Abstract
The major immunogenic protein capsid (Cap) of porcine circovirus type 2 (PCV2) is critical to induce neutralizing antibodies and protective immune response against PCV2 infection. This study was conducted to investigate the immune response of recombinant adenovirus expressing PCV2b Cap and C-terminal domain of Yersinia pseudotuberculosis invasin (Cap-InvC) fusion protein in pigs. The recombinant adenovirus rAd-Cap-InvC, rAd-Cap and rAd were generated and used to immunize pigs. The phosphate-buffered saline was used as negative control. The specific antibodies levels in rAd-Cap-InvC and ZJ/C-strain vaccine groups were higher than that of rAd-Cap group (p < 0.05), and the neutralization antibody titer in rAd-Cap-InvC group was significantly higher than those of other groups during 21–42 days post-immunization (DPI). Moreover, lymphocyte proliferative level, interferon-γ and interleukin-13 levels in rAd-Cap-InvC group were increased compared to rAd-Cap group (p < 0.05). After virulent challenge, viruses were not detected from the blood samples in rAd-Cap-InvC and ZJ/C-strain vaccine groups after 49 DPI. And the respiratory symptom, rectal temperature, lung lesion and lymph node lesion were minimal and similar in the ZJ/C-strain and rAd-Cap-InVC groups. In conclusion, our results demonstrated that rAd-Cap-InvC was more efficiently to stimulate the production of antibody and protect pigs from PCV2 infection. We inferred that InvC is a good candidate gene for further development and application of PCV2 genetic engineering vaccine.
Keywords: Swine, circovirus, adenovirus vaccines, immunization, capsid protein
INTRODUCTION
Porcine circovirus type 2 (PCV2) is an essential pathogen of porcine circovirus-associated diseases (PCVADs) and caused great economic loss in pig breeding [1,2,3]. The main approach to control and prevents PCV2 infection is vaccination in pig industry [4]. At present, commercial inactivated vaccines based on PCV2 have been shown to be effective to decrease mortality and increase growth parameters in commercial pig herds. However, inactivated vaccines-induced immune responses are slow and weak, thus lead to inadequate protection [5]. In some farms, pigs immunized with PCV2 inactivated vaccines still have PCV2 infection even onset [6]. Genetic engineering vaccines could prevent multiple viruses attack and induce strong immune responses [7,8,9]. It has been reported that adenovirus vaccines have many advantages including simple preparation, high antibody titer, durable immune stimulation and high safety [10,11]. PCV2 capsid (Cap) protein is the major immunogenic structural protein of PCV2 and is responsible for neutralizing antibodies and inducing protective immunity [12]. Therefore, Cap protein is the first choice for the research of PCV2 genetic engineering vaccines.
Several studies have demonstrated that recombinant adenovectors expressing Cap protein of PCV2 could induce the production of the specific antibody [11,13,14,15]. However, the poor immune responses of recombinant adenovirus with Cap protein limit its application in PCVADs control and prevention. Several adjuvants and genes were selected as immunity enhancers, such as Intron A and woodchuck hepatitis virus posttranscriptional regulatory element, CD40 ligand (CD40L), and granulocyte-macrophage colony-stimulating factor (GMCSF), interferon (IFN)-γ, and so on [10,16,17]. In addition, Yersinia pseudotuberculosis invasin (Inv) protein has been reported to enhance cellular ability to capture antigens and improve immune responses of vaccines [18,19]. Our previous study indicated that adenovirus expressing classical swine fever virus E2-C-terminal domain of Y. pseudotuberculosis invasin (InvC) fusion protein could enhance the immune protective effects [20].
In this study, we attempted to establish a method to enhance immune protection of PCV2 adenovirus vaccine in Pigs. The recombinant adenovirus including PCV2b Cap gene and InvC gene was constructed to co-express both the antigen and the immune enhancing factor, and the animal immune experiment was carried out to evaluate the immune effect.
MATERIALS AND METHODS
Plasmids, virus and cells
Adenovirus shuttle vector pAdTrack-CMV and recombinant plasmid T-PCV2 inserted PCV2b full-length genomic sequence were provided by the Laboratory of Veterinary Public Hygiene and Food Safety of College of Veterinary Medicine, Northwest A&F University. pAd-(Gly4Ser)3-InvC was kindly provided by Dr. En-qi Du, the College of Veterinary Medicine of Northwest A&F University. Recombinant adenoviruses were cultured and propagated in human embryonic kidney293 (HEK293) cell line. PCV2b inactivated vaccine (ZJ/C strain) is a commercially available vaccine manufactured by Zhejiang Shihua Nuobeiwei Biotechnology. The virulent PCV2 strain SD-3 (GenBank No. EU366323) was used for challenge experiment.
Construction of recombinant adenovirus
Recombinant plasmids pAd-(Gly4Ser)3-InvC and T-PCV2 were used as templates to obtain InvC gene and Cap gene by polymerase chain reaction (PCR) amplification with the specific primers (Table 1). Recombinant adenoviral vector pAdTrack-CMV-Cap was constructed by inserting Cap gene into BglII and SalI restriction enzymes site of pAdTrack-CMV. InvC gene was amplified and cloned into pAdTrack-CMV-Cap to construct pAdTrack-CMV-Cap-InvC. A secretory signal peptide was inserted into upstream of Cap gene. All ligation products were transformed into DH5α. pAdTrack-CMV-Cap-InvC was linearized with PmeI enzyme digestion to obtain linearized homologous recombinant adenovirus vector pAd-Cap-InvC.
