Abstract
Background
Fatigue is reported to occur in up to 92% of patients with multiple sclerosis (MS) and has been described as the most debilitating of all MS symptoms by 28% to 40% of MS patients.
Objectives
To assess whether carnitine (enteral or intravenous) supplementation can improve the quality of life and reduce the symptoms of fatigue in patients with MS‐related fatigue and to identify any adverse effects of carnitine when used for this purpose.
Search methods
A literature search was performed using Cochrane MS Group Trials Register (09 September 2011), Cochrane Central Register of Controlled Trials (CENTRAL) "The Cochrane Library 2011, issue 3", MEDLINE (PubMed) (1966‐09 September 2011), EMBASE (1974‐09 September 2011), and www.clinicaltrials.gov for ongoing trials retrieval. Reference lists of review articles and primary studies were also screened. A hand search of the abstract book of recent relevant conference symposia was also conducted. Personal contact with MS experts and a manufacturer (Source Naturals, United States) of carnitine formulation was contacted to determine if they knew of other clinical trials. No language restrictions were applied.
Selection criteria
Full reports of published and unpublished randomized controlled trials and quasi‐randomized trials of any carnitine intervention in adults affected by multiple sclerosis with a clinical diagnosis of fatigue associated with multiple sclerosis were included.
Data collection and analysis
Data from the eligible trials was extracted and coded using a standardized data extraction form and entered into RevMan 5. Discrepancies were to be resolved by discussion with a third reviewer, however this was not necessary.The quality items to be assessed were method of randomization, allocation concealment, blinding (participants, investigators, outcome assessors and data analysis), intention‐to‐treat analysis and completeness of follow up.
Main results
The search identified one ongoing randomized, placebo‐controlled, cross‐over trial (expected completion 2013) and one completed randomized, active‐comparator, cross‐over trial. In the completed study, adult patients with relapsing‐remitting and secondary progressive MS were exposed to both acetyl L‐carnitine 2 grams daily and amantadine 200 mg daily The effects of carnitine on fatigue are unclear. There was no difference between carnitine and amantadine for the number of patients withdrawing from the study due to an adverse event (relative risk ratio 0.20; 95% confidence interval 0.03 to 1.55) and no patients experienced a serious adverse event in either treatment group. Mortality and quality of life were not reported.
Authors' conclusions
There is insufficient evidence that carnitine for the treatment of MS‐related fatigue offers a therapeutic advantage over placebo or active comparators. Results of the ongoing trial are eagerly anticipated in order to provide clarity.
Keywords: Adult, Humans, Acetylcarnitine, Acetylcarnitine/therapeutic use, Amantadine, Amantadine/therapeutic use, Fatigue, Fatigue/drug therapy, Multiple Sclerosis, Multiple Sclerosis/complications, Randomized Controlled Trials as Topic, Vitamin B Complex, Vitamin B Complex/therapeutic use
Plain language summary
Carnitine for fatigue in patients with multiple sclerosis (MS)
Fatigue is commonly associated with multiple sclerosis and leads to significant disability and loss of quality of life. Several kind of interventions have been carried out, but definitive evidence on their relative efficacy and tolerability are not available. As quite recently it has been hypothesised a relationship between low carnitine levels in the blood and fatigue, and several studies showed that supplementation with carnitine produced improvement in fatigue symptoms. The Authors decided to perform a systematic review to assess the possible efficacy of carnitine in improving fatigue and to identify any adverse events in relapsing‐remitting and secondary progressive MS patients. Most of the studies did not meet the inclusion criteria of methodological quality, but one with the enrollement of 36 patients for 12‐month intervention with carnitine associated with amantadine, another drug commonly used to improve fatigue.
From this report, it is unclear if carnitine supplementation in MS patients improves fatigue, reduces the disability that results from fatigue or improves quality of life. It is also unclear if the use of carnitine for MS related fatigue is safe.
Background
Description of the condition
Multiple sclerosis (MS) is an idiopathic inflammatory condition that involves demyelination and assonal degeneration of neurons in the central nervous system. The condition may lead to progressive neurological disability and is associated with psychological dysfunction that worsens over decades (Sa 2008). Fatigue is reported to occur in up to 92% of patients with MS (Branas 2000) and has been described as the most debilitating of all MS symptoms by 28% to 40% of MS patients (Freal 1985, Krupp 1988, Murray 1985). A survey of 233 MS patients in the UK, fatigue was listed as one of the top three symptoms of MS along with bladder/bowel problems and balance problems (UK MS 1997). The consequences of MS fatigue have a significant impact on the life of a patient. A recent study determined that fatigue and depression in MS patients are independent predictors of impaired quality of life (QOL), while physical disability due to MS only had a mild impact on QOL (Janardhan 2002). It is also known that fatigue, in this setting, persists through the entire course of the disease and any changes are related to changes in mood status, not with worsening physical disability (Tellez 2006). MS fatigue has been shown to effect many physical and social life conditions such as an inability to maintain employment (Jackson 1991), negative impacts on social function and physical function (Fisk 1994, Iriarte 1999), cognitive impairment (Fisk 1994), and worsening other MS symptoms (Freal 1985).
Description of the intervention
MS fatigue is defined as a "subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities" (PVA 1998). MS fatigue is characterized by tiredness that manifests in many ways. The tiredness is usually disproportional to the amount of exertion, comes on easily, does not subside with rest or a good night's sleep, can be present throughout the day despite the absence of exertion, and is triggered by heat (Kos 2007).
The exact pathogenesis of MS fatigue is not known, but a couple of proposed mechanisms include the following: 1) an autoimmune process involving cytokine activation; or 2) demyelination of neurons in central pathways responsible for sustained neural activity (Racke 2004, Schwid 2002). It can be said, however, that the true etiology is likely multifactorial.
