Abstract
Background
A macular hole is an anatomic opening in the retina that develops at the fovea. Macular holes can be seen in highly myopic eyes or following ocular trauma, but the great majority are idiopathic. Pars plana vitrectomy was introduced to treat full‐thickness macular holes, which if left untreated have a poor prognosis since spontaneous closure and visual recovery are rare.
Vitrectomy is a surgical technique involving the removal of the vitreous body that fills the eye. The surgeon inserts thin cannulas into the eyes through scleral incisions to relieve traction exerted by the vitreous or epiretinal membranes to the central retina and to induce glial tissue to bridge and close the hole.
Objectives
The primary objective of this review was to examine the effects of vitrectomy for idiopathic macular hole on visual acuity. A secondary objective was to investigate anatomic effects on hole closure and other dimensions of visual function, as well as to report on adverse effects recorded in included studies.
Search methods
We searched the Cochrane Eyes and Vision Group Trials Register (4 March 2015), the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 2), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to March 2015), EMBASE (January 1980 to March 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2015), the Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S) (January 1980 to March 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 4 March 2015.
Selection criteria
We included randomised controlled trials comparing vitrectomy (with or without internal limiting membrane peeling) to no treatment (that is observation) for macular holes.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently extracted the data. We estimated best corrected visual acuity and macular hole closure at 6 to 12 months of follow‐up.
Main results
Three studies provided data on the comparison between vitrectomy and observation in eyes with macular hole and visual acuity less than 20/50. Two studies, conducted in the USA and published in 1996 and 1997, used a similar protocol and included participants with stage II macular hole (42 eyes randomised, 36 analysed, number of participants not reported) or participants with stage III/IV hole (129 eyes of 120 participants, 115 eyes in analyses). The third study, conducted in the UK and published in 2004, included 185 eyes of 174 participants with full‐thickness macular hole (41 eyes with stage II holes and 74 eyes with stage III/IV holes in analyses). Studies were of good quality for randomisation and allocation concealment, whereas visual acuity measurement was unmasked.
At 6 to 12 months, visual acuity was improved by about 1.5 Snellen lines (‐0.16 logMAR, 95% confidence intervals ‐0.23 to ‐0.09 logMAR, 270 eyes, moderate‐quality evidence). The chances of macular hole closure at 6 to 12 months were greatly increased using vitrectomy, yielding an odds ratio of 31.4 (95% confidence intervals 14.9 to 66.3, 265 eyes, high‐quality evidence; raw sum data: 76% vitrectomy, 11% observation). Vitrectomy was beneficial both in smaller (stage II) and in larger (stage III/IV) macular holes.
The largest study reported that cataract surgery was needed in about half of cases at two years after operation and that retinal detachment occurred in about 5% of operated eyes.
Authors' conclusions
Vitrectomy is effective in improving visual acuity, resulting in a moderate visual gain, and in achieving hole closure in people with macular hole. However, these results may not apply to modern surgery due to technological improvements in vitrectomy techniques.
Keywords: Humans, Visual Acuity, Cataract Extraction, Cataract Extraction/statistics & numerical data, Randomized Controlled Trials as Topic, Retinal Detachment, Retinal Detachment/epidemiology, Retinal Perforations, Retinal Perforations/surgery, Vitrectomy, Vitrectomy/adverse effects, Vitrectomy/methods, Watchful Waiting
Plain language summary
Vitrectomy for idiopathic macular hole
Background A macular hole is an opening in the retina (the layer at the back of the eye that is sensitive to light) that develops at the fovea (the part of the eye that is responsible for sharp vision) and causes a small dark spot in the central vision, often preventing those with the condition from recognising very small objects, and particularly from reading ordinary print. Macular holes can be seen in people with highly myopic eyes (who cannot see clearly in the distance) or following ocular trauma, but in the great majority of cases the cause is unknown (idiopathic).
Pars plana vitrectomy has been used for more than a decade to treat full‐thickness macular holes, which if left untreated cause a blind spot in central vision that only rarely improve naturally. Vitrectomy is a surgical technique involving the removal of the vitreous body (the clear gel that fills the eye). The surgeon inserts thin tubes called cannulas into the eyes through scleral (white part of the eye) incisions or incision of the eye wall to relieve traction exerted by the vitreous to the central retina and close the hole. The objective of this review was to examine the effects on visual acuity of vitrectomy for idiopathic macular hole.
Study characteristics We included three studies, published between 1996 and 2004 and conducted in the USA and the UK, including 270 eyes in analyses, comparing vitrectomy and observation after 6 or 12 months. The evidence is current as of March 2015.
Key results Vitrectomy improved visual acuity in participants with macular hole by about 1.5 lines of a standard distance acuity chart. Macular hole closure was much more likely with vitrectomy compared to observation, with mean closure rates of 76% versus 11%, respectively.
Cataract surgery was common in operated eyes. In the largest study, retinal detachment occurred in the months following vitrectomy in about 5% of cases.
Quality of the evidence The evidence was of moderate quality, as the visual acuity measurement was unmasked.
Conclusion Vitrectomy is effective in improving visual acuity, resulting in a moderate visual gain, and in achieving hole closure in people with macular hole. However, as vitrectomy technology has improved since the included trials were conducted, with use of a smaller incision and outpatient care, the results of this review may not apply to modern surgery.
Summary of findings
Summary of findings for the main comparison. Vitrectomy compared to observation for idiopathic macular hole.
| Vitrectomy compared to observation for idiopathic macular hole | ||||||
| Patient or population: participants with idiopathic macular hole Settings: Intervention: vitrectomy Comparison: observation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation | Vitrectomy | |||||
| Mean visual acuity (logMAR) at 6‐12 months Follow‐up: 6‐12 months | The mean visual acuity (logMAR) at 6‐12 months in the control groups was 0.6 to 0.9 logMAR | The mean visual acuity (logMAR) at 6‐12 months in the intervention groups was ‐0.16 logMAR better (‐0.23 to ‐0.09) | ‐ | 270 eyes (3 studies) | ⊕⊕⊕⊝ moderate1 | ‐ |
| Hole closure at 6‐12 months Follow‐up: 6‐12 months | 110 per 1000 | 796 per 1000 (649 to 891) | OR 31.4 (14.9 to 66.3) | 265 (3 studies) | ⊕⊕⊕⊕ high2 | ‐ |
| Reading acuity at 6 months | The mean reading acuity at 6 months in the control groups was 0.8 to 1.0 logMAR | The mean reading acuity at 6 months in the intervention groups was ‐0.17 better (‐0.26 to ‐0.07 better) | ‐ | 154 (2 studies) | ⊕⊕⊝⊝ low1,3 | ‐ |
| Reading speed (word/minute) at 6 months | The mean reading speed (word/minute) at 6 months in the control groups was 36 words/minute | The mean reading speed (word/minute) at 6 months in the intervention groups was 5.6 lower (14.42 lower to 3.22 higher) | ‐ | 118 (1 study) | ⊕⊕⊝⊝ low1,3 | ‐ |
| Hole recurrence | Data on macular hole recurrence after initially successful surgery could not be extracted from the studies. | |||||
| Adverse outcomes |
Retinal detachment:Freeman 1997 reported no cases; Ezra 2004 described the retinal detachment rate for the vitrectomy groups only (vitrectomy alone or with serum), with values of 7/124 (5.6%), most detachments occurring in the first 6 weeks. Symptomatic visual field defects (Ezra 2004) in the inferotemporal sector were found in 4/124 eyes (3.2%), with 15 eyes (12.1%) having asymptomatic defects. Re‐operation (Ezra 2004): 18 (14.5%) of the surgical eyes, with subsequent hole closure in 78% of cases. |
|||||
| Costs | Cost data were not available in the included studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No or unclear masking of participant, physician, or outcome assessor (‐1)
2 No downgrade, as a result of no or unclear masking of outcome assessor in one study (‐1), but upgraded because of strong effect (+1) 3 Wide confidence interval (‐1)
Background
Description of the condition
A macular hole is an anatomic opening in the retina that develops at the fovea. Macular holes can be seen in highly myopic eyes or following ocular trauma, but the great majority are idiopathic (Ho 1998).
Idiopathic macular hole is a rather common retinal disease affecting elderly people. The Beaver Dam Study found full‐thickness macular holes to be prevalent in 0.3% of the population, with prevalence rates increasing from 0% in those between 43 and 54 years of age to 0.8% in people aged 75 years or more (Klein 1994).
In a case‐control study, 72% of idiopathic macular holes occurred in women and more than 50% occurred in people 65 to 74 years old. The study found that only 3% were occurred in people under the age of 55 years (de Bustros 1994).
The five‐year risk for developing a full‐thickness macular hole in the fellow eye of a person with a full‐thickness macular hole in one eye is about 10% to 15% (Ezra 1998; Freeman 1997; Kim 1996).
The pathogenesis of idiopathic full‐thickness macular holes is believed to involve anteroposterior traction, tangential traction, or both, exerted by the posterior vitreous cortex at the fovea (Azzolini 2001; Gass 1988; Gaudric 1999; Tanner 2001).
