Abstract
Background
Efficacy of light therapy for non‐seasonal depression has been studied without any consensus on its efficacy.
Objectives
To evaluate clinical effects of light therapy in comparison to the inactive placebo treatment for non‐seasonal depression.
Search methods
We searched the Depression Anxiety & Neurosis Controlled Trials register (CCDANCTR January 2003), comprising the results of searches of Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1966 ‐), EMBASE (1980 ‐), CINAHL (1982 ‐), LILACS (1982 ‐), National Research Register, PsycINFO/PsycLIT (1974 ‐), PSYNDEX (1977 ‐), and SIGLE (1982 ‐ ) using the group search strategy and the following terms: #30 = phototherapy or ("light therapy" or light‐therapy). We also sought trials from conference proceedings and references of included papers, and contacted the first author of each study as well as leading researchers in the field.
Selection criteria
Randomised controlled trials comparing bright light with inactive placebo treatments for non‐seasonal depression.
Data collection and analysis
Data were extracted and quality assessment was made independently by two reviewers. The authors were contacted to obtain additional information.
Main results
Twenty studies (49 reports) were included in the review. Most of the studies applied bright light as adjunctive treatment to drug therapy, sleep deprivation, or both. In general, the quality of reporting was poor, and many reviews did not report adverse effects systematically. The treatment response in the bright light group was better than in the control treatment group, but did not reach statistical significance. The result was mainly based on studies of less than 8 days of treatment. The response to bright light was significantly better than to control treatment in high‐quality studies (standardized mean difference (SMD) ‐0.90, 95% confidence interval (CI) ‐1.50 to ‐0.31), in studies applying morning light treatment (SMD ‐0.38, CI ‐0.62 to ‐0.14), and in sleep deprivation responders (SMD ‐1.02, CI ‐1.60 to ‐0.45). Hypomania was more common in the bright light group compared to the control treatment group (risk ratio 4.91, CI 1.66 to 14.46, number needed to harm 8, CI 5 to 20).
Authors' conclusions
For patients suffering from non‐seasonal depression, light therapy offers modest though promising antidepressive efficacy, especially when administered during the first week of treatment, in the morning, and as an adjunctive treatment to sleep deprivation responders. Hypomania as a potential adverse effect needs to be considered. Due to limited data and heterogeneity of studies these results need to be interpreted with caution.
Plain language summary
Light treatment for non‐seasonal depression
The reviewers conclude that the benefit of light treatment is modest though promising for non‐seasonal depression. The short‐term treatment as well as light administered in the morning and with concomitant sleep deprivation in sleep deprivation responders appear to be most beneficial for treatment response. Hypomania as a potential adverse effect needs to be considered. Due to limited data and heterogeneity of studies these results need to be interpreted with caution.
Background
Depressive disorders are disabling, recurring illnesses that affect every society. It has been estimated that 20‐48% of the population will be affected by a mood disorder at least once in a lifetime (Cassem 1995; Kessler 1996). Major depression is estimated to be the fourth most important cause of loss in disability‐adjusted life years (Murray 1996), but in the future it may be the first cause in developed countries.
Etiologic theories have linked both disordered physiology and psychology to disordered mood (Dubovsky 1999). One of the biological factors, disruption in biological rhythms (circa dies; about a day), has been suggested to play a causal role in mental illness, particularly in affective disorders (Kripke 1981; Goodwin 1982).
Both biological treatments and psychological treatments can usually be applied in the treatment of depression. Antidepressant medication has become the predominant form of biological treatment. The response rate has been considered to be only slightly better than the response for placebo treatment (Mulrow 1999; Khan 2000). In the beginning of the treatment, the response usually takes two to eight weeks or even more. Moreover, adverse effects of antidepressant drugs can limit acceptability. Effects of psychotherapy appear largely similar in magnitude to those of antidepressants (Elkin 1989).
Administration of bright light for treatment of a mood disorder with recurrent annual depressive episodes, seasonal affective disorder (SAD), has been shown to be effective. Light therapy has become a treatment of choice for SAD (Lam 1999), though a formal Cochrane review is not yet available. Efficacy of light therapy for non‐seasonal depression has been studied less, but a substantial number of small controlled trials are now available. The mechanism of action of light is not yet completely understood. Light is a potent phase‐shifting agent of circadian rhythms and acts on melatonin secretion and metabolism. Artificial bright light has also been reported useful for treating sleep disorders (Campbell 1998; Chesson 1999), seasonal lethargy (Partonen 2000), premenstrual depression (Parry 1998), bulimia (Lam 1998), adaptation to timezone (Cole 1989) and work‐shift changes (Eastman 1999).
The minimal intensity of artificial light that appears necessary for an antidepressant effect in SAD is 2500 lux for two hours, or alternatively, a brighter light exposure of 10,000 lux for 30 minutes (Tam 1995). Bright light appears to be safe and side effects are mild, if the light does not contain substantial energy in the ultraviolet spectrum (Rosenthal 1989). For patients with bipolar disorder, light therapy is most safely administered with mood stabilizers because of the risk of mania (Kripke 1998). In SAD light has been shown to be most effective when administered in the morning (Terman 2001). Both morning and evening light have been used for non‐seasonal depression, but there is no consensus of the optimal timing of the treatment. In addition to efficacy and timing of light therapy for non‐seasonal depression, several issues such as the length of light treatment and preventive aspects are not yet fully understood. There have been interesting reports on combined treatment of light with antidepressant medication (Beauchemin 1997) and with sleep deprivation (Neumeister 1996).
Objectives
The main objective was to evaluate clinical effects of light therapy in comparison to the inactive placebo treatment for non‐seasonal depression.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria All relevant randomized controlled trials (RCTs).
Exclusion criteria 1. Quasi‐randomized studies. Quasi‐randomized studies were determined as studies in which a method of allocating participants to different forms of care that is not truly random; for example, allocation by date of birth, day of the week, medical record number, month of the year, or the order in which participants are included in the study; and 2. Controlled clinical studies (CCTs).
Types of participants
Inclusion criteria People with a diagnosis of non‐seasonal depression, irrespective of gender or age. In addition to major depressive disorder, we included dysthymia, minor depression, bipolar disorder, and other depressive conditions that were the primary focus of treatment in the study. Depression was being diagnosed according to Research Diagnostic Criteria (RDC), Diagnostic and Statistical Manual (DSM) criteria, or other validated diagnostic instruments, or was being assessed for levels of depressive symptoms through self‐rated or clinician‐rated validated instruments.
Exclusion criteria 1. Seasonal depression, such as Seasonal Affective Disorder (SAD) and Sub‐Syndromal Seasonal Affective Disorder (Sub‐SAD). As some studies might include both seasonal and non‐seasonal depressive patients, these studies were not included if more than 20% of the cases in a sample suffered from seasonal symptomatology (SAD or Sub‐SAD). If the number of cases with seasonal depression was not more than 20% in a study sample, these patients were included with non‐seasonal patients in the analysis. Even though we did not expect results to be influenced by seasonality of minor extent, sensitivity analysis were undertaken to evaluate the effect of these studies; and 2. Premenstrual Dysphoric Disorder (PMDD).
Types of interventions
1. All forms of bright light therapy, in terms of timing, intensity, and duration of light exposure and the device being used. Bright light could be administered either alone, or concomitant with antidepressant drug therapy, with sleep deprivation, or with both adjunctive treatments, so long as light and placebo were administered randomly and the concomitant therapies were not adjusted or biased according to light/placebo assignment; and 2. Inactive placebo treatment (dim light or other inactive treatment).
Types of outcome measures
Principal outcomes of interest were: 1. Depression symptom level. This is usually measured using a variety of rating scales, for example, clinician‐rated scales such as the Hamilton Rating Scales for Depression (HRSD) (Hamilton 1960), and self‐rating scales such as the Beck Depression Inventory (BDI) (Beck 1961). Symptom levels may be presented as continuous (mean and Standard Deviation [SD]) or dichotomous outcomes (remission/recovery vs. non‐remission/non‐recovery); 2. Adverse effects, particularly mania, the elevation of mood, eye irritation, and headache. These are usually presented as dichotomous outcomes (adverse effect yes/no); 3. Acceptability of treatment as assessed indirectly by the number of persons dropping out of the studies; and 4. Deterioration in mental state or relapse during treatment.
Information were also sought regarding other outcomes including (objective or subjective measures): 1. Overall clinical improvement; 2. Quality of life; 3. Cost effectiveness; and 4. Long‐term follow‐up.
All outcomes were grouped by time, i.e., duration of treatment ‐ short term (up to one week), medium term (eight days to eight weeks) and long term (more than eight weeks). An overall analysis was also performed. In crossover studies, only the first treatment phase prior to crossover (first arm) was included.
Search methods for identification of studies
1. Electronic databases: See: Collaborative Review Group search strategy The Depression Anxiety & Neurosis Controlled Trials register (CCDANCTR December 2002)), comprising the results of searches of Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL (1982 ‐), EMBASE (1980 ‐), LILACS (1982 ‐), MEDLINE (1966 ‐), National Research Register, PsycINFO/PsycLIT (1974 ‐), PSYNDEX (1977 ‐), and SIGLE (1982 ‐ ) was searched using the following terms:
Intervention = phototherapy or ("light therapy" or light‐therapy);
2. Reference lists: we searched all references of articles selected for further relevant trials;
3. Conference proceedings: we sought studies from conference proceedings if available;
4. Authors: we contacted the first author of each study as well as leading researchers in the field regarding additional information and unpublished trials.
Data collection and analysis
Study selection Two reviewers (AT and either DK or TE) independently inspected all study citations of published and unpublished trials identified by the searches to assess their relevance to this review. Full reports of the studies of agreed relevance were obtained. When disagreement occurred, the full article was acquired for further inspection. If there was disagreement with the inspection of the report, this was resolved by discussion and further information was sought from the authors when needed.
If the report did not comment on randomization or double blindness in allocation, and additional information could not be obtained from the authors, the study was categorized as 'not randomized' and excluded from the analysis.
Quality assessment Two independent reviewers (AT and either DK or TE) assessed the methodological quality of the selected trials using the criteria based on the guidelines included in the Cochrane Collaboration Handbook (Mulrow 1997). Should disagreements have arisen, resolution was attempted by discussion. If this was not possible and further information was needed to clarify into which category to allocate the trial, data were not entered and the trial was allocated to the list of those awaiting assessment. A rating was given for each trial based on the three quality categories. These criteria are based on the evidence of a strong relationship between the potential for bias in the results and the allocation concealment and are defined as below:
A. Low risk of bias (adequate allocation concealment); B. Moderate risk of bias (intermediate, some doubt about the results); and C. High risk of bias (inadequate allocation concealment).
Only trials in Category A or B were included in the review. Randomized studies as well as double‐blind studies with no further information on randomization process were included in Category B.
Addressing publication bias Data from all identified and selected trials were entered into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. If appropriate, funnel plot asymmetries (suggesting potential publication bias) were investigated by visual inspection and formal statistical tests (Egger 1997).
Data extraction Two reviewers (AT and either DK or TE) independently extracted data from selected trials using data extraction forms. If disputes arose, resolution was attempted by discussion. If further information was necessary to resolve the dilemma, data were not entered until the authors were contacted and additional information was obtained.
Data synthesis The data was synthesized using Review Manager 4.2.1. software. Outcomes were assessed using continuous (for example, outcome figures of a depression scale) or dichotomous measures (for example, 'no important changes' or 'important changes' in a person's behavior, adverse effects information).
1. Continuous data Where trials reported continuous data, as a minimum standard, the instrument that has been used to measure outcomes had to have established validity, for instance, to have been published in a peer‐reviewed journal. The following minimum standards for instruments were set: the instrument shall either be a) self‐report, or b) completed by an independent rater or relative (not the therapist); and the instrument should be a global assessment of an area of functioning. Continuous data were reported as presented in the original studies, without making any assumptions about those lost to follow‐up. Whenever possible, the opportunity was taken to make direct comparisons between trials that used the same measurement instrument to quantify specific outcomes. For continuous data, reviewers calculated weighted mean differences (WMDs). Where continuous data were presented from different scales rating the same outcomes, the reviewers applied standardized mean differences (SMDs).
2. Dichotomous data Where dichotomous outcomes were presented, the cut‐off points designated by the authors as representing 'clinical improvement' were identified and used to calculate relative risks (RR) and 95% confidence intervals (95% CIs). These cut‐off points are, however, often defined quite differently, and only those studies that had used similar cut‐off points (e.g., 20% reduction in scores or 50% reduction in scores) were combined into a single pooled estimate. For undesirable outcomes an RR that is less than one indicates that the intervention was effective in reducing the risk of that outcome. As a measure of effectiveness, the number needed to treat (NNT) or the number needed to harm (NNH) statistic was calculated together with its confidence interval. Where patients were lost to follow‐up at the end of the study, it was assumed that they had had a poor outcome and once they were randomized they were included in the analysis (last observation carried forward analysis). If patients had dropped out after randomization due to non‐compliance, lack of efficacy, relapse, or for unknown reason, it was assumed that those cases also had failed to improve. However, it needs to be acknowledged that categorizing these drop‐out subjects as "failures" might overestimate the number of subjects with poor outcome.
Fixed effect model and random effects model For both dichotomous and continuous data, a fixed effect model was used to analyze data, but if significant heterogeneity (p<0.05) was found, a supplementary random effects model was computed. A random effects model will tend to give a more conservative estimate, but the results from the two models should agree when the between‐study variation is estimated to be zero.
Parametric tests and non‐parametric data Data on outcomes are not normally distributed. To avoid applying parametric tests to non‐parametric data the following standards were selected for all data derived from continuous measures before inclusion: 1. Standard deviations (SDs) and means were reported in the paper or were obtainable form the authors; and 2. SD, when multiplied by two, was less than the mean, as otherwise the mean is unlikely to be an appropriate measure of the center of distribution (Altman 1996).
Data that did not meet the standards were not planned to be entered into a meta‐analysis (which assumes a normal distribution) but reported in the "Other data" tables.
Although in our protocol we stated that we would only use data that met our criteria 1. and 2., during the analysis, given the scarcity of results, we decided to include also data which did not meet the criterion 2. We performed an additional sensitivity analysis to study the effect of this procedure and have indicated this in the Results section.
Post‐intervention scores (data at endpoint) were used in the meta‐analysis. Because change scores take into account pre‐existing differences between groups at baseline, mean change scores and SDs were extracted where available and pooled where appropriate.
Graphs In all cases the data were entered into the Review Manager in such a way that in graphs the area to the left of the 'line of no effect' indicates a favorable outcome for the relevant intervention.
Subgroup analyses and heterogeneity Investigation of sources of heterogeneity was performed with the sub‐group analysis. We investigated whether: 1. Trials studying long term treatment effects differed in their results from trials evaluating short term treatment; 2. Trials using inpatients differed in their results from trials using outpatients; 3. Trials using concomitant sleep deprivation differed in their results from trials not using sleep‐deprivation; 4. Trials using concomitant drug treatment differed in their results from trials not using drug treatment; 5. Trials with bright light treatment in the morning differed in their results with trials administering light in the evening, at night, or at various times of a day; 6. Trials using a light box differed in their results from trials using some other lighting device; 7. Trials with higher intensity of light treatment (> 2500 lux) differed in their results from trials with lower intensity of light; 8. Trials with longer duration of light treatment differed in their results with trials with shorter duration of light; and 9. Trials using very old or very young subjects differed in their results from trials using adult subjects.
Sensitivity analyses were performed to exclude the studies including following conditions: 1. Studies of lower methodological quality; 2. Mixed study sample of non‐seasonal and seasonal patients; and 3. Robustness of the findings based on dichotomous outcomes in which it was assumed that drop‐outs are treatment failures.
As heterogeneity was found in many statistical analyses, a random effects model was also applied as an additional sensitivity analysis for the results from the fixed effect model. Both of the results are described in the Results section.
Excluded studies All excluded studies were listed with the reason for exclusion.
Results
Description of studies
1. Excluded studies Twenty‐five studies were excluded, either because they were not randomized trials (15 studies), more than 20% of the participants were suffering from seasonal depression or seasonal difficulties (3 studies), participants were not clinically diagnosed to have depression (1 study), interventions were not standardized (2 studies), active treatment was combined with two other treatments not balanced in the placebo group (1 study), control treatment was also clearly active (in one study the control treatment being 2500 lux bright light and in another study exercise), or the comparison was between depressive patients with atypical symptoms and patients with classical symptoms (1 study). The table Characteristics of excluded studies describes the details of exclusion as follows: if the study was eligible by its allocation method (randomized), then information on participants has been listed. If the participants have fit our criteria, then the reason for exclusion has been the intervention of the study.
2. Included studies We identified 20 studies for inclusion in this review, dating from between 1983 and 2002. In two studies, randomization was performed after sleep deprivation for responders and nonresponders separately. Both of these studies were separated to two individual studies according to the randomization procedure (Neumeister 1996a; Neumeister 1996b; Fritzsche 2001a; Fritzsche 2001b). One study (Bloching 2000) has been reported as conference abstracts only with additional data supplied by the author. Two of the studies (Schuchardt 1992; Sumaya 2001) have provided insufficient information at the moment, and the authors have been contacted for additional data.
Length of trials Thirteen studies presented data on 'short term' treatment (up to one week). Two studies lasted only for one day, either one single hour (Kripke 1983) or one night (Giedke 1989). Seven studies fell into the 'medium term' (eight days to eight weeks) category, the longest ones being of 4 weeks in duration (Schuchardt 1992; Holsboer 1994). In one of these medium term studies (Holsboer 1994) administration of bright light was decreased to three times per week during the last three weeks of treatment. None of the trials fulfilled our criterion for the 'long term' category (more than eight weeks).
Participants Seventeen studies reported on participants mostly suffering from major depressive disorder. Ten studies had both unipolar and bipolar patients in their sample. Inclusion and exclusion criteria varied among studies (see Characteristics of Included Studies table). Participants were more likely to be female than male (60% versus 40%, respectively), and had a mean age of 50 years. Assessment of seasonality either in exclusion criteria or in use of seasonality scales was commented on in 13 out of 20 studies. As almost all patients had major depressive disorders, by definition this excludes the existence of SAD and subsyndromal SAD.
Setting Almost all studies took place in the hospital or long‐term care facility. Only two studies (Loving 2002; Schuchardt 1992) assessed outpatients. None of the studies were multicenter. Only five studies (Mackert 1990; Yamada 1995; Fritzsche 2001a; Fritzsche 2001b; Benedetti 2003) reported on the time of the year when the study was performed.
Study size Study size ranged from 115 people (108 completers) (Colombo 2000) to 6 people (Neumeister 1996b), with a mean size of 31. The total number of patients from the 20 studies that provided data for this review was 620.
Interventions Bright light therapy was administered in a wide range of intensities (from 400 lux to 10,000 lux), several colors such as white (active), green (active), red (control) and yellow (control) wave lengths, and at different times in a day. The duration of active treatment varied between 30 minutes (Benedetti 2003; Loving 2002) and the whole night, i.e., eight (van den Burg 1990) or nine hours (Giedke 1989). Duration/brightness in relation to efficacy was not assessed in any of the studies. Eleven studies administered bright light in the morning: one of these studies (Yamada 1995) had used morning light to one group and evening light to another group of their patients. There was only one study (Holsboer 1994) that had used evening light only. Two studies (Neumeister 1996a; Neumeister 1996b) used both morning and evening treatments, and two studies (Giedke 1989; van den Burg 1990) used the whole night light treatment. Inactive placebo treatment was almost always dim light, mostly red (10 studies) and varied in intensity between 25 to 500 lux. One study described the use of a negative ion generator as inactive treatment (Benedetti 2003). The device for light therapy was usually a light box, but also other lighting approaches were used. Three studies (Giedke 1989; van den Burg 1990; Holsboer 1994) described dim illumination in a room as inactive treatment.
Light was administered adjunctive to sleep deprivation in nine studies, and in two additional studies (Kripke 1983; Kripke 1987) the participants were awakened before their usual wake up time for light treatment. One study (Benedetti 2003) reported that active treatment group patients were awakened 1 1/2 hours earlier than patients in the control group, which makes the groups slightly unequal to compare and can be considered as a minor additional sleep deprivation for patients in the active treatment group. As early awakening was not intended as an additional treatment as such and was not designed to be an active treatment, this study was not excluded from the concomitant analysis. Standardized adjunctive pharmacotherapy was applied in seven studies, and in ten studies, concomitant drug treatment of the participants was kept unchanged. One study (Colombo 2000) had applied both sleep deprivation and standardized drug treatment (lithium) in the study design. In another study (Holsboer 1994), the sleep deprivation intervention could not be included in the evaluation, as the intervention groups were not comparable. Only two studies (Mackert 1990; Yamada 1995) had applied bright light only, without sleep deprivation or pharmacotherapy.