Various non‐pharmacological and pharmacological treatments have been described in the literature. Definitive evidence of their relative efficacy and tolerability is unavailable. Non pharmacological interventions studied include aerobic exercise regimens (Mostert 2002) and a case series describing improved fatigue symptoms in eight patients that used cooling suits (Flensner 2002). Drug therapies for the treatment of MS fatigue that have been studied are amantadine (Pucci 2007), pemoline (Krupp 1995, Weinshenker 1992, Cylert Insert), modafinil (Rammohan 2002, Zifko 2002), potassium channel‐blocking aminopyridine (Solari 2002), and Prokarin (blend of histamine and caffeine) (Gillson 2002).
How the intervention might work
Research has suggested a relationship between fatigue and serum carnitine levels Lebrun 2006). Carnitine is thought to improve fatigue through involvement in several cellular level mechanisms such as acting as essential component in mitochondrial energy production (Graziano 2002, Peluso 2000, Sayed‐Ahmed 1999), involvement in the synthesis of acetylcholine in the brain (Helman 2005), and via anti‐inflammatory and anti‐oxidant properties (Laviano 2006).
Carnitine supplementation has been used effectively to treat chronic fatigue syndrome, chemotherapy induced fatigue, fatigue in centenarians, and fatigue in a variety of neurological diseases (Plioplys 1997, Malaguarnera 2007, Pistone 2003). One study in particular demonstrated improvements in fatigue in fibromyalgia patients who received carnitine (Rossini 2007). In addition, a pilot study demonstrated that celiac patients who used carnitine for six months had significant improvements in fatigue versus placebo (Ciacci 2007). There seems to be a relationship between low serum carnitine levels and fatigue in otherwise normally healthy individuals and in those with Multiple Sclerosis (Lebrun 2006)However this has been contested by some researchers (Fukazawa 1996).
Why it is important to do this review
This review is important for two reasons:
1) Carnitine is thought to improve fatigue through involvement in several cellular level mechanisms such as acting as essential component in mitochondrial energy production (Graziano 2002, Peluso 2000, Sayed‐Ahmed 1999), involvement in the synthesis of acetylcholine in the brain (Helman 2005), and via anti‐inflammatory and anti‐oxidant properties (Laviano 2006).
2) There seems to be a relationship between serum carnitine levels and fatigue in otherwise normally healthy individuals and in those with Multiple Sclerosis. Studies have demonstrated that in patients suffering from fatigue, total and free acylcarnitine levels declined with worsening of fatigue (Plioplys 1997, Cruciani 2004). In these studies, supplementation with carnitine produced improvement in fatigue symptoms. Similarly, patients treated with medications for MS have been shown to have lower than normal serum carnitine levels (Marthaler 1999). Supplementation with carnitine to achieve normal plasma levels, in a cohort study of MS patients compared to non‐MS controls, demonstrated a reduction in fatigue intensity in up to 63% of MS patients (Lebrun 2006). In a randomized crossover trial versus amantadine, carnitine supplementation was shown to be superior in terms of improvement of fatigue symptoms in MS patients (Tomassini 2004). However, to date, there are no published systematic reviews of randomized trials specifically assessing the efficacy and safety of carnitine for use in MS patients with fatigue.
Objectives
To assess whether carnitine (enteral or intravenous) supplementation can improve the quality of life and reduce the symptoms of fatigue in patients with MS‐related fatigue. In addition the aim is to identify any adverse effects of carnitine when used in patients with MS fatigue.
Methods
Criteria for considering studies for this review
Types of studies
Full reports of published and unpublished randomized parallel‐group controlled trials, randomized cross‐over and quasi‐randomized trials of any carnitine intervention in adults with a clinical diagnosis of fatigue associated with multiple sclerosis were included.
Types of participants
Patients with definite MS (i.e Patients diagnosed as having clinically definite or probable MS according to Schumacher (Schumacher 1965), Poser (Poser 1983), the older McDonald criteria (Polman 2005), or the revised McDonald criteria (McDonald 2011) (Table 1) and associated fatigue (no strict definition of fatigue is required).
Types of interventions
Any formulation of carnitine versus placebo or other drugs
Types of outcome measures
Primary outcomes
MS fatigue as measured by MFIS Fatigue Severity Scale (FSS), MS‐specific FSS (MFSS), Modified Fatigue Impact Scale (MFIS), and Visual Analogue Scale (VAS)
Total adverse events
Withdrawals due to adverse events
Secondary outcomes
All cause mortality
Total serious adverse events
Multiple Sclerosis Quality of Life (MSQOL)‐54
Search methods for identification of studies
A systematic search without language restrictions was conducted using the optimally sensitive strategy developed for the Cochrane Collaboration to identify all relevant published and unpublished randomised controlled trials. (Lefebvre 2008). For additional information about the Group's search strategy please see: Cochrane Multiple Sclerosis Group.
Electronic searches
We searched the following databases:
The Cochrane Multiple Sclerosis Group Trials Register (September 09, 2011)
The Cochrane Central Register of Controlled Trials (CENTRAL) "The Cochrane Library"(Issue 2, 2011) (Appendix 1)
MEDLINE (PubMed) (January 1966 to 09 September 2011) (Appendix 2)
EMBASE (EMBASE.com) (1974 to 09 September 2011) (Appendix 3)
www.clinicaltrials.gov on 09 September 2011
Searching other resources
Reference lists of all available review articles and primary studies were also screened.
A hand search of the abstract book of recent symposia of European Committed Therapies and Rehabilitation in MS, Italian Neurological Society, European Federation of Neurological Sciences, and American Academy of Neurology was also conducted (19 June 2009).
Personal contact with MS experts was carried out to determine other potential sources of information. No language restrictions were applied to the search.