Macular hole formation typically evolves over a period of weeks or months through a series of stages first described biomicroscopically by Gass (Gass 1988; Gass 1995).
Stage IA: central yellow spot, loss of foveolar depression, no vitreofoveolar separation.
Stage IB: yellow ring with bridging interface, loss of foveolar depression, no vitreofoveolar separation.
Stage II: eccentric oval, crescent or horseshoe retinal defect inside edge of yellow ring, central round retinal defect with rim of elevated retina, with or without prefoveolar opacity.
Stage III: central round retinal defect ≥ 400 microns diameter, no Weiss’s ring, rim of elevated retina, with or without prefoveolar opacity.
Stage IV: central round retinal defect, rim of elevated retina, Weiss’s ring, with or without prefoveolar opacity.
Since its introduction, optical coherence tomography (OCT) has been recognised as an extremely useful tool for making or confirming diagnoses of macular hole, as well as for defining the stage of the lesion (Hee 1995).
A detailed staging based on OCT observations was recently proposed (Altaweel 2003).
Stage IA: foveal splitting, a pseudocyst visible on OCT prior to the clinically recognised yellow spot.
Stage IB: pseudocyst enlargement and extension to the outer retina with roof intact.
Stage IIA: full‐thickness macular hole (diameter < 400 microns) with posterior hyaloid face remaining attached to roof of pseudocyst.
Stage IIB: full‐thickness macular hole (diameter < 400 microns) with operculum.
Stage III: full‐thickness macular hole (diameter > 400 microns) with surrounding thickened retina including intraretinal cystoid spaces. The perifoveal and prefoveal hyaloid is separated from the macular retina.
Stage IV: a stage III hole with a complete posterior vitreous detachment. OCT often cannot visualise the posterior hyaloid because it is too anterior.
The prognosis of untreated full‐thickness macular holes is poor. Approximately 5% will have 20/50 visual acuity or better; 55% to 58% will have visual acuity of 20/100 or better; and approximately 40% will have visual acuity of 20/200 or worse (Casuso 2001; Chew 1999; Hikichi 1993; Lewis 1996; Morgan 1985). About 75% of stage II macular holes progress to a full‐thickness stage III or stage IV macular hole (Guyer 1992; Hikichi 1995a; Hikichi 1995b; Kim 1995; Kim 1996).
The fellow eyes of people with macular holes have an approximately 10% to 20% risk of developing a macular hole, especially if the hyaloid remains attached (Lewis 1996).
Description of the intervention
In 1991, Kelly et al introduced the use of pars plana vitrectomy to treat macular holes (Kelly 1991). Vitrectomy is a surgical technique involving the removal of the vitreous body that fills the eye using thin cannulas that are inserted into the eyes through scleral incisions. Pars plana vitrectomy is a vitrectomy with separation of the vitreous from the retinal surface, and may include removal (peeling) of any fibrous membranes adherent to the retina (epiretinal membranes), filling the eye with long‐acting gas (tamponade), and positioning of the patient strictly face‐down for the first days after the operation. The surgical objective is to relieve traction exerted by the vitreous or by epiretinal membranes to the central retina and to induce glial tissue to bridge and close the hole. The effectiveness of face‐down positioning is the subject of another Cochrane review (Solebo 2011), while that of internal limiting membrane (ILM) peeling has been demonstrated by yet another review (Spiteri Cornish 2013). To achieve reproducible, complete, and atraumatic ILM peeling, the use of a vital dye (indocyanine green or trypan blue) or triamcinolone has been advocated to facilitate its clear identification (Da Mata 2001; Li 2003; Lochhead 2004; Perrier 2003; Spiteri Cornish 2013; Teba 2003; Wolf 2003). Intraoperative adjuvants such as transforming growth factor‐2 (Glaser 1992; Smiddy 1993; Thompson 1998), serum (Banker 1999; Kusaka 1997, Ligget 1995), an absorbable partially cross‐linked gelatin (collagen) plug (Peyman 1997), thrombin‐activated fibrinogen (Olsen 1998), plasmin (Margherio 1998; Trese 2000), thrombin (Olsen 1998; Vine 1996), and a plasma‐thrombin mixture (Blumenkranz 2001) have been studied. Other variations in surgical technique include retinal tamponade by different methods at the end of macular hole surgery to help flatten the macular hole and achieve closure, such as using octafluoropropane, sulfur hexafluoride (Mulhern 2000; Thompson 1996; Thompson 1998), or air tamponade (Brooks 2000; Park 1999). Silicone oil has been recommended for people who cannot be positioned postoperatively or who need to travel in aeroplanes soon after surgery (Goldbaum 1998; Pertile 1999).
Surgical techniques and outcomes have improved over the last two decades, with many case series now reporting primary anatomical closure following conventional surgery in more than 90% of eyes with full‐thickness macular holes (Margherio 2000; Polk 1996; Smiddy 1997).
Why it is important to do this review
Although vitrectomy for macular hole is now well established, a systematic review of these treatment modalities is useful to patients, ophthalmologists, and other professionals involved in providing eye health care in order to assess the role of vitrectomy in the treatment of macular holes and to outline the differences between the different techniques, should there be any.
Objectives
The primary objective of this review was to examine the effects of vitrectomy for idiopathic macular hole on visual acuity. A secondary objective was to investigate anatomic effects on hole closure and other dimensions of visual function, as well as to report on adverse effects recorded in included studies.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) and quasi‐RCTs. Quasi‐RCTs are trials in which the methods of allocating people to interventions are not truly random, such as date of birth, day of the week, etc.
Types of participants
Participants were people affected by any stage of idiopathic macular hole.
Types of interventions
We included studies in which vitrectomy (with or without ILM peeling) was compared to no treatment (that is observation). We considered that a sham procedure was not feasible or ethical when compared with vitreous surgery.
Types of outcome measures
Primary outcomes
Best corrected visual acuity on a continuous scale after at least one year of follow‐up (plus or minus six months).
Secondary outcomes
Best corrected visual acuity recorded yearly after 12 months.
Anatomic closure of macular hole at the first intervention.
Rate of macular hole recurrences.
Contrast sensitivity, reading acuity and speed, or any other validated measures of visual function as available in the studies; these were expressed on a continuous scale.
Quality of life measures: any validated measurement scale that aims to measure the impact of visual function loss on quality of life of participants.
Economic data: we had planned to perform comparative cost analysis if data were available.
Adverse effects
Any reported adverse outcomes, particularly retinal detachment.
We based the conclusions of this review on the primary outcome 'best corrected visual acuity' in order to minimise the impact of reporting biases and multiplicity of outcome measures.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Eyes and Vision Group Trials Register (4 March 2015), the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 2), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to March 2015), EMBASE (January 1980 to March 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2015), the Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S) (January 1980 to March 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 4 March 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), CPCI‐S (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7) and the ICTRP (Appendix 8).
Searching other resources
We handsearched the reference lists of the included trials for other possible trials but found no additional studies.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Selection of studies
Two review authors independently selected the studies for inclusion. The review authors examined the titles and abstracts of all reports identified by the electronic searches and handsearching and classified them as (a) definitely include, (b) unsure, or (c) definitely exclude. The review authors obtained full‐text copies of the titles and abstracts classified as (a) definitely include and (b) unsure and reclassified them as (1) included, (2) awaiting assessment, or (3) excluded. We contacted the authors of studies classified as (2) awaiting assessment for further clarification and re‐assessed them as more information became available. In particular, we contacted the leading author of Freeman 1997 for additional data on randomisation and masking procedures. We excluded studies identified by both review authors as (3) excluded, and we documented these in the review. We included studies identified as (1) included and assessed these for risk of bias. The review authors were unmasked to the report authors, institutions, and trial results during this assessment. Disagreements between the two review authors were resolved by referral to a third review author.
Data extraction and management
Two review authors independently extracted the data for the primary and secondary outcomes onto paper data extraction forms developed by the Cochrane Eyes and Vision Group. We resolved discrepancies by discussion.
One review author entered the data into Review Manager (RevMan 2014), and another review author checked the data.
Assessment of risk of bias in included studies
Two review authors independently assessed the included trials for bias according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed the following parameters: sequence generation; allocation concealment; masking (blinding) of outcome assessors (masking of participants and study personnel was not possible for a surgical procedure); incomplete outcome data; and selective outcome reporting. We evaluated these items for each outcome measure or class of outcome measure as specified in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions. As reported in the Handbook, other sources of bias were: risk of bias related to the specific study design used; trial stopped early due to some data‐dependent process (including a formal stopping rule); an extreme baseline imbalance; or the study was claimed to have been fraudulent.
We used guidance contained in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) and also guidance available on publications listed in the GRADE website to prepare the Table 1.
If the information available in the published trial reports was inadequate to assess methodological quality, we contacted the trial authors for clarification. Specifically, we obtained unpublished information from Freeman 1997 on randomisation generation and allocation concealment.
