Abstract
Background
Hip fracture occurs predominantly in older people, many of whom are frail and undernourished. After hip fracture surgery and rehabilitation, most patients experience a decline in mobility and function. Anabolic steroids, the synthetic derivatives of the male hormone testosterone, have been used in combination with exercise to improve muscle mass and strength in athletes. They may have similar effects in older people who are recovering from hip fracture.
Objectives
To examine the effects (primarily in terms of functional outcome and adverse events) of anabolic steroids after surgical treatment of hip fracture in older people.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (10 September 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2013 Issue 8), MEDLINE (1946 to August Week 4 2013), EMBASE (1974 to 2013 Week 36), trial registers, conference proceedings, and reference lists of relevant articles. The search was run in September 2013.
Selection criteria
Randomised controlled trials of anabolic steroids given after hip fracture surgery, in inpatient or outpatient settings, to improve physical functioning in older patients with hip fracture.
Data collection and analysis
Two review authors independently selected trials (based on predefined inclusion criteria), extracted data and assessed each study's risk of bias. A third review author moderated disagreements. Only very limited pooling of data was possible. The primary outcomes were function (for example, independence in mobility and activities of daily living) and adverse events, including mortality.
Main results
We screened 1290 records and found only three trials involving 154 female participants, all of whom were aged above 65 years and had had hip fracture surgery. All studies had methodological shortcomings that placed them at high or unclear risk of bias. Because of this high risk of bias, imprecise results and likelihood of publication bias, we judged the quality of the evidence for all primary outcomes to be very low.
These trials tested two comparisons. One trial had three groups and contributed data to both comparisons. None of the trials reported on patient acceptability of the intervention.
Two very different trials compared anabolic steroid versus control (no anabolic steroid or placebo). One trial compared anabolic steroid injections (given weekly until discharge from hospital or four weeks, whichever came first) versus placebo injections in 29 "frail elderly females". This found very low quality evidence of little difference between the two groups in the numbers discharged to a higher level of care or dead (one person in the control group died) (8/15 versus 10/14; risk ratio (RR) 0.75, 95% confidence interval (CI) 0.42 to 1.33; P = 0.32), time to independent mobilisation or individual adverse events. The second trial compared anabolic steroid injections (every three weeks for six months) and daily protein supplementation versus daily protein supplementation alone in 40 "lean elderly women" who were followed up for one year after surgery. This trial provided very low quality evidence that anabolic steroid may result in less dependency, assessed in terms of being either dependent in at least two functions or dead (one person in the control group died) at six and 12 months, but the result was also compatible with no difference or an increase in dependency (dependent in at least two levels of function or dead at 12 months: 1/17 versus 5/19; RR 0.22, 95% CI 0.03 to 1.73; P = 0.15). The trial found no evidence of between‐group differences in individual adverse events.
Two trials compared anabolic steroids combined with another nutritional intervention ('steroid plus') versus control (no 'steroid plus'). One trial compared anabolic steroid injections every three weeks for 12 months in combination with daily supplement of vitamin D and calcium versus calcium only in 63 women who were living independently at home. The other trial compared anabolic steroid injections every three weeks for six months and daily protein supplementation versus control in 40 "lean elderly women". Both trials found some evidence of better function in the steroid plus group. One trial reported greater independence, higher Harris hip scores and gait speeds in the steroid plus group at 12 months. The second trial found fewer participants in the anabolic steroid group were either dependent in at least two functions, including bathing, or dead at six and 12 months (one person in the control group died) (1/17 versus 7/18; RR 0.15, 95% CI 0.02 to 1.10; P = 0.06). Pooled mortality data (2/51 versus 3/51) from the two trials showed no evidence of a difference between the two groups at one year. Similarly, there was no evidence of between‐group differences in individual adverse events. Three participants in the steroid group of one trial reported side effects of hoarseness and increased facial hair. The other trial reported better quality of life in the steroid plus group.
Authors' conclusions
The available evidence is insufficient to draw conclusions on the effects, primarily in terms of functional outcome and adverse events, of anabolic steroids, either separately or in combination with nutritional supplements, after surgical treatment of hip fracture in older people. Given that the available data points to the potential for more promising outcomes with a combined anabolic steroid and nutritional supplement intervention, we suggest that future research should focus on evaluating this combination.
Keywords: Aged; Aged, 80 and over; Female; Humans; Male; Anabolic Agents; Anabolic Agents/adverse effects; Anabolic Agents/therapeutic use; Androgens; Androgens/adverse effects; Androgens/therapeutic use; Frail Elderly; Hip Fractures; Hip Fractures/rehabilitation; Hip Fractures/surgery; Nandrolone; Nandrolone/adverse effects; Nandrolone/analogs & derivatives; Nandrolone/therapeutic use; Nandrolone Decanoate; Postoperative Care; Postoperative Care/methods; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Anabolic steroids for improving recovery after hip fracture in older people
Why anabolic steroids might help after a hip fracture
Hip fracture occurs mainly in older people, many of whom are frail. After surgery for their hip fracture, most patients suffer a loss of muscle mass and strength. Despite rehabilitation, most patients experience a long‐term decline in mobility and function. Anabolic steroids, the synthetic derivatives of the male hormone testosterone, have been used in combination with exercise to improve muscle mass and strength in athletes. This review considers the evidence for the use of anabolic steroids aimed at improving outcomes after hip fracture in older people.
Description of the studies included in the review
We searched the medical literature until September 2013 and found three relevant studies that included a total of 154 women over the age of 65 years who had had hip surgery. Two studies were conducted in Sweden and one in Canada. The studies tested two comparisons. One study had three groups and contributed data to both comparisons.
Quality of the evidence There were only three studies available and all three were small and at high risk of bias. We therefore judged the quality of the evidence to be very low, which means that we are uncertain how reliable the evidence is.
Summary of the evidence
Two very different studies compared anabolic steroid versus control (no anabolic steroid or placebo). One study conducted in the hospital ward compared weekly anabolic steroid injections versus placebo injections in 29 "frail elderly females". This study found no evidence that anabolic steroid resulted in better function, as measured by numbers discharged to a higher level of care or dead, or the time to mobilisation. The second study compared steroid injections given every three weeks for six months plus daily protein supplementation versus daily protein supplementation alone in 40 "lean elderly women". This study provided some evidence that anabolic steroids may result in better function, but they may also make no difference or result in worse function. Neither study found a difference in the incidence of individual adverse events in the two groups.
Two studies compared anabolic steroids combined with another nutritional intervention ('steroid plus') versus control (no 'steroid plus'). One study compared anabolic steroid injections every three weeks for 12 months in combination with daily supplement of vitamin D and calcium versus calcium only in 63 women who were living independently at home. The other study compared anabolic steroid injections every three weeks for six months and daily protein supplementation versus control in 40 "lean elderly women". Both studies found some evidence of better function in the steroid plus group. Pooled mortality data from the two studies showed no difference between the two groups at one year. Similarly, there was no evidence of between‐group differences in individual adverse events. Three participants in the steroid group of one study reported side effects of hoarseness and increased facial hair. The other study reported better quality of life in the steroid plus group. None of the studies reported on patient acceptability of the intervention.
Conclusions
The quality of the evidence was very low, meaning that we are very uncertain about the direction and size of effect. Thus we are unable to say if anabolic steroids, either separately or in combination with nutritional supplements, improve recovery after hip fracture surgery in older people. Given that the results available point to the potential for more promising outcomes with a combined anabolic steroid and nutritional supplement intervention, we suggest that future research should focus on evaluating this combination.
Background
Description of the condition
Fracture of the proximal femur (known widely as hip fracture) is a common cause of morbidity and mortality in older people. About 40% to 50% of women and 13% to 22% of men are at risk of having an osteoporotic fracture in their lifetime (Dennison 2006). With the rise in life expectancy, the prevalence of hip fracture is expected to increase (Cooper 1992; Gullberg 1997). There is worldwide variation in the incidence of hip fracture. While it is already an established health problem in the West, it is increasingly recognised as a growing problem in Asia (Mithal 2009).
Surgical management is the mainstay of the treatment for hip fracture. This is generally followed by inpatient rehabilitation, with or without extension to an outpatient rehabilitation programme (Marks 2003). Despite treatment, functional recovery after hip fracture is often incomplete, with many people who were walking independently before their hip fracture losing their independence afterwards (Osnes 2004). This negatively impacts on their quality of life (Adachi 2001). By six to 12 months after a hip fracture, between 22% and 75% of people have not recovered their pre‐fracture ambulatory or functional status (Cummings 1988; Koval 1995). People sustaining hip fracture use extensive health system resources (Braithwaite 2003), and many patients require continued support and care services. After their initial treatment, people who have had a hip fracture are at high risk for re‐hospitalisation (Wolinsky 1997), refracture (Johnell 1985) and institutionalisation (Rosell 2003; Tajeu 2013). Older adults have five‐ to eight‐fold increased risk of dying during the first three months after hip fracture (Haentjens 2010). There is also some evidence that functional recovery after hip fracture is the main determinant of long‐term mortality (Dubljanin‐Raspopović 2013).
Description of the intervention
Following surgical treatment of hip fracture, a wide range of therapies are used to promote functional recovery (SIGN 2009). Some of these have specific goals such as restoration of mobility and independence in basic activities of daily living. This review focuses on the use of anabolic steroids for restoring and maintaining function after hip fracture surgery.
Anabolic steroids are a group of synthetic hormones, related to the male hormone testosterone, that promote the storage of protein and the growth of tissue (anabolism) (Dorland 2007). Their use has been demonstrated to have a positive effect in the treatment of diverse clinical conditions, including the treatment of anaemia in renal disease patients (Navarro 2002;Teruel 1996), osteoporosis (specifically bone density), cachexia (muscle wasting) in people with chronic illness (Johns 2009), and improving muscle mass and strength in older people (Snyder 1999). Women show an age‐related decline in endogenous androgen levels, which might influence the development of osteoporosis (Zofkova 2000). A double‐blind randomised controlled trial showed better mobility and less pain in people with vertebral fractures after treatment with anabolic steroids compared with a vitamin D analogue, alphacalcidol (Lyritis 1994).
Anabolic steroids come in different preparations, which can be given various ways (e.g. orally, skin patches, intramuscular injections), started at different times (prior to, or at any stage of recovery after hip fracture surgery) and can be administered for different lengths of time.
How the intervention might work
Patients with hip fractures are often elderly, frail and undernourished (Bachrach‐Lindström 2000; Lumbers 2001). They may undergo a catabolic state (Patterson 1992), which leads to chronic muscle wasting and reduced muscle strength. This can affect mobility and result in falls. Loss of muscle mass and lean body weight contribute to generalised weakness, an impaired immune response and slower wound healing. Anabolic steroids provide some benefit in other conditions associated with increased catabolic rates such as burns, chronic obstructive airway disease and acquired immune deficiency syndrome (AIDS) (Berger 1996).
There is also good reason to combine the use of anabolic steroids with nutritional supplementation. Protein energy malnutrition occurs in 30% to 50% of people who sustain a hip fracture (Lumbers 1996; Ponzer 1999). Postoperative hip fracture rehabilitation is facilitated by improving the nutritional intake of the patient (Delmi 1990). A Cochrane review concluded that some evidence exists for the beneficial effects of nutritional supplementation after hip fracture (Avenell 2006). There is some evidence that combining testosterone and nutritional supplementation for undernourished older people reduces both the number of people hospitalised and the duration of hospital admissions (Chapman 2009).