Outcomes In addition to general mental state outcome assessment, we analyzed clinician‐rated and self‐rated mental state separately. In part of the studies self‐rating instruments were the only method to evaluate the outcome status of the participants (van den Burg 1990; Moffit 1993; Colombo 2000; Sumaya 2001; Loving 2002), and these scores were used to assess the general mental state. Deterioration in mental health or relapse during the treatment was assessed by dichotomous scales. Acceptability of treatment was measured indirectly by patients dropping out of the study. Adverse effects in detail were evaluated by dichotomous scales and by continuous symptom scales. Abbreviations of the tests are explained in the footnotes of the Characteristics of Studies tables.
Almost all studies reported stringent criteria for the diagnosis of depression: only one study did not report on diagnostic criteria (Kripke 1983), and one study assessed the level of depressive symptoms through a self‐rated validated instrument, the Geriatric Depression Scale (Sumaya 2001).
Improvement of condition was dichotomously defined in three studies as percentage or number of respondents with 50% reduction in HDRS (Holsboer 1994; Benedetti 2003; Loving 2002). As one study (Prasko 2002) applied an additional criterion of HDRS scores less than 8 to improvement of condition, this study was not included in the meta‐analysis of outcome of improvement, but reported in the Other data table. Studies of another group (Fritzsche 2001a; Fritzsche 2001b) had also applied the same additional criterion to the 50% reduction definition, but as they only had used the cutpoint to determine sleep deprivation responders and nonresponders before randomization, this dichotomization could not be included in the analysis.
Outcome scales Details of scales that provided usable data are described below.
As some of the studies had applied several rating scales to assess the depressive mental state, the reviewers made their choice for inclusion of data in the meta‐analysis as follows: firstly, priority was given to the most commonly used rating scales HDRS and BDI. Secondly, if the authors had used the rating scale to determine the treatment response or expressed their preference over scales by the order of presenting the results, the following choices were made: AMS (Bloching 2000), M‐S (Giedke 1989), D‐S (Mackert 1990) and D‐S (Holsboer 1994). If follow‐up scores were available even if a different outcome scale had been used, the scores were utilized in the analysis: AMS (van den Burg 1990).
Mental state scales Hamilton Depression Rating Scale (HDRS) (Hamilton 1960) is an observer scale and is designed to be used on patients already diagnosed as suffering from affective disorder. The scale contains 17 or 21 variables measured on either a five‐point or a three‐point scale. Among the variables are: depressed mood, suicide, work and interests, retardation, agitation, gastrointestinal symptoms, general somatic symptoms, hypochondriasis, loss of insight, and loss of weight. Higher scores indicate more symptoms.
Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979) is a semi‐structured symptom scale that is measuring the severity of depression. The twelve items cover the eight clinical features listed in the DSM‐III‐R definition of major depressive disorder. Scoring of either 0 to 3 (with operational criteria) or 0 to 6 (with undefined intermediate steps) can be used. Higher scores indicate more severe depression.
Adjective Mood Scale (AMS), a.k.a. Befindlichkeits‐Skala (Bf‐S) (von Zerssen 1983) is a self‐rated mood scale that is measuring subjective impairment. There are 28 items which are scored from 0 (not depressed) to 56 (severely depressed), and it is particularly suited for frequent use at short intervals.
Depression Scale (D‐S) (von Zerssen 1986) is another self‐rated instrument that is measuring depression.
Beck Depression Inventory (BDI) (Beck 1961) is a self‐rated symptom scale that assesses the severity of depressive states. There are 21 items which are scored 0 to 3, based on the degree of the symptoms. Higher scores indicate more severe symptoms.
Geriatric Depression Scale (GDS) (Yesavage 1983) is a self‐rated instrument that assesses depression in geriatric population. The 30‐item instrument consists of yes/no format assessments of cognitive complaints, self‐image, energy and motivation, future/past orientation, agitation, and social behavior. Higher scores indicate more severe symptoms.
Global assessment scales Clinical Global Impressions (CGI) (Guy 1976) is a rating instrument that assesses severity of illness. It consists of three global scales (items), of which two items, 'Severity of illness' and 'Global improvement', are rated on a seven‐point scale; while the third, 'Efficacy index', requires a rating of the interaction of a therapeutic effectiveness and adverse reactions. Lower scores indicate decreased severity and/or greater recovery.
Visual Analogue Scale (VAS) (Aitken 1969) is a rating instrument for global assessment of a particular item or the severity of illness. The instrument has a millimeter scale from 0 to 100, where 0 stands for an optimally healthy condition and 100 for a very severe condition of illness. In assessment of mood, 100 denotes extremely happy feelings. This rating scale was used to measure subjective mood levels in four studies (Mackert 1990; Holsboer 1994; Bloching 2000; Colombo 2000).
Adverse effect scales Complaint List (C‐S) (von Zerssen 1986) is a self‐rated instrument that assesses various symptoms. Specific items such as fatigue, nausea, irritability, inner restlessness, restless feeling in the legs, excessive need of sleep, insomnia, trembling, and neck and shoulder pain were used to represent side‐effects that have been described in the literature. Higher scores indicate more symptoms.
Fisher's Somatic Symptom/Undesired Effect Checklist (FSUCL) (CIPS 1986) is a semi‐structured clinician‐rated symptom scale that evaluates adverse effects. It consists of six different facets of adverse effect items (central nervous system related, 5 items; gastrointestinal complaints, 6 items; vegetative, 5 items; neurological, 7 items; headache, 1 item; cardiovascular, 2 items) with a total of 26 items. Each item is scored on a 4‐point scale, with 0 indicating absence and 3 indicating serious severity. Higher scores indicate more severe symptoms.
3. Studies awaiting assessment One study (Deltito 1991) has evaluated the intensity of light therapy in patients with non‐seasonal depression, but the outcome measures have reported light therapy contrasted between bipolar and unipolar patients. To assess the difference between bright and dim light intervention, the authors have been contacted for further details, but no reply has been received as yet.
4. Ongoing studies One study (Goel 2001) reports an ongoing study on bright light and negative ion treatment in patients with chronic depression, and the other (Zirpoli 2002) is evaluating the sensitivity of melatonin to light suppression and light treatment in depressed and non‐depressed children.
Risk of bias in included studies
Randomization All included studies described themselves as randomized, but presented little methodological detail to elaborate on the truly random nature of allocation. Three studies (Moffit 1993; Kripke 1987; Benedetti 2003) had used the method of block randomization. One study (Schuchardt 1992) had used a randomized list, but only two studies (Moffit 1993; Benedetti 2003) described a truly random method of allocation (computer generated randomization with no stratification; sealed envelopes). Apart from these studies, no other studies described a method that would prevent foreknowledge of allocation.
Blinding of assessment Blinding of assessment in administration of light therapy is more difficult than in studies with drug intervention, since the active treatment due to its brightness looks dissimilar to the control treatment. Subjects cannot fail to perceive the treatment and cannot be literally blind to treatment, though they may not know which is intended as the active treatment. Eleven studies described double blind assessment, i.e., a patient and an experimenter blind to the details of light treatment. It needs to be understood that these studies attempted to conceal from patients which was the active treatment, but patients were certainly not blind to the brightness of the light, and therefore, no patient was 'blind'. Four studies were single blind, and in two of them (Colombo 2000; Benedetti 2003) explained that raters could not keep themselves blind due to patients' questions. In five studies blinding was not stated. Patients' expectations were studied in two studies only (Mackert 1990; Kripke 1992), and this issue was commented on but not studied in two more studies (Colombo 2000; Benedetti 2003).
Data reporting As studies frequently presented data on graphs and by p‐values, raw data were not always available for synthesis. Standard deviations (SDs) were not routinely reported in all studies. If the participants of the studies had been dropping out from the study after randomization, data reporting was not always sufficient in terms of the reasons for dropping out or the group that the participants had belonged to. The method of 'last observation carried forward' was not declared in the studies except for in one personal communication (Kripke 1992). It remained unclear if the studies had applied a true 'intention to treat' analysis, as numbers of patients at endpoint results (when reported) rarely matched those reported at baseline. In four out of five studies with a crossover design (Kripke 1983; Kripke 1987; Giedke 1989; van den Burg 1990), the data of the first arm were available and made meta‐analysis approach possible. The authors of the fifth crossover study (Sumaya 2001) have been contacted for additional information.
Apart from two category A studies (Moffit 1993; Benedetti 2003), all other studies were located in the quality category B (randomized but concealment of allocation unclear). In almost one third of the studies, the numbers of patients allocated to each treatment group were identical. When allocating by chance this is improbable unless block randomization has been used. The studies did not comment on this.
Effects of interventions
The search The Cochrane Depression, Anxiety and Neurosis Group's register found 160 records (January 2003). After two reviewers independently screening the searches, altogether 33 possible citations were identified. The evaluation of the full reports of the search, screening the report references, conference proceedings available, and correspondence with authors of identified studies yielded 49 reports of 20 separate trials judged to fulfill the inclusion criteria of the review. Most of the excluded studies were non‐randomized, open studies. According to the study protocol, the studies evaluating light therapy for seasonal depression were beyond this review and will be included in another Cochrane review.
General comments If there were several active intervention groups in the study, we grouped together all the experimental groups (active treatment group) and compared them collectively with the control group. To evaluate the effect of inclusion of studies with possible non‐normal distribution in the analysis, we evaluated primary mood rating scale endpoint scores with studies with normal distribution only, i.e. following the original criteria of our protocol 'SD multiplied by two being less than a mean'. However, as the result (details given below) was similar to that of a whole group, all outcomes have been analyzed without exclusion of studies in which normality cannot be assumed. In dichotomous variables we categorized drop‐out subjects as "failures". It needs to be kept in mind that this categorization we made might have overestimated the number of subjects with poor outcome.
To evaluate the effect of inclusion of studies that did not meet strict criteria of normal distribution, we excluded the studies in which the mean was less than SD multiplied by two (Kripke 1983; Giedke 1989; Holsboer 1994; Yamada 1995; Fritzsche 2001a; Benedetti 2003; Loving 2002; Prasko 2002). Using the primary mood rating scale endpoint score, the outcome result of the studies that were normally distributed did not differ from that of the whole group.
Overall quality In general, the quality of reporting was poor. All but two trials reported the randomization procedure without adequate information on allocation concealment. Blinding procedures were also generally inadequately described. Many studies did not report the number of drop‐outs and did not specify reasons for drop‐out. The trials did not report if intention‐to‐treat analysis was performed. The meta‐analysis results are based on 18 studies, because the mean outcome scores and SDs of two studies (Schuchardt 1992; Sumaya 2001) were not available at the time of preparation of the review. Both of these studies had reported significant benefits of bright light.
Specific comments
Global state The information of patients with no clinical improvement/deterioration by dichotomous CGI criteria could be extracted in none of the studies. Continuous CGI endpoint scores showed that, based on a small medium term study (Prasko 2002), there was a trend for the control treatment being more effective than bright light.
Mental state Treatment response, analyzed by primary mood rating scale endpoint scores and using a fixed effect model, was significantly better in the bright light group compared to the control treatment group (18 studies, 505 patients, standardized mean difference (SMD) ‐0.20, CI ‐0.38 to ‐0.01). A negative standardized mean difference means that the bright light group was better than the control group. This finding was mainly due to the significant benefit of short term treatment of seven days or less (12 studies, 367 patients, fixed effect model: SMD ‐0.23, CI ‐0.44 to ‐0.02). Medium term treatment did not show any significant superiority of bright light (6 studies, 138 patients, fixed effect model: SMD ‐0.10, CI ‐0.45 to 0.24). Since significant heterogeneity was found, the more conservative random effects model was also examined. According to the evaluation with this model, both short term studies and the total study effects were no longer statistically significant in favoring bright light over control treatment (short term studies: SMD ‐0.27, CI ‐0.64 to 0.10; total group: SMD ‐0.22, CI ‐0.52 to 0.09). Excluding the outlier detected in one of the short term studies (Loving 2002) had little effect on short term outcome (12 studies, 366 patients, fixed effect model: SMD ‐0.24, CI ‐0.45 to ‐0.02; random effects model: SMD ‐0.28, CI ‐0.65 to 0.09) or the all‐studies results (18 studies, 504 patients, fixed effect model: SMD ‐0.20, CI ‐0.38 to ‐0.02; random effects model: SMD ‐0.23, CI ‐0.53 to 0.08). Six studies in which the change score data of primary mood rating scales including SDs were available (Kripke 1983; Kripke 1987; Kripke 1992; Bloching 2000; Colombo 2000; Loving 2002) were also significantly in favor of bright light based on a fixed effect model but not based on a random effects model (6 studies, 198 patients; fixed effect model: SMD ‐0.35, CI ‐0.64 to ‐0.06; random effects model: SMD ‐0.46, CI ‐1.10 to 0.18).
Examining studies with clinician‐rated treatment responses showed a similar significant benefit for bright light in short term studies (9 studies, 258 patients, fixed effect model: SMD ‐0.35, CI ‐0.61 to ‐0.10) and in the total group (14 studies, 376 patients, fixed effect model: SMD ‐0.23, CI ‐0.44 to ‐0.01), whereas in the medium term studies there was no significant difference in the treatment effect between bright light and control treatment groups (5 studies, 118 patients, fixed effect model: SMD 0.04, CI ‐0.33 to 0.42). A more conservative evaluation using the random effects model was in line with previous comparisons but statistical significance was lost (short term: SMD ‐0.40, CI ‐0.90 to 0.10, the total group: SMD ‐0.23, CI ‐0.61 to 0.15). In self‐rated responses, the treatment effects of bright light and control treatments were close to equal with a fixed effect model approach (short term studies: 9 studies, 320 patients, SMD ‐0.02, CI ‐0.24 to 0.20; medium term studies: 3 studies, 68 patients, SMD ‐0.11, CI ‐0.60 to 0.37; total group: 12 studies, 388 patients, SMD ‐0.04, CI ‐0.24 to 0.17).
There were only two short term studies (Mackert 1990; Yamada 1995) that had applied bright light only, i.e. the patients were not exposed to sleep deprivation or other adjunctive treatments and did not receive any medication. The treatment response, evaluated by a fixed effect model, was better for bright light than for control treatment (2 studies, 69 patients, SMD ‐0.64, CI ‐1.14 to ‐0.14). With a more conservative random effects model the result was in line with a fixed effect model approach but did not reach statistical significance (SMD ‐0.73, CI ‐1.58 to 0.12).
According to the criterion of 50% decrease in the HDRS score, there was no difference between groups: 20 out of 39 patients (51%) in the bright light group and 17 out of 32 patients (53%) in the control treatment group were not improved (3 studies, 71 patients, relative risk (RR) 0.94, CI 0.61 to 1.46). One study (Prasko 2002) used a more conservative criterion of the definition of improvement (50% improvement and a score less than 8). In their study sample 9 out of 13 patients (69%) in the bright light group and 7 out of 10 patients (70%) in the control treatment group were not improved.
Only a few short term studies (Yamada 1995; Colombo 2000; Loving 2002) had analyzed the deterioration in mental state or relapse of the participants during treatment. These studies showed a trend of the occurrence of less deterioration/relapses in the bright light group compared to findings in the control treatment group, but the result was not statistically significant (3 studies, 120 patients, RR 0.40, CI 0.12 to 1.31). None of the medium term studies provided information on this outcome.
The baseline scores for interventions in each study are presented in Other data tables.
Adverse effects One study that used concomitant trimipramine drug treatment reported on adverse effects in detail (Holsboer 1994), and several other studies gave short notes on adverse effects during the study. Six studies gave information on the occurrence of mania, and in the only study that had detected patients suffering from mania (Colombo 2000), the condition was more frequent in the control treatment group. Evaluation of hypomania was reported in seven studies, in which 19 out of 118 participants in the bright light group and 3 out of 101 patients in the control group developed hypomania (7 studies, 219 patients, RR 4.91, CI 1.66 to 14.46, number needed to harm (NNH) 8, CI 5 to 20). It needs to be acknowledged that categorizing the drop‐out subjects as "failures" might overestimate the number of subjects with this adverse effect as well as with other poor outcomes. Headache was slightly more frequent in the bright light group compared to control treatment group, but did not reach statistical significance (3 studies, 109 patients, RR 2.26, CI 0.91 to 5.59). None of the patients had experienced disturbed sleep. Sleep onset difficulties were more frequent in the bright light group, although this information was reported in one study only (Kripke 1992). Agitation, headache, blurred vision and eye irritation were slightly though statistically non‐significantly more common in the bright light group than in the control group (agitation: 2 studies, 89 patients, RR 3.22, CI 0.95 to 10.89; headache: 2 studies, 109 patients, RR 2.26, CI 0.9 to 5.59; blurred vision: 2 studies, 89 patients, RR 2.22, CI 0.73 to 6.78; eye irritation: 2 studies, 68 patients, RR 3.53, CI 0.97 to 12.88). Other isolated adverse effects did not show any preference over either of the treatment groups. Two studies (Mackert 1990; Holsboer 1994) had applied a structured symptom scale for adverse effects: the short term study (Mackert 1990) did not find any significant difference between treatment groups, whereas the medium term study (Holsboer 1994) showed slightly but not statistically significantly more adverse effects in the bright light group than in the control treatment group.
Acceptability of treatment Acceptability of treatment, analyzed by the number of patients dropping out of the study, did not show any significant difference between the groups (16 studies, 453 patients, RR 1.35, CI 0.60 to 3.07).
Quality of life, cost effectiveness and follow up These issues were not evaluated in the included studies. Follow up of the mood scores was evaluated in 5 studies only (Giedke 1989; van den Burg 1990; Kripke 1992; Holsboer 1994; Benedetti 2003) and it was short (between 2 days and 2 weeks). These studies did not show any statistically significant superiority of bright light over control treatment (5 studies, 189 patients, SMD 0.15, CI ‐0.14 to 0.44). Mortality No mention of mortality or permanent injuries was made in any of the studies. The studies in which all the participants had completed an assigned treatment enabled us to conclude indirectly that no deaths occurred.
Subgroup and sensitivity analyses Short term results were slightly though not statistically significantly better than medium term results. As there were no long term studies available, long term and short term treatment effects could not be compared.
Comparison between inpatient and outpatient studies could not be performed, since there was only one study that gave outcome scores on outpatient treatment for the meta‐analysis. To evaluate the effect of sleep deprivation procedure, we created the following subgroups: concomitant sleep deprivation (9 studies, 266 patients), unclear sleep deprivation (2 studies, 21 patients), and no sleep deprivation (6 studies, 167 patients). Treatment responses between studies with patients who underwent sleep deprivation and those who did not showed that with a fixed effect model approach sleep deprivation studies showed a non‐significant trend to favor for bright light over control treatment (9 studies, 266 patients, SMD ‐0.22, CI ‐0.47 to 0.22), whereas in studies without sleep deprivation bright light was significantly better than control treatment (6 studies, 167 patients, SMD ‐0.34, ‐0.66 to ‐0.02). When a random effects model was applied with the latter group, the significance was lost (SMD ‐0.36, CI ‐0.99 to 0.26). If patients were awakened 1‐to‐2 hours before wake‐up time, there was no difference between bright light and control treatment groups based on a fixed effect model approach (2 studies, 21 patients, SMD 0.39, CI ‐0.50 to 1.28). In studies in which both bright light and sleep deprivation were applied, a fixed model approach revealed that the sleep deprivation responders had a statistically significantly better response to bright light than to control treatment (4 studies, 63 patients, SMD ‐1.02, CI ‐1.60 to ‐0.45). The result remained significant even though a more conservative random effects model was applied (SMD ‐1.24, CI ‐2.45 to ‐0.03). This finding was mainly due to short term studies (a fixed effect model approach: 3 studies, 43 patients, SMD ‐1.84, CI ‐2.60 to ‐1.07), and remained statistically significant even when a random effects model was applied (SMD ‐1.84, CI ‐2.60 to ‐1.07). In a medium term study there was no difference in response between bright light and control treatments (1 study, 20 patients, SMD 0.07, CI ‐0.81 to 0.95). The sleep deprivation non‐responders showed no significant difference in response between bright light and control treatments according to a fixed model approach (3 studies, 45 patients, SMD ‐0.25, CI ‐0.85 to 0.36).
A great majority of studies had applied concomitant drug treatment (14 studies, 329 patients) whereas only a few studies had no drug treatment (4 studies, 134 patients). Evaluation of concomitant drug therapy showed that in studies with patients receiving concomitant pharmacotherapy, bright light showed a statistically significant efficacy over control treatment with a fixed effect model approach (14 studies, 329 patients, SMD ‐0.25, CI ‐0.47 to ‐0.02), but significance was lost when a random effects model was applied (SMD ‐0.24, CI ‐0.61 to 0.12). Studies with patients not receiving concomitant drug therapy showed a statistically non‐significant trend of response to bright light over control treatment (4 studies, 134 patients, SMD ‐0.18, CI ‐0.53 to 0.17).
