Abstract
Chronic mitral valve disease (CMVD) is the most common cardiovascular disease in dogs, causing decreased cardiac output that results in poor tissue perfusion and tissue damage to kidneys, pancreas, and other organs. The purpose of this study was to evaluate the relationships between heart disease severity and N-terminal pro B-type natriuretic peptide (NT-proBNP) and lipase in dogs with CMVD, as well as to evaluate longitudinal changes in these values. A total of 84 dogs participated in this 2015 to 2017 study. Serum values of NT-proBNP and lipase were analyzed; radiography was used to measure the vertebral heart score and assess various echocardiographic values. NT-proBNP showed a strong positive correlation with increasing stage of heart disease; lipase showed a mild positive correlation with heart disease stage. When the three values (NT-proBNP, lipase and month) were continuously measured at 6-month intervals, all showed a correlation with the increasing length of the disease.
Keywords: NT-proBNP, lipase, mitral valve disease, dog
INTRODUCTION
Chronic mitral valve disease (CMVD) progresses chronically over the years through progressive valve degeneration and worsening of symptoms. Risk factors for rapid progression include the quantity of mitral regurgitation, age, and changes in valvular severity [1,2].
The N-terminal proB-type natriuretic peptide (NT-proBNP) has received considerable interest as a dog heart biomarker in recent years. NT-proBNP while regarded as a biomarker of heart disease, also exhibits a high degree of diversity among individuals, according to recent studies [3,4,5,6]. Regardless, longitudinal evaluation of NT-proBNP is reported to be effective in dogs with CMVD [7].
Reduced cardiac output can lead to poor tissue perfusion and secondary tissue congestion, which can damage multiple organs. In humans, many patients undergoing cardiac surgery exhibit a variety of pancreatic lesions, ranging from lethal necrotizing pancreatitis to subclinical hyperamylasemia. These lesions are associated with a variety of factors, among which the most important factor is hypoperfusion of the pancreas caused by hypotension [8]. These results imply that severe ischemic conditions over a short period of time can lead to severe pancreatic damage, which occurs rapidly and steadily, but, typically, it is subclinical. Such ischemic injury of the pancreas acts as a trigger for acute or chronic pancreatitis [9]. In humans, ischemia/hypoxia in the pancreas is the leading cause of pancreatitis [10,11,12]; however, the prevalence of pancreatic damage in CMVD has not been extensively studied in veterinary medicine [13]. In one study of dogs with CMVD, canine pancreatic lipase levels increased with increasing International Small Animal Cardiac Health Council (ISACHC) stage; to our knowledge, there have been no pertinent studies published [13].
This study was performed to evaluate the relationships among NT-proBNP, lipase, Vertebral Heart Scale (VHS), Left Ventricular End-diastolic Internal Dimension, Normalized for Body Weight (LVEDDN), Left Atrium to Aorta Ratio (LA/Ao), and the mitral valve E and E′ wave velocity ratio (E/E′) in dogs with CMVD and to analyze longitudinal changes in these values, as well as to compare individual values at various stages of the disease.
MATERIALS AND METHODS
Animals
The study population comprised dogs that visited the veterinary clinic at Chungnam National University from August 2015 to December 2017. Prior to the study, the owners of all included dogs provided informed consent for participation in this study. A total of 84 dogs participated in this prospective observational study (Table 1). Subdivided by breeds, the participating dogs were as follows: Shih-tzu (n =26), Maltese (n = 22), Yorkshire terrier (n = 12), Poodle (n = 6), Cocker spaniel (n = 3), Pekingese (n = 3), Beagle (n =3), Schnauzer (n = 2), Dachshund (n =2), Italian greyhound (n =1), Chihuahua (n =1), Miniature pinscher (n =1), Pungsan dog (n =1), and Mongrel (n = 1). Ten dogs comprised the normal control group; these dogs ranged in size from small to medium (weight: 3.5–10.1 kg), were between 3 and 18 years of age, and exhibited no cardiovascular or systemic disease (as determined by physical examination, blood chemistry and complete blood count analysis, chest and abdomen radiography, and echocardiography). Unlike the control group, dogs with myxomatous degeneration of the mitral valve were classified as the CMVD group; these dogs were categorized on the basis of their CMVD stage as proposed by the American College of Veterinary Internal Medicine (ACVIM) consensus. The diagnosis of CMVD was based on clinical symptoms, blood chemistry and complete blood count analysis, chest radiography, and echocardiography.