Table 1. Primers for polymerase chain reaction amplification used in this study.
| Gene | Sequence (5′–3′) | Size (bp) | Accession No. |
|---|---|---|---|
| PCV2b Cap | F: GAAGATCTATGACGTATCCAAGGAGGCG | 702 | KM924367.1 |
| R: ACGCGTCGACTTAAGGGTTAAGTGG | |||
| InvC | F: ACGCGTCGACGGATCCGGATCAGGTTCAG | 664 | HE805230.1 |
| R: CCCAAGCTTCTATATTGCCAGCGCACAG | |||
| PCV2 SD-3 | F: AAGGGCTGGGTTATGGTATGT | 353 | EU366323 |
| R: CGCTGGAGAAGGAAAAATGG |
Recombinant adenoviruses packaging
The recombinant plasmids pAdTrack-CMV-Cap-InvC, pAdTrack-CMV-Cap and pAdTrack-CMV were linearized with the PacI restriction endonuclease and transfected into HEK293 cells by Lipofectamine 2000 Transfection Reagent (Invitrogen, USA) to produce 3 recombinant adenovirus rAd-Cap-InvC, rAd-Cap and rAd, respectively. Purified recombinant adenovirus was diluted as ten-times step and successively inoculated into 96-well plates which have paved cells from 10−3 to 10−13. There were 8 duplications for each dilatability and 100 μL/well. Then they were cultured at 37°C with 5% CO2 for 5 days. At last, viral 50% tissue culture infectious dose (TCID50) was calculated according to Reed-Muench method [21].
Western blot analysis of the protein expression
The recombinant adenoviruses rAd-Cap-InvC, rAd-Cap and rAd were inoculated into pig kidney (PK-15) cell line at 20 multiplicity of infection for 48 h, respectively. Cells were harvested and treated with protein lysis buffer for 10 min on ice. Cell lysates were centrifuged at 10,000 g for 5 min at 4°C. The protein samples were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked with 50 g/L skim milk before incubated with rabbit anti-PCV2 Cap antibody (gifted by Dr. Zeng-jun Lu, Lanzhou Veterinary Research Institute, Chinese Academy of Agriculture Sciences) which used as the primary antibodies, followed by mouse anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (Santa Cruz Biotechnology, USA) as the secondary antibodies. A mouse anti-porcine glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Life Span Biosciences, USA) was used as an internal reference. Signals were visualized by enhanced chemiluminescence Western blotting analysis system (Thermo Fisher Scientific, USA).
Immunization and challenge
The recombinant adenovirus rAd-Cap-InvC, rAd-Cap and rAd were purified and concentrated by using Adenovirus purification mini kit (Biomiga, USA) under the manufacturer's instruction. The adenovirus titer was adjusted to 1010 TCID50/mL. Twenty 6-week-old female piglets (without PCV2 antibody) were randomly divided into 4 groups. Group A, B and C were inoculated with 1 mL recombinant adenovirus rAd-Cap-InvC, rAd-Cap, or rAd (1010 TCID50/piglet) by intramuscular inoculation, respectively. Group D were immunized with 1-dose commercial inactivated vaccine (ZJ/C-strain) and served as the positive control. Group E was vaccinated with PBS as the negative control. Booster immunization was performed on 14 DPI, and the virus dosages and immune methods were same as the primary immunization. Finally, all animals were challenged with 5 × 105 TCID50 of PCV2 SD-3 per pig on 28 DPI. Following the challenge, the pigs were monitored daily and all surviving pigs were euthanized after the experiment.
All animal procedures were approved by the Animal Care and Use Committee of Northwest A&F University (approval No. NWAFU 20131017/02). Piglets were from the Ecological Breeding Base of Northwest A&F University. All efforts were made to minimize animal suffering.
Clinical evaluation
Following PCV2 SD-3 inoculation, the pigs were monitored daily for physical conditions and the clinical respiratory disease score was given to each piglet with the method described previously [22]. Rectal temperatures were recorded daily from 28 DPI through 42 DPI. Observers were blinded to vaccination status.
Detection of PCV2 specific antibody by enzyme-linked immunosorbent assay (ELISA)
Blood samples of each piglet was collected individually on 0, 14, 21, 35, and 42 DPI. Then separated serum was used to test the PCV2 antibody level according to the manufacturer's instruction of Antibody Test Kit for PCV2 (ELISA) (Shenzhen Finder Biotech., China). Antibody levels were presented as the values of optical density (OD450).
Virus neutralization assay
The serum samples were serially diluted in 2-fold with a starting dilution of 1:2. Then 100 μL of PCV2 SD-3 (200 TCID50) was mixed with equal volume of serum and incubated for 1 h at 37 °C. The serum-virus mixture was added into PK15 and incubated in 5% CO2 at 37 °C for 72 h. The cell monolayer was were fixed with cold 80% acetone (50 μL/well) for 20 min. The cells were incubated with anti-PCV2 antibody diluted at 1:1,000 with PBS for 1 h at 37°C, and followed by staining with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Boshide, China). All cells were washed 3 times with PBS and read under a fluorescent microscope. Cells with FITC fluorescent staining were recorded as infected. The antibody titers were expressed as the reciprocal of the highest serum dilution at ≥ 80% fluorescent focus reduction.
Lymphocytes proliferation assay by MTT
Fresh peripheral blood was collected at 28 DPI before the challenge. Then the blood samples were diluted with saline at 1:1 and added to the upper layer of lymphocyte separation medium. lymphocytes were collected at the white lymphocyte layer in the middle layer of the separation medium after centrifuging at 1,500 g for 15 min. Lymphocytes were seeded into a 96-well plate and cultured in Roswell Park Memorial Institute (RPMI) 1640 containing 10% fetal calf serum and Cap protein (20 μg/mL) at 37°C for 72 h. Without Cap protein group was served as negative control. Then, 20 μL of 5 mg/mL MTT (3-[4,5-dimethylthiazol-2-y]-2,5-diphenyltetrasodiumbromide tetrazolium; Sigma, USA) was added into each well and incubated for 4 h. Dimethyl sulfoxide was added 100 mL per well to stop the reactions. The stimulation index = mean OD570 of Cap stimulated cells/mean OD570 of unstimulated cells.