Measures of treatment effect
In our data analysis we followed the guidelines set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We calculated a mean difference for continuous outcomes. We planned to calculate a summary risk ratio (RR) if dichotomous outcomes were summarised, but we discovered difficulties in the interpretation of RR when the control risk varied. We therefore chose to use the odds ratio (OR) as the measure of association. We also derived the risk difference in the Table 1.
We expressed the primary outcome 'best corrected visual acuity' on a continuous scale, as functional secondary outcomes (near acuity and reading speed). We recorded macular hole closure and as a dichotomous variable.
Unit of analysis issues
We had considered that the unit of analysis needed to be the individual participant. We originally planned to present studies with more than 10% of participants providing both eyes to analyses as a subgroup versus other studies. However, since we included only three studies in this review, and bearing in mind that macular hole is mostly a unilateral disease, we decided to present data with eyes as the unit of analysis. We acknowledged that studies included participants with bilateral macular hole, but considered that this is a rare condition and bias is negligible (20 of 294, 6.8% participants with macular hole in both eyes in Freeman 1997 and Ezra 2004, overall).
Dealing with missing data
Where data were missing due to participant dropout, we conducted a primary analysis based on participants with complete data (available case analysis). We considered that missing outcome data was not a problem if loss to follow‐up was balanced in the study arms and causes of loss to follow‐up were documented and judged to be unrelated to outcome in both study arms (Higgins 2011b). When causes of missing data were not available and sufficient studies were found, we had originally planned to use Stata 2011 user‐written function 'metamiss' to take into account missing data and conduct sensitivity meta‐analyses, as per White 2008. However, metamiss can only be used for dichotomous data, such as hole closure. As we explain later, we found a limited amount of missing data: 9 of 174 participants (5%) were lost in Ezra 2004 at 24 months; 11 of 129 participants (8.5%) were lost in Freeman 1997 at 6 months; and 6 of 36 eyes (16.7%) were lost in Kim 1996 at 12 months. We assumed that this rather low overall rate of missing data would not cause bias given the large effect observed with vitrectomy.
Assessment of heterogeneity
We looked for clinical heterogeneity by examination of the study details, then tested for statistical heterogeneity between trial results using the Chi2 test and the I2 value (Deeks 2011). We classified heterogeneity using the following I2 values.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
We also reported I2 values with 95% confidence intervals (CI) as computed using Stata 12 'heterogi' command.
We had originally planned that if we found only one trial providing data for one comparison, we would conduct a sensitivity analysis on the robustness of trial results based on a recent publication by Borm 2009. These authors presented the epidemiological and statistical framework of the estimate of effect by a single trial. They suggested that, assuming a heterogeneity I2 value of 0.25, 0.50, or 0.75 in a hypothetical meta‐analysis including the trial, the robustness of the results of a single trial can be assessed by applying an inflation factor of 1.15, 1.41, or 2.00, respectively, to the 95% CI (on a log scale if the measure of effect is RR or OR). However, in the review phase we decided to comment on the results with no formal statistical simulation since three studies informed the analyses on the most important outcomes (mean visual acuity and hole closure), which informed our conclusions.
Assessment of reporting biases
We planned to assess asymmetry of the funnel plot to identify publication bias if we found at least 10 studies, but we only included three studies in this review.
We also investigated selective outcome reporting by undertaking an 'outcome matrix' and classifying missing outcomes according to the classification presented in Table 2 (adapted from a list provided by Paula Williamson at a Cochrane training workshop on selective outcome reporting bias; Edinburgh, March 2009). We limited this presentation to the primary outcome.
1. Selective outcome reporting matrix: primary outcome 'mean visual acuity'.
| Follow‐up | ||||
| Study | 3 months | 6 months | 12 months | 24 months |
| Kim 1996 | G | reported | reported | F |
| Freeman 1997 | G | reported | F (6 months used) |
F |
| Ezra 2004 | reported | reported | reported | reported |
Missing outcomes classification adapted from a list provided by Paula Williamson at a Cochrane training workshop on selective outcome reporting bias; Edinburgh, March 2009: A: States outcome analysed but only reported that P value > 0.05, i.e. not significant. B: States outcome analysed but only reported that P value < 0.05. C: Clear that outcome was analysed but insufficient data presented to be included in meta‐analysis or full tabulation. D: Clear that outcome was analysed but no results reported. E: Clear that outcome was measured (e.g. includes structurally related outcomes) but not necessarily analysed. F: States that outcome was not measured. G: Not mentioned, but clinical judgement says likely to have been measured. H: Not mentioned, but clinical judgement says unlikely to have been measured. I: Other (to be specified)
Data synthesis
In the protocol phase, we had planned to use the following criteria to synthesise the data. If there was no substantial statistical heterogeneity and no clinical heterogeneity between the trials, we would combine the results in a meta‐analysis using a random‐effects model. We would use a fixed‐effect model if the number of trials was three or less. In case of substantial statistical (that is I2 greater than 50%) or clinical heterogeneity, we planned not to combine study results but to present a narrative or tabulated summary, provided that the estimate of heterogeneity (that is I2 CI) was measured with acceptable precision, owing to a sufficient amount of studies being found. In case substantial statistical heterogeneity were detected (that is a high I2 value), we would pool the results of the studies only if examination of the forest plot indicated that the individual trial results were all consistent in the direction of the effect (that is the RR and CI largely fell on one side of the null line). However, we found only three studies and considered that we could not estimate heterogeneity precisely in this case, so we based our assessment on the graphical exploration of the forest plot.
Studies reported visual acuities in logMAR. We had planned to convert Early Treatment Diabetic Retinopathy Study (ETDRS) letter scores to logMAR for calculations where these were reported. In any studies in which Snellen (decimal) visual acuity was measured by non‐ETDRS or non‐logarithmic charts, we had planned to extract data only if calculations were based on logMAR transformed data and the back‐transformed to decimal for reporting. We did not intend to use studies in which means and standard deviations were computed using decimal visual acuity in meta‐analyses, but to summarise their results in the discussion.
As discussed above, we originally planned to use RRs in meta‐analyses of dichotomous outcomes such as hole closure, but then found RRs were not suitable. They are in fact difficult to interpret when the control risk is rather variable and the effect is very large, such as in this case (for example a RR greater than 5 has very different meaning if the control risk ranges from 4% to 12% across studies, such as in this review). To further support our choice, we found the use of OR substantially reduced heterogeneity from 77% using RR (P equals 0.01) to 27% using OR (P equals 0.25) regarding the outcome hole closure, and we preferred this measure of effect.
Subgroup analysis and investigation of heterogeneity
If we found enough studies comparing vitrectomy to observation, we planned to conduct subgroup analyses using the following subgroups:
Macular hole stage
Use of adjuvants during surgery, such as vital dyes or triamcinolone
Type of vitreous tamponade, i.e. air versus gas or silicone oil
ILM peeling or no peeling
Small gauge (23 or 25 gauge) surgery or conventional 20 gauge surgery
We had planned to conduct subgroup analyses only if there were two or more studies in each subgroup. We were able to perform subgroup analyses by hole stage (II versus III/IV), due to the fact that Ezra 2004 provided within‐study subgroup data by macular hole stage.
Sensitivity analysis
We wanted to conduct sensitivity analyses to determine the impact of exclusion of studies with lower methodological quality, unpublished studies, quasi‐randomised trials, and industry‐funded studies, but this was not possible due to sparse data.
Results
Description of studies
Results of the search
The electronic database search yielded a total of 1504 references (Figure 1). The Trials Search Co‐ordinator removed 371 duplicates, and we screened the remaining 1133 reports to identify potentially relevant studies. We obtained three full‐text copies of reports of studies for further investigation (Ezra 2004; Freeman 1997, Kim 1996). These three studies met the inclusion criteria, and we included them in the review.
1.
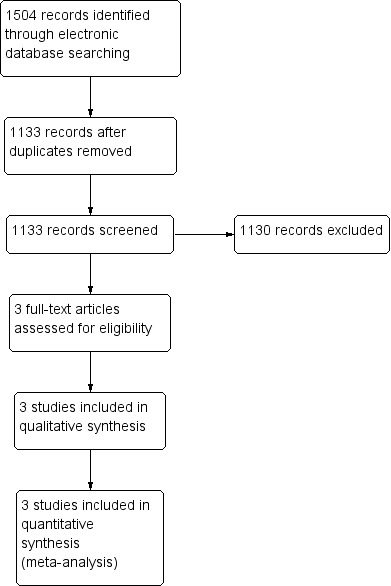
Results from searching for studies for inclusion in the review.
Included studies
We included three studies in the review: Ezra 2004, Freeman 1997, and Kim 1996. See the 'Characteristics of included studies' table for more information.
The studies provided data on the comparison between vitrectomy and observation in eyes with macular hole and visual acuity less than 20/50. Kim 1996 and Freeman 1997 were conducted in the USA, used a similar protocol, and included people with stage II macular hole (Kim 1996: 42 eyes randomised, 36 analysed, number of participants not reported) or people with stage III/IV hole (Freeman 1997: 129 eyes of 120 participants, 115 eyes in analyses). Ezra 2004 was conducted in the UK and included 185 eyes of 174 participants with full‐thickness macular hole (41 eyes with stage II holes and 74 eyes with stage III/IV holes in analyses).
