Abstract
Background
Chronic suppurative otitis media (CSOM) causes ear discharge and impairs hearing.
Objectives
Assess topical antibiotics (excluding steroids) for treating chronically discharging ears with underlying eardrum perforations (CSOM).
Search methods
The Cochrane Ear, Nose and Throat Disorders Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 1, 2005), MEDLINE (January 1951 to March 2005), EMBASE (January 1974 to March 2005), LILACS (January 1982 to March 2005), AMED (1985 to March 2005), CINAHL (January 1982 to March 2005), OLDMEDLINE (January 1958 to December 1965), PREMEDLINE, metaRegister of Controlled Trials (mRCT), and article references.
Selection criteria
Randomised controlled trials; any topical antibiotic without steroids, versus no drug treatment, aural toilet, topical antiseptics, or other topical antibiotics excluding steroids; participants with CSOM.
Data collection and analysis
One author assessed eligibility and quality, extracted data, entered data onto RevMan; two authors inputted where there was ambiguity. We contacted investigators for clarifications.
Main results
Fourteen trials (1,724 analysed participants or ears). CSOM definitions and severity varied; some included otitis externa, mastoid cavity infections and other diagnoses. Methodological quality varied; generally poorly reported, follow‐up usually short, handling of bilateral disease inconsistent. Topical quinolone antibiotics were better than no drug treatment at clearing discharge at one week: relative risk (RR) was 0.45 (95% confidence interval (CI) 0.34 to 0.59) (two trials, N = 197). No statistically significant difference was found between quinolone and non‐quinolone antibiotics (without steroids) at weeks one or three: pooled RR were 0.89 (95% CI 0.59 to 1.32) (three trials, N = 402), and 0.97 (0.54 to 1.72) (two trials, N = 77), respectively. A positive trend in favour of quinolones seen at two weeks was largely due to one trial and not significant after accounting for heterogeneity: pooled RR 0.65 (0.46 to 0.92) (four trials, N = 276) using the fixed‐effect model, and 0.64 (95% CI 0.35 to 1.17) accounting for heterogeneity with the random‐effects model. Topical quinolones were significantly better at curing CSOM than antiseptics: RR 0.52 (95% CI 0.41 to 0.67) at one week (three trials, N = 263), and 0.58 (0.47 to 0.72) at two to four weeks (four trials, N = 519). Meanwhile, non‐quinolone antibiotics (without steroids) compared to antiseptics were more mixed, changing over time (four trials, N = 254). Evidence regarding safety was generally weak.
Authors' conclusions
Topical quinolone antibiotics can clear aural discharge better than no drug treatment or topical antiseptics; non‐quinolone antibiotic effects (without steroids) versus no drug or antiseptics are less clear. Studies were also inconclusive regarding any differences between quinolone and non‐quinolone antibiotics, although indirect comparisons suggest a benefit of topical quinolones cannot be ruled out. Further trials should clarify non‐quinolone antibiotic effects, assess longer‐term outcomes (for resolution, healing, hearing, or complications) and include further safety assessments, particularly to clarify the risks of ototoxicity and whether quinolones may result in fewer adverse events than other topical treatments.
Keywords: Humans; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Anti‐Infective Agents, Local; Anti‐Infective Agents, Local/therapeutic use; Chronic Disease; Developing Countries; Hearing Disorders; Hearing Disorders/drug therapy; Hearing Disorders/etiology; Otitis Media, Suppurative; Otitis Media, Suppurative/complications; Otitis Media, Suppurative/drug therapy; Quinolones; Quinolones/therapeutic use; Randomized Controlled Trials as Topic; Tympanic Membrane Perforation; Tympanic Membrane Perforation/complications; Tympanic Membrane Perforation/drug therapy
A Cochrane systematic review assessing topical antibiotics without steroids for treating chronically discharging ears with underlying eardrum perforations, in participants of any age
Chronic suppurative otitis media (CSOM) is an infection of the middle ear with pus and a persistent perforation in the eardrum. It is a common cause of preventable hearing impairment, particularly in low and middle‐income countries. This review assesses topical antibiotics (without steroids), to clarify whether they are better than no treatment or aural toilet (cleaning of the ear discharge), or treatment with topical antiseptics and to identify which antibiotic is best. Fourteen randomised controlled trials were included (1,724 analysed participants or ears); most were poorly reported, and some included a range of diagnoses.
Quinolone antibiotic drops (considered to be the 'gold standard' topical antibiotics) are better than no drug treatment or antiseptics at drying the ear. The effects of non‐quinolone antibiotics (without steroids) when compared to antiseptics are less clear. Studies were also inconclusive regarding any differences between quinolone and non‐quinolone antibiotics, although indirect evidence suggests a benefit of quinolones cannot be ruled out. Less is known about longer‐term outcomes (producing a dry ear in the long term, preventing complications, healing the eardrum, and improving hearing), or about treating complicated CSOM. The evidence in these trials about safety is also weak. More research is needed to assess whether there may be fewer adverse events with topical quinolones than with alternative topical treatments.
Background
Chronically discharging ears associated with underlying persistent eardrum perforations (chronic suppurative otitis media, CSOM) are a common cause of preventable hearing impairment in low and middle‐income countries (McPherson 1997; WHO 1998). CSOM usually occurs in the first five years of life (although it often persists into adulthood), and is related to poor socio‐economic conditions. Therefore, while this review aims to address the global perspective of CSOM, much of the information discussed here relates to low‐income settings and may differ in developed countries (e.g. age distribution).
What is CSOM?
CSOM is one of several types of otitis media (infection of the middle ear). The World Health Organization (WHO) defines CSOM as "a stage of ear disease in which there is chronic infection of the middle ear cleft, a non‐intact tympanic membrane (i.e. perforated eardrum) and discharge (otorrhoea), for at least the preceding two weeks" (WHO 1998), although this could more strictly be considered a childhood definition. Perforations and infection can be in one ear (unilateral) or both (bilateral). A variety of underlying pathologies can cause CSOM including: an acute episode of acute otitis media that has burst the ear drum and not settled within two weeks; a recurrent episode of acute otitis media in an ear with a perforation from a previous episode of acute otitis media; or an ear with a persistent perforation with active chronic otitis media with metaplastic changes to the mucosa of the middle ear and mastoid air cell system (Browning 2003, personal correspondence). In adults, the majority of patients are likely to have CSOM with a perforation that will not spontaneously heal.
What are the effects of CSOM?
Hearing impairment, aside from the disability from recurrent ear discharge, is the most frequent effect of CSOM. A school survey in Kenya found 63% of ears with CSOM had more than 30 decibels (dB) hearing loss, compared to only 3.4% of ears without outer or middle ear pathology (Hatcher 1995). Hearing impairment due to otorrhoea and a perforated eardrum will usually improve as the disease resolves. However, untreated CSOM may result in permanent hearing loss due to damage to the ossicles which transmit sound vibrations from the eardrum to the cochlea. Because otitis media occurs mostly in children during preschool years, the years in which the most dynamic phase of speech and language development occurs, there is concern that the associated hearing deficits may result in speech and language delays or permanent learning disabilities, as well as disturbances in behaviour (Klein 2000).
In addition to hearing impairment (with its associated consequences), complications of otitis media can often result in death or severe disability, especially in low‐income countries (WHO 2000), where immunity, housing conditions, and access to medical services are often poorer than in high income settings. The infection may extend and spread to the head and neck structures and to the brain. Intracranial infections include meningitis, abscesses, hydrocephalus, or thrombosis of the lateral venous sinus (from suppuration within the mastoid causing clots occluding the lumen of the vessel) (Ludman 1997). Alternatively complications may be extracranial, such as subperiosteal abscess (superficial accumulations of pus that have broken the bony mastoid cortex), facial paralysis, cholesteatoma (a destructive formation of layers of keratinising epithelium, accumulating in the middle ear and mastoid (Bluestone 1996), also described as 'active squamous (epithelial) chronic otitis media (Browning 1997)), labyrinthitis (extension to the labyrinth through the round window), or acute mastoiditis (spread of the infection to the mastoid air cells), which may spread further due to necrosis of the bony wall of the cells resulting in further life‐threatening complications (Dhillon 1999; Ludman 1997).
How much of a burden is CSOM?
Around 91% of the burden of otitis media (all types) and nearly all related deaths occur in low and middle‐income countries (World Bank 1993; WHO 2002). Reliable data on prevalence of CSOM are uncommon. One study estimated it at 1.1% in Kenyan school children (Hatcher 1995) and a review of school and community‐based studies reported a prevalence between 0.4% and 6.1% in low and middle‐income countries (Berman 1995). Data from the World Health Organization and World Bank suggest the global burden of otitis media has dropped dramatically since 1990, to approximately 6000 deaths (0.01% of all deaths) and 1,474,000 disability adjusted life years (DALYs) lost (0.1% of all DALYs) worldwide in 2001 (WHO 2002). Most of these deaths are likely to be due to chronic otitis media and its complications, because acute otitis media is usually a self‐limiting infection (Acuin 2004).
Although most of the background literature cited in this review relates to children, reliable and generalisable data for the global burden in children are not readily available; the WHO estimates therefore quoted here are for both adults and children.
What are the causes of CSOM?
The causes and risk factors associated with CSOM are unclear, and few studies have examined these for CSOM. Instead authors have extrapolated results of studies for acute otitis media and otitis media with effusion to CSOM. However these studies often have conflicting findings, and there is no proven correlation between the various host and environmental factors associated with CSOM and the factors associated with acute otitis media and otitis media with effusion. Despite this, some important factors that may be associated with CSOM include: environmental factors such as inadequate treatment (of CSOM and acute otitis media), poor access to medical care, poor socioeconomic conditions, season, exposure to tobacco smoke, overcrowding, attendance at day care centres, lack of breastfeeding, or poor nutrition or hygiene; and host factors such as altered immunity and underlying diseases (e.g. HIV/AIDS (Barnett 1992; Singh 2003), frequent upper respiratory tract infections), early onset of otitis media in the first months of life, and family history of otitis media. Some populations are at increased risk of developing CSOM, and have high rates reported, including certain ethnic groups (such as Native American tribes of Apache and Navajo, Australian Aborigines, and Inuits of Canada, Greenland and Alaska), and individuals with anatomical defects (e.g. cleft palate or submucous cleft), altered physiological defences (Eustachian tube dysfunction) or Down's syndrome (Bluestone 1998).
What management approaches are there?
The aims of treatment are to stop the discharge (and to eradicate infection), to heal the tympanic membrane, improve hearing, prevent the common problems of recurrent or new infections, and to prevent potentially life‐threatening complications. Treatment options for uncomplicated CSOM include dry mopping, ear wicking, gentle syringing, or suctioning, to clean the ear discharge (aural toilet); systemic antibiotics (e.g. oral antibiotic preparations, or intravenous antibiotics); and topical treatment with either antiseptics or antibiotics, sometimes with steroids. If complications develop, surgery is usually required to remove the infected tissue from the middle ear and mastoid air cells, and possibly repair the damaged eardrum and ossicles. Each of these treatments will be considered in the following Cochrane reviews:
aural toilet: aural toilet versus no treatment or various methods of aural toilet
systemic antibiotic treatment: systemic antibiotics versus no treatment or aural toilet, or various methods of systemic antibiotics
topical antiseptics: topical antiseptic versus no treatment or aural toilet, or various topical antiseptics
topical antibiotics without steroids (THIS REVIEW): topical antibiotic, versus no treatment or aural toilet, topical antiseptics or various topical antibiotics, excluding steroids
systemic versus topical treatments: any systemic treatment against any topical treatment excluding steroids
systemic or topical steroids: steroids, as monotherapy or combination therapy, versus no treatment or aural toilet, topical antiseptics, topical antibiotics, or systemic antibiotics
surgical treatment: surgery versus no treatment or any other treatment
A report of a WHO/Ciba Foundation workshop held in 1996, recommends administration of topical (and/or systemic) antibiotics as well as dry mopping/wicking, since wicking alone is suggested to be ineffective (as found by Smith 1996) (WHO 1998). However, the WHO guidelines still currently recommend treating CSOM by using wicking to dry the ear alone (and a five‐day follow‐up).
Topical treatment with antibiotics
A number of topical antibiotics have been used in the treatment of CSOM. However, concern exists regarding their ability to penetrate the middle ear and mastoid cavities as well as their activity against the causative bacteria (usually gram‐negative). There also remains controversy and uncertainty about the possible ototoxic effect, in particular of topical aminoglycoside antibiotics (by damaging the hair cells in the basal turn of the cochlea), particularly where the eardrum is not intact. The newer 4‐quinolone antibiotics (e.g. ciprofloxacin) are widely considered to be the 'gold standard' topical antibiotics, but are expensive and not generally available as ototopical preparations in many countries. Topical antibiotics may be superior to topical antiseptics, although this needs investigating, particularly as topical antiseptics are cheap and easily available, and are thus widely used in many low and middle‐income countries.
Objectives
To assess the effects of topical antibiotics (excluding steroids) for chronically discharging ears with underlying eardrum perforations (CSOM) in participants of any age.
Methods
Criteria for considering studies for this review
Types of studies
Individual randomised controlled trials. Cluster randomised controlled trials.
Types of participants
People of any age with a diagnosis of CSOM as defined by the trial authors.
Types of interventions
Intervention: topical (aural) antibiotics without steroids. Comparator: no intervention; aural toilet; placebo; other topical antibiotics without steroids; antiseptics. (Treatments containing steroids will not be included here, but will be considered in a separate Cochrane review, as indicated above).
Types of outcome measures
Primary outcomes
Resolution of CSOM at two to four weeks, and after four weeks, according to the investigators' criteria
Secondary outcomes
Healing of perforation at two to four weeks, and after four weeks
Time to resolution of CSOM as defined by the investigators
Improvement in hearing threshold, as measured by audiometry at two to four weeks, and after four weeks
Time to re‐appearance of discharge and perforation after its previous resolution
Adverse events that
a) are fatal, life‐threatening, require inpatient hospitalisation or prolongation of existing hospitalisation, or result in persistent or significant disability/incapacity, such as permanent hearing loss, tinnitus or vertigo (Karbwang 1999; UMC 2003) b) result in withdrawal or discontinuation of treatment c) any other adverse events, such as ear pain, ear canal reactions and transient dizziness
Where outcomes (resolution of discharge, healing of the tympanic membrane, and hearing threshold) are reported at several time‐points within the ranges above, we took the last reported result.