Cytokines release assay by ELISA
Lymphocytes were separated as described above were seeded into a 96-well plate and cultured in RPMI-1640. Then, 2 μg Cap protein was added into each well. The supernatants were collected after 72 h culture. The levels of IFN-γ and IL-13 were tested by porcine IFN-γ and IL-13 ELISA Kits (SenBeiJia Biological Techology, China) according to the manufacturer's instruction.
Detection of PCV2 in blood by real-time PCR (RT-PCR)
Serum samples were collected on 28, 35, 42, 49, and 56 DPI. Virus DNA was extracted from serum samples by DNA extraction kits (Tiangen, China). The PCV2 genomic DNA was detected by RT-PCR as reported previously [23]. Briefly, The PCR primers for detecting PCV2 SD-3 was shown in Table 1. Quantitative RT-PCR was carried out with a SYBR ExScriptTM kit (Takara, China) according to the manufacturer's instructions. Reactions were performed in an iQ5 RT-PCR Detection System (Bio-Rad, USA) under the following conditions: 5 min at 95°C and 40 cycles of 5 sec at 95°C, 10 sec at 60°C, and 30 sec at 72°C. PCV2-T plasmid was quantitated by NanoDrop 2000 spectrophotometer and served to generate a standard curve. The absolute copy number of viral genomes per milliliter in serum was determined and presented as log 10 genomic copies/mL ± standard error of mean (SEM).
Pathologic examination
All piglets were euthanized and necropsied at 56 DPI. The superficial inguinal lymph node and lungs were collected at necropsy. Macroscopic lung lesions were given a score to estimate the volume percentage of the lung affected by pneumonia as previously described [24]. And a gross lymph node lesion score was given to each piglet: 0 = normal; 1 = mild enlargement to 1.1–1.5 times normal size; 2 = moderate enlargement to 1.6–2 times normal size; 3 = moderate enlargement to 2.1–3 times normal size; 4 = severe enlargement to 3.1–5 times normal size.
Statistical analysis
All data were presented as the mean ± SEM. Statistical analyses were performed by one-way analysis of variance and lysergic acid diethylamide test using SPSS 19.0 (SPSS, USA). A value of p < 0.05 was considered as significantly statistical difference.
RESULTS
The expression of Cap-InvC fusion protein
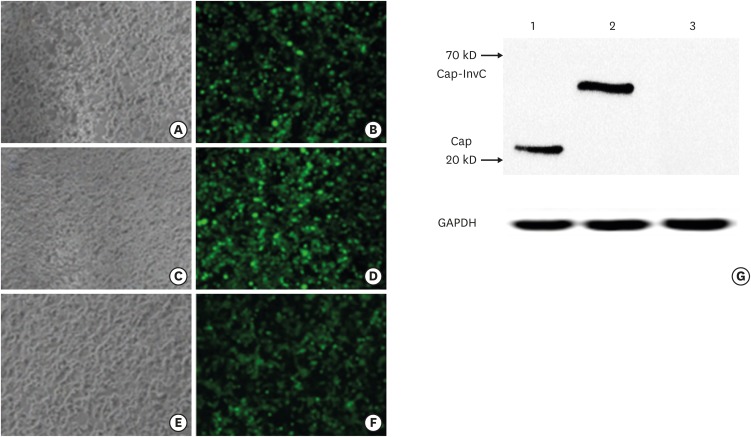
The recombinant adenoviruses rAd, rAd-Cap and rAd-Cap-InvC were generated and used to infect PK-15 cells. The expression of enhanced green fluorescent protein could be observed under inverted fluorescence Microscope after 48 infection (Fig. 1A-E). Protein of Cap-InvC and Cap was detected by Western blot. As shown in Fig. 1G, the protein bands of rAd-Cap-InvC and rAd-Cap were shown at approximately 54 kD and 28 kD, and no band was observed in rAd infected cells. The band of GAPDH could be observed in all the 3 recombinant adenoviruses infected cells. This result indicated that the Cap-InvC fusion protein and Cap protein could be expressed by rAd-Cap-InvC and rAd-Cap, respectively.
Fig. 1. The recombinant adenoviruses rAd-Cap-InvC, rAd-Cap and rAd infected PK-15 cells. The expression of enhanced green fluorescent protein could be observed under inverted fluorescence microscope in rAd (A, B), rAd-Cap (C, D) and rAd-Cap-InvC (E, F) infected PK-15 cells. (G) Western blot of Cap-InvC and Cap in the infected PK-15 cells. Lanes 1–3: rAd-Cap, rAd-Cap-InvC, and rAd infected cells. GAPDH was used as an internal reference.
Cap, protein capsid; InvC, C-terminal domain of Yersinia pseudotuberculosis invasin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PK, pig kidney.
Clinical evaluation
Respiratory symptom was characterized by tachypnea and labored abdominal respiration, but no coughing was observed. Other clinical symptoms noted in individual pigs in PBS and rAd groups included anorexia and diarrhea. The mean clinical respiratory scores in ZJ/C-strain and rAd-Cap-InvC groups were significantly lower than those in PBS treated group from 30 DPI to 42 DPI (p < 0.05). Respiratory symptom and rectal temperature were minimal and similar in the ZJ/C-strain and rAd-Cap-InvC groups (Table 2). But dyspnea and tachypnea after being stressed were observed in group rAd and PBS from 30 DPI to 42 DPI. During the same period, rectal temperatures of the 2 groups were above normal. And mean rectal temperature of group PBS and rAd was peaked at 40.7°C at 36 DPI and 34 DPI, respectively (Table 2).
