Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in the Western world. It is more prevalent in male gender, and with increasing age, obesity, and insulin resistance. Besides weight loss, there are limited treatment options. The use of anti-diabetic medications has been studied with mixed results. In this review, we discuss the use of anti-diabetic medications in the management of NAFLD with a specific focus on sodium-glucose cotransporter 2 inhibitors. We shed light on the evidence supporting their use in detail and discuss limitations and future directions.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Sodium-glucose cotransporter 2 inhibitors, Liver cirrhosis, Diabetes
Core tip: Non-alcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in the Western world. NAFLD is associated with obesity and insulin resistance. Weight loss is the cornerstone of therapy with no other proven pharmacologic therapy, Sodium-glucose co-transporter 2 (SGLT2) inhibitors may play a role in preventing and treating NAFLD. SGLT2 inhibitors reduce hepatic steatosis, steatohepatitis, and fibrosis in patients with NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of liver disease in Western countries[1] and its prevalence worldwide is increasing substantially. NAFLD constitutes a spectrum of liver disease that extends from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH), a more progressive form of the disease that can lead to advanced fibrosis or cirrhosis. The worldwide prevalence is approximately 10%-35%[2]. In the United States, it is estimated that NAFLD affects more than 20% of the population[3,4]. Cardiovascular disease remains the most common cause of death in patients with NAFLD[5].
The underlying pathophysiology of NAFLD is not fully understood, but genetics and insulin resistance seem to play key roles[6]. Certain risk factors have been identified in NAFLD. Gender, age, ethnicity, and the presence of obesity or type 2 diabetes mellitus (T2DM) are differentially associated with NAFLD. Males are affected more often than females with approximately a 2:1 ratio. Most patients are diagnosed in their 40 s and 50 s. Studies demonstrate a higher prevalence in Hispanics, medium prevalence in Caucasians, and relatively low prevalence amongst blacks[7]. Certain genetic polymorphisms (i.e., PNPLA-3 and TM6SF2) have also been implicated in the disease process leading to more progressive form of NAFLD[8].
The multifaceted pathophysiologic nature of NAFLD has challenged the development of targeted therapeutic strategies for this growing disease. Thus far, weight loss is the most effective therapy with 3%-5% weight loss resulting in improvement of liver transaminases and reversal of steatosis[9,10], and 7%-10% weight reduction resulting in reversal of abnormal histologic features[11].
Pharmacologic therapies for NAFLD have not yet gained widespread use, mainly due to the poor quality of evidence supporting their use. Evaluated medications include those with anti-oxidative effects (Vitamin E)[12], anti-inflammatory effects (Ursodeoxycholic acid)[13], lipid-lowering effects (Atorvastatin)[14], anti-diabetic medications, and other nutritional supplements (Omega-3 fatty acids)[15].
In this review, we focus on the use of anti-diabetic agents in the treatment of NAFLD, more specifically on the newly emerging class of Sodium-glucose co-transporter 2 (SGLT2) inhibitors. We shed light on the evidence supporting their use in detail and discuss future directions.
SEARCH CRITERIA
MEDLINE search was conducted using the keywords “SGLT2 inhibitors” and “NAFLD” OR “NASH” and all the studies were included. There were no excluded articles. The studies were mainly focused on the role of SGLT2 inhibitors in NAFLD and were included up to December 2018.
ANTI-DIABETICS IN NAFLD
A cornerstone in the management of NAFLD is treating concomitant diabetes mellitus. The relationship between NAFLD and type-2 diabetes mellitus (T2DM) is well established and is often relayed as a bidirectional relationship. There is an association between the prevalence of NAFLD and T2DM, as multiple prospective observational studies shown NAFLD independently increases the incidence of T2DM[16-21]. In one study, NAFLD was independently associated with impaired glucose metabolism[22]. Previous reports show a high prevalence of NAFLD in patients with T2DM[23,24]. T2DM was also associated with worsening NAFLD and progression to NASH and hepatocellular carcinoma (HCC)[25-27]. The underlying mechanisms between NAFLD and T2DM is complicated, but stems from the critical role the liver plays in regulating glucose and lipid metabolism, where the inciting event is thought to be a fat-associated chronic low-grade inflammatory response[28,29]. As there is overwhelming evidence that NAFLD and T2DM share a common pathogenesis[30], the treatment of T2DM had been suggested as an important key in the management of NAFLD.