Adverse effects, often dose‐related, from anabolic steroids include changes in voice, growth of facial hair in women, hair loss, acne, oedema, thromboembolic events and liver damage.
Why it is important to do this review
Despite advances in surgical treatments, hip fractures continue to have a large impact on older people and society because they often result in disability and institutionalisation (Fierens 2006; Osnes 2004). Anabolic steroids may have a role in improving outcomes and recovery, and allowing greater independence in these patients. It is important to assess the evidence for the use of these drugs in this predominantly elderly and frail population.
Objectives
To examine the effects (primarily in terms of functional outcome and adverse events) of anabolic steroids after surgical treatment of hip fracture in older people.
The following main comparisons were intended, set in the context of usual or conventional care.
Anabolic steroid versus no anabolic steroid or a placebo control.
Anabolic steroid in conjunction with other intervention (either nutrition or exercise or both) versus no anabolic steroid plus same other intervention or a placebo control.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of anabolic steroid treatment following surgery for hip fracture. We planned also to include trials that used quasi‐randomisation (e.g. allocation by date of birth or hospital record number) or cluster randomisation (e.g. by hospital ward). The failure to use an intention‐to‐treat approach to analysis was not a reason for exclusion.
Types of participants
We considered older people with any type of hip fracture that was surgically treated. It was anticipated that a large proportion of these people would be older than 65 years of age. We did not exclude trials that included younger participants if the mean age minus one standard deviation was greater than 65 years. Participants younger than 65 years, or with multi‐trauma or with pathological fractures, would have been included provided they made up less than 25% of the total sample size, and there was adequate randomisation of these participants to intervention and control groups.
Types of interventions
The intervention assessed was anabolic steroids, administered enterally (orally, nasogastric or via percutaneous gastrostomy tubes), parenterally (intramuscular routes) or via alternative routes such as transdermal. The intervention could start prior to or at any stage of recovery after hip fracture surgery, but interventions that were pre‐surgical only were excluded. The duration of administration could vary and it was acceptable for it to continue until the end of the rehabilitation phase. We compared the administration of anabolic steroids with the provision of no intervention or a placebo intervention. It was envisaged that usual or conventional care would be provided to all trial participants. Such care, as described below, could comprise nutrition or exercise or both.
We included the following comparisons, set in the context of usual or conventional care.
Anabolic steroid versus no anabolic steroid or a placebo control. This included studies where nutrition or exercise or both were provided to both groups in the comparison.
Anabolic steroid plus 'other intervention', where this was either nutrition or exercise or both, versus no anabolic steroid plus same other intervention or a placebo control.
Types of outcome measures
Primary outcomes
The primary outcome was function: for example, independence in mobility and activities of daily living. We gave preference to validated, patient‐reported outcome measures. We also sought data on adverse events including mortality, hospital readmission and complications from the use of anabolic steroids.
Secondary outcomes
Secondary outcomes were patients' perceived quality of life, adherence and acceptability of the intervention, objective assessments of body composition, nutritional indices, muscle strength and the use of resources such as length of hospital stay.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (10 September 2013), the Cochrane Central Register of Controlled Trials (The Cochrane Library, 2013 Issue 8), MEDLINE (1946 to August Week 4 2013), MEDLINE In‐Process & Other Non‐Indexed Citations (9 September 2013) and EMBASE (1974 to 2013 Week 36). We also searched Current Controlled Trials and the WHO international Clinical Trials Registry Platform for ongoing and recently completed trials (September 2013). We did not impose any restrictions based on language or publication status.
In MEDLINE (OvidSP), the subject specific search was combined with the sensitivity‐maximizing version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). Search strategies for the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE are shown in Appendix 1.
Searching other resources
We assessed the American Orthopaedic Trauma Association's annual meetings (1996 to 2012) by handsearching the table of contents of the meeting proceedings. We also searched reference lists of relevant articles.
Data collection and analysis
The intended methodology for data collection and analysis was described in our published protocol (Farooqi 2010).
Selection of studies
Two review authors (VF and MvdB) independently screened titles and abstracts of records identified from database searches for possible inclusion. We retrieved the full text of potentially relevant citations. From the full text, we selected trials that met the selection criteria for inclusion. We sought further information from trial authors where necessary. A third author (MC) moderated any disagreement. We documented the reasons for exclusion.
Data extraction and management
Two review authors (VF and MvdB) independently performed data extraction using a piloted form. The data collected included study design characteristics, study population, interventions, outcome measures, and length of follow‐up. We attempted to contact trial authors for clarification when necessary. Disagreements were resolved by a third review author (MC).
Assessment of risk of bias in included studies
Two review authors (VF and MvdB) independently assessed risk of bias using a piloted version of The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). Disagreements were resolved by discussion. We assessed random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other biases, including those associated with major baseline imbalance and early stopping. We rated each domain as either 'low risk'; 'unclear risk' or 'high risk' of bias.
Measures of treatment effect
We analysed results both at short‐term (six months or less) and longer‐term (more than six months) intervals. We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. We calculated mean differences with 95% CIs for continuous outcomes. We had planned to use standardised mean differences for pooling continuous outcomes based on differences in scores or scales.
Unit of analysis issues
The unit of randomisation in these trials was the individual patient. However, we would have considered randomised trials where the unit of randomisation was another entity, such as a hospital ward. If possible, appropriate adjustments would have been made before presenting data from such trials if the trial authors had not adjusted for clustering. We planned to seek advice on the interpretation and presentation of the results from such trials from the statistical editors of the Cochrane Bone, Joint and Muscle Trauma Review Group.
Dealing with missing data
We attempted to contact the authors of included trials for missing data. Where appropriate, we intended to perform intention‐to‐treat analysis to include all people randomised. However, where drop‐outs were identified, the actual denominators of participants contributing data at the relevant outcome assessment were used. When possible, in future updates, we will investigate the effect of drop‐outs and exclusions by conducting worst‐ and best‐case scenario sensitivity analyses. The 'best‐case' scenario is when all participants with missing outcomes in the experimental intervention group are assigned a good outcome, and all those with missing outcomes in the control intervention group a bad outcome; the 'worst‐case' scenario is the converse. We will continue to be alert to potential mislabelling or non‐identification of standard errors and standard deviations. Additionally, unless missing standard deviations can be derived from CIs, P values or standard errors, we will not assume values in order to present these in the analyses.
Assessment of heterogeneity
We planned to assess heterogeneity by visual inspection of the forest plot (analysis) and consideration of the Chi² test for heterogeneity and the I² statistic (Higgins 2003). We interpreted I² values as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
If at least 10 trials had contributed data to a forest plot, we would have considered preparing a funnel plot to assess publication bias. We investigated selective outcome reporting by comparing the study outcomes with those routinely presented for similar studies and also by comparing the methods section with the results reported in the trials.
Data synthesis
Where considered appropriate, we pooled results of comparable groups of trials. We used the fixed‐effect model and 95% CIs. We would have considered using the random‐effects model, especially where there was unexplained heterogeneity. It was anticipated that we would pool data even if heterogeneity was high. For continuous outcomes, where outcomes were reported using different scales or instruments but assessing the same dimension, the results were to be pooled using the standardised mean difference. Mindful of unit of analysis issues, we intended to pool the data from cluster‐randomised trials using the generic inverse variance.
Subgroup analysis and investigation of heterogeneity
If sufficient data are available in future, we will conduct subgroup analyses to determine whether the primary outcomes vary according to gender and route of steroid administration. We will use the test for subgroup differences available in Review Manager (RevMan 2014) to determine if the results for subgroups are statistically significantly different.
Sensitivity analysis
Had sufficient data been available, we would have performed sensitivity analyses to explore the effects of important sources of bias, such as whether allocation was concealed, in the included studies.
Assessment of the quality of the evidence
We used the GRADE approach to assess the quality of evidence related to the primary outcomes listed in the Types of outcome measures (Schunemann 2011, section 12.2). Where there is sufficient evidence in future to merit the preparation of 'Summary of findings' tables, we will develop these for the main comparisons.
Results
Description of studies
Results of the search
The search was completed in September 2013. We screened a total of 1290 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (8 records); Cochrane Central Register of Controlled Trials (CENTRAL) (113), MEDLINE (598), EMBASE (480), the WHO International Clinical Trials Registry Platform (27) and Current Controlled Trials (64). We identified six potentially eligible studies, published in 10 reports. On full review of their texts, we included three trials (Hedstrom 2002 (two reports); Sloan 1992;Tidermark 2004 (four reports)) and excluded two (Beringer 1986; Naessen 2008). One trial (NCT00280267), while completed, has yet to be published (see the Characteristics of ongoing studies).
A flow diagram summarising the study selection process is shown in Figure 1.
1.
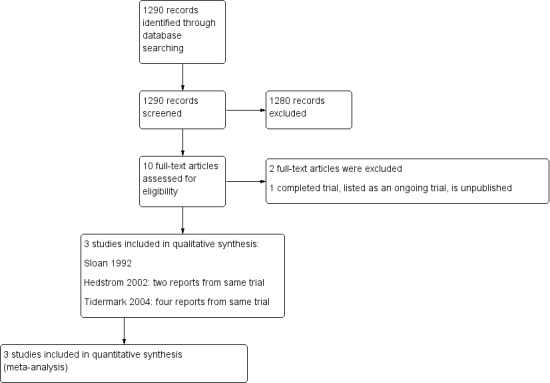
Study flow diagram
Included studies
We included three trials (Hedstrom 2002; Sloan 1992; Tidermark 2004), all of which investigated the effects of anabolic steroids on recovery from hip fracture surgery in older people aged 65 years or over. The three trials included a total of 154 female participants (Table 1). Sloan 1992 also included eight males but provided only a brief summary of the results of this small subgroup. For completeness, we present the results for adverse events for this subgroup in this review.
1. Demographics of included participants.
| Study | Number of participants | Mean (SD) age (years) | Mean (SD) weight (kg) | Cognitive status | Pre‐injury function | Nutritional status |
| Hedstrom 2002 | 63 | 80.5 (6.3) | 60.8 (11.0) | Dementia excluded | Independent living status | BMI < 24 |
| Sloan 1992 | 29 (of 31) | 82 (6.5) | 57.8 (9.9) | Severe dementia excluded | Extended care home residents excluded only | No information |
| Tidermark 2004 | 60 | 82.9 (5.4) | 53.3 (8.8) | Severe cognitive impairment excluded | Independent living status | BMI < 24 |
All study participants described above were females. However, no other details and very limited results were provided for eight male participants in Sloan 1992
BMI: body mass index
SD: standard deviation
Summaries of the three trials are given below. For further details, please see the Characteristics of included studies.
Hedstrom 2002 compared nandrolone decanoate (steroid) at the dose of 25 mg intramuscularly every third week for one year and a daily supplement of 1alpha‐hydroxylated vitamin D3 (0.25 g) plus calcium (500 mg) versus calcium (500 mg) alone (control group). This study, conducted in Sweden, included 63 women (mean age 80.5 years) who were living independently at home after hip fracture surgery. Follow‐up assessments were at 3, 6, 9 and 12 months.