Table 2. Average CRS and rectal temperature in different treatment groups.
| Groups | DPI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 28 | 30 | 32 | 34 | 36 | 38 | 40 | 42 | ||
| rAd | |||||||||
| CRS | 0 | 1.7 ± 0.37† | 2.4 ± 0.24† | 3.6 ± 0.24† | 3.6 ± 0.24† | 3.4 ± 0.24† | 2.6 ± 0.24† | 2.4 ± 0.24† | |
| RT | 39.1 ± 0.16 | 39.9 ± 0.15 | 40.4 ± 0.22 | 40.7 ± 0.31 | 40.3 ± 0.29 | 39.9 ± 0.10 | 39.8 ± 0.07 | 39.8 ± 0.2 | |
| rAd-Cap | |||||||||
| CRS | 0 | 0.8 ± 0.20*,† | 1.4 ± 0.24‡ | 1.6 ± 0.24‡ | 1.6 ± 0.24‡ | 1.4 ± 0.24‡ | 1.4 ± 0.24‡ | 1.2 ± 0.20‡ | |
| RT | 39.0 ± 0.17 | 39.7 ± 0.07 | 39.7 ± 0.23 | 39.9 ± 0.13 | 39.8 ± 0.13 | 39.6 ± 0.09 | 39.6 ± 0.08 | 39.5 ± 0.2 | |
| rAd-Cap-InvC | |||||||||
| CRS | 0 | 0.6 ± 0.24* | 0.6 ± 0.24* | 0.8 ± 0.20* | 0.6 ± 0.24* | 0.6 ± 0.24* | 0.5 ± 0.24* | 0.4 ± 0.24* | |
| RT | 39.0 ± 0.18 | 39.4 ± 0.15 | 39.5 ± 0.15 | 39.5 ± 0.15 | 39.2 ± 0.17 | 39.2 ± 0.10 | 39.2 ± 0.16 | 39.0 ± 0.1 | |
| ZJ/C-strain vaccine | |||||||||
| CRS | 0 | 0.4 ± 0.24* | 0.4 ± 0.24* | 0.4 ± 0.24* | 0.6 ± 0.24* | 0.4 ± 0.24* | 0.3 ± 0.24* | 0.4 ± 0.24* | |
| RT | 38.8 ± 0.13 | 39.3 ± 0.16 | 39.2 ± 0.17 | 39.2 ± 0.14 | 39.1 ± 0.17 | 39.1 ± 0.12 | 39.1 ± 0.12 | 38.9 ± 0.2 | |
| PBS | |||||||||
| CRS | 0 | 1.6 ± 0.40† | 2.4 ± 0.24† | 3.6 ± 0.51† | 3.6 ± 0.24† | 3.2 ± 0.20† | 2.8 ± 0.37† | 2.6 ± 0.24† | |
| RT | 38.8 ± 0.12 | 39.9 ± 0.16 | 40.4 ± 0.31 | 40.5 ± 0.22 | 40.7 ± 0.30 | 40.2 ± 0.23 | 39.8 ± 0.12 | 39.8 ± 0.1 | |
CRS, clinical respiratory score; RT, rectal temperature; DPI, days post-immunization; Cap, protein capsid; InvC, C-terminal domain of Yersinia pseudotuberculosis invasin; PBS, phosphate-buffered saline.
*,†,‡The mean CRSs with different superscripts are significantly different from each other (p < 0.05).
Detection of antibody in recombinant adenovirus immunized piglets
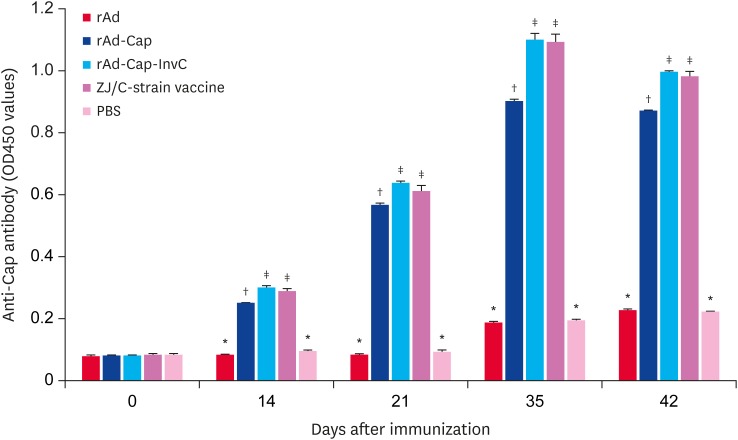
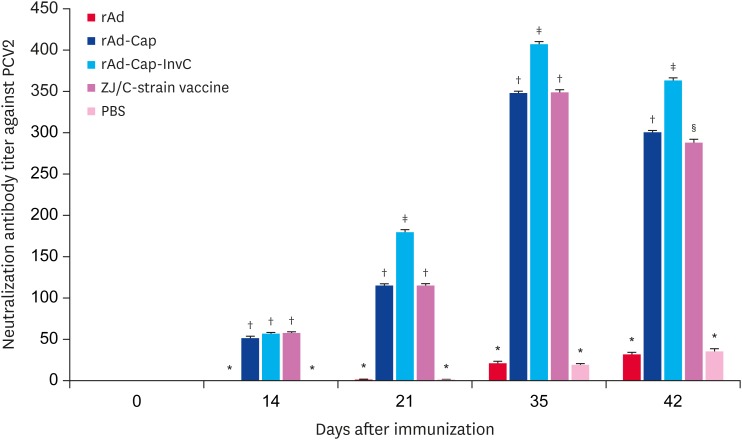
PCV2 specific antibodies were measured by ELISA following vaccination with rAd, rAd-Cap, rAd-Cap-InvC, ZJ/C-strain vaccine, and PBS. As shown in Fig. 2, PCV2 specific antibodies levels increased in rAd-Cap, rAd-Cap-InvC, and ZJ/C-strain vaccine groups after 14 DPI following booster immunization. After challenge, the antibodies levels peaked at 35 DPI. And the antibodies levels in rAd-Cap-InvC and ZJ/C-strain vaccine groups were higher than that of rAd-Cap (p < 0.05). There was no difference in the specific antibody levels between rAd and PBS groups (p > 0.05). PCV2 neutralization antibody titers were shown in Fig. 3. The overall change trend of the neutralization antibody titers was similar with that of PCV2 specific antibody. However, the neutralization antibody titer in rAd-Cap-InvC group was significantly higher than those of other groups from 21 DPI to 42 DPI. These results demonstrated that rAd-Cap-InvC was more efficiently to stimulate the production of neutralization antibody against PCV2 in pigs.