Table 1. Characteristics of each group according to ACVIM stage.
| Characteristics | ACVIM stage | |||||
|---|---|---|---|---|---|---|
| Control | ACVIM B1 | ACVIM B2 | ACVIM C | ACVIM D | ||
| Total (n = 84) | 10 | 15 | 12 | 20 | 27 | |
| Age (yr) | 12.50 ± 3.27 | 12.13 ± 2.61 | 12.92 ± 3.67 | 14.72 ± 1.86 | 13.64 ± 2.27 | |
| Weight (kg) | 6.20 ± 2.40 | 5.52 ± 4.49 | 6.16 ± 1.69 | 5.66 ± 3.88 | 5.85 ± 2.09 | |
| BCS (9pt) | 5.30 ± 0.67 | 5.00 ± 0.84 | 4.67 ± 0.88 | 4.96 ± 0.84 | 4.73 ± 0.83 | |
| Sex (M/F) | M (6), F (4) | M (6), F (9) | M (4), F (8) | M (10), F (10) | M (15), F (12) | |
| Breeds | ||||||
| Beagle | 3 | |||||
| Chihuahua | 1 | |||||
| Cocker spaniel | 1 | 2 | ||||
| Dachshund | 2 | |||||
| Italian greyhound | 1 | |||||
| Maltese | 1 | 3 | 6 | 4 | 8 | |
| Miniature pinscher | 1 | |||||
| Mongrel | 1 | |||||
| Pekingese | 1 | 1 | 1 | |||
| Poodle | 2 | 4 | ||||
| Pungsan dog | 1 | |||||
| Schnauzer | 1 | 1 | ||||
| Shih-tzu | 6 | 1 | 7 | 12 | ||
| Yorkshire terrier | 1 | 2 | 2 | 6 | 1 | |
Values are presented as mean ± standard deviation.
ACVIM, American College of Veterinary Internal Medicine; BCS, body condition score; M, male; F, female.
Dogs of the CMVD stage C group were prescribed drugs such as benazepril (0.25–0.5 mg/kg, twice a day), furosemide (1–2 mg/kg, twice a day), spironolactone (1–2 mg/kg, twice a day), pimobendan (0.3 mg/kg, twice a day), sildenafil (1–2 mg/kg, twice a day), or theophylline (7–10 mg/kg, twice a day), on the basis of disease severity. Dogs of the CMVD stage D group that did not respond to general treatment were prescribed drugs such as torsemide (0.2 mg/kg, twice a day) or chlorothiazide (0.2–0.4 mg/kg, twice a day).
Analysis of serum NT-proBNP and lipase concentration
To minimize the effect of feeding, all dogs were fasted for 12 h after which blood samples were collected from the jugular vein, placed in a serum separating tube, and at 30 min after collection, were centrifuged at 2,000 × g for 10 min. Several serum chemistries including lipase level were measured by using a serum chemistry instrument (BS-330; Mindray, China); the remaining samples were stored at −80°C until NT-proBNP measurements were obtained. Serum NT-proBNP levels were measured by using a reference laboratory (IDEXX Laboratories, Korea); frozen serum samples were slowly melted at room temperature before analysis.
Echocardiography
Before echocardiography, VHS was determined by using chest radiography and echocardiography; that process was performed in accordance with recommended standards for dogs. M-mode, two-dimensional echocardiography, as well as Doppler and tissue Doppler imaging (TDI) were performed in left and right lateral recumbent postures. The left ventricular end-diastolic diameter (VIDd) and the left ventricular dimension at systole (LVIDs) were measured in M-mode. Fractional shortening (FS) values and LVEDDN were quantified based on the obtained LVIDd and LVIDs values. Left atrium (LA) and proximal aorta (Ao) diameters were measured via two-dimensional echocardiography, and the LA/Ao ratio was quantified. The velocity of the E wave in the mitral valve was measured by Doppler imaging in the left apical four-chamber view. The velocity of the E′ wave in the mitral valve annulus was measured by TDI; the E/E′ ratio was then quantified.