Search methods for identification of studies
The Trials Search Co‐ordinator of the Cochrane Ear, Nose and Throat Group carried out an independent search in August 2003 and March 2005.
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). Trials reported in conference proceedings or on posters have not been sought for this review, but will be sought for inclusion in an update of this review.
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register (code SR‐ENT), and the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library Issue 1, 2005, for relevant trials up to March 2005. Full details of the Cochrane Ear, Nose and Throat Disorders Group methods and the journals handsearched are published in The Cochrane Library in the section on Collaborative Review Groups.
CENTRAL was searched using the terms shown in Appendix 1.
Using the CENTRAL search terms, in combination with the search strategy for identifying trials developed by The Cochrane Collaboration (Clarke 2003), we also searched the following databases:
(1) MEDLINE (January 1951 to March 2005) (2) EMBASE (January 1974 to March 2005) (3) LILACS (www.bireme.br; January 1982 to March 2005) (4) AMED (1985 to March 2005) (5) CINAHL (January 1982 to March 2005) (6) OLDMEDLINE (January 1958 to December 1965) (7) PREMEDLINE (8) NNR (9) ZETOC
We searched the following potential sources of trials:
metaRegister of Controlled Trials (mRCT accessible via the Internet: http://controlled‐trials.com/mrct/)
Cochrane Ear, Nose and Throat Disorders Group Trials Register for any relevant abstracts from conference proceedings
Reference lists of all articles/trials identified by the above methods (includes searching of bibliographies for relevant citations)
Previous published Cochrane review, 'Interventions for chronic suppurative otitis media' (Acuin 1998)
Other previously published (systematic) reviews: 'Chronic suppurative otitis media', in Clinical Evidence (Acuin 2004), and 'Systematic Review of Existing Evidence and Primary Care Guidelines on the Management of Otitis Media (Middle Ear Infection) in Aboriginal and Torres Strait Islander Populations, March 2001 (Couzos 2001)
DARE using issues 2 and 3 of Tthe Cochrane Library 2003 ‐ searched for systematic reviews
We will explore the following potential sources of trials for future updates of this review:
Other previously published (systematic) reviews identified by the above search strategy
Organisations and individual researchers working in the field of otitis media (including authors of published trials and other experts who may know about additional trials)
Pharmaceutical companies (see published notes for list of companies contacted) to locate additional studies, unpublished data, confidential reports, and raw data of published trials
Data collection and analysis
Selection of studies
Carolyn Macfadyen (CM) and Jose Acuin (JA) independently reviewed the titles and abstracts identified by the search strategy to identify potentially relevant trials.
CM retrieved the full papers for all potentially relevant studies, and assessed their eligibility to be included in the review using an eligibility form based on the stated inclusion criteria. We identified multiple publications from the same data set and reported these as one trial. Where outcomes are not reported, we contacted the author of the paper for this information, as the data may have been collected but not reported. We excluded studies that do not meet the inclusion criteria for this review and stated the reason in the 'Characteristics of excluded studies' table. Where necessary, we contacted the study authors for clarification.
JA and Carrol Gamble (CG) provided a second opinion on trials CM had selected for inclusion, and the three authors resolved any disagreements through discussion.
Data extraction and management
CM extracted data of study characteristics, including methods, participants, interventions, and outcomes, and recorded these on standard forms. JA provided further information where this had been obtained from authors of trials included in the previous review 'Interventions for chronic suppurative otitis media' (Acuin 1998). In studies where data are insufficient or missing, we contacted the authors of the original studies for additional data and/or verification of methods, to clarify any uncertainties about the data and the way in which they were collected, and to try to obtain missing data.
CSOM can occur in one or both ears for each participant, which means participants can be counted more than once if ears are used as the unit of analysis. Where outcomes were reported in number of ears only, we also attempted to obtain the values for number of participants; numbers of ears were used where this information could not be obtained. We checked the data and resolved any discrepancies by referring to the trial report, through discussion.
Where possible we extracted data to allow an intention‐to‐treat analysis (i.e. the analysis should include all the participants in the groups to which they were originally randomly assigned). If the number randomised and the numbers analysed were inconsistent, we calculated a per cent loss‐to‐follow‐up and reported this information in Table 6. For binary outcomes, we recorded total number of participants (or ears, where participant numbers were unavailable) and number with the event in each group of the trial. For continuous outcomes, for each group, we extracted the number of participants, and the arithmetic means and standard deviations. If the data were reported using geometric means, we planned to extract standard deviations on the log scale. We planned to extract medians and ranges, and report these in additional tables if any trials reported these.
Table 1.
Methodological quality of included studies
| Study ID | Sequence generation | Alloc. concealment | Balance at baseline? | Blinding | Follow‐up |
| Browning 1983a | Unclear Treatment allocation described as random, using random numbered list kept by the pharmacist, who dispensed the medication, but method of sequence code generation was not stated. The choice of antibiotic depended on sensitivity results of ear discharge isolates. Participants with Pseudomonas species isolated were randomised to topical antibiotics or antiseptics only ‐ not to oral antibiotics due to resistance. Did not discuss whether randomisation was stratified by the different diagnostic groups (and results not reported separately in trial report). | Unclear Medication was supplied in the clinic by the pharmacist using the random numbered list, after eligibility assessments by the clinicians, who were blinded (except for antiseptic). Whether treatment was concealed from the pharmacist is not discussed. | Unclear Baseline characteristics (including the distribution of participants with and without modified radical mastoidectomy) not reported. | Single blind Allocation was kept blinded from the clinicians, with the necessary exception of antiseptic, given by the otologist after aural toilet. | Inadequate 32% dropout: 24/75 participants were defaulters or non‐compliers (ie used <75% of the medication). (Numbers excluded not given by treatment group or diagnosis). Results given for the 51 participants who complied with treatment only (38 on topical antibiotics or antiseptics, included in this review). |
| Clayton 1990 | Unclear Treatment allocation described as random, with medication codes; but no further details of sequence generation method were given. Did not discuss whether randomisation was stratified by the different diagnostic groups ‐ therefore included all participants in this review. | Adequate Patients were randomly allocated treatment in a double‐blind fashion. Medication codes were known only by the pharmacy. | No The following imbalances were reported for analysed participants, after exclusions (due to failure to attend clinic or comply with treatment; exclusions were not reported by treatment group): Significant bias (P=0.0002) for more mildest disease score (score 1) in the antiseptic group (1/60) than antibiotic (11/42) ‐ scores not presented by diagnosis; More ears with otitis externa (significant) and central perforations (not reported as significant) were analysed in the antibiotic group than antiseptic (see Table 02 for numbers). | Double blinded | Inadequate Dropout rates (numbers of randomised ears that were excluded; not reported by treatment group): 25% (5/20) central perforations; 30% (9/30) mastoid cavity infections; 25.8% (23/89) otitis externa ; 26.6% (37/139) total. Patients who failed to attend clinics or to comply with treatment were excluded. |
| Fradis 1997 | Unclear Participants were randomised to treatment, with coded bottles, but method of sequence code generation not described. Participants with otorrhoea in both ears were given two different bottles. | Adequate All numbered treatment bottles were similar in appearance. Treatment bottles and code were retained in the hospital pharmacy; only the pharmacy department head knew what each bottle contained. | Yes Groups comparable for age and bacteriology (although slight imbalance in numbers of positive cultures: 18 ciprofloxacin, 16 tobramycin, 12 antiseptic/placebo). No information was given for treatment groups of cases of surgical perforation or where no perforation could be seen. | Double blind The treating physician and participants were blinded to treatment. The treatment code was only broken at the end of the study to summarise the results of the investigation. | Adequate Adequate for ears, which were the unit of analysis, but slightly inadequate for participants: 6/60 (10%) randomised ears (6/51 participants, ie 12%) were unavailable for follow‐up after three weeks. Excluded numbers by treatment group: Ciprofloxacin: 1/20 ears (5%) Tobramycin: 2/20 ears (10%) 1% Burow aluminium acetate: 3/20 ears (15%) |
| Gyde 1978 | Adequate Used Taves' method of minimisation, with type of infection as the principal criteria. Minimisation is an alternative method to stratified randomisation for treatment allocation. It is sometimes recommended for small sample sizes, and can incorporate more prognostic factors (Scott 2002). Unclear whether 'type of infection' relates to diagnosis or bacteriology ‐ therefore included all participants in this review. | Unclear Allocation concealment not mentioned, except that solutions were called 'A' (TSP) and 'B' (gentamicin) (trial was described as double blind). Taves' minimisation approach can lead to predictability of treatment allocation in some situations. | Yes Ears were balanced accross treatments for diagnosis (equal numbers per treatment) before and after crossover. Balanced for age, sex, and diagnosis, with no great differences betwen the seriousness of infections in the two groups, according to the trialists. These results are for all ears including crossed‐over cases, so some participants are counted twice (bilateral disease or crossover) and 1 was counted 4 times (bilateral disease and crossed over). But there was more bilateral disease in the TSP group (7/43 participants = 16.3%) than on gentamicin (2/48=4.2%), for all participants before crossover. | Double‐blind The solutions were called 'A' (TSP) and 'B' (gentamicin) to conserve the double blind. The treatment provider/outcome assessor was blinded until after the analysis of the results. Participants were also blinded. | Unclear No withdrawal was reported ‐ numbers analysed are the same as the reported numbers eligible for the study. But participants unable to continue the proposed length of treatment or return for follow‐up visits were excluded. Appears to be only per protocol population reported and analysed; number of excluded noncompliers was not reported. |
| Gyde 1981 | Unclear Participants were randomly assigned to one treatment group using coded medications, but method of generation of random codes was not described. Did not discuss whether randomisation was stratified by the different diagnostic groups ‐ therefore included all participants in this review. | Adequate Medications were coded and identically labelled, and used sequentially starting with the lowest number; no number was skipped or used more than once. | Mostly Comparable across treatment groups (for ears) for diagnosis. Overall results for age and bacteria were also comparable (not broken down by diagnosis). But there was a slightly higher proportion of males in the TP group (17/33 ears, 51.5%) than the TSP group (25/35 ears, 71.4%). | Double blind | Unclear No withdrawal was reported ‐ number analysed is the same as the reported number of ears eligible for the study. But participants unable to continue the proposed length of treatment or return for follow‐up visits were excluded. Appears to be only per protocol population reported and analysed; number of excluded noncompliers was not reported. |
| Jaya 2003 | Unclear Treatment allocation described as random, with coded bottles, but method of generation of random codes was not described. | Adequate Both drugs were coloured identically and were dispensed in identical bottles, labelled with code numbers only ‐ given to participants after baseline assessments. | Mostly Comparable for a range of demographic and disease related (prognostic) factors, except more participants receiving PVP‐I were male (10/19, 52.6%), than on ciprofloxacin (4/21, or 19.0%). Other prognostic criteria were assessed and found to have no significant effect on the outcomes for the compared treatments. | Double blinded The randomisation code was only decoded at the end of the study. | Adequate 10% dropout rate: 4/40 randomised participants were excluded by week 4 (1/21 ciprofloxacin; 3/19 PVP‐I ). Reasons for exclusion from the analysis were not given. |
| Kasemsuwan 1997 | Unclear Treatment allocation was described as random, with coded medications, but method of generation of random codes was not specified. | Adequate Participants were randomly allocated treatment in a double blind method. The medication codes were known only in the pharmaceutical laboratory. | Unclear Baseline characteristics not reported for each group. | Double blind | Inadequate 30% dropout: 15/50 participants were excluded due to lack of attendance. Participants who received other antibiotics during the trial were also excluded. |
| Kaygusuz 2002 | Unclear Treatment allocation described as random, but randomisation method was not stated. | Unclear Allocation concealment not reported ‐ nor was blinding. | Yes for bacteriology Baseline characteristics only reported for infective organism, which was comparable accross groups. | Unclear Blinding not mentioned. | Adequate No loss to follow‐up or exclusions were reported. 80 participants were reported as included in the study and analysed. |
| Lorente 1995 | Unclear Treatment allocation described as randomised, but randomisation method was not stated. | Unclear Allocation concealment methods were not stated ‐ but trial was double blind. | Yes Comparable for age sex, otorrhoea, itching, stinging, pain, irritation, and tinnitus. | Double‐blind | Unclear Not clear how many people were originally randomised into the trial. (No exclusion was reported.) |
| Macfadyen 2005 | Adequate Treatment was prepared according to computer generated block randomisation schedule, stratified by school. | Adequate. Treatment was identical in appearance, and identically labeled and packaged in treatment boxes, identifiable by school name and trial number only. Treatment packs remained sealed until sequentially allocated to a child. | Yes Comparable for age*, sex, duration of current episode*, proportion of bilateral cases*, size of perforation*, cause of current episode, whether previous ear treatment had ever been used, and hearing level. * Logistic regression adjusting for these factors did not significantly alter the treatment effect. | Double blind Participants, care‐givers, and outcome assessors remained blind to the treatment allocated throughout the study. | Adequate Number with missing data and so excluded from analysis for resolution of discharge at four weeks: 33/427 (7.7%) total; 20/216 (9%) ciprofloxacin; 13/211 (6%) boric acid. Some of these were due to missing data for participants seen at that visit. The rest were due to withdrawals or lost to follow up: 25/427 (5.9%) total; 16/216 (7.4%) ciprofloxacin (15 lost to follow‐up; 1 consent withdrawn); 9/211 (4.3%) boric acid (all lost to follow‐up). All analyses followed the Intention To Treat principle. |
| Tutkun 1995 | Unclear Participants were randomly divided into two groups but randomisation method was not stated. | Unclear Allocation concealment not reported ‐ nor was blinding. | Unclear? Only reported baseline data for bacteriology ‐ comparable apart from "normal flora" isolated in the ciprofloxacin group only (4 cases; excluded from hearing analyses in Ozagar 1997. | Unclear Blinding not mentioned. | Unclear It is not clear how many people were originally randomised into the trial ‐ an unreported number of non‐compliers (participants who did not use the topical solutions regularly and those who had taken any other medication during the study period) were excluded from study. 4 participants were excluded from the hearing analysis (and clinical response in Ozagar 1997). |
| van Hasselt 1997 | Unclear Described as a randomised trial in 2002 paper, but randomisation method was not stated. | Unclear Allocation concealment not reported ‐ nor was blinding. | Unclear Baseline characteristics were not provided. | Unclear Blinding not mentioned. | Inadequate 28% dropout: 27/96 participants did not complete the trial. Ofloxacin: 8% dropout (1/12 participants) Neomycin/polymyxin B: 21% dropout (8/38 participants) Acetic acid/spirit: 39% dropout (18/46 participants) Only children completing the trial fully (ie attended at both review dates) were included in the analysis. |
| van Hasselt 1998 | Unclear Described as a randomised trial, but randomisation method was not stated. | Unclear Allocation concealment not reported ‐ but trial was double‐blind. | Unclear Baseline characteristics were not provided. | Double‐blind | Adequate (except week 8) Number excluded from the analysis: Week 1: 12/151 ears (8%); Week 2: 13/151 ears (9%) ; Week 8: 23/151 ears (15%). 'Defaulting ears' were excluded. |
| van Hasselt 2002 | Unclear Described as a randomised trial, but randomisation method was not stated. | Unclear Allocation concealment not reported ‐ but trial was double‐blind. | Unclear Baseline characteristics were not provided. | Double‐blind | Unclear Not clear how many people were originally randomised into the trial. |
Assessment of risk of bias in included studies
CM assessed the methodological quality of all the trials identified as eligible for inclusion. CG reviewed trials where there was any ambiguity about the methods used, and JA provided further information where this had already been obtained from authors of trials included in the previous review 'Interventions for chronic suppurative otitis media' (Acuin 1998). Any disagreements were resolved through discussion. Where necessary, we contacted the study authors for further clarification.