Fig. 2. Detection of porcine circovirus type 2-specific antibody levels in pigs immunized with different adenoviruses by enzyme-linked immunosorbent assay. The data were presented as the mean ± standard error of mean of the OD450 values.
Cap, protein capsid; InvC, C-terminal domain of Yersinia pseudotuberculosis invasin; PBS, phosphate-buffered saline.
*,†,‡Column with different superscripts are significantly different from each other (p < 0.05).
Fig. 3. Neutralization antibody activity assay in pigs immunized with different adenoviruses. The neutralization antibody titers were expressed as the reciprocal of the highest serum dilution at ≥ 80% fluorescent focus reduction.
PCV2, porcine circovirus type 2; Cap, protein capsid; InvC, C-terminal domain of Yersinia pseudotuberculosis invasin; PBS, phosphate-buffered saline.
*,†,‡,§Column with different superscripts are significantly different from each other (p < 0.05).
Lymphocyte proliferation and cytokine detection in pigs
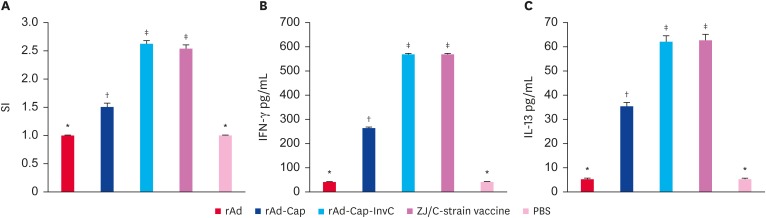
Lymphocyte proliferative levels in rAd-Cap, rAd-Cap-InvC and ZJ/C-strain vaccine vaccinated pigs were significantly higher than those of rAd and PBS treated pigs, and rAd-Cap-InvC and ZJ/C-strain vaccine groups were higher than that of rAd-Cap group (p < 0.05, Fig. 4A). The levels of IFN-γ and IL-13 in lymphocyte from rAd-Cap, rAd-Cap-InvC, and ZJ/C-strain vaccine groups were higher than those in rAd and PBS groups (p < 0.05, Fig. 4B and C). The rAd-Cap-InvC and ZJ/C-strain vaccine groups showed higher IFN-γ and IL-13 levels than other groups (p < 0.05, Fig. 4B and C).
Fig. 4. lymphocyte proliferation was analyzed by MTT after immunized with different adenoviruses (A). The SI = mean OD570 of Cap stimulated cells/mean OD570 of unstimulated cells. The IFN-γ (B) and IL-13 (C) levels were analyzed by enzyme-linked immunosorbent assay.
SI, stimulation index; IFN, interferon; IL, interleukin; Cap, protein capsid; InvC, C-terminal domain of Yersinia pseudotuberculosis invasin; PBS, phosphate-buffered saline.
*,†,‡Column with different superscripts are significantly different from each other (p < 0.05).
Protection of immunized pigs from virulent challenge
Serum samples were collected on 28, 35, 42, 49, and 56 DPI and used to detect the viremia by RT-PCR. As shown in Table 3, there were no viruses could be detected in all groups on 28 DPI. After virulent challenge, all pigs in the rAd and PBS groups were identified as viremia. However, viruses were not detected from the blood samples in rAd-Cap-InvC and ZJ/C-strain vaccine groups after 49 DPI. In group rAd-Cap, some pigs were viremia during 35–49 DPI, but viruses were not detected after 56 DPI. This result indicated that rAd-Cap-InvC could effectively protect the immunized animals from PCV2 infection.
Table 3. Detection of PCV2 in blood by RT-PCR after challenge.
| Groups | Days after immunization | ||||
|---|---|---|---|---|---|
| 28 | 35 | 42 | 49 | 56 | |
| rAd | 0/5 | 5/5 (7.13 ± 0.27) | 5/5 (7.37 ± 0.21) | 5/5 (7.12 ± 0.25) | 5/5 (6.58 ± 0.24) |
| rAd-Cap | 0/5 | 4/5 (5.01 ± 0.05) | 2/5 (5.01 ± 0.05) | 1/5 (3.37 ± 0.00) | 0/5 |
| rAd-Cap-InvC | 0/5 | 2/5 (4.40 ± 0.28) | 0/5 | 0/5 | 0/5 |
| ZJ/C-strain vaccine | 0/5 | 2/5 (4.70 ± 0.33) | 1/5 (3.96 ± 0.00) | 0/5 | 0/5 |
| PBS | 0/4 | 4/4 (6.68 ± 0.29) | 4/4 (6.87 ± 0.31) | 4/4 (6.40 ± 0.40) | 4/4 (6.05 ± 0.26) |
Data presented as number of pigs that were RT-PCR positive/total number of pigs (log 10 viral genomic copies/mL ± standard error of mean).
PCV2, porcine circovirus type 2; RT-PCR, real-time polymerase chain reaction; Cap, protein capsid; InvC, C-terminal domain of Yersinia pseudotuberculosis invasin; PBS, phosphate-buffered saline.