Sloan 1992 included 31 women (mean age 82 years) who had been treated surgically for hip fracture. They compared weekly nandrolone decanoate (steroid) intramuscular injections 2 mg/kg versus a placebo group. The steroid injections were given weekly until discharge from hospital or four weeks, whichever came first. Follow‐up assessments were weekly until four weeks or discharge, whichever came first.
Tidermark 2004 was conducted in Sweden and involved 60 women (mean age 83 years) who had hip fracture surgery. The purpose was to evaluate the effects of a protein‐rich liquid supplementation, alone or in combination with the anabolic steroid nandrolone decanoate. The participants were randomised to the following groups.
Steroid plus group: nandrolone decanoate (Deca‐Durabol 25 mg every three weeks) + protein‐rich formula (Fortimel 200 ml/day, 20 g protein/day) + daily calcium (1 g) and vitamin D (CalchichewD3 800 IE).
Control group (protein supplementation): protein‐rich formula (Fortimel) + daily calcium + vitamin D (doses as above).
Control group: daily calcium and vitamin D (doses as above).
Follow‐up assessments were at six and 12 months. The Tengstrand 2007 publication was a post hoc analysis of Tidermark 2004 and thus not a separate trial.
Excluded studies
We excluded two studies. The primary reason for excluding Beringer 1986, which compared the relative effects on calcitonin secretion of anabolic steroid (Stanozolol) versus oral calcium in 20 women with hip fracture, was the lack of a no treatment control. We excluded Naessen 2008 because it included only healthy women without hip fracture. For more details, please see the Characteristics of excluded studies.
Risk of bias in included studies
Summaries of our risk of bias assessment of the included studies are presented in Figure 2 and Figure 3.
2.
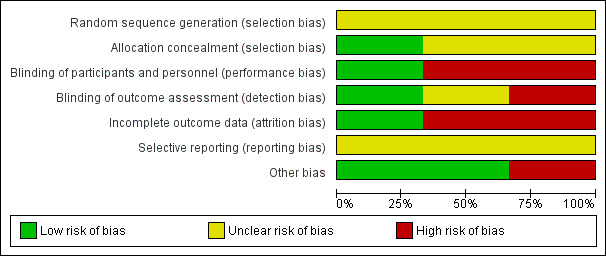
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
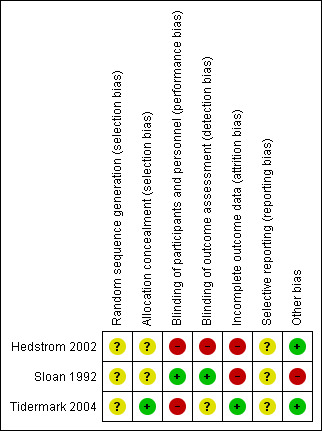
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
None of the trials gave details of their method of random sequence generation and we therefore judged all three trials as being at unclear risk of bias for this item.
Two trials (Hedstrom 2002; Tidermark 2004) used sealed envelopes; Tidermark 2004 further described these as opaque and was thus considered to be at low risk of selection bias relating to successful concealment of allocation. The risk of selection bias in Hedstrom 2002 was judged as unclear. The same applied for Sloan 1992, which did not disclose their method of allocation concealment.
Blinding
Hedstrom 2002 did not describe blinding of either participants, investigators or outcome assessors and we judged this trial to be at high risk of both performance and detection bias. Sloan 1992 did not provide details of blinding but we considered this trial to be at low risk of performance and detection bias given that it was clearly reported to be a double‐blind, placebo‐controlled study. Tidermark 2004 was 'open label' and at high risk of performance bias; however, although it did not describe blinding, there was independent outcome assessment, which we judged put it at 'unclear' rather than high risk of detection bias.
Incomplete outcome data
Only Tidermark 2004 was at low risk of attrition bias as it used an intention‐to‐treat analysis and gave a participant flow diagram, illustrating losses at 12 months follow‐up. While both Hedstrom 2002 and Sloan 1992 documented the reasons for drop‐outs in their trials, the reporting of losses was incomplete and unclear in Hedstrom 2002. The added failure to add in the denominators in the tables makes this trial at high risk of attrition bias. Similarly, we judged Sloan 1992 as being at high risk of attrition bias, reflecting both the large percentage of participants with missing data at four weeks (39% = 12/31) (patients being discharged from their inpatient stay) and an imbalance between the two groups in those available at four weeks (12/16 versus 7/15). This is a consequence of a flawed study design where no efforts were made to obtain post‐discharge outcomes.
Selective reporting
Published trial registration information or protocols were not available for any of the three trials. However, all the outcomes that were listed in the methods sections were reported in the results. All three trial reports had aspects that appeared to indicate post hoc reporting decisions and thus we judged them as being unclear for reporting bias. Hedstrom 2002 reported functional outcomes using the Katz Index but provided the median and range and it was not clear if they intended to report the three‐month results. Tidermark 2004 used the same scale (the Katz Index) as Hedstrom 2002 but did not report means and standard deviations. Instead they chose to present the results in a graph format without giving actual values. They also reported significant differences among groups in terms of the quality of life scores using EQ‐5D index but again presented the results as a graph. We judged Sloan 1992 to be at unclear risk of bias because of the incomplete reporting of the results of the male participants and the lack of clarity about whether they intended to report males and females separately from the outset.
Other potential sources of bias
We judged Hedstrom 2002 and Tidermark 2004 to be at low risk of other bias, specifically bias relating to major imbalances in baseline characteristics and early stopping. Baseline characteristic data for Sloan 1992 provided for 29 of the 31 female participants (none were provided for the male participants) showed the participants in the steroid group had lower functional ability and were more dependent in activities of daily living than in the placebo group. We judged this trial as being at high risk of other bias.
Effects of interventions
Anabolic steroid versus no anabolic steroid or a placebo intervention
Sloan 1992 compared anabolic steroid with placebo intervention, reporting results for 17 participants at four weeks follow‐up. Tidermark 2004 compared anabolic steroid and protein supplementation versus protein supplementation alone, reporting results for 35 participants at 6 and 12 months follow‐up. The large difference in the timing of follow‐up of these two trials meant that pooling data for common outcomes was inappropriate.
Primary outcomes
Functional independence
One person in the control group of Sloan 1992 died in hospital. Similar numbers of participants in the two groups of Sloan 1992 were discharged to a higher level of care. The results of the combined outcome of death or discharge to a higher level of care ('more dependent or dead at hospital discharge') are shown in Analysis 1.1 (8/15 versus 10/14, risk ratio (RR) 0.75, 95% CI (confidence interval) 0.42 to 1.33; P = 0.32).
1.1. Analysis.
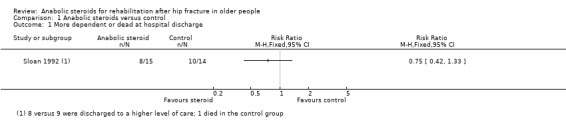
Comparison 1 Anabolic steroids versus control, Outcome 1 More dependent or dead at hospital discharge.
One person in the control group (nutrition only) of Tidermark 2004 died within one year. Tidermark 2004 based their functional assessment on the results of the Katz Index and used graphs to display the results grouped by independence in five or all six activities of daily living functions versus dependence in bathing and at least one other function. Fewer participants in the anabolic steroid group were either dependent in at least two functions or dead at six months (0/17 versus 3/19; RR 0.16, 95% CI 0.01 to 2.87; P = 0.21) and at 12 months (1/17 versus 5/19; RR 0.22, 95% CI 0.03 to 1.73; P = 0.15) (Analysis 1.2); neither result was statistically significant.
1.2. Analysis.
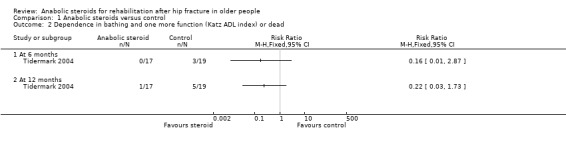
Comparison 1 Anabolic steroids versus control, Outcome 2 Dependence in bathing and one more function (Katz ADL index) or dead.
Although the anabolic steroid group in Sloan 1992 took a longer time to stand with support (steroid: 3.1 days versus control: 2.3 days; MD 0.80 days, 95% CI ‐0.59 to 2.19 days; P = 0.26), and five days longer on average to mobilise independently (steroid: 14.5 days versus control: 9.3 days; MD 5.20 days, 95% CI ‐1.68 to 12.08 days; P = 0.14), neither of the differences between the two groups was statistically significant (Analysis 1.3).
1.3. Analysis.
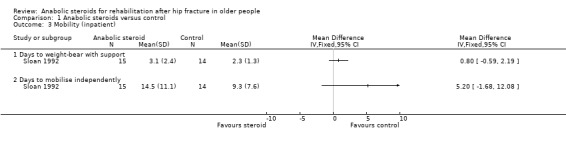
Comparison 1 Anabolic steroids versus control, Outcome 3 Mobility (inpatient).
Adverse events
One participant in the control group of each trial died within the follow‐up period (Analysis 1.4). Since the number of participants experiencing an adverse event was not available, details of the individual events are described below and displayed in Analysis 1.5. This is restricted to female participants only.
1.4. Analysis.
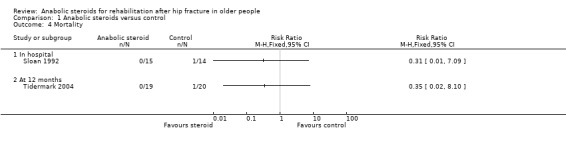
Comparison 1 Anabolic steroids versus control, Outcome 4 Mortality.
1.5. Analysis.
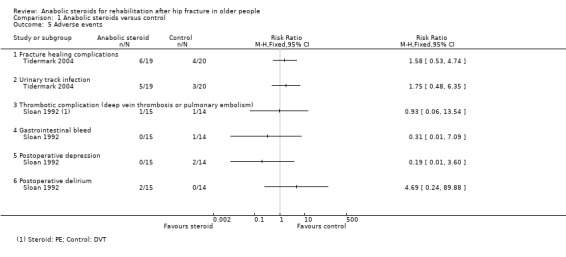
Comparison 1 Anabolic steroids versus control, Outcome 5 Adverse events.
Sloan 1992 withdrew one participant in the steroid group because of the doubling of the serum liver enzymes after their second injection (liver function tests were normal within the next week) and one participant in the placebo group because of nightmares. Of the 15 remaining in the steroid group, there was one case of pulmonary embolism and two cases of delirium within one week post‐surgery. In the 14 participants in the control group, there was one case of deep venous thrombosis, one gastrointestinal bleed and two participants developed post‐operative depression. There was a total of 10 units of blood transfused to participants in the steroid group and seven units to participants in the placebo group.
Of the eight male participants in Sloan 1992, one of the three steroid group participants required a transurethral prostatectomy after developing a urinary obstruction. One participant in each group became depressed. In the control group, one participant had a deep venous thrombosis and one had a syncopal event.
In Tidermark 2004, fracture healing complications were recorded in six participants of the steroid group versus four of the control group; and urinary tract infection was reported for five versus three participants. A slight and transient elevation of serum calcium was noted in six participants of the steroid group.