The time of the day for bright light treatment was evaluated by categorizing the studies into the following groups: morning light (11 studies, 297 patients), evening light (2 studies, 43 patients), all‐night light (2 studies, 80 patients), both morning and evening light (2 studies, 20 patients), and various times of light treatment (2 studies, 65 patients). Based on a fixed effect model approach, the effect of morning light was statistically significantly better than that of control treatment (11 studies, 297 patients, SMD ‐0.38, CI ‐0.62 to ‐0.14), whereas the treatment administered at other times of the day didn't show any superiority over control treatment. Even with a more conservative random effects model the response to morning light treatment remained statistically significantly better than the response to the control treatment (SMD ‐0.43, CI ‐0.82 to ‐0.05).
When the combination of concomitant sleep deprivation and morning bright light were evaluated, the treatment response with morning light plus concomitant sleep deprivation showed a statistically non‐significant trend for bright light over control treatment based on a fixed model approach (5 studies, 166 patients, SMD ‐0.28, SMD ‐0.59 to 0.03). Using the same fixed effect model, morning light without concomitant sleep deprivation was statistically significantly more effective than control treatment (5 studies, 124 patients, SMD ‐0.53, CI ‐0.91 to ‐0.16), and the result remained statistically significant even when a more conservative random effects model was applied (SMD ‐0.62, CI ‐1.24 to ‐0.01).
A combination of concomitant drug therapy and morning light was applied in half of the studies (9 studies, 243 patients), whereas morning light without any drug therapy was rare (2 studies, 54 patients). Evaluation of the effect of combination of concomitant drug and morning bright light showed that there was no difference between the two morning light conditions with or without pharmacotherapy. With a fixed effect model approach, both conditions were statistically significantly in favor of bright light over control treatment (combination treatment: 9 studies, 243 patients, SMD ‐0.32, CI ‐0.60 to ‐0.08; light only: 2 studies, 54 patients, SMD ‐0.57, CI ‐1.14 to ‐0.01), but with a more conservative random effects model the statistical signficance was lost (combination treatment: SMD ‐0.36, CI ‐0.79 to 0.07; light only: SMD ‐1.03, ‐2.63 to 0.58).
The majority of studies had used a light box (12 studies, 275 patients) whereas another device was used in only a few studies (5 studies, 157 patients). In studies using a light box, a fixed effect model approach showed that bright light was more effective than the control treatment (12 studies, 275 patients, SMD ‐0.50, CI ‐0.75 to ‐0.25), and the statistical significance remained even when a random effects model was applied (SMD ‐0.47, CI ‐0.86 to ‐0.08). If other devices, e.g. lighted rooms, were used, there was a trend for control treatment being better than light treatment but the result did not reach statistical significance (5 studies, 157 patients, SMD 0.21, CI ‐0.11 to 0.52).
There was no difference in contrasts between bright light and control treatment groups in terms of intensity of bright light (more than 2500 lux: 8 studies, 198 patients; 2500 lux maximum: 8 studies, 133 patients) or duration of light exposure (more than one hour: 13 studies, 368 patients; one hour or less: 4 studies, 123 patients).
As only one of the two studies assessing geriatric patients could provide rating scale scores for the meta‐analysis, and none of the studies had evaluated young patients, the issue of age could not be evaluated as yet.
Studies with a higher methodological quality rating (category A) showed unequivocal superiority of bright light over control treatment (2 studies, 50 patients, SMD ‐0.90, CI ‐1.50 to ‐0.31), even with a more conservative random effects model approach (SMD ‐0.90, CI ‐1.50 to ‐0.31). The statistical significance of studies with lower methodological quality (category B) was weaker and did not reach statistical significance when analyzed with a fixed effect model (16 studies, 455 patients, SMD ‐0.12, CI ‐0.31 to 0.07).
Two studies (Fritzsche 2001a, Fritzsche 2001b) had recruited a small number of seasonal patients also. The treatment response to bright light was not better in these studies than in studies that had applied non‐seasonal patients only.
Robustness of findings was tested in dichotomous outcomes in which it was assumed that drop‐outs were treatment failures. If drop‐outs of unknown reason were not considered as treatment failures, the result changed in none of the reanalyses in comparison to the primary analyses.
Patient expectations Assessment of patient expectations was reported in two studies only (Mackert 1990; Kripke 1992).
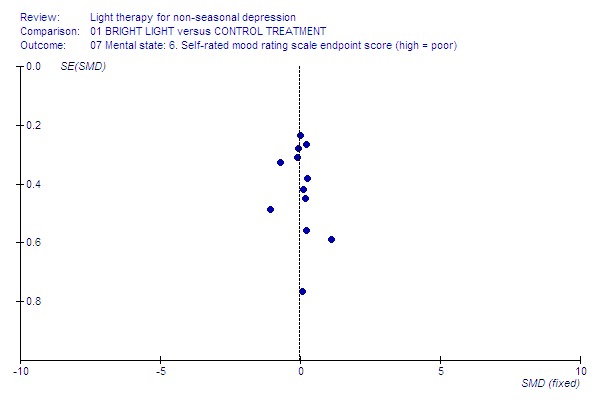
Funnel plot for publication bias In some of the comparisons with only one study it was not possible to undertake the proposed funnel plot for publication bias. For the outcomes for which the funnel plots were possible (see Additional figures in Figures Figure 1; Figure 2; Figure 3; Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12; Figure 13; Figure 14; Figure 15), visual inspection did not show any suggestion of asymmetry.
1.
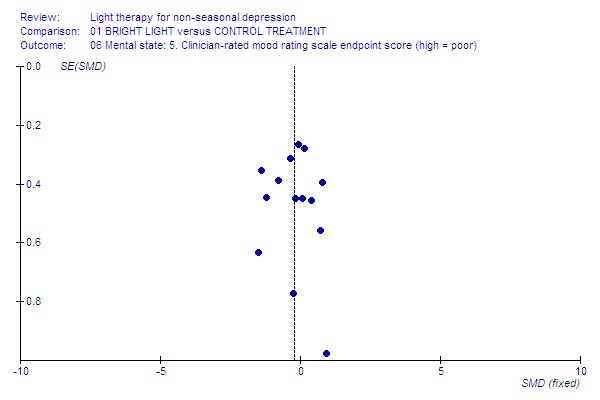
Clinician‐rated.
2.
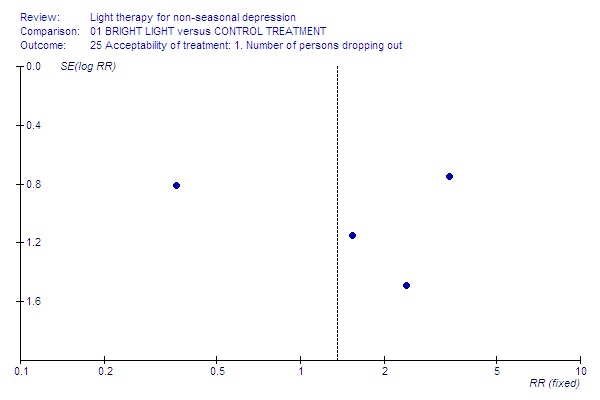
Drop‐outs.
3.
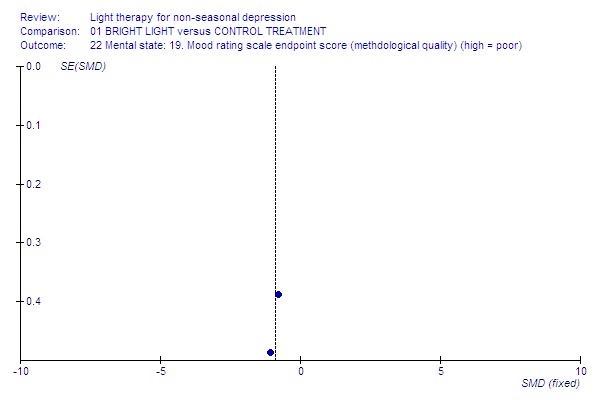
High‐quality studies.
4.
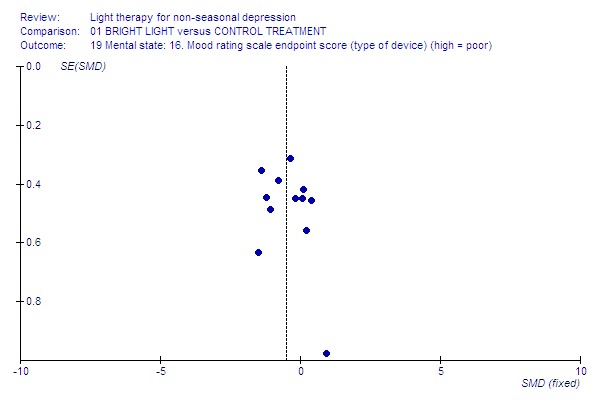
Light box.
5.
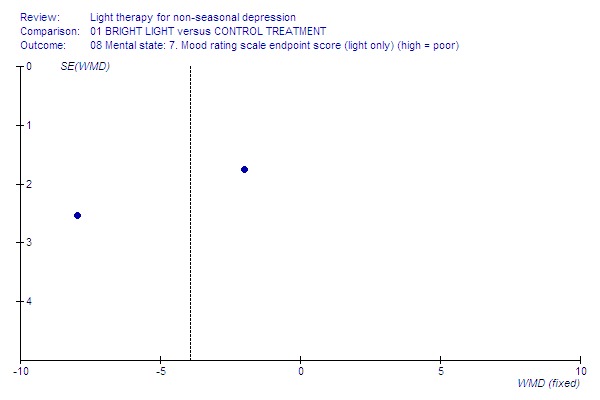
Light only.
6.
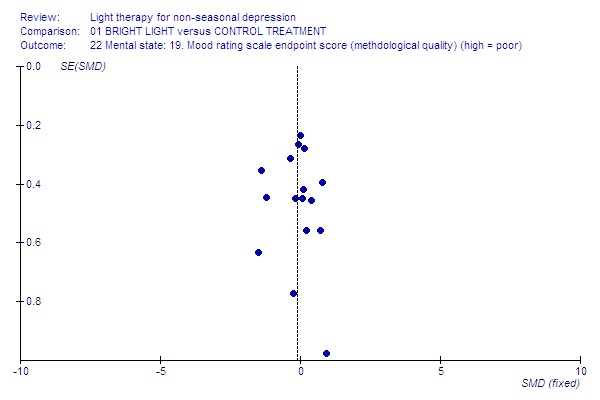
Low‐quality studies.
7.
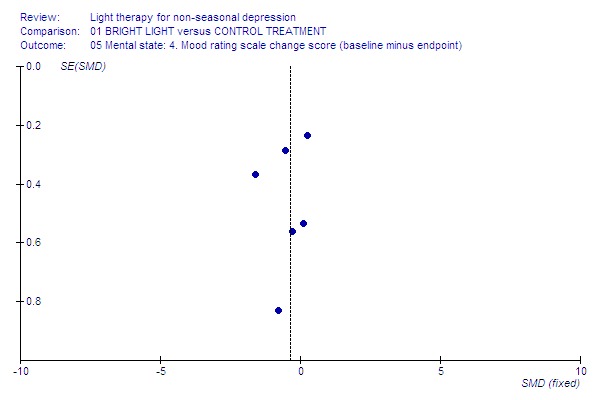
Mood change.
8.
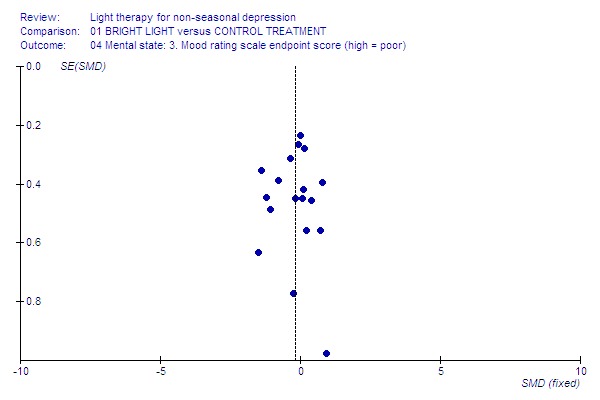
Mood endpoint.
9.
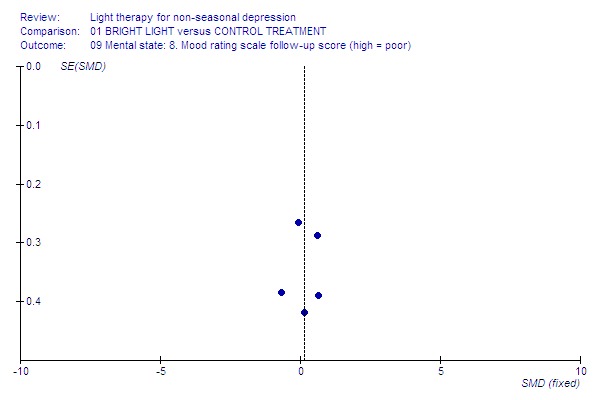
Mood follow‐up.
10.

Morning light.
11.

Non‐seasonals only.
12.
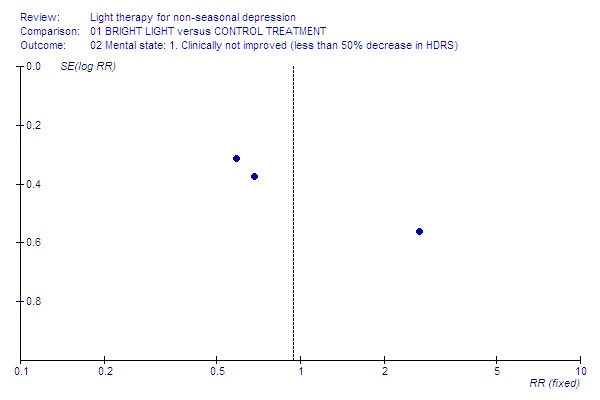
Not improved.
13.
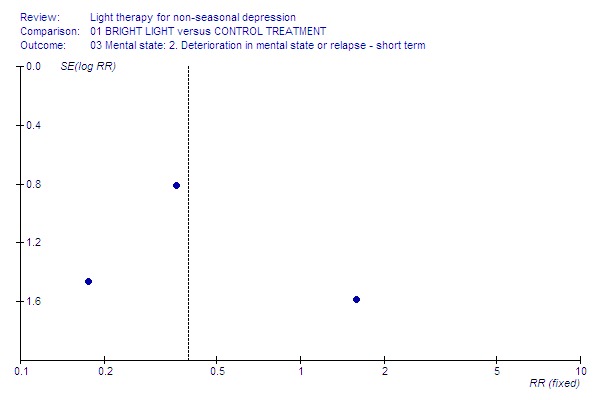
Relapse.
14.
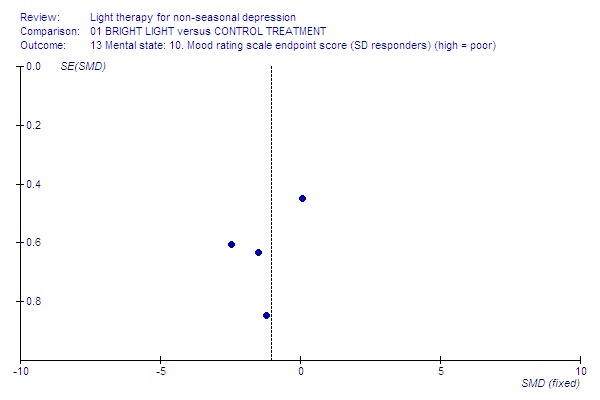
SD responders.
15.
Self‐rated.
Discussion
General comments The studies that were identified were generally of short duration, small (underpowered) and failed to report many outcomes in sufficient detail to allow pooling of all possible data.
This review benefited from extensive searches of the worldwide literature regarding light therapy as well as from personal contacts to the authors and other experts in the field. Previous research on the topic has been based on fewer studies: a recent systematic review on phototherapy for mood disorders (Gaynes 2003) had included four studies only and concluded that light was efficacious with effect sizes equivalent to those from antidepressant pharmacotherapy trials, whereas a previous meta‐analysis (Thompson 2002) had included four studies and failed to show efficacy. One more review (Kripke 1998) that had identified six studies showed that bright light treatment was effective especially as adjunctive treatment. Another major strength of our systematic review was that we only included randomized studies for non‐seasonal depression in our analysis. Light therapy for seasonal depression will be studied in a forthcoming Cochrane review.
A major problem of this meta‐analysis is that the bright light treatment studies were not fully blind. Blinding of assessment in administration of light therapy is more difficult than in studies with drug intervention, since the active treatment due to its brightness looks dissimilar to the control treatment. Subjects cannot fail to perceive the treatment and cannot be literally blind to treatment, though they may not know which is the active treatment. This might be an issue causing bias towards the benefit of active treatment. Another weakness is that for several outcomes there was either minimal or no data from high quality randomized trials. Small numbers and therefore lack of power might have made many findings being prone to type II error (a masking of a real effect), and several important hypotheses were unanswered with confidence. Many of the papers lacked important information such as details about the population, randomization procedure, and number of dropouts. Several studies were reported more than once, including preliminary results and sub‐samples with post‐hoc analysis.
The design of bright light treatment studies was very variable, and surprisingly few studies had assessed bright light as the only intervention (Mackert 1990; Yamada 1995). The number and size of trials was particularly poor for analyzing clinical global improvement, deterioriation in mental state or relapse during treatment, and adverse effects. Change scores were available in six studies and follow‐up of the outcome in five studies only.
It was possible to perform intention to treat analyses by assuming that people who left early had negative outcomes. This procedure can introduce a bias that would make the treatment arms similar if they have an equal number of dropouts, or exaggerate their divergence if they have a differential dropout rate.
Generalizability of findings Concerning the generalizability of the main results of this review, patients in the trials seemed to be similar to those seen in clinical practice, in terms of presence or absence of concurrent major depression, duration of illness, settings, and age groups.
Reporting on concealment of allocation Randomization and blindness were not well reported. The studies usually declared only randomization protocol but did not report how this procedure was performed. If blindness was declared, it was not always reported who was blind. Due to the nature of bright light treatment, it is difficult to keep patients blinded to treatment choice, even though they might have equal expectations towards active and control treatment. Very few studies had studied patients' expectations towards the treatment (Mackert 1990; Kripke 1992).
The quality of included studies was in 'category B' in all but two studies reaching 'category A' (Benedetti 2003, Moffit 1993), suggesting that even these results that are presented in this systematic review might be prone to biases and an overestimate of effect. Poor reporting of the process and outcomes of the trials was common. Some studies had to be excluded due to a lack of information about possible randomization and the number of people randomized to various treatment arms. Using a more detailed reporting would have enabled us more data for inclusion in this review.
Global impression Global status was very poorly reported, and did not show any statistically significant benefit of either treatment option over the other one.
Mental state Treatment response analyzed by primary mood rating scale endpoint scores and using a conservative statistical approach was modestly though not statistically significantly better in the bright light group compared to the control treatment group. This finding was mainly due to the result based on short term studies. Analyzing the few studies which employed the criterion of 50% decrease in the HDRS score, there was no significant difference between groups, whereas those patients that were treated with bright light showed a trend towards less deterioration in mental state or relapse during the treatment than those receiving control treatment.
Adverse effects Hypomania and sleep onset difficulties were more prevalent in patients receiving bright light treatment (showing a risk of one out of 8 patients to develop hypomania in the bright light group). Agitation, headache, blurred vision and eye irritation showed also a trend to be more prevalent in the bright light group. It needs to be considered that the study providing the most comprehensive data for adverse effects (Holsboer 1994) was evaluating bright light treatment adjunct to trimipramine, a tricyclic antidepressant with anticholinergic properties which might influence pupil size. Hence the adverse effects reported in this review might not be 'pure' effects caused by bright light itself. Also, although the study was randomized, the group receiving bright light had significantly poorer prognostic factors, such as duration of illness, at baseline. One more reason for a possible bias might be lack of reporting of adverse effects by most of the studies. Also, it needs to be acknowledged that categorizing the drop‐out subjects as "failures" might overestimate the number of subjects with adverse effects.
Acceptability of treatment Based on limited evidence from our meta‐analysis, bright light and control treatment seemed to be equally acceptable, as evidenced by the overall dropout rates from the study.
Quality of life, economic evaluation and long term studies Randomized studies assessing the quality of life of patients receiving bright light therapy or economic evaluation of the treatment were not found.
Mortality Despite the association of depression with suicide and deliberate self harm, these outcomes were not reported.