A United States manufacturer (Source Naturals) of carnitine formulation was contacted to determine if they could provide information regarding existing clinical trials or ongoing clinical trials of carnitine for MS‐related fatigue.
Data collection and analysis
Selection of studies
Two reviewers independently reviewed the results of the search to select articles for full test retrieval. The selected papers were assessed independently for inclusion in the review. Any disagreements were discussed with a third reviewer and agreement was reached by consensus.
Data extraction and management
Data from eligible trials was extracted and coded using a standardized data extraction form and entered into RevMan 5. Details coded include origin of publication; study design; study population such as age, socio‐economic status, stage of MS, and other concurrent medications. Number of participants, dosage and duration of intervention, details regarding outcomes, and duration of follow up were also encoded.
Assessment of risk of bias in included studies
The quality of studies to be included was assessed independently without blinding to authorship or journal using the checklist developed for the Cochrane MS Group. Discrepancies were to be resolved by discussion with a third reviewer however this was not necessary. The quality items to be assessed were method of randomization, allocation concealment, blinding (participants, investigators, outcome assessors and data analysis), intention‐to‐treat analysis and completeness of follow up.
Quality checklist
Allocation concealment A. Adequate ‐ Randomization method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study B. Unclear ‐ Randomization stated but no information on method used is available C. Inadequate ‐ Method of randomization used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group.
Blinding Blinding of investigators: Yes/No/not stated Blinding of participants: Yes/No/not stated Blinding of outcome assessor: Yes/No/not stated Blinding of data analysis: Yes/No/not stated The above are considered not blinded if the treatment group can be identified in > 20% of participants because of the side effects of treatment.
Intention‐to‐treat Yes ‐ Specifically reported by authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment Yes ‐ Not stated but confirmed on study assessment No ‐ Not reported and lack of intention‐to‐treat analysis confirmed on study assessment (Patients who were randomized were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation) No ‐ Stated but not confirmed upon study assessment Not stated
Completeness of follow up Percent of participants excluded or lost to follow up. Authors of the included trial were contacted to supply missing information and to resolve uncertainties regarding quality characteristics however further details were not provided.
Measures of treatment effect
For dichotomous outcomes, results were expressed as relative risk (RR) with 95% confidence intervals (CI). Data was pooled using the fixed‐effect model but the random‐effects model was also analyzed if statistical heterogeneity was detected (using a chi squared test on N‐1 degrees of freedom, with an alpha of 0.1 used for statistical significance and with the I2 test) and to ensure robustness of the model chosen and susceptibility to outliers.
Where continuous scales of measurement were used to assess the effects of treatment on fatigue, the weighted mean difference (WMD) was used, or the standardized mean difference (SMD) if different scales had been used. Heterogeneity was also analyzed using a chi squared test on N‐1 degrees of freedom, with an alpha of 0.1 used for statistical significance and with the I2 test.
Data synthesis
In the event that more than one trial was included, and these trials differed with respect to the identified trial quality indicators, sensitivity analyses was performed to test the assumption that the presence or absence of trial quality indicators (e.g. whether or not allocation concealment was done) had a bearing on the intervention effect estimates (e.g. is the overall intervention effect estimate different than that in trials with adequate allocation concealment).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis would have been used to explore possible sources of heterogeneity (e.g. participants, intervention dose/frequency/route of administration, comparator, and study quality). Heterogeneity among participants could be related to age, time since diagnosis of MS, or the type of MS (i.e relapsing‐remitting versus progressive forms of MS). Heterogeneity in treatments could be related to prior agent (s) used, concurrent MS immunotherapies used, and the agent, dose and duration of therapy for fatigue.
Adverse effects were tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used. Where possible, the risk difference with 95% CI was also calculated for each adverse effect, either compared to no treatment or to another agent.
Results
Description of studies
Results of the search
The initial search of databases identified 12 citations. A review of titles and abstracts was conducted by two reviewers independently (MW, AT) which identified one trial that met inclusion criteria (Tomassini 2004). Consensus between the two reviewers was not reached concerning the inclusion of one paper (Lebrun 2006). This paper was retrieved and a third reviewer determined that it was to be excluded because patients were not randomized to treatment.
The present update (September 2011) identified 3 additional references. After examination of the titles and abstracts, independently done by two review authors (AT, GR), all references were excluded because they did not match inclusion criteria and were clearly ineligible except for one ongoing trial (Ouallett 2010) which is expected to be published in 2013.
Included studies
The Tomassini 2004 study was a randomized cross‐over trial of acetyl L‐carnitine 2 grams daily (divided in 2 doses) and amantadine 200 mg daily (divided in 2 doses) in adult patients with relapsing‐remitting and secondary progressive MS. No information was provided on the criteria used to make the diagnosis of MS. Patients received randomized treatment for 3 months and then went through a 3‐month wash‐out period after which they were given 3 months of the alternate treatment. This was followed by another 3‐month wash‐out period. Follow‐up visits and assessments were done at the end of each 3‐month study period. Thirty‐six patients were enrolled and randomized with 30 patients completing the 12‐month study period. The authors reported that no serious adverse events occurred during any treatment phase of the study. Statistically significant improvement in fatigue severity was reported in favour of acetyl L‐carnitine as measured by the Fatigue Severity Scale (FSS). It is difficult to interpret the way the data was reported in the published trial report. For example, authors state that there was a "significant difference (p=0.039) between the FSS reduction after ALCAR (carnitine) and the slight FSS increase after amantadine. The data for this effect is presented in a graph (Figure 2 in the published report) with no raw numbers. It appears that the absolute FSS change with carnitine is ‐0.2 points (range ‐0.45 to +0.1 points) and that with amantadine is +0.15 points (range ‐0.1 to +0.4 points). It should be noted that the range of changes overlaps between carnitine and amantadine. Also, approximating from the graph, it appears that the difference in absolute change in FSS between the groups in 0.35 points in favour of carnitine, This must be viewed with caution as the authors themselves note that a 0.5 reduction is clinically relevant which implies that a difference between treatments of less that 0.5 points is not relevant (as is the case with the aforementioned effects).
