (n = 8), Candida tropicalis (n = 1), Candida glabrata (n = 1) and Cryptococcus neoformans (n = 8). However, only 5 patients, among 15 SFP patients received anti-fungal therapy. All patients received third-generation cephalosporin (cefotaxime/ceftriaxone). The SFP patients had a 1-mo mortality rate of 73.3% (11/15). The median time to death was 2 d (range, 0-20 d)[10].
Karvellas et al[11] utilized the Cooperative Antimicrobial Therapy of Septic Shock (CATSS) database and carried out a nested cohort study to determine the appropriate antimicrobial management in cirrhotic patients with SBP-associated septic shock. The Cooperative Antimicrobial Therapy of Septic Shock (CATSS) database collected data on septic shock patient from 28 medical centers in Canada, the United States, and Saudi Arabia. There were 126 cirrhotic SBP associated-septic shock patients included in the study from CATSS database. Of these, 11 patients had concomitant SFP; Candida albicans (9/11) and Candida tropicalis (1/11) and Candida glabrata (1/11). Nine patients (81.8%) were treated with antifungal therapy. All SFP patients died during the course of their hospital stay[11].
Lahmer et al[2] performed a retrospective cross-sectional study by reviewing medical records of cirrhotic critically ill patients with SBP. Of 205 patients included in the study, 20 (10%) patients were identified with SFP. Majority of the patients had Candida spp.: C. albicans (n = 12), C. glabrata (n = 3), C. krusei (n = 3), C. Kefyr (n = 2), C. parapsilosis (n = 1), C. tropicalis (n = 1). Antifungal therapy was given to 30% (n = 6) patients. Mortality rate was 90% (n = 18) patients[2].
Quality assessment
Quality assessment of the included studies was performed according to NHLBI quality assessment tools (Tables 2 and 3)[8]. All the cross-sectional and cohort studies were of high-quality while the case-control study was of fair quality[1,2,6,9-11]. None of the studies described sample size or power estimates. Low sample size was the major limitation in all studies (n ≤ 25). Only 2 studies did not define SFP. Four studies; Hwang et al[10], Gravito-Soares et al[6], Lahmer et al[2], and Bremmer et al[1], had primary outcomes of SFP in patients with cirrhosis. The other two studies had the following primary outcomes in cirrhotic patients: Karvellas et al[11] had a primary outcome of SBP; and Hassan et al[9], had a primary outcome of invasive fungal infection. All the studies included patient baseline characteristics and risk factors, fungal pathogens, management with anti-fungal therapy (except Hassan et al[9]) and mortality.
Table 2.
Quality assessment of included observational cohort and cross-sectional studies according to NHBLI Quality Assessment Tool
| Hwang et al[10], 2014 | Hassan et al[9], 2014 | Karvellas et al[11], 2015 | Bremmer et al[1], 2015 | Lahmer et al[2], 2016 | |
| 1 Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes |
| 2 Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes |
| 3 Was the participation rate of eligible persons at least 50%? | Yes | Yes | Yes | Yes | Yes |
| 4 Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | Yes |
| 5 Was a sample size justification, power description, or variance and effect estimates provided? | No | No | No | No | No |
| 6 For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | Yes | Yes | Yes | Yes | Yes |
| 7 Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Yes | Yes | Yes | Yes | Yes |
| 8 For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | NA | NA | Yes | NA | NA |
| 9 Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes |
| 10 Was the exposure(s) assessed more than once over time? | No | No | No | No | No |
| 11 Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes |
| 12 Were the outcome assessors blinded to the exposure status of participants? | No | No | No | No | No |
| 13 Was loss to follow-up after baseline 20% or less? | Yes | Yes | Yes | Yes | Yes |
| 14 Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | No | Yes | Yes | Yes | Yes |
| Rating | Good | Good | Good | Good | Good |
Table 3.