Statistical analysis
Statistical analysis was performed by using SPSS Statistics 24.0.0 (IBM Corp., USA). Age, weight, NT-proBNP, lipase, Symmetric Dimethylarginine (SDMA), Blood Urea Nitrogen (BUN), creatinine, VHS, LVEDDN), LA/Ao, Fractional Shortening (FS), and E/E′ values are presented as means and standard deviations for each ACVIM stage group. The stage group values were compared by using repeated-measures analysis of variance (ANOVA). NT-proBNP and lipase were measured at 6-month intervals in 14 dogs of stage C or higher. A comparative analysis of these values was also performed by using repeated-measures analysis of variance ANOVA. In addition, the degrees of correlation between month and NT-proBNP, as well as month and lipase were measured by determining Pearson's coefficient of correlation. The degrees of correlation with the ACVIM stage, NT-proBNP, lipase, SDMA, BUN, creatinine, VHS, LVEDD, LA/Ao, FS, and E/E′ were also measured by using Pearson's coefficient of correlation. At the p < 0.05 level, the differences between the stage group values and the correlations between values were considered significantly different.
RESULTS
Ten dogs were included in the normal control group (weight: 3.5–10.1 kg), while the ACVIM stage B1 group comprised 15 dogs (2.6–21 kg), stage B2 group comprised 12 dogs (2.25–6.7 kg), stage C group comprised 20 dogs (2.5–14.3 kg), and stage D group comprised 27 dogs (2.3–10.1 kg). Most were small breed dogs (2.25–21 kg). To distinguish stages B1 and B2, at least two of the following three diagnostic imaging results needed to be satisfied: LA/AO ratio ≥ 1.6, VHS ≥ 10.7, and LVEDDN ≥ 1.7.
Dogs with obesity, renal failure or hypertension, which may affect NT-proBNP levels were excluded from assessment. The mean NT-proBNP level increased with increasing ACVIM stage. When comparing the values between groups, the stage B1 and B2 group values did not differ from that of the control group; in contrast, the stage C group NT-proBNP value significantly differed from that of the control and B1 groups, and the stage D group value differed from the control, B1, B2, and C group (p < 0.05, Table 2). The values of VHS, LA/Ao, and E/E' significantly increased in ACVIM stage D group (Table 2).
Table 2. The values of NT-proBNP, lipase, kidney panel and echocardiographic measures for control dogs and dogs with CMVD.
| Characteristics | Control (n = 10) | ACVIM B1 (n = 15) | ACVIM B2 (n = 12) | ACVIM C (n = 20) | ACVIM D (n = 27) |
|---|---|---|---|---|---|
| NT-proBNP (pmol/L) | 462.60 ± 176.73 | 517.83 ± 222.41 | 690.00 ± 197.06 | 2,154.05 ± 919.81*,† | 4,400.22 ± 2,173.95*,†,‡,§ |
| Lipase (U/L) | 94.80 ± 35.38 | 127.62 ± 45.65 | 154.25 ± 38.77 | 226.67 ± 204.71 | 380.94 ± 417.36 *,† |
| SDMA (μg/dL) | 10.00 ± 3.05 | 12.18 ± 2.08 | 13.40 ± 4.39 | 13.53 ± 4.47 | 14.45 ± 5.86* |
| BUN (mg/dL) | 17.52 ± 8.80 | 25.50 ± 13.22 | 20.10 ± 10.01 | 23.43 ± 9.24 | 24.17 ± 21.51* |
| Creatinine (mg/dL) | 0.72 ± 0.22 | 0.83 ± 0.37 | 0.88 ± 0.24 | 0.87 ± 0.22 | 0.90 ± 0.56* |
| VHS | 9.71 ± 0.67 | 10.10 ± 0.51 | 10.55 ± 0.23* | 10.75 ± 0.51* | 11.26 ± 0.85*,† |
| LVEDDN | 1.54 ± 0.15 | 1.58 ± 0.22 | 1.72 ± 0.17* | 1.75 ± 0.32* | 1.79 ± 0.18 |
| LA/Ao | 1.40 ± 0.17 | 1.55 ± 0.27 | 1.75 ± 0.38 | 2.01 ± 0.33*,† | 2.02 ± 0.45*,† |
| FS (%) | 55.12 ± 6.09 | 51.67 ± 13.38 | 56.45 ± 12.00 | 60.84 ± 12.30 | 60.57 ± 12.93 |
| E/E′ | 9.84 ± 1.45 | 11.28 ± 1.92 | 11.52 ± 1.82 | 12.56 ± 2.69 | 15.67 ± 3.50*,†,‡ |
Values are presented as mean ± standard deviation.
ACVIM, American College of Veterinary Internal Medicine; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SDMA, symmetric dimethylarginine; BUN, blood urea nitrogen; VHS, vertebral heart size; LVEDDN, left ventricular end-diastolic diameter, normalized for body weight; LA/Ao, left atrium to aorta ratio; FS, fractional shortening. E/E' ratio, the mitral valve E and E' wave velocity ratio.