In addition, 70% (21/30 patients) reported a reduction in FSS during carnitine treatment and 43% (13/30) reported a reduction in FSS with amantadine however this difference was not statistically significant (p=0.073). The authors note a 0.5 point reduction in FSS is considered clinically relevant but do not cite justification for this cut‐off. They do report that 29% of carnitine patients and 21% of amantadine patients reported at least a 0.5 point decrease in FSS, however this difference was not statistically significant (p=0.054).
No other significant differences between treatments were reported (with respect to secondary outcomes: Fatigue Impact Scale, Beck Depression inventory, and Social Experience Checklist). The authors of this trial suggest that acetyl L‐carnitine is a reasonable treatment option for MS‐related fatigue. These results and conclusions should be interpreted with caution (please refer to the "Risk of Bias Table" for details).
In an updated search an ongoing trial ( Ouallett 2010 ) was identified. Based on the registered protocol, and in correspondence with the lead author, this trial will likely meet the inclusion criteria and will be completed in 2013. This trial is a randomized, cross‐over trial in adults with MS comparing carnitine to placebo. The primary objective of this trial is to measure the impact of carnitine administration on fatigue using the Modified Fatigue Impact Scale. Future updates of this review will include the results of Ouallett 2010.
Excluded studies
The Lebrun 2006 study was excluded as it was a prospective open‐labelled study which recruited 240 consecutive patients hospitalised in MS clinic. Controls were not MS patients and did not complain of fatigue.
Risk of bias in included studies
See Risk of Bias table.
Effects of interventions
The effects of carnitine on fatigue (as measured by validated fatigue scores) in MS patients is not clear based on the 1 included crossover RCT (Tomassini 2004). As noted above, there were no statistically significant differences in the number of patients that had improved FSS scores or had a 0.5 point improvement (out of a total 63 points) in FSS between carnitine and amantadine in Tomassini 2004. The authors report a significant difference in absolute change in FSS in favour of carnitine and amantadine of an approximate magnitude of 0.35 points which is likely not clinically relevant.
The authors of the study did not report total adverse events nor did they report on the number of patients in each group with at least one adverse event. There was no difference between carnitine and amantadine for the number of patients withdrawing from the study due to an adverse event (see Figure 1) relative risk ratio 0.20; 95% confidence interval 0.03 to 1.55 (Analysis 1.1). Mortality and quality of life were not reported in the trial.
1.
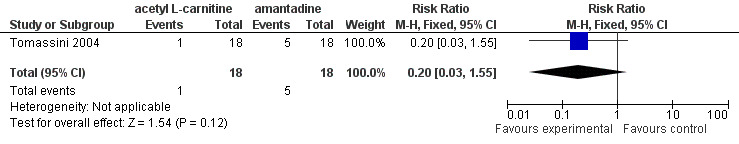
Forest plot of comparison: 1 acetyl L‐carnitine versus amantadine, outcome: 1.1 Withdrawal due to adverse events.
1.1. Analysis.
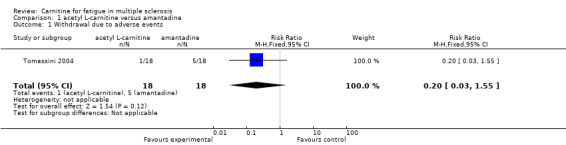
Comparison 1 acetyl L‐carnitine versus amantadine, Outcome 1 Withdrawal due to adverse events.
Discussion
Summary of main results
Our initial search identified only one RCT that was included in our review. The present update identified one ongoing RCT (Ouallett 2010) that might meet our inclusion criteria and will likely be completed in 2013.
There are still many unanswered questions regarding the role of carnitine for the treatment of MS‐related fatigue. The unresolved issues stem from a lack of evidence versus placebo for the comparator. In other words carnitine has been compared to amantadine but there is insufficient evidence that amantadine is superior to placebo. Hypothetically, then it is possible that a trial of carnitine versus amantadine may show carnitine is better for fatigue, but both carnitine and amantadine may in fact be "worse" than placebo. In addition the (in)appropriateness of cross‐over design for fatigue, weak data analysis, and the clinical relevance of fatigue scale measurement changes make the results from Tomassini 2004 of limited clinical value.
Despite the concerns raised about the methodology and analysis used in Tomassini 2004, if one were to believe the reported results, it appears that there are no clinically significant differences in fatigue between patients who receive carnitine versus amantadine.
Overall completeness and applicability of evidence
The data and carnitine's applicability to treating MS fatigue are questionable for several reasons. First, in the Tomassini 2004 RCT (Tomassini 2004), amantadine was chosen as the comparator. There is no evidence from RCTs that amantadine improves fatigue in MS patients (Pucci 2007). It would first be important to know that amantadine improves fatigue versus placebo before it should have been chosen as a clinically relevant comparator. As such, the findings of this trial are of debatable clinical importance. The appropriate comparator should have been placebo with the possible addition of an amantadine comparator arm. The Ouallett 2010 RCT is a randomized cross‐over trial of carnitine versus placebo, hence will address the aforementioned issues when published in 2013.
Second, the RCT employed a cross‐over design. As noted in the risk of bias table there were a number of methodology issues that were not clear based on the published report that would be needed before the data could be sufficiently interpreted. Contact was made with the authors, however it was not possible to attain the details which made interpretation difficult. Also cross‐over designs are best suited for stable medical conditions. Fatigue in MS has not been shown to be stable, rather its severity progresses with time. This is especially important as Tomassini 2004 enrolled patients with relapsing‐remitting MS which is inherently unstable.