We assessed the methodological quality of the trials in terms of generation of allocation sequence, allocation concealment, blinding and inclusion of randomised participants. We classified generation of allocation sequence, allocation concealment, and inclusion of randomised participants as adequate, inadequate and unclear as outlined by Juni 2001. Blinding is classified as double blind, single blind, or open.
Data synthesis
CM entered data into Review Manager 4.2. In studies that enrolled people with otitis externa, draining surgical cavities or acute otitis media, as well as CSOM, we only included the results for just CSOM participants if the authors reported accounting for diagnosis at randomisation (e.g. stratified by diagnosis) and presented results by diagnosis. Where information was not reported regarding whether alternative diagnostic groups were accounted for at randomisation, we included all participants (groups may be unbalanced, and decisions by trialists to report subgroups may have been linked to trend). We also included all participants where separate results were not available. See Table 7 for details. For crossover trials, we have only taken the results before participants were crossed over to the alternative treatment, where possible; otherwise results for all participants combining pre and post‐crossover data were used.
Table 2.
Participant eligibility criteria, including CSOM diagnostic criteria
| Study ID | CSOM diagnosis | Otitis exclusions | Other elig criteria | Disease duration | Bacteriology? | Mucosal appearance? | Other diagnoses? | Other diags: sep? |
| Browning 1983a | Active chronic otitis media including previous modified radical mastoidectomy participants. | 1) Cholesteatoma 2) Aural polyp | Non‐otitis inclusion criteria: 1) Over 16 years old Exclusions ‐ see otitis exclusions; no other exclusions were specified. | Not specified ‐ but all participants were secondary referrals | Yes. Sensitivity of isolated aerobic flora determined the choice of antibiotic. Participants with Pseudomonas spp were not randomised to oral (systemic) antibiotic. | Not reported | 19/51 (i.e. 37%) analysed participants had previously undergone modified radical mastoidectomy (not provided by treatment group). NB: only 38/51 on topical antibiotics or antiseptics included in this review (2 of 3 treatment groups) | No |
| Clayton 1990 | Otorrhoea caused by: a) discharging central perforation; b) mastoid cavity; c) otitis externa. | 1) Acute middle ear disease 2) Cholesteatoma 3) Clinical evidence of fungal ear infection | Exclusion criteria ‐ treatment related: 1) Topical or systemic antibiotic use in previous three weeks; 2) History of drug sensitivity to any of the agents used in the study. | Not specified ‐ but excluded acute middle ear disease | Indicated; Not required for inclusion: 11.7% had no bacteria isolated. | Yes: scoring system of severity included presence and degree of oedema obscuring the tympanic membrane or mastoid cavity, or of oedematous mucosa lining the mastoid cavity. | Included otorrhoea from mastoid cavity and otitis externa. a) Discharging central perforation: 15 ears + 5 excluded b) Mastoid cavity: 21 ears + 9 excluded c) Otitis externa: 66 ears + 23 excluded Participants who failed to attend clinic or comply with treatment were excluded (numbers not given by treatment group). | Not separate in review: randomisation not reported to stratify by diagnosis. But results were given by diagnosis separately in trial publication. |
| Fradis 1997 | CSOM was defined as a chronic inflammation of the middle ear and mastoid process with a perforated tympanic membrane and discharge. All cases had purulent disease. | 1) Prior middle‐ear surgery 2) Suspected cholesteatoma | Other inclusion criteria: 1) Signed consent 2) Discontinued all other medications two weeks before study entry. Exclusion criteria ‐ treatment related 1) History of allergy to aminoglycosides or fluoroquinolone derivatives. Exclusion criteria ‐ other 1) General health problems 2) Younger than 18 years (range was 18 to 73 years) | Disease duration: mean (range): 24 months (1 to 240 months). | Indicated; Not required for inclusion: 23/60 cultures were negative for organisms before treatment. | In 8/60 ears granulation tissue meant no perforation could be seen | a) 3/60 ears: surgical perforations; b) 8/60 ears: no perforation seen due to granulation tissue | No |
| Gyde 1978 | 1) Otorrhoea from: a) recurrent benign chronic otitis media with tympanic membrane perforation (CSOM); b) postoperative infections (mastoidectomy or tympanoplasty) and mastoid cavity infections; c) subacute otitis media (with small or medium perforation); d) external otitis. | 1) Otorrhoea not caused by bacteria 2) 'Unsafe' ears (active atticoantral disease, accompanied by cholesteatoma with adjacent area extension) | Inclusion criteria ‐ other: 1) Adults or children ‐ includes paediatric cases (under 12 years old), and geriatric cases (over 60 years old). 2) Informed consent Exclusion criteria ‐ treatment related: 1) Known allergy to any components of the trial drugs. 2) Use of high doses of local (topical) corticosteroid preparations. 3) Previous use of ototoxic drug therapy. 4) Use of general or topical antibiotics within two weeks before study entry. 5) Unable to continue the proposed length of treatment or return for follow‐up visits due to distance or inconvenience. Exclusion criteria ‐ other: 1) Pregnant or lactating. 2) Infants under 2 months old. | Not specified | Indicated with results; Required for inclusion. | Not reported | Total 100 analysed ears before crossover; 12 ears crossed over to the alternative treatment (8/50 TSP to gentamicin; 4/50 gentamicin to TSP): a) 50 CSOM (25 TSP, 25 gentamicin); 7 ears then crossed over (2 from gentamicin to TSP, 5 from TSP to gentamicin) b) 10 postoperative and mastoid cavity infections (5 TSP, 5 gentamicin); 3 ears then crossed over (3 from TSP to gentamicin) c) 16 subacute otitis media (8 TSP, 8 gentamicin); 2 ears then crossed over (2 from gentamicin to TSP) d) 24 external otitis (12 TSP; 12 gentamicin) 0 ears crossed over. | Not separate in review: type of infection was the principal criteria for randomisation (minimisation), but unclear whether this relates to diagnosis or bacteriology. But results were given by diagnosis separately in trial publication (for number of ears). |
| Gyde 1981 | 1) Otorrhoea from a) recurrent otitis media with tympanic membrane perforation (CSOM); b) infected mastoid cavities and post‐operative tympanoplasties; c) subacute otitis media; d) external otitis. 2) Presence of gram‐negative or gram‐positive organisms from a specified list. | 1) Acute otitis media 2) 'Unsafe' ears (active atticoantral disease, with adjacent area extension) | 13/68 ears were paediatric (< 12 years old), 12/68 ears were geriatric (> 70 years old). Concurrent medications, such as analgesics and decongestants etc were allowed. Exclusion criteria ‐ treatment related: 1) History of sensitivity to any components of the trial drugs. 2) Use of extensive ototopical corticosteroid preparations within the past four weeks. 3) Previous use of ototoxic drug therapy. 4) Use of oral or parenteral antibiotics within two weeks before study entry. 5) Unable to continue the proposed length of treatment or return for follow‐up visits due to distance or inconvenience. Exclusion criteria ‐ other: 1) Pregnant or lactating. 2) Tuberculosis, fungal or viral diseases. | Disease duration (for all diagnoses) ranged from just under 1 month to 5 years. | Indicated; Required for inclusion: Presence of gram‐negative or gram‐positive organisms from a specified list. | Not reported | Numbers for analysed ears (68 total: 35 TSP, 33 TP) a) 27 CSOM (13 TSP, 14 TP); b) 6 infected mastoid cavities and post‐operative tympanoplasties (2 TSP, 4 TP); c) 4 subacute otitis media (2 TSP, 2 TP); d) 31 external otitis (18 TSP, 13 TP). | Not separate in review: randomisation not reported to stratify by diagnosis. But results were given by diagnosis separately in trial publication. |
| Jaya 2003 | Actively discharging CSOM with moderate to large central perforation (i.e. active CSOM‐tubotympanic disease). | 1) Cholesteatoma 2) Aural polyp 3) Impending complications of CSOM | Other inclusion criteria: 1) Older than 10 years Exclusion criteria ‐ treatment related: 1) Known allergy to iodine or fluoroquinolone 2) Prior systemic or topical antibiotic therapy within 10 days of study entry Exclusion criteria ‐ other 1) Debilitating illness (e.g. diabetes mellitis, tuberculosis, renal failure or AIDS) | Total duration of disease: </= 5 years: n = 13 > 5 years: n = 27 Current episode of discharge was < 2 weeks for many participants: <1 week: n = 15 1 to 4 weeks: n = 20 > 4 weeks: n = 5 The trialists reported that this duration did not significantly alter the outcome in both treatment groups. | Indicated; Not required for inclusion: 8/40 swabs did not yield potential bacterial pathogen | Status of middle ear at baseline (number of participants): 20 hyperemic 18 pale and edematous 2 granular | None specified ‐ see otitis exclusions | n/a |
| Kasemsuwan 1997 | 1) Perforated tympanic membrane for longer than 3 months; 2) Mucopurulent otorrhoea (Assessed on microscopic examination) | 1) Cholesteatoma 2) Underlying diseases | Other inclusion criteria: 1) Adult (age range was 21 to 66) Exclusion criteria ‐ treatment related: 1) Receiving antibiotics in the previous two weeks and during the study. Exclusion criteria ‐ other: 1) Pregnant women 2) Underlying diseases | Perforated tympanic membrane for longer than 3 months | Indicated; Not required for inclusion: no bacteria were isolated for 26% of participants | Not reported | Not specified ‐ see otitis exclusions | n/a |
| Kaygusuz 2002 | Chronic suppurative otitis media with: 1) perforated eardrum, and 2) discharge in the ears for > 3 months | 1) Suspected or confirmed cholesteatoma (on otoscopy) 2) Previous history of ear surgery | Other inclusion criteria: None reported other than adults with CSOM (age range was 18 to 60 years, mean, (SD) 31 (+/‐11.5) years) Exclusion criteria ‐ treatment related: 1) Allergy to aminoglycosides or fluoroquinolones Exclusion criteria ‐ other: 1) Under 18 years old 2) General health problems | Discharge for > 3 months (average duration was 44.4 years +/‐ 40.9 months; range 3 to 10 years) | Indicated; Not required for inclusion: 5.8% of pre‐treatment cultures were negative | Not reported | Not specified ‐ see otitis exclusions. | n/a |
| Lorente 1995 | Simple chronic otitis media in the suppurative phase (CSOM): purulent otitis of > 3 months duration, with perforated tympanic membrane. | 1) Cholesteatoma or attic disease 2) Otomycosis 3) Bilateral hypoacusia higher than 60 dB | Inclusion criteria ‐ other: 1) Age 18 to 65 (mean 42 years) 2) Either gender 3) No evidence of physical or psychological (psychiatric) illness, with laboratory tests (biochemical and haematological) within reference ranges 4) Consent to participate Exclusion criteria ‐ treatment related: 1) Topical or systemic antibiotic use in the previous 48 hours 2) Allergy to quinolones or aminoglycosides Exclusion criteria ‐ other: 1) Pregnant or breast feeding 2) Severe kidney or liver deficiency | Purulent otitis of > 3 months duration | Indicated: Not specified in inclusion criteria: 304 microorganisms isolated before treatment were reported | Not reported | Not specified ‐ see otitis exclusions | n/a |
| Macfadyen 2005 | 1) Purulent aural discharge for 14 days or longer, with pus seen in the external canal on otoscopy 2) Perforated tympanic membrane (on otoscopy) | Other ear problems: 1) Pre‐existing disease, e.g. otomycosis, polyp, impacted foreign body, or previous ear surgery 2) Complicated otitis media, e.g. cholesteatoma, mastoiditis, requires treatment other than study treatment 3) Anatomical abnormalities | Inclusion criteria ‐ other: 1) School children (enrolled at Kisumu rural primary schools visited) 2) Age five years or older (range was 4 to 19 years) 3) Informed consent (parental consent for affected children; school staff and parent‐teacher‐association representatives consented to school participation). Exclusion criteria ‐ treatment related: 1) Treated for ear infection or received antibiotics for any other disorder in the previous 2 weeks 2) Allergy to study drugs. | Purulent aural discharge for 14 days or longer | Assessed but results not provided Not required for inclusion | Not assessed | No ‐ see otitis exclusions | n/a |
| Tutkun 1995 | History of purulent otorrhoea lasting > 1 year | Cholesteatoma | Inclusion criteria ‐ treatment related: 1) All participants stopped taking any medication at least 10 days prior to treatment. Inclusion criteria ‐ other: 1) Informed consent. Exclusion criteria ‐ treatment related: 1) History of allergy to fluoroquinolone derivatives or aminoglycosides, or to topical agents 2) Did not use the topical solutions regularly, or had taken any other medication during the study. Exclusion criteria ‐ other: 1) Younger than 9 years 2) History of general health problems. | History of purulent otorrhoea lasting > 1 year | Indicated Not specified in inclusion criteria: 4 participants had normal flora | Not reported | Not specified | n/a |
| Van Hasselt 1997 | CSOM ('typically... affected ears were filled with mucoid pus.... Most perforations were medium or large. Granulations were present in most cases') | Not reported | Inclusion criteria ‐ other: Children Exclusion criteria: Not reported. | Not specified | Not specified | Not reported | Not specified | n/a |
| Van Hasselt 1998 | > 2 months CSOM for inclusion as an active ear (CSOM not described) | Not reported | Participants were mainly children. Exclusion criteria: 1) Uncooperative with suction cleaning | > 2 months CSOM | Not specified | Not reported | Not specified | n/a |
| Van Hasselt 2002 | CSOM (not described) | Not reported | Other eligibility criteria: Not reported | Not specified | Not specified | Not reported | Not specified | n/a |
For binary data, we combined trials using relative risks (RR) and 95% confidence intervals (CI). We combined trials with continuous data using the weighted mean difference (WMD) and its 95% confidence interval. Where data have been reported using medians and ranges, or there is evidence of skewed data, we reported medians and ranges where possible (dividing mean by the standard deviation (SD); results of < 1.64 indicate a positive skew). If continuous data were reported using geometric means, we combined the findings on a log scale and report on the original scale.