Types of participants
Ezra 2004 included 185 eyes of 174 participants with full‐thickness macular hole stage II‐IV, with visual acuity less than 20/60, duration less than 9 months, and positive Watzke‐Allen test result.
Freeman 1997 included 120 participants with stage III and IV macular holes, visual acuity less than 20/50, decreasing subjective vision, hole tissue loss at least 400 micron in diameter, and positive Watzke‐Allen test result.
Kim 1996 included 42 eyes enrolled in the Vitrectomy for Macular Hole Study with stage II macular hole. Inclusion criteria were: eccentric macular hole diameter between 100 and 650 micron, centric diameter less than 300 micron and a dark yellow ring, positive Watzke‐Allen test result.
Types of interventions
Ezra 2004 randomised eyes to three arms: (a) observation, (b) vitrectomy, and (c) vitrectomy plus autologous serum. We used only the vitrectomy group as the intervention group and did not consider data for the vitrectomy plus serum arm.
Freeman 1997 randomised eyes to vitrectomy or observation.
Kim 1996 randomised eyes to vitrectomy or observation.
Types of outcomes
Ezra 2004 did not define primary and secondary outcomes. Outcomes reported in the study at 12 and 24 months were anatomic closure of the hole and visual acuity.
Freeman 1997 did not define primary and secondary outcomes. Outcomes reported in the study at six months were for measures of best‐corrected visual function, standardised photographic evaluation of the extent of hole closure, evaluation of lens opacification, and adverse events.
Kim 1996 did not define primary and secondary outcomes. Outcomes reported in the study at 6 and 12 months were measures of visual function, including our primary outcome visual acuity, standardised photographic evaluation of the extent of hole closure, evaluation of lens opacification, and adverse events.
Excluded studies
We did not exclude any studies after reviewing the full text. In addition, we could not find any ongoing studies comparing vitrectomy with observation in people with macular hole, which was as expected due to the established nature of this surgical intervention.
Risk of bias in included studies
We have summarised the 'Risk of bias' assessment in Figure 2.
2.
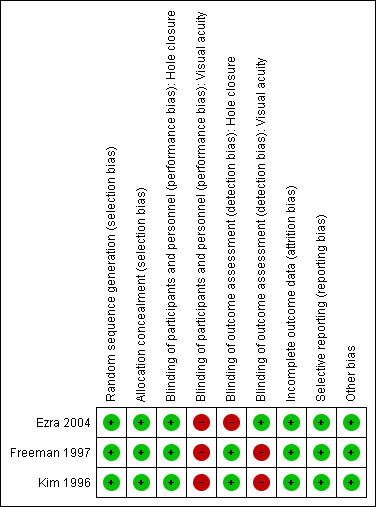
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were at low risk of bias regarding random sequence generation and allocation concealment.
Blinding
The main issue in all studies concerned lack of masking of participants to treatment assignment, which is unavoidable for a surgical intervention. Moreover, Ezra 2004 did not mask investigators assessing the recurrence of the macular hole and Freeman 1997 and Kim 1996 did not mask visual acuity examiners.
Incomplete outcome data
Missing data in the control and treatment arm were 3% and 5% in Ezra 2004, 11% and 6% in Freeman 1997, and 13% and 19% in Kim 1996, respectively. Although no study reported on causes of loss to follow‐up, Freeman 1997 presented baseline data for participants lost versus not lost to follow‐up, finding little differences. Taking all these features into account, we did not consider missing data a significant source of bias.
Selective reporting
Only Freeman 1997 and Ezra 2004 reported a prespecified primary endpoint, and it coincided with our primary outcome measure of mean visual acuity. Table 2 shows that mean visual acuity was reported consistently only at six months. In fact, we had to use 6‐month data for Freeman 1997 in the primary analysis at 12 months since the follow‐up was shorter in this study, which was consistent with our original protocol. Furthermore, visual acuity in vitrectomised and control eyes was relatively stable after six months in Kim 1996 and Ezra 2004.
Other potential sources of bias
Finally, Freeman 1997 and Ezra 2004 reported that a small number of participants (6.8%) had both eyes included in the study, and Kim 1996 did not report whether eyes and participants differed. We considered this to not be a problem since macular hole is generally a unilateral disease.
Effects of interventions
See: Table 1
All data were available in published reports. We have summarised results for important outcomes in the Table 1.
Primary outcome
At 6 months in Freeman 1997 or 12 months in Ezra 2004 and Kim 1996, visual acuity was improved by about 1.5 Snellen lines (pooled estimate ‐0.16 logMAR; 95% CI ‐0.23 to ‐0.09, 3 studies with 270 eyes, Analysis 1.1; Figure 3). There was no statistical heterogeneity in this analysis (I2 equals 0%), and the point estimates of effect were about the same.
1.1. Analysis.
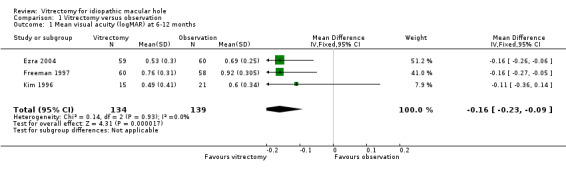
Comparison 1 Vitrectomy versus observation, Outcome 1 Mean visual acuity (logMAR) at 6‐12 months.
3.
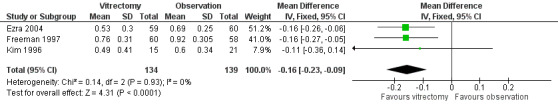
Forest plot of comparison: 1 Vitrectomy versus observation, outcome: 1.1 Mean visual acuity (logMAR) at 6 to 12 months.
Secondary outcomes
Ezra 2004 found a similar improvement of about 1.5 lines of visual acuity at 3 months, and an improvement of 2.5 lines at 24 months (Analysis 1.2; Analysis 1.3), which they related to cataract extraction, a procedure that is more common in the second year.
1.2. Analysis.
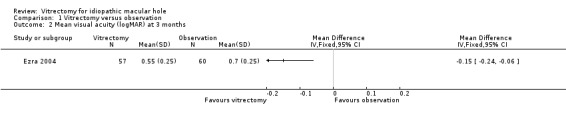
Comparison 1 Vitrectomy versus observation, Outcome 2 Mean visual acuity (logMAR) at 3 months.
1.3. Analysis.
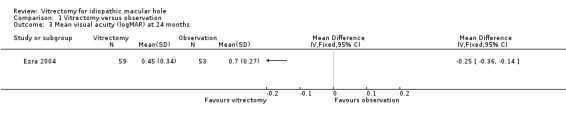
Comparison 1 Vitrectomy versus observation, Outcome 3 Mean visual acuity (logMAR) at 24 months.
Vitrectomy greatly increased the chances of macular hole closure at 6 to 12 months (OR 31.4; 95% CI 14.9 to 66.3, 265 eyes, Analysis 1.4; Figure 4). The success rate in the vitrectomy arm versus the observation arm was in fact 80% versus 29% in Kim 1996 (stage II holes), 69% versus 4% in Freeman 1997 (stage III/IV holes), and 82% versus 12% in Ezra 2004 (mixed stage), respectively.
1.4. Analysis.
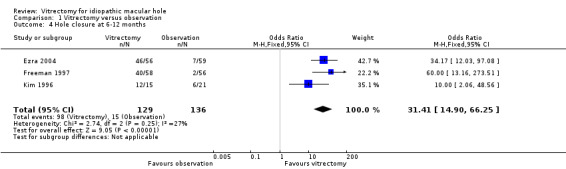
Comparison 1 Vitrectomy versus observation, Outcome 4 Hole closure at 6‐12 months.
4.
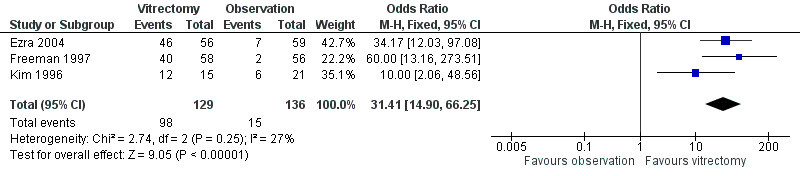
Forest plot of comparison: 1 Vitrectomy versus observation, outcome: 1.4 Hole closure at 6 to 12 months.
Kim 1996 and Freeman 1997 reported reading acuity at six months, which was better in the vitrectomy arm than in the observation arm by ‐0.17 logMAR (95% CI ‐0.07 to ‐0.26, Analysis 1.5). Freeman 1997 also reported reading speed at six months, which was better in the vitrectomy arm than in the observation arm (Analysis 1.6), although the improvement was not statistically significant.
1.5. Analysis.
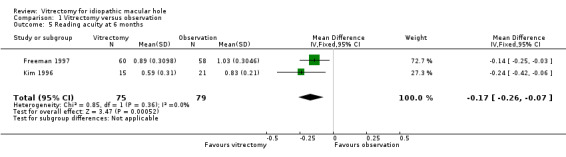
Comparison 1 Vitrectomy versus observation, Outcome 5 Reading acuity at 6 months.