METFORMIN
Metformin is the most commonly used medication in the management of T2DM. It reduces hepatic glucose production and promotes skeletal muscle glucose uptake. Given the pathogenesis of NAFLD and T2DM, multiple investigations have been carried out regarding its use in NASH. However, a meta-analysis published in 2010 demonstrated that metformin failed to improve hepatic steatosis, inflammation, hepatocyte ballooning, Alanine aminotransferase (ALT) levels, liver fibrosis, or body mass index (BMI) in subjects with simple steatosis or biopsy-proven NASH[31]. As a result, metformin is not recommended for use in NAFLD, even in patients with T2DM.
THIAZOLIDINEDIONES
Thiazolidinediones are PPAR-gamma agonists that enhance insulin sensitivity[32]. A study investigating the effect of pioglitazone on patients with NASH but without T2DM showed a significant reduction in ALT levels and improvement in histological features of NAFLD such as steatosis, inflammation, and hepatocyte ballooning when compared to placebo[33], however it did not slow down the progression of hepatic fibrosis[33].
INCRETIN-BASED THERAPY
GLP-1 agonists are incretin-based therapies that are used in the management of T2DM by promoting glucose-dependent insulin secretion[34]. An investigation comparing liraglutide and placebo in patients with NASH showed that liraglutide led to a significant resolution of steatosis as determined by an end-of-treatment liver biopsy[35]. It was also shown to slow down the progression to fibrosis[35].
Dipeptidyl-peptidase 4 (DPP-4) inhibitors, such as sitagliptin, inhibit the degradation of incretins, which in turn stimulate secretion of insulin in patients with T2DM. They have been shown to have extra-pancreatic effects, including protective effects on hepatocytes against diet-induced steatosis and ultimately NAFLD[36]. Not only do they prevent the development of NAFLD, but they seem to exert an effect in treating it by influencing the serum levels of ALT, Aspartate aminotransferase (AST) and gamma-GT[37]. They were also found to be safe in patients with T2DM and NAFLD, and had been suggested as a potential mono-therapeutic agent for NAFLD[38]. However, there are yet to be randomized controlled trials showing their therapeutic effects in NAFLD.
SGLT2 INHIBITORS
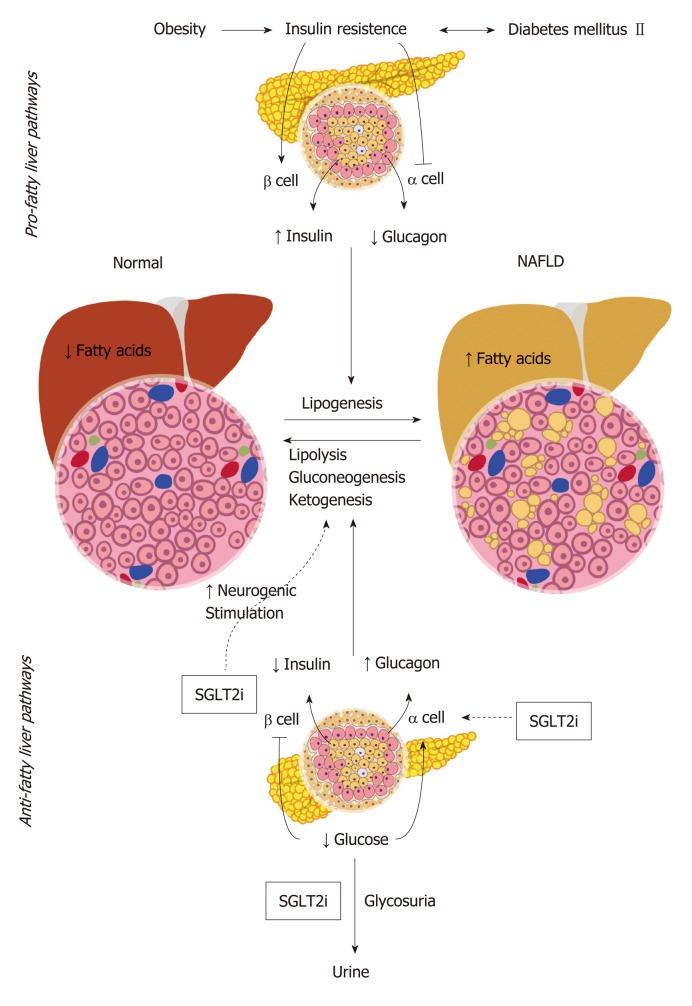
SGLT2 inhibitors are a class of drugs that inhibit glucose reabsorption in the kidney via inhibition of the SGLT channels which are primarily located in the proximal convoluted tubules epithelial cells, thus promoting glucosuria. Their mechanism of action is independent of insulin secretion making the use of these drugs useful in patients with limited pancreatic beta cell activity. The hypothesized mechanism of SGLT2 inhibitors in NAFLD stems from their glycosuric effect leading to total loss of energy which results in increased pancreatic secretion of glucagon while suppressing insulin secretion. SGLT2 inhibitors also work as alpha-cells secretagogues by directly stimulating glucagon release via neuronal stimulation[39]. This mild hyper-glucagonemic state induces hepatic gluconeogenesis, ketogenesis and lipolysis, leading to an overall reduction in the amount of fatty acids. Furthermore, SGLT2 inhibitors exert a direct neurogenic effect that enhances gluconeogenesis and lipolysis in the liver[40]. The sum of such effects leads to reduction in hepatic steatosis and halts the progression of NAFLD (Figure 1). Albeit being of the same group of medications, different SGLT2 inhibitors demonstrated different effects on NAFLD. In the following section, we discuss the evidence that supports the use of different members of this family of drugs in SGLT2.