Quality assessment of included case-control studies according to NHBLI Quality Assessment Tool
| Gravito-Soares et al[6], 2017 | |
| 1 Was the research question or objective in this paper clearly stated? | Yes |
| 2 Was the study population clearly specified and defined? | Yes |
| 3 Did the authors include a sample size justification? | No |
| 4 Were controls selected or recruited from the same or similar population that gave rise to the cases (including the same timeframe)? | Yes |
| 5 Were the definitions, inclusion and exclusion criteria, algorithms or processes used to identify or select cases and controls valid, reliable, and implemented consistently across all study participants? | No |
| 6 Were the cases clearly defined and differentiated from controls? | Yes |
| 7 If less than 100 percent of eligible cases and/or controls were selected for the study, were the cases and/or controls randomly selected from those eligible? | NA |
| 8 Was there use of concurrent controls? | No |
| 9 Were the investigators able to confirm that the exposure/risk occurred prior to the development of the condition or event that defined a participant as a case? | Yes |
| 10 Were the measures of exposure/risk clearly defined, valid, reliable, and implemented consistently (including the same time period) across all study participants? | Yes |
| 11 Were the assessors of exposure/risk blinded to the case or control status of participants? | No |
| 12 Were key potential confounding variables measured and adjusted statistically in the analyses? If matching was used, did the investigators account for matching during study analysis? | Yes |
| Rating | Fair |
DISCUSSION
Based on our review, the prevalence of SFP anywhere from 2%-10% (Table 1). This is in keeping with a prior meta-analysis which documented a 4.28% prevalence[12]. The reasons for this low prevalence are several most important of which are low index of suspicion leading to a delay in carrying out appropriate diagnostic work up, and longer period of time required for fungal growth. Despite a lower prevalence than SBP, this systematic review confirms that SFP patients with cirrhosis have a high in-hospital mortality (33.3%-100%) and 1-mo mortality (50%-73.3%).
Our systematic review suggests several reasons for the high mortality rates in cirrhotic patients with SFP. Patients with SFP had 3.6 times higher risk of admissions to ICU with severe sepsis/septic shock as compared to SBP patients[6]. Higher mortality rates were observed in patients with high Charlson Comorbidity Index, Model for ESLD and APACHE II scores. Another unique observation was a significantly higher 1-mo mortality in patients who did not undergo liver transplantation compared to patients who underwent liver transplantation. Hence, antifungal therapy in SFP patients could be utilized as a bridging therapy to liver transplantation[1]. Furthermore, a high rate of mortality was noted in patients with SFP who were treated empirically for suspected SBP. After empirical treatment for suspected SBP was initiated, the condition of most of the SFP patients deteriorated resulting in death, even when treated with antifungal agents[10]. In a systemic review suggested that lack of improvement within 48 h after admission is linked to an increased risk of SFP. Hence, fungi should be sought as potential pathogens in cases of ceftriaxone or cefotaxime-resistant SP[13] which occurs in approximately 7%-17% of cirrhotic patients[6].
Understanding the microbiology, diagnosis, and treatment of SFP may lower the associated mortality. Our systematic review provides insight into the microbiology of SFP. Fungi are saprophytes that are common commensal organisms of the skin and mucous membranes[14]. Significant fungal colonization occurs when antibiotics are used for the prevention of SBP in patients with ascites as a result of reduction in the intestinal bacterial flora. This subsequently leads to translocation across the damaged gastrointestinal tract mucosa into the peritoneal activity, causing peritonitis[4]. This effect is enhanced in the setting of immunosuppression and malnutrition which is common in ESLD[5]. Fungi are much larger in size (Candida spp. 10-12 µm) than bacteria including E. coli (0.3-1 µm and K. pneumoniae (0.6-6 µm) hence a higher gut permeability is required for fungal translocation[1,10]. This explains why SFP is likely limited to those individuals who experience the greatest hit to their innate immunity and those with advanced cirrhosis.