Gross lesions of lung and lymph node
Lung lesions in ZJ/C-strain and rAd-Cap-InvC groups were similar in type and extent (Table 4). The mean estimated amount of lung affected by pneumonia at 56 DPI was 0.87% and 1.6% for group ZJ/C-strain and rAd-Cap-InvC, respectively. The mean gross lung lesion score of group PBS and rAd was 18.5% and 17.4%, respectively. The scores of the 2 groups were significantly higher than those of group ZJ/C-strain and rAd-Cap-InvC (p < 0.05). Lymph node lesion in PBS group was generally similar to that observed in the rAd group. Superficial inguinal lymph nodes were often 2–5 times normal size in the 2 groups. And the gross lymph node lesions of the 2 groups were obviously higher than the group ZJ/C-strain and rAd-Cap-InvC (p < 0.05, Table 4). But there was no significant difference between group ZJ/C-strain and group rAd-Cap-InvC.
Table 4. Lung and lymph node score in different treatment groups.
| Groups | Lung (%) | Lymph node |
|---|---|---|
| rAd | 17.4 ± 1.1† | 3.75 ± 0.25† |
| rAd-Cap | 8.4 ± 0.74*,† | 2.33 ± 0.33† |
| rAd-Cap-InvC | 1.6 ± 0.16* | 0.75 ± 0.25* |
| ZJ/C-strain vaccine | 0.87 ± 0.2* | 0* |
| PBS | 18.5 ± 0.52† | 3.67 ± 0.33† |
Cap, protein capsid; InvC, C-terminal domain of Yersinia pseudotuberculosis invasin; PBS, phosphate-buffered saline.
*,†The mean lesion scores with different superscripts are significantly different from each other (p < 0.05).
DISCUSSION
The current commercial PCV2 vaccines are inactivated virus vaccines. A lot of clinical practice and laboratory tests have proved that the specific antibodies induced from PCV2 inactivated vaccines can effectively inhibit the multiplication of PCV2 and reduce the morbidity of PCVAD. However, inactivated vaccines are easy to trigger allergic reaction in animal bodies. Moreover, there is a risk of virulence return once these vaccines were not inactivated completely, and it is hard to distinguish immunization from natural infection. Consequently, the genetic engineering vaccines with high antibody titer and safety are preferred to be used to protect swine from PCV2 infection. Adenoviral vector is one of the important vectors of genetic engineering vaccine. It has many advantages, such as the high infection efficiency, high virus titers, stable expression and no integration property [25]. And it is widely used in the research of genetic engineering vaccines and gene therapy for many serious diseases.
In the present study, our results demonstrated that the recombinant adenovirus expressing Cap-InvC fusion protein could effectively stimulate the immune response to protect piglets against PCV2 infection. And a higher neutralization antibody titer was tested in the piglets immunized by rAd-Cap-InvC in compared with those immunized by inactivated vaccine (p < 0.05, Fig. 4). And also significantly lower clinical respiratory score, rectal temperature, lesion of lung and lymph node were observed in piglets treated with rAd-Cap-InvC than those of piglets in group PBS, rAd-Cap and rAd (Tables 2 and 4).
Cap protein of PCV2 has been used as a crucial immunogenicity protein in the research of PCV2 genetic engineering vaccines. However, people found individual application of Cap protein could not stimulate good immune protection. Therefore, many vaccines were designed with Cap protein and some proteins or cytokines to enhance immune responses. Zhang et al. [26] constructed the recombinant adenovirus co-expressing protein of PCV2 Cap and GM-CSF, which can make pigs obtain specific antibodies against PCV2, and the immunized pigs had better feed conversion ratio than the non-immunized group [27]. In a previous study, Ad-CD40L-Cap-GMCSF was constructed by inserting porcine (CD40L and GMCSF into recombinant adenovirus Ad-Cap, the results showed that CD40L and GMCSF can synergistically enhance the protective immune response of PCV2 adenovirus vaccine, and the 2 factors have synergistic effects on lymphocyte proliferation and Th1 cytokine production [16]. The recombinant adenovirus consisted of PCV2 Cap protein and herpetic stomatitis virus G protein can induce specific humoral and cellular immunity in mice [28]. There were some researchers using immune factors to promote the level of immune response. Genmei et al. [10] linked adenovirus vectors with Cap gene and IFN-γ gene, then acquired Cap-IFN-γ fusion protein. This virus can stimulate higher immune response than that simple expressing Cap protein.
As an immunity enhancer, Inv protein has been confirmed to enhance cellular ability to capture antigens and improve immune effects of vaccines [19,29]. Inv gene encodes invasion which binds tightly to β1-integrin and expresses on cell surface [30]. Over expression of Inv protein in cells could sufficiently induce β1-integrin production [31,32,33]. InvC containing 192 amino acids of C-terminal domain of invasin protein is essential to bind with β1-integrin and plays an important role in strengthening immune stimulation [18,34]. InvC gene fragment is small so that it can be easily manipulated and modified [19]. In this study, InvC gene was fused with PCV2 Cap gene to enhance the immune effects of Cap gene. The neutralization antibody titer in rAd-Cap-InvC group was significantly higher than those of rAd-Cap and ZJ/C-strain vaccine groups (p < 0.05). This result indicated that rAd-Cap-InvC was more efficiently to stimulate the production of neutralization antibody against PCV2 in pigs. Lymphocyte proliferation and cytokine levels are 2 important indexes of cellular immune response. The binding of InvC to β1-integrin receptors of host cells can induces activation of NF-κB and MAP kinases, and thereafter promotes the production of proinflammatory cytokines and T cell responses. Our results showed that lymphocyte proliferative levels and IFN-γ and IL-13 levels of rAd-Cap-InvC and ZJ/C-strain vaccine groups were higher than those of rAd-Cap group (Fig. 4). These results indicated that recombinant adenovirus with InvC could induce higher cellular immune response. This result indicated that rAd-Cap-InvC was better than the inactivated vaccine based on the neutralization antibody titer level. Although the commercial inactivated vaccine has very good efficacy, the complex components of inactivated vaccine might trigger allergic reaction and cause harmful immune reactions. However, the efficacy of rAd-Cap-InvC in commercial pig herds needs to be further investigated.