Figure 1.
Mechanism of action of Sodium-glucose co-transporter-2 inhibitors in non-alcoholic fatty liver disease. Obesity-induced insulin resistance leading to diabetes are the major risk factors for non-alcoholic fatty liver disease (NAFLD). The increase in insulin secretion and inhibition of glucagon secretion by the islet cells in the pancreas ultimately leads to the stimulation of lipogenesis, ultimately shifting the balance towards hepatic steatosis and NAFLD. Sodium-glucose co-transporter inhibitors primary effect is inducing glycosuria causing lowering of the blood glucose levels. This inhibits the secretion of insulin and stimulates glucagon secretion, causing a higher insulin-to-glucagon ratio, which increases the lipolytic, gluconeogenetic, and ketogenetic pathways. This results in reduction in the hepatic steatosis in NAFLD.
CANAGLIFLOZIN
Canagliflozin is the most commonly prescribed SGLT2 inhibitors for patients with T2DM. In animal models of NAFLD, canagliflozin used in high-fat diet fed mice reduced ALT levels and prevented the development of cirrhosis as evident by reduced steatosis on histologic examination[41]. Canagliflozin also showed favorable outcomes when pitted against sitagliptin, a DPP4-inhibitor, in the management of Japanese patients with biopsy-proven NAFLD[42]. It demonstrated reductions in BMI, fasting blood glucose, body weight, HbA1c, and ALT levels[42]. It is worth noting that the study was a retrospective cohort study and the results could not be directly attributed to canagliflozin[42]. Canagliflozin used for 24 wk in patients aged 20-64 years with biopsy-proven NAFLD complicated with T2DM showed significant reductions in BMI, fasting blood glucose, waist circumference, ferritin level, gamma-glutamyltransferase (GGT) level, and type IV collagen 7S[43]. Furthermore, there was a decrease in the NAFLD score in all patients included in the study[43]. However, the study was a single center, single arm study and only involved 5 patients. Hence, extrapolation to the general population was difficult[43]. A systemic analysis pooled the results of 4 studies in which canagliflozin was used for 26 or 52 wk vs placebo or sitagliptin, and showed significant reductions in HbA1c, body weight, ALT, AST, alkaline phosphatase and gamma-glutamyl transferase. The favorable changes in liver function tests were attributed to reductions in HbA1c and body weight[44]. In western-diet fed murine models, canagliflozin showed significant improvements in hyperglycemia, hyperinsulinemia and liver function tests as early as 8 wk after initiation, and significant improvements in hepatic fibrosis after 20 wk of treatment. There was additionally a significant reduction in the number of liver tumors after 1 year of canagliflozin treatment[45]. More recent evidence emerged on the positive effect of canagliflozin with a human study demonstrating significant reductions in hepatic steatosis, hepatocyte ballooning, fibrosis, and inflammation after 24 wk of treatment in patients with T2DM and NAFLD[46]. Another prospective cohort study also demonstrated significant reductions in ALT, AST, GGT, triglycerides, HbA1c, and body weight[47].