In keeping with the literature, this systematic review shows that Candida albicans is the most frequent fungal infectious agent isolated from ascitic fluid cultures, followed by candida glabrata, candida parapsilosis, candida krusei, and candida tropicalis. A recent study described a shift towards increasing prevalence of candida glabrata and candida parapsilosis infections in cirrhotic patients[15]. Cryptococcus neoformans, Aspergillus spp. and Fusarium have also been isolated though less commonly than candidal spp[2,5]. One of the possible explanations as to why candida is more common in patients with SFP as compared to other fungi such as cryptococcus is probably related to the size difference between these pathogenic organisms[10]. Cryptococcal spp. have diameters up to 20 µm which limit their migration across the intestinal wall[16]. Fungal infections are often polymicrobial with bacterial colonization occurring in 32%-74% of SFP cases[13].
This systematic review also provides data on risk factors for SFP. The findings of our review reveal that SFP is more commonly seen in patients with Child Pugh Class C liver cirrhosis and those with MELD score beyond 30 points[13]. Higher bilirubin levels, blood urea nitrogen levels, low ascitic fluid protein (< 1 g/dL), antibiotic prophylaxis against SBP and hepatorenal syndrome (HRS) are other potential risk factors that have been described in literature[6,11]. It has been speculated that prophylactic antibiotics alter the normal intestinal flora and cause an excessive growth of fungi and is considered one of the pathophysiological mechanisms for the development of fungal peritonitis and dissemination[17]. Furthermore, patients with corticosteroid use, prolonged antimicrobial use, central venous catheter, total parenteral nutrition, high APACHE score, renal replacement therapy, or malnutrition are more susceptible to opportunistic fungal infections[18-20]. Renal failure is associated with impaired cell mediated immunity and defective granulocyte-macrophage function, which are the dominant host defenses against fungal pathogens[9]. Nosocomial development of SP was also found to be a risk factor for SFP[10,12,13]. As a result of commensal colonization of mucocutaneous membranes, percutaneous inoculation of fungi can commonly occur in patients with refractory ascites who undergo routine paracentesis[9]. Other invasive procedures such as colonoscopy, urinary catheterization and nasogastric intubation have also been identified as risk factors for SFP[6].
This systematic review also sheds light on the risk factors associated with increased mortality from SFP. These factors include severity of liver disease as measured by higher MELD or Child-Pugh score C, recent antibacterial prophylaxis, presence of HRS, low ascitic protein concentration, high acute physiology, and Chronic Health Evaluation II (APACHE II) score and presence of septic shock[12]. In a retrospective cohort study of 241 cirrhotic patients with invasive candidiasis, multivariate analysis demonstrated septic shock (odds ratio 3.2, CI: 1.7-6, P < 0.001) as the most significant predictor of mortality[15].
Given the high mortality related to SFP, early diagnosis and treatment are essential to improving outcomes for patients with SFP. Newer diagnostic tests like pan-fungal PCR assay and 1,3 beta-D-Glucan are not only more sensitive in detecting fungi in peritoneal fluid but also help in early identification of SFP by shortening the time to diagnosis[21,22]. In patients with risk factors for SFP, our systematic review supports early testing of peritoneal fluid with these assays. In addition, laboratorial parameters such as leukocyte count, procalcitonin or C-reactive are too non-specific for SFP and hence are not very useful in SFP diagnosis[2]. It is important to note that ascitic lactate dehydrogenase, blood WBC count, blood urea nitrogen and predominance of lymphocytes in ascitic fluid were significantly higher in SFP compared with patients with SBP and might provide a clue to diagnosis[6,9,23].
