Abstract
The composition of the gastrointestinal microorganisms in poultry is closely associated with the host and its environment. In this study, using 16S rRNA and metagenomic techniques, we comprehensively analyzed the structure and diversity of the cecal microbiota of broiler chickens (BC) and laying hens (LH). The 16S rRNA sequencing analysis showed Firmicutes, Bacteroidetes, and Proteobacteria were the main cecal bacterial phyla in BC and LH. However, at the genus level, LH had a greater abundance of Bacteroides (P < 0.05), Rikenellaceae_RC9_gut_group (P < 0.01), Phascolarctobacterium (P < 0.05), Desulfovibrio (P < 0.05), Prevotellaceae_UCG-001 (P < 0.05), and unclassified_o_Bacteroidales (P < 0.05), whereas BC had a greater abundance of Alistipes (P < 0.05), Rikenella (P < 0.05), Ruminococcaceae_UCG-005 (P < 0.05), Lachnoclostridium (P < 0.05), and unclassified_f_Ruminococcaceae (P < 0.05). It is particularly noteworthy that the genus Desulfovibrio was significantly more abundant in the LH cecum than in the BC cecum (P < 0.05). A metagenomic analysis showed that the annotations in the LH dataset were significantly more abundant than in the BC dataset, and included replication, recombination and repair, energy production and transformation, cell wall/membrane/envelope biogenesis, and amino acid transport and metabolism-related functions (P < 0.05). This study indicates that microbial genotypic differences in chickens of the same species can cause changes in the abundances of the gut microbiota, but do not alter the structural composition or major functional characteristics of the gut microbiota.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1834-1) contains supplementary material, which is available to authorized users.
Keywords: 16S rRNA sequencing, Metagenomic sequencing, Cecal microbiota, Broiler chicken, Laying hen, Microbe composition, Function
Introduction
More than 1000 species are found in the chicken intestine (Chambers and Gong 2011). The gut microbiota is an important metabolic organ, and the bacterial intestinal microbiota is considered extremely important in chicken (Wen et al. 2019), as it plays important roles in the feed conversion ratio (Cui et al. 2017), nutrient absorption (Pan and Yu 2014), the immune system (Yeoman et al. 2012), and the prevention of intestinal pathogen colonization of the intestine (Clavijo and Vives Florez 2018). Therefore, a comprehensive understanding of the composition and diversity of the intestinal microbiota of laying hens (LH) and broiler chickens (BC) and the functions of their intestinal bacteria is essential.
The animal intestine is a dynamic ecosystem. Both the host and the external environment affect the diversity of gut microbes. It is generally believed that the host′s diet, age, and genotype are important factors affecting this diversity. Molecular biology studies have shown that the host genotype is closely related to the structure of the intestinal microbiota of an organism, and the host colonies of different hosts have individual specificity (Garcia-Mazcorro et al. 2012). When studying the effects of the host genetic background on the composition of the intestinal microbiota, Zhao found that chicken intestinal microbes differ according to the host genotype (Zhao et al. 2013). Another study showed that the diversity of the intestinal microbiota is more similar between closely related mice than between distantly related mice (Hufeldt et al. 2010).
Early studies of the gut microbiota were mainly performed with traditional culture techniques or traditional biological methods (Zhu et al. 2002; van der Wielen et al. 2002). The workload was large and the accuracy was low, which limited the study of the complex intestinal microbiota. In recent years, with the development of biological technologies based on 16S rRNA, metagenomic research methods have made it possible to understand the structure of the intestinal microbiota. New generation high-throughput sequencing technology has become the platform predominantly used to study the intestinal microbiota (Qin et al. 2011) and can be used for the parallel sequencing of multiple samples, greatly improving the sequencing efficiency and ensuring the accuracy of the results.
In this study, Roman hens and AA broiler chickens were used as the research objects. With 16S rRNA and metagenomic techniques, we comprehensively analyzed the structure and diversity of the cecal microbiota of BC and LH, and determined the similarities and differences in the functions of the microbiota. Our purpose was to understand the effects of different host types on the microbiotal composition in the chicken cecum and its functions, to clarify the diversity and functional differences in the chicken intestinal microbiota, and to better understand the structural composition and functional roles of the chicken intestinal microbiota as a comprehensive and scientific reference for future management strategies and microbial research.