Subgroup analyses Long term evaluation studies were missing. Thus it was not possible to evaluate whether long term treatment effects differed in their results from trials evaluating short term treatment. There was a trend for studies evaluating short term effects to show a slightly more beneficial effect than studies evaluating medium term studies. These studies do indicate that bright light may be effective in as little as one week.
As only two studies (Schuchardt 1992; Loving 2002) used the outpatient setting in their study and only one of them provided outcome scores for the meta‐analysis, the inpatient versus outpatient studies were not compared.
Light therapy trials using concomitant sleep deprivation showed that bright light was more beneficial than the control treatment for sleep deprivation responders. Mainly the finding was due to short term studies. In studies with sleep deprivation nonresponders, there was no difference between bright light and control treatment groups.
There was a trend for bright light being more effective in those patients receiving concomitant drug therapy compared to the group without drug therapy, but with a more conservative approach the studies applying concomitant drug treatment lost statistical significance.
Administration of bright light in the morning showed that light treatment was more beneficial than control treatment, whereas light treatment given at other times of the day or night did not show any statistically significant benefit over the control treatment group. Morning light treatment without concomitant sleep deprivation was slightly more effective than the combination of light treatment and sleep deprivation. The efficacy of morning bright light over control treatment was equal in groups with and without concomitant drug therapy.
Trials using the light box showed that bright light treatment was more effective than control treatment compared to the results of trials using other devices.
Trials with higher intensity of light treatment (> 2500 lux) did not differ in their results from trials with lower intensity of light.
Trials with longer duration of light treatment did not differ in their results from trials with shorter duration of light, though duration and intensity may have been confounded.
Only two trials (Moffit 1993; Sumaya 2001) had studied geriatric subjects, and outcome scores of the first treatment arm were still missing in one of them; none of the trials had used very young subjects. Hence, it was not possible to evaluate whether trials using very old or very young subjects differed in their results from trials using adult subjects. However, the study with very old subjects that already has been included (Moffit 1993) did report positive results.
Based on two high quality studies only (Moffit 1993; Benedetti 2003), studies with higher methodological quality showed a more significant efficacy of bright light compared to control treatment than studies with lower methodological quality.
The treatment response to bright light was not better in two studies with both seasonal and non‐seasonal patients (Fritzsche 2001a; Fritzsche 2001b) than in studies that had applied non‐seasonal patients only.
Authors' conclusions
Implications for practice.
1. For clinicians
Our general conclusion is that the benefit of light treatment is modest though promising for non‐seasonal depression. Although the clinical efficacy of bright light over control treatment was modest, light administered in the morning or among sleep deprivation responders was beneficial for treatment response. In general, the main effect was found in short term studies, which might indicate a more rapid action than with antidepressant drugs. A light box might be a preferable device to administer bright light. Hypomania as a possible adverse effect needs to be considered. Our findings need to be interpreted with caution, because in bright light treatment studies truly blindness is very difficult to achieve, as well as because included studies were from various settings, short and medium term only and very heterogeneous in treatment methods, patient groups, and outcomes.
A wide range of durations and intensities of bright light were applied. High versus low daily duration and intensity of light did not show any superiority over each other. It also needs to be remembered that previous research has shown that these variables are interrelated and possibly confounding, i.e., the higher the intensity, the shorter the duration is effective.
Our review covered all forms of non‐seasonal depression. The benefit of bright light in specific forms of depression and in various age groups was not possible to evaluate sufficiently. In particular, there was an absence of RCTs for more than four weeks of treatment. Long term effects of light treatment in both therapeutic and maintenance indications should be evaluated in future light treatment trials.
2. For people with depression
Bright light administered in the morning is likely to benefit in the treatment of non‐seasonal depression. Most of the studies have used it as an adjunct therapy, and especially people who respond to sleep deprivation might benefit from bright light. In general, short term effect of bright light was slightly better than longer term effect. A light box is an effective device to administer light treatment. More information is needed regarding various forms of depression, different age groups, and the therapeutic value of light for treatment and maintenance purposes.
3. For policy makers
There are no data regarding the long term effect of bright light therapy in non‐seasonal depression, or the impact of bright light on health service utilization and costs. For example, it was not possible to evaluate whether bright light treatment shortens hospital length of stay, though brighter hospital rooms have been associated with shorter duration of hospitalization in two studies (Beauchemin 1996; Benedetti 2001), which could not be included in the meta‐analysis due to variability in their interventions.
This review highlights the need for funding agencies, industry, and regulatory authorities to collaborate to ensure that future clinical trials utilize the information from previous studies on this topic. These agencies should commission or access existing evidence that closely examines treatment issues for other conditions treated with bright light. They should then ensure that this information informs the design of clinical trials at the planning stages. Such an intervention would be likely to decrease any real or perceived bias regarding the tolerability and efficacy studies of bright light in the future.
Implications for research.
The majority of existing randomized studies were short term, in‐hospital trials focusing on clinical outcomes. Short studies may underestimate both adverse effects and global efficacy.
More trials are clearly needed to assess the advantages and disadvantages of bright light treatment, especially in outpatient settings. Trials need to be of longer duration than existing ones to find out the enduring effect of bright light on both the acute symptoms and on the chronic illness and its impact on a person's life. Concurrent economic evaluations are required to assess the cost implications of this treatment in clinical practice, both for the patients themselves and the health care system.
Light trials need improvement in concealment of allocation, randomization and blinding in a rigorous manner. These procedures should be reported sufficiently to allow the critical reader to be sure that each of these potential sources of bias are dealt with. The impossibility of achieving true double‐blindness in these trials is outlined above so an honest acknowledgement of these problems will lead to more rigorous and believable research evidence. Patient expectation questionnaires should be used.
Studies should be planned to cover special target populations of non‐seasonal depression such as treatment‐resistant depression, different types of depression, first episode of depression, child and adolescent as well as old age depressive symptoms to give information on the results in each subgroup of patients. More information is needed on the timing of light as well as the benefit of adjunctive treatments such as drugs or sleep deprivation.
Bright light treatment should be evaluated by trying to find an optimal intervention for the patient in terms of time of day of bright light, duration and intensity of treatment. At least the studies of longer duration should extend to the patient's own environment where the effect and limitations caused by the treatment are more clearly seen.
Investigators should be encouraged to use widely accepted rating scales, with acceptable validity and reliability. Systematic symptom rating scales encourage researchers to report data on a continuous form, but rating scales that evaluated adverse effects are often not normally distributed and may be problematic in the data analysis. Where possible, additional use of dichotomous outcome measures is to be encouraged. Dichotomous outcomes such as relapse, discontinuation, and readmission may be of direct relevance to clinicians and policy makers. These outcome measures could be collected at no extra cost to experimenters.
Reasons for discontinuation should be reported in detail. Absence of occurrence of deaths and serious life‐threatening adverse effects should routinely be reported explicitly. 'Intention to treat' analysis should be undertaken and reported in sufficient detail to allow the reader to be sure that it is in fact what took place. 'Last observation carried forward' or other methods should be used to include the patient data of as many participants as possible into the endpoint data analysis.
The adverse effect profile is likely to affect not only safety but also longer‐term compliance and quality of life. The inclusion of outcome measures such as quality of life, satisfaction with treatment, relapse and readmission would also allow meaningful economic evaluation to be presented.
Data should preferably be presented in tables with means and standard deviations and including the actual number of patients studied. In cases where binary outcomes can be used, these should be encouraged, provided that relevant cut‐off points can be presented. Data change between baseline and endpoint stages would be informative, but endpoint scores are needed to make inter‐study comparisons more accessible.
Feedback
Feedback‐ October 2006
Summary
We noted that there was extreme heterogeneity for the lower quality studies in fig. 35, e.g. Bloching had an SMD of ‐1.38 (‐2.07 to ‐0.68), i.e. a very large, significantly beneficial effect, whereas Holsboer had an SMD of 0.80 (0.02 to 1.57), i.e. a large, significantly harmful effect. The distance between the two non‐overlapping confidence intervals is also very large, 0.70. This suggests that one of these estimates is unlikely to be correct.
We also noted that in Holsboer?s table 2, after week 5‐6, the values are given as means 14.07 and 8.54, and SDs 5.83 and 8.35, whereas you reported means 14.50 and 8.64 and SDs 5.59 and 8.38. We were unable to find the data you entered in Holsboer?s table 2.
Reply
In response to the first point made:
In our Methods section we have stated as follows:
"Although in our protocol we stated that we would only use data that met our criteria 1. and 2., during the analysis, given the scarcity of results, we decided to include also data which did not meet the criterion 2. We performed an additional sensitivity analysis to study the effect of this procedure and have indicated this in the Results section."
The funnel plot of mood endpoint scores (Fig 8) did not show any asymmetry.
In response to the second point made:
The values of the comment are obtained from one publication that states that the endpoint values are mean of week 5/ week 6 ratings. In another publication (Holsboer's thesis in German) we found the endpoint values that are obtained immediately after the cessation of light therapy, at Day 35. We preferred to use these endpoint values because in other included studies as well we have used endpoint values immediately after the end of intervention.
Contributors
Gøtzsche PC, Hróbjartsson A, Marić K, Tendal B Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 2 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 3 January 2004 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review is in the process of being updated. We hope to publish the updated version in Issue 2, 2008.
Acknowledgements
We would like to thank the CCDAN editorial base for valuable help and support, especially for advice on the search strategy and the search for this review, and Professor John Geddes and Dr Kristian Wahlbeck for helpful comments on the protocol. The following colleagues have provided invaluable help in retrieving data for the review and are acknowledged: Sonia Ancoli‐Israel, Francesco Benedetti, Susan Benloucif, Benedikt Bloching, Maria Corral, Henner Giedke, Namni Goel, Siegfrid Kasper, Raymond Lam, Richard Loving, Donald Moss, Alexander Neumeister, Barbara Parry, Jan Prasko, Alexander Putilov, Martina Reide, Isabel Sumaya, Lukasz Swiecicki, Anna Wirz‐Justice, Naoto Yamada, Gina Zirpoli.
Data and analyses
Comparison 1. BRIGHT LIGHT versus CONTROL TREATMENT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1. CGI endpoint score (high = poor) ‐ medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Mental state: 1. Clinically not improved (less than 50% decrease in HDRS) | 3 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.61, 1.46] |
| 2.1 Short term | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.43] |
| 2.2 Medium term | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.62, 1.77] |
| 3 Mental state: 2. Deterioration in mental state or relapse ‐ short term | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.31] |
| 4 Mental state: 3. Mood rating scale endpoint score (high = poor) | 18 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.01] |
| 4.1 Short term | 12 | 367 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.44, ‐0.02] |
| 4.2 Medium term | 6 | 138 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.45, 0.24] |
| 5 Mental state: 4. Mood rating scale change score (baseline minus endpoint) | 6 | 198 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.64, ‐0.06] |
| 6 Mental state: 5. Clinician‐rated mood rating scale endpoint score (high = poor) | 14 | 376 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.44, ‐0.01] |
| 6.1 Short term | 9 | 258 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.61, ‐0.10] |
| 6.2 Medium term | 5 | 118 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.33, 0.42] |
| 7 Mental state: 6. Self‐rated mood rating scale endpoint score (high = poor) | 12 | 388 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.24, 0.17] |
| 7.1 Short term | 9 | 320 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.24, 0.20] |
| 7.2 Medium term | 3 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.60, 0.37] |
| 8 Mental state: 7. Mood rating scale endpoint score (light only) (high = poor) | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐3.93 [‐6.75, ‐1.11] |
| 9 Mental state: 8. Mood rating scale follow‐up score (high = poor) | 5 | 189 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.14, 0.44] |
| 9.1 Short term | 3 | 131 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.11, 0.58] |
| 9.2 Medium term | 2 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.58, 0.50] |
| 10 Mental state: 9. Mood rating scale endpoint score (concomitant SD) (high = poor) | 17 | 454 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.43, ‐0.04] |
| 10.1 Concomitant SD | 9 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.47, 0.02] |
| 10.2 Unclear SD (1‐to‐2 hours before wakeup time) | 2 | 21 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.50, 1.28] |
| 10.3 No SD | 6 | 167 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.66, ‐0.02] |
| 13 Mental state: 10. Mood rating scale endpoint score (SD responders) (high = poor) | 4 | 63 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.60, ‐0.45] |
| 13.1 Short term | 3 | 43 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐2.60, ‐1.07] |
| 13.2 Medium term | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.81, 0.95] |
| 14 Mental state: 11. Mood rating scale endpoint score (SD nonresponders) (high = poor) | 3 | 45 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.85, 0.36] |
| 14.1 Short term | 2 | 25 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.14, 0.53] |
| 14.2 Medium term | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐1.06, 0.70] |
| 15 Mental state: 12. Mood rating scale endpoint score (concomitant drug) (high = poor) | 18 | 463 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.42, ‐0.04] |
| 15.1 Concomitant drug | 14 | 329 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.47, ‐0.02] |
| 15.2 No drug | 4 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.53, 0.17] |
| 16 Mental state: 13. Mood rating scale endpoint score (time of day of bright light) (high = poor) | 18 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.36, ‐0.00] |
| 16.1 Morning light | 11 | 297 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.62, ‐0.14] |
| 16.2 Evening light | 2 | 43 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.25, 1.01] |
| 16.3 All‐night light | 2 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.45, 0.42] |
| 16.4 Both morning and evening light | 2 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.83, 0.25] |
| 16.5 Various times | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.24, 0.75] |
| 17 Mental state: 14. Mood rating scale endpoint score (concomitant SD and morning light) (high = poor) | 10 | 290 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.62, ‐0.14] |
| 17.1 Concomitant SD, morning light | 5 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.59, 0.03] |
| 17.2 No SD, morning light | 5 | 124 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.91, ‐0.16] |
| 18 Mental state: 15. Mood rating scale endpoint score (concomitant drug and morning light) (high = poor) | 11 | 297 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.62, ‐0.14] |
| 18.1 Concomitant drug, morning light | 9 | 243 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] |
| 18.2 No drug, morning light | 2 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.14, ‐0.01] |
| 19 Mental state: 16. Mood rating scale endpoint score (type of device) (high = poor) | 17 | 432 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.43, ‐0.03] |
| 19.1 Light box | 12 | 275 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.75, ‐0.25] |
| 19.2 Other device | 5 | 157 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.11, 0.52] |
| 20 Mental state: 17. Mood rating scale endpoint score (intensity of bright light) (high = poor) | 16 | 431 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.46, ‐0.06] |
| 20.1 Higher than 2500 lux | 8 | 198 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.55, 0.04] |
| 20.2 Not more than 2500 lux | 8 | 233 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.53, 0.00] |
| 21 Mental state: 18. Mood rating scale endpoint score (duration of bright light) (high = poor) | 17 | 491 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.40, ‐0.04] |
| 21.1 More than 1 hour | 13 | 368 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.45, ‐0.03] |
| 21.2 Not more than 1 hour | 4 | 123 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.53, 0.19] |
| 22 Mental state: 19. Mood rating scale endpoint score (methdological quality) (high = poor) | 18 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.01] |
| 22.1 Higher quality studies (Category A) | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.50, ‐0.31] |
| 22.2 Lower quality studies (Category B) | 16 | 455 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.31, 0.07] |
| 23 Mental state: 20. Mood rating scale endpoint score (mixed study sample) (high = poor) | 18 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.01] |
| 23.1 Both non‐seasonal and seasonal patients | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.68, 0.57] |
| 23.2 Non‐seasonal patients only | 16 | 465 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.40, ‐0.02] |
| 24 Mental state: 21. Primary mood rating scale baseline scores | Other data | No numeric data | ||
| 24.1 Baseline scores | Other data | No numeric data | ||
| 25 Acceptability of treatment: 1. Number of persons dropping out | 16 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.60, 3.07] |
| 25.1 Short term | 10 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.55, 3.20] |
| 25.2 Medium term | 6 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.16, 14.66] |
| 26 Adverse effects: 1. Cardiovascular system related | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26.1 Hypotension | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Adverse effects: 2. Endocrinologic system related | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 27.1 Sweating | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28 Adverse effects: 3. Gastrointestinal system related | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Constipation | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.40] |
| 28.2 Decreased appetite | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.46] |
| 28.3 Diarrhea | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 67.91] |
| 28.4 Dry mouth | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.45] |
| 28.5 Increased appetite | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.21] |
| 28.6 Nausea | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 67.91] |
| 28.7 Salivation | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.8 Stomach pain | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.82] |
| 29 Adverse effects: 4. Mood related | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 Hypomania | 7 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [1.66, 14.46] |
| 29.2 Mania | 6 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.07, 1.76] |
| 30 Adverse effects: 5. Nervous system related | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Agitation | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.95, 10.89] |
| 30.2 Confusion | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.39 [0.78, 14.69] |
| 30.3 Disorientation | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.55] |
| 30.4 Headache | 3 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.91, 5.59] |
| 30.5 Restlessness | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.6 Sedation | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.82] |
| 30.7 Vertigo | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 95.61] |
| 31 Adverse effects: 6. Sleep related | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 Disturbed sleep | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Sleep onset difficulties | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [1.13, 19.31] |
| 32 Adverse effects: 7. Urinary system related | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 32.1 Miction complaints | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 Adverse effects: 8. Vision related | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 Blurred vision | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.73, 6.78] |
| 33.2 Eye irritation | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.97, 12.88] |
| 34 Adverse effects: 9. Complaint List or FSUCL endpoint score (high = poor) | 2 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.26, 0.68] |
| 34.1 Short term | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.63, 0.59] |
| 34.2 Medium term | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐0.19, 1.33] |
| 35 Death | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 Short term | 8 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Medium term | 5 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
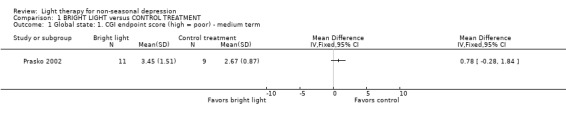
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 1 Global state: 1. CGI endpoint score (high = poor) ‐ medium term.
1.2. Analysis.
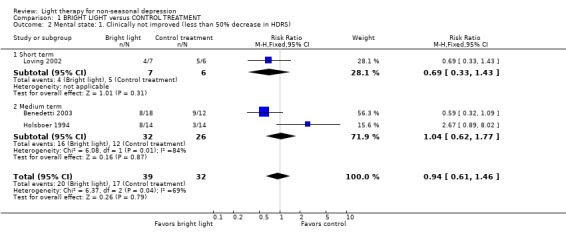
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 2 Mental state: 1. Clinically not improved (less than 50% decrease in HDRS).
1.3. Analysis.
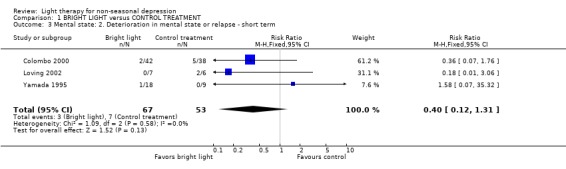
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 3 Mental state: 2. Deterioration in mental state or relapse ‐ short term.
1.4. Analysis.
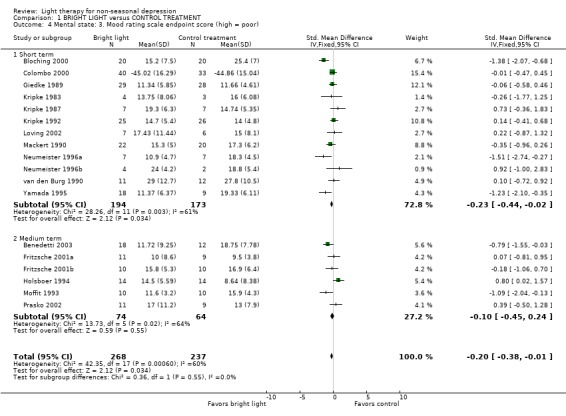
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 4 Mental state: 3. Mood rating scale endpoint score (high = poor).
1.5. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 5 Mental state: 4. Mood rating scale change score (baseline minus endpoint).
1.6. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 6 Mental state: 5. Clinician‐rated mood rating scale endpoint score (high = poor).
1.7. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 7 Mental state: 6. Self‐rated mood rating scale endpoint score (high = poor).
1.8. Analysis.
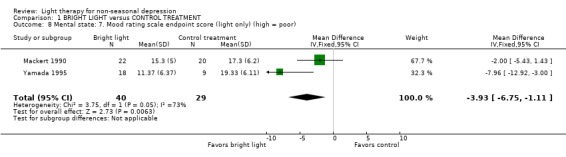
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 8 Mental state: 7. Mood rating scale endpoint score (light only) (high = poor).