Despite its high mortality, the results from this systematic review show suboptimal utilization of antifungal therapy utilization in cirrhotic SFP patients, ranging from 33.3% to 81.8%. In addition, we note that only a small percent was treated using appropriate systemic antifungal therapy. Based on the contemporary microbiology of SFP, our review supports the use of echinocandins as initial therapy with tailoring after culture results are available. Echinocandins are preferred over fluconazole in septic shock due to their lower overall toxicity and high tolerability. They are associated with lower hepatoxicity compared to fluconazole. Once patients become clinically stable, antimicrobial therapy is de-escalated from echinocandins to fluconazole[15,24-27]. Furthermore, empiric treatment is essential for reducing risk of mortality in SFP as fungal recovery using routine culture methods is associated with significant delays. Our review shows that 73% of patients receiving antifungal therapy experienced a median lag in treatment of 3 d until yeast was isolated from ascitic fluid cultures with the average time from SFP diagnosis to death was 2 d[1,10]. This further supports the use of empiric broad-spectrum antifungals while awaiting culture results in those who are most at risk. Limited and low-quality data exists regarding the appropriate time for initiation of empiric antifungal treatment. Based on this systematic review and current literature, it is reasonable to use antifungal therapy in critically ill cirrhotics with ascites who fail to recover within 48 h of receiving broad spectrum antibiotics, in those patients who are at increased risk of developing infections. For instance, patients on immunosuppressants or antibiotics for a long time, those with invasive vascular access devices, patients on total parenteral nutrition or renal replacement therapy or those who have high APACHE scores and are malnourished. These patients are at higher risk of mortality from SFP if mis-diagnosed[4,18]. However, given the low overall incidence of SFP, empiric antifungal therapy is generally not recommended in patients with CA SP.
In conclusion, SFP is not an uncommon complication in cirrhotic patients and associated with high mortality both in the hospital and at 1 mo. High clinical suspicion is required particularly in those with higher MELD and Child Pugh scores who fail to improve despite appropriate antibiotic treatment. Antifungal therapy is inappropriately used and currently underutilized. Our review suggests rapid initiation of antifungal therapy in the presence of septic shock and failure to respond to broad spectrum antibiotic regimen. It also highlights the need for further studies that will inform the timing and choice of anti-fungal use in patients at high-risk for SFP. Finally, our review also shows that liver transplantation is a possible outcome for those with SFP with low risk for short-term recurrence and acceptable 1-mo mortality rates.
ARTICLE HIGHLIGHTS
Research background
Spontaneous fungal peritonitis (SFP) is a devastating and underestimated complication of end stage liver disease (ESLD) which is defined as fungal infection of the ascitic fluid and the presence of ascitic neutrophil count of > 250 cells/mL. The combination of cirrhosis and critical illness causes acquired immunodeficiency leading to increased risk of developing SFP. There is limited literature regarding clinical course, risk factors, management and outcomes of SFP particularly in critically ill patients. With this study, we have compiled a systematic review of available data on SFP.
Research motivation
When compared to spontaneous bacterial peritonitis, SFP is less well recognized and is associated with higher mortality rates. In many cases, the clinical importance of isolating Candida from abdominal cultures is unknown and therapeutic approaches are largely undefined. Furthermore, the epidemiology and outcomes of patients with SFP have only been reported sporadically in literature. Hence, by performing a systematic review we aimed to increase the available knowledge regarding SFP.
Research objectives
The main objective of the study was to determine the prevalence of fungal micro-organisms and describe the risk factors, management and mortality rates of SFP in critically ill patients with cirrhosis.
Research methods
This is a systematic review of available studies identified using PubMed, EMBASE, Cochrane Central Register of Controlled Trials and Scopus databases. Inclusion criteria were intervention trials and observation studies describing the association between SFP and cirrhosis. The primary outcome was in-hospital, 1-mo, and 6-mo mortality rates of SFP in cirrhotic patients. Secondary outcomes were fungal microorganisms identified and anti-fungal medications utilized for the management of SFP. The National Heart, Lung and Blood Institute quality assessment tools were used to assess internal validity and risk of bias for each included study.
Research results
Six observational studies were included in this systematic review. A total of 82 patients with SFP were identified in these studies. Candida albicans was the predominant fungal pathogen in majority of the cases (48-81.8%) followed by Candida krusei (15%-25%) and Candida glabrata (6.66%-20%). Antifungal therapy in SFP patients was utilized in 33.3% to 81.8% cases. The in-hospital mortality ranged from 33.3% to 100%, whereas 1-mo mortality ranged between 50% and 73.3%.
Research conclusions
SFP is not an uncommon complication associated with a worse prognosis in cirrhotic patients, particularly those with higher MELD and Child Pugh scores who fail to improve despite appropriate antibiotic treatment. Our study also showed that antifungal therapy is currently underutilized. Rapid initiation of antifungal therapy in the presence of septic shock and failure to respond to broad spectrum antibiotic regimen is crucial in the management of SFP.