IPRAGLIFLOZIN
Ipragliflozin used in high fat diet fed murine models that had streptozocin nicotanamide-induced T2DM showed improvement in glucose tolerance, blood glucose, insulin, and lipid levels[48]. Moreover, there were reductions in hepatic steatosis and liver levels of oxidative stress biomarkers as well as improvement in aminotransferase levels after 4 wk of treatment[48]. Another murine based study demonstrated similar results by demonstrating improvement in insulin resistance, free fatty acids, AST and ALT levels, and liver fat content with an 8 wk course of ipragliflozin[49]. Murine models fed a choline-deficient l-amino acid-defined diet developed liver triglyceride increase, liver fibrosis, and mild inflammation[50]. These changes were prevented with 5 wk of ipragliflozin therapy which suggests that SGLT2 inhibitors might play a role in the prevention of hepatic fibrosis[50]. In human subjects, ipragliflozin used for 16 wk in patients with T2DM showed significantly reduced fatty liver index, fasting plasma glucose, HbA1c, body weight, visceral adipose tissue, and subcutaneous tissue and fat mass[51]. When ipragliflozin was compared to pioglitazone, a PPAR agonist, in patients with T2DM, similar effects were observed with regards to blood glucose, HbA1c, liver to spleen ratio, AST and ALT levels. There was a significantly reduced body weight and fat area with ipragliflozin[52]. The co-administration of ipragliflozin with incretin-based drugs such as GLP-1 analogs or DPP-4 inhibitors showed significant reductions in HbA1c, body weight, serum ALT levels, and fibrosis-4 index[53]. The most important aspect observed here is that ALT levels were not normalized with incretin-based therapies until combined with iprafliglozin, which suggests a synergistic effect between incretin-based therapies and SGLT2 inhibitors[53]. In a larger multicenter prospective study involving patients with T2DM and NAFLD, ipragliflozin administration for 24 wk showed significant reductions in HbA1c, AST, ALT, body weight, and steatosis[54]. It further suggests that SGLT2 inhibitors can help in the management of patients with T2DM with metabolic syndrome[55].
DAPAGLIFLOZIN
Dapagliflozin is a highly selective competitive inhibitor of SGLT2. In genetic murine models of obesity and diabetes, such as db/db, dapagliflozin was shown to improve markers of liver injury such as MPO and reactive oxygen species[56]. Even in diet-induced obesity, dapagliflozin showed decreased serum ALT, AST, hepatic lipid accumulation, and hepatic fibrosis in mice that were fed western diet compared to low-fat diet. Dapagliflozin also attenuated the western diet-mediated increases in body weight, plasma glucose, plasma triglycerides, and renal fibrosis[57]. This suggests that dapagliflozin can be used for reversal of hepatic steatosis associated with NAFLD, even in humans. Indeed, the use of dapagliflozin and empagliflozin demonstrated a significant reduction in ALT levels in patients with T2DM. This change was independent of HbA1c and fasting glucose levels[58]. Dapagliflozin also showed significant improvement in BMI, AST levels, ALT levels, fasting plasma glucose and HbA1c when used for 24 wk in patients with biopsy-proven NASH and T2DM[59]. More recently, a study investigating the use of dapagliflozin for 24 wk in patients with T2DM and NASH showed a significant reduction in ALT and GGT levels as well as significant improvement in liver stiffness measurement[60]. Dapagliflozin was also found to significantly reduce hepatic steatosis and attenuate severe liver fibrosis in patients with T2DM and NAFLD[60]. A randomized double-blind placebo-controlled trial involving 84 patients with T2DM and NAFLD demonstrated significant reduction of liver fat content with combination dapagliflozin and n-3 carboxylic acid for 12 wk. Dapagliflozin monotherapy also decreased hepatic injury biomarkers and as mentioned earlier, ALT, AST, GGT and body weight[61].
EMPAGLIFLOZIN
Empagliflozin, vs combination empagliflozin and linagliptin, a DPP-IV inhibitor, vs placebo demonstrated that empagliflozin monotherapy reduced the severity on NASH at 21 d in NASH mouse-models[62]. Furthermore, the combination of em-pagliflozin and linagliptin led to reduction in body weight and liver collagen deposition i.e., fibrosis indicating a probable synergistic effect upon coad-ministration[62]. The E-LIFT trial which involved patients with T2DM and NAFLD, showed that empagliflozin in addition to standard diabetes management causes a significant reduction in liver fat content and ALT and a non-significant difference in GGT and AST levels[63]. A subgroup analysis from the EMPA-REG trial showed significant reduction in ALT independently of changes in HbA1c or body weight[64].