Quinolone and non‐quinolone topical antibiotic treatments are not combined across trials. This was done to avoid counting patients in the antiseptic arm twice for trials that compared a quinolone with a non‐quinolone and an antiseptic. There is also clinical interest in the difference in effectiveness of quinolones compared to non‐quinolones, as quinolones are thought to be more effective and safer but more expensive.
Where heterogeneity is considered to be present, and it remained clinically appropriate to combine data, we also used the DerSimonian and Laird random‐effects model, and reported both fixed‐effect and random‐effects results.
The primary analysis is of all eligible studies. If a sufficient number of trials is available for future updates (not available for each comparison in this version of the review), we will explore whether heterogeneity can be explained using subgroup analyses or meta‐regression for the following factors: age (under 16, and adults 16 years or older), associated mopping (dividing studies into those with some form of ear toilet, and those without), co‐interventions (dividing studies into those with treatment comparison alone, and those with treatment comparison in combination with other co‐interventions), and methodological quality (initially excluding studies of the poorest quality). Sensitivity analyses will also be used to explore methodological quality (notably adequate concealment), and trial design (e.g. cluster randomisation). We will display the results for each sensitivity analysis according to the subgroups within each methods category.
The sensitivity analysis will include the following, as outlined in the statistical guidelines in the Cochrane Ear, Nose and Throat Group 'Guidelines for Reviewers' (CochraneENTGuideline updated November 2000).
(1) Repeat the analysis excluding unpublished studies (if any). (2) Repeat the analysis excluding studies of the lowest quality (already done if there is heterogeneity). (3) If there are one or more very large studies, we will repeat the analysis excluding these, to investigate how much they dominate the results. (4) Repeat the analysis excluding studies where people with CSOM are only a subgroup of the participants included in the study, for example, those that enrol people with otitis externa, draining surgical cavities or acute otitis media, as well as CSOM.
Within this version of the review, trials where people with CSOM are only a subgroup of the participants included in the study are identified ‐ see Table 7.
For this version of the review, we visually examined forest plots, in conjunction with the chi2 test, using a 5% level of statistical significance, and the I2 statistic. The I2 statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). A value greater than 50% may be considered substantial heterogeneity (Deeks 2004). There were insufficient trials to investigate publication bias using funnel plots; this may be done in further updates of the review.
Results
Description of studies
Search results
The electronic searches identified 649 citations in August and September 2003, plus 698 citations in March 2005, including four unpublished trials. Three additional trials were already known to the authors: van Hasselt 1997; van Hasselt 1998; van Hasselt 2002. Macfadyen 2005 was also already known to the authors. Trials with duplicate publications were identified and referred to under the main trial publication. Full texts of 127 trials were reviewed, and 14 included as eligible for this review ‐ see breakdown of numbers below. We attempted to include all relevant studies regardless of language.
127 trials: full texts obtained for eligibility assessment (117 from the above sources, plus ten from reference lists and other searches). Of these, 85 were in English only, three had English and non‐English publications, and 39 were in non‐English languages only.
105 trials excluded: 74 English only, one English and non‐English language, 30 only available in non‐English language.
Eight trials awaiting assessment: two English only [Nawasreh 2001 is awaiting a response from the author as to whether this is the same trial as another trial already included in this review (Tutkun 1995); McKelvie 1975 is awaiting full assessment for probable inclusion], and six trials only available in non‐English language, awaiting translation to determine eligibility.
14 trials included: nine English only, two English and non‐English language, three only available in non‐English language.
The Characteristics of excluded studies table outlines reasons for excluding studies following review of their full texts. The Characteristics of included studies table provides information on the included trials; also see tables: Table 6 (Methodological quality of included studies); Table 8 (Bilateral Disease: Numbers for Ears versus Participants); Table 7 (Participant eligibility criteria, including CSOM diagnostic criteria); Table 9 (Intervention regimens used); Table 10 (Outcomes assessed).
Table 3.
Bilateral disease: numbers for ears versus participants
| Study ID | # of particpants | # of ears | % bilateral cases | Handling bilat cases | Results: pt or ears? |
| Browning 1983a | 75 randomised ‐ all treatments; not reported by group 51 analysed: 18 topical antibiotics 20 topical antiseptics * 13 systemic antibiotics; not included in this review * 19/51 (37%) were post modified radical mastoidectomy ‐ numbers and results were not presented separately. | Not reported | Not reported | One ear was chosen at random by the pharmacist (method of choice unknown). | Participants |
| Clayton 1990 | Not reported | Randomised ears: 139 total: 20 central perforation; 30 mastoid cavity; 89 otitis externa Completed (analysed) + excluded cases: Total: 102 completed (42 antiseptic; 60 antibiotic) +37 excluded; Central perforation: 15 completed (4 antiseptic; 11 antibiotic) + 5 excluded; Mastoid cavity: 21 completed (13 antiseptic; 8 antibiotic) + 9 excluded; Otitis externa: 66 completed (25 antiseptic; 41 antibiotic) + 23 excluded | Not reported | Unclear ‐ report for ears only, but baseline characteristics table uses term 'patients' instead, although totals match number of ears reported in text. | Ears? But baseline characteristics table uses term 'patients' (text uses 'ears'). |
| Fradis 1997 | 51 randomised participants; 45 analysed | 60 randomised ears (20 per treatment group) 54 analysed ears: 19 ciprofloxacin 18 tobramycin 17 Burow aluminium acetate | Randomised bilateral rates: 9/51 (17.6%) Analysed bilateral rates: 9/41 (20.0%). | Treated both ears (each ear given a different bottle in bilateral cases), and report number of ears. | Ears |
| Gyde 1978 | Randomised participants: not reported Analysed participants before crossover (all diagnoses reported only): 91 total; 43 TSP; 48 gentamicin. Analysed participants crossed over to other treatment (all diagnoses): 11/91 total; 7/43 from TSP to gentamicin; 4/48 from gentamicin to TSP. NB the trialists reported the combined crossover plus pre‐crossover numbers. | Randomised ears: not reported Analysed ears before crossover: 100 total (50 TSP; 50 gentamicin); 50 CSOM (25 TSP; 25 gentamicin); 10 post‐operative and mastoid cavity infections (5 TSP; 5 gentamicin); 16 subacute otitis media (8 TSP; 8 gentamicin); 24 otitis externa (12 TSP; 12 gentamicin). Analysed ears crossed over to other treatment: 12/100 total (8/50 from TSP to gentamicin; 4/50 from gentamicin to TSP); 7/50 CSOM (5/25 from TSP to gentamicin; 2/25 from gentamicin to TSP); 3/10 post‐operative and mastoid cavity infections (3/5 from TSP to gentamicin); 2/16 subacute otitis media (2/8 from gentamicin to TSP); 0/24 otitis externa. NB the trialists reported combined pre‐ plus post‐crossover numbers. Where possible, we have used pre‐crossover cases only. | Randomised participants: rates not reported Analysed participants bilateral rates (all diagnoses only): Before crossover: Total: 9/91 (9.9% bilateral); TSP: 7/43 (16.3% bilateral); Gentamicin: 2/48 (4.2% bilateral). Crossed over to alternative treatment: Total: 1/11 (9% bilateral); TSP to gentamicin: 1/7 (14% bilateral); Gentamicin to TSP: 0/4 (0% bilateral). ie this crossover participant is counted 4 times in the combined figures presented by the trialists (twice per treatment). | Both ears treated and analysed separately ‐ reported number of ears | Ears |
| Gyde 1981 | 60 participants were eligible and included in the analyses Not available by treatment group or diagnosis. | Eligible ears included in the analyses: 68 total (35 TSP; 33 TP); 27 CSOM (13 TSP; 14 TP); 6 post‐operative and mastoid cavity infections (2 TSP; 4 TP); 4 subacute otitis media (2 TSP; 2 TP); 31 otitis externa (18 TSP; 13 TP) | Total: 8/60 (13.33%) Not available by treatment group or diagnosis. | Treated both eligible ears and report number of ears. | Ears |
| Jaya 2003 | 40 randomised participants (21 ciprofloxacin; 19 PVP‐I ) 36 assessed at week 4 (20 ciprofloxacin; 16 PVP‐I) | Not reported; but only 40 ear swabs taken at baseline | Not reported | Unclear ‐ reported at participant level. | Participants |
| Kasemsuwan 1997 | Main text states participants: 50 randomised 35 completed study and analysed (19 ciprofloxacin; 16 normal saline) | Abstract states ears: 50 ears randomised 35 completed study and analysed | Not reported | Unclear ‐ used term 'ears' in abstract, but mostly 'patients' in main text. | Participants? 'Participant' in main text, but used term 'ears' in abstract. |
| Kaygusuz 2002 | 80 participants included and analysed (20 per treatment group; i.e. 40 for this review) | 103 ears included (not reported by treatment group) | 23/80 (28.75%) | Appears to have taken success when both ears had resolved only (treatment was successful when there was no discharge). | Participants |
| Lorente 1995 | 308 analysed participants (159 ciprofloxacin; 149 gentamicin) Numbers originally randomised, not provided | Not reported | Not reported | Not reported | Participants |
| Macfadyen 2005 | 427 randomised participants (216 ciprofloxacin; 211 boric acid) 394 analysed for resolution at four weeks (196 ciprofloxacin; 198 boric acid) | 533 randomised ears (264 ciprofloxacin; 269 boric acid) | Rates for randomised participants: Total: 106/427 (25%) Ciprofloxacin: 48/216 (22%) Boric acid: 58/211 (27%) | Report at participant level. Both ears treated and assessed for bilateral cases ‐ success when both ears had resolved or healed. Sensitivity analysis conducted ‐ success for bilateral cases when either or both ears resolved or healed. Audiometry results were averaged over the study ear(s) for one overall reading. | Participants |
| Tutkun 1995 | 44 analysed participants (24 ciprofloxacin; 20 gentamicin) 40 analysed for hearing (20 ciprofloxacin; 20 gentamicin) (Ozagar 1994) | Not reported | Not reported | Unclear ‐ reported at participant level; bilateral disease or numbers of ears not discussed | Participants |
| van Hasselt 1997 | 96 children randomised: 12 ofloxacin; 38 neomycin/polymyxin B 46 acetic acid/spirit 69 children completed and included in analysis: 11 ofloxacin 30 neomycin/polymyxin B 28 acetic acid/spirit | Number ears randomised: not reported 93 ears included in analysis: (or 88?) 14 ofloxacin 40 neomycin/polymyxin B (35 in Van Hasselt 2002 paper) 39 acetic acid/spirit. | Randomised rates: not available Analysed participants: Total: 24/69 (34.78%) Ofloxacin: 3/11 (27.27%) Neomycin/polymyxin B: 10/30 (33.33%) (or 5/30, 16.67% from Van Hasselt 2002 publication) Acetic acid/spirit: 11/28 (39.29%) | Treated both ears and report % ears dry. CBM Report: provided number and proportion of ears dry and wet at each visit. 2002 paper: reported proportion of ears dry at two weeks. | Ears |
| van Hasselt 1998 | 107 participants randomised Number analysed: not reported | 151 randomised ears Analysed ears: 139 ears at week 1 138 ears at week 2: 32 ofloxacin twice daily; 39 ofloxacin once weekly; 36 neomycin polymyxin B twice daily; 31 neomycin polymyxin B once weekly. 128 ears at week 8. | Total randomised: 44/107 (41.12%) Analysed participants: not available | Report proportion of analysed ears dry; numbers of ears per group reported for week two only. | Ears |
| van Hasselt 2002 | Not reported | 253 analysed ears: 79 ofloxacin + HPMC 91 povidone iodine + HPMC 83 HPMC | Not reported | Reported % of number of ears dry. | Ears |
Table 4.