Materials and methods
Animals and sample collection
The purpose of this study was to investigate the differences in the composition and functions of the cecal microbiota in AA broiler chickens (BC) and Roman laying hens (LH). The broilers are characterized by white feathers and excellent meat quality, and Roman laying hens by a high egg production rate, high feed conversion rate, and good egg quality. All the chicken were hatched on the same day and reared in a poultry facility under standardized conditions of a 20:4 h light:dark cycle at 19–28 °C, with free access to water and a corn-soybean-based diet. The birds included in our study were not administered any antibiotics or other veterinary drugs. We randomly selected 10 similarly sized 120-day-old Roman hens and AA broiler chickens from a slaughterhouse (in Hefei, Anhui Province, China). They were immediately dissected with sterile scissors to aseptically remove the intestines from the abdominal cavity. The contents of the hindgut were gently squeezed out. The cecal contents were collected aseptically, rapidly injected into liquid nitrogen, and stored at − 80 °C before analysis. The study was performed in accordance with the Chinese Laboratory Animal Administration Act of 1988. Before the experiments, the research protocol was reviewed and approved by the Research Ethics Committee of Anhui Agricultural University. Permission was obtained from all the managers of the chicken farms involved before the samples were collected.
DNA extraction and sequencing
Microbial DNA was extracted with the QIAamp Fast DNA Stool Mini Kit, according to the manufacturer’s protocol. The final DNA concentration and purity were determined with a NanoDrop 2000 UV–Vis spectrophotometer (Thermo Scientific, Wilmington, USA). The detection well of A Nanodrop 2000 spectrophotometer (Thermo, USA) was cleaned with buffer AE until the blank control was 0.0 ng/uL. The sample number was entered and check the sample concentration checked. An optical density ratio of OD260/280 = 1.8–2.0 indicated optimal DNA purity. A higher value may indicate RNA contamination, a lower value indicates protein contamination. A ratio of OD260/230 > 2 also indicates DNA purity, whereas a lower value can indicate humic acid pollution. After the test was completed, the Nanodrop detection hole was washed three times with ddH2O, These data are the output. The DNA quality was confirmed with 1.0% agarose gel electrophoresis. The V3–V4 hypervariable regions of the bacterial 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) on a PCR thermocycler system (GeneAmp 9700, ABI, USA), and the PCR products were then separated electrophoretically on and extracted from a 2.0% agarose gel, further purified with a AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and quantified with a QuantiFluor™-ST fluorometer (Promega, USA), according to the manufacturers’ protocols.
Equimolar amounts of the purified amplicons were pooled and paired-end sequenced (2 × 300) on the Illumina MiSeq platform (Illumina, San Diego, CA, USA), with standard protocols (Majorbio Bio-Pharm Technology Co., Ltd, Shanghai, China). The raw FASTQ files were demultiplexed and quality-filtered with Trimmomatic, and then merged using FLASH, with the following criteria: (1) reads that were truncated at any site, with an average quality score of < 20 over a 50-bp sliding window; (2) primers were exactly matched, two nucleotide mismatches were allowed, and reads containing ambiguous bases were removed; and (3) sequences overlaps > 10 bp were merged according to the overlap sequence. Sequences with ≥ 97% similarity were clustered into operational taxonomic units (OTUs) with UPARSE (version 7.1; http://drive5.com/uparse/), and chimeric sequences were identified and removed with UCHIME. The taxonomy of each 16S rRNA gene sequence was analyzed with the RDP Classifier algorithm (http://rdp.cme.msu.edu/) against the Silva (SSU123) 16S rRNA database, using a confidence threshold of 70%.
We used DNA that was fragmented to 300 bp with a Covaris M220 focused ultrasonicator (Gene Company Limited, China) to construct the paired-end library, which was prepared with the TruSeq™ DNA Sample Prep Kit (Illumina). Adapters containing the full complement of sequencing primer hybridization sites were ligated to the blunt-ended fragments, and paired-end sequencing was performed on the Illumina HiSeq 4000 platform (Illumina Inc.) at Majorbio Bio-Pharm Technology Co., Ltd (Shanghai, China) with the HiSeq 3000/4000 PE Cluster Kit and HiSeq 3000/4000 SBS Kits, according to the manufacturer’s instructions (www.illumina.com). The 3′ and 5′ ends were then stripped with SeqPrep (https://github.com/jstjohn/SeqPrep), and the low-quality reads (length < 50 bp, quality value < 20, or N bases) were removed with Sickle (https://github.com/najoshi/sickle). The reads were aligned to the chicken genome (https://www.ncbi.nlm.nih.gov/genome/?term=chicken) with BWA (http://bio-bwa.sourceforge.net), and any hit that associated with a read with a corresponding reads was removed. We used a de-Bruijn-graph-based assembler (SOAPdenovo, http://soap.genomics.org.cn, version 1.06) for the short reads (Li et al. 2008), and the K-mers, varying from 1/3 to 2/3 of the read length, were tested for each sample. We evaluated the quality and quantity of the scaffolds generated by each assembly and selected the best K-mer, i.e. the K-mer that yielded the minimum scaffold number and maximum values for N50 and N90. The scaffolds > 500 bp in length were then extracted and broken into contigs, without gaps, for gene prediction and annotation.