REMOGLIFLOZIN
Mice placed on a high fat diet for 11 wk followed by administration of remogliflozin or placebo for 4 wk resulted in significant reduction of ALT and ALT levels. Both liver weight and hepatic triglyceride content were significantly reduced. Furthermore, when compared to canagliflozin and dapagliflozin, remogliflozin had a significantly higher effect with regards to oxygen radial absorbance capacity. This study demonstrated that remogliflozin had clear significant effects on mice with NAFLD and NASH[65]. Similar studies are yet to occur in humans.
LUSEOGLIFLOZIN
Mice models receiving streptozotocin and nicotinamide to reduce insulin secretion followed by administration of luseogliflozin or placebo exhibited reductions in ALT levels along with reduction in the increase of collagen deposition in the treatment group[66]. A human-based study in which luseogliflozin was compared to metformin in patients with type 2 diabetes and NAFLD demonstrated significantly lower liver-to-spleen ratio, visceral fat, HbA1c, and BMI with luseogliflozin after 6 months of use[67]. Another prospective study showed significant reductions in ALT, AST, BMI, and GGT levels after 24 wk of therapy in patients with T2DM and NAFLD[68].
POTENTIAL ANTITUMORIGENIC EFFECTS OF SGLT2I
One of the dreadful complications of NAFLD is the development of HCC, which appears to be increasing[69], regardless of the presence cirrhosis. One in vitro study showed that the effects of canagliflozin on HCC showing effects that include antiproliferation, cellular arrest, and apoptosis of cancer cells[70]. Such effects were also shown to decrease HCC tumor burden in a murine xenograft model of human HCC. Interestingly, those effects were glycemic-state independent[70]. Although the data supporting the antitumorigenic effects of SGLT2 inhibitors is limited, it is potentially a promising medication in preventing HCC in patients with NAFLD. Since normal and cancer colonic tissue express SGLT2, in one case report of colon cancer with liver metastasis, treatment with dapagliflozin in combination with cetuximab showed substantial response to therapy[71]. Although such results remain in need of validation, they show the potential of SGLT2 inhibitors in the carcinogenesis that could not only be HCC-specific.
ADVERSE REACTIONS DUE TO SGLT2 INHIBITORS
There have been a few reported side effects with regards to SGLT2 inhibitors use, namely vulvovaginal candidiasis and urinary tract infections[72], hypotension[73] through osmotic diuresis causing hypovolemia, acute kidney injury[74] likely secondary to hypoperfusion of the kidneys in the setting of hypovolemia, bone fractures[75], increased risk of amputation[76], and euglycemic diabetic ketoacidosis[77]. Although the mechanisms of SGLT2 inhibitors ketoacidosis is not fully understood, the food and drug administration (FDA) has recognized it as an important side effect to watch for, especially in patient with type-1 diabetes mellitus[78]. Monitoring of kidney function is essential during treatment particularly in those taking concomitant diuretics and other medications that predispose to hypovolemia and acute kidney injury[79]. A major potentially lethal rare consequence of SGLT2 inhibitors use is the development of Fournier’s gangrene. However, it has only been reported in 12 cases, but was serious enough the FDA issued an official warning statement for clinicians to be aware of[80]. Further, it is important to acknowledge that SGLT2 inhibitors were only FDA-approved as recently as 2013[81], and as such there is ongoing research for their long-term safety profile[79] (Table 1).
Table 1.
Sodium-glucose co-transporter inhibitors and their use in non-alcoholic fatty liver disease
| SGLT2 inhibitor | ALT | AST | GGT | Bilirubin | BMI | Steatosis | Inflam-mation | Fibrosis | HCC | Adverse effects | Study organism | Ref. |
| Canagli-flozin | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | Urogenital tract fungal infections, DKA, amputat-ions, bone fractures | Human | [44,46,76] |
| Ipraglif-lozin | ↓ | - | - | NR | ↓ | ↓ | ↓ | ↓ | NR | Urinary tract infections | Human/Mouse | [49,51] |
| Dapagli-flozin | ↓ | ↓ | ↓ | NR | ↓ | ↓ | ↓ | NR | NR | Urogenital tract infections, bladder cancer, DKA, amputat-ions | Human | [59,61,76,81] |
| Empagl-iflozin | ↓ | - | - | - | ↓ | ↓ | ↓ | ↓ | NR | Genital tract infections, DKA | Human/Mouse | [62-64] |
| Remogl-iflozin | ↓ | ↓ | NR | NR | ↓ | ↓ | ↓ | NR | NR | Urogenital tract fungal infections | Mouse | [65] |
| Luseogl-iflozin | ↓ or - | ↓ | ↓ | NR | ↓ | ↓ | ↓ | ↓ or - | NR | Vaginal itching, dehydra-tion | Human/Mouse | [66-68] |
SGLT2: Sodium-glucose co-transporter 2; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGT: Gamma-glutamyltransferase; BMI: Body mass index; HCC: Hepatocellular carcinoma; DKA: Diabetic ketoacidosis; NR: Not reported; ↓: Decreases; ↑: Increases; -: No change.