As far as analysis of the data was concerned there were several key issues that deserve mention. Firstly, fatigue score changes before and after were computed for each treatment and washout period when changes should be calculated relative to the first baseline measurement. So computed changes may be contaminated by group differences in fatigue severity at the multiple baseline assessments. Secondly, the multiple baseline approach does not support the authors conclusion of no carryover effects which was based upon a finding of no significant baseline‐endpoint differences in the washout conditions. Thirdly, additional testing for sequencing effects was conducted, however the form of the original data was not preserved (i.e continuous data was converted to categorical) thereby reducing sensitivity to detect sequence differences. Therefore it is impossible to determine if the changes in fatigue were truly due to differences in therapy in each treatment, carryover effects or simply changes in fatigue over time.
In terms of threats to internal validity of data in the Tomassini trial, bias may exist as fatigue relapse was not distributed equally across periods and sequence randomizations. A general pattern of improved fatigue is noted with L‐carnitine treatment and also for each of the washout periods, with worsening fatigue associated with Amantadine. This pattern suggests that there was a general improvement in fatigue over time, but that Amantadine treatment worsened the condition. These design and analysis issues suggest poor internal validity of the study and also that a crossover trial design is likely not appropriate for treatment studies of fatigue.
Quality of the evidence
Overall the included study attempted to answer an important clinical question, however several major issues in the conduct, analysis and reporting of the trial make interpretation problematic.
Authors' conclusions
Implications for practice.
There is insufficient evidence at this time to determine if carnitine has any clinically important, positive impact on fatigue in MS patients. In addition, there is insufficient evidence that the use of carnitine to treat fatigue in MS patients is safe. Clinicians should advise their patients that the effect (both beneficial and harmful) of carnitine to treat fatigue associated with MS remains unknown at this time.
Implications for research.
To really prove or disprove carnitine’s role in treating MS related fatigue, research is needed based on good design principles (i.e. randomized controlled trials using a parallel group prospective design, patients randomized into at least two arms (carnitine and placebo), employing double blind/double‐dummy procedures, testing the success of blinding after the trial, have adequate allocation concealment, a trial duration of at least one year, and outcomes that should include those listed in our hierarchy of outcomes that was used in this review). The ongoing Ouallett 2010 RCT incorporates design similar to this and will provide key information when published in 2013.
In addition, it is essential that minimally clinically important differences (MCID) be identified for common fatigue measurement scales using distribution or anchor‐based methods. Without identified MCID for these outcome measures, clinicians will not be able to use the data in practice from the above mentioned RCTs.
What's new
| Date | Event | Description |
|---|---|---|
| 28 October 2011 | New citation required but conclusions have not changed | Added citation in ongoing studies section (to be published 2013) Quallett 2010 |
| 9 September 2011 | New search has been performed | Search updated to September 09, 2011 |
Acknowledgements
Cochrane Multiple Sclerosis Review Group
Deirdre Beecher for her help in updating the search.
Stephen Adams (Cochrane Hypertension Group) for help retrieving studies
Jean‐Christrophe Ouallett for information regarding his ongoing RCT (Ouallett 2010)
Appendices
Appendix 1. CENTRAL (Issue 3, 2011)
#1"multiple sclerosis" #2MeSH descriptor Multiple Sclerosis explode all trees #3"Demyelinating disease*" #4MeSH descriptor Demyelinating Diseases, this term only #5"transverse myelitis" #6MeSH descriptor Myelitis, Transverse, this term only #7"neuromyelitis optica" #8"optic neuritis" #9MeSH descriptor Optic Neuritis explode all trees #10"encephalomyelitis acute disseminated" #11MeSH descriptor Encephalomyelitis, Acute Disseminated, this term only #12"devic" #13(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14MeSH descriptor Fatigue explode all trees #15MeSH descriptor Muscle Fatigue explode all trees #16MeSH descriptor Fatigue Syndrome, Chronic explode all trees #17fatigue #18"chronic fatigue syndrome" #19muscle AND fatigue #20"chronic fatigue" #21(#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20) #22MeSH descriptor Carnitine explode all trees #23carnitine #24l‐carnitine #25acetylcarnitine #26acetyl‐l‐carnitine #27alcar #28branigen OR medosan OR bicarnesine OR R‐isomer #29(#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28) #30(#13 AND ( #21 AND #29 ))
Appendix 2. MEDLINE (PubMed) (From January 1966‐ 09 September 2011)
(((("Multiple Sclerosis"[mh]) OR ("Myelitis, Transverse"[mh:noexp]) OR ("Demyelinating Diseases"[mh:noexp]) OR ("Encephalomyelitis, Acute Disseminated"[mh:noexp]) OR ("Optic Neuritis"[mh])) OR ((("multiple sclerosis") OR ("neuromyelitis optica") OR ("transverse myelitis") OR (encephalomyelitis) OR (devic) OR ("optic neuritis")) OR ("demyelinating disease*") OR ("acute disseminated encephalomyelitis"))) AND (((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT ((animals[mh]) NOT ((animals[mh]) AND (human[mh]))))) AND (((carnitine) OR (bicarnesine) OR (l‐carnitine) OR ("vitamin bt") OR (acetylcarnitine) OR (acetyl‐l‐carnitine) OR ("levocarnitine acetyl") OR (acetylcarnitine) OR (R‐isomer) OR (Alcar) OR (branigen) OR (medosan)) AND (("Fatigue"[Mesh]) OR ("Muscle Fatigue"[Mesh]) OR ("Fatigue Syndrome, Chronic"[Mesh]) OR ("chronic fatigue syndrome") OR (fatigue) OR (muscle AND fatigue) OR ("chronic fatigue")))