1.9. Analysis.
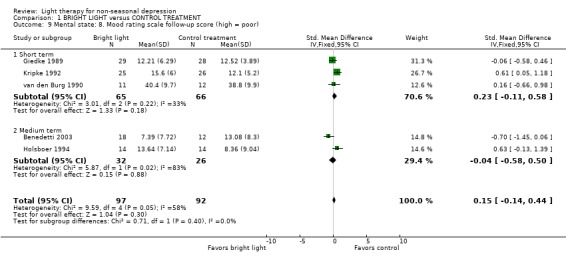
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 9 Mental state: 8. Mood rating scale follow‐up score (high = poor).
1.10. Analysis.
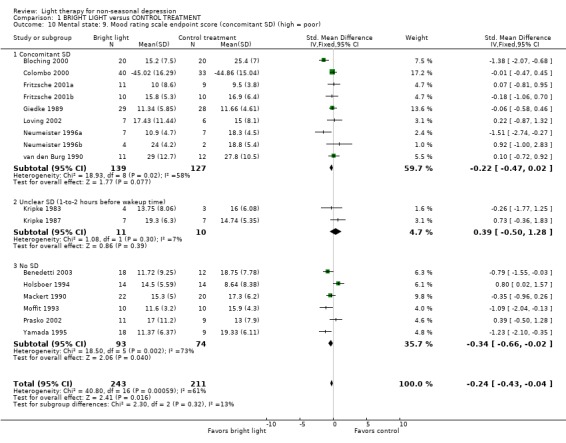
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 10 Mental state: 9. Mood rating scale endpoint score (concomitant SD) (high = poor).
1.13. Analysis.
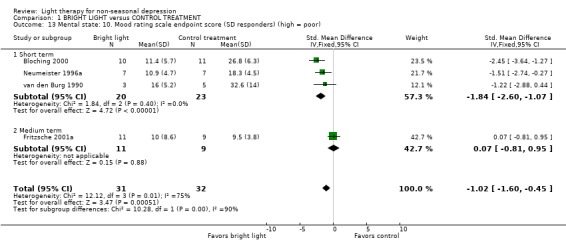
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 13 Mental state: 10. Mood rating scale endpoint score (SD responders) (high = poor).
1.14. Analysis.
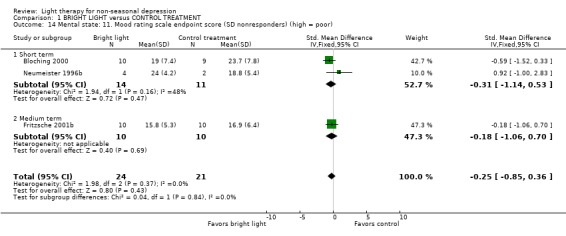
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 14 Mental state: 11. Mood rating scale endpoint score (SD nonresponders) (high = poor).
1.15. Analysis.
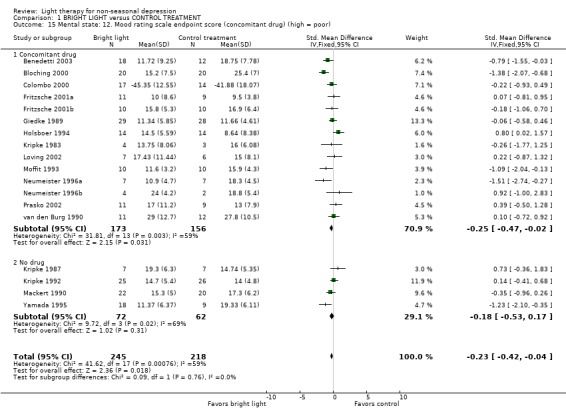
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 15 Mental state: 12. Mood rating scale endpoint score (concomitant drug) (high = poor).
1.16. Analysis.
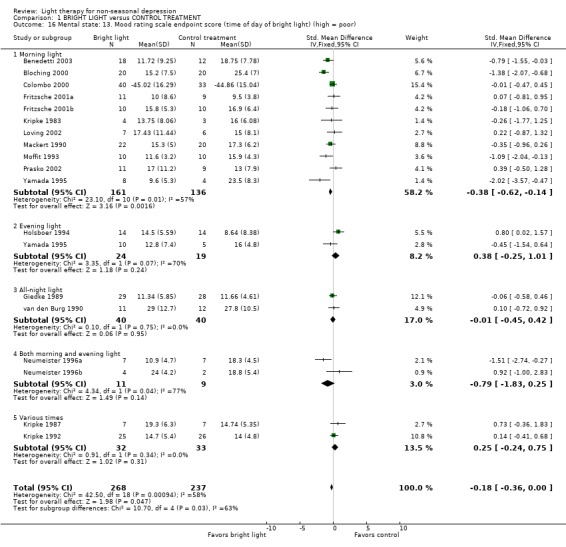
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 16 Mental state: 13. Mood rating scale endpoint score (time of day of bright light) (high = poor).
1.17. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 17 Mental state: 14. Mood rating scale endpoint score (concomitant SD and morning light) (high = poor).
1.18. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 18 Mental state: 15. Mood rating scale endpoint score (concomitant drug and morning light) (high = poor).
1.19. Analysis.
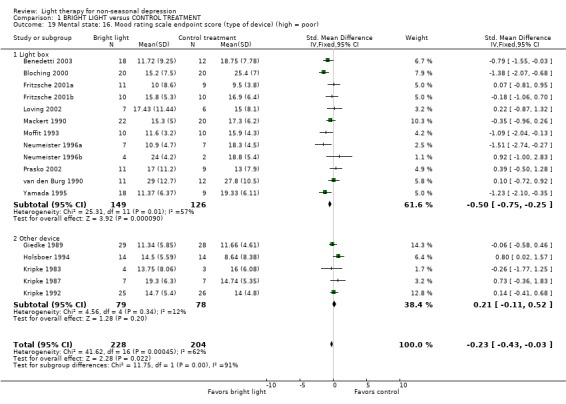
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 19 Mental state: 16. Mood rating scale endpoint score (type of device) (high = poor).
1.20. Analysis.
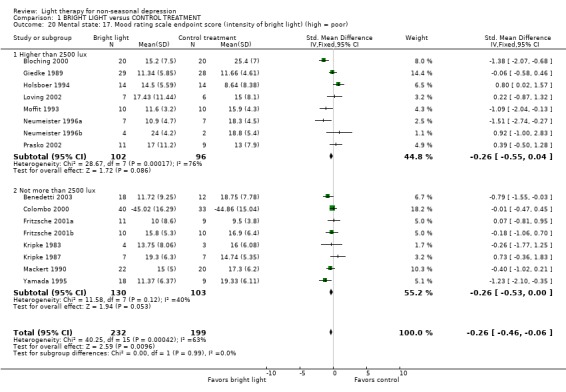
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 20 Mental state: 17. Mood rating scale endpoint score (intensity of bright light) (high = poor).
1.21. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 21 Mental state: 18. Mood rating scale endpoint score (duration of bright light) (high = poor).
1.22. Analysis.
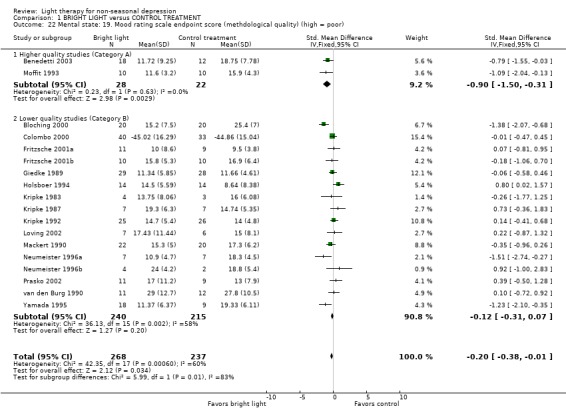
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 22 Mental state: 19. Mood rating scale endpoint score (methdological quality) (high = poor).
1.23. Analysis.
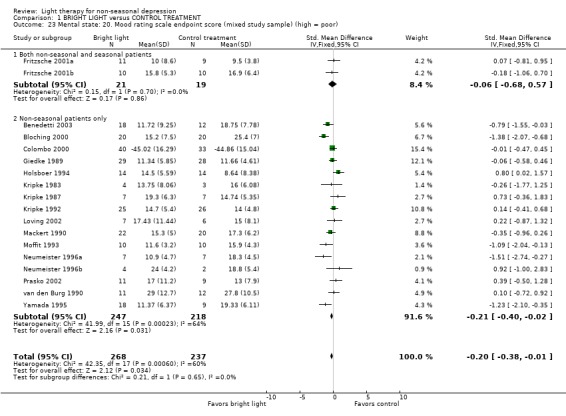
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 23 Mental state: 20. Mood rating scale endpoint score (mixed study sample) (high = poor).
1.24. Analysis.
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 24 Mental state: 21. Primary mood rating scale baseline scores.
| Mental state: 21. Primary mood rating scale baseline scores | |||
|---|---|---|---|
| Study | Bright light | Control treatment | Comments |
| Baseline scores | |||
| Benedetti 2003 | N = 18 Mean: 23.72 SD: 6.90 | N = 12 Mean: 22.58 SD: 4.89 | Rating scale: HDRS |
| Benedetti 2003 | |||
| Bloching 2000 | N = 20 Mean: 27.3 SD: 5.5 | N = 20 Mean: 29.4 SD: 6.2 | Rating scale: HDRS |
| Bloching 2000 | |||
| Colombo 2000 | N = 40 Mean: 22.43 SD: 12.68 | N = 33 Mean: 26.27 SD: 13.35 | Rating scale: VAS (mean of three daily ratings) |
| Colombo 2000 | |||
| Fritzsche 2001a | N = 11 Mean: 24.0 SD: 7.0 | N = 9 Mean: 19.8 SD: 5.2 | Rating scale: HDRS |
| Fritzsche 2001a | |||
| Fritzsche 2001b | N = 10 Mean: 21.5 SD: 5.4 | N = 10 Mean: 21.5 SD: 6.2 | Rating scale: HDRS |
| Fritzsche 2001b | |||
| Giedke 1989 | N = 29 Mean: 16.67 SD: 4.12 | N = 28 Mean: 16.32 SD: 2.93 | Rating scale: HDRS |
| Giedke 1989 | |||
| Holsboer 1994 | N = 14 Mean: 22.69 SD: 5.25 | N = 14 Mean: 26.02 SD: 6.37 | Rating scale: HDRS (17‐item) |
| Holsboer 1994 | |||
| Kripke 1983 | N = 4 Mean: 16.50 SD: 9.04 | N = 3 Mean: 15.67 SD: 8.14 | Rating scale: HDRS |
| Kripke 1983 | |||
| Kripke 1987 | N = 7 Mean: 21.06 SD: 7.47 | N = 7 Mean: 17.18 SD: 4.31 | Rating scale: HDRS |
| Kripke 1987 | |||
| Kripke 1992 | N = 25 Mean: 18.3 SD: 5.2 | N = 26 Mean: 15.2 SD: 4.3 | Rating scale: HDRS (17‐item) |
| Kripke 1992 | |||
| Loving 2002 | N = 7 Mean: 24.0 SD: 9.85 | N = 6 Mean: 18.8 SD: 7.17 | Rating scale: HDRS (17‐item; self‐rated, part of SIGH‐SAD‐SR) |
| Loving 2002 | |||
| Mackert 1990 | N = 22 Mean: 19.5 SD: 4.1 | N = 20 Mean: 19.1 SD: 4.2 | Rating scale: HDRS |
| Mackert 1990 | |||
| Moffit 1993 | N = 10 Mean: 17.8 SD: 5.1 | N = 10 Mean: 16.3 SD: 4.4 | Rating scale: GDS |
| Moffit 1993 | |||
| Neumeister 1996a | N = 7 Mean: 11.1 SD: 3.1 | N = 7 Mean: 17.7 SD: 3.9 | Rating scale: HDRS (modified 17‐item version) |
| Neumeister 1996a | |||
| Neumeister 1996b | N = 4 Mean: 18.0 SD: 7.1 | N = 2 Mean: 18.8 SD: 2.9 | Rating scale: HDRS (modified 17‐item version) |
| Neumeister 1996b | |||
| Prasko 2002 | N = 11 Mean: 23.0 SD: 6.4 | N = 9 Mean: 24.7 SD: 3.8 | Rating scale: HDRS |
| Prasko 2002 | |||
| van den Burg 1990 | N = 11 Mean: 33.2 SD: 10.5 | N = 12 Mean: 28.4 SD: 9.5 | Rating scale: BDI |
| van den Burg 1990 | |||
| Yamada 1995 | N = 18 Mean: 17.6 SD: 4.7 | N = 9 Mean: 21.7 SD: 5.8 | Rating scale: HDRS |
| Yamada 1995 | |||
1.25. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 25 Acceptability of treatment: 1. Number of persons dropping out.
1.26. Analysis.
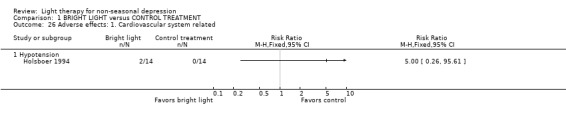
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 26 Adverse effects: 1. Cardiovascular system related.
1.27. Analysis.
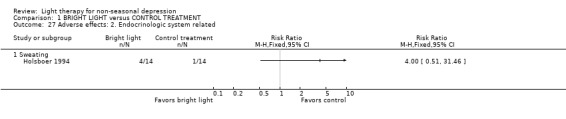
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 27 Adverse effects: 2. Endocrinologic system related.
1.28. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 28 Adverse effects: 3. Gastrointestinal system related.
1.29. Analysis.
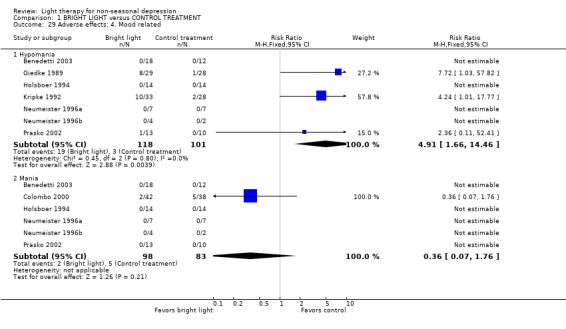
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 29 Adverse effects: 4. Mood related.
1.30. Analysis.
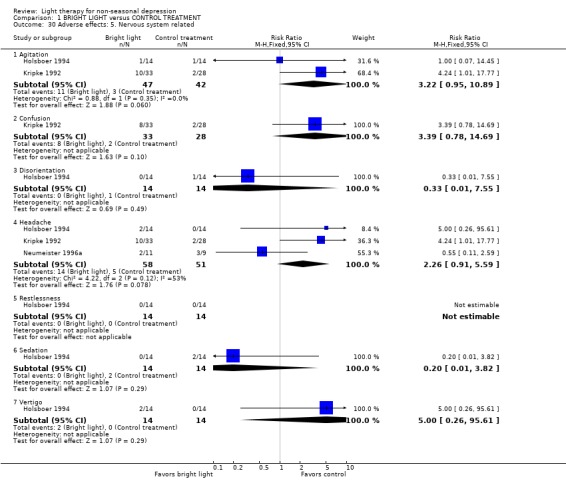
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 30 Adverse effects: 5. Nervous system related.
1.31. Analysis.
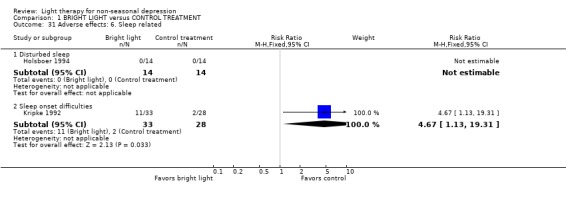
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 31 Adverse effects: 6. Sleep related.
1.32. Analysis.
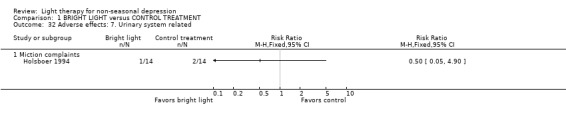
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 32 Adverse effects: 7. Urinary system related.
1.33. Analysis.

Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 33 Adverse effects: 8. Vision related.
1.34. Analysis.
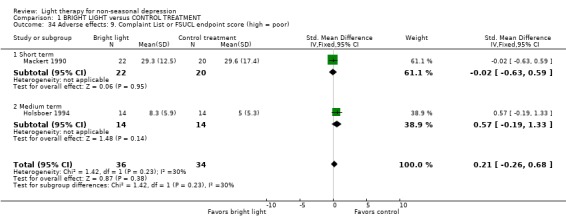
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 34 Adverse effects: 9. Complaint List or FSUCL endpoint score (high = poor).
1.35. Analysis.
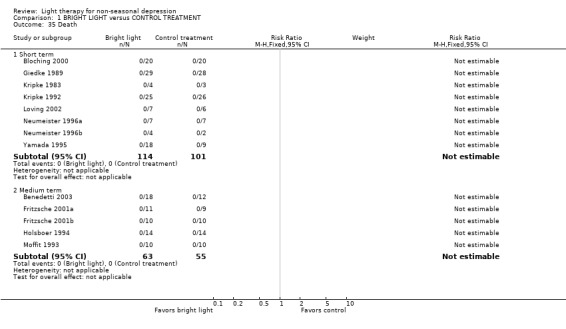
Comparison 1 BRIGHT LIGHT versus CONTROL TREATMENT, Outcome 35 Death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benedetti 2003.
| Methods | Allocation: randomized, 'computer generated randomization with no stratification', 'in a 3:2 manner'. Blinding: single blind, 'raters could not keep themselves blind due to patients' questions'. Duration: 4 weeks (light treatment the first 2 weeks). | |
| Participants | Diagnosis: Major depressive disorder without psychotic features. Major depression (N = 21), bipolar (N = 9). DSM‐IV. Inclusion criteria: absence of following conditions: other diagnoses on Axis I, mental retardation on Axis II, pregnancy, history of epilepsy, major medical or neurological disorder, treatment with long‐active neuroleptic drugs within 3 months, treatment with neuroleptics or irreversible MAOIs within the last month, history of drug or alcohol dependency or abuse within 6 months. N = 30. Age: mean 54.3 years. Sex: F 24, M 6. History: duration of illness mean 13.4 years. Setting: inpatients. | |
| Interventions | 1. Green light (400 lux in the morning for 30 minutes) + citalopram 40 mg/day. N = 18. 2. Deactivated negative ion generator (in the morning 1.5 hours after the optimal timing for light) + citalopram 40 mg/day. N = 12. Device: Sunnex green light box. | |
| Outcomes | Clinical improvement (50% reduction HDRS).
Mental state (HDRS).