CONCLUSION
Limited pharmacologic options with proven efficacy makes the treatment of NAFLD challenging. Apart from weight-loss, there are few pharmacologic treatment options. However, recent emerging evidence of the use of SGLT2-inhibitors in patients with NAFLD is promising. Those agents have shown to improve levels of serum transaminases, decrease steatosis, prevent cirrhosis and HCC, and reduce body weight. They are also gaining wide popularity due to their anti-diabetic effect and potential cardiovascular benefits. However, prior to establishing the use of those agents clinically, further studies including randomized controlled trials should be conducted.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Peer-review started: March 25, 2019
First decision: June 3, 2019
Article in press: June 27, 2019
P-Reviewer: Jamali R, Malnick SDH S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
Contributor Information
Amr Dokmak, Division of Medicine, St. Elizabeth’s Medical Center, Brighton, MA 02135, United States; Tufts University School of Medicine, Boston, MA 02111, United States.
Mohammad Almeqdadi, Division of Medicine, St. Elizabeth’s Medical Center, Brighton, MA 02135, United States; Tufts University School of Medicine, Boston, MA 02111, United States.
Hirsh Trivedi, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA 02215, United States.
Sandeep Krishnan, Tufts University School of Medicine, Boston, MA 02111, United States; Division of Gastroenterology, St. Elizabeth’s Medical Center, Brighton, MA 02135, United States. sandeep.krishnan@steward.org.
References
- 1.Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci. 2013;1281:106–122. doi: 10.1111/nyas.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol. 2006;40:745–752. doi: 10.1097/00004836-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28 Suppl 4:64–70. doi: 10.1111/jgh.12271. [DOI] [PubMed] [Google Scholar]
- 7.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, Singal AG. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:198–210.e2. doi: 10.1016/j.cgh.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dongiovanni P, Anstee QM, Valenti L. Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr Pharm Des. 2013;19:5219–5238. doi: 10.2174/13816128113199990381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 12.Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J Non-alcoholic Steatohepatitis Clinical Research Network (NASH CRN) Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 14.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–77. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 15.Parker HM, Johnson NA, Burdon CA, Cohn JS, O'Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940–2944. doi: 10.2337/dc07-0792. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Park JY, Lee KU, Kim JH, Kim HK. Fatty liver is an independent risk factor for the development of Type 2 diabetes in Korean adults. Diabet Med. 2008;25:476–481. doi: 10.1111/j.1464-5491.2008.02410.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamada T, Fukatsu M, Suzuki S, Wada T, Yoshida T, Joh T. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol. 2010;25:352–356. doi: 10.1111/j.1440-1746.2009.05998.x. [DOI] [PubMed] [Google Scholar]
- 19.Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1093–1097. doi: 10.1210/jc.2010-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae JC, Rhee EJ, Lee WY, Park SE, Park CY, Oh KW, Park SW, Kim SW. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care. 2011;34:727–729. doi: 10.2337/dc10-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35:717–722. doi: 10.2337/dc11-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, Wasada T. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 23.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 24.Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM, Strachan MW Edinburgh Type 2 Diabetes Study Investigators. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139–1144. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 26.Silverman JF, O'Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, Norris HT, Caro JF. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–1355. [PubMed] [Google Scholar]