Sequencing data analysis
The 16S rRNA data and their richness were investigated with the Quantitative Insights Into Microbial Ecology 1.9.0 software (QIIME; http://qiime.org). The Illumina adapters and primers were removed from the raw sequences. The trimmed forward and reverse sequences were combined (Eren et al. 2013). These sequences were clustered into OTUs (97% similarity) with UCLUST (Edgar 2010). The reference OTU sequences were taxonomically assigned with the UCLUST Consensus Taxon Assigner (DeSantis et al. 2006) against the Greengenes database (McDonald et al. 2012), with a 0.5 confidence threshold, and identified to the species level. Rarefied OTUs were used to measure the bacterial richness from the total lengths of the phylogenetic branches (Faith and Baker 2006) and the relative proportions of rare sequences (Chao1) (Neufeld and Mohn 2005). The unweighted UniFrac distances (Chang et al. 2011) were used to compare the bacterial communities depending on the chicken breed. Based on the sample information, a redundancy analysis (RDA) with clustered OTUs was used to compare the chicken breed with the bacterial community structures using the R statistical software version 3.3.0 (Stanley et al. 2013). To assess whether the two chicken-breed-specific microbiomes were significantly distinguished, we used a nonparametric statistical test, analysis of similarity (ANOSIM). The significance of differences between groups was determined with permutations (n = 999) using the vegan package in the R statistical software. Using the Mothur software, we analyzed the co-occurrence of the genera by calculating the C-scores, and Spearman’s rank correlations of the 50 most abundant genera were calculated. A network analysis of those genera with rho > 0.6 and P < 0.01 was visualized with Cytoscape (version 3.4.0).
The open reading frames (ORFs) in each metagenomic sample were predicted with MetaGene (http://metagene.cb.k.u-tokyo.ac.jp/) (Noguchi et al. 2006), and the predicted ORFs with lengths > 100 bp were extracted and translated to amino acid sequences using the National Center for Biotechnology Information (NCBI) translation table (http://www.ncbi.nlm.nih.gov/Taxonomy/taxonomyhome.html/index.cgi?chapter=tgencodes#SG). The sequences from gene sets with 95% sequence identity (90% coverage) (Fu et al. 2012) were clustered as a nonredundant gene catalogue with CD-HIT (http://www.bioinformatics.org/cd-hit/). After quality control, the reads were mapped to the representative genes with 95% identity using SOAPaligner (http://soap.genomics.org.cn/), and the gene abundance in each sample was evaluated.
We used BLASTP (version 2.2.28 + ; http://blast.ncbi.nlm.nih.gov/Blast.cgi) (Altschul et al. 1997) for taxonomic annotation, by aligning the nonredundant gene catalogues against the NCBI nonredundant (NR) database, with an e-value cutoff of 1e-5. An analysis of the ORF annotations [Clusters of Orthologous Groups (COG)] was performed with BLASTP against the eggNOG database (v4.5) (Jensen et al. 2008), with an e-value cutoff of 1e-5. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation was performed with a BLASTP search (version 2.2.28 +) against the KEGG database (http://www.genome.jp/kegg/) (Xie et al. 2011), with an e-value cutoff of 1e-5. Carbohydrate-active enzyme annotations were made with hmmscan (http://hmmer.janelia.org/search/hmmscan) against the CAZy database v5.0 (http://www.cazy.org/), with an e-value cutoff of 1e-5.
Statistical analyses
The operational taxonomic units (OTUs) were clustered with UPARSE (version 7.1 http://drive5.com/uparse) with a 97% similarity cutoff and UCHIME was used to identify and remove chimeric sequences. The taxonomy of each 16S rRNA gene sequence was analyzed against the SILVA 119 16S rRNA database with the RDP classifier (http://rdp.cme.msu.edu) using a 70% confidence threshold. Alpha diversity estimates of OTUs with 97% similarity, including diversity (Shannon), richness (Chao), and Good’s coverage indices, were calculated and a rarefaction curve analysis with Mothur (version 1.30.2; www.mothur.org) was performed. PCoA was performed based on the Bray–Curtis distance matrix calculated with the OTU information from each sample. To show the difference between the samples, an averaging method was used with the ape package in R. Differences in the microorganismal compositions at the phylum and genus levels in hens of the two breeds were tested with analysis of variance (ANOVA), and we used the statistical analysis of metagenomic profiles (STAMP) probability model to identify biologically relevant differences between metagenomic communities.
Data deposition
The raw 16S rRNA gene sequences are accessible through Sequence Read Archive (SRA) study accession number SRP139155, and the raw shotgun metagenomic data are accessible through SRA study accession number SRP158884.