Physiological monitoring (ECG, lab tests). Unable to use ‐ Mental state (ZDRS, VAS ‐ no mean scores and SD available). |
|
| Notes | Light adjunct to pharmacotherapy. Groups not totally comparable (active treatment patients were awaked earlier). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bloching 2000.
| Methods | Allocation: randomized, 'balanced parallel design'. Blinding: not stated. Duration: 7 days (followed by one night of LPSD). | |
| Participants | Diagnosis: Depressive disorder. Major depression (N = 35). DSM‐IV. Inclusion criteria: HDRS (21‐item) 15 or more. N = 40. Age: mean 53 years. Sex: F 24, M 16. Setting: inpatients. | |
| Interventions | 1. Bright light (minimum 2500 lux in the morning for 2 hours). N = 20. 2. Dim light (100 lux in the morning for 2 hours). N = 20. Device: light box. | |
| Outcomes | Mental state (AMS, HDRS, VAS). | |
| Notes | Light after LPSD of one night. Pharmacotherapy unchanged. Assesses LPSD respondents and nonrespondents separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Colombo 2000.
| Methods | Allocation: randomized. Blinding: single blind, 'raters could not keep themselves blind due to patients' questions'. Duration: 7 days (TSD treatments in 3 nights, each separated by recovery night sleep, light at 3 AM during the TSD night and in the morning of the recovery night). | |
| Participants | Diagnosis: Depressive episode. Bipolar disorder (N = 115). DSM‐IV. Inclusion criteria: HDRS (21‐item) more than 18. Absence of following conditions: other Axis I diagnosis, mental retardation on Axis II, pregnancy, history of epilepsy, major medical and neurological disorders, history of drug or alcohol dependency or abuse within the last 6 months. Exclusion criteria: long‐acting neuroleptic drugs in the last 6 months before admission, neuroleptics or irreversible MAOIs in the previous month. N = 115 (108 completers). Age: mean 45.8 years (completers). Sex: 72 F, 36 M. History: duration of illness mean 16.2 years (completers). Setting: inpatients. | |
| Interventions | 1. Bright white light (2500 lux in the morning for 60 minutes) + TSD + lithium. N = 17. 2. Bright white light (2500 lux in the morning for 60 minutes) + TSD. N = 23. 3. Red light (150 lux in the morning for 60 minutes) + TSD + lithium. N = 14. 4. Red light (150 lux in the morning for 60 minutes) + TSD. N = 19. 5. No additional light (ambient light 80 lux) + TSD + lithium. N = 15. 6. No additional light (ambient 80 lux) + TSD. N = 20. Device: not stated. | |
| Outcomes | Mental state (VAS). Physiological monitoring (ECG, lab). | |
| Notes | Light adjunct to either TSD plus pharmacotherapy or TSD alone. Stabilizing medication, if any, kept constant. Only interventions with light (1.‐4.) included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fritzsche 2001a.
| Methods | Allocation: randomized, 'TSD respondents and nonrespondents randomized separately'. Blinding: double blind, 'rater blind'. Duration: light therapy 14 days (duration of study 16 days). Light starting the 3rd day after TSD. TSD respondents. | |
| Participants | Diagnosis: Major depressive disorder. Recurrent (N = 14, single episode N = 3, depressive episode of bipolar I (N = 3). DSM‐IV. Inclusion criteria: TSD responders. HDRS (21‐item) 16 or more. Exlusion criteria: mood disorder due to organic reasons or with opthalmological disorders. N = 20. Age: mean 46.5 years. Sex: F 13, M 7. Setting: inpatients. | |
| Interventions | 1. Bright white light (2500 lux in the morning for 2 hours). N = 11. 2. Dim red light (50 lux in the morning for 2 hours). N = 9. Device: light box. | |
| Outcomes | Mental state (HDRS). Unable to use ‐ Clinical improvement (50% reduction HDRS ‐ no data available). Mental state (Bf‐S ‐ no mean and SD available). |
|
| Notes | Light after TSD of one night. Pharmacotherapy unchanged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fritzsche 2001b.
| Methods | Allocation: randomized, 'TSD respondents and nonrespondents randomized separately'. Blinding: double blind, 'rater blind'. Duration: light therapy 14 days (duration of study 16 days). Light starting on the 3rd day after TSD. TSD nonrespondents. | |
| Participants | Diagnosis: Major depressive disorder. Recurrent (N = 11), single episode (N = 9). DSM‐IV. Inclusion criteria: TSD nonresponders. HDRS (21‐item) 16 or more. Exclusion criteria: mood disorder due to organic reasons or with opthalmological disorders. N = 20. Age: mean 47.8 years. Sex: F 13, M 7. Setting: inpatients. | |
| Interventions | 1. Bright white light (2500 lux in the morning for 2 hours). N = 10. 2. Dim red light (50 lux in the morning for 2 hours). N = 10. Device: light box. | |
| Outcomes | Mental state (HDRS). Unable to use ‐ Clinical improvement (50% reduction HDRS ‐ no data available). Mental state (Bf‐S ‐ no mean and SD available). |
|
| Notes | Light after TSD of one night. Pharmacotherapy unchanged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Giedke 1989.
| Methods | Allocation: randomized, 'balanced crossover design'. Blinding: not stated. Duration: 1 day. | |
| Participants | Diagnosis: Major depressive disorder (N = 53), schizo‐affective disorder depressive type (N = 2), minor depression (N = 2. RDC, ICD‐9. Inclusion criteria: depressive symptoms due to organic or abuse reasons, HDRS (17‐item) 15 or more. Exclusion criteria: suicidality, productive schizo‐affective or schizophrenic psychosis, current physical illness. N = 57. Age: mean 47.6 years. Sex: F 44, M 13. History: duration of illness mean 11 years. Setting: inpatients. | |
| Interventions | 1. Bright light (5000 lux during the TSD night for 9 hours) + TSD. N = 29. 2. Normal room light (less than 300 lux during the SD for 9 hours). N = 28. Device: fluorescent light tubes. | |
| Outcomes | Mental state (D‐S, HDRS ‐ 13 item, M‐S). | |
| Notes | Light adjunct to TSD. Pharmacotherapy unchanged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Holsboer 1994.
| Methods | Allocation: randomized, 'randomly assigned to three treatment modalities'. Blinding: double blind, 'raters blind to treatment modality'. Duration: 6 weeks (light treatment 4 weeks). | |
| Participants | Diagnosis: Major depressive disorder. First episode (N = 12), recurrent (N = 23), bipolar (N=6), dysthymia (N = 1). DSM‐III‐R. Inclusion criteria: HDRS (17‐item) 18 or more. Exclusion criteria: medical illness, hormone replacement therapy. N = 42. Age: mean 52.1 years. Sex: F 20, M 22. History: duration of illness mean 7.7 years. Setting: inpatients. | |
| Interventions | 1. Bright light (5000 lux 5.30‐7.30 PM for 2 hours) + trimipramine. N = 14. 2. LPSD + trimipramine. N = 14. 3. Trimipramine. N = 14. Device: light box. | |
| Outcomes | Clinical improvement (50% reduction HDRS). Mental state (HDRS, MADRS, D‐S, VAS). Adverse effects (FSUCL). Physiological monitoring (ECG, lab, neuroendocrinological measurements). | |
| Notes | Light adjunct to pharmacotherapy. Only interventions 1. and 3. compared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kripke 1983.
| Methods | Allocation: randomized, 'counterbalanced crossover'. Blinding: not stated. Duration: 1 day. | |
| Participants | Diagnosis: Major depressive disorder (N = 8), bipolar II (N = 1), schizoaffective (N = 1), dysthymia (N = 1). N = 12. Age: not stated. Sex: not stated. Setting: inpatients. | |
| Interventions | 1. Bright white light (2500 lux 5‐6 AM for 1 hour). N = 4. 2. Dim red light (25 lux 5‐6 AM for 1 hour). N = 3. 3. Dim red light (25 lux 2‐3 AM for 1 hour). N = 5. Device: fluorescent bulbs in a frame. | |
| Outcomes | Mental state (HDRS, BDI). | |
| Notes | Light only. Minor SD (1‐to‐2 hours before wakeup time). About half of the sample on pharmacotherapy. Only interventions 1. and 2. compared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kripke 1987.
| Methods | Allocation: randomized, 'blocked randomization', crossover. Blinding: double blind, 'rater blind'. Duration: 5 days. | |
| Participants | Diagnosis: Major depressive disorder (N = 4), bipolar disorder (N=1), endogenous depression (N = 6), schizo‐affective disorder (N = 2), minor depressive disorder (N = 1). RDC. Inclusion criteria: HDRS (24‐item) and BDI at least 15. Exclusion criteria: psychotropic drugs. N = 15 (14 completers). Age: mean 46 years. Sex: F 1, M 14. Setting: inpatients. | |
| Interventions | 1. Bright light (1500‐2500 lux for 6 patients 5‐6 AM for 1 hour, for 8 patients 5‐6 AM and 9‐10 PM for 2 hours). N = 7. 2. Dim red light (50 lux for 6 patients 5‐6 AM for 1 hour, for 8 patients 5‐6 AM and 9‐10 PM for 2 hours). N = 7. Device: ceiling mounted lights. | |
| Outcomes | Mental state (HDRS, BDI, circadian self‐rating). | |
| Notes | Light only. Minor SD (1‐to‐2 hours before wakeup time). No pharmacotherapy except for one patient. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kripke 1992.
| Methods | Allocation: randomized, 'randomly assigned', 'for the last 16 subjects stratified by baseline Hamilton scores'. Blinding: double blind, 'rater blind'. Duration: 1 week. | |
| Participants | Diagnosis: Major depressive disorder or bipolar disorder. Major depression recurrent (N = 27) or single episode (N = 12), dysthymia (N = 13), atypical bipolar (N = 6), bipolar depressed (N = 4), bipolar manic (N = 1), cyclothymic (N = 1). DSM‐III. Inclusion criteria: adults, no psychotropic drugs for 10 days, no other drugs that might affect the treatment. HDRS (24‐item) and BDI at least 15. Exclusion criteria: trend toward seasonality. N = 61 (51 completers). Age: mean 48 years (completers). Sex: F 1, M 50. Setting: inpatients. | |
| Interventions | 1. Bright white light (2000‐3000 lux for 5 patients 1 hour in the morning and 1 hour in the evening, thereafter 3 hours in the evening). N = 25. 2. Dim red light (50 lux for 7 patients one hour in the morning and 1 hour in the evening, thereafter 3 hours in the evening). N = 26. Device: ceiling mounted lights. | |
| Outcomes | Mental state (HDRS, BDI, circadian self‐rating). Adverse effects (patient reports). Patient expectation (patient reports). | |
| Notes | Light only. Minor SD (1‐to‐2 ‐hours before wakeup time) for 25% of the patients. No pharmacotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Loving 2002.
| Methods | Allocation: randomized. Blindness: single blind, 'rater not blind'. Duration: 1 week (followed by one night of LPSD). | |
| Participants | Diagnosis: Major depressive disorder. DSM‐IV. Exclusion criteria: seasonal trait. N = 13. Age: mean 44 years. Sex: F 11, M 2. Setting: outpatients. | |
| Interventions | 1. Bright white light (10,000 lux in the morning for 30 minutes). N = 7. 2. Dim red light (100 lux in the morning for 30 minutes). N = 6. Device: light box. | |
| Outcomes | Mental state (self‐rated HDRS ‐ 17‐item) as part of the SIGH‐SAD‐SR). | |
| Notes | Light after LPSD of one night. Pharmacotherapy and supportive psychotherapy unchanged. One outlier in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mackert 1990.
| Methods | Allocation: randomized. Blindning: double blind, 'rater blind'. Duration: 7 days. | |
| Participants | Diagnosis: Major depressive disorder. RDC, ICD‐9. Exclusion criteria: seasonal depression, ophthalmological or oculomotor disorder, acute suicidality, IQ lower than 90. N = 42. Age: mean 54.2 years. Sex: F 34, M 8. Setting: inpatients. | |
| Interventions | 1. Bright white light (2500 lux 7‐9 AM for 2 hours). N = 22. 2. Dim red light (50 lux 7‐9 AM for 2 hours). N = 20. Device: light box. | |
| Outcomes | Mental state (HDRS, D‐S, D‐S', VAS, AMDP).
Adverse effects (C‐ L).
Patient expectation (subjective initial response).
Physiological monitoring (ECG, lab). Global improvement (CGI). |
|
| Notes | Light only. No pharmacotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moffit 1993.
| Methods | Allocation: randomized, 'sealed envelopes', 'block of 4', 'stratified on severity of depression'. Blinding: single blind, 'patients blind'. Duration: 10 days. | |
| Participants | Diagnosis: Depression. RDC. Inclusion criteria: 50 years or older, needing care for medical and/or psychiatric conditions. Exclusion criteria: severe visual impairment, history of ocular photosensitivity, red‐green color blindness, organic aggressive syndrome, bipolar disorder, schizophrenia, MMSE 15 or less. N = 20. Age: mean 73.9 years. Sex: not known. Setting: nursing home patients. | |
| Interventions | 1. Bright light (2500 lux 10‐12 AM for 2 hours). N = 10. 2. Dim red light (50 lux 10‐12 AM for 2 hours). N = 10. Ddevice: Light box. | |
| Outcomes | Mental state (GDS). Unable to use ‐ Cognitive function (MMSE ‐ no SD available). |
|
| Notes | Light only. Pharmacotherapy unchanged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Neumeister 1996a.
| Methods | Allocation: randomized. Blindning: double blind, 'raters blind to light condition'. Duration: 1 week, light therapy followed by one night of LPSD. LPSD responders. | |
| Participants | Diagnosis: Major depressive disorder (N = 14). DSM‐IV. Inclusion criteria: HDRS (17‐item) at least 40% reduction after LPSD. Exclusion criteria: seasonal pattern, mood disorders due to general medical condition, retinal disorders. N = 14. Age: mean 47.1 years. Sex: F 8, M 6. History: duration of illness mean 10.1 years. Setting: inpatients. | |
| Interventions | 1. Bright white light (3000 lux 7‐9 AM and 5‐7 PM). N = 7. 2. Dim light (100 lux (7‐9 AM, 5‐7 PM). N = 7. Device: light box. | |
| Outcomes | Mental state (HDRS ‐ 17‐item modified). Adverse effects. | |
| Notes | Light after LPSD of one night. Pharmacotherapy unchanged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Neumeister 1996b.
| Methods | Allocation: randomized. Blindning: double blind, 'raters blind to light condition'. Duration: 1 week, light therapy followed by one night of LPSD. LPSD nonresponders. | |
| Participants | Diagnosis: Major depressive disorder (N = 5), bipolar disorder (N = 1). DSM‐IV. Inclusion criteria: HDRS (17‐item) less than 40% reduction after LPSD. Exclusion criteria: seasonal pattern, mood disorders due to general medical condition, retinal disorders. N = 6. Age: mean 48.7 years. Sex: F 6, M 0. History: duration of illness mean 11.7 years. Setting: inpatients. | |
| Interventions | 1. Bright white light (3000 lux 7‐9 AM and 5‐7 PM). N = 4. 2. Dim light (100 lux 7‐9 AM and 5‐7 PM). N = 2. Device: light box. | |
| Outcomes | Mental state (HDRS ‐ 17‐item modified). Adverse effects. | |
| Notes | Light after LPSD of one night. Pharmacotherapy unchanged. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Prasko 2002.
| Methods | Allocation: randomized. Blindning: double blind. Duration: 3 weeks. | |
| Participants | Diagnosis: Major depressive disorder. DSM‐III‐R. Inclusion criteria: 20‐60 years, no seasonal pattern, at least 2 episodes of major depression in life time, and at least one episode during the last 2 years, at least one episode in another season, HDRS (21‐item) more than 20. Exclusion criteria: bipolar depression, panic disorder, alcoholism or drug abuse, antisocial or histrionic personality disorder, schizophrenia, organic brain impairment, mental retardation, physical illness or medical contranindications for imipramine, endocrine disease history, pregnancy, drugs causing depression during the past month, eye diseases. N = 34 (29 completers). Age: mean 42.6 years (completers). Sex: F 22, M 12. Setting: inpatients. | |
| Interventions | 1. Bright light (5000 lux 6‐8 AM) + imipramine 150 mg/day. N = 11. 2. Bright light (5000 lux 6‐8 AM) + imipramine‐like placebo. N = 9. 3. Dim red light (500 lux 6‐8 AM) + imipramine 150 mg/day. N = 9. Device: light box. Bright light device: | |
| Outcomes | Mental state (HDRS ‐ 21‐item, MADRS, BDI). Global state (CGI). | |
| Notes | Light adjunct to pharmacotherapy. Only interventions 1. and 3. compared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schuchardt 1992.
| Methods | Allocation: randomized, 'randomized list'. Blinding: double blind, 'blind raters'. Duration: 4 weeks. | |
| Participants | Diagnosis: Major depressive disorder. DSM‐III‐R. Exclusion criteria: seasonal pattern. N = 40. Age: not known. Sex: not known. Setting: outpatients. | |
| Interventions | 1. Bright light (2500 lux between 8 AM and 8 PM for 2 hours) + fluoxetine 20mg/day. N = not known. 2. Dim light (300 lux between 8 AM and 8 PM for 2 hours) + fluoxetine 20 mg/day. N = not known. Device: light box. | |
| Outcomes | Unable to use ‐ Mental state (HDRS, hypomania scales ‐ no mean scores and SD available). Authors contacted. | |
| Notes | Light adjunct to pharmacotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sumaya 2001.
| Methods | Allocation: randomized, crossover. Blinding: not stated. Duration: 5 days. | |
| Participants | Diagnosis: moderate to severe depression based on pretest score (GDS). Inclusion criteria: mentally autonomous state. Exclusion criteria: primary degenerative dementia, multi‐infarct dementia, photosensitizing medications, use of antidepressants, retinal problems. N = 11 (10 completers). Age: mean 83.8 years. Sex: F 7, M 4. Setting: long‐term care facility. | |
| Interventions | 1. Bright light (10,000 lux between 9 AM and 12:30 PM for 30 minutes). N = 4. 2. Dim light (300 lux between 9 AM and 12:30 PM for 30 minutes). N = 3. 3. No treatment. N = 4. Device: light box. | |
| Outcomes | Unable to use ‐ Mental state (GDS ‐ no mean scores and SD of the first phase of the crossover design available). Adverse effects (patient reports). Authors contacted. | |
| Notes | Light only. Only interventions 1. and 2. compared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
van den Burg 1990.
| Methods | Allocation: randomized, crossover. Blinding: not stated. Duration: 2 days (two separate nights with simultaneous TSD and light intervention, separated by one recovery night). | |
| Participants | Diagnosis: Major depression (N = 21), atypical bipolar disorder (N = 1), atypical depression without seasonality (N = 1). DSM‐III. Inclusion criteria: BDI more than 16 and interview. N = 28 (23 completers). Age: mean 47.2 years (completers). Sex: F 11, M 12. Setting: inpatients. | |
| Interventions | 1. Bright light (2000 lux from 11 PM to 7 AM for 8 hours) + TSD during two nights. N = 11. 2. Dim light (60 lux from 11 PM to 7 AM for 8 hours) + TSD during two nights. N = 12. Device: light box (bright light intervention), dimly lit room (control). | |
| Outcomes | Mental state (BDI, AMS). | |
| Notes | Light adjunct to TSD. Pharmacotherapy unchanged. Information on dropout groups insufficient. Results on completers only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yamada 1995.
| Methods | Allocation: randomized. Blinding: double blind, 'psychiatrist blind'. Duration: 7 days. | |
| Participants | Diagnosis: Major depressive disorder (N = 17), bipolar disorder (N = 10). DSM‐III‐R. Inclusion criteria: female in luteal phase or postmenopause. Exclusion criteria: seasonal pattern, acute suicidality, neurological or somatic illness, medications. N = 27. Age: mean 47.6 years. Sex: F 18, M 9. Setting: inpatients. | |
| Interventions | 1. Bright light (2500 lux 6‐8 AM or 6‐8 PM for two hours). N = 18. 2. Dim yellow light (500 lux 6‐8 AM or 6‐8 PM for 2 hours). N = 9. Device: light box. | |
| Outcomes | Mental state (HDRS ‐ 21‐item). Physiological monitoring (lab, body temperature). | |
| Notes | Light only. No pharmacotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
General abbreviations: ECG ‐ Electrocardiography F ‐ Female lab ‐ Laboratory LPSD ‐ Late partial sleep deprivation M ‐ Male MAOI ‐ Monoamine oxidase inhibitor SD ‐ Sleep deprivation TSD ‐ Total sleep deprivation
Diagnostic tools: ICD‐9 ‐ International Classification of Diseases, ninth revision DSM‐III ‐ Diagnostic and Statistical Manual of Mental Disorders, third edition DSM‐III‐R ‐ Diagnostic and Statistical Manual of Mental Disorders, third edition, revised DSM‐IV ‐ Diagnostic and Statistical Manual of Mental Disorders, fourth edition RDC ‐ Research Diagnostic Criteria
Global effect scales: CGI ‐ Clinical Global Impressions
Mental state scales: AMS ‐ Adjective Mood Scale (von Zerssen) AMDB ‐ Arbeitsgemeinschaft für Methodik und Dokumentation in der Psychiatrie BDI ‐ Beck Depression Inventory Bf‐S ‐ Befindlichkeits‐Skala (von Zerssen) D‐S ‐ Depression‐Skala (von Zerssen) HDRS ‐ Hamilton Depression Rating Scale MARDS ‐ Montgomery‐Asberg Depression Rating Scale M‐S ‐ Mood scale (von Zerssen) SIGH‐SAD‐SR ‐ Structured Interview Guide for the Hamilton Depression Rating Scale ‐ Seasonal Affective Disorder Version ‐ Self Report ZDRS ‐ Zung Depression Rating Scale
Adverse effect scales: C‐L ‐ Complaint List FSUCL ‐ Fischer's Somatic Symptom/Undesired Effect Checklist
Other scales: VAS ‐ Visual Analogue Scale
Cognitive‐psychomotor functions: MMSE ‐ Mini‐Mental State Examination
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beauchemin 1996 | Allocation: randomized. Participants: patients with non‐seasonal depression. Interventions: bright (sunlight) vs dim rooms (in brightly lit rooms variability in intensity of light (both at different times of a day and on sunny/cloudy days). |
| Beauchemin 1997 | Allocation: randomized. Participants: patients with non‐seasonal depression. Interventions: 10,000 lux vs 2,500 lux (also lower experimental level of light intensity is high and clearly active). |
| Benedetti 2001 | Allocation: randomized. Participants: patients with non‐seasonal depression. Interventions: bright (sunlight) vs dim rooms (in brightly lit rooms variability in intensity of light (both at different times a day and on sunny/cloudy days). |
| Benloucif 2002 | Allocation: not randomized. |
| Brown 2001 | Allocation: randomized. Participants: women with non‐seasonal depressive symptoms. Interventions: Tri‐modal intervention (walk+outdoor light+vitamin) vs placebo vitamin. |
| Corral 2001 | Allocation: randomized. Participants: postpartum depressives, more than 20% of the patients with seasonal depression. |
| Dietzel 1986 | Allocation: not randomized, case‐control design. |
| Gordijn 1998 | Allocation: not randomized, case‐control, crossover design. |
| Heim 1988 | Allocation: randomization not stated, crossover design. Participants: patients with cyclothymic axial syndrome. Intervention: bright light vs sleep deprivation. |
| Kasper 1989 | Allocation: balanced randomized. Participants: general population with seasonal difficulties of varying degrees. |
| Kripke 1981 | Allocation: quasi‐randomized. |
| Köhler 1989 | Allocation: randomization not stated. Participants: patients with seasonal depression. |
| Leibenluft 1995 | Allocation: non‐randomized, crossover, longitudinal comparison. |
| Martin 2001 | Allocation: randomized. Participants: nursing home patients, no clinical diagnosis of depression. |
| Neudorfer 1989 | Allocation: randomized. Participants: patients with non‐seasonal depression. Interventions: light therapy for depressive patients with atypical symptoms vs for depressive patients with classical symptoms. |
| Oren 2002 | Allocation: not randomized, open trial. |
| Pinchasov 2000 | Allocation: randomized. Participants: patients with seasonal or non‐seasonal depression. Interventions: bright light vs exercise (both active treatments). |
| Prasko 1988a | Allocation: randomization not stated. Authors contacted, no response as yet. |
| Prasko 1988b | Allocation: randomization not stated. Authors contacted, no response as yet. |
| Reide 1994 | Allocation: quasi‐randomized (alternation). |
| Stewart 1990 | Allocation: randomization not stated. Participants: More than 20% of the patients with seasonal depression. Contrast between patients with seasonal affective disorder and patients with atypical depression. |
| Stinson 1990 | Allocation: not randomized, open trial. |
| Thalén 2001 | Allocation: not randomized. |
| Wehr 1985 | Allocation: randomized. Participants: more than 20% of patients with seasonal depression. |
| Yerevanian 1986 | Allocation: not randomized, open trial. |
Characteristics of ongoing studies [ordered by study ID]
Goel 2001.
| Trial name or title | Bright light and negative ion treatment in patients with chronic depression. |
| Methods | |
| Participants | Diagnosis: Major depressive disorder (DSM‐IV). Inclusion criteria: medically healthy. No other Axis I disorders. Multicenter (Wesleyan University, the New York State Psychiatric Institute). |
| Interventions | 1. Bright light (10000 lux). N = 11. 2. Negative air ion exposure. N = 9. 3. Inactive treatment. N = unknown. |
| Outcomes | SIGH‐SAD. |
| Starting date | 2001. |
| Contact information | Dr. N. Goel, Wesleyan University, Middletown, CT 06459, USA. E‐mail: ngoel@wesleyan.edu. |
| Notes | Allocation: randomized.