Intervention regimens used
| Study ID | Intervention | Strength | Dose and frequency | Duration | Ear Toilet | Concurrent meds |
| Do topical antibiotic eardrops work? | ||||||
| Quinolone versus placebo: | ||||||
| Kasemsuwan 1997 | 1) Quinolone: ciprofloxacin in saline solution 2) Placebo: normal saline solution | 1) Ciprofloxacin: 250 microgram/mL 2) Saline: n/a | Both treatments: 5 drops three times daily. | Both treatments: At least 7 days. | Both groups: Ear cleaning at each visit (treatment days 1, 4 and 7). | Excluded if received antibiotics in the previous two weeks or during the study. |
| van Hasselt 2002 | 1) Quinolone: ofloxacin in HPMC 2) Placebo: HPMC HPMC = hydroxypropyl methyl‐cellulose (hypromellose) ‐ a treatment delivery vehicle. | 1) 0.075% Ofloxacin in 1.5% HPMC 2) 1.5% HPMC | All treatments: 1 single topical application after suction cleaning. | Both treatments: Once only. | Both groups: Suction cleaning once. | Not reported. |
| Which antibiotic eardrops work best? | ||||||
| Non‐quinolone versus non‐quinolone | ||||||
| Gyde 1978 | 1) Non‐quinolone: trimethoprim‐sulfacetamide‐polymyxin B (TSP) (Burroughs Wellcome Ltd). 2) Non‐quinolone: gentamicin sulphate (Garamycine) | 1) 0.1% TSP (Trimethoprim 1 mg/mL; Polymyxin B 10,000 U/mL; Sulfacetamide 5mg/mL). 2) 0.3% gentamycin sulphate | Both treatments: 8 drops twice daily (morning and evening). | Both treatments: Depended on clinical and bacteriological response: Up to 3 weeks initially, plus 3 weeks if required. Failed ears or not dry at 6 months crossed over to the other treatment for 3 weeks; 6 month follow‐up as before: 11 participants (12 ears; 7 CSOM ears). Average duration (including crossovers) (days): All successfully treated ears: TSP 19.3 days (range 7 to 32) (N = 46); gentamicin 21.7 days (range 10 to 37) (N = 51). CSOM: TSP 16.4, gentamicin 22.8; Post‐operative or mastoid infections: TSP 24.0, gentamicin 24.3; Subacute otitis media: TSP 21.3, gentatmicin 22.7 Otitis externa: TSP 17.1, gentamicin 17.5. | Both groups: Suction and dry mopping before each treatment application. Pneumatic otoscope sometimes also used to ensure the eardrops reached the middle ear and mastoid cavity. | Two‐week washout period if used any antibiotics before study entry. Excluded if used high doses of ototopical corticosteroids, or ever used ototoxic drug therapy. |
| Gyde 1981 | 1) Non‐quinolone: Trimethoprim‐ sulfacetamide‐ polymyxin B (TSP) 2) Non‐quinolone: Trimethoprim‐ polymyxin B (TP) | 1) 0.1% TSP 2) 0.1% TP Concentrations of treatment components: Trimethoprim 1 mg/mL; Polymyxin B 10,000 U/mL; Sulfacetamide 5 mg/mL. | Both treatments: 8 drops twice daily (morning and night). | Both treatments: Up to 14 days maximum, depending on response to treatment and bacterial culture results. | Both groups: Suction and dry mopping before each treatment application. Pneumatic otoscope sometimes also used to ensure the eardrops reached the middle ear and mastoid cavity. | Two week washout period if used any antibiotics before inclusion. Excluded if used extensive ototopical corticosteroids within 4 weeks, or ever used ototoxic drug therapy. Concurrent medications such as analgesics and decongestants etc were allowed. |
| Quinolone versus non‐quinolone | ||||||
| Fradis 1997 | 1) Quinolone: ciprofloxacin hydrochloride solution 2) Non‐quinolone: tobramycin | 1) Ciprofloxacin: Not specified 2) Tobramycin: Not specified | All treatments: 5 drops, 3 time daily. | All treatments: 3 weeks. | Not specified. | All other medication discontinued two weeks before study entry. (34 participants previously received systemic antibiotics; 12 had used neomycin‐polymyxin B eardrops without success). |
| Kaygusuz 2002 | 1) Quinolone: ciprofloxacin hydrochloride 2) Non‐quinolone: tobramycin | 1) 0.3% Ciprofloxacin hydrochloride 2) 0.3% Tobramycin | All treatments: 2 drops 3 times daily. | All treatments: 3 weeks. | All groups: Aspiration once daily. | Not reported. |
| Lorente 1995 | 1) Quinolone: ciprofloxacin 2) Non‐quinolone: gentamicin | 1) 0.3% Ciprofloxacin 2) 0.3% Gentamicin | Both treatments: 5 drops (0.75 mg) 3 times daily. | Both treatments: 8 days (awaiting confirmation that this was not continued to day 30). | Not specified. | Excluded if received antibiotic treatment (topical or systemic) in the last 48 hours. |
| Tutkun 1995 | 1) Quinolone: ciprofloxacin hydrochloride 2) Non‐quinolone: gentamicin sulfate | 1) Ciprofloxacin hydrochloride: 200 microgram/mL 2) Gentamicin sulfate: 5 mg/mL | Both treatments: 5 drops 3 times daily. | Both treatments: 10 days. | Not specified. | All other medication stopped at least 10 days prior to treatment. Excluded if used any other medication during the study. |
| van Hasselt 1997 | 1) Quinolone: ofloxacin (Exocin R from Allergan) 2) Non‐quinolone: neomycin‐polymyxin B | 1) 0.3% Ofloxacin; 2) 0.5% Neomycin, 0.1% polymyxin B | All treatments: 3 drops, 3 times daily. | All treatments: 2 weeks. | All groups: Suction cleaning at the beginning of the trial, and at weeks 1 and 2 visits. | Not reported. |
| VH 1998 daily | 1) Quinolone: ofloxacin 2) Non‐quinolone: neomycin‐ polymyxin B | 1) 0.3% Ofloxacin 2) Neomycin‐ polymyxin B: not specified | Both treatments: 6 drops twice daily. | All treatments: 2 weeks. | All groups: Once weekly suction cleaning. | Not reported. |
| VH 1998 weekly | 1) Quinolone: ofloxacin 2) Non‐quinolone: neomycin‐ polymyxin B | 1) 0.3% Ofloxacin 2) Neomycin‐ polymyxin B: not specified | Both treatments: Once weekly. | All treatments: 2 weeks. | All groups: Once weekly suction cleaning. | Not reported. |
| Do topical antibiotics work better than topical antiseptics? | ||||||
| Non‐quinolone versus antiseptic | ||||||
| Browning 1983a | 1) Non‐quinolone: chloramphenicol (Chloromycetin), or gentamicin (Genticin) (self treated). Choice of antibiotics depended on sensitivity of bacterial isolated at baseline. 2) Antiseptics: insufflation of boric acid and iodine powder after aural toilet (otologist treated). | 1) Antibiotics: not specified 2) Antiseptic: not specified | 1) Topical Antibiotics (self treat): a) Chloramphenicol: 1 or 2 drops, 3 times daily; b) gentamicin: 3 or 4 drops, 4 times daily. 2) Antiseptic: once weekly (insufflation after aural toilet, by otologist) (dose not reported). | All treatments: 4 weeks. | All groups: Weekly aural toilet by otologist, using microscopic vision and suction aspiration when necessary. | Not reported. |
| Clayton 1990 | 1) Non‐quinolone: gentamicin sulphate 2) Antiseptic: aluminium acetate | 1) 0.3% Gentamicin sulphate 2) 8% Aluminium acetate | All treatments: 5 drops, 3 times daily. | All treatments: 3 weeks. | All groups: Self mopping before each treatment administration. | No antibiotics in the preceding 3 weeks. |
| Fradis 1997 | 2) Non‐quinolone: tobramycin 3) Antiseptic: Burow aluminium acetate solution | 2) Tobramycin: Not specified 3) 1% Aluminium acetate (weak antiseptic, used as a "placebo" by the trialists). | All treatments: 5 drops, 3 times daily. | All treatments: 3 weeks. | Not specified. | All other medication discontinued two weeks before study entry. (34 participants previously received systemic antibiotics; 12 had used neomycin‐polymyxin B eardrops without success). |
| van Hasselt 1997 | 2) Non‐quinolone: neomycin‐polymyxin B 3) Antiseptic: acetic acid in spirit and glycerin | 2) 0.5% Neomycin, 0.1% polymyxin B 3) 2% Acetic acid in 25% spirit and 30% glycerin | All treatments: 3 drops, 3 times daily. | All treatments: 2 weeks. | All groups: Suction cleaning at the beginning of the trial, and at weeks 1 & 2 visits. | Not reported. |
| Quinolone versus antiseptic | ||||||
| Fradis 1997 | 1) Quinolone: ciprofloxacin hydrochloride solution 3) Antiseptic: Burow aluminium acetate solution | 1) Ciprofloxacin: Not specified 3) 1% Aluminium acetate (weak antiseptic, designated as the "placebo group" by the trialists). | All treatments: 5 drops, 3 time daily. | All treatments: 3 weeks. | Not specified. | All other medication discontinued two weeks before study entry. (34 participants previously received systemic antibiotics; 12 had used neomycin‐polymyxin B eardrops without success). |
| Jaya 2003 | 1) Quinolone: ciprofloxacin hydrochloride 2) Antiseptic: povidone (polyvinyl pyrrolidone)‐ iodine (PVP‐I, Betadine) | 1) 0.3% Ciprofloxacin hydrochloride 2) 5% Povidone iodine | All treatments: 3 drops three times daily. | All treatments: 10 days. | Both groups: Dry mopping before each treatment occasion. Aural suctioning performed before initial treatment for all participants, and at subsequent weekly visits if discharge was present. | No prior systemic or topical antibiotic therapy within 10 days of study entry. |
| Macfadyen 2005 | 1) Quinolone: ciprofloxacin hydrochloride (Ciloxan, from Alcon) 2) Antiseptic: boric acid in alcohol (powder from UK but manufactured locally) | 1) 0.3% Ciprofloxacin hydrochloride 2) 2% Boric acid in 45% alcohol | Both treatments: Drops given twice daily (morning registration and lunch time). Child‐to‐child treatment: older children trained to clean and treat infected ears (at school), under supervision of trained teachers. | Both treatments: 10 consecutive school days (ie excluding weekends). | Both groups: Dry mopping before each treatment administration. Dry mop only for weeks 2‐4 if persistent discharge at week 2. | Excluded if treated for ear infection or received antibiotics for any other disorder in the previous 2 weeks. |
| van Hasselt 1997 | 1) Quinolone: ofloxacin (Exocin R, from Allergan) 3) Antiseptic: acetic acid in spirit and glycerin | 1) 0.3% Ofloxacin 3) 2% Acetic acid in 25% spirit and 30% glycerin | All treatments: 3 drops, 3 times daily. | All treatments: 2 weeks. | All groups: Suction cleaning at the beginning of the trial, and at weeks 1 & 2 visits. | Not reported. |
| van Hasselt 2002 | 1) Quinolone: ofloxacin in HPMC 2) Antiseptic: povidone iodine in HPMC HPMC = hydroxypropyl methyl‐cellulose (hypromellose) ‐ a treatment delivery vehicle. | 1) 0.075% Ofloxacin in 1.5% HPMC 2) 1% Povidone iodine in 1.5% HPMC | All treatments: 1 single topical application after suction cleaning. | Both treatments: Once only. | All groups: Suction cleaning once. | Not reported. |
Table 5.