Research perspectives
Future large-scale, prospective studies aimed at identifying the ideal timing and choice of anti-fungal therapy in patients at high-risk for developing SFP are needed. Also, research efforts should aim at determining appropriate non-cultural tests for SFP in order to improve the rapidity of diagnosis.
ACKNOWLEDGEMENTS
We would like to thank Iris Kovar-Gough for helping with the search strategy for the study.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None declared in agreement with all co-authors.
Peer-review started: May 29, 2019
First decision: June 10, 2019
Article in press: July 5, 2019
P-Reviewer: Garbuzenko DV, Gencdal G S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
、
Contributor Information
Tooba Tariq, Department of Internal Medicine, Western Michigan University, Kalamazoo, MI 49008, United States. toobatariq31@gmail.com.
Furqan B Irfan, College of Osteopathic Medicine, Michigan State University, WEast Lansing, MI 48824, United States.
Mehdi Farishta, Department of Internal Medicine, Western Michigan University, Kalamazoo, MI 49008, United States.
Brian Dykstra, Department of Pulmonary and Critical Care Medicine, Western Michigan University, Kalamazoo, MI 49008, United States.
Eric Martin Sieloff, Department of Internal Medicine, Western Michigan University, Kalamazoo, MI 49008, United States.
Archita P Desai, Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, IN 46202, United States.
References
- 1.Bremmer DN, Garavaglia JM, Shields RK. Spontaneous fungal peritonitis: a devastating complication of cirrhosis. Mycoses. 2015;58:387–393. doi: 10.1111/myc.12321. [DOI] [PubMed] [Google Scholar]
- 2.Lahmer T, Brandl A, Rasch S, Schmid RM, Huber W. Fungal Peritonitis: Underestimated Disease in Critically Ill Patients with Liver Cirrhosis and Spontaneous Peritonitis. PLoS One. 2016;11:e0158389. doi: 10.1371/journal.pone.0158389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheer TA, Runyon BA. Spontaneous bacterial peritonitis. Dig Dis. 2005;23:39–46. doi: 10.1159/000084724. [DOI] [PubMed] [Google Scholar]
- 4.Maraolo AE, Buonomo AR, Zappulo E, Scotto R, Pinchera B, Gentile I. Unsolved Issues in the Treatment of Spontaneous Peritonitis in Patients with Cirrhosis: Nosocomial Versus Community-acquired Infections and the Role of Fungi. Rev Recent Clin Trials. 2019;14:129–135. doi: 10.2174/1574887114666181204102516. [DOI] [PubMed] [Google Scholar]
- 5.Fiore M, Leone S. Spontaneous fungal peritonitis: Epidemiology, current evidence and future prospective. World J Gastroenterol. 2016;22:7742–7747. doi: 10.3748/wjg.v22.i34.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravito-Soares M, Gravito-Soares E, Lopes S, Ribeiro G, Figueiredo P. Spontaneous fungal peritonitis: a rare but severe complication of liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1010–1016. doi: 10.1097/MEG.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 8.National Heart, Lung, and Blood Institute. Development and use of quality assessment tools. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Google Scholar]
- 9.Hassan EA, Abd El-Rehim AS, Hassany SM, Ahmed AO, Elsherbiny NM, Mohammed MH. Fungal infection in patients with end-stage liver disease: low frequency or low index of suspicion. Int J Infect Dis. 2014;23:69–74. doi: 10.1016/j.ijid.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SY, Yu SJ, Lee JH, Kim JS, Yoon JW, Kim YJ, Yoon JH, Kim EC, Lee HS. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014;33:259–264. doi: 10.1007/s10096-013-1953-2. [DOI] [PubMed] [Google Scholar]