- 27.Noureddin M, Rinella ME. Nonalcoholic Fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis. 2015;19:361–379. doi: 10.1016/j.cld.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 31.Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32:1211–1221. doi: 10.1111/j.1365-2036.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 32.Phielix E, Szendroedi J, Roden M. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact. Trends Pharmacol Sci. 2011;32:607–616. doi: 10.1016/j.tips.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jespersen MJ, Knop FK, Christensen M. GLP-1 agonists for type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol. 2013;9:17–29. doi: 10.1517/17425255.2013.731394. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 36.Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, Amo K, Aoki K, Morimoto C, Takeda E, Terauchi Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60:1246–1257. doi: 10.2337/db10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, Terauchi Y, Nakajima A. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103–2105. doi: 10.5754/hge11263. [DOI] [PubMed] [Google Scholar]
- 38.Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan H, Naeshiro N, Honda Y, Miyaki D, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Aikata H, Chayama K. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepatogastroenterology. 2014;61:323–328. [PubMed] [Google Scholar]
- 39.Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B, Pattou F. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 40.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabil SL, Mahmoud NM. Canagliflozin protects against non-alcoholic steatohepatitis in type-2 diabetic rats through zinc alpha-2 glycoprotein up-regulation. Eur J Pharmacol. 2018;828:135–145. doi: 10.1016/j.ejphar.2018.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, Hara T, Okajima A, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Kanemasa K, Yasui K, Imai S, Shimada K, Itoh Y. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1072–1078. doi: 10.1111/hepr.12834. [DOI] [PubMed] [Google Scholar]
- 43.Akuta N, Watanabe C, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Suzuki Y, Suzuki F, Ikeda K, Kumada H. Effects of a sodium-glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: Preliminary prospective study based on serial liver biopsies. Hepatol Commun. 2017;1:46–52. doi: 10.1002/hep4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25–32. doi: 10.1016/j.diabet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N, Yamaguchi S, Ogasawara N, Katoh M, Itoh M, Suganami T, Ogawa Y. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8:2362. doi: 10.1038/s41598-018-19658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akuta N, Kawamura Y, Watanabe C, Nishimura A, Okubo M, Mori Y, Fujiyama S, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki F, Suzuki Y, Arase Y, Ikeda K, Kumada H. Impact of sodium glucose cotransporter 2 inhibitor on histological features and glucose metabolism of non-alcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Res. 2019;49:531–539. doi: 10.1111/hepr.13304. [DOI] [PubMed] [Google Scholar]
- 47.Itani T, Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes Sci Pract. 2018;4:477–482. doi: 10.1002/osp4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Honda Y, Imajo K, Kato T, Kessoku T, Ogawa Y, Tomeno W, Kato S, Mawatari H, Fujita K, Yoneda M, Saito S, Nakajima A. The Selective SGLT2 Inhibitor Ipragliflozin Has a Therapeutic Effect on Nonalcoholic Steatohepatitis in Mice. PLoS One. 2016;11:e0146337. doi: 10.1371/journal.pone.0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashizaki-Someya Y, Kurosaki E, Takasu T, Mitori H, Yamazaki S, Koide K, Takakura S. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol. 2015;754:19–24. doi: 10.1016/j.ejphar.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Takase T, Nakamura A, Miyoshi H, Yamamoto C, Atsumi T. Amelioration of fatty liver index in patients with type 2 diabetes on ipragliflozin: an association with glucose-lowering effects. Endocr J. 2017;64:363–367. doi: 10.1507/endocrj.EJ16-0295. [DOI] [PubMed] [Google Scholar]
- 52.Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364–1372. doi: 10.2337/dc17-0518. [DOI] [PubMed] [Google Scholar]
- 53.Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of Ipragliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor, as a Second-line Treatment for Non-Alcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus Who Do Not Respond to Incretin-Based Therapies Including Glucagon-like Peptide-1 Analogs and Dipeptidyl Peptidase-4 Inhibitors. Clin Drug Investig. 2016;36:313–319. doi: 10.1007/s40261-016-0383-1. [DOI] [PubMed] [Google Scholar]