Appendix 3. EMBASE (EMBASE.com) (From 1974‐ 09 September 2011)
(((('carnitine'/exp OR 'carnitine')) OR (('acetylcarnitine'/exp OR 'acetylcarnitine')) OR (bicarnesine:ab,ti) OR ('l carnitine':ab,ti) OR ('vitamin bt':ab,ti) OR (acetyl‐l‐carnitine:ab,ti) OR (alcar:ab,ti) OR (branigen:ab,ti) OR (medson:ti,ab)) AND ((('fatigue'/exp OR 'fatigue')) OR (('chronic fatigue syndrome'/exp OR 'chronic fatigue syndrome')) OR ('muscle fatigue':ab,ti) OR ('chronic fatigue':ab,ti) OR (fatigue:ab,ti))) AND ((('encephalomyelitis'/exp) OR ('demyelinating disease'/exp) OR ('multiple sclerosis'/exp) OR ('myelooptic neuropathy'/exp) OR ('multiple sclerosis':ab,ti) OR ('neuromyelitis optica':ab,ti) OR (encephalomyelitis:ab,ti) OR (devic:ti,ab)) AND (('crossover procedure'/exp) OR ('controlled clinical trial'/exp) OR ('double blind procedure'/exp) OR ('single blind procedure'/exp) OR ('randomized controlled trial'/exp) OR (random*:ab,ti) OR (factorial*:ab,ti) OR (crossover:ab,ti) OR (cross:ab,ti AND over:ab,ti) OR (placebo*:ab,ti) OR ('double blind':ti,ab) OR ('single blind':ab,ti) OR (assign*:ab,ti) OR (alloact*:ab,ti) OR (volunteer*:ab,ti))) AND [humans]/lim AND [embase]/lim
Data and analyses
Comparison 1. acetyl L‐carnitine versus amantadine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to adverse events | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Tomassini 2004.
| Methods | Single‐centre, double‐blind, randomized, cross‐over | |
| Participants | 1. >18 years old, relapsing‐remitting with an EDSS score of 1.0‐3.5 OR secondary progressive MS with an EDSS score 4.0‐7.0 AND FSS greater than 4 2. RRMS should have had interferon beta treatment for at least 1 year 3. SPMS patients were not undergoing disease‐modifying therapies 4. None of the patients being treated with medications known to influence MS‐related fatigue 5. All medications were discontinued at least 3 months before the beginning of the study 6. No persons experienced a relapse or were treated with steroids within the 8 weeks prior to the study |
|
| Interventions | ALCAR (acetyl L‐carnitine) 2 grams orally divided twice daily for 3 months Amantadine 200 mg orally divided twice daily for 3 months No details on crossover design |
|
| Outcomes | Primary: Self‐report of the Fatigue Severity Scale (FSS) Secondary: Fatigue Impact Scale (FIS), Beck Depression Inventory (BDI), Social Experiences Checklist (SEC) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described nor was test for success conducted |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention was made of data for the 6 patients that were not included in the analysis or how there missing data was dealt with |
| Selective reporting (reporting bias) | Low risk | Stated they would measure changes in FSS, FIS, BDI, and SEC and they did |
| Other bias | High risk | Changes before and after were computed for each period when changes should be calculated relative to the first baseline measurement. So computed changes may be contaminated by group differences at the multiple baseline assessments |
| Relevant inclusion criteria (FSS score entry criteria) | Low risk | |
| Access to individual patient data | High risk | Author was contacted to provide individual patient data. Original data was no longer available |
| Reporting of timing of patients that relapsed? | Unclear risk | Bias may exist as relapse was not distributed equally across periods and sequence randomizations |
| Cross‐over design suitable for condition? | High risk | Cross‐over design is not suitable for conditions that are unstable (i.e fatigue has not been demonstrated to be stable especially in relapsing‐remitting MS patients) |
| Assessment of carry‐over effects? | Unclear risk | A test for sequencing effects was conducted however the form of the original data was not preserved (i.e continuous data was converted to categorical) thereby reducing sensitivity to detect sequence differences |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lebrun 2006 | Patients were not randomized to treatment |
Characteristics of ongoing studies [ordered by study ID]
Ouallett 2010.
| Trial name or title | Efficacy of L‐carnitine Versus Placebo in the Treatment of Fatigue in Multiple Sclerosis (FACTSEP) |
| Methods | Randomized, prospective, cross‐over, double‐blind (subject, investigator) |
| Participants | Adults >18 years, with relapsing remitting (RR) secondary progressive (SP) or primary progressive multiple sclerosis according to McDonald 2005 MS diagnostic criteria |
| Interventions | Experimental: L‐Carnitine 2g oral solution , twice per day (morning/evening), during 3 months Control: Placebo oral solution, twice per day (morning/evening), during 3 months |
| Outcomes | Fatigue: MFIS, FSS, Fatigue visual analogue scale SEP59 (Multiple Sclerosis Quality Of Life scale) Follow‐up: 3 and 9 months |
| Starting date | June 2010 |
| Contact information | jean‐christophe.ouallet@chu‐bordeaux.fr |
| Notes | Currently enrolling participants with expected completion in 2013 |
Differences between protocol and review
Manufacturers of carnitine were too numerous therefore not all of them were contacted in order to identify trials for inclusion. One large US manufacturer was selected randomly and they were not able to provide any information regarding additional studies.
Contributions of authors
Aaron M Tejani performed screening of identified trials for possible inclusion, data extraction, data analysis, and participated in writing of the final report.
Rae Spiwak resolved discrepancies in identifying trials for analysis, participated in the statistical analysis of data, and contributed to the writing of the final report.
Michael Wasdell performed screening of identified trials for possible inclusion, data extraction, data analysis, and participated in writing of the final report.
Greg Rowell provided expertise in developing a sensitive search strategy, screening of identified trials for possible inclusion for the 2011 update, and contributed to the writing of the full report.