Secondary outcomes
Patients' perceived quality of life
Tidermark 2004 captured patient‐rated health‐related quality of life (HRQoL) during the week preceding the injury using the EuroQol (EQ‐5D). They performed a logistic regression analysis in order to evaluate factors of importance for changes in the HRQoL. Randomisation to the combined anabolic steroid and protein supplement group (steroid group) was reported to be associated with an increased odds ratio for improved HRQoL at the end of the six month intervention period (P < 0.05). These results were presented as a graph only and no baseline values, changes in scores or means or standard deviations were given in textual format or a table.
Adherence and acceptability of the intervention
All but one participant in the steroid group of Sloan 1992 received at least four doses of the anabolic steroid. In Tidermark 2004, one patient withdrew consent following randomisation, but prior to receiving the intervention. The intramuscular nandrolone injections were administered by a research nurse in patients' own homes while the compliance regarding the protein‐rich formula and calcium/vitamin D was verified with interviews and logbooks. The adherence for steroid administration was 100%.
Neither trial recorded patient acceptability of the intervention nor, in Sloan 1992, of the weekly injection.
Objective assessments of body composition
On average, participants in the steroid group in Sloan 1992 were lighter and had lower lean body mass (measured in three ways) and smaller mid‐arm muscle circumference than participants on placebo group at baseline; a difference that persisted in the follow‐up data (Analysis 1.6).
1.6. Analysis.
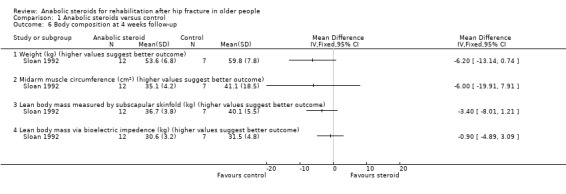
Comparison 1 Anabolic steroids versus control, Outcome 6 Body composition at 4 weeks follow‐up.
Tidermark 2004 showed smaller declines in body weight and lean body mass (measured using dual energy X‐ray absorptiometry) at 12 months in the steroid group; the differences between the two groups did not reach statistical significance (Analysis 1.7).
1.7. Analysis.
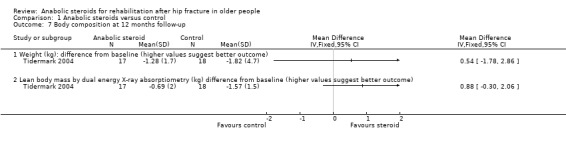
Comparison 1 Anabolic steroids versus control, Outcome 7 Body composition at 12 months follow‐up.
Nutritional indices
Both Sloan 1992 and Tidermark 2004 reported changes in albumin as a marker for nutritional improvement during recovery from hip fracture. Neither trial found a difference between the two groups (Analysis 1.8). None of the differences in three other nutritional indices reached statistical significance in Sloan 1992.
1.8. Analysis.
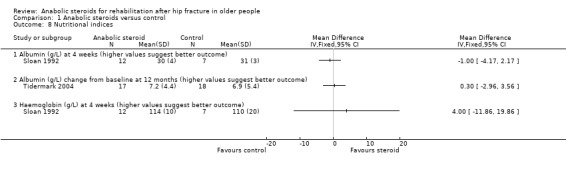
Comparison 1 Anabolic steroids versus control, Outcome 8 Nutritional indices.
Muscle strength
Sloan 1992 and Tidermark 2004 both reported on hand grip strength as a measure of muscle strength. Neither study found a statistically significant difference between the groups at final follow‐up (four weeks: Sloan 1992; one year: Tidermark 2004) (Analysis 1.9).
1.9. Analysis.
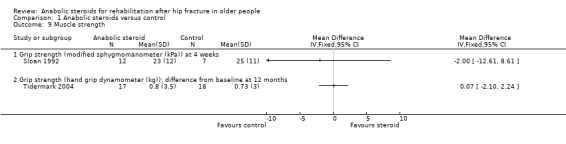
Comparison 1 Anabolic steroids versus control, Outcome 9 Muscle strength.
Use of resources such as length of hospital stay
With the exception of length of hospital stay data, neither trial reported resource use or cost outcomes. In Sloan 1992, the steroid group stayed in hospital an average of five days longer than those in the placebo group (steroid: 45 days versus control: 40 days; MD 5.00 days, 95% CI ‐11.46 to 21.46 days; P = 0.55); the difference between the two groups was not statistically significant (Analysis 1.10). Tidermark 2004 reported that the difference in length of hospital stay in the first year after surgery was not significant (median (range): 18 (8 to 51) days versus 20 (5 to 356) days).
1.10. Analysis.
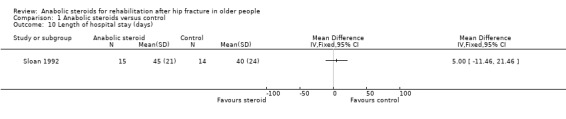
Comparison 1 Anabolic steroids versus control, Outcome 10 Length of hospital stay (days).
Anabolic steroid plus other intervention ('steroid plus') versus no anabolic steroid plus some other intervention or a placebo control
The two trials (Hedstrom 2002; Tidermark 2004) in this comparison differed in their co‐interventions. Hedstrom 2002 compared anabolic steroid plus vitamin D plus calcium (steroid plus group) with calcium alone (control group); and reported results for an estimated 44 participants at 12 months follow‐up. Tidermark 2004 compared anabolic steroid and protein supplementation (steroid plus group) versus no steroid or protein supplementation (control group); and reported results for 34 patients at follow‐up.
Primary outcomes
Functional independence
Hedstrom 2002 used the Katz index to assess the level of independence in activities of daily living. Both the steroid plus group and the control group had equal medians at both six and 12 months (median = 6); however, the range was narrower and towards improved independence at 12 months in the steroid plus group (reported P = 0.05; Analysis 2.1).
2.1. Analysis.
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 1 Katz index of ADL (Score 0 to 6; 6 = completely independent, 0 = totally dependent).
| Katz index of ADL (Score 0 to 6; 6 = completely independent, 0 = totally dependent) | ||||
|---|---|---|---|---|
| Study | Assessment (best estimate N given) | Steroid plus nutrition group | Control group | Reported P value |
| Hedstrom 2002 | 6 months: median (range); N | 6 (5 to 6); N = 26 | 6 (5 to 6); N = 24 | 0.7 |
| Hedstrom 2002 | 12 months: median (range); N | 6 (5 to 6); N = 21 | 6 (2 to 6); N = 23 | 0.05 |
Tidermark 2004 based their functional assessment on the results of the Katz Index and used graphs to display the results grouped by independence in five or all six activities of daily living functions versus dependence in bathing and at least one more function. Fewer participants in the anabolic steroid group were either dependent in at least two functions, including bathing, or dead at six months (0/17 versus 8/16; RR 0.06, 95% CI 0.00 to 0.89; P = 0.04) and 12 months (1/17 versus 7/18; RR 0.15, 95% CI 0.02 to 1.10; P = 0.06) (Analysis 2.2). One person in the control group had died within 12 months.
2.2. Analysis.
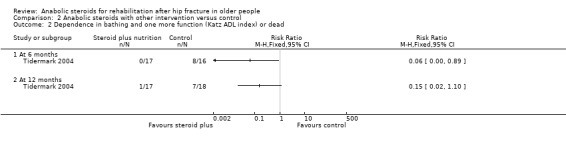
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 2 Dependence in bathing and one more function (Katz ADL index) or dead.
Hedstrom 2002 reported significantly higher Harris hip scores in the steroid plus group at six months (median 86 versus 72; reported P = 0.006) and 12 months (median 88.5 versus 79; reported P = 0.04) (Analysis 2.3), suggesting a better outcome for this group.
2.3. Analysis.
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 3 Harris hip score (100 point scale; higher score = better outcome).
| Harris hip score (100 point scale; higher score = better outcome) | ||||
|---|---|---|---|---|
| Study | Assessment (best estimate N given) | Steroid plus nutrition group | Control group | Reported P value |
| Hedstrom 2002 | 6 months: median (range); N | 86 (59 to 100); N = 26 | 72 (42 to 91); N = 24 | 0.006 |
| Hedstrom 2002 | 12 months: median (range); N | 88.5 (64 to 100); N = 21 | 79 (48 to 98); N = 23 | 0.04 |
Gait speed
Hedstrom 2002 reported significantly higher gait speeds in the steroid plus group at both six months (P = 0.007) and 12 months (P = 0.009) post‐intervention (Analysis 2.4).
2.4. Analysis.
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 4 Gait speed (s/30m).
| Gait speed (s/30m) | ||||
|---|---|---|---|---|
| Study | Assessment (best estimate N given) | Steroid plus nutrition group | Control group | Reported P value |
| Hedstrom 2002 | 6 months: median (range); N | 34 (20 to 70); N = 26 | 50 (20 to 145); N = 24 | 0.007 |
| Hedstrom 2002 | 12 months: median (range); N | 30 (20 to 90); N = 21 | 46 (25 to 250); N = 23 | 0.009 |
Adverse events
Hedstrom 2002 reported four deaths, two in each group, up to 12 months follow‐up (Analysis 2.5). Seven participants, four in the steroid plus group and three in the control group developed pseudarthrosis or avascular necrosis and underwent arthroplasty subsequently (Analysis 2.6). Three participants of the steroid group had side effects such as hoarseness or increased facial hair.
2.5. Analysis.
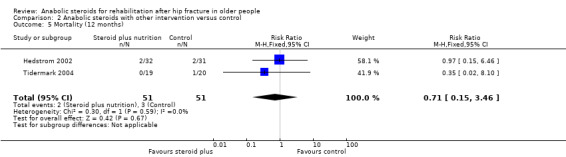
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 5 Mortality (12 months).
2.6. Analysis.
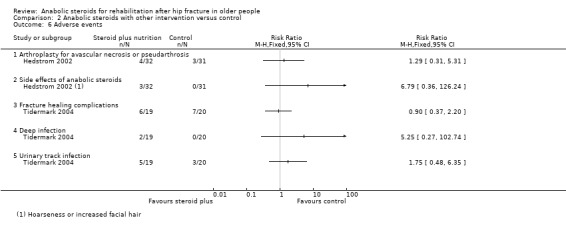
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 6 Adverse events.
In Tidermark 2004, one person died in the control group. Fracture healing complications were recorded in six participants of the steroid plus group versus seven of the control group; and urinary tract infection was reported for five versus three participants (Analysis 2.6). A slight and transient elevation of serum calcium was noted in six participants of the steroid group. Two participants in the steroid group developed deep infection.
Secondary outcomes
Patients' perceived quality of life
Tidermark 2004 captured patient‐rated HRQoL during the week preceding the injury using the EuroQol (EQ‐5D). A logistic regression analysis was performed in order to further evaluate factors of importance for changes in the HRQoL. Randomisation to the steroid plus group was associated with an increased odds ratio for improved HRQoL at the end of the six month intervention period (P < 0.05). These results were presented as a graph only with no presentation of actual values, means or standard deviations.
Adherence and acceptability of the intervention
Hedstrom 2002 stated that participants who discontinued treatment "were analysed by intention‐to‐treat", but gave only limited information relating to adherence. Four people, two in each group, declined to participate in the study after their hip surgery and two others (group not stated) appeared to have stopped due to ill health at seven and eight months. There were several others lost to follow‐up, including four deaths. Aside from one person allocated in the steroid plus group who withdrew her consent, Tidermark 2004 reported 100% compliance for the anabolic steroid administration and that, based on interviews and a logbook, there appeared to be "no indications of inadequate compliance" regarding the protein supplementation or vitamin D and calcium interventions.