Results
16S rRNA profiling of BC and LH
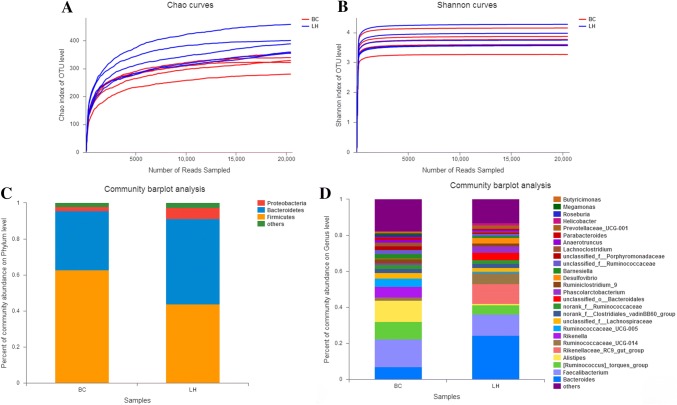
Cecal samples from chickens in each group were used to evaluate the microbiotal compositions of LH and BC with a 16S rRNA sequence analysis. After quality control, we obtained a total of 400,638 reads, with an average of 40,063 reads/sample. The average sequence length was 435 bp (Table S1). The Shannon curve and the Chao index were used to evaluate the sequencing date for all samples, As the number of bands obtained by sequencing increased, the number of OUTs identified also increased (Fig. 1a). The Shannon and Chao biodiversity curves tended to plateau, suggesting that the obtained sequencing data obtained were sufficient to cover the large majority of microorganisms in the samples (Fig. 1a, b, Table S2). In addition, the average rarefaction curves showed a significant difference between the cecal microbiota of the LH and BC chickens (Fig. 1).
Fig. 1.
16S rRNA profiling of LH and BC samples. Panel a shows the average rarefaction curve representing the variation in the Chao diversity index at increasing sequencing depths for the LH and BC samples. Panel b shows the average rarefaction curve representing the variation in the Shannon diversity index at increasing sequencing depths for the LH and BC samples. Panel c shows the average taxonomic compositions at the phylum level for the LH and BC samples with a bar plot. The legend d shows the average relative abundance of each genus in all samples
16S rRNA profiling analysis of gut microbiotal compositions of BC and LH
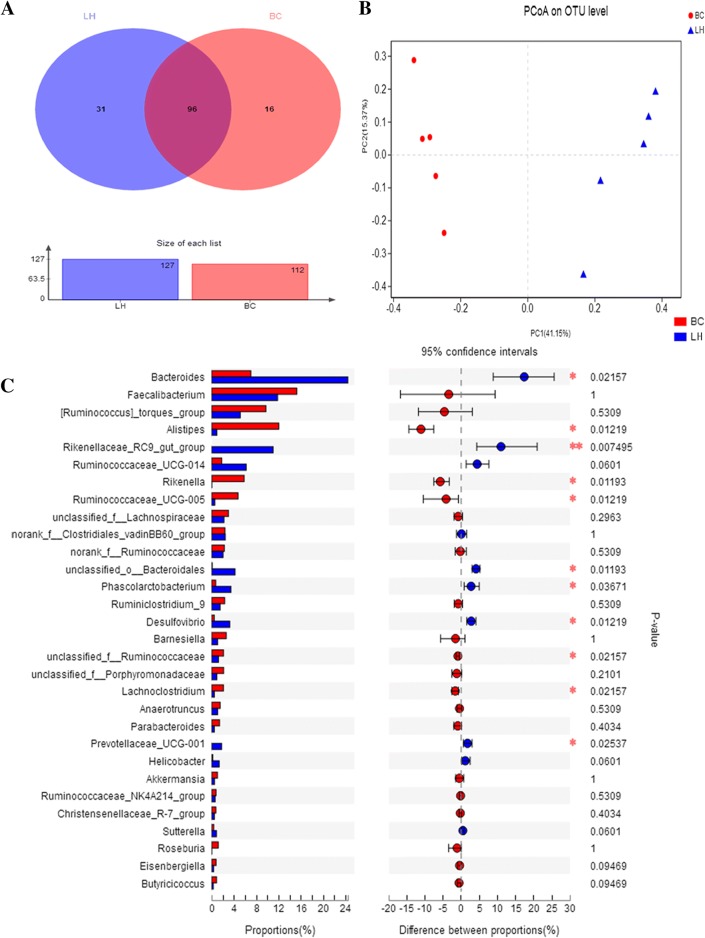
At the phylum level, greater bacterial diversity was observed in the LH ceca than in the BC ceca (Fig. 1c). The three most abundant phyla in BC were Firmicutes (62.7%), Bacteroidetes (33.0%), and Proteobacteria (2.1%). In LH, Bacteroidetes was overwhelmingly predominant, with an average relative abundance of 43.8%, followed by Firmicutes (47.5%) and Proteobacteria (6.1%). Sequences that could not be classified into any known group and were detected at low levels were grouped as “others.” These results show that each sample had its own characteristic distribution at the phylum level. We also compared the chicken intestinal microbiotal compositions of the BC and LH groups at the genus level. The most abundant genus in BC was Faecalibacterium (15.2% relative abundance), followed by Alistipes (12.0% relative abundance), Ruminococcus torques (9.7% relative abundance), and Bacteroides (7.0% relative abundance). In contrast, Bacteroides (24.4% relative abundance), Faecalibacterium (11.8% relative abundance), Rikenellaceae_RC9_gut_group (11.0% relative abundance), and Ruminococcus torques (5.1% relative abundance) predominated in the LH cecal microbiome. Interestingly, the Rikenellaceae_RC9_gut_group was not found in the BC group, and only a few members of Alistipes (0.9%) were detected in the LH group (Fig. 1d, Table S3). We identified 143 taxa, 96 of which appeared to be present in all samples, whereas 31 and 16 were uniquely present in the cecal samples from LH and BC, respectively (Fig. 2a).