1.6. Analysis.
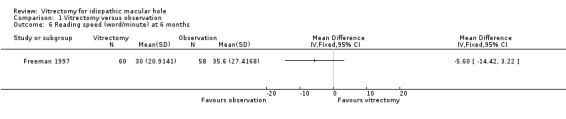
Comparison 1 Vitrectomy versus observation, Outcome 6 Reading speed (word/minute) at 6 months.
Harms
The most commonly reported event was cataract, referred to as nuclear sclerosis score with no reference to the measurement tool. Freeman 1997 reported values of 2.4 (standard deviation (SD): 0.1) and 1.3 (SD: 0.1) respectively for the vitrectomy and control groups at six months. Ezra 2004 reported a progression of nuclear sclerosis from 0.9 (SD: 0.4) at baseline to 1.9 (SD: 0.7) at six months and 2.3 (SD: 0.9) at 24 months, and cataract surgery rates of 19% at one year and 53% at two years, but did not give the control figures. Kim 1996 reported five (33%) events: one cataract and four abnormalities of retinal pigment epithelium.
Freeman 1997 did not report retinal detachment, which is a severe, expected adverse event of vitrectomy. Freeman 1997 recorded one case of endophthalmitis.
Ezra 2004 described the retinal detachment rate for the vitrectomy groups only (vitrectomy alone or with serum), with values of 7 of 124 (5.6%), most detachments occurring in the first 6 weeks. Symptomatic visual field defects in the inferotemporal sector were found in 4 of 124 eyes (3.2%), with 15 eyes (12.1%) having asymptomatic defects. A second operation was needed in 18 (14.5%) of the surgical eyes, with subsequent hole closure in 78% of cases.
Predictors of clinical outcome
Ezra 2004 reported within‐study subgroup data, finding a treatment benefit both for smaller and more recent holes (stage II) and for larger holes (stage III and IV), although smaller holes in the control group closed spontaneously in 5 out of 24 participants. Kim 1996 was conducted on stage II holes, and Freeman 1997 used a similar protocol including stage III/IV holes. We could therefore conduct Analysis 1.7, confirming that a benefit was found both for stage II and for stage III/IV holes and demonstrating no significant subgroup difference.
1.7. Analysis.
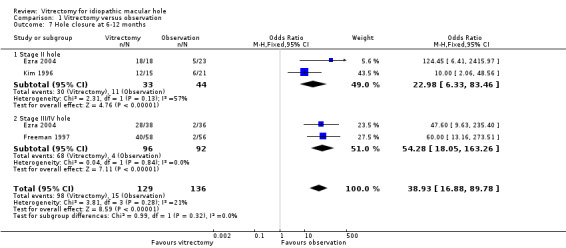
Comparison 1 Vitrectomy versus observation, Outcome 7 Hole closure at 6‐12 months.
Quality of the evidence
The quality of the evidence for the primary outcome was moderate due to lack of masking of visual acuity in two studies (‐1 for risk of bias). For secondary outcomes, hole closure assessment was unmasked in one study, but there was no quality downgrade because treatment effect was strong. Additionally, the quality of the evidence for key secondary outcomes was low due to the uncertainty of the estimates (‐1 for imprecision). See Table 1.
Discussion
Summary of main results
We found three RCTs, conducted almost 10 years apart, that showed similar benefits with vitrectomy for macular hole compared to observation. The studies included participants with visual acuity lower than 20/50 and at stages II to IV. At 6 or 12 months, visual acuity was improved by about 1.5 Snellen lines in the studies comparing vitrectomy and observation groups, and the chances of macular hole closure were increased by more than four times, with hole closure occurring on average in about 76% versus 11% in the vitrectomy and observation arms, respectively.
We found a benefit regardless of hole stage, although smaller holes were reported to have more chance of improvement than larger holes (about 90% versus 70% closed with vitrectomy). We must also consider that smaller holes may have more chance of spontaneous closure than larger holes.
Overall completeness and applicability of evidence
The included studies were conducted about 10 and 20 years ago, and vitrectomy techniques have improved since, particularly with the introduction of small‐gauge vitrectomy and ILM peeling. Other technical improvements include the use of a dye to stain ILM, the type of tamponade used, and the position of the patient after the surgery. Other Cochrane reviews have previously investigated these topics (Solebo 2011; Spiteri Cornish 2013).
The included studies used different methods to evaluate hole closure. While in Ezra 2004 the hole closure was evaluated by one observer by means of fundoscopy, photography, and fluorescein angiography, in Kim 1996 and Freeman 1997 the closure was graded by an independent photograph‐reading centre. Modern research uses optical coherence tomography to assess macular hole closure more objectively and precisely. We also suggest that predictors of hole closure and surgical complications can be studied in larger non‐comparative studies, since there is little spontaneous variation of average visual acuity in untreated macular holes.
Other sources of heterogeneity were not a problem in this review, particularly different follow‐up length, since visual function was found stable from 6 to 12 months in the largest study.
We did not find RCTs on surgical treatment of stage I holes. Pharmacological alternatives, that is intravitreal ocriplasmin (Stalmans 2012), are available to treat specific types of vitreomacular traction syndrome, whether associated with small macular holes or not.
The main severe complications of vitrectomy we recorded in our review was iatrogenic retinal detachment, which occurred in about 5% of cases in the largest and most recent study, but this risk was imprecisely estimated due to the small sample size. A more recent, large series of small gauge vitrectomies for epiretinal membrane and macular hole found a lower detachment rate of 2.4% in 656 holes and 1.2% in 1206 epiretinal membranes (1.7% overall) (Rizzo 2010).
The studies included in this review used vitrectomy techniques that did not permit the study of many of the predictors of visual recovery after macular hole surgery. In a recent analysis from a multicentre database study in the UK, a total of 1078 eyes from 1045 participants were followed for a median follow‐up of 0.6 years. The study found an improvement of 0.3 logMAR (3 Snellen lines) in about half of participants at six months and almost 60% at one year, while less than 10% deteriorated by 0.30 logMAR units, suggesting improved visual outcomes with modern vitrectomy techniques (Jackson 2013; Steel 2013). A review of non‐comparative studies reported that macular hole stage and size, symptom duration and preoperative visual acuity, and several morphological parameters with OCT could be predictors of better visual outcome (Kusuhara 2014). Although based on earlier techniques, our review did not find the OR of hole closure closure differed in stage II compared to stage III/IV macular holes.
Quality of the evidence
The quality of the evidence was moderate for primary efficacy outcome as we found that masking of outcome assessors was not attempted or was unclear in all the studies. The quality of the evidence was low for harms such as retinal detachment, which cannot be estimated precisely in RCTs of this size.
Potential biases in the review process
Some secondary outcomes were not measured and reported similarly or consistently between the studies.
Agreements and disagreements with other studies or reviews
We found two Cochrane systematic reviews that did not compare vitrectomy to observation as in the current review, but wanted to explore the benefit of different surgical and postsurgical approaches, as described below.
Solebo 2011 aimed to evaluate the evidence of the impact of postoperative face‐down positioning on the outcome of surgery for macular hole and included three RCTs (Guillaubey 2008; Lange 2012; Tadayoni 2011). Even if there was insufficient evidence from which to draw firm conclusions about the impact of postoperative face‐down positioning on the outcome of surgery for macular hole, two of the RCTs suggested a benefit in larger holes, but none demonstrated evidence of a benefit in smaller holes.
Spiteri Cornish 2013 aimed to determine whether ILM peeling improves anatomical and functional outcomes of macular hole surgery compared with the no‐peeling technique and included four RCTs (Christensen 2009; Kwok 2005; Lois 2011; Tadayoni 2009). Although the authors found no evidence of a benefit of ILM peeling in terms of the primary outcome (visual acuity at six months), ILM peeling appears to be superior as it offers more favourable cost effectiveness by increasing the likelihood of primary anatomical closure, subsequently decreasing the likelihood of further surgery, with no differences in unwanted side effects compared with no peeling.
Authors' conclusions
Implications for practice.
Vitrectomy is effective in improving visual acuity, resulting in a moderate visual gain, and in achieving hole closure in people with macular hole. However, these results may not apply to modern surgery, since vitrectomy techniques have improved since the studies were conducted, particularly with the introduction of small‐gauge vitrectomy and ILM peeling. Other technical improvements include the use of a dye to stain ILM, the type of tamponade used, and the position of the patient after the surgery (Solebo 2011; Spiteri Cornish 2013).
Implications for research.
A systematic review comparing small‐gauge to conventional vitrectomy in people with macular hole is needed.