Neither trial reported directly on the acceptability of the intervention to the participants.
Objective assessments of body composition
The data for the various measures relating to body composition presented in Hedstrom 2002 are shown in Analysis 2.7. No measures of variability were reported. The differences between the two groups in the changes in the body mass index or skinfold thickness between groups at six and 12 months were generally statistically not significant. There was a significantly greater increase in muscle volume in the steroid plus group at six months (P = 0.004) and at 12 months (P = 0.002) in the non‐operated leg. The operated leg results also favoured the steroid plus group (P = 0.01 at both follow‐ups). Hedstrom 2002 calculated relative muscle mass to demonstrate that these differences were not due to intracellular fluid accumulation, a known side‐effect of anabolic steroid treatment.
2.7. Analysis.
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 7 Body composition.
| Body composition | ||||
|---|---|---|---|---|
| Study | Assessment | Steroid plus nutrition group | Control group | Reported P value |
| BMI (kg/m²) | ||||
| Hedstrom 2002 | 6 months: mean change | ‐1.3 | ‐1.0 | "NS" (> 0.05) |
| Hedstrom 2002 | 12 months: mean change | ‐1.6 | ‐0.5 | "NS" (> 0.05) |
| Muscle volume operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | ‐0.1 | ‐7.1 | 0.01 |
| Hedstrom 2002 | 12 months: mean change | 3 | ‐6.3 | 0.01 |
| Muscle volume non‐operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | 12.6 | 8.6 | 0.004 |
| Hedstrom 2002 | 12 months: mean change | 14.1 | 8.1 | 0.002 |
| Relative muscle mass operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | 339 | ‐25 | 0.03 |
| Hedstrom 2002 | 12 months: mean change | 617 | ‐161 | 0.002 |
| Relative muscle mass non‐operated leg (cm²) | ||||
| Hedstrom 2002 | 6 months: mean change | 836 | 573 | 0.02 |
| Hedstrom 2002 | 12 months: mean change | 1049 | 434 | 0.0005 |
| Triceps skinfold (mm) | ||||
| Hedstrom 2002 | 6 months: mean change | ‐1.6 | 0.5 | 0.04 |
| Hedstrom 2002 | 12 months: mean change | ‐1.6 | 0.6 | "NS" (> 0.05) |
Tidermark 2004 found no statistically significant differences between the two groups in the decline in body weight and lean body mass (measured using dual energy X‐ray absorptiometry) at 12 months (Analysis 2.8).
2.8. Analysis.
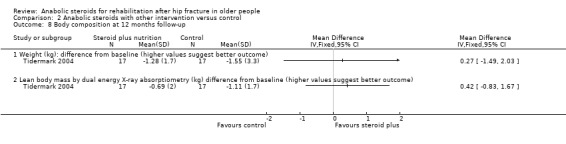
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 8 Body composition at 12 months follow‐up.
Nutritional indices
Tidermark 2004 found no statistically significant difference between the two groups in changes in albumin at 12 months (Analysis 2.9).
2.9. Analysis.
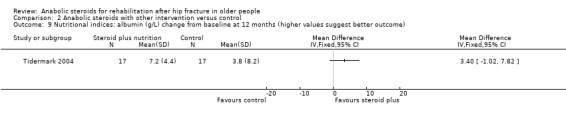
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 9 Nutritional indices: albumin (g/L) change from baseline at 12 months (higher values suggest better outcome).
Muscle strength
Tidermark 2004 reported on hand grip strength as a measure of muscle strength. Although the results favoured the steroid plus group, the difference between the two groups in grip strength at one year did not reach statistical significance (P = 0.16) (Analysis 2.10).
2.10. Analysis.
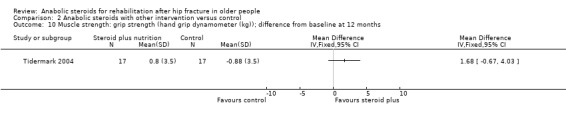
Comparison 2 Anabolic steroids with other intervention versus control, Outcome 10 Muscle strength: grip strength (hand grip dynamometer (kg)); difference from baseline at 12 months.
Use of resources such as length of hospital stay
With the exception of length of hospital stay data for Tidermark 2004, neither trial reported resource use or costs outcomes. Tidermark 2004 reported that the difference in length of hospital stay in the first year after surgery was not significant (median (range): 18 (8 to 51) days versus 27 (5 to 197) days).
Discussion
Summary of main results
The aim of this review was to evaluate the effect of anabolic steroids on functional outcome after surgical treatment of hip fracture in older people. We included three heterogeneous randomised controlled trials, involving a total of 154 female patients after hip fracture surgery. As well as being small trials, all three had methodological shortcomings that placed them at high or unclear risk of bias. One trial had three groups and contributed data to the two main comparisons listed in our Objectives. Reflecting the high risk of bias of the included trials, the imprecision of the results and the risk of publication bias, we concluded that the evidence for all primary outcomes is of very low quality, which means that we are very uncertain about the results.
Anabolic steroid versus control (no anabolic steroid or a placebo intervention)
The two trials that compared anabolic steroid with control tested this in very different settings and patient populations. Sloan 1992, a 'pilot' trial, compared anabolic steroid injections versus placebo injections given weekly until discharge from hospital or four weeks, whichever came first, in 31 "frail elderly females" (mean age 82 years). Tidermark 2004 compared anabolic steroid injections every three weeks for six months and daily protein supplementation versus daily protein supplementation alone, in 40 "lean elderly women" (mean age 82 years) who were followed up for one year post‐surgery. These differences and the incompatible outcome measurement, including timing of follow‐up, precluded pooling of data. Sloan 1992 provided very low quality evidence of little difference between the two groups in the combined outcome of discharge to a higher level of care or dead, in the restoration of mobility (time to weight‐bear; time to mobilise independently) or in individual adverse events. It is plausible that the tendency in the steroid group to take longer to weight‐bear and walk independently could relate to the poorer pre‐fracture function of this group. One participant in this group withdrew after a doubling of serum liver enzymes. Tidermark 2004 provided very low quality evidence that anabolic steroid may result in less dependency, assessed in terms of being either dependent in at least two functions or dead at six and 12 months, but the result was also compatible with no difference or an increase in dependency. Tidermark 2004 found no evidence of between‐group differences in adverse events (fracture healing complications; urinary tract infection). Both trials reported nearly complete compliance with the administration of the steroid injection. Neither trial reported on patient acceptability of the intervention.
Anabolic steroid plus nutritional co‐intervention (steroid plus) versus control (no anabolic steroid plus nutritional co‐intervention)
Although the study design and patient population of two trials that compared anabolic steroid plus a nutritional co‐intervention versus control were sufficiently similar to warrant pooling, this was possible only for one‐year mortality. Hedstrom 2002 compared anabolic steroid injections every three weeks for 12 months and a daily supplement of vitamin D versus control in 63 women (mean age 80.5 years) who were living independently at home after hip fracture surgery. Tidermark 2004 compared anabolic steroid injections every three weeks for six months and daily protein supplementation versus control in 40 "lean elderly women" (mean age 83 years) who were followed up for one year post‐surgery.
Although data could not be pooled, both trials found some very low quality evidence of better function in the steroid plus group. One trial reported a narrower distribution of Katz scores towards greater independence in the steroid plus group at 12 months. The same trial reported significantly higher Harris hip scores and gait speeds for both six months and 12 months follow‐up times in the steroid plus group. The second trial found fewer participants in the anabolic steroid group were either dependent in at least two functions, including bathing, or dead at six months and 12 months (one person in the control group had died within 12 months). Pooled mortality data from the two trials showed no evidence of a difference between the two groups at one year. Similarly, there was no evidence of between‐group differences in adverse events. However, Hedstrom 2002 reported that three participants (9.4%) of the steroid group had side effects (hoarseness or increased facial hair) of anabolic steroids while Tidermark 2004 reported better quality of life in the steroid plus group. Tidermark 2004 also reported nearly complete compliance with the administration of the steroid injection. Neither trial reported on patient acceptability of the intervention.
Overall completeness and applicability of evidence
All three trials were small and pooling of data was undertaken for one outcome (mortality) from two trials only.
Since hip fractures occur predominantly in older females, the restriction to older females in the three trials is acceptable although it limits applicability. A further limitation is that all three trials excluded patients with severe cognitive impairment or dementia, which is present in around 25% to 33% of the typical hip fracture population. Other considerations include that Sloan 1992 was an inpatient study, with a more representative population in the hospital setting, whereas Hedstrom 2002 was an outpatient trial that included only people who were able to live independently in their own home after recovery from hip fracture surgery.
As well as differing in their populations, the trials differed in their interventions, co‐interventions, control groups, durations and outcome assessment. Sloan 1992 used weekly nandrolone decanoate intramuscular injections at a dose of 2 mg/kg until discharge or four weeks, whichever came first. In contrast, Hedstrom 2002 and Tidermark 2004 used 25 mg intramuscular nandrolone injections every three weeks for 12 and six months, respectively. These two trials also used steroids in combination with either protein supplement or Vitamin D. Combining interventions adds complexity to the interpretation as interaction between interventions could be responsible for the effects, or the effect could be solely attributed to the nutritional intervention other than the anabolic steroid. The variability in the duration and dosages of anabolic steroids makes it difficult to establish what doses should be tested in future studies. Cummings 1988 and Koval 1995 have documented the deterioration in function following hip fracture and the deterioration in the control group in Tidermark 2004 at six months is not unexpected.
There was a lack of information on what constituted "standard rehabilitation" in Sloan 1992, but the average length of hospital stay in this trial is noticeably longer than that reported in a recent study carried out in a nearby hospital (43 versus 24 days) in Vancouver (Lefaivre 2009). Similarly, the prolonged treatment regimen in both Hedstrom 2002 and Tidermark 2004 is not customary in many countries.
The follow‐up until discharge in Sloan 1992 resulted in bias because of the missing data for participants at the four‐week time point. The presentation of outcome data relating to dependence was inadequate in all three trials. In Sloan 1992, this was reported as a change in the level of care but it is unclear how permanent this change was and what exactly this meant. Hedstrom 2002 provided inadequate data to assess their conclusions, while Tidermark 2004 provided dichotomised data that may hide more subtle variation. Side effects of anabolic steroids were reported in the three trials, the most serious one occurring in a male patient in Sloan 1992. These trials were too small to get any meaningful measure of the harms of anabolic steroid treatment. However, Hedstrom 2002 reported three participants with well known side effects (hoarseness and facial hair) of anabolic steroids in females, and this points to the importance of recording patient acceptability of this intervention, which was not done in these trials.
Quality of the evidence
Overall, this review presents very low quality evidence for the effects of anabolic steroids for rehabilitation of hip fractures in older people. Each of three small trials were at high risk of bias in one or more domains evaluated in our assessment. Despite the use of a placebo control group in Sloan 1992, there was a major baseline imbalance in participant characteristics that could have affected the findings of this trial. Post‐randomisation exclusions and failure to follow‐up patients after hospital discharge also reduces the quality of the evidence on the use of anabolic steroids in the inpatient setting. The two studies (Tidermark 2004 and Hedstrom 2002) that reported positive results, favouring the use of anabolic steroid in combination with nutritional interventions, were not adequately blinded as no placebo injections were given to the control group. The interaction between interventions could be responsible for the effects, or the effect could be solely attributed to the nutritional intervention other than the anabolic steroid. As well as downgrading for limitations in design and implementation (risk of bias), we downgraded the evidence for imprecision (all three trials) and publication bias (all three trials), reflecting the very small numbers of published trials on this topic.