*p < 0.05, difference from control group; †p < 0.05, difference from ACVIM B1; ‡p < 0.05, difference from ACVIM B2; §p < 0.05, difference from ACVIM C.
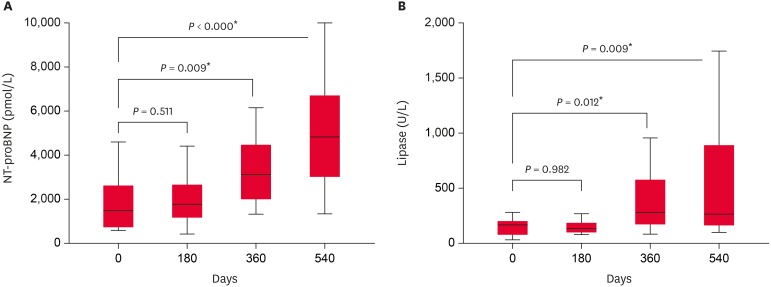
Fourteen dogs with stage C or higher were examined for NT-proBNP and lipase values at 180-day intervals (Fig. 1). The levels of two markers at day 180 did not differ from those at day 0. At days 360 and 540, levels of NT-proBNP and lipase significantly differ from those at day 0 (p < 0.05). When the correlations between time and NT-proBNP and lipase levels were examined (Table 3), NT-proBNP was moderately correlated with time (R=0.556). Finally, we measured the correlation coefficients (R) between ACVIM stage, NT-proBNP, lipase, renal markers and imaging results (Table 4). ACVIM stage and NT-proBNP showed a strong correlation (R=0.729); NT-proBNP and lipase were also correlated (R=0.484).
Fig. 1. The results of NT-proBNP and lipase levels measured at 180-day intervals in 14 dogs of ACVIM stage C or higher. NT-proBNP, N-terminal pro-B-type natriuretic peptide; ACVIM, American College of Veterinary Internal Medicine.
Table 3. When 14 dogs of the ACVIM stage C or more were checked at 6-month intervals, the correlation coefficient between NT-proBNP, lipase concentration and month.
| Characteristics | Month | NT-proBNP | Lipase | |||
|---|---|---|---|---|---|---|
| R | p* | R | p* | R | p* | |
| Month | 0.556 | <0.001 | 0.449 | <0.001 | ||
| NT-proBNP | 0.556 | <0.001 | 0.409 | 0.001 | ||
| Lipase | 0.449 | <0.001 | 0.409 | 0.001 | ||
ACVIM, American College of Veterinary Internal Medicine; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
*The p values were measured by Pearson's coefficient of correlation analysis.
Table 4. Correlation coefficient of ACVIM stage, NT-proBNP and lipase concentration to other measurements.
| Characteristics | ACVIM stage | NT-proBNP | Lipase | |||
|---|---|---|---|---|---|---|
| R | p* | R | p* | R | p* | |
| ACVIM stage | - | - | 0.729 | <0.001 | 0.370 | 0.001 |
| NT-proBNP | 0.729 | <0.001 | - | - | 0.484 | <0.001 |
| Lipase | 0.370 | 0.484 | 0.484 | <0.001 | - | - |
| SDMA | 0.787 | <0.001 | 0.755 | <0.001 | 0.634 | <0.001 |
| BUN | 0.646 | <0.001 | 0.659 | <0.001 | 0.457 | <0.001 |
| Creatinine | 0.548 | <0.001 | 0.655 | <0.001 | 0.600 | <0.001 |
| VHS | 0.620 | <0.001 | 0.491 | <0.001 | 0.208 | 0.143 |
| LVEDDN | 0.356 | 0.011 | 0.502 | 0.006 | 0.346 | 0.031 |
| LA/Ao | 0.521 | <0.001 | 0.550 | 0.001 | 0.224 | 0.279 |
| FS | 0.217 | 0.138 | 0.321 | 0.030 | 0.079 | 0.596 |
| E/E′ | 0.611 | <0.001 | 0.539 | <0.001 | 0.392 | 0.012 |
ACVIM, American College of Veterinary Internal Medicine; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SDMA, symmetric dimethylarginine; BUN, blood urea nitrogen; VHS, vertebral heart size; LVEDDN, left ventricular end-diastolic diameter, normalized for body weight; LA/Ao, left atrium to aorta ratio; FS, fractional shortening; E/E′, the mitral valve E and E′ wave velocity ratio.