Shabita Nathwani aided in the retrieval and organization of trials, performed data extraction, and aided in the writing of the final report.
Sources of support
Internal sources
Fraser Health Research Administration and Development Office, Canada.
External sources
Cochrane Multiple Sclerosis Review Group, Italy.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Tomassini 2004 {published data only (unpublished sought but not used)}
- Tomassini V, Pozzilli C, Onesti E, Pasqualetti P, Marinelli F, Pisani A, et al. Comparison of the effects of acetyl L‐carnitine and amantadine for the treatment of fatigue in multiple sclerosis: results of a pilot, randomised, double‐blind, crossover trial. Journal Neurological Sciences 2004;218(1‐2):103‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Lebrun 2006 {published data only}
- Lebrun C, Alchaar H, Candito M, Bourg V, Chatel M. Levocarnitine administration in multiple sclerosis patients with immunosuppressive therapy‐induced fatigue. Multiple Sclerosis 2006;12(3):321‐4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Ouallett 2010 {published and unpublished data}
- Efficacy of L‐carnitine Versus Placebo in the Treatment of Fatigue in Multiple Sclerosis (FACTSEP). Ongoing study June 2010.
Additional references
Branas 2000
- Branas P, Jordan RE, Fry‐Smith A, Burls A, Hyde CJ. Treatments for fatigue in multiple sclerosis: a rapid and systematic review. Health Technology Assessment 2000;4:27. [PubMed] [Google Scholar]
Ciacci 2007
- Ciacci C, Peluso G, Iannoni E, Sinisgalchi M, Iovino P, Rispo A, et al. L‐Carnitine in the treatment of fatigue in adult celiac disease patients: a pilot study. Digestive Liver Disease 2007;39(10):922‐8. [DOI] [PubMed] [Google Scholar]
Cruciani 2004
- Cruciani RA, Dvorkin E, Homel P, Culliney B, Malamud S, Shaiova L, et al. L‐carnitine supplementation for the treatment of fatigue and depressed mood in cancer patients with carnitine deficiency: a preliminary analysis. Annals of the New York Academy of Sciences 2004;1033:168‐76. [DOI] [PubMed] [Google Scholar]
Cylert Insert
- Cylert (package insert). Abbott Laboratories: East Chicago, Illinois 1999.
Fisk 1994
- Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Canadian Journal of Neurological Sciences 1994;21:9‐14. [PubMed] [Google Scholar]
Flensner 2002
- Flensner G, Lindencrona C. The cooling‐suit: case studies of its influence on fatigue among eight individuals with multiple sclerosis. Journal of Advanced Nursing 2002;37:541‐50. [DOI] [PubMed] [Google Scholar]
Freal 1985
- Freal JE, Kraft GH, Coryell JK. Symptomatic fatigue in multiple sclerosis. Archives of Physical Medicine and Rehabilitation 1984;65:135‐8. [PubMed] [Google Scholar]
Fukazawa 1996
- Fukazawa T, Sasaki H, Kikuchi S, Hamada T, Tashiro K. Serum carnitine and disabling fatigue in multiple sclerosis. Psychiatry and Clinical Neurosciences 1996;50(6):323‐325. [DOI] [PubMed] [Google Scholar]
Gillson 2002
- Gillson G, Richard TL, Smith RB, Wright JV. A double‐blind pilot study of the effect of Prokarin on fatigue in multiple sclerosis. Multiple Sclerosis 2002;8:30‐5. [DOI] [PubMed] [Google Scholar]
Graziano 2002
- Graziano F, Bisonni R, Catalano V, Silva R, Rovidati S, Mencarini E, et al. Potential role of levocarnitine supplementation for the treatment of chemotherapy‐induced fatigue in non‐anaemic cancer patients. British Journal of Cancer 2002;86:1854‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helman 2005
- Helman A. Carnitine for Alzheimer's Disease. Arbor Clinical Nutrition Updates 2005;230:1‐2. [Google Scholar]
Iriarte 1999
- Iriarte J, Katsamakis G, Castro P. The fatigue descriptive scale (FDS): a useful tool to evaluate fatigue in multiple sclerosis. Multiple Sclerosis 1999;5:10‐16. [DOI] [PubMed] [Google Scholar]
Jackson 1991
- Jackson MF, Quaal C, Reeves MA. Effects of multiple sclerosis on occupational and career patterns multiple sclerosis on occupational and career patterns. Axone 1991;13:16‐17. [PubMed] [Google Scholar]
Janardhan 2002
- Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. Journal of Neurological Sciences 2002;205:51‐8. [DOI] [PubMed] [Google Scholar]
Kos 2007
- Kos D, Kerckhofs E, Nagels G, D'hooghe MB, Llsbroukx S. Origin of Fatigue in Multiple Sclerosis: Review of the Literature. Neurorehabilitation and Neural Repair 2008;22:91‐100. [DOI] [PubMed] [Google Scholar]
Krupp 1988
- Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Archives of Neurology 1988;45:435‐7. [DOI] [PubMed] [Google Scholar]
Krupp 1995
- Krupp LB, Coyle PK, Doscher C, Miller A, Cross AH, Jandorf L, et al. Fatigue therapy in multiple sclerosis: results of a double‐blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology 1995;45:1956‐61. [DOI] [PubMed] [Google Scholar]
Laviano 2006
- Laviano A, Mequid MM, Guijarro A, Muscaritoli M, Cascino A, Preziosa I, et al. Antimyopathic Effects of Carnitine and Nicotine. Current Opinion in Clinical Nutrition and Metabolic Care 2006;9(4):442‐8. [DOI] [PubMed] [Google Scholar]
Lebrun 2006
- Lebrun C, Alchaar H, Candito M, Bourg V, Chatel M. Levocarnitine administration in multiple sclerosis patients with immunosuppressive therapy‐induced fatigue. Multiple Sclerosis 2006;12:321‐4. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Malaguarnera 2007