In conclusion, we successfully constructed the recombinant adenovirus rAd-Cap-InvC co-expressing PCV2 Cap protein and immune enhancement factor InvC. Our results showed that the recombinant adenoviruses rAd-Cap-InvC could increase the neutralization antibody titer and protect pigs against PCV2 infection. Therefore, we inferred that InvC gene was an alternative immunologic stimulator for genetic engineering vaccine.
Footnotes
Funding: This work was financially supported by the National Natural Science Foundation of China (Project No. 31672580), Key Agricultural Science and Technology Extension and Demonstration Projects in Shaanxi Province (Project No. ZDKJ-2014-33), Shaanxi Agricultural Science and Technology Innovation and Research Project (Project No. 2015NY177), Shaanxi Science and Technology Research and Development Plan Project (Project No. 2012K02-03).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Zhang Z, Guo K.
- Funding acquisition: Guo K, Zhang Z.
- Methodology: Zhang Z.
- Writing - original draft: Luo Y, Zhang Z.
- Writing - review & editing: Zhang Y.
References
- 1.Afghah Z, Webb B, Meng XJ, Ramamoorthy S. Ten years of PCV2 vaccines and vaccination: Is eradication a possibility? Vet Microbiol. 2017;206:21–28. doi: 10.1016/j.vetmic.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Palinski R, Piñeyro P, Shang P, Yuan F, Guo R, Fang Y, Byers E, Hause BM. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J Virol. 2016;91:e01879-16. doi: 10.1128/JVI.01879-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo HW, Han K, Park C, Chae C. Clinical, virological, immunological and pathological evaluation of four porcine circovirus type 2 vaccines. Vet J. 2014;200:65–70. doi: 10.1016/j.tvjl.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Shin MK, Yoon SH, Kim MH, Lyoo YS, Suh SW, Yoo HS. Assessing PCV2 antibodies in field pigs vaccinated with different porcine circovirus 2 vaccines using two commercial ELISA systems. J Vet Sci. 2015;16:25–29. doi: 10.4142/jvs.2015.16.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y, Guo L, Hou W, Guo C, Zhang W, Ma X, Ma L, Song X. The adjuvant activity of epimedium polysaccharide-propolis flavone liposome on enhancing immune responses to inactivated porcine circovirus vaccine in mice. Evid Based Complement Alternat Med. 2015;2015:972083. doi: 10.1155/2015/972083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai SL, Chen SN, Xu ZH, Tang MH, Wang FG, Li XJ, Sun BB, Deng SF, Hu J, Lv DH, Wen XH, Yuan J, Luo ML, Wei WK. Porcine circovirus type 2 in China: an update on and insights to its prevalence and control. Virol J. 2014;11:88–101. doi: 10.1186/1743-422X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo X, Zhu Q, Pan X, Yang X, Qiao H, Wang S, Chen H. Immunogenicity of a recombinant pseudorabies virus coexpressing ORF2 gene of PCV2 and porcine IL-18 gene in mice. Wei Sheng Wu Xue Bao. 2016;56:120–129. [PubMed] [Google Scholar]
- 8.Hu G, Wang N, Yu W, Wang Z, Zou Y, Zhang Y, Wang A, Deng Z, Yang Y. Generation and immunogenicity of porcine circovirus type 2 chimeric virus-like particles displaying porcine reproductive and respiratory syndrome virus GP5 epitope B. Vaccine. 2016;34:1896–1903. doi: 10.1016/j.vaccine.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Piñeyro PE, Kenney SP, Giménez-Lirola LG, Heffron CL, Matzinger SR, Opriessnig T, Meng XJ. Expression of antigenic epitopes of porcine reproductive and respiratory syndrome virus (PRRSV) in a modified live-attenuated porcine circovirus type 2 (PCV2) vaccine virus (PCV1-2a) as a potential bivalent vaccine against both PCV2 and PRRSV. Virus Res. 2015;210:154–164. doi: 10.1016/j.virusres.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Genmei L, Manlin L, Ruiai C, Hongliang H, Dangshuai P. Construction and immunogenicity of recombinant adenovirus expressing ORF2 of PCV2 and porcine IFN gamma. Vaccine. 2011;29:8677–8682. doi: 10.1016/j.vaccine.2011.08.118. [DOI] [PubMed] [Google Scholar]
- 11.Roques E, Girard A, Gagnon CA, Archambault D. Antibody responses induced in mice immunized with recombinant adenovectors expressing chimeric proteins of various porcine pathogens. Vaccine. 2013;31:2698–2704. doi: 10.1016/j.vaccine.2013.03.068. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Wang X, Bai J, Ma T, Li Z, Li Y, Jiang P. Construction and immunogenicity of recombinant porcine circovirus-like particles displaying somatostatin. Vet Microbiol. 2013;163:23–32. doi: 10.1016/j.vetmic.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Xu D, Wang Z, Du Q, Chang L, Zhao X, Huang Y, Tong D. Immunogenicity evaluation of modified adenovirus vaccines expressing porcine circovirus type 2 capsid protein in pigs. Viral Immunol. 2017;30:111–119. doi: 10.1089/vim.2016.0086. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Jiang P, Li Y, Jiang W, Dong X. Protection of pigs against post-weaning multisystemic wasting syndrome by a recombinant adenovirus expressing the capsid protein of porcine circovirus type 2. Vet Microbiol. 2007;121:215–224. doi: 10.1016/j.vetmic.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Jiang W, Jiang P, Li Y, Feng Z, Xu J. Construction and immunogenicity of recombinant adenovirus expressing the capsid protein of porcine circovirus 2 (PCV2) in mice. Vaccine. 2006;24:3374–3380. doi: 10.1016/j.vaccine.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Huang Y, Du Q, Wang Z, Chang L, Zhao X, Tong D. CD40 ligand and GMCSF coexpression enhance the immune responses and protective efficacy of PCV2 adenovirus vaccine. Viral Immunol. 2016;29:148–158. doi: 10.1089/vim.2015.0109. [DOI] [PubMed] [Google Scholar]