Fig. 2.
Microbial diversity in BC and LH groups. Panel a shows a Venn diagram illustrating the total, unique, and shared numbers of genera predicted from the BC and LH datasets. Panel b shows the phylogenetic differences in the cecal microbiotas of BC and LH. Two-dimensional OUT-abundance-based principal coordinates analysis (PCoA). Panel c shows an extended error bar plot that demonstrates differences between the 30 most abundant genera in BC and LH. A positive difference in the mean relative abundance indicates that the genus was overrepresented in BC, whereas a negative abundance indicates that the genus was more abundant in LH. The Wilcoxon rank-sum test within STAMP was used to identify the genera that differed significantly in abundance between the groups (confidence interval method). The abscissa indicates the percentage abundance of a species in the sample, and the ordinate indicates the species name at different classificatory levels. Different colors indicate different groups. The rightmost is the P value: *P < 0.05; **P < 0.01
Analysis of the relationships between bacterial communities of BC and LH
To measure the relationships between the microbiotal communities of the two groups, we analyzed their β-diversity based on the ordination of the distance matrix generated with the Bray–Curtis complementary algorithm. A clear demarcation of the bacterial assemblages from BC and LH was apparent along principal coordinates axis 1 (PCoA1) in the PCoA plot (Fig. 2b). This was confirmed with ANOSIM (P = 0.008, R = 0.396) when the LH- and BC-derived datasets were compared (Table S3). The results of a comparative analysis of the compositions and average relative abundances of the 30 most abundant genera (relative abundance > 1%) in the LH and BC cecal microflora showed that LH had most-abundant Bacteroides (P < 0.05), Rikenellaceae_RC9_gut_group (P < 0.01), Phascolarctobacterium (P < 0.05), Desulfovibrio (P < 0.05), Prevotellaceae_UCG-001 (P < 0.05), and unclassified_o_Bacteroidales (P < 0.05), and the BC group had most-abundant Alistipes (P < 0.05), Rikenella (P < 0.05), Lachnoclostridium (P < 0.05), and unclassified_f_Ruminococcaceae (P < 0.05) (Fig. 2c).
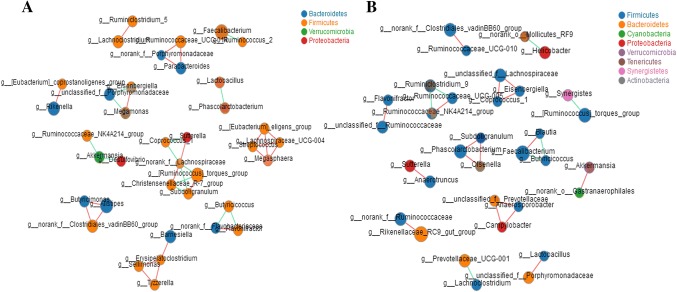
The analysis of β-diversity demonstrated a difference in the structures of the cecum intestinal microbiota of BC and LH. At the genus level, the results of a correlation network study showed that the cecum microfloral networks of BC and LH differed. The relationships within the cecal microbe community of LH were closer than those of BC, especially between Firmicutes and Bacteroidetes (Fig. 3a), whereas there was a relatively simple relationship in BC, mainly driven by Firmicutes (Fig. 3b).
Fig. 3.
Network analysis of the cecal microbiota in BC and LH at the genus level. Size of the node indicates the abundance and the color of the connecting edge indicates the interaction type: co-presence, white; mutual exclusion, yellow. Its thickness indicates the weight of the interaction. Panels a and b show the BC and LH species correlation networks (relative abundances of the 50 most-abundant species), respectively
Shotgun metagenomic analysis of cecal microbiotas of BC and LH
To evaluate the overall genetic content of the cecal microbiotas of the chickens, we selected the metagenomes of two samples representing BC or LH. The selection of these samples was based on the 16S rRNA microbiological profiling data. Sequencing the metagenomes of these two samples produced a total of 101,472,628 raw reads, which were filtered to exclude host DNA and by quality, which resulted in 100,538,293 filtered reads that were used for further analysis. The taxonomic distribution predicted from the 16S rRNA profiling analysis (Fig. 1c) was compared with that derived from the metagenomics data (Fig. S1), and showed that the structures of the BC and LH cecal microflora were identical.