Outcomes assessed
| Study ID | CSOM Resolution | Healing | Time to resolution | Time to reappearance | Hearing improvement | Safety | Other outcomes? | Other notes | Participant or ears? |
| Browning 1983a | 1) Participants with active, mucoid or inactive ears after 4 weeks of treatment. | Participant | |||||||
| Clayton 1990 | 1) Improved ears (i.e. dry or discharge improved by a score of 2 or more) at treatment days 9 and 21. Scoring system for severity of infection: degree of otorrhoea and of oedema or oedematous mucosa. Numbers with complete cure not reported. Presented results for day 21 only. | 1) Antibiotic or antiseptic resistant bacterial strains at treatment days 9 and 21. (Reported resistant strains for 21 days.) 2) Compliance to treatment, monitored at assessments every 3 days. | Resolution reported as 'improved' or 'not improved'; complete cure not reported. Excluded participants who failed to attend clinics or failed to comply with treatment (exclusions per diagnosis reported but not by treatment group). | Unclear ‐ ears? | |||||
| Fradis 1997 | 1) Clinical efficacy: cessation of otorrhoea on microscopic examination 24 hours after end of 3 weeks treatment. Reported as cure, improvement or failure ‐ improvement has been grouped with failure for this review. | ** 1) Hearing assessment: audiology assessed before and 24 hours after 3 weeks treatment. Results were not reported. ** | 1a) Bacteriological efficacy: eradication, persistence or superinfection, 24 hours after end of treatment; Reported pre and post‐treatment microorganisms isolated (including fungi, Candida). 1b) Sensitivity of pre and post (24 hrs)‐treatment bacteria to ciprofloxacin and tobramycin. | Ears | |||||
| Gyde 1978 | 1) Ears with clinical cure or failure at 6 months: Success: dry ear within 3 weeks of treatment, or sufficiently improved after 3 weeks that 3 weeks further therapy allowed the discharge to stop; with negative post‐treatment culture; and no return of the same strain of causative organism within 6 months of stopping treatment. Failure: evidence of exudate after 3 weeks, with a positive culture and little hope of further improvement. Failures at 6 months were allocated the alternative treatment, and followed‐up for 6 months as before. Results at 6 months, before crossover, have been taken for this review. | 1) Mean duration of treatment (days). Reported for successful ears only; includes crossed over treatment times. | 1) Relapse | 1) Hearing assessment in 50 participants: pre and post‐treatment audiometry, with a follow‐up audiogram by a certified audiologist. Results for average hearing loss before and after treatment, average improvement, and variation in improvement, are reported for each treatment group and treatment + 'intervention' (unclear what this is ‐ possibly surgery). Post‐treatment observations were 3 to 12 months after medical treatment or surgery, to detect any delayed ototoxicity. Audiometry (or an assessment of impedence, or both) assessed for a subset of 50 subjects only. Results were not reported separately by diagnosis; all analysed cases are included in this review. | 1) Ototoxicity: pre and post‐treatment audiometry, with a follow‐up audiogram by a certified audiologist. Post‐treatment observations were 3 to 12 months after medical treatment or surgery, to detect any delayed ototoxicity. Audiometry (or an assessment of impedence, or both) assessed for a subset of 50 subjects only (not reported separately by diagnosis). 2) Adverse reactions: open‐ended question with further questioning for intensity of reaction. No specific or suggestive questions were asked. | 1) Bacteriology ‐ reported cure, improvement or failure according to pre‐treatment bacteria cultured; Results include crossed over cases. | Negative bacterial culture was included in the definition of cure. | Ears | |
| Gyde 1981 | 1) Ears with clinical cure or failure at 3 months: Success: dry ear clinically within 3 weeks of treatment, with negative post‐treatment culture, and no return of the same strain of causative organism within 3 months of stopping treatment. Failure: evidence of exudate after 3 weeks, with a positive culture and little hope of further improvement. | ** 1) Hearing assessment: pre and post‐treatment audiometry, with a follow‐up audiogram by a certified audiologist. Only reported negative findings in relation to ototoxicity ‐ see safety column.** | 1) Ototoxicity: pre and post‐treatment audiometry, with a follow‐up audiogram by a certified audiologist. 2) Adverse reactions: open‐ended question with further questioning for intensity of reaction. No specific or suggestive questions were asked. | 1) Bacteriology ‐ reported cure or failure according to pre‐treatment bacteria cultured. | Negative bacterial culture was included in the definition of cure. Participants were examined at Days 7 and 14, and 3 months after completing therapy to detect any delayed toxicity. Clarification has been sought regarding the timings of the outcomes (2 weeks, 3 weeks or 3 months). | Ears | |||
| Jaya 2003 | 1) State of discharge (actively discharging or inactive) on microscopy. Assessed at study start and weeks 1, 2, 3, and 4 (results presented for all 4 weeks) | ** 1) Hearing assessment: pure tone audiometry (PTA) measured at study start and week 4. Only stated a lack of cases with deteriorated hearing on PTA; improvement not reported ** | 1) Pure tone audiometry (PTA) measured at study start and week 4. Reported lack of cases with deteriorated hearing on PTA only. 2) Ototoxic effects or allergy to the drug: specific inquiries for symptoms at weeks 1, 2, 3, and 4. | 1) Bacteriology and sensitivity of pus culture at study start, and week 4 (if still discharging). | Participant | ||||
| Kasemsuwan 1997 | 1) Clinical response (no discharge on microscopic examination) after 7 days. | ** 1) Hearing assessment: audiometric measurements before treatment and within 48 hours post therapy. Only presented results in relation to ototoxicity, and not improvement in hearing.** | 1) Adverse effects of medication: recorded at each visit (treatment days 1, 4 and 7) 2) Ototoxicity: audiometric measurements assessed before treatment and within 48 hours post‐treatment. | 1) Bacteriological response after 7 days (eradication; only performed aerobic culture). Sensitivity analysis also conducted. | Unclear ‐ participants? | ||||
| Kaygusuz 2002 | 1) Clinical response ‐ subjective assessment according to scale of discharge: complete improvement (no discharge); partial improvement (moist/wet); or unsuccessful (discharge filled the middle ear or external canal). Partial improvement has been classified as failure for this review. Participants were assessed daily for three weeks. Results were reported for Days 7, 14 and 21. | ** Not reported as an outcome, although a lack of additional side‐effects when adding steroid to treatment, was reported in the discussion.** | 1) Bacteriology before and after treatment, and resistance of pre‐treatment cultures to ciprofloxacin and tobramycin. | Participant | |||||
| Lorente 1995 | 1) Number and proportion of participants with dry ears (presence or absence of otorrhoea, assessed on otoscopy) at Day 8. | **1) Hearing assessment: audiometry to detect deterioration in hearing in relation to ototoxicity; Not reported improvement or degree of change. ** | 1) Ototoxicity: assessment of audiometry results and report of tinnitus to detect deterioration in hearing, and of balance to detect damage to the labyrinth. 2) Adverse events: local (pain, pruritis, stinging etc) and general. | 1) Participants with negative bacterial cultures (pre and post‐treatment bacteriology assessed); only reported proportions, not numbers. 2) Changes in mean scores for otorrhoea, pruritus (itching), stinging, pain, irritation and tinnitus at days 8 and 30. Scoring was on a scale of 0 (absent) to 3 (severe). | All outcomes were assessed at visits on Days 0 (before treatment), 8 and 30. Telephone contact on Day 3, to collect details of adverse events only. | Participant | |||
| Macfadyen 2005 | 1) Resolution of aural discharge on otoscopy at two and four weeks (resolution at two weeks was the primary outcome). | 1) Healing of the tympanic membrane on otoscopy at 2 and 4 weeks. | 1) Change in hearing threshold (pure tone audiometry) from baseline at 2 and 4 weeks. Hearing threshold in dB HL was averaged across 0.5, 1, 2 and 4 kHz. Unilateral cases: 1 single reading taken from the diseased ear; Bilateral disease: calculated average across both ears. Presented results for mean improvements in hearing threshold, and the difference in improvement between treatments, controlling for baseline threshold with analysis of covariance (ANCOVA). Adding background noise as an additional covariate did not change the conclusions. Also described hearing impairment levels using WHO classifications; considered changes between levels using Fishers exact test. | 1) Adverse event probe ‐ reported adverse events of interest only (local symptoms of pain, irritation and bleeding on mopping) at weeks 2 and 4. | Participants were followed‐up at weeks 2 and 4; reported results for both visits. All analyses followed the intention‐to‐treat principle. For bilateral cases, success was defined as when both ears resolved or healed; sensitivity analysis used success for bilateral cases when either or both ears resolved or healed. | Participant | |||
| Tutkun 1995 | 1) Clinical success 24 hours after 10 days treatment: cessation of otorrhoea on otoscopy and eradication of microorganisms in post‐treatment culture (aerobic culture only). | Differences between average pre‐treatment and post‐treatment (24 hours after 10 days treatment) mean values of: 1) speech reception thresholds; 2) speech discrimination scores; 3) pure tone thresholds for air conduction (dB HL, at 250 to 8000 Hz); 4) pure tone thresholds for bone conduction (dB HL, at 500 Hz to 4000 Hz). Also: 6) Pre‐treatment and post‐treatment air‐bone gaps (dB HL at 500 Hz to 4000 Hz (dB HL). For all hearing analyses, only averages have been reported, with no standard deviation or other indicators of variation or range of results. | 1) Ototoxicity: pure tone threshold, and speech discrimination scores assessed before and 24 hours post‐treatment. 2) Safety: absence of side‐effects reported in results but not specified as an outcome for assessment. | 1) Sensitivity to gentamicin and ciprofloxacin of aerobic culture taken 24 hours before treatment and 24 hours after completing treatment. | Participants were assessed on Days 2, 5, 7 and 10. Final assessment 24 hours after completing 10 days treatment. Negative bacterial culture was included in the definition of cure. | Participant | |||
| van Hasselt 1997 | 1) Dry or wet ear after 1 and 2 weeks. | Results for cure at week 2 were discrepant between the 1997 CBM report (see figures and results text) and 2002 paper (see results text) for acetic acid and neomycin‐ polymyxin B. | Ears | ||||||
| van Hasselt 1998 | 1) Resolution of otorrhoea at weeks 1, 2 (end of treatment) and week 8: inactive when completely dry middle ear; active when otorrhoea still present. Presented % ears cured at each visit, but only reported numbers per group at week 2. | 1) Medicine cost per healed ear after week 8. | Ears | ||||||
| van Hasselt 2002 | 1) Dry ears after 1 week. | Ears |
Age, setting and location (for included studies)
See the Characteristics of included studies table for details.
Ages varied:
Three studies were primarily in children, four included children and adults, five were in adults only, and two did not specify. Details are reported in the 'Characteristics of included studies table and Table 7.
Setting:
Two trials were community‐based, and mainly in children: Macfadyen 2005 (Kenyan primary schools, using child‐to‐child treatment) and van Hasselt 1998 (rural Malawi). Two further trials in rural Malawi did not specify the setting (van Hasselt 1997 and van Hasselt 2002).
Eight were hospital‐based (outpatient). Another two did not report the setting but appear to be based either in a hospital or private clinic (Gyde 1978; Gyde 1981).
Location:
Six trials in high‐income countries (UK, Canada, Spain and Israel).
Eight were in low or middle‐income countries (Thailand, Turkey, India, Kenya and Malawi).
Diagnostic criteria for included participants
Table 7 provides eligibility criteria and more detailed CSOM diagnostic criteria. Definitions used for CSOM varied, particularly for duration, and also whether positive bacterial culture or changes in mucosal appearance were assessed and/or required ‐ see Table 7. We accepted the authors' definitions when assessing eligibility.
Three trials included otitis externa and draining mastoid cavities; all analysed and presented results separately, but two did not report how or whether randomisation was stratified by diagnosis (Clayton 1990; Gyde 1981) and although one stated that type of infection was the principal criterion for randomisation by minimisation, it is not clear whether this relates to diagnosis or bacteriology (Gyde 1978); all participants are therefore included in this review. Browning 1983a also included participants with draining mastoid cavities, and Fradis 1997 reported surgical perforations in three ears although prior middle‐ear surgery was an exclusion criterion; neither trial reported the results separately, and all participants are also included in this review. Table 7 provides further details with numbers involved.
Interventions
Table 9 provides details of the treatment regimens used in the trials. Treatment regimens (strength, dose, frequency of dose, duration, and treatment provider) and associated aural toilet were equivalent for all comparisons within each trial, unless specified below.
Ten trials tested non‐quinolone antibiotics:
seven tested aminoglycoside alone (five gentamicin, two tobramycin);
two tested aminoglycoside‐polymyxin combination (neomycin‐polymyxin B);
one tested chloramphenicol;
two tested trimethoprim‐sulphacetamide‐polymyxin B (TSP);
one tested trimethoprim‐polymyxin B (TP).
In one trial, the choice of antibiotic (gentamicin or chloramphenicol) depended on baseline bacteriology (Browning 1983a).
Ten trials tested quinolone antibiotics:
seven tested ciprofloxacin;
three tested ofloxacin (although van Hasselt 2002 used a single weak dose in a treatment delivery vehicle).
Seven trials tested antiseptics:
two tested aluminium acetate (although Fradis 1997 used a weak strength as the "placebo" group);
one tested boric acid + iodine powder;
one tested boric acid in alcohol;
one tested acetic acid in spirit and glycerin;
two tested povidone iodine (although van Hasselt 2002 used a single weak dose in a treatment delivery vehicle).
Two trials tested placebo treatments:
one tested normal saline solution;
one tested a treatment delivery vehicle with no treatment, hydroxypropyl methyl‐cellulose (hypromellose, HPMC).
Most treatments were self‐administered two or three times daily, for between seven days and three weeks, usually with ear toilet at least once (by the participant or clinician). However, Browning 1983a compared daily antibiotics (self‐treated) with weekly antiseptics (given by the otologist), both for four weeks. See Table 9 for further exceptions and trial details. One trial included a crossover to the alternative treatment for failures at six months (Gyde 1978); we have only used results before crossover where possible. Nine trials reported a treatment‐free period before study entry (washout usually 10 days or two weeks), for antibiotics or other specified medicines. See Table 9 for details and exceptions. Two trials also excluded participants who received antibiotics or any other treatment during the study.
Outcomes
The 'Characteristics of included studies' table indicates which review outcomes were covered by each trial. Table 10 describes the definitions used by the trials, for each review outcome, and how and when outcomes were measured and reported.
Treatment failure (persistent discharge) ‐ 14 trials:
All trials reported CSOM resolution for inclusion in the primary outcome of this review. However, definitions varied, and four only reported results before two weeks, not at or after two weeks ‐ see Table 10. Where trials reported separate categories for 'cure' and 'improvement', or 'active' and 'mucoid', we have classed 'improvement' or 'mucoid' as failure along with any other cases of failure reported. One trial (Clayton 1990) only categorised cases as improved or not improved ‐ for this trial, improvement has been classed as success.
Healing of the perforation ‐ one trial:
Numbers with complete healing at two and four weeks are presented for Macfadyen 2005.
Time to resolution of CSOM ‐ no trials:
However, Gyde 1978 reported the mean duration of treatment, for successfully treated ears, but this includes crossed over times.
Improvement in hearing threshold ‐ three trials:
See tables 7 to 11 for Gyde 1978 (Table 11), Tutkun 1995 (Table 12, Table 13, and Table 14), and Macfadyen 2005 (Table 15 and results text). Gyde 1978 and Tutkun 1995 also provided a summary statement for ototoxicity (see Table 16).
Table 6.
Gyde 1978 Hearing analysis measured in dB (TSP versus gentamicin at 3‐12 months
| Intervention | Number examined | Number improved | Ave dB loss before | Ave dB loss after Rx | Average hearing gain | dB variation in gain |
| Topical non‐quinolone antibiotic: | ||||||
| Trimethoprim‐polymyxin‐B‐sulfacetamide (TSP) | 19 (any diagnosis) | 17 | 31.3 | 13.5 | 17.8 | ? to 43 |
| TSP + intervention (not specified) | 25 (any diagnosis) | 24 | 35.7 | 16.1 | 20.0 | ‐6 to 53 |
| Topical non‐quinolone antibiotic: | ||||||
| Gentamicin | 26 (any diagnosis) | 23 | 35.9 | 19.6 | 16.4 | 0 to 35 |
| Gentamicin + intervention (not specified) | 22 (any diagnosis) | 20 | 37.9 | 19.6 | 18.2 | ‐3 to 34 |
Table 7.
Tutkun 1995 Hearing analysis: pre and post‐treatment pure tone thresholds (dB HL
| Frequency | Ciprofloxacin ‐ air | Ciprofloxacin ‐ bone | Gentamicin ‐ air | Gentamicin ‐ bone |
| 250 Hz | ||||
| Difference (dB HL) | ‐1.2 | 2.0 | ||
| 500 Hz | ||||
| Pre‐treatment (dB HL) | 38.3 | 14.5 | 34.8 | 11.8 |
| Post‐treatment (dB HL) | 35.4 | 12.7 | 31.7 | 13.3 |
| Difference (dB HL) | +2.9 | +1.8 | +3.1 | ‐1.5 |
| 1000 Hz | ||||
| Pre‐treatment (dB HL) | 32.3 | 13.3 | 35.8 | 10.1 |
| Post‐treatment (dB HL) | 31.7 | 10.4 | 30.3 | 14.0 |
| Difference (dB HL) | +0.6 | +2.9 | +5.5 | ‐3.9 |
| 2000 Hz | ||||
| Pre‐treatment (dB HL) | 30.5 | 13.1 | 31.5 | 12.5 |
| Post‐treatment (dB HL) | 29.5 | 14.0 | 27.5 | 14.6 |
| Difference (dB HL) | +1.0 | ‐0.9 | +4.0 | ‐2.1 |
| 4000 Hz | ||||
| Pre‐treatment (dB HL) | 36.0 | 22.2 | 37.6 | 23.9 |
| Post‐treatment (dB HL) | 31.3 | 19.5 | 40.6 | 24.6 |
| Difference (dB HL) | +4.7 | +2.7 | ‐3.0 | ‐0.7 |
| 8000 Hz | ||||
| Difference (dB HL) | +7.7 | +4.0 |
Table 8.