- Malaguarnera M, Callamerri L, Gargante MP, Vacante M, Colonna V, Motta M. L‐Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. American Journal of Clinical Nutrition 2007;86:1738‐44. [DOI] [PubMed] [Google Scholar]
Marthaler 1999
- Marthaler NP, Visarius T, Kupfer A, Lauterburg BH. Increased urinary losses of carnitine during ifosfamide chemotherapy. Cancer Chemotherapy Pharmacology 1999;44:170‐2. [DOI] [PubMed] [Google Scholar]
McDonald 2011
- Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292‐302. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mostert 2002
- Mostert S, Kesselring J. Effects of a short‐term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Multiple Sclerosis 2002;8:161‐8. [DOI] [PubMed] [Google Scholar]
Murray 1985
- Murray TJ. Amantadine therapy for fatigue in multiple sclerosis. Canadian Journal of Neurological Sciences 1985;12:251‐4. [DOI] [PubMed] [Google Scholar]
Peluso 2000
- Peluso G, Nicolai R, Reda E, Benatti P, Barbarisi A, Calvani M. Cancer and anticancer therapy‐induced modifications on metabolism mediated by carnitine system. Journal of Cell Physiology 2000;182:339‐50. [DOI] [PubMed] [Google Scholar]
Pistone 2003
- Pistone G, Marino AD, Leotta C, Dell'Arte S, Finocchario G, Malaguarnera M. Levocarnitine administration in elderly subjects with rapid muscle fatigue. Drugs Aging 2003;20:761‐7. [DOI] [PubMed] [Google Scholar]
Plioplys 1997
- Plioplys AV, Plioplys S. Amantadine and L‐carnitine treatment of chronic fatigue syndrome. Neuropsychobiology 1997;35:16‐23. [DOI] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic Criteria for Multiple Sclerosis: 2005 revisions to the Macdonald Criteria. Annals of Neurology 2005;59(4):840‐6. [DOI] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, Donald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for Multiple Sclerosis: guide‐lines for research protocols. Annals of Neurology 1983;13(3):227‐31. [DOI] [PubMed] [Google Scholar]
Pucci 2007
- Pucci E, Branas P, D'Amico R, Giuliani G, Solari A, Taus C. Amantadine for fatigue in multiple sclerosis. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002818.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
PVA 1998
- Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence‐Based Management Strategies for Fatigue in Multiple Sclerosis. Paralyzed Veterans of America 1998.
Racke 2004
- Racke MK, Hawker K, Frohman EM. Fatigue in Multiple Sclerosis: is the picture getting simpler or more complex?. Archives of Neurology 2004;61:176‐7. [DOI] [PubMed] [Google Scholar]
Rammohan 2002
- Rammohan KW, Rosenberg JH, Lynn DJ, Blumenfeld AM, Pollak CP, Nagaraja HN. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two‐centre phase 2 study. Journal of Neurologu, Neurosurgery and Psychiatry 2002;72:179‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossini 2007
- Rossini M, Munno O, Valentini G, Bianchi G, Cacace E, et al. Double‐blind, multicenter trial comparing acetyl l‐carnitine with placebo in the treatment of fibromyalgia patients. Clinical and Experimental Rheumatology 2007;25(2):182‐8. [PubMed] [Google Scholar]
Sa 2008
- Sa, Jose Maria. Psychological aspects of multiple sclerosis. Clinical Neurology and Neurosurgery November 2008;110(9):868‐877. [DOI] [PubMed] [Google Scholar]
Sayed‐Ahmed 1999
- Sayed‐Ahmed MM, Shaarawy S, Shouman SA, Osman AMM. Reversal of doxorubicin‐induced cardiac metabolic damage by L‐carnitine. Pharmacological Research 1999;39:289‐95. [DOI] [PubMed] [Google Scholar]
Schumacher 1965
- Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, McDowell, et al. Problems of experimental trials of therapy in Multiple Sclerosis: Report by the panel of the evaluation of experimental trials in Multiple Sclerosis.. Annals of the New York Academy of Sciences 1965;122:552‐68. [DOI] [PubMed] [Google Scholar]
Schwid 2002
- Schwid S, Covington M, Segal B, Goodman A. Fatigue in multiple sclerosis: Current understanding and future direction. Journal of rehabilitation Research and Development 2002;39:211‐24. [PubMed] [Google Scholar]
Solari 2002
- Solari A, Uitdehaag B, Giuliani G, Pucci E, Taus C. Aminopyridines for symptomatic treatment in multiple sclerosis. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001330] [DOI] [PubMed] [Google Scholar]
Tellez 2006
- Tellez N, Rio J, Tintore M, Nos C, Galan I, Montalban X. Fatigue in Multiple Sclerosis Persists Over Time: a longitudinal study. Journal of Neurology 2006;253:1466‐70. [DOI] [PubMed] [Google Scholar]
UK MS 1997
- United Kingdom Multiple Sclerosis Society. Symptom Management Survey. United Kingdom Multiple Sclerosis Society 1997.
Weinshenker 1992
- Weinshenker BG, Penman M, Bass B, Ebers GC, Rice GPA. A double‐blind, randomized crossover trial of pemoline in fatigue associated with multiple sclerosis. Neurology 1992;42:1468‐71. [DOI] [PubMed] [Google Scholar]
Zifko 2002
- Zifko UA, Rupp M, Schwarz S, Zipko HT, Maida EM. Modafinil in treatment of fatigue in multiple sclerosis. Results of an open‐label study. Journal of Neurology 2002;249:983‐7. [DOI] [PubMed] [Google Scholar]