Functional characterization of the chicken cecal microbiomes
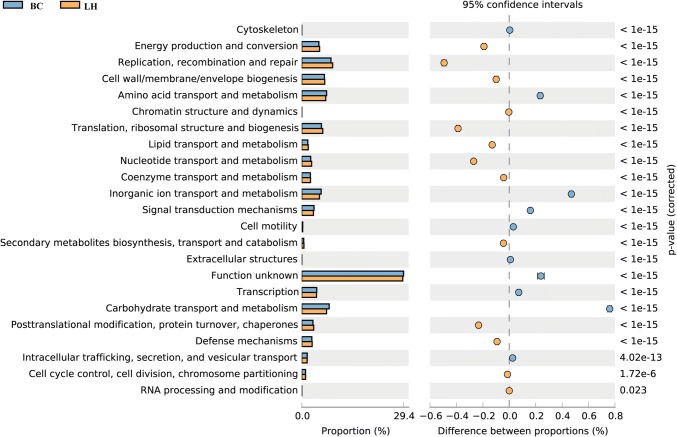
The functional classification of the ORFs based on the COG data obtained from the assembled metagenomic datasets enabled the detection of significant differences in the relative abundance of the COG functional categories between the two datasets. The richness of annotation in the LH dataset was significantly greater than that in the BC. The categories replication, recombination and repair, energy production and conversion, cell wall/membrane/envelope biogenesis, and amino acid transport and metabolism were the most strongly represented in both datasets (P < 0.05; Fig. 4).
Fig. 4.
COG functional classification and differences in COG abundance
Based on the corresponding relationships between the species and functions in the samples, a correlation analysis of the species and functional contribution relative abundances was performed. At the functional category level, in the BC group, we noted that the main functional contributions made by Firmicutes (> 49%), Bacteroidetes (> 29%), and Proteobacteria (> 2%) were metabolism, cellular processes and signals, and information storage and processing functions. In the LH group, these functions were also enriched in the phyla Firmicutes (> 36%), Bacteroidetes (> 41%), and Proteobacteria (> 6%). Interestingly, in the BC group, the functional contribution of Verrucomicrobia species was greater than 6% but was barely detectable in the LH group. In the LH group, we noted that the functional contribution of Actinobacteria species was > 1.5%, whereas the functional contribution of this species in the BC group was < 1% (Fig. 5a). In the analysis of species and their COG contributions, we annotated the functions of the Akkermansia species in BC as Function01: COG0534 (MATE efflux family protein, defense mechanisms, cellular processes and signaling), Function02: ENOG410XNMH (histidine kinase, signal transduction mechanisms, cellular processes and signaling), Function03: COG1132 [(ABC) transporter, defense mechanisms, cellular processes and signaling], and Function04: COG3250 (carbohydrate transport and metabolism), and their contributions were greater than in LH. Akkermansia species were not detected in LH. The main functional contributions of Alistipes and Bacteroides were Function05: ENOG410XNNV (TonB dependent receptor, inorganic ion transport and metabolism). Other species made functional contributions in the first four functions. In the LH group, Alistipes and Bacteroides contributed to the function of the main species (Fig. 5b).
Fig. 5.
Species and functional contribution analysis. a Species contribution relationship of category functions at the phylum level. b Species contribution relationship of NOG functions at the gene level
Discussion
Metagenomics can provide a phylogenetic description and functional analysis of gastrointestinal microbial species. The high-throughput sequencing technology plays a major role in animal gastrointestinal microbial research. The data obtained with this sequencing technology are more intuitive and comprehensive than those obtained with other molecular biology techniques (Shaufi et al. 2015). Intestinal microorganisms are closely associated with the feeding, energy metabolism, and health status of their animal hosts. The composition of the animal intestinal microbiota is affected by the environment and the host genes. High-throughput sequencing analyses have shown that the intestinal microbiota can contain different taxa even within a single species (Zhou et al. 2016; Mancabelli et al. 2016). In this study, Firmicutes and Bacteroidetes dominated the microbiomes of both the BC and LH chickens at the phylum level, but there were differences in abundance. This is basically consistent with the results of other research (Zhao et al. 2013; Wilkinson et al. 2017).