- 11.Karvellas CJ, Abraldes JG, Arabi YM, Kumar A Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747–757. doi: 10.1111/apt.13135. [DOI] [PubMed] [Google Scholar]
- 12.Fiore M, Chiodini P, Pota V, Sansone P, Passavanti MB, Leone S, Aurilio C, Pace MC. Risk of spontaneous fungal peritonitis in hospitalized cirrhotic patients with ascites: a systematic review of observational studies and meta-analysis. Minerva Anestesiol. 2017;83:1309–1316. doi: 10.23736/S0375-9393.17.12034-1. [DOI] [PubMed] [Google Scholar]
- 13.Shizuma T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: A literature review. World J Hepatol. 2018;10:254–266. doi: 10.4254/wjh.v10.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould D. Diagnosis, prevention and treatment of fungal infections. Nurs Stand. 2011;25:38–47; quiz 48. doi: 10.7748/ns2011.04.25.33.38.c8464. [DOI] [PubMed] [Google Scholar]
- 15.Bassetti M, Peghin M, Carnelutti A, Righi E, Merelli M, Ansaldi F, Trucchi C, Alicino C, Sartor A, Toniutto P, Wauters J, Laleman W, Tascini C, Menichetti F, Luzzati R, Brugnaro P, Mesini A, Raviolo S, De Rosa FG, Lagunes L, Rello J, Dimopoulos G, Colombo AL, Nucci M, Vena A, Bouza E, Muñoz P, Tumbarello M, Losito R, Martin-Loeches I, Viscoli C. Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: a multicenter study. Intensive Care Med. 2017;43:509–518. doi: 10.1007/s00134-017-4717-0. [DOI] [PubMed] [Google Scholar]
- 16.Gazzoni AF, Oliveira Fde M, Salles EF, Mayayo E, Guarro J, Capilla J, Severo LC. Unusual morphologies of Cryptococcus spp. in tissue specimens: report of 10 cases. Rev Inst Med Trop Sao Paulo. 2010;52:145–149. doi: 10.1590/s0036-46652010000300006. [DOI] [PubMed] [Google Scholar]
- 17.Choi SH, Soo Kim Y, Chung JW, Choo EJ, Kwak YG, Lee YS, Kim MN, Woo JH, Ryu J, Kim NJ. Clinical significance of untreated Candida species isolated from ascites in cirrhotic patients. Scand J Infect Dis. 2004;36:649–655. doi: 10.1080/00365540410020866. [DOI] [PubMed] [Google Scholar]
- 18.Nadim MK, Durand F, Kellum JA, Levitsky J, O'Leary JG, Karvellas CJ, Bajaj JS, Davenport A, Jalan R, Angeli P, Caldwell SH, Fernández J, Francoz C, Garcia-Tsao G, Ginès P, Ison MG, Kramer DJ, Mehta RL, Moreau R, Mulligan D, Olson JC, Pomfret EA, Senzolo M, Steadman RH, Subramanian RM, Vincent JL, Genyk YS. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol. 2016;64:717–735. doi: 10.1016/j.jhep.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Righi E. Management of bacterial and fungal infections in end stage liver disease and liver transplantation: Current options and future directions. World J Gastroenterol. 2018;24:4311–4329. doi: 10.3748/wjg.v24.i38.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runyon BA AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen MH, Wissel MC, Shields RK, Salomoni MA, Hao B, Press EG, Shields RM, Cheng S, Mitsani D, Vadnerkar A, Silveira FP, Kleiboeker SB, Clancy CJ. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin Infect Dis. 2012;54:1240–1248. doi: 10.1093/cid/cis200. [DOI] [PubMed] [Google Scholar]
- 23.Bal CK, Bhatia V, Khillan V, Rathor N, Saini D, Daman R, Sarin SK. Spontaneous cryptococcal peritonitis with fungemia in patients with decompensated cirrhosis: Report of two cases. Indian J Crit Care Med. 2014;18:536–539. doi: 10.4103/0972-5229.138161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JL, Chang CH, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother. 2010;54:2409–2419. doi: 10.1128/AAC.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 26.Fiore M, Andreana L, Leone S. Preemptive therapy of spontaneous fungal peritonitis. Hepatology. 2016;64:997–998. doi: 10.1002/hep.28448. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]