Potential biases in the review process
Although we conducted extensive searches and were careful and systematic in our screening processes, it is possible that we may have failed to identify studies, especially those that are unpublished. Additionally, we may have missed published trials that are only listed in other databases such as CINAHL and LILACS. We were unsuccessful in our attempts to get further information and data on the included trials. Deviations and changes from protocol described in Differences between protocol and review were few and unlikely to bias the review findings.
Agreements and disagreements with other studies or reviews
To our knowledge there have been no previous systematic reviews on the use of anabolic steroids for recovery after hip fracture surgery. There is some moderate quality evidence showing improved outcomes in patients with hip fractures when nutritional supplementation has been used (Avenell 2010), which points to the importance of considering anabolic steroids as part of a package of interventions aimed at improving recovery. We have not identified cohort studies monitoring the short‐term or long‐term adverse effects of anabolic steroids in older people.
Authors' conclusions
Implications for practice.
The available evidence is insufficient to draw conclusions on the effects, primarily in terms of functional outcome and adverse events, of anabolic steroids, either separately or in combination with nutritional supplements, after surgical treatment of hip fracture in older people.
Implications for research.
The importance of identifying effective and safe interventions, applied either alone or in combination, that improve the poor outcome of people recovering from hip fracture continues. Further randomised trials are warranted. As the available data from this review points to the potential for more promising outcomes with a combined anabolic steroid and nutritional supplement intervention, we suggest that future research should focus on the use of this combination. The potential toxicity of anabolic steroids and adverse effects (particularly in females) is a barrier to further research, and also deters clinicians from prescribing these drugs. This points to the crucial need to collect data on adverse effects and also on patient acceptability of the intervention.
One area that requires investigation is the effect of anabolic steroids in male hip fracture patients who may have normal or low androgen profiles. This is important, as even though the population is smaller than female hip fractures, the outcomes after hip fracture in males are worse.
Functional outcomes, such as independence in mobility and daily activities, and monitoring of adverse events are important clinical outcomes for older people who are recovering from hip fracture and should be the primary outcomes of future hip fracture trials. As recommended in Crotty 2010, a core set of functional outcomes should be used in hip fracture trials with strict adherence to reporting standards to obtain consistent data that allows for more robust analysis in meta‐analyses.
Acknowledgements
The authors are grateful for valuable comments and help from Lesley Gillespie, Helen Handoll, Amy Kavanagh and Ronald Koretz on drafts of the protocol, and to Ian Chapman for his contribution as a co‐author to the protocol. The authors are grateful also to Lesley Gillespie for her assistance with developing the search strategies. The authors are grateful for valuable comments and help from Fulvia Baldassarre, Anna Gagliardi, Joanne Elliott, Lindsey Elstub, Helen Handoll, Laura MacDonald and Haris Vasiliadis on drafts of the review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (Wiley Online Library)
#1 MeSH descriptor: [Femoral Fractures] explode all trees (1184) #2 ((hip* or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular* or ((femur* or femoral*) near/3 (neck or proximal or head))) near/4 fracture*) (2541) #3 #1 or #2 (2659) #4 MeSH descriptor: [Steroids] explode all trees (36182) #5 MeSH descriptor: [Androgens] explode all trees (526) #6 MeSH descriptor: [Anabolic Agents] explode all trees (259) #7 anabolic near/1 steroid* (227) #8 androgen* near/1 anabolic (58) #9 etiocholanolone or androst* or prasterone or stanolone or testosterone or methyltestosterone or metribolone or ethylestrenol or fluoxymesterone or mesterolone or methandriol or methandrostenolone or methenolone or nandrolone or norethandrolone or oxandrolone or oxymetholone or stanozolol or trenbolone or amafolone or atromid or benorterone or boldenone or calusterone or danazol or drostanolone or etiocholanone or mestanolone or mibolerone or testololactone or hydroxyandrost* or epiandrosterone or oxotestosterone or oxoandrostenedione (6312) #10 #4 or #5 or #6 or #7 or #8 or #9 (37878) #11 #3 and #10 in Trials (113)
MEDLINE (OvidSP)
1 exp Femoral Fractures/ (29353) 2 ((hip* or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular* or ((femur* or femoral*) adj3 (neck or proximal or head))) adj4 fracture*).mp. (25085) 3 1 or 2 (35370) 4 exp Steroids/ (710010) 5 exp Androgens/ (82963) 6 exp Anabolic Agents/ (12095) 7 (anabolic adj1 steroid*).mp. (3830) 8 (androgen* adj1 anabolic).mp. (1261) 9 (etiocholanolone or androst* or prasterone or stanolone or testosterone or methyltestosterone or metribolone or ethylestrenol or fluoxymesterone or mesterolone or methandriol or methandrostenolone or methenolone or nandrolone or norethandrolone or oxandrolone or oxymetholone or stanozolol or trenbolone or amafolone or atromid or benorterone or boldenone or calusterone or danazol or drostanolone or etiocholanone or mestanolone or mibolerone or testololactone or hydroxyandrost* or epiandrosterone or oxotestosterone or oxoandrostenedione).mp. (112744) 10 or/4‐9 (742213) 11 3 and 10 (1155) 12 Randomized controlled trial.pt. (384981) 13 Controlled clinical trial.pt. (89120) 14 randomized.ab. (300266) 15 placebo.ab. (161737) 16 Drug therapy.fs. (1748887) 17 randomly.ab. (212565) 18 trial.ab. (316255) 19 groups.ab. (1352336) 20 or/12‐19 (3382653) 21 exp Animals/ not Humans/ (4027008) 22 20 not 21 (2899484) 23 11 and 22 (598)
EMBASE (OvidSP)
1 exp Hip Fracture/ (28832) 2 ((hip* or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular* or ((femur* or femoral*) adj3 (neck or proximal or head))) adj4 fracture*).mp. (37173) 3 1 or 2 (38355) 4 exp Steroid Therapy/ (65380) 5 exp Androgen/ (147750) 6 exp Anabolic Agent/ (22104) 7 (anabolic adj1 steroid*).mp. (4260) 8 (androgen* adj1 anabolic).mp. (1507) 9 (etiocholanolone or androst* or prasterone or stanolone or testosterone or methyltestosterone or metribolone or ethylestrenol or fluoxymesterone or mesterolone or methandriol or methandrostenolone or methenolone or nandrolone or norethandrolone or oxandrolone or oxymetholone or stanozolol or trenbolone or amafolone or atromid or benorterone or boldenone or calusterone or danazol or drostanolone or etiocholanone or mestanolone or mibolerone or testololactone or hydroxyandrost* or epiandrosterone or oxotestosterone or oxoandrostenedione).mp. (156319) 10 or/4‐9 (245815) 11 3 and 10 (1234) 12 Randomized controlled trial/ (357881) 13 Clinical trial/ (892653) 14 Controlled clinical trial/ (405007) 15 Randomization/ (63369) 16 Single blind procedure/ (18204) 17 Double blind procedure/ (119927) 18 Crossover procedure/ (38360) 19 Placebo/ (237582) 20 Prospective study/ (248905) 21 ((clinical or controlled or comparative or placebo or prospective* or randomi#ed) adj3 (trial or study)).tw. (738968) 22 (random* adj7 (allocat* or allot* or assign* or basis* or divid* or order*)).tw. (178166) 23 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).tw. (165101) 24 (cross?over* or (cross adj1 over*)).tw. (70352) 25 ((allocat* or allot* or assign* or divid*) adj3 (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)).tw. (229163) 26 RCT.tw. (12530) 27 or/12‐26 (1901953) 28 Case Study/ or Abstract Report/ or Letter/ (924227) 29 27 not 28 (1863905) 30 11 and 29 (480)
WHO International Clinical Trials Registry Platform
Advanced search option
Condition: hip% OR femur% OR femoral
Intervention: anabolic OR steroid% OR androgen OR etiocholanolone OR androst% OR prasterone OR stanolone OR testosterone OR methyltestosterone OR metribolone OR ethylestrenol OR fluoxymesterone OR mesterolone OR methandriol OR methandrostenolone OR methenolone OR nandrolone OR nORethandrolone OR oxandrolone OR oxymetholone OR stanozolol OR trenbolone OR amafolone OR atromid OR benORterone OR boldenone OR calusterone OR danazol OR drostanolone OR etiocholanone OR mestanolone OR mibolerone OR testololactone OR hydroxyandrost% OR epiandrosterone OR oxotestosterone OR oxoandrostenedione
Recruitment status: all
Current Controlled Trial
(femoral OR femur OR hip OR hips) AND (fracture OR fractures) AND (anabolic ) [All Registers]
Data and analyses
Comparison 1. Anabolic steroids versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 More dependent or dead at hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Dependence in bathing and one more function (Katz ADL index) or dead | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mobility (inpatient) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Days to weight‐bear with support | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Days to mobilise independently | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 In hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Fracture healing complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Urinary track infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Thrombotic complication (deep vein thrombosis or pulmonary embolism) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Gastrointestinal bleed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Postoperative depression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Postoperative delirium | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Body composition at 4 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Weight (kg) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Midarm muscle circumference (cm²) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Lean body mass measured by subscapular skinfold (kg) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Lean body mass via bioelectric impedence (kg) (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Body composition at 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Weight (kg): difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Lean body mass by dual energy X‐ray absorptiometry (kg) difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Nutritional indices | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Albumin (g/L) at 4 weeks (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Albumin (g/L) change from baseline at 12 months (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Haemoglobin (g/L) at 4 weeks (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Muscle strength | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Grip strength (modified sphygmomanometer (kPa)) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Grip strength (hand grip dynamometer (kg)); difference from baseline at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Length of hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Anabolic steroids with other intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Katz index of ADL (Score 0 to 6; 6 = completely independent, 0 = totally dependent) | Other data | No numeric data | ||
| 2 Dependence in bathing and one more function (Katz ADL index) or dead | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Harris hip score (100 point scale; higher score = better outcome) | Other data | No numeric data | ||
| 4 Gait speed (s/30m) | Other data | No numeric data | ||
| 5 Mortality (12 months) | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.15, 3.46] |
| 6 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Arthroplasty for avascular necrosis or pseudarthrosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Side effects of anabolic steroids | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Fracture healing complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Deep infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Urinary track infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Body composition | Other data | No numeric data | ||
| 7.1 BMI (kg/m²) | Other data | No numeric data | ||
| 7.2 Muscle volume operated leg (cm²) | Other data | No numeric data | ||
| 7.3 Muscle volume non‐operated leg (cm²) | Other data | No numeric data | ||
| 7.4 Relative muscle mass operated leg (cm²) | Other data | No numeric data | ||
| 7.5 Relative muscle mass non‐operated leg (cm²) | Other data | No numeric data | ||
| 7.6 Triceps skinfold (mm) | Other data | No numeric data | ||
| 8 Body composition at 12 months follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Weight (kg): difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Lean body mass by dual energy X‐ray absorptiometry (kg) difference from baseline (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Nutritional indices: albumin (g/L) change from baseline at 12 months (higher values suggest better outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Muscle strength: grip strength (hand grip dynamometer (kg)); difference from baseline at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hedstrom 2002.