Analysis of the differences in the microbiotal compositions of the LH and BC groups at the genus level showed that LH had a greater abundance of Bacteroides (P < 0.05), Rikenellaceae_RC9_gut_group (P < 0.01), Rikenellaceae_UCG_014 (P < 0.01), Phascolarctobacterium (P < 0.05), Desulfovibrio, and Prevotellaceae_UCG_001 (P < 0.05), whereas BC was richer in Alistipes (P < 0.05), Rikenella (P < 0.05), Ruminococcaceae_UCG-005 (P < 0.05), unclassified_f_Ruminococcaceae (P < 0.05) and Lachnoclostridium (P < 0.05) (Fig. 2c). Most intestinal microbes do not directly affect the host, but their metabolites do. The metabolites of the intestinal microbiota include various kinds of vitamins, amino acids, short-chain fatty acids, and various gases (carbon dioxide, ammonia, methylene, ammonia, indole), and they also maintain the pH of the intestinal environment. Changes in the microbiotal structure can alter the metabolites produced, thereby affecting the host. Bacteroides produces glassic acid and acetic acid when carbon sources are abundant, and propionic acid when carbon sources are sparse (Macfarlane and Macfarlane 2003), Prevoella mainly produces succinic acid, which improves glucose homeostasis (De Vadder et al. 2016), and also enhances the inflammatory response (Carlsson et al. 2013). The main fermentation product of Bacteroides and Phascolarctobacterium is propionic acid, which inhibits fat synthesis in the liver (De Vadder et al. 2016) and reduces the host’s food intake. Alistipes is the main producer of bacterial short-chain fatty acids (Li et al. 2016). It is worth noting that the genus Desulfovibrio was significantly more abundant in the LH cecum than in the BC cecum (P < 0.05), and Desulfovibrio reportedly degrades intestinal mucin, a component of viscoelastic mucus. This will weaken or damage the intestinal barrier, which may directly expose the intestinal epithelial cells to the intestinal microbiota (Song et al. 2018).
The intestinal microecosystem performs many functions, which change as the composition of the microbiotal community changes. In the present study, the analysis of the overall functional microbial profiles of BC and LH indicated that the gut bacteria in chickens have high metabolic activities. These results are consistent with a preliminary study of the gut metagenomes of chicken (Mancabelli et al. 2016). Many bacterial functions involve the metabolic pathways for amino acids, carbohydrates, energy, and lipids. The functional profiles of the microbiomes of LH and BC showed many differences, suggesting that not only the bacterial compositions but their functions are important to closely related animals. For example, functions related to carbohydrate metabolism, lipid metabolism, amino acid metabolism, and glycan biosynthesis and metabolism were significantly more abundant in LH than in BC.
Conclusions
Collectively, Firmicutes, Bacteroidetes, and Proteobacteria were the main cecal bacterial phyla in BC and LH Faecalibacterium, Ruminococcus torques and Alistipes were more abundant in BC, and Bacteroides and Rikenellaceae_RC9_gut_group were more abundant in LH. The main functional annotations of species were replication, recombination and repair, energy production and transformation, cell wall/membrane/envelope biogenesis, and amino acid transport and metabolism-related functions. These results indicate that microbial genotypic differences in chickens of the same species can cause changes in the abundance of the gut microbiota, but do not alter the structural composition and major functional differences in the gut microbiota.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the National Science Foundation of China (grant no. 31772707) for supporting the high-throughput sequencing. The collection of the experimental samples was supported by the Integration and Demonstration of Quality and Safety Control Technology for Green Ecological Livestock and Poultry Products Industry Chain (grant no. 1604a0702033) and the Animal Food Quality and Safety Control, Anhui Province 115 Industry Innovation Team. We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Author contributions
SL conceived and designed the experiments; ZQ and SS performed the experiments, and these authors contributed equally to this work; ZQ analyzed the data; SS and JT contributed reagents/materials/analysis tools, SS and ZQ wrote the paper. All authors critically read and contributed to the manuscript and approved the final version.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
This study was performed in accordance with the Chinese Laboratory Animal Administration Act of 1988. Before the experiments, the research protocol was reviewed and approved by the Research Ethics Committee of Anhui Agricultural University. Permission was obtained from all managers of the chicken farms studied before the samples were collected.
Contributor Information
Zhao Qi, Email: 879237477@qq.com.
Shuiqin Shi, Email: shishuiqin0511@163.com.
Jian Tu, Email: tujian1980@126.com.