Tutkun 1995 Hearing analysis: pre and post‐treatment air‐bone gaps (in dB HL)
| Group | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz |
| Ciprofloxacin | ||||
| Pre‐treatment (dB HL) | 25.0 | 23.0 | 18.0 | 15.7 |
| Post‐treatment (dB HL) | 24.5 | 18.2 | 15.7 | 12.3 |
| Gentamicin | ||||
| Pre‐treatment (dB HL) | 21.4 | 25.7 | 17.1 | 15.1 |
| Post‐treatment (dB HL) | 22.5 | 20.0 | 16.4 | 17.8 |
Table 9.
Tutkun 1995 Hearing analysis: pre and post‐treatment SRTs and SDSs
| Assessment | Ciprofloxacin | Gentamicin | student's t‐test p |
| Speech Reception Threshold (SRT) (dB HL) | |||
| Pre‐treatment | 34.3 | 34.4 | |
| Post‐treatment | 30.8 | 30.2 | |
| Difference (dB HL) | +3.5 | +4.2 | P > 0.01 |
| Speech Discrimination Score (SDS) | |||
| Pre‐treatment | 96.3% | 96.0% | |
| Post‐treatment | 96.6% | 98.4% | |
| Difference (%) | ‐0.3% | ‐2.4% | P > 0.01 |
Table 10.
Macfadyen 2005 Hearing analysis: average change from baseline at 2 and 4 weeks
| Week | Treatment Group | N | dB Mean Improve (SD) | Dif 95%CI: cip‐boric | p |
| (ANCOVA controlling for baseline audio level) | (ANCOVA controlling for baseline audio level) | ||||
| 2 weeks | Ciprofloxacin Boric acid | 201 202 | 4·32 (11·18) 2·69 (11·67) | 2·17 (0·09 to 4·24) | 0·0410 |
| 4 weeks | Ciprofloxacin Boric acid | 196 194 | 5·42 (11·03) 2·63 (12·18) | 3·43 (1·34 to 5·52) | 0·0014 |
Table 11.
Safety
| Study ID | Treatment comparison | Allergic reaction | Ototoxicity | Other AE |
| Gyde 1978 | 1) Topical non‐quinolone antibiotic: trimethoprim‐polymyxin B‐sulfacetamide eardrops. 2) Topical non‐quinolone antibiotic: gentamicin 0.3% eardrops | The treatments did not cause any allergic reactions. | The results of audiometry (tested on 50 subjects, 3 to 12 months after treatment or additional surgery) showed no signs of ototoxicity either immediatly after the treatment , or later on, for either treatments (see separate table for results). The majority of the participants tested showed an improvement of the auditory acuteness, and hearing results remained stable in all cases examined during the 3 to12 month post‐observation period. | There were no unfavourable reactions nor side effects following the administration of either treatment. The participants did not complain about pain, itching or of burn. The treatment did not cause allergic reactions not excessive fungal growth. |
| Gyde 1981 | 1) Topical non‐quinolone antibiotic: trimethoprim‐polymyxin B‐sulfacetamide eardrops. 2) Topical non‐quinolone antibiotic: trimethoprim‐polymyxin B eardrops. | There were no signs of ototoxicity. [From abstract only ‐ data not presented] | There were no incidences of side‐effects/local sensitivity, or fungal infection overgrowth. [From abstract and discussion only ‐ data not presented] | |
| Jaya 2003 | 1) Topical quinolone: 0.3% ciprofloxacin hydrochloride eardrops. 2) Topical antiseptic: 5% povidone‐iodine eardrops. | No patients developed allergic manifestations. | No patients developed ototoxic effects. There was no deterioration of hearing as assessed by pure‐tone audiometry. | |
| Kasemsuwan 1997 | 1) Topical quinolone: ciprofloxacin in saline eardrops. 2) Topical placebo: normal saline eardrops. | No worsening of audiological measurements (taken 24 hours after completing treatment) was detected. | No medical side‐effects related to the topical medication were detected in the study. | |
| Lorente 1995 | 1) Topical quinolone: ciprofloxacin eardrops 0.3% 2) Topical non‐quinolone: gentamicin 0.3% | One case (on gentamicin) of slight auditory disorder was detected on audiometry; the loss was considered to be only slight and without clinical relevance by the trialists. One case of loss of balance was reported before treatment in a patient randomised to ciprofloxacin; this disappeared during the course of treatment. A joint analysis of the action of both treatments showed no statistically significant effects on the audiometry results (P = 0.21). | Both treatments were reported to be very well tolerated with respect to general side effects. No cases of superinfection with fungus (Candida albicans or Aspergillus) were observed. | |
| Macfadyen 2005 | 1) Topical quinolone: ciprofloxacin eardrops (after dry mopping) 2) Topical antiseptic: boric acid in alcohol eardrops (after dry mopping) | For adverse events of ear pain, irritation and bleeding on mopping, there was a higher frequency in the boric acid group (30/206) than in the ciprofloxacin group (17/210). | ||
| Tutkun 1995 | 1) Topical quinolone: ciprofloxacin eardrops 2) Topical non‐quinolone: gentamicin eardrops | Audiometric evaluation yielded no evidence of ototoxicity as reflected by the pure tone threshold and speech discrimination scores in either group. Instead, hearing thresholds were slightly better than pre‐treatment levels in both groups (Ozagar 1997). | There were no side effects. |
Five other trials assessed hearing, but reported no results (Fradis 1997) or only a summary statement regarding worsening in hearing or ototoxicity (four trials). Table 16 (Safety) provides information regarding worsened hearing, where available.
Time to reappearance of discharge and perforation after its previous resolution ‐ no trials:
However, Gyde 1978 did report relapses.
Adverse events ‐ seven trials:
Results of ototoxicity assessments and other adverse events collected are reported in Table 16 (Safety). One further trial did not report safety in the outcomes, but did report a lack of additional side‐effects when adding steroid to treatment, in the discussion (Kaygusuz 2002).
Other outcomes assessed but not specified in the protocol for this review ‐ 10 trials:
See Table 10 for details of these outcomes, which have not been analysed in this review.
Sources of Support
Pharmaceutical company support:
Three trials (Gyde 1978; Gyde 1981; Lorente 1995).
Trusts and charities:
Three trials (Macfadyen 2005; van Hasselt 1997; van Hasselt 1998).
Not mentioned:
Eight trials.
Risk of bias in included studies
Table 6 provides details of the methodological quality of included studies.
Allocation
Six trials were "adequate": these trials were also double‐blind.
Eight trials were "unclear": allocation concealment was not discussed (four trials were described as double blind and one single blind).
Blinding
Ten trials were double‐blinded.
One trial was single‐blinded (except the antiseptic group, which was given by the clinicians, after aural toilet; Browning 1983a).
Three trials did not report on blinding.
Incomplete outcome data
For the primary outcome, resolution or treatment failure up to four weeks: Five trials were "adequate" (> 90% included): One trial reported following the intention‐to‐treat principle for the analyses (Macfadyen 2005). One trial was adequate for ears, the unit of analysis (analysed 54/60 = 90%), but slipped to 88% (45/51) for participants (Fradis 1997). One trial did not report any loss to follow‐up or exclusions (Kaygusuz 2002). Five trials were "unclear": These trials did not report numbers initially randomised or excluded from the analyses: three gave reasons but not numbers; two did not discuss at all.
Four trials were "inadequate": Overall rates for participants or ears analysed per trial were 68% to 75%. However follow‐up rates for one trial varied widely between treatment groups (61% to 92%) (van Hasselt 1997).
Main reasons specified for exclusion were: Lack of attendance (loss to follow‐up) (most trials); participants who did not adhere to study treatment (five trials); participants who received antibiotics or any other treatment during the study (two trials); missing data (one trial); or consent withdrawn (one trial). See Table 6.
Other potential sources of bias
Sequence generation
Two trials were "adequate": Gyde 1978, Macfadyen 2005.
Twelve trials were "unclear": described as randomised (five mentioned randomisation codes or coded treatments), but did not discuss how the sequence was generated, or how different diagnoses were accounted for during randomisation.
Balance of baseline characteristics across groups
This was not reported in five trials. The rest reported at least one characteristic by treatment group ‐ see Table 6 for details.
For the five trials that included a range of diagnoses, three reported numbers for each across treatment groups: two of these were mostly balanced (for ears) (Gyde 1978; Gyde 1981), but one was not (Clayton 1990) ‐ see Table 6. The other two trials did not report baseline numbers or results for each diagnosis separately by treatment group (Browning 1983a; Fradis 1997).
Bilateral disease
Trials were inconsistent in handling of bilateral disease, and have analysed and presented results either by ear or by participant. Table 8 provides detailed information regarding bilateral disease, and reporting of ears versus participants, for each trial. Where available the number of participants with bilateral disease for each trial is also presented. Clarification has been requested from the authors for those where the unit of analysis is not clear, and for further information on handling bilateral cases.
Results for ears ‐ six trials:
For bilateral disease each ear was treated and analysed as a separate case. Rates of bilateral disease in these trials, where available, varied widely from 9.9% of analysed participants (4.2% on gentamicin) in Gyde 1978, to 41.1% of all randomised participants in van Hasselt 1998. See Table 8.
Results for participants ‐ six trials:
Only two trials specified how bilateral cases were handled (Browning 1983a and Macfadyen 2005) ‐ see Table 8.
Unit of analysis unclear ‐ two trials:
Bilateral disease was also not discussed (Clayton 1990 and Kasemsuwan 1997).
Where available, we have reported results for number of participants rather than ears; otherwise results for number of ears have been taken. The concern is that a trial analysed by ears rather than number of participants will have an underestimated standard error (SE) and therefore receive an inappropriate increased weight in a meta‐analysis. We will use the information in Table 8 to attempt to address the issues of inflated weighting for trials reporting numbers of ears, in a later update of this review.
Effects of interventions
See also tables (Table 8 for details regarding bilateral disease in each trial, Table 9 for details of the treatment regimens used for each of the sets of comparisons discussed below, and Table 10 for the definitions and timings of the outcomes assessed by trialists for each outcome category). Data relating to adverse events and ototoxicity have been summarised in Table 16 for trials that mentioned this outcome. We have only presented outcomes below that the trial authors have reported ‐ see Table 10 for other outcomes. Clarification is being sought from the authors where uncertainties exist in the data and where outcomes are not reported. These responses will be incorporated in subsequent updates of the review.
There were insufficient trials in each comparison to consider exploring heterogeneity or the sensitivity analyses outlined above; these will be considered in a subsequent update of the review if there are sufficient trials. Meanwhile, trials where people with CSOM are only a subgroup of the participants included in the study are identified in this review ‐ see Table 7.
Do Topical Antibiotics Without Steroids Work?
Quinolone versus no treatment
Two trials compared quinolones with placebo; all groups received aural toilet at least once (Kasemsuwan 1997; van Hasselt 2002). Follow‐up in both trials was for one week only; the authors have been contacted to request longer follow‐up data, if available. Results for van Hasselt 2002 refer to number of ears, but it is not clear whether the results for Kasemsuwan 1997 refer to ears or participants; further details have been requested.
Treatment failure (persistent discharge)
(Analysis 1.1) Results were only provided at one week ‐ see figure. The pooled RR is 0.45 (95% CI 0.34 to 0.59). However, there is a large amount of heterogeneity present (I2 = 72.5% and chi2 P value = 0.06), possibly because Kasemsuwan 1997 used daily applications of ciprofloxacin versus saline solution while van Hasselt 2002 used only one single application of ofloxacin in HPMC versus HPMC alone. The random‐effects pooled result is 0.34 (95% CI 0.12 to 0.94).
Analysis 1.1.
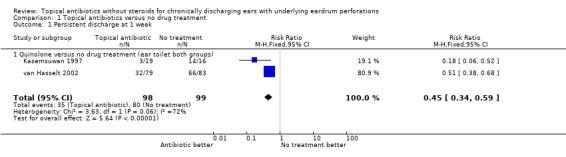
Comparison 1 Topical antibiotics versus no drug treatment, Outcome 1 Persistent discharge at 1 week.
Improvement in hearing threshold
One trial measured audiometry (Kasemsuwan 1997) before treatment and within 48 hours of completing seven days treatment. Data for these results were not provided but have been requested. The trial report provides a summary statement only '...no worsening of audiometry measurements related to this topical medication was detected in this study'. van Hasselt 1997 did not mention this outcome and a request has been made for any available results.
Adverse Events
Data relating to adverse events have been summarised in Table 16. van Hasselt 2002 did not mention this outcome and a request has been made to the authors for any available results.
Non‐quinolone versus no treatment
No trials were identified.
Which Topical Antibiotics (Without Steroids) Work Best?
Comparisons between topical non‐quinolone antibiotics without steroids
Two trials compared non‐quinolones. Gyde 1978 compared trimethoprim‐sulfacetamide‐polymyxin B (TSP) with gentamicin, while Gyde 1981 compared TSP with trimethoprim‐polymyxin B (TP). Table 9 gives more details of the treatment regimens. Both trials reported results for numbers of ears (Table 8).
Treatment failure
(Analysis 2.1) Results for Gyde 1978 at six months (before crossover) found no significant difference between TSP and gentamicin: RR was 2.00 (95% CI 0.64 to 6.22). Meanwhile Gyde 1981 at three months showed a significant effect in favour of TSP over TP: RR was 0.29 (95% CI 0.11 to 0.80). However, both trials included participants with a range of diagnoses (see Table 8 and Table 7), and cure rates varied between diagnoses; the figure shows the results for all participants. No meta‐analysis has been undertaken for these trials due to different comparator drugs within the "non‐quinolones only" comparison.
Analysis 2.1.
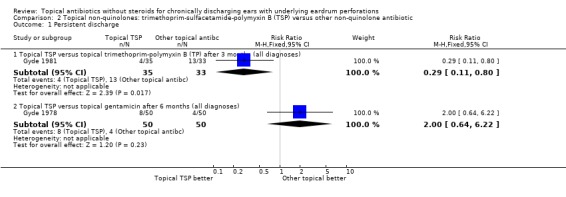
Comparison 2 Topical non‐quinolones: trimethoprim‐sulfacetamide‐polymyxin B (TSP) versus other non‐quinolone antibiotic, Outcome 1 Persistent discharge.