Blindness: single‐blind.
Duration: unknown. Supported by NIH Grant and a Wesleyan Project Grant. |
Zirpoli 2002.
| Trial name or title | The sensitivity of melatonin to light suppression and light treatment in depressed and non‐depressed children. |
| Methods | |
| Participants | Diagnosis: Depression (DSM‐IV). Inclusion criteria: age 7‐18, no medication, no psychotic symptoms, no bipolar diagnosis, no recent history of substance abuse. |
| Interventions | 1. Morning light (7‐10 years: 2500 lux; 11‐18 years: 5000 lux). N = 11. 2. Evening light (age and light intensity as above). N = 10. 3. Dim red evening light (10 lux). N = 12. |
| Outcomes | SIGH‐SAD (both child and parent evaluation). CDI. Child Daily Mood Ratings. Expectation Questionnaire. Horne‐Ostberg Morningness‐Eveningness Questionnaire. Child psychiatrist evaluation. |
| Starting date | 1995. |
| Contact information | Dr. G. Zirpoli, Department of Psychiatry, University of California San Diego. E‐mail: gzirpoli@ucsd.edu. |
| Notes | Allocation: randomized.
Blindness: single‐blind.
Crossover study.
Duration: unknown. Supported in part by grants from the St.Giles Foundation, NARSAD, The Stanley Foundation, NIMH, NIH. |
Diagnostic tools: DSM‐IV = Diagnostic and Statistical Manual of Mental Disorders, fourth edition
Mental state scales: CDI = Child Depression Inventory HDRS = Hamilton Depression Rating Scale SIGH‐SAD = Structured Interview Guide for the Hamilton Depression Rating Scale‐SAD version
Contributions of authors
Arja Tuunainen ‐ initiated the study, wrote the initial protocol, took primary responsibility for data collection for the review with co‐reviewers, undertook data management for the review, was responsible for analysis and interpretation of the data with co‐reviewers, and wrote the review in collaboration with co‐reviewers. Arja Tuunainen is the guarantor of the protocol.
Daniel F. Kripke ‐ participated in conception and design of the study, commented on the initial protocol, collaborated with data collection, analysis, and interpretation, and participated in writing the review.
Takuro Endo ‐ commented on the initial protocol, collaborated with data collection, analysis, and interpretation, and participated in writing the review.
Sources of support
Internal sources
University of Helsinki, Finland.
Helsinki University Central Hospital (HUCH), Finland.
University of California San Diego (UCSD), USA.
Hokkaido University Graduate School of Medicine, Sapporo, Hokkaido, Japan.
External sources
Finnish Office of Health Care Technology Assessment (FinOHTA), Finland.
NIH/NIA (AG 12364), USA.
Declarations of interest
Arja Tuunainen ‐ None known.
Daniel F. Kripke ‐ No ownership interest in makers of lighting devices or light treatment. Past collaboration on grants with Apollo Lighting Systems and Synchrony Applied Health Sciences. Contribution of light boxes from Sunbox.
Takuro Endo ‐ None known.
Edited (no change to conclusions)
References
References to studies included in this review
Benedetti 2003 {published and unpublished data}
- Benedetti F, Colombo C, Pontiggia A, Bernasconi A, Florita M, Smeraldi E. Morning light treatment hastens the antidepressant effect of citalopram: a placebo‐controlled trial. Journal of Clinical Psychiatry 2003;64(6):648‐53. [DOI] [PubMed] [Google Scholar]
Bloching 2000 {published and unpublished data}
- Bloching B, Dechêne C, Täschner KL. Bright light stabilizes the antidepressant effect of late partial sleep deprivation. Society of Light Treatment and Biological Rhythms Abstracts. 2001.
- Bloching B, Dechêne C, Täschner KL. Outlasting antidepressant effect of late partial sleep deprivation by bright light therapy. Journal of Sleep Research 2000;9(Suppl 1):21. [Google Scholar]
Colombo 2000 {published and unpublished data}
- Benedetti F, Colombo C, Barbini B, Lucca A, Campori E, Cigala Fulgosi M, et al. Light and lithium to sustain the rapid effects of total sleep deprivation in bipolar depression. European Neuropsychopharmacology 2001;11(Suppl 2):52. [Google Scholar]
- Benedetti F, Colombo C, Barbini B, Smeraldi E. Light treatment effects in bipolar disorder. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 2001.
- Colombo C, Benedetti F, Barbini B, Campori E, Cigala Fulgosi M. Light, lithium and sleep phase advace to sustain the rapid effects of total sleep deprivation in bipolar depression. The World Journal of Biological Psychiatry 2001;2(Suppl 1):352. [Google Scholar]
- Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, Smeraldi E. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Research 2000;95(1):43‐53. [DOI] [PubMed] [Google Scholar]
Fritzsche 2001a {published data only}
- Fritzsche M, Heller R, Hill H, Kick H. Sleep deprivation as a predictor of response to light therapy in major depression. Journal of Affective Disorders 2001;62(3):207‐15. [DOI] [PubMed] [Google Scholar]
- Heller R, Fritzsche M, Hill H, Kick H. Sleep deprivation as a predictor of response to light therapy in major depression [Schlafentzug als prädiktor für das ansprechen auf lichttherapie bei major depression]. Fortschritte der Neurologie Psychiatrie 2001;69(4):156‐63. [DOI] [PubMed] [Google Scholar]
Fritzsche 2001b {published data only}
- Fritzsche M, Heller R, Hill H, Kick H. Sleep deprivation as a predictor of response to light therapy in major depression. Journal of Affective Disorders 2001;62(3):207‐15. [DOI] [PubMed] [Google Scholar]
- Heller R, Fritzsche M, Hill H, Kick H. Sleep deprivation as a predictor of response to light therapy in major depression [Schlafentzug als prädiktor für das ansprechen auf lichttherapie bei major depression]. Fortschritte der Neurologie Psychiatrie 2001;69(4):156‐63. [DOI] [PubMed] [Google Scholar]
Giedke 1989 {published and unpublished data}
- Bloching B. Lässt sich die antidepressive wirkung des schlafentzugs in hellem licht verbessern?. Tübingen: Druck Köhler, 1994. [Google Scholar]
- Giedke H, Bloching B. Therapeutic sleep deprivation in a brightly lit room. In: Horne J editor(s). Sleep '88. Stuttgart: Gustav Fischer Verlag, 1989:245‐7. [Google Scholar]
Holsboer 1994 {published data only}
- Holsboer‐Trachsler E. Neurobiologische und psychopatologische verlaufsmessungen bei depressionstherapie: trimipramin, schlafentzug und licht. Bibliotheca Psychiatrica, No. 166. Freiburg: Karger, 1994. [PubMed] [Google Scholar]
- Holsboer‐Trachsler E, Hemmeter U, Hatzinger M, Seifritz E, Gerhard U, Hobi V. Sleep deprivation and bright light as potential augmenters of antidepressant drug treatment ‐ neurobiological and psychometric assessment of course. Journal of Psychiatric Research 1994;28(4):381‐99. [DOI] [PubMed] [Google Scholar]
- Holsboer‐Trachsler E, Stohler R, Hatzinger M, Gerhard U, Hobi V, Witz‐Justice A. Bright light and sleep deprivation improve cognitive psychomotor performance in major depression. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1990:28.
- Müller MJ, Seifritz E, Hatzinger M, Hemmeter U, Holsboer‐Trachsler E. Side effects of adjunct light therapy in patients with major depression. European Archives in Psychiatry and Clinical Neuroscience 1997;247(5):252‐8. [DOI] [PubMed] [Google Scholar]
Kripke 1983 {published and unpublished data}
- Kripke DF, Risch SC, Janowsky DS. Lighting up depression. Psychopharmacology Bulletin 1983;19(3):526‐30. [PubMed] [Google Scholar]
Kripke 1987 {published and unpublished data}
- Kripke DF, Gillin JC, Mullaney DJ, Risch SC, Janowsky DJ. Five‐day bright light treatment of major depressive disorders. Sleep Research 1985;14:132. [Google Scholar]
- Kripke DF, Gillin JC, Mullaney DJ, Risch SC, Janowsky DS. Treatment of major depressive disorders by bright white light for 5 days. In: Halaris A editor(s). Chronobiology and Psychiatric Disorders. New York: Elsevier, 1987:207‐18. [Google Scholar]
- Kripke DF, Mullaney DJ, Gillin JC, Risch SC, Janowsky DS. Phototherapy of non‐seasonal depression. In: Shagass C, Josiassen RC, Bridger WH, Weiss KJ, Stoff D, Simpson GM editor(s). Biological Psychiatry 1985. Elsevier Science Publishing Co, 1986:993‐5. [Google Scholar]
Kripke 1992 {published and unpublished data}
- Kripke DF, Gillin JC, Mullaney DJ. Bright light benefit unrelated to REM latency. 142nd Annual Meeting of the American Psychiatric Association, New Research Program and Abstracts. 1989:137.
- Kripke DF, Gillin JC, Mullaney DJ. Depressive nonseasonal response to bright light. 141st Annual Meeting of the American Psychiatric Association. 1988:96.
- Kripke DF, Mullaney DJ, Gillin JC, Risch SC, Janowsky DS. Phototherapy of non‐seasonal depression. In: Shagass C, Josiassen RC, Bridger WH, Weiss KJ, Stoff D, Simpson GM editor(s). Biological Psychiatry 1985. Elsevier Science Publishing Co, 1986:993‐5. [Google Scholar]
- Kripke DF, Mullaney DJ, Klauber MR, Risch SC, Gillin JC. Controlled trial of bright light for nonseasonal major depressive disorders. Biological Psychiatry 1992;31(2):119‐34. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Mullaney DJ, Savides TJ, Gillin JC. Phototherapy for nonseasonal major depressive disorders. In: Rosenthal NE, Blehar NC editor(s). Seasonal Affective Disorders and Phototherapy. New York: Guilford, 1989:342‐56. [Google Scholar]
Loving 2002 {published and unpublished data}
- Loving RT, Kripke DF, Shuchter SR. Bright light augmentation of antidepressant medication. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1999; Vol. 11:33.
- Loving RT, Kripke DF, Shuchter SR. Bright light augments antidepressant effects of medication and wake therapy. Depression and Anxiety 2002;16(1):1‐3. [DOI] [PubMed] [Google Scholar]
Mackert 1990 {published data only}
- Baumgartner A, Volz HP, Campos‐Barros A, Stieglitz RD, Mansmann U, Mackert A. Serum concentrations of thyroid hormones in patients with nonseasonal affective disorders during treatment with bright and dim light. Biological Psychiatry 1996;40(9):899‐907. [DOI] [PubMed] [Google Scholar]
- Mackert A, Volz HP, Stieglitz RD, Müller‐Oerlinghausen B. Effect of bright light on non‐seasonal depressive disorder. Pharmacopsychiatry 1990;23(3):151‐4. [DOI] [PubMed] [Google Scholar]
- Mackert A, Volz HP, Stieglitz RD, Müller‐Oerlinghausen B. Light treatment of non‐seasonal affective disorder. Pharmacopsychiatry 1989;22:206. [Google Scholar]
- Mackert A, Volz HP, Stieglitz RD, Müller‐Oerlinghausen B. Phototherapy in nonseasonal depression. Biological Psychiatry 1991;30(3):257‐68. [DOI] [PubMed] [Google Scholar]
- Rao ML, Müller‐Oerlinghausen B, Mackert A, Stieglitz RD, Strebel B, Volz HP. The influence of phototherapy on serotonin and melatonin in non‐seasonal depression. Pharmacopsychiatry 1990;23(3):155‐8. [DOI] [PubMed] [Google Scholar]
- Rao ML, Müller‐Oerlinghausen B, Mackert A, Strebel B, Stieglitz RD, Volz HP. Blood serotonin, serum melatonin and light therapy in healthy subjects and in patients with nonseasonal depression. Acta Psychiatrica Scandinavica 1992;86(2):127‐32. [DOI] [PubMed] [Google Scholar]
- Volz HP, Mackert A, Stieglitz RD, Müller‐Oerlinghausen B. Are there differential effects of phototherapy on chronobiological parameters of endogenous depressives?. Pharmacopsychiatry 1989;22:220. [Google Scholar]
- Volz HP, Mackert A, Stieglitz RD, Müller‐Oerlinghausen B. Diurnal variations of mood and sleep disturbances during phototherapy in major depressive disorder. Psychopathology 1991;25(4):238‐46. [DOI] [PubMed] [Google Scholar]
- Volz HP, Mackert A, Stieglitz RD, Müller‐Oerlinghausen B. Effect of bright white light therapy on non‐seasonal depressive disorder. Journal of Affective Disorders 1990;19(1):15‐21. [DOI] [PubMed] [Google Scholar]
- Volz HP, Mackert A, Stieglitz RD, Müller‐Oerlinghausen B. Nebenwirkungen der phototherapie bei nichtsaisonal depressiven patienten. In: Gaebel W, Laux G editor(s). Biologische Psychiatrie Synopsis 1990/91. Berlin: Springer Verlag, 1992:363‐5. [Google Scholar]
- Votz HP, Mackert A, Stieglitz RD. Side‐effects of phototherapy in nonseasonal depressive disorder. Pharmacopsychiatry 1991;24(4):141‐3. [DOI] [PubMed] [Google Scholar]
Moffit 1993 {published data only}
- Moffit MT. Bright light treatment of late‐life depression. Dissertation thesis 1993.
- Moffit MT, Ancoli‐Israel S. Bright light treatment of late‐life depression. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1993:41.
Neumeister 1996a {published and unpublished data}
- Neumeister A, Goessler R, Lucht M, Kapitay T, Bamas C, Kasper S. Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biological Psychiatry 1996;39(1):16‐21. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Goessler R, Lucht M, Kasper S. Combination of sleep deprivation and light therapy. 150th Annual Meeting of the American Psychiatric Association. 1997:112.
- Neumeister A, Stastny J, Praschak‐Rieder N, Willeit M, Kasper S. Light treatment in depression (SAD, s‐SAD & non‐SAD). In: Holick MF, Jung EG editor(s). Biologic Effects of Light 1998. Boston: Kluwer Adacemic Publishers, 1999:409‐16. [Google Scholar]
Neumeister 1996b {published and unpublished data}
- Neumeister A, Goessler R, Lucht M, Kapitany T, Bamas C, Kasper S. Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biological Psychiatry 1996;39(1):16‐21. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Goessler R, Lucht M, Kasper S. Combination of sleep deprivation and light therapy. 150th Annual Meeting of the American Psychiatric Association. 1997:112.
- Neumeister A, Stastny J, Praschak‐Rieder N, Willeit M, Kasper S. Light treatment in depression (SAD, s‐SAD & non‐SAD). In: Holick MF, Jung EG editor(s). Biologic Effects of Light 1988. Boston: Kluwer Academic Publishers, 1999:409‐16. [Google Scholar]
Prasko 2002 {published data only}
- Prasko J, Baudis P, Klaschka J, Lestina J, Novotná D, Ondráková I, et al. Bright light therapy in patients with recurrent nonseasonal uniplar major depressive disorder ‐ double blind study. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1995; Vol. 7:48.
- Prasko J, Baudis P, Lestina J, Novotná D, Kosová J, Ondrácková I. Double‐blind study of bright light therapy and imipramine for major depressive disorder. Abstracts of the X World Congress of Psychiatry, Madrid, Spain. 1996; Vol. 2:248.
- Prasko J, Horacck J, Klaschka J, Kosova J, Ondrackova I, Sipek J. Bright light therapy and/or imipramine for inpatients with recurrent non‐seasonal depression. Neuroendocrinology Letters 2002;23(2):109‐13. [PubMed] [Google Scholar]
Schuchardt 1992 {published data only}
- Kasper S, Ruhrmann S, Schuchardt HM. The effects of light therapy in treatment indications other than seasonal affective disorder (SAD). In: Holick MF, Jung EG editor(s). Biologic Effects of Light 1993. Berlin: Walter de Gruyter & Co, 1994:206‐18. [Google Scholar]
- Schuchardt HM, Kasper S. Lichttherapie in der psychiatrischen praxis. Fortschritte der Neurologie Psychiatrie 1992;60(S2):193‐4. [Google Scholar]
- Schuchardt HM, Kasper S, Ruhrmann S. Is light therapy able to enhance the anti‐depressant effect of fluoxetine in patients with non‐seasonal major depression?. Pharmacopsychiatry 1993;26:201. [Google Scholar]
Sumaya 2001 {published and unpublished data}
- Sumaya IC, Rienzi BM, Deegan JF II, Moss DE. Bright light treatment decreases depression in institutionalized older adults: a placebo‐controlled crossover study. Journal of Gerontology: Medical Sciences 2001;56A(6):M356‐60. [DOI] [PubMed] [Google Scholar]
van den Burg 1990 {published data only}
- Burg W, Bouhuys AL, Hoofdakker RH, Beersma DGM. Sleep deprivation in bright and dim light: antidepressant effects on major depressive disorder. Journal of Affective Disorders 1990;19(2):109‐17. [DOI] [PubMed] [Google Scholar]
Yamada 1995 {published data only}
- Yamada N, Martin‐Iverson MT, Daimon K, Tsujimoto T, Takahashi S. Clinical and chronobiological effects of light therapy on nonseasonal affective disorders. Biological Psychiatry 1995;37(12):866‐73. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beauchemin 1996 {published data only}
- Beauchemin KH, Hays P. Sunny hospital rooms expedite recovery from severe and refractory depressions. Year Book of Psychiatry and Applied Mental Health. Vol. 7, Mosby‐Year Book Inc, 1998:214‐5. [Google Scholar]
- Beauchemin KM, Hays P. Sunny hospital rooms expedite recovery from severe and refractory depressions. Journal of Affective Disorders 1996;40(1‐2):49‐51. [DOI] [PubMed] [Google Scholar]
Beauchemin 1997 {published data only}
- Beauchemin KM, Hays P. Phototherapy is a useful adjunct in the treatment of depressed in‐patients. Acta Psychiatrica Scandinavica 1997;95(5):424‐7. [DOI] [PubMed] [Google Scholar]
Benedetti 2001 {published data only}
- Benedetti F, Colombo C, Barbini B, Campori E, Smeraldi E. Morning sunlight reduces lenght of hospitalization in bipolar depression. Journal of Affective Disorders 2001;62(3):221‐3. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C, Barbini B, Smeraldi E. Light treatment effects in bipolar disorder. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 2001.