- 17.Li DL, Huang Y, Chang LL, Du Q, Chen Y, Wang TT, Luo XM, Zhao XM, Tong DW. Modified recombinant adenoviruses increase porcine circovirus 2 capsid protein expression and induce enhanced immune responses in mice. Acta Virol. 2016;60:271–280. doi: 10.4149/av_2016_03_271. [DOI] [PubMed] [Google Scholar]
- 18.Bühler OT, Wiedig CA, Schmid Y, Grassl GA, Bohn E, Autenrieth IB. The Yersinia enterocolitica invasin protein promotes major histocompatibility complex class I- and class II-restricted T-cell responses. Infect Immun. 2006;74:4322–4329. doi: 10.1128/IAI.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisano F, Kochut A, Uliczka F, Geyer R, Stolz T, Thiermann T, Rohde M, Dersch P. In vivo-induced InvA-like autotransporters Ifp and InvC of Yersinia pseudotuberculosis promote interactions with intestinal epithelial cells and contribute to virulence. Infect Immun. 2012;80:1050–1064. doi: 10.1128/IAI.05715-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Ning P, Lin Z, Liang W, Kang K, He L, Zhang Y. Co-expression of the C-terminal domain of Yersinia enterocolitica invasin enhances the efficacy of classical swine-fever-vectored vaccine based on human adenovirus. J Biosci. 2015;40:79–90. doi: 10.1007/s12038-014-9495-z. [DOI] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 22.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 23.Xu XG, Zhao HN, Zhang Q, Ding L, Li ZC, Li W, Wu HY, Chuang KP, Tong DW, Liu HJ. Oral vaccination with attenuated Salmonella enterica serovar typhimurium expressing Cap protein of PCV2 and its immunogenicity in mouse and swine models. Vet Microbiol. 2012;157:294–303. doi: 10.1016/j.vetmic.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Kim CH, Han K, Seo HW, Oh Y, Park C, Kang I, Chae C. Comparative efficacy of commercial Mycoplasma hyopneumoniae and porcine circovirus 2 (PCV2) vaccines in pigs experimentally infected with M. hyopneumoniae and PCV2. Vaccine. 2011;29:3206–3212. doi: 10.1016/j.vaccine.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Beach NM, Meng XJ. Efficacy and future prospects of commercially available and experimental vaccines against porcine circovirus type 2 (PCV2) Virus Res. 2012;164:33–42. doi: 10.1016/j.virusres.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Qian P, Peng B, Shi L, Chen H, Li X. A novel subunit vaccine co-expressing GM-CSF and PCV2b Cap protein enhances protective immunity against porcine circovirus type 2 in piglets. Vaccine. 2015;33:2449–2456. doi: 10.1016/j.vaccine.2015.03.090. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Lu Y, Liu D, Wei Y, Guo L, Wu H, Huang L, Liu J, Liu C. Enhanced Th1-biased immune efficacy of porcine circovirus type 2 Cap-protein-based subunit vaccine when coadministered with recombinant porcine IL-2 or GM-CSF in mice. Appl Microbiol Biotechnol. 2015;99:1155–1163. doi: 10.1007/s00253-014-6167-8. [DOI] [PubMed] [Google Scholar]
- 28.Ye Y, Cheng X, Zhang J, Tong T, Lin W, Liao M, Fan H. Induction of robust immunity response in mice by dual-expression-system-based recombinant baculovirus expressing the capsid protein of porcine circovirus type 2. Virol J. 2013;10:316–324. doi: 10.1186/1743-422X-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blome S, Moß C, Reimann I, König P, Beer M. Classical swine fever vaccines-state-of-the-art. Vet Microbiol. 2017;206:10–20. doi: 10.1016/j.vetmic.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Anderson JC, Clarke EJ, Arkin AP, Voigt CA. Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol. 2006;355:619–627. doi: 10.1016/j.jmb.2005.10.076. [DOI] [PubMed] [Google Scholar]
- 31.Critchley-Thorne RJ, Stagg AJ, Vassaux G. Recombinant Escherichia coli expressing invasin targets the Peyer's patches: the basis for a bacterial formulation for oral vaccination. Mol Ther. 2006;14:183–191. doi: 10.1016/j.ymthe.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Gillenius E, Urban CF. The adhesive protein invasin of Yersinia pseudotuberculosis induces neutrophil extracellular traps via β1 integrins. Microbes Infect. 2015;17:327–336. doi: 10.1016/j.micinf.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Yoshikawa Y, Ashida H, Iwai H, Toyotome T, Matsui H, Sasakawa C. High vaccine efficacy against shigellosis of recombinant noninvasive Shigella mutant that expresses Yersinia invasin. J Immunol. 2006;177:4709–4717. doi: 10.4049/jimmunol.177.7.4709. [DOI] [PubMed] [Google Scholar]
- 34.Bonaventura A, Liberale L, Carbone F, Vecchié A, Diaz-Cañestro C, Camici GG, Montecucco F, Dallegri F. The pathophysiological role of neutrophil extracellular traps in inflammatory diseases. Thromb Haemost. 2018;118:6–27. doi: 10.1160/TH17-09-0630. [DOI] [PubMed] [Google Scholar]