Acknowledgements
We thank Catey Bunce and Mario Romano for comments on the protocol of this review and Marie Diener‐West, Kurt Spiteri Cornish and Jennifer Evans for comments on the full review. We thank Anupa Shah for her assistance throughout the review development. The Cochrane Eyes and Vision Group created and executed the electronic searches. We also thank Professor Freeman for providing data on his study.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Retinal Perforations #2 macula* near/3 hole* #3 (#1 OR #2) #4 MeSH descriptor Vitrectomy #5 vitrectom* #6 PPV #7 (#4 OR #5 OR #6) #8 (#3 AND #7)
Appendix 2. MEDLINE search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp retinal perforations/ 14. (macula$ adj3 hole$).tw. 15. or/13‐14 16. exp vitrectomy/ 17. vitrectom$.tw. 18. PPV.tw. 19. or/16‐18 20. 15 and 19 21. 12 and 20
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. retina tear/ 34. (macula$ adj3 hole$).tw. 35. or/33‐34 36. exp vitrectomy/ 37. vitrectom$.tw. 38. PPV.tw. 39. or/36‐38 40. 35 and 39 41. 32 and 40
Appendix 4. LILACS search strategy
macula$ and hole$ and vitrectom$ or PPV
Appendix 5. Web of Science CPCI‐S search strategy
#5 #1 AND #4 #4 #2 OR #3 #3 TS=PPV #2 TS=vitrectom* #1 TS=macula* hole
Appendix 6. metaRegister of Controlled Trials search strategy
macula hole AND vitrectomy
Appendix 7. ClinicalTrials.gov search strategy
macula hole AND vitrectomy
Appendix 8. ICTRP search strategy
macula hole AND vitrectomy
Data and analyses
Comparison 1. Vitrectomy versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean visual acuity (logMAR) at 6‐12 months | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.23, ‐0.09] |
| 2 Mean visual acuity (logMAR) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean visual acuity (logMAR) at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Hole closure at 6‐12 months | 3 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 31.41 [14.90, 66.25] |
| 5 Reading acuity at 6 months | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.26, ‐0.07] |
| 6 Reading speed (word/minute) at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Hole closure at 6‐12 months | 3 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 38.93 [16.88, 89.78] |
| 7.1 Stage II hole | 2 | 77 | Odds Ratio (M‐H, Fixed, 95% CI) | 22.98 [6.33, 83.46] |
| 7.2 Stage III/IV hole | 2 | 188 | Odds Ratio (M‐H, Fixed, 95% CI) | 54.28 [18.05, 163.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ezra 2004.
| Methods | Randomised clinical trial conducted in the UK | |
| Participants | 185 eyes of 174 participants with full‐thickness macular hole Inclusion criteria: symptom duration < 9 months, visual acuity 20/60, positive Watzke‐Allen test result, full‐thickness macular hole stage II, III, or IV | |
| Interventions | 3 arms: (a) observation, (b) vitrectomy, (c) vitrectomy plus autologous serum | |
| Outcomes | Predefined: anatomic closure of the hole and visual acuity | |
| Notes | Authors declare no relevant conflict of interest. Funding sources reported: grant GREZ0195 from the Guide Dogs for the Blind Association, London, England, and grant EZRE0311 from the Stringer Bequest to the Special Trustees of Moorfields Eye Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation schedule involved computer generated randomizations cards using the 'block' method" |
| Allocation concealment (selection bias) | Low risk | Quote: "Serially numbered, sealed opaque envelopes were used to conceal individual allocations. Assignment occurred at the end of the entry visit, when the envelope was opened. All envelopes were held and opened by the study coordinator (M.D.), who acted as executor and held the allocation sequence code. The executor, allocation schedule generators, assessors, and surgeon were separate individuals." |
| Blinding of participants and personnel (performance bias) Hole closure | Low risk | Unmasked, but hole closure is an anatomic outcome and no such bias is expected |
| Blinding of participants and personnel (performance bias) Visual acuity | High risk | Unmasked, and visual acuity is a subjective outcome that can be influenced by participants' knowledge of allocation status |
| Blinding of outcome assessment (detection bias) Hole closure | High risk | Quote: "One observer, using the funduscopic, photographic, and fluorescein angiography, assessed the hole status. As far as funduscopic assessment was concerned, it was not possible to postoperatively mask between surgery and observation, as examination alone would reveal whether a vitrectomy had been performed. However, the assessor was masked as far as vitrectomy versus vitrectomy plus serum, as it was not possible to distinguish between them on clinical grounds." Comment: judgement could be biased |
| Blinding of outcome assessment (detection bias) Visual acuity | Low risk | Quote: "The assessor of visual acuity (A.M.) was masked to the allocation status." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months: 2, 3, and 1 participants were lost in observation, vitrectomy, and vitrectomy plus serum groups respectively. At 24 months: 3, 3, and 3 participants were lost in observation, vitrectomy, and vitrectomy plus serum groups respectively. |
| Selective reporting (reporting bias) | Low risk | Predefined primary outcomes |
| Other bias | Low risk | A small number of participants (11) had both eyes included in the study. No other source of bias. |
Freeman 1997.
| Methods | A multicentre, randomised clinical trial in USA. Study began in June 1992 and was published in 1997 | |
| Participants | 129 eyes of 120 participants with stage III and IV macular holes; relevant inclusion criteria were visual acuity less than 20/50, decreasing subjective vision, hole tissue loss at least 400 micron in diameter, positive Watzke‐Allen test result | |
| Interventions | Vitrectomy versus observation | |
| Outcomes | 4 measures of best‐corrected visual function, standardised photographic evaluation of the extent of hole closure, evaluation of lens opacification, and adverse events at 6 months | |
| Notes | No conflict of interest declaration reported in the manuscript. Funding sources: potential conflict of interest related to funding by Alcon Inc, Fort Worth, Texas, as surgical device producer. Other sources: grant from Research to Prevent Blindness Inc, New York, NY; and EY‐03040 Core Grant from National Eye Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation code delivered in an envelope only after the participant had been judged to be eligible and agreed to enter the study |
| Blinding of participants and personnel (performance bias) Hole closure | Low risk | Unmasked, but hole closure is an anatomic outcome and no such bias is expected |
| Blinding of participants and personnel (performance bias) Visual acuity | High risk | Unmasked, and visual acuity is a subjective outcome that can be influenced by participants' knowledge of allocation status |
| Blinding of outcome assessment (detection bias) Hole closure | Low risk | Independent photograph‐reading centre |
| Blinding of outcome assessment (detection bias) Visual acuity | High risk | Unmasked visual acuity examiners |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6 months, 4/64 eyes versus 7/65 eyes were lost in vitrectomy group and observation group, respectively |
| Selective reporting (reporting bias) | Low risk | A protocol was not available, nor were predefined outcomes declared. However, hole closure and mean logMAR visual acuity are key expected outcomes in this research field. |
| Other bias | Low risk | No other source of bias |
Kim 1996.
| Methods | A multicentre, randomised clinical trial in USA conducted between 1991 and 1994 | |
| Participants | 42 of 213 eyes enrolled in the Vitrectomy for Macular Hole Study had stage II macular hole. Inclusion criteria: Eccentric macular hole diameter between 100 and 650 micron, centric macular hole diameter less than 300 micron and a dark yellow ring, positive Watzke‐Allen test result | |
| Interventions | Vitrectomy versus observation | |
| Outcomes | 4 measures of best‐corrected visual function, standardised photographic evaluation of the extent of hole closure, evaluation of lens opacification, and adverse events at 6 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Each center opened a sealed envelope containing the randomization card for a given patient after informed consent was signed". |
| Blinding of participants and personnel (performance bias) Hole closure | Low risk | Unmasked, but hole closure is an anatomic outcome and no such bias is expected |
| Blinding of participants and personnel (performance bias) Visual acuity | High risk | Unmasked, and visual acuity is a subjective outcome that can be influenced by participants' knowledge of allocation status |
| Blinding of outcome assessment (detection bias) Hole closure | Low risk | Masked reading of the fundus photographs. Two photograph readers read fundus photographs. Where the 2 principal photograph readers were not in complete agreement, the images were reviewed by the principal investigator and by a fundus photographer reader from an outside institution. |
| Blinding of outcome assessment (detection bias) Visual acuity | High risk | Unmasked visual acuity examiners |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months 2/15 and 4/21 were lost in vitrectomy group and observation group, respectively |
| Selective reporting (reporting bias) | Low risk | A protocol was not available, nor were predefined outcomes. However, hole closure and mean logMAR visual acuity are key expected outcomes in this research field. |
| Other bias | Low risk | No other source of bias |
Differences between protocol and review
Our unit of analysis changed from participants to eyes.
We used odds ratio rather than risk ratio to meta‐analyse data on macular hole closure, for reasons explained in the text.