Shaowen Li, Phone: 15755101655, Email: shwli@ahau.edu.cn.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson AH, Yakymenko O, Olivier I, Hakansson F, Postma E, Keita AV, Soderholm JD. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013;48(10):1136–1144. doi: 10.3109/00365521.2013.828773. [DOI] [PubMed] [Google Scholar]
- Chambers JR, Gong J. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res Int. 2011;44(10):3149–3159. doi: 10.1016/j.foodres.2011.08.017. [DOI] [Google Scholar]
- Chang Q, Luan Y, Sun F. Variance adjusted weighted UniFrac: a powerful beta diversity measure for comparing communities based on phylogeny. Bmc Bioinform. 2011;12(118):1–14. doi: 10.1186/1471-2105-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V, Vives Florez MJ. Non-invited review the gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult Sci. 2018;97(3):1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wang Q, Liu S, Sun R, Zhou Y, Li Y. Age-related variations in intestinal microflora of free-range and caged hens. Front Microbiol. 2017;8(1310):1–10. doi: 10.3389/fmicb.2017.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016;24(1):151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. doi: 10.1128/aem.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Eren AM, Vineis JH, Morrison HG, Sogin ML. A filtering method to generate high quality short reads using illumina paired-end technology. Plos One. 2013;8(6):e66643. doi: 10.1371/journal.pone.0066643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith DP, Baker AM. Phylogenetic diversity (PD) and biodiversity conservation: some bioinformatics challenges. Evolutionary Bioinform. 2006;2:121–128. [PMC free article] [PubMed] [Google Scholar]
- Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mazcorro JF, Dowd SE, Poulsen J, Steiner JM, Suchodolski JS. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiologyopen. 2012;1(3):340–347. doi: 10.1002/mbo3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufeldt MR, Nielsen DS, Vogensen FK, Midtvedt T, Hansen AK. Variation in the Gut microbiota of laboratory mice is related to both genetic and environmental factors. Comp Med. 2010;60(5):336–342. [PMC free article] [PubMed] [Google Scholar]
- Jensen LJ, Julien P, Kuhn M, von Mering C, Muller J, Doerks T, Bork P. eggNOG: automated construction and annotation of orthologous groups of genes. Nucleic Acids Res. 2008;36:D250–D254. doi: 10.1093/nar/gkm796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sung CYJ, Lee N, Ni Y, Pihlajamaki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA. 2016;113(9):E1306–E1315. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- Mancabelli L, Ferrario C, Milani C, Mangifesta M, Turroni F, Duranti S, Lugli GA, Viappiani A, Ossiprandi MC, van Sinderen D, Ventura M. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ Microbiol. 2016;18(12):4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6(3):610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld JD, Mohn WW. Unexpectedly high bacterial diversity in arctic tundra relative to boreal forest soils, revealed by serial analysis of ribosomal sequence tags. Appl Environ Microbiol. 2005;71(10):5710–5718. doi: 10.1128/aem.71.10.5710-5718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H, Park J, Takagi T. MetaGene: prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006;34(19):5623–5630. doi: 10.1093/nar/gkl723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5(1):108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Li D, Yang R. Next-generation sequencing technologies and the application in microbiology—a review. Wei Sheng Wu Xue Bao Acta Microbiol Sin. 2011;51(4):445–457. [PubMed] [Google Scholar]
- Shaufi MAM, Sieo CC, Chong CW, Gan HM, Ho YW. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathogens. 2015;7(4):1–12. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Wang W, Shen B, Jia H, Hou Z, Chen P, Sun Y. Pretreatment with probiotic Bifico ameliorates colitis-associated cancer in mice: transcriptome and gut flora profiling. Cancer Sci. 2018;109(3):666–677. doi: 10.1111/cas.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ. Highly variable microbiota development in the chicken gastrointestinal tract. Plos One. 2013;8(12):e84290. doi: 10.1371/journal.pone.0084290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wielen P, Keuzenkamp DA, Lipman LJA, van Knapen F, Biesterveld S. Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb Ecol. 2002;44(3):286–293. doi: 10.1007/s00248-002-2015-y. [DOI] [PubMed] [Google Scholar]
- Wen CL, Yan W, Sun CJ, Ji CL, Zhou QQ, Zhang DX, Zheng JX, Yang N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019;13(6):1422–1436. doi: 10.1038/s41396-019-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson TJ, Cowan AA, Vallin HE, Onime LA, Oyama LB, Cameron SJ, Gonot C, Moorby JM, Waddams K, Theobald VJ, Leemans D, Bowra S, Nixey C, Huws SA. Characterization of the microbiome along the gastrointestinal tract of growing turkeys. Front Microbiol. 2017;8:1–11. doi: 10.3389/fmicb.2017.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li C-Y, Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman CJ, Chia N, Jeraldo P, Sipos M, Goldenfeld ND, White BA. The microbiome of the chicken gastrointestinal tract. Anim Health Res Rev. 2012;13(1):89–99. doi: 10.1017/S1466252312000138. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang G, Siegel P, He C, Wang H, Zhao W, Zhai Z, Tian F, Zhao J, Zhang H, Sun Z, Chen W, Zhang Y, Meng H. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci Rep. 2013;3:1163. doi: 10.1038/srep01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jiang X, Yang C, Ma B, Lei C, Xu C, Zhang A, Yang X, Xiong Q, Zhang P, Men S, Xiang R, Wang H. Cecal microbiota of tibetan chickens from five geographic regions were determined by 16S rRNA sequencing. Microbiologyopen. 2016;5(5):753–762. doi: 10.1002/mbo3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Zhong TY, Pandya Y, Joerger RD. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl Environ Microbiol. 2002;68(1):124–137. doi: 10.1128/AEM.68.1.124-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.