*The p values were measured by Pearson's coefficient of correlation analysis.
DISCUSSION
This study focused on differences and changes in NT-proBNP and lipase levels according to the stage and duration of heart disease. Knowledge of NT-proBNP level is very helpful in monitoring cardiac patients. In addition to visualizing the degree of ventricular stretch in cardiac patients, it enables evaluation of the survival rate among hospitalized human and dogs [2,14,15,16].
Similar to the NT-proBNP result, the lipase level was also significantly high, compared with that of the control group, in all ACVIM stages. Stages C and D levels were higher than those in both the control and B1 groups. Importantly, there have been no studies regarding lipase differences according to heart disease stage; thus, more studies are needed in the future.
During our periodic evaluation of heart disease patients (every 6 month intervals), we observed that NT-proBNP and lipase levels increased with time. Notably, those values were significantly higher at 1 year after baseline measurement. Other studies have examined the subject-specific variability of NT-proBNP in dogs [4,17]. However, those studies were not conducted at 6-month intervals, as used in the present study; rather, the measurements were obtained at weekly weekly intervals for up to 3 or 7weeks. Thus, the other studies could not confirm longer-term longitudinal changes in NT-proBNP levels in dogs.
When the respective correlations between time and NT-proBNP and lipase in patients with heart disease were analyzed, NT-proBNP and lipase levels showed moderate positive correlations with increasing time. Thus, periodic monitoring of NT-proBNP and lipase levels in patients with heart disease may be helpful in determining the progression of heart disease. When the correlations between ACVIM stage and NT-proBNP and lipase levels were analyzed, NT-proBNP showed a strong correlation with ACVIM stage, whereas lipase showed only a moderate correlation. In another study, the stage of heart disease was reported to be moderately correlated with NT-proBNP level [18]; this difference may have been due to differences in heart disease stage classification in the two studies. The correlations between NT-proBNP and month as well as between NT-proBNP and lipase, were moderate. These results suggest that pancreas marker levels, such as lipase level, are not strongly correlated with NT-proBNP level, a direct biomarker of heart disease.
A number of imaging values should be measured in heart disease patients [19]. When classified as an ISACHC stage, there was a significant correlation between imaging values and the stage of heart disease [18]. Among these, VHS, LVEDDN, LA/Ao, FS, and E/E′ were monitored in the present study. ACVIM stage C or D were significantly correlated with VHS, LA/Ao and E/E' ratio.
In humans, ischemic injury occurs when tissue hypoperfusion occurs, thus resulting in pancreatitis [9,10]. Therefore, in this study, we aimed to observe changes in pancreatic marker levels according to the degree of heart disease. The pancreatic marker levels were moderately correlated with the stage of heart disease and the NT-proBNP level, indicating that secondary pancreatitis may occur when heart disease progresses; however, the correlation is not strong.
The limitations of this study include the following: First, the number of dogs in each group was small, increasing the risk of false negative results in the study. Further studies are needed with larger study populations to obtain more accurate data. Second, dogs with ACVIM stage B2 or higher were surveyed while they were using cardiac medications. However, the influences on pancreatic and renal dysfunction by the cardiac medications were not assessed in this study.
In conclusion, increasing NT-proBNP levels showed a strong correlation with increasing ACVIM stage. While the increase in lipase levels showed a mild correlation with increasing ACVIM stage. NT-proBNP and lipase levels were moderately correlated. In addition, when those two values were continuously measured at 6-month intervals, they showed mild to moderate correlations with the increasing duration of CMVD.
Footnotes
Conflict of Interest: The authors declare no conflicts of interests.
- Conceptualization: Park JS, Song KH.
- Investigation: Park JS, Park JH.
- Methodology: Park JH.
- Resources: Park JS.
- Supervision: Song KH.
- Validation: Seo KW.
- Writing - original draft: Park JS, Song KH.
- Writing - review & editing: Park JS; Seo KW, Song KH.