Contributions of authors
Conceiving the review: FG, GV Designing the review: FG, GV Co‐ordinating the review: GV
Data collection for the review
Designing search strategies: Cochrane Eyes and Vision Group Editorial Base
Undertaking manual searches: CE, YCY
Screening search results: MP, FG, CME, YCY, GV
Organising retrieval of papers: FG, MP, GV
Screening retrieved papers against inclusion criteria: MP, CME, YCY, GV
Appraising quality of papers: MP, FG, CME, YCY, GV, SR
Extracting data from papers: MP, FG, GV
Writing to authors of papers for additional information: MP, FG, GV
Providing additional data about papers: YCY
Obtaining and screening data on unpublished studies: FG, GV
Data management for the review
Entering data into RevMan/checking data in RevMan: MP, FG, GV
Analysis of data: MP, YCY, GV
Interpretation of data
Providing a methodological perspective: GV
Providing a clinical perspective: MP, FG, CME, YCY, SR
Providing a policy perspective: FG, CME, YCY, SR
Providing a consumer perspective: not applicable
Writing the review: MP, FG, GV, SR Providing general advice on the review: MP, FG, CME, YCY, GV, SR
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
None
New
References
References to studies included in this review
Ezra 2004 {published data only}
- Ezra E, Gregor ZJ, Moorfields Macular Hole Study Group Report No. 1. Surgery for idiopathic full‐thickness macular hole: two‐year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Moorfields Macular Hole Study Group Report No. 1. Archives of Ophthalmology 2004;122(2):224‐36. [DOI] [PubMed] [Google Scholar]
Freeman 1997 {published data only}
- Freeman WR, Azen SP, Kim JW, el‐Haig W, Mishell DR 3rd, Bailey I, et al. Vitrectomy for the treatment of full‐thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. Vitrectomy for Treatment of Macular Hole Study Group. Archives of Ophthalmology 1997;115(7):11‐21. [DOI] [PubMed] [Google Scholar]
Kim 1996 {published data only}
- Kim JW, Freeman WR, Azen SP, el‐Haig W, Klein DJ, Bailey IL. Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Vitrectomy for Macular Hole Study Group. American Journal of Ophthalmology 1996;121(6):605‐14. [DOI] [PubMed] [Google Scholar]
Additional references
Altaweel 2003
- Altaweel M, Ip M. Macular hole: improved understanding of pathogenesis, staging, and management based on optical coherence tomography. Seminars in Ophthalmology 2003;18(2):58‐66. [DOI] [PubMed] [Google Scholar]
Azzolini 2001
- Azzolini C, Patelli F, Brancato R. Correlation between optical coherence tomography data and biomicroscopic interpretation of idiopathic macular hole. American Journal of Ophthalmology 2001;132(3):348‐55. [DOI] [PubMed] [Google Scholar]
Banker 1999
- Banker AS, Freeman WR, Azen SP, Lai MY. A multicentered clinical study of serum as adjuvant therapy for surgical treatment of macular holes. Vitrectomy for Macular Hole Study Group. Archives of Ophthalmology 1999;117(11):1499‐502. [DOI] [PubMed] [Google Scholar]
Blumenkranz 2001
- Blumenkranz MS, Ohana E, Shaikh S, Chang S, Coll G, Morse LS, et al. Adjuvant methods in macular hole surgery: Intraoperative plasma‐thrombin mixture and postoperative fluid‐gas exchange. Ophthalmic Surgery and Lasers 2001;32(3):197‐207. [PubMed] [Google Scholar]
Borm 2009
- Borm GF, Lemmers O, Fransen J, Donders R. The evidence provided by a single trial is less reliable than its statistical analysis suggests. Journal of Clinical Epidemiology 2009;62(7):711‐5. [DOI] [PubMed] [Google Scholar]
Brooks 2000
- Brooks HL Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000;107(10):1939‐48. [DOI] [PubMed] [Google Scholar]
Casuso 2001
- Casuso LA, Scott IU, Flynn HW Jr, Gass JD, Smiddy WE, Lewis ML, et al. Long‐term follow‐up of unoperated macular holes. Ophthalmology 2001;108(6):1150‐5. [DOI] [PubMed] [Google Scholar]
Chew 1999
- Chew EY, Sperduto RD, Hiller R, Nowroozi L, Seigel D, Yanuzzi LA, et al. Clinical course of macular holes: the Eye Disease Case‐Control Study. Archives of Ophthalmology 1999;117(2):242‐6. [DOI] [PubMed] [Google Scholar]
Christensen 2009
- Christensen UC, Krøyer K, Sander B, Larsen M, Henning V, Villumsen J. Value of internal limiting membrane peeling in surgery for idiopathic macular hole stage 2 and 3: a randomised clinical trial. British Journal of Ophthalmology 2009;93(8):1005‐15. [DOI] [PubMed] [Google Scholar]
Da Mata 2001
- Mata AP, Burk SE, Riemann CD, Rosa Jr RH, Snyder ME, Petersen MR, et al. Indocyanine green‐assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology 2001;108(7):1187‐92. [DOI] [PubMed] [Google Scholar]
de Bustros 1994
- Bustros S. Vitrectomy for prevention of macular holes. Results of a randomized multicenter clinical trial. Vitrectomy for Prevention of Macular Hole Study Group. Ophthalmology 1994;101(6):1055‐9. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ezra 1998
- Ezra E, Wells JA, Gray RH, Kinsella FM, Orr GM, Grego J, et al. Incidence of idiopathic full‐thickness macular holes in fellow eyes. A 5‐year prospective natural history study. Ophthalmology 1998;105(2):353‐9. [DOI] [PubMed] [Google Scholar]
Gass 1988
- Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Archives of Ophthalmology 1988;106(5):629‐39. [DOI] [PubMed] [Google Scholar]
Gass 1995
- Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. American Journal of Ophthalmology 1995;119(6):752‐9. [DOI] [PubMed] [Google Scholar]
Gaudric 1999
- Gaudric A, Haouchine B, Massin P, Paques M, Blain P, Erginay A. Macular hole formation: new data provided by optical coherence tomography. Archives of Ophthalmology 1999;117(6):744‐51. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Glaser 1992
- Glaser BM, Michels RG, Kuppermann BD, Sjaarda RN, Pena RA. Transforming growth factor‐beta 2 for the treatment of full‐thickness macular holes. A prospective randomized study. Ophthalmology 1992;99(7):1162‐73. [DOI] [PubMed] [Google Scholar]
Goldbaum 1998
- Goldbaum MH, McCuen BW, Hanneken AM, Burgess SK, Chen HH. Silicone oil tamponade to seal macular holes without position restrictions. Ophthalmology 1998;105(11):2140‐8. [DOI] [PubMed] [Google Scholar]
Guillaubey 2008
- Guillaubey A, Malvitte L, Lafontaine PO, Jay N, Hubert I, Bron A, et al. Comparison of face‐down and seated position after idiopathic macular hole surgery: a randomized clinical trial. American Journal of Ophthalmology 2008;146(1):128‐34. [DOI] [PubMed] [Google Scholar]
Guyer 1992
- Guyer DR, Bustros S, Diener‐West M, Fine SL. Observations on patients with idiopathic macular holes and cysts. Archives of Ophthalmology 1992;110(9):1264‐8. [DOI] [PubMed] [Google Scholar]
Hee 1995
- Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Schuman JS, et al. Optical coherence tomography of macular holes. Ophthalmology 1995;102(5):748‐56. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hikichi 1993
- Hikichi T, Trempe CL. Risk of decreased visual acuity in full‐thickness idiopathic macular holes. American Journal of Ophthalmology 1993;116(6):708‐12. [DOI] [PubMed] [Google Scholar]
Hikichi 1995a
- Hikichi T, Yoshida A, Akiba J, Konno S, Trempe CL. Prognosis of stage 2 macular holes. American Journal of Ophthalmology 1995;119(5):571‐5. [DOI] [PubMed] [Google Scholar]
Hikichi 1995b
- Hikichi T, Yoshida A, Akiba J, Trempe CL. Natural outcomes of stage 1, 2, 3, and 4 idiopathic macular holes. British Journal of Ophthalmology 1995;79(6):517‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 1998
- Ho AC, Guyer DR, Fine SL. Macular hole. Survey of Ophthalmology 1998;42(5):393‐416. [DOI] [PubMed] [Google Scholar]
Jackson 2013
- Jackson TL, Donachie PH, Sparrow JM, Johnston RL. United Kingdom National Ophthalmology Database study of vitreoretinal surgery: report 2, macular hole. Ophthalmology 2013;120(3):629‐34. [DOI] [PubMed] [Google Scholar]
Kelly 1991
- Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Archives of Ophthalmology 1991;109(5):654‐9. [DOI] [PubMed] [Google Scholar]
Kim 1995
- Kim JW, Freeman WR, el‐Haig W, Maguire AM, Arevalo JF, Azen SP, et al. Baseline characteristics, natural history, and risk factors to progression in eyes with stage 2 macular holes. Results from a prospective randomized clinical trial. Vitrectomy for Macular Hole Study Group. Ophthalmology 1995;102(12):1818‐28. [DOI] [PubMed] [Google Scholar]
Klein 1994
- Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Transactions of the American Ophthalmological Society 1994;92:403‐25. [PMC free article] [PubMed] [Google Scholar]
Kusaka 1997