Benloucif 2002 {published data only}
- Benloucif S, Ortiz R, Orbeta L, Keng M, Janssen I, Zee PZ. Evening light normalizes phase but morning light improves daytime performance in older adults. Sleep 2002;25:A59. [Google Scholar]
Brown 2001 {published data only}
- Brown MA, Goldstein‐Shirley J, Robinson J, Casey S. The effects of a multi‐modal intervention trial of light, exercise, and vitamins on women's mood. Women & Health 2001;34(3):93‐112. [DOI] [PubMed] [Google Scholar]
Corral 2001 {published data only}
- Corral M. Bright light therapy for postpartum depression. Canadian Psychiatric Association's Annual General Meeting Syllabus. 2002:84.
- Corral M. Non‐pharmacological treatments for postpartum depression: light therapy. Archives of Women's Mental Health. 2001; Vol. 3, issue Suppl 2:3.
- Corral M, Xanthoula K. Bright light therapy in the treatment of postpartum depression. World Psychiatric Association Meeting Abstracts. 2002:189‐90.
- Corral MR, Patton S, Kostaras X. Bright light treatment of postpartum depression. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 2001.
Dietzel 1986 {published data only}
- Dietzel M, Saletu B, Lesch OM, Sieghart W, Schjerve M. Light treatment in depressive illness. Polysomnographic, psychometric and neuroendocrinological findings. European Neurology 1986;25(Suppl 2):93‐103. [DOI] [PubMed] [Google Scholar]
Gordijn 1998 {published data only}
- Gordijn MC, Beersma DG, Korte HJ, Hoofdakker RH. Light therapy in depressed patients and controls: effects on sleep. Sleep‐wake research in the Netherlands. Leiden: Dutch Society for Sleep‐Wake Research, 1992; Vol. 3:61.
- Gordijn MC, Beersma DG, Korte HJ, Hoofdakker RH. Light therapy in non‐seasonal, depressed patients and controls: effects on sleep. Sleep Research 1991;20A:534. [Google Scholar]
- Gordijn MC, Beersma DG, Korte HJ, Hoofdakker RH. Testing the hypothesis of a circadian phase disturbance underlying depressive mood in nonseasonal depression. Journal of Biological Rhythms 1998;13(2):132‐47. [DOI] [PubMed] [Google Scholar]
Heim 1988 {published data only}
- Heim M. On the effectiveness of bright‐light therapy in cyclothymic axial syndromes examined in a cross‐over study against partial deprivation of sleep [Zur effizienz der bright‐light‐therapie bei zyklothymen achsensyndromen ‐ eine cross‐over‐studie gegenüber partiellem schlafentzug]. Psychiatrie Neurologie medical Psychologie 1988;40(5):269‐77. [PubMed] [Google Scholar]
Kasper 1989 {published data only}
- Kasper S, Rogers SL, Madden PA, Joseph‐Vanderpool JR, Rosenthal NE. The effects of phototherapy in the general population. Journal of Affective Disorders 1990;18(3):211‐9. [DOI] [PubMed] [Google Scholar]
- Kasper S, Rogers SL, Yancey A, Schulz PM, Skwerer RG, Rosenthal NE. Phototherapy in individuals with and without subsyndromal seasonal affective disorder. Archives of General Psychiatry 1989;46(9):837‐44. [DOI] [PubMed] [Google Scholar]
Kripke 1981 {published and unpublished data}
- Kripke DF. Photoperiodic mechanisms for depression and its treatment. In: Perris C, Struwe G, Jansson B editor(s). Biological Psychiatry 1981. Elsevier/North‐Holland Biomedical Press, 1981:1249‐52. [Google Scholar]
- Kripke DF, Risch SC, Janowsky D. Bright white light alleviates depression. Psychiatry Research 1983;10(2):105‐12. [DOI] [PubMed] [Google Scholar]
Köhler 1989 {published data only}
- Köhler WK, Pelzer A, Schmidt KP, Carella AB, Pflug B. Bright light and DIM light in the therapy of depression: effects on the circadian system and clinical results. Pharmacopsychiatry 1989;46:837‐44. [Google Scholar]
Leibenluft 1995 {published data only}
- Leibenluft E, Turner EH, Feldman‐Naim S, Matthews J, Wehr TA, Rosenthal NE. Bright light in rapid‐cycling bipolar disorder. 150th Annual Meeting of American Psychiatric Association, San Diego, USA. 1997:111‐2.
- Leibenluft E, Turner EH, Feldman‐Naim S, Schwartz PJ, Wehr TA, Rosenthal NE. Light therapy in patients with rapid cycling bipolar disorder: preliminary results. Psychopharmacology Bulletin 1995;31(4):705‐10. [PubMed] [Google Scholar]
Martin 2001 {published data only}
- Martin JL, Marler MM, Schochat T, Ancoli‐Israel S. Does light therapy improve depression in severe Alzheimer's disease?. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 2001.
Neudorfer 1989 {published data only}
- Neudorfer C, Schwitzer J, Schifferle I, Blecha HG, Meise U, Friedrich H, et al. Light therapy for non‐seasonal affective disorders with hypersomnia. Pharmacopsychiatry 1989;22:210‐1. [Google Scholar]
Oren 2002 {published data only}
- Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, et al. An open trial of morning light therapy for treatment of antepartum depression. American Journal of Psychiatry 2002;159(4):666‐9. [DOI] [PubMed] [Google Scholar]
- Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, et al. Morning light treatment for antepartum depression. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1999; Vol. 11:7.
Pinchasov 2000 {published and unpublished data}
- Pinchasov BB, Shurgaja AM, Grischin OV, Putilov AA. Effects of midday kinesitherapy and light therapy on mood, physical performance, and oxygen consumption in women with depression. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1997; Vol. 9:16.
- Pinchasov BB, Shurgaja AM, Grischin OV, Putilov AA. Mood and energy regulation in seasonal and non‐seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Research 2000;94(1):29‐42. [DOI] [PubMed] [Google Scholar]
Prasko 1988a {published and unpublished data}
- Prasko J. The acceleration of antidepressant's effects by using phototherapy in endogenous depression. Psychopharmacology 1988;96(Suppl):398. [Google Scholar]
- Prasko J, Foldmann P, Prasková H, Zindr V. Hastened onset of the effect of antidepressive drugs when using three types of timing of intensive white light [Urychlení nástupu úcinku antidepresiv pri pouzití trí druhu nacasování intenzívního bílého svetla]. Ceskoslovenska Psychiatrie 1988;84(6):373‐83. [PubMed] [Google Scholar]
- Prasko J, Goldmann P, Zindr R, Zindr V. Hastening the onset of action of tricyclic antidepressants by using bright white light [Urychlení nástupu úcinku tricyklických antidepresiv uzitím jasného bílého svetla]. Ceskoslovenska Psychiatrie 1987;83(6):376‐84. [PubMed] [Google Scholar]
Prasko 1988b {published and unpublished data}
- Prasko J. The acceleration of antidepressant's effects by using phototherapy in endogenous depression. Psychopharmacology 1988;96(Suppl):398. [Google Scholar]
- Prasko J, Foldmann P, Prasková H, Zindr V. Hastened onset of the effect of antidepressive drugs when using three types of timing of intensive white light [Urychlení nástupu úcinku antidepresiv pri pouzití trí druhu nacasování intensívního bílého svetla]. Ceskoslovenska Psychiatrie 1988;84(6):373‐83. [PubMed] [Google Scholar]
- Prasko J, Goldmann P, Zindr R, Zindr V. Hastening the onset of action of tricyclic antidepressants by using bright white light [Urychlení nástupu úcinku tricyklických antidepresiv uzitím jasného bílého svétla]. Ceskoslovenska Psychiatrie 1987;83(6):376‐84. [PubMed] [Google Scholar]
Reide 1994 {published and unpublished data}
- Reide M, Göhlert C. Lichttherapie in der behandlung depressive psychosen. Wiss Zeitschrift der Hubboldt‐Univerität zu Berlin. R. Medizin 1992;41(2):95‐8. [Google Scholar]
- Reide M, Göhlert C. Light therapy in the treatment of nonseasonal major depressive disorder. In: Holick MF, Jung EG editor(s). Biologic effects of light 1993. Berlin: Walter de Gruyter & Co, 1994:281‐6. [Google Scholar]
Stewart 1990 {published data only}
- Stewart JW, Quitkin FM, Terman M, Terman JS. Is seasonal affective disorder a variant of atypical depression?. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1989:22.
- Stewart JW, Quitkin FM, Terman M, Terman JS. Is seasonal affective disorder a variant of atypical depression? Differential response to light therapy. Psychiatry Research 1990;33(2):121‐8. [DOI] [PubMed] [Google Scholar]
Stinson 1990 {published data only}
- Stinson D, Thompson C. Clinical experience with phototherapy. Journal of Affective Disorders 1990;18(2):129‐35. [DOI] [PubMed] [Google Scholar]
Thalén 2001 {published data only}
- Thalén BE. Light treatment in seasonal and nonseasonal depression: diagnostic, clinical and neuroendocrine studies. Stockholm: Repro Print, 1996. [Google Scholar]
- Thalén BE, Kjellman BF, Moerkrid L, Wibom R, Wetterberg L. Light treatment in seasonal and nonseasonal depression. Acta Psychiatrica Scandinavica 1995;91(5):352‐60. [DOI] [PubMed] [Google Scholar]
- Thalén BE, Moerkrid L, Wetterberg L, Kjellman BF. Clinical and neuroendocrinological studies of the effect of light treatment in seasonal and nonseasonal depression. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 2001.
Wehr 1985 {published data only}
- Wehr TA, Rosenthal NE, Sack DA, Gillin JC. Antidepressant effects of sleep deprivation in bright and dim light. Acta Psychiatrica Scandinavica 1985;72(2):161‐5. [DOI] [PubMed] [Google Scholar]
Yerevanian 1986 {published data only}
- Yerevanian BI, Anderson JL, Grota LJ, Bray M. Effects of bright incandescent light on seasonal and nonseasonal major depressive disorder. Psychiatry Research 1986;18(4):355‐64. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Deltito 1991 {published data only}
- Deltito JA, Moline M, Pollak C, Curran MJ. The effect of bright light treatment on non‐SAD unipolar and bipolar spectrum depressed patients. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 1989:118.
- Deltito JA, Moline M, Pollak C, Martin LY, Maremmani I. Effects of phototherapy on non‐seasonal unipolar and bipolar depressive spectrum disorders. Journal of Affective Disorders 1991;23(4):231‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Goel 2001 {published and unpublished data}
- Goel N, Terman JS, Macchi MM, Stewart JW, Terman M. Bright light and negative ion treatments in patients with chronic depression. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 2001.
Zirpoli 2002 {published and unpublished data}
- Zirpoli G, Newton RP, Heyneman EK, Haynes P, Mostofi N, Tan JA, et al. The sensitivity of melatonin to light suppression and light treatment in depressed and non‐depressed children. Chronobiology International 2002;19(5):997‐8. [Google Scholar]
- Zirpoli G, Newton RP, Heyneman EK, Haynes P, Mostofi N, Tan JA, et al. The sensitivity of melatonin to light suppression and light treatment in depressed and non‐depressed children. Society of Light Treatment and Biological Rhythms (SLTBR) Abstracts. 2002; Vol. 14:30.
Additional references
Aitken 1969
- Aitken RCB. Measuring of feeling using visual analogue scales. Proceedings of the Royal Society of Medicine 1969;62:989‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beauchemin 1997
- Beauchemin KM, Hays P. Phototherapy is a useful adjunct in the treatment of depressed in‐patients. Acta Psychiatrica Scandinavica 1997;95(5):424‐7. [DOI] [PubMed] [Google Scholar]
Beauchemin 1998
- Beauchemin KH, Hays P. Sunny hospital rooms expedite recovery from severe and refractory depressions. Year Book of Psychiatry and Applied Mental Health. Vol. 7, Mosby‐Year Book Inc, 1998:214‐5. [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Brickenkamp 1962
- Brickenkamp R. Aufmerksamkeits‐Belastungstest d2. Göttingen: Hogrefe, 1962. [Google Scholar]
Campbell 1998
- Campbell SS. Bright light treatment of sleep maintenance insomnia and behavioral disturbance. In: Lam RW editor(s). Seasonal affective disorder and beyond: Light treatment for SAD and Non‐SAD conditions. Washington, DC: American Psychiatric Press, 1998:289‐304. [Google Scholar]
Cassem 1995
- Cassem EH. Depressive disorders in the medically ill: an overview. Psychosomatics 1995;36(2):S2‐10. [DOI] [PubMed] [Google Scholar]
Chesson 1999
- Chesson AL Jr, Littner M, Davila D, Anderson WM, Grigg‐Damberger M, Hartse K, et al. Practice parameters for the use of light therapy in the treatment of sleep disorders. Standards of Practice Committee, American Academy of Sleep Medicine. Sleep 1999;22(5):641‐60. [DOI] [PubMed] [Google Scholar]
CIPS 1986
- Collegium International Psychiatriae Scalarum (CIPS). Collegium International Psychiatriae Scalarum (CIPS). Weinheim: Beltz, 1986. [Google Scholar]
Cole 1989
- Cole RJ, Kripke DF. Amelioration of jet lag by bright light treatment: effects on sleep consolidation. Sleep Research 1989;18:411. [Google Scholar]
Dubovsky 1999
- Dubovsky SL, Buzan R. Mood disorders. In: Hales RE, Yudofsky SC, Talbott JA editor(s). Textbook of psychiatry. 3rd Edition. Washington, DC: American Psychiatric Press, 1999:479‐565. [Google Scholar]
Eastman 1999
- Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Annals of Medicine 1999;31(2):87‐98. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elkin 1989
- Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, et al. National Institute of Mental Health treatment of depression collaborative research program. General effectiveness of treatments. Archives of General Psychiatry 1989;46(11):971‐82. [DOI] [PubMed] [Google Scholar]
First 1995
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM‐IV. New York: New York State Psychiatric Institute, Biometrics Research, 1995. [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [DOI] [PubMed] [Google Scholar]
Gaynes 2003
- Gaynes BN, Ekstrom D, Hamer RM, Jacobsen FM, Nemeroff CB, Suppes P, et al. Phototherapy: systematic review of the evidence. American Psychiatric Association 2003 Annual Meeting, San Francisco, CA, USA. New research abstracts. 2003:152.
Goodwin 1982
- Goodwin FK, Wirz‐Justice A, Wehr TA. Evidence that the pathophysiology of depression and the mechanism of antidepressant drugs both involve alterations in circadian rhythms. Advances in Biochemical Psychopharmacology 1982;32:1‐11. [PubMed] [Google Scholar]
Guy 1976
- Guy W. Early Clinical Drug Evaluation Unit (ECDEU) assessment manual for psychopharmacology. Publication no 76‐338. Rockville, MD: National Institute of Mental Health, 1976. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 1996
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM‐III‐R major depressive disorder in the general population: results from the US National Comorbidity Survey. British Journal of Psychiatry ‐ Supplementum 1996;30:17‐30. [PubMed] [Google Scholar]
Khan 2000
- Khan A, Warner Ha, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials. Archives of General Psychiatry 2000;57(4):311‐28. [DOI] [PubMed] [Google Scholar]
Kripke 1981
- Kripke DF. Photoperiodic mechanisms for depression and its treatment. In: Perris C, Struwe G, Jansson B editor(s). Biological psychiatry. Elsevier‐North Holland: Biomedical Press, 1981:1249‐52. [Google Scholar]
Kripke 1998
- Kripke DF. Light treatment for nonseasonal depression: speed, efficacy, and combined treatment. Journal of Affective Disorders 1998;49(2):109‐17. [DOI] [PubMed] [Google Scholar]
Lam 1998
- Lam RW, Goldner EM. Seasonality of bulimia nervosa and treatment with light therapy. In: Lam RW editor(s). Seasonal affective disorder and beyond. Washington, DC: American Psychiatric Press, 1998:193‐220. [Google Scholar]
Lam 1999
- Lam RW, Levitt AJ. Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. Vancouver , CA: Clinical and Academic Publishing, 1999:1‐160. [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to changes. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman A. Cochrane Collaboration Handbook [Updated 1 March 1997]. The Cochrane Library [database on disk and CDROM].. Oxford: Update Software, 1997. [Google Scholar]
Mulrow 1999
- Mulrow CD, Williams JW Jr, Trivedi M, Chiquette E, Aguilar C, Cornell JE, et al. Treatment of Depression: Newer Pharmacotherapies. Evidence Report/Technology Assessment No. 7. (Prepared by the San Antonio Evidence‐based Practice Center based at The University of Texas Health Science Center at San Antonio under Contract 290‐97‐0012). AHCPR Publication No. 99‐E014. Rockville, MD: Agency for Health Care Policy and Research, 1999. [Google Scholar]
Murray 1996
- Murray CJ, Lopez AD. Evidence‐based health policy ‐ lessons from the global burden of disease study. Science 1996;274(5288):740‐3. [DOI] [PubMed] [Google Scholar]
Müller 1977
- Müller A. Aufmerksamkeits‐Prüf‐Gerät. APG. Handanweisung. Homburg: Saar, 1977. [Google Scholar]
Neumeister 1996
- Neumeister A, Goessler R, Lucht M, Kapitany T, Bamas C, Kasper S. Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biological Psychiatry 1996;39(1):16‐21. [DOI] [PubMed] [Google Scholar]
Parry 1998
- Parry B. Light therapy of premenstrual depression. In: Lam RW editor(s). Seasonal affective disorder and beyond. Washington, DC: American Psychiatric Press, 1998:173‐91. [Google Scholar]
Partonen 2000
- Partonen T, Lonnqvist J. Bright light improves vitality and alleviates distress in healthy people. Journal of Affective Disorders 2000;57(1‐3):55‐61. [DOI] [PubMed] [Google Scholar]
Priebe 1987
- Priebe S. Early subjective reactions predicting the outcome of hospital treatment in depressive patients. Acta Psychiatrica Scandinavica 1987;76(2):134‐8. [DOI] [PubMed] [Google Scholar]
Rosenthal 1989
- Rosenthal NE. Light therapy. In: Gabbard GO editor(s). Treatments of Psychiatric Disorders. Vol. 1, Washington, DC: APA Press, 1989:1263‐73. [Google Scholar]
Tam 1995
- Tam EM, Lam RW, Levitt AJ. Treatment of seasonal affective disorder: a review. Canadian Journal of Psychiatry 1995;40(8):457‐66. [DOI] [PubMed] [Google Scholar]
Terman 2001
- Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Archives of General Psychiatry 2001;58(1):69‐75. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson C. Light therapy in the treatment of seasonal and non‐seasonal affective disorders: a meta‐analysis of randomised controlled trials. In: Partonen T, Magnusson A editor(s). Seasonal affective Disorder. Practice and Research. Oxford: Oxford University Press, 2001:149‐58. [Google Scholar]
von Luckner 1985
- Luckner N von, Maurer M, Kuny S, Woggon B, Dittrich A. Comparison of AMP system and Comprehensive Psychopathological Rating Scale with regard to contents. Neuropsychobiology 1985;13(3):117‐20. [DOI] [PubMed] [Google Scholar]
von Zerssen 1983
- Zerssen BD, Koeller DM. Die Befindlichkeits‐Skala. Parallelform Bf‐S und Bf‐S. Weinheim: Beltz Test, 1983. [Google Scholar]
von Zerssen 1986
- Zerssen D. Clinical self‐rating scales (CSRS) of the Munich psychiatric information system (Psychis München). In: Sartorius N, Ban TA editor(s). Assessment of depression. Berlin: Springer Verlag, 1986:271‐303. [Google Scholar]
Williams 1990
- Williams JBW, Link MJ, Rosenthal NE, Terman M. Seasonal affective disorder assessment tools packet. In: Terman M editor(s). SLTBR 1988‐90: The Complete Works. New York: SLTBR, 1990:207‐242. [Google Scholar]
Yesavage 1983
- Yesavage JA, Brink TL, Rose TL, Adey M. The geriatric depression rating scale: comparison with other self‐report and psychiatric rating scales. In: Crook T, Ferris S, Bartus R editor(s). Assessment in geriatric psychopahrmacology. New Haven: Marc Powles Associates Inc., 1983:153‐67. [Google Scholar]
Zung 1965
- Zung WW. A self rating depression scale. Archives of General Psychiatry 1965;12:63‐70. [DOI] [PubMed] [Google Scholar]