| Methods | Randomised controlled trial | |
| Participants | 63 women (mean age 80.5 years) aged over 65 years who had been treated surgically (internal fixation) for a hip fracture, living independently in their homes Inclusion criteria: living independently in own home after hip fracture surgery, aged over 65 years Exclusion criteria: treatment with bone active drugs; metabolic disease that could affect the bone density; smoker; fracture of contralateral hip; cerebrovascular disease affecting mobility; liver, thyroid or renal dysfunction; alcohol abuse; or dementia |
|
| Interventions | Steroid group: nandrolone decanoate (25 mg intramuscular every 3 weeks) + 1alpha‐hydroxylated VitD3 (alphacalcidol 0.25 µg daily) + calcium (500 mg daily), N = 32 Control group: calcium only, N = 31 Intervention period: 12 months Analysis: 25 versus 26 at 12 months, but see Notes |
|
| Outcomes | Follow‐up: assessments at 3, 6, 9 and 12 months (data from 6 and 12 months) 1. Katz index (activities of daily living) 2. Harris hip score 3. Gait speed 4. Pain 5. Mortality 6. Adverse events 7. Body composition 8. Bone densitometry (not reported in review) |
|
| Setting | Community setting, Danderyd, Sweden | |
| Notes | The numbers followed up at 6 and 12 months were provided but the group allocation was unclear for two patients excluded because of ill health, and (although likely) whether these were the same two patients excluded because of progressive cholangitis and primary hyperparathyroidism, respectively. Additionally, seven patients followed for one year who had arthroplasty for pseudarthrosis or avascular necrosis were not included in the assessment of clinical function. Thus there is some uncertainty about the denominators for these outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating random sequences not described |
| Allocation concealment (selection bias) | Unclear risk | "The patients were allocated by sealed envelopes to receive either treatment for one year with nandrolone decanoate (25 mg intramuscularly every third week), a daily supplement of 1alpha‐hydroxylated vitamin D3 (alphacalcidol 0.25 g) and calcium (500 mg), or to receive calcium only" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | While drop‐outs are described and numbers available at follow‐up are stated, there is a lack of clarity regarding the exclusions of some participants (e.g. "Two patients were subsequently excluded because of poor health") and numbers available for the clinical assessments. The authors state that "the patients who discontinued treatment were analysed by intention to treat, except for two who were followed up for seven and eight months". It is likely that these were the same two patients, but it is not clear |
| Selective reporting (reporting bias) | Unclear risk | The method section does not describe a protocol for the trial but all the outcomes mentioned in the methods section were reported in the results. The method of reporting the results and whether there was an original intention to report results at 3 and 9 months was not clarified |
| Other bias | Low risk | No signs of baseline differences between the two groups. The trial was carried out during the intended study period with no early termination |
Sloan 1992.
| Methods | Randomised, double‐blind, placebo‐controlled pilot study | |
| Participants | 39 participants (31 female and 8 male) aged over 65 years treated surgically (internal fixation or hemiarthoplasty) for hip fracture. The study reported characteristics of 29 females (mean age 82 years) Inclusion criteria: "frail elderly" patients with hip fractures, written informed consent obtained Exclusion criteria: aged under 65, in extended care (highest level of nursing care) prior to admission, had severe dementia impeding participation, severe medical illnesses, hormone responsive conditions such as breast cancer or liver disease |
|
| Interventions | Steroid group: nandrolone decanoate (2 mg/kg weekly) + standard rehabilitation, N = 19 Control group: placebo + standard rehabilitation, N = 20 Intervention period for duration of hospital stay or until 4 weeks, whichever came first Analysis: 15 versus 14 (females only) |
|
| Outcomes | Follow‐up: assessments weekly until 4 weeks or discharge, whichever came first 1. 'Rehabilitation end points': days to weight‐bear with support; days to mobilise independently; length of hospital stay 2. In hospital mortality and discharge level of care 3. Adverse events 4. Body composition 5. Nutritional indices 6. Muscle strength 7. Cognitive function |
|
| Setting | Inpatient setting, University Hospital Vancouver, Canada | |
| Notes | The study report presented detailed results for the female participants and a brief incomplete summary of the results for the male participants. We report only on the adverse events for this second group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double‐blind: "Subjects were randomized to therapy with either nandrolone decanoate 2 mg/kg or placebo in a double‐blind fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated double‐blind: "Subjects were randomized to therapy with either nandrolone decanoate 2 mg/kg or placebo in a double‐blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐outs described: Two early withdrawals (1 in the steroid group had abnormal liver function tests; 1 in the control group had nightmares) were excluded from the analyses. Data from 10 out of 29 remaining participants were missing at week four as these patients were discharged prior to the completion of this assessment; there was an imbalance between the two groups (12 versus 7) available for analysis at 4 weeks |
| Selective reporting (reporting bias) | Unclear risk | The methods section does not describe any pre‐agreed protocol for the trial but all the outcomes mentioned in the methods section were reported in the results. It was not clear that there was a prior intention to present the full results only for the female participants |
| Other bias | High risk | There was imbalance, based on recorded activities of daily living scores, between the groups in functional independence in favour of the control group. The steroid group tended to be lighter and have a lower lean body mass |
Tidermark 2004.
| Methods | Randomised controlled trial | |
| Participants | 60 women (mean age 82.9 years) aged over 70 years treated surgically (internal fixation) for an acute femoral neck fracture Inclusion criteria: aged over 70 years, body mass index (BMI) less than or equal to 24 kg/m², without severe cognitive dysfunction, independent living status and independent walking capability with or without walking aids Exclusion criteria: fracture not suitable for internal fixation, i.e. pathological fractures or were displaced fractures older than 24 hours at the time of arrival at the emergency room; rheumatoid arthritis; or radiographic osteoarthritis |
|
| Interventions | Steroid group: nandrolone decanoate (Deca‐Durabol 25 mg every 3 weeks) + protein‐rich formula (Fortimel 200ml/day, 20 g protein/day) + daily calcium (1g) and vitamin D (CalchichewD3 800 IE), N = 20 Protein supplementation group: protein‐rich formula (Fortimel) + daily calcium + vitamin D, N = 20 Control group: daily calcium and vitamin D, N = 20 Intervention period: 6 months Analysis: 17 versus 18 versus 17 at 12 months |
|
| Outcomes | Follow‐up: assessments at 6 and 12 months 1. Katz index (activities of daily living) 2. EQ‐5D 3. Mobility (data not shown in trial report) 4. Mortality 5. Fracture healing complications 6. Urinary tract infections 7. Nutritional indices 8. Body composition 9. Length of hospital stay |
|
| Setting | Soder Hospital then in the community, Stockholm, Sweden | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described |
| Allocation concealment (selection bias) | Low risk | "The patients were randomised, using opaque, sealed envelopes, to open treatment during 6 months" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was an open label study with no blinding of participants. Of note, however, is that the research nurse visited all patients "in order to minimise differences between treatments" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "A research nurse not involved in the surgery or clinical decisions assessed all clinical variables." No mention of blinding, however |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient flowchart provided: "One patient in the PR/N group withdrew her consent after inclusion and was accordingly excluded from the follow‐up. Two patients (3%) died and 5 (8%) were lost to follow‐up, giving a total of 52 out of 59 patients (88%) available at the final follow‐up (Fig. 1)." Intention‐to‐treat analysis not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No description of protocol provided in the text of the review but the outcomes mentioned in the methods section were all reported in the results. Actual figures of the Katz Index were not provided. Instead the results were dichotomised into functionally independent and functionally dependent groups and the results were presented as a graph, which makes it impossible to pool results in meta‐analysis. EQ‐5D scores were also presented as graph only using linear regression. No mean scores or mean change scores were provided. Posthoc analysis was performed but was not intended and it was published separately |
| Other bias | Low risk | No signs of baseline differences among the groups and no signs of early termination of the trial |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beringer 1986 | The focus of this study was on the role of calcitonin in the aetiology of postmenopausal osteoporosis. The comparison, anabolic steroid versus calcium, did not meet the inclusion criteria in the review. |
| Naessen 2008 | The focus of this study was postural balance function in older women. However, the study population was healthy females with no hip fracture; i.e. wrong population. |
Characteristics of ongoing studies [ordered by study ID]
NCT00280267.
| Trial name or title | Testosterone therapy after hip fracture in elderly women |
| Methods | Randomised, double‐blinded, placebo‐controlled prospective study to determine the feasibility, tolerability and safety of six months of testosterone therapy in community‐dwelling, physically frail, elderly female hip fracture patients |
| Participants | Planned recruitment: 27 female hip fracture patients, using objective criteria for testosterone deficiency and frailty |
| Interventions | Evaluate two dosages of testosterone, administered as a 0.5% topical gel: a supraphysiologic dosage versus physiologic replacement dosage |
| Outcomes | Primary outcomes: serum testosterone levels, drug compliance, symptoms and side effects during the six months of treatment Secondary outcomes:
|
| Starting date | Start date: August 2004 |
| Contact information | Ellen F. Binder, Washington University School of Medicine, Division of Geriatrics and Nutritional Science |
| Notes | Completed: December 2006 The author was contacted via email and has confirmed that the data are still unpublished. Results were requested but have not been provided by the author |
Differences between protocol and review
The Background was updated with the addition of more up‐to‐date supporting references.
In our risk of bias assessment we split the blinding domain into two as per updated guidance (Higgins 2011). Contrary to our intentions implied in our protocol, we assessed risk of bias associated with blinding and incomplete outcome data for all outcomes together rather than separately by individual or the main classes of outcomes.
The limited data available from Sloan 1992 meant that we were unable to analyse the impact of drop‐outs on the final result and to adjust results by conducting worst‐ or best‐case scenario sensitivity analysis.
We clarified the basis for interpreting I² values in Assessment of heterogeneity.
We used the GRADE approach to assess the quality of evidence.
Contributions of authors
Vaqas Farooqi contributed to the conception and design of the protocol. He searched electronic databases and conference proceedings, screened titles and abstracts of references identified by the search, selected and assessed trials, extracted trial and outcome data, guided the interpretation of the data, and contributed to and approved the final manuscript of the review.
Maayken van den Berg screened titles and abstracts of references identified by the search, located, selected and assessed trials, extracted trial and outcome data, entered the data, assessed the methodological quality of selected trials, guided and carried out the statistical analyses, helped with the interpretation of the data, and contributed to and approved the final manuscript of the review.
Ian Cameron reviewed the text and provided feedback on the review and interpretation of results.
Maria Crotty contributed to conception and design, provided feedback on the review and interpretation of results and is the guarantor of this review.
Sources of support
Internal sources
-
Repatriation General Hospital, Australia.
Infrastructure to support the review authors affiliated with this institution
-
Rehabilitation Studies Unit, Sydney Medical School, The University of Sydney, Australia.
Infrastructure to support the review author affiliated with this institution
-
The University of Adelaide, Australia.