- Kusaka S, Sakagami K, Kutsuna M, Ohashi Y. Treatment of full‐thickness macular holes with autologous serum. Japanese Journal of Ophthalmology 1997;41(5):332‐8. [DOI] [PubMed] [Google Scholar]
Kusuhara 2014
- Kusuhara S, Negi A. Predicting visual outcome following surgery for idiopathic macular holes. Ophthalmologica 2014;231(3):125‐32. [DOI] [PubMed] [Google Scholar]
Kwok 2005
- Kwok AK, Lai TY, Wong VW. Idiopathic macular hole surgery in Chinese patients: a randomized study to compare indocyanine green assisted internal limiting membrane peeling with no internal limiting membrane peeling. Hong Kong Medical Journal 2005;11(4):259‐66. [PubMed] [Google Scholar]
Lange 2012
- Lange C, Membrey L, Ahmad N, Wickham L, Maclaren R, Solebo L, et al. Pilot randomized controlled trial of face‐down positioning following macular hole surgery. Eye 2012;26(2):272‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1996
- Lewis ML, Cohen SM, Smiddy WE, Gass JD. Bilaterality of idiopathic macular holes. Graefe's Archive for Clinical and Experimental Ophthalmology 1996;234(4):241‐5. [DOI] [PubMed] [Google Scholar]
Li 2003
- Li K, Wong D, Hiscott P, Stanga P, Groenewald C, McGalliard J. Trypan blue staining of internal limiting membrane and epiretinal membrane during vitrectomy: visual results and histopathological findings. British Journal of Ophthalmology 2003;87(2):216‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ligget 1995
- Liggett PE, Skolik DS, Horio B, Saito Y, Alfaro V, Mieler W. Human autologous serum for the treatment of full‐thickness macular holes. A preliminary study. Ophthalmology 1995;102(7):1071‐6. [DOI] [PubMed] [Google Scholar]
Lochhead 2004
- Lochhead J, Jones E, Chui D, Lake S, Karia N, Patel CK, et al. Outcome of ICG‐assisted ILM peel in macular hole surgery. Eye 2004;18(8):804‐8. [DOI] [PubMed] [Google Scholar]
Lois 2011
- Lois N, Burr J, Norrie J, Vale L, Cook J, McDonald A, et al. Internal limiting membrane peeling versus no peeling for idiopathic full thickness macular hole: a pragmatic randomised controlled trial. Investigative Ophthalmology and Visual Science 2011;52(3):1586‐92. [DOI] [PubMed] [Google Scholar]
Margherio 1998
- Margherio AR, Margherio RR, Hartzer M, Trese MT, Williams GA, Ferrone PJ. Plasmin enzyme‐assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology 1998;105(9):1617‐20. [DOI] [PubMed] [Google Scholar]
Margherio 2000
- Margherio RR, Margherio AR, Williams GA, Chow DR, Banach MJ. Effect of perifoveal tissue dissection in the management of acute idiopathic full‐thickness macular holes. Archives of Ophthalmology 2000;118(4):495‐8. [DOI] [PubMed] [Google Scholar]
Morgan 1985
- Morgan CM, Schatz H. Idiopathic macular holes. American Journal of Ophthalmology 1985;99(4):437‐44. [DOI] [PubMed] [Google Scholar]
Mulhern 2000
- Mulhern MG, Cullinane A, Cleary PE. Visual and anatomical success with short‐term macular tamponade and autologous platelet concentrate. Graefe's Archive for Clinical and Experimental Ophthalmology 2000;238(7):577‐83. [DOI] [PubMed] [Google Scholar]
Olsen 1998
- Olsen TW, Sternberg P Jr, Capone A Jr, Martin DF, Lim JI, Grossniklaus HE, et al. Macular hole surgery using thrombin‐activated fibrinogen and selective removal of the internal limiting membrane. Retina 1998;18(4):322‐9. [DOI] [PubMed] [Google Scholar]
Park 1999
- Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal‐limiting membrane peeling and intravitreous air. Ophthalmology 1999;106(7):1392‐7. [DOI] [PubMed] [Google Scholar]
Perrier 2003
- Perrier M, Sebag M. Trypan blue‐assisted peeling of the internal limiting membrane during macular hole surgery. American Journal of Ophthalmology 2003;135(6):903‐5. [DOI] [PubMed] [Google Scholar]
Pertile 1999
- Pertile G, Claes C. Silicone oil vs. gas for the treatment of full‐thickness macular hole. Bulletin de la Societe Belge d Ophtalmologie 1999;274:31‐6. [PubMed] [Google Scholar]
Peyman 1997
- Peyman GA, Daun M, Greve MD, Yang D, Wafapoor H, Rifai A. Surgical closure of macular hole using an absorbable macular plug. International Ophthalmology 1997;21(2):87‐91. [DOI] [PubMed] [Google Scholar]
Polk 1996
- Polk TD, Smiddy WE, Flynn HW Jr. Bilateral visual function after macular hole surgery. Ophthalmology 1996;103(3):422‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rizzo 2010
- Rizzo S, Belting C, Genovesi‐Ebert F, Bartolo E. Incidence of retinal detachment after small‐incision, sutureless pars plana vitrectomy compared with conventional 20‐gauge vitrectomy in macular hole and epiretinal membrane surgery. Retina 2010;30(7):1065‐71. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Smiddy 1993
- Smiddy WE, Glaser BM, Thompson JT, Sjaarda RN, Flynn HW Jr, Hanham A, et al. Transforming growth factor‐beta 2 significantly enhances the ability to flatten the rim of subretinal fluid surrounding macular holes. Preliminary anatomic results of a multicenter prospective randomized study. Retina 1993;13(4):296‐301. [PubMed] [Google Scholar]
Smiddy 1997
- Smiddy WE, Pimentel S, Williams GA. Macular hole surgery without using adjunctive additives. Ophthalmic Surgery and Lasers 1997;28(9):713‐7. [PubMed] [Google Scholar]
Solebo 2011
- Solebo AL, Lange CA, Bunce C, Bainbridge JW. Face‐down positioning or posturing after macular hole surgery. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008228.pub2] [DOI] [PubMed] [Google Scholar]
Spiteri Cornish 2013
- Spiteri Cornish K, Lois N, Scott N, Burr J, Cook J, Boachie C, et al. Vitrectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full‐thickness macular hole (FTMH). Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009306.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stalmans 2012
- Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. The New England Journal of Medicine 2012;367(7):606‐15. [DOI] [PubMed] [Google Scholar]
Stata 2011 [Computer program]
- StataCorp. Stata Statistical Software. Version Release 12. Texas: StataCorp, 2011.
Steel 2013
- Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond) 2013;27 Syppl 1:S1‐212013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tadayoni 2009
- Tadayoni R, Creuzot‐Garcher C, Korobelnik JF. Internal limiting membrane peeling for large macular holes: a randomized, multicentric, and controlled clinical trial. Investigative Ophthalmology and Visual Science 2009;50:ARVO E‐Abstract 5206. [Google Scholar]
Tadayoni 2011
- Tadayoni R, Vicaut E, Devin F, Creuzot‐Garcher C, Berrod JP, Mer Y, et al. A randomized controlled trial of alleviated positioning after small macular hole surgery. Ophthalmology 2011;118(1):150‐5. [DOI] [PubMed] [Google Scholar]
Tanner 2001
- Tanner V, Chauhan DS, Jackson TL, Williamson TH. Optical coherence tomography of the vitreoretinal interface in macular hole formation. British Journal of Ophthalmology 2001;85(9):1092‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teba 2003
- Teba FA, Mohr A, Eckardt C, Wong D, Kusaka S, Joondeph BC, et al. Trypan blue staining in vitreoretinal surgery. Ophthalmology 2003;110(12):2409‐12. [DOI] [PubMed] [Google Scholar]
Thompson 1996
- Thompson JT, Smiddy WE, Glaser BM, Sjaarda RN, Flynn HW Jr. Intraocular tamponade duration and success of macular hole surgery. Retina 1996;16(5):373‐82. [DOI] [PubMed] [Google Scholar]
Thompson 1998
- Thompson JT, Smiddy WE, Williams GA, Sjaarda RN, Flynn HW Jr, Margherio RR, et al. Comparison of recombinant transforming growth factor‐beta‐2 and placebo as an adjunctive agent for macular hole surgery. Ophthalmology 1998;105(4):700‐6. [DOI] [PubMed] [Google Scholar]
Trese 2000
- Trese MT, Williams GA, Hartzer MK. A new approach to stage 3 macular holes. Ophthalmology 2000;107(8):1607‐11. [DOI] [PubMed] [Google Scholar]
Vine 1996
- Vine AK, Johnson MW. Thrombin in the management of full thickness macular holes. Retina 1996;16(6):474‐8. [DOI] [PubMed] [Google Scholar]
White 2008
- White IR, Higgins JP, Wood AM. Allowing for uncertainty due to missing data in meta‐analysis‐‐part 1: two‐stage methods. Statistics in Medicine 2008;27(5):711‐27. [DOI] [PubMed] [Google Scholar]
Wolf 2003
- Wolf S, Reichel MB, Wiedemann P, Schnurrbusch UE. Clinical findings in macular hole surgery with indocyanine green‐assisted peeling of the internal limiting membrane. Graefe's Archive for Clinical and Experimental Ophthalmology 2003;241(7):589‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Giansanti 2011
- Giansanti F, Virgili G, Eandi CM, Yap YC. Vitrectomy for idiopathic macular hole. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD009080] [DOI] [PMC free article] [PubMed] [Google Scholar]