References
- 1.Atkins C, Bonagura J, Ettinger S, Fox P, Gordon S, Haggstrom J, Hamlin R, Keene B, Luis-Fuentes V, Stepien R. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142–1150. doi: 10.1111/j.1939-1676.2009.0392.x. [DOI] [PubMed] [Google Scholar]
- 2.Borgarelli M, Savarino P, Crosara S, Santilli RA, Chiavegato D, Poggi M, Bellino C, La Rosa G, Zanatta R, Haggstrom J, Tarducci A. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120–128. doi: 10.1111/j.1939-1676.2007.0008.x. [DOI] [PubMed] [Google Scholar]
- 3.Boswood A, Dukes-McEwan J, Loureiro J, James RA, Martin M, Stafford-Johnson M, Smith P, Little C, Attree S. The diagnostic accuracy of different natriuretic peptides in the investigation of canine cardiac disease. J Small Anim Pract. 2008;49:26–32. doi: 10.1111/j.1748-5827.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 4.Kellihan HB, Oyama MA, Reynolds CA, Stepien RL. Weekly variability of plasma and serum NT-proBNP measurements in normal dogs. J Vet Cardiol. 2009;11(Suppl 1):S93–S97. doi: 10.1016/j.jvc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds CA, Brown DC, Rush JE, Fox PR, Nguyenba TP, Lehmkuhl LB, Gordon SG, Kellihan HB, Stepien RL, Lefbom BK, Meier CK, Oyama MA. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: the PREDICT cohort study. J Vet Cardiol. 2012;14:193–202. doi: 10.1016/j.jvc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Prošek R, Sisson DD, Oyama MA, Solter PF. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B-type natriuretic factor, endothelin, and cardiac troponin-I. J Vet Intern Med. 2007;21:238–242. doi: 10.1892/0891-6640(2007)21[238:dcandi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Wolf J, Gerlach N, Weber K, Klima A, Wess G. Lowered N-terminal pro-B-type natriuretic peptide levels in response to treatment predict survival in dogs with symptomatic mitral valve disease. J Vet Cardiol. 2012;14:399–408. doi: 10.1016/j.jvc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Svensson LG, Decker G, Kinsley RB. A prospective study of hyperamylasemia and pancreatitis after cardiopulmonary bypass. Ann Thorac Surg. 1985;39:409–411. doi: 10.1016/s0003-4975(10)61945-5. [DOI] [PubMed] [Google Scholar]
- 9.Kyogoku T, Manabe T, Tobe T. Role of ischemia in acute pancreatitis. Hemorrhagic shock converts edematous pancreatitis to hemorrhagic pancreatitis in rats. Dig Dis Sci. 1992;37:1409–1417. doi: 10.1007/BF01296012. [DOI] [PubMed] [Google Scholar]
- 10.Sakorafas GH, Tsiotos GG, Sarr MG. Ischemia/reperfusion-induced pancreatitis. Dig Surg. 2000;17:3–14. doi: 10.1159/000018793. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabbahy ME, Vaidya VS. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip Rev Syst Biol Med. 2011;3:606–618. doi: 10.1002/wsbm.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han D, Choi R, Hyun C. Canine pancreatic-specific lipase concentrations in dogs with heart failure and chronic mitral valvular insufficiency. J Vet Intern Med. 2015;29:180–183. doi: 10.1111/jvim.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 15.Moonarmart W, Boswood A, Luis Fuentes V, Brodbelt D, Souttar K, Elliott J. N-terminal pro B-type natriuretic peptide and left ventricular diameter independently predict mortality in dogs with mitral valve disease. J Small Anim Pract. 2010;51:84–96. doi: 10.1111/j.1748-5827.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 16.Troughton R, Michael Felker G, Januzzi JL., Jr Natriuretic peptide-guided heart failure management. Eur Heart J. 2014;35:16–24. doi: 10.1093/eurheartj/eht463. [DOI] [PubMed] [Google Scholar]
- 17.Ruaux C, Scollan K, Suchodolski JS, Steiner JM, Sisson DD. Biologic variability in NT-proBNP and cardiac troponin-I in healthy dogs and dogs with mitral valve degeneration. Vet Clin Pathol. 2015;44:420–430. doi: 10.1111/vcp.12268. [DOI] [PubMed] [Google Scholar]
- 18.Choi BS, Moon HS, Seo SH, Hyun C. Evaluation of serum cystatin-C and symmetric dimethylarginine concentrations in dogs with heart failure from chronic mitral valvular insufficiency. J Vet Med Sci. 2017;79:41–46. doi: 10.1292/jvms.16-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chetboul V, Tissier R. Echocardiographic assessment of canine degenerative mitral valve disease. J Vet Cardiol. 2012;14:127–148. doi: 10.1016/j.jvc.2011.11.005. [DOI] [PubMed] [Google Scholar]