Infrastructure to support the review author affiliated with this institution
External sources
No sources of support supplied
Declarations of interest
Vaqas Farooqi: none known. Maayken EL Van den Berg: none known. Ian D Cameron: none known. Maria Crotty: is an investigator on two clinical trials of an unrelated drug sponsored by pharmaceutical companies (Eli Lily Pty Ltd and Allergan Eli Lily).
New
References
References to studies included in this review
Hedstrom 2002 {published data only}
- Hedström M, Sjöberg K, Brosjö E, Åström K, Sjöberg H, Dalén N. Changes in soft tissue body composition during treatment with anabolic steroids in women with hip fracture ‐ a prospective randomized study on 64 female patients. Acta Orthopaedica Scandinavica 1998;Suppl 280(69):31. [Google Scholar]
- Hedström M, Sjöberg K, Brosjö E, Åström K, Sjöberg H, Dalén N. Positive effects of anabolic steroids, vitamin D and calcium on muscle mass, bone mineral density and clinical function after a hip fracture: a randomised study of 63 women. Journal of Bone and Joint Surgery ‐ British Volume 2002;84(4):497‐503. [DOI] [PubMed] [Google Scholar]
Sloan 1992 {published data only}
- Sloan JP, Wing P, Dian L, Meneilly GS. A pilot study of anabolic steroids in elderly patients with hip fractures. Journal of the American Geriatrics Society 1992;40(11):1105‐11. [DOI] [PubMed] [Google Scholar]
Tidermark 2004 {published data only}
- Carlsson P, Tidermark J, Ponzer S, Soderqvist A, Cederholm T. Food habits and appetite of elderly women at the time of a femoral neck fracture and after nutritional and anabolic support. Journal of Human Nutrition and Dietetics 2005;18(2):117–20. [DOI] [PubMed] [Google Scholar]
- Tengstrand B, Cederholm T, Söderqvist A, Tidermark J. Effects of protein‐rich supplementation and nandrolone on bone tissue after a hip fracture. Clinical Nutrition 2007;26(4):460‐5. [DOI] [PubMed] [Google Scholar]
- Tidermark J. Quality of life and femoral neck fractures. Acta Orthopaedica Scandinavica 2003;74(309):1‐42. [PubMed] [Google Scholar]
- Tidermark J, Ponzer S, Carlsson P, Soderqvist A, Brismar K, Tengstrand B, et al. Effects of protein‐rich supplementation and nandrolone in lean elderly women with femoral neck fractures. Clinical Nutrition 2004;23(4):587‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beringer 1986 {published data only}
- Beringer TR, Ardill J, Taggart HM. Effect of calcium and stanozolol on calcitonin secretion in patients with femoral neck fracture. Bone and Mineral 1986;1(4):289‐95. [PubMed] [Google Scholar]
Naessen 2008 {published data only}
- Naessen T, Lindmark B, Larsen HC, van Os S, Larsson M. Tibolone low dose (1.25 mg/d) therapy and postural balance in elderly women. Maturitas 2009;62(1):72‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00280267 {unpublished data only}
- NCT00280267. Testosterone therapy after hip fracture in elderly women. www.clinicaltrials.gov/ct2/show/NCT00280267 (accessed 19 February 2013).
Additional references
Adachi 2001
- Adachi JD, Loannidis G, Berger C, Joseph L, Papaioannou A, Pickard L, et al. The influence of osteoporotic fractures on health‐related quality of life in community‐dwelling men and women across Canada. Osteoporosis International 2001;12(11):903‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Avenell 2006
- Avenell A, Handoll HHG. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001880.pub4] [DOI] [PubMed] [Google Scholar]
Avenell 2010
- Avenell A, Handoll HHG. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001880.pub5] [DOI] [Google Scholar]
Bachrach‐Lindström 2000
- Bachrach‐Lindström M, Johansson T, Unosson M, Ek AC, Wahlström O. Nutritional status and functional capacity after femoral neck fractures: a prospective randomized one‐year follow‐up study. Aging 2000;12(5):366‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berger 1996
- Berger JR, Pall L, Hall CD, Simpson DM, Berry PS, Dudley R. Oxandrolone in AIDS‐wasting myopathy. AIDS 1996;10(14):1657‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Braithwaite 2003
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. Journal of American Geriatrics Society 2003;51(3):364‐70. [DOI] [PubMed] [Google Scholar]
Chapman 2009
- Chapman IM, Visvanathan R, Hammond AJ, Morley JE, Field JB, Tai K, et al. Effect of testosterone and a nutritional supplement, alone and in combination, on hospital admissions in undernourished older men and women. American Journal of Clinical Nutrition 2009;89(3):880‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cooper 1992
- Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: A world‐wide projection. Osteoporosis International 1992;2(6):285‐9. [PUBMED: 1421796] [DOI] [PubMed] [Google Scholar]
Crotty 2010
- Crotty M, Unroe K, Cameron I, Miller M, Ramirez G, Couzner L. Rehabilitation interventions for improving physical and psychosocial functioning after hip fracture in older people. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007624.pub3] [DOI] [PubMed] [Google Scholar]
Cummings 1988
- Cummings SR, Phillips SL, Wheat ME, Black D, Goosby E, Wlodarczyk D, et al. Recovery of function after hip fracture. The role of social supports. Journal of the American Geriatrics Society 1988;36(9):801‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Delmi 1990
- Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 1990;335(8696):1013‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dennison 2006
- Dennison E, Mohamed MA, Cooper C. Epidemiology of osteoporosis. Rheumatic Disease Clinics of North America 2006;32:617–29. [DOI] [PubMed] [Google Scholar]
Dorland 2007
- Dorland WAN (editor). Dorland's Medical Dictionary for Health Consumers. Saunders, an imprint of Elsevier, Inc, 2007. [Google Scholar]
Dubljanin‐Raspopović 2013
- Dubljanin‐Raspopović E, Marković‐Denić L, Marinković J, Nedeljković U, Bumbaširević M. Does early functional outcome predict 1‐year mortality in elderly patients with hip fracture?. Clinical Orthopedics and Related Research 2013;471(8):2703‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fierens 2006
- Fierens J, Broos PL. Quality of life after hip fracture. Acta Chirurgica Belgica 2006;106(4):393‐6. [DOI] [PubMed] [Google Scholar]
Gullberg 1997
- Gullberg B, Johnell O, Kanis JA. World‐wide projections for hip fracture. Osteoporosis International 1997;7(5):407‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haentjens 2010
- Haentjens P, Magaziner J, Colón‐Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Annals of Internal Medicine 2010;152(6):380‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnell 1985
- Johnell O, Nilsson BE. Hip fracture and accident disposition. Acta Orthopaedica Scandinavica 1985;56(4):302‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johns 2009
- Johns K, Beddall MJ, Corrin RC. Anabolic steroids for the treatment of weight loss in HIV‐infected individuals. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005483] [DOI] [PubMed] [Google Scholar]
Koval 1995
- Koval KJ, Skovron ML, Aharonoff GB, Meadows SE, Zuckerman JD. Ambulatory ability after a hip fracture. A prospective study in geriatric patients. Clinical Orthopaedics and Related Research 1995;(310):150‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Lefaivre 2009
- Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. Journal of Bone and Joint Surgery ‐ British Volume 2009;91(7):922‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for Studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lumbers 1996
- Lumbers M, Driver LT, Howland RJ, Older MWJ, William CM. Nutritional status and clinical outcome in elderly female surgical orthopaedic patients. Clinical Nutrition 1996;15(3):101‐7. [EMBASE: 1996180532] [DOI] [PubMed] [Google Scholar]
Lumbers 2001
- Lumbers M, New SA, Gibson S, Murphy MC. Nutritional status in elderly female hip fracture patients: comparison with an age‐matched home living group attending day centres. British Journal of Nutrition 2001;85(6):733‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lyritis 1994
- Lyritis GP, Androulakis C, Magiasis B, Charalambaki Z, Tsakalakos N. Effect of nandrolone decanoate and 1‐alpha‐hydroxy‐calciferol on patients with vertebral osteoporotic collapse. A double‐blind clinical trial. Bone and Mineral 1994;27(3):209‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marks 2003
- Marks R, Allegrante JP, MacKenzie CR, Lane JM. Hip fractures among the elderly: causes, consequences and control. Aging Research Reviews 2003;2:57‐93. [DOI] [PubMed] [Google Scholar]
Mithal 2009
- Mithal A, Dhingra V, Beizing LE, International Osteoporosis Foundation. The Asian audit: Epidemiology, costs and burden of osteoporosis in Asia. www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_2009.pdf (accessed 01 Sept 2014).
Navarro 2002
- Navarro JF, Mora C, Macia M, Garcia J. Randomized prospective comparison between erythropoietin and androgens in CAPD patients. Kidney International 2002;61(4):1537‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Osnes 2004
- Osnes EK, Lofthus CM, Meyer HE, Falch JA, Nordsletten L, Cappelen I. Consequences of hip fracture on activities of daily life and residential needs. Osteoporosis International 2004;15:567–74. [DOI] [PubMed] [Google Scholar]
Patterson 1992
- Patterson BM, Cornell CN, Carbone B, Levine B, Chapman D. Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. Journal of Bone and Joint Surgery ‐ American Volume 1992;74(2):251‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Ponzer 1999
- Ponzer S, Tidermark J, Brismar K, Soderqvist A, Cederholm T. Nutritional status, insulin‐like growth factor‐1 and quality of life in elderly women with hip fractures. Clinical Nutrition 1999;18(4):241‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosell 2003
- Rosell PA, Parker MJ. Functional outcome after hip fracture. A 1‐year prospective outcome study of 275 patients. Injury 2003;34(7):529‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2009
- Scottish Intercollegiate Guidelines Network (SIGN). Guideline 56: Prevention and management of hip fracture in older people. A national clinical guideline. www.sign.ac.uk/guidelines/fulltext/111/index.html 2009 (accessed 17 September 2014).
Snyder 1999
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. Journal of Clinical Endocrinology and Metabolism 1999;84(8):2647‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tajeu 2013
- Tajeu GS, Delzell E, Smith W, Arora T, Curtis JR, Saag KG, et al. Death, debility, and destitution following hip fracture. Journals of Gerontology 2014;69(3):346‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tengstrand 2007
- Tengstrand B, Cederholm T, Söderqvist A, Tidermark J. Effects of protein‐rich supplementation and nandrolone on bone tissue after a hip fracture. Clinical Nutrition 2007;26(4):460‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teruel 1996
- Teruel JL, Aguilera A, Marcen R, Navarro Antolin J, Garcia Otero G, Ortuno J. Androgen therapy for anaemia of chronic renal failure. Indications in the erythropoietin era. Scandinavian Journal of Urology and Nephrology 1996;30(5):403‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolinsky 1997
- Wolinsky FD, Fitzgerald JF, Stump TE. The effect of hip fracture on mortality, hospitalization, and functional status: a prospect study. American Journal of Public Health 1997;87(3):398‐403. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zofkova 2000
- Zofkova I, Bahbouh R, Hill M. The pathophysiological implications of circulating androgens on bone mineral density in a normal female population. Steroids 2000;65(12):857‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Farooqi 2010
- Farooqi V, Cameron ID, Chapman I, Couzner L, Crotty M. Anabolic steroids for rehabilitation after hip fracture in older people. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008887] [DOI] [PMC free article] [PubMed] [Google Scholar]