- 54.Miyake T, Yoshida S, Furukawa S, Sakai T, Tada F, Senba H, Yamamoto S, Koizumi Y, Yoshida O, Hirooka M, Kumagi T, Niiya T, Miyaoka H, Masanori A, Matsuura B, Hiasa Y. Ipragliflozin Ameliorates Liver Damage in Non-alcoholic Fatty Liver Disease. Open Med (Wars) 2018;13:402–409. doi: 10.1515/med-2018-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawata T, Iizuka T, Iemitsu K, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, Kanamori A, Takeda H, Ito S, Kikuchi T, Amemiya H, Kaneshiro M, Mokubo A, Takuma T, Machimura H, Tanaka K, Asakura T, Kubota A, Aoyanagi S, Hoshino K, Ishikawa M, Matsuzawa Y, Obana M, Sasai N, Kaneshige H, Minagawa F, Saito T, Shinoda K, Miyakawa M, Tanaka Y, Terauchi Y, Matsuba I. Ipragliflozin Improves Glycemic Control and Decreases Body Fat in Patients With Type 2 Diabetes Mellitus. J Clin Med Res. 2017;9:586–595. doi: 10.14740/jocmr3038w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang L, Wu Y, Tian M, Sjöström CD, Johansson U, Peng XR, Smith DM, Huang Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313:E563–E576. doi: 10.1152/ajpendo.00086.2017. [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, Levi M. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee PCH, Gu Y, Yeung MY, Fong CHY, Woo YC, Chow WS, Tan K, Lam KSL. Dapagliflozin and Empagliflozin Ameliorate Hepatic Dysfunction Among Chinese Subjects with Diabetes in Part Through Glycemic Improvement: A Single-Center, Retrospective, Observational Study. Diabetes Ther. 2018;9:285–295. doi: 10.1007/s13300-017-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. Curr Ther Res Clin Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, Iijima M, Takekawa H, Usui I, Hiraishi H, Aso Y. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 61.Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923–1934. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45. doi: 10.1186/s13098-016-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 64.Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61:2155–2163. doi: 10.1007/s00125-018-4702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakano S, Katsuno K, Isaji M, Nagasawa T, Buehrer B, Walker S, Wilkison WO, Cheatham B. Remogliflozin Etabonate Improves Fatty Liver Disease in Diet-Induced Obese Male Mice. J Clin Exp Hepatol. 2015;5:190–198. doi: 10.1016/j.jceh.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiang S, Nakatsu Y, Seno Y, Fujishiro M, Sakoda H, Kushiyama A, Mori K, Matsunaga Y, Yamamotoya T, Kamata H, Asano T. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr. 2015;7:104. doi: 10.1186/s13098-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, Kawai H, Ohashi N, Mori A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438–442. doi: 10.1111/dom.13061. [DOI] [PubMed] [Google Scholar]
- 68.Sumida Y, Murotani K, Saito M, Tamasawa A, Osonoi Y, Yoneda M, Osonoi T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective, single-arm trial (LEAD trial) Hepatol Res. 2019;49:64–71. doi: 10.1111/hepr.13236. [DOI] [PubMed] [Google Scholar]
- 69.Charlton M. Risk of Hepatocellular Carcinoma in Patients With Nonalcoholic Steatohepatitis. Gastroenterol Hepatol (NY) 2018;14:247–249. [PMC free article] [PubMed] [Google Scholar]
- 70.Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, Furukawa M, Kawaratani H, Kitade M, Moriya K, Namisaki T, Yoshiji H. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142:1712–1722. doi: 10.1002/ijc.31193. [DOI] [PubMed] [Google Scholar]
- 71.Okada J, Matsumoto S, Kaira K, Saito T, Yamada E, Yokoo H, Katoh R, Kusano M, Okada S, Yamada M. Sodium Glucose Cotransporter 2 Inhibition Combined With Cetuximab Significantly Reduced Tumor Size and Carcinoembryonic Antigen Level in Colon Cancer Metastatic to Liver. Clin Colorectal Cancer. 2018;17:e45–e48. doi: 10.1016/j.clcc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weir MR, Januszewicz A, Gilbert RE, Vijapurkar U, Kline I, Fung A, Meininger G. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich) 2014;16:875–882. doi: 10.1111/jch.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.INVOKANA (canagliflozin) Tablets 48. Available from: https://www.netdoctor.co.uk/medicines/diabetic/a8900/invokana-canagliflozin. [Google Scholar]
- 75.Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2016;101:157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ueda P, Svanström H, Melbye M, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Pasternak B. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365. doi: 10.1136/bmj.k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38:1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Research C for DE and Drug Safety and Availability - FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections [Internet] [cited 2019 Feb 12]; Available from: https://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. [Google Scholar]
- 79.Filippas-Ntekouan S, Filippatos TD, Elisaf MS. SGLT2 inhibitors: are they safe? Postgrad Med. 2018;130:72–82. doi: 10.1080/00325481.2018.1394152. [DOI] [PubMed] [Google Scholar]
- 80.Research C for DE and Drug Safety and Availability-FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes [Internet] [cited 2019 Feb 12]; Available from: https://www.fda.gov/Drugs/DrugSafety/ucm617360.htm. [Google Scholar]
- 81.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]