Time to resolution of CSOM; and Time to re‐appearance of discharge and perforation after its previous resolution
No trials reported these outcomes, but two related outcomes reported by Gyde 1978 were mean duration of treatment (for ears successfully treated only, including pre and post‐crossover durations), and occurrence of relapses. The average successful treatment duration (for all diagnoses) was 19.3 days for TSP (range 7 to 32, N = 46), and 21.7 days for gentamicin (range 10 to 37, N = 51). There were no cases of relapse within the six to 12 month follow‐up period.
Improvement in hearing threshold
Both trials reported recording this outcome, although Gyde 1981 did not provide results. The results for Gyde 1978 are shown in Table 11. In a summary statement, the authors reported that "the majority of the participants tested showed an improvement of the auditory acuteness, and hearing results remained stable in all cases examined during the 3 to 12 month post‐observation period".
Adverse Events
Data relating to adverse events have been summarised in Table 16.
Quinolone versus non‐quinolone antibiotics without steroids
Six trials compared a quinolone antibiotic with a non‐quinolone antibiotic, with follow‐up at weeks one to three: Lorente 1995 (day eight); Tutkun 1995 (day 11); Fradis 1997 (week three); Kaygusuz 2002 (weeks one, two and three); van Hasselt 1997 (weeks one and two), and van Hasselt 1998 (week two). van Hasselt 1998 also presented results at weeks one and eight (as percentage of ears cured), but did not report numbers analysed per group; this information has been requested from the authors. van Hasselt 1998 had four treatment groups: the non‐quinolone antibiotic delivered once weekly or twice daily and the same for the quinolone groups. For this trial we have entered data comparing the once weekly treatment regimens separately to the twice daily treatment regimens. Table 9 indicates the treatment regimens used for each trial. Three trials reported results for ears (Fradis 1997; van Hasselt 1997; van Hasselt 1998); the other three reported for participants. Table 8 provides further details.
Treatment failure
(Analysis 3.1) The figure shows the results at weeks one, two and three separately, and combined for weeks two to three. No statistically significant difference was found between quinolones and non‐quinolones at weeks one or three: RR were, 0.89 (95% CI 0.59 to 1.32) at week one and 0.97 (95% CI 0.54 to 1.72) at week three. However, a difference in favour of quinolones was seen at week two with a pooled RR of 0.65 (95% CI 0.46 to 0.92). Pooling the results for weeks two to three gives a RR of 0.76 (95% CI 0.55 to 1.04).
Analysis 3.1.
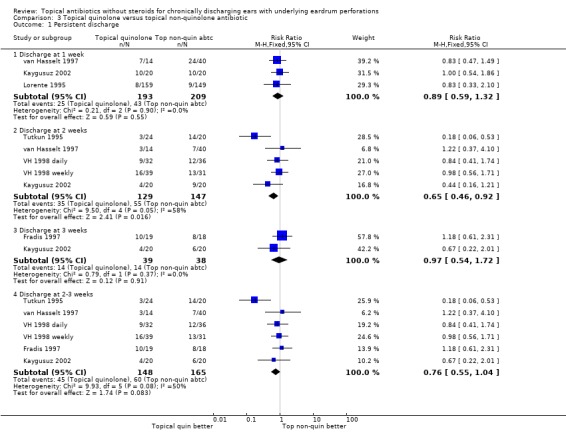
Comparison 3 Topical quinolone versus topical non‐quinolone antibiotic, Outcome 1 Persistent discharge.
Results suggest moderate heterogeneity for results at two weeks, and for the overall two to three week results, mainly caused by Tutkun 1995, which received 28% and 26% of the weight for results at two weeks and two to three weeks, respectively. The random‐effects analyses are RR 0.64 (95% CI 0.35 to 1.17) for two weeks, and 0.78 (95% CI 0.48 to 1.26) for two to three weeks. Excluding Tutkun 1995 gives pooled estimates detecting no difference between quinolone and non‐quinolone antibiotics with a fixed RR of 0.84 (95% CI 0.57 to 1.23) (I2 = 0%, chi2 P = 0.53) at two weeks, and overall results at two to three weeks of 0.96 (95% CI 0.68 to 1.35) (I2 = 0%, chi2 P = 0.90) (figure not provided).
The two week data for van Hasselt 1997 were discrepant between an internal report (CBM 1997) and a published report in 2002, which summarised the results in an introductory paragraph. The figure shows the results taken from CBM 1997. The results from the paper van Hasselt 2002 are 3/14 (21%) with failure on quinolone (ofloxacin) and 6/35 (17%) with failure on non‐quinolone (neomycin‐polymyxin B) (RR is 1.25 (95% CI 0.36 to 4.32)). However, the pooled results for failure at two weeks and for two to three weeks do not change: RR is 0.65 (95% CI 0.46 to 0.92) (I2 = 58.0%, chi2 P = 0.05) and 0.76 (95% CI 0.55 to 1.04) (I2 = 49.7%, chi2 P = 0.08) respectively using the fixed‐effect model; or 0.64 (0.35 to 1.17) at two weeks and 0.78 (0.48 to 1.27) for two to three weeks using the random‐effects model. The results when Tutkun 1995 is excluded also remain unchanged (except heterogeneity for two to three weeks pooled results: I2 =0%, chi2 P = 0.89). (Figure not provided for results using van Hasselt 2002 paper.)
Improvement in hearing threshold
Three trials mentioned this outcome. Fradis 1997 measured audiometry before treatment and after treatment but did not provide any results. Lorente 1995 recorded audiometry measurements but only reported in a summary statement that one case (on gentamicin) of slight auditory disorder was detected on audiometry; the trialists considered this loss was only slight and without clinical relevance. A joint analysis of the two treatments was reported to show no statistically significant effects on the audiometry results, but details are not provided. Follow‐up on this trial was for eight days only; however, results have been requested for completeness. Tutkun 1995 looked at pre‐treatment and post‐treatment measurements for pure tone air and bone thresholds, air‐bone gaps, speech reception threshold, and speech discrimination scores. These results are presented in tables: Table 12 for pre and post‐treatment pure tone thresholds (in dB HL); Table 13 for pre and post‐treatment air‐bone gaps between 500 and 4000 Hz (in dB HL); and Table 14 for pre and post‐treatment speech reception thresholds (SRTs in dB HL) and speech discrimination scores (SDSs). No standard deviations were reported but have been requested. Bone conduction hearing thresholds were slightly elevated after treatment with gentamicin (non‐quinolone); however, the authors reported that none of the differences in any of the assessments were significant (student's t‐test P > 0.01).
Adverse Events
Only Lorente 1995 and Tutkun 1995 mentioned this outcome ‐ data relating to adverse events have been summarised in Table 16.
Comparisons between topical quinolone antibiotics
No trials were identified that compared different topical quinolone antibiotics against one another.
Comparisons between different treatment regimens of the same drug (e.g. method of delivery, or dose or frequency) have not been analysed in this review (e.g. excluded studies Kiris 1998 and de Miguel 1999; or included study, van Hasselt 1998; see other comparisons).
Do Topical Antibiotics Without Steroids Work Better Than Antiseptics?
Non‐quinolone versus antiseptic
Four trials compared a non‐quinolone with an antiseptic (van Hasselt 1997; Clayton 1990; Fradis 1997; Browning 1983a). Follow‐up was at two, three, three and four weeks, respectively. van Hasselt 1997 also reported results at one week. Table 9 indicates the treatment regimens used by each trial, with potential sources of heterogeneity. Browning 1983a reported results for participants, while Fradis 1997 and van Hasselt 1997 presented by ears. Clayton 1990 appears to report results for ears, although this is not clear in the paper and clarification has been requested. See Table 8 for more details.
Treatment failure
(Analysis 4.1) The figure shows results at weeks one and two to four ordered by length of follow‐up. Note that Clayton 1990 reports a success as an improvement and not a resolution. Browning 1983a; Clayton 1990 and Fradis 1997 included participants with mastoid cavities or surgical perforations, while Clayton 1990 also included otitis externa (see Table 8 and Table 7). Only Clayton 1990 presented results separately, but the authors did not specify whether the diagnostic groups were stratified for during randomisation; all participants are included in the figure.
Analysis 4.1.
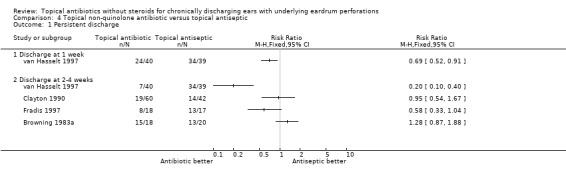
Comparison 4 Topical non‐quinolone antibiotic versus topical antiseptic, Outcome 1 Persistent discharge.
Only one trial found a significant effect in favour of non‐quinolone antibiotics (van Hasselt 1997 at one and two weeks); Fradis 1997 also found a non‐significant result in favour of antibiotic at week three, although the antiseptic was a weak concentration used as a "placebo" group by the trialists. The possibility of any benefit decreased over time, and one trial found a non‐significant trend in favour of antiseptics by week four (Browning 1983a). Results have not been pooled, due to the significant heterogeneity observed in these results, which may be partly explained by the length of follow‐up and possibly also diagnostic criteria.
The two week data for van Hasselt 1997 were discrepant between an internal report (CBM 1997) and a published report in 2002, which summarised the results in an introductory paragraph. The figure shows the results taken from CBM 1997. The results from the paper van Hasselt 2002 are 6/35 (17%) with failure on non‐quinolone antibiotic, and 35/39 (90%) with failure on antiseptic (RR was 0.19 (95% CI 0.09 to 0.40)). Clarification has been requested from the author.
Improvement in hearing threshold
Fradis 1997 stated measurements were taken but did not provide results; these have been requested. None of the other authors mentioned this outcome and a request has been made for any available results.
Quinolone versus antiseptic
Five trials compared a quinolone antibiotic with an antiseptic (van Hasselt 2002; van Hasselt 1997; Fradis 1997; Jaya 2003; Macfadyen 2005). The longest length of follow‐up for each of these trials was one, two, three, four and four weeks respectively. The antiseptic in Fradis 1997 was a weak concentration used as a "placebo" group by the trialists. Table 9 indicates the treatment regimens used by each trial. Jaya 2003 and Macfadyen 2005 report results for participants; the rest report numbers of ears. See Table 8 for more details.
Treatment failure
(Analysis 5.1) The figure shows the results for week one, and weeks two to four ordered by length of follow‐up. van Hasselt 2002 presented at week one only. The results indicate that quinolones are superior to antiseptics, with pooled results at one week of RR 0.52 (95% CI 0.41 to 0.67), and at two to four weeks of 0.58 (95% CI 0.47 to 0.72).
Analysis 5.1.
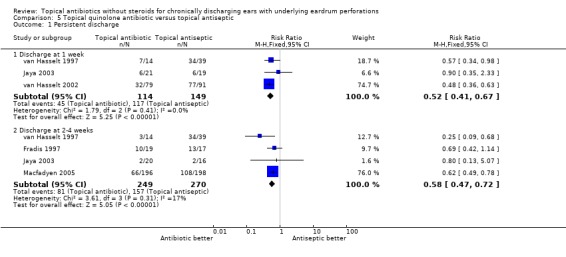
Comparison 5 Topical quinolone antibiotic versus topical antiseptic, Outcome 1 Persistent discharge.
Macfadyen 2005 dominated the results at two to four weeks with 76% of the weight, due to its large size and narrow confidence intervals. Excluding Macfadyen 2005 increases the heterogeneity and confidence intervals with a non‐significant effect after accounting for heterogeneity: the pooled results at two to four weeks become RR 0.46 (95% CI 0.28 to 0.75) using the fixed‐effect model, but 0.50 (95% CI 0.22 to 1.16) using the random‐effects model (I2 = 53.3%, chi2 P = 0.12) (figure not provided).
The two week data for van Hasselt 1997 were discrepant between an internal report (CBM 1997) and a published report in 2002, which summarised the results in an introductory paragraph. The figure shows the results taken from CBM 1997. The results from the paper van Hasselt 2002 are 3/14 (21%) with failure on quinolone antibiotic and 35/39 (90%) with failure on antiseptic (RR 0.24 (95% CI 0.09 to 0.65)). If these results are used then the pooled result becomes RR 0.58 (95% CI 0.47 to 0.71) (figure not provided). Clarification has been requested from the author.
Healing of the perforation
(Analysis 5.2) Only one trial looked at healing of the perforation at two and four weeks (Macfadyen 2005). Results, expressed as RR, are 1.06 (95%CI 0.52 to 2.13) at two weeks, and 1.54 (95%CI 0.91 to 2.61) in favour of ciprofloxacin over boric acid at four weeks.
Analysis 5.2.
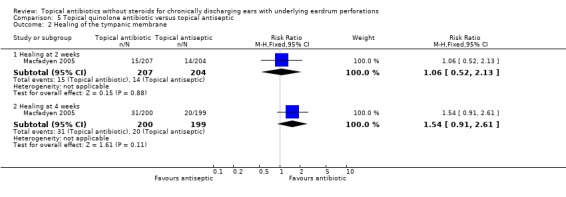
Comparison 5 Topical quinolone antibiotic versus topical antiseptic, Outcome 2 Healing of the tympanic membrane.
Improvement in hearing threshold
Three trials reported this outcome. Fradis 1997 and Jaya 2003 measured audiometry before treatment and after treatment but did not provide any results. These have been requested. However Jaya 2003 provided a summary statement of results: "There is no deterioration of hearing as assessed by pure tone audiometry".
Macfadyen 2005 showed greater hearing improvement on ciprofloxacin than boric acid at two and four weeks. Hearing threshold was averaged across four frequencies, 0.5, 1, 2 and 4 kHz (for one single reading for diseased ear, or averaged across the two ears in bilateral cases). Results from ANCOVA, controlling for baseline audio level, for the difference in mean improvement are: 2.17 (95%CI 0.09 to 4.24) at two weeks, and 3.43 (95%CI 1.34 to 5.52) in favour of ciprofloxacin at four weeks ‐ see Table 15.
Adverse Events
Only two trials mentioned this outcome: Jaya 2003 (audiometry only) and Macfadyen 2005 ‐ data relating to adverse events (and ototoxicity) have been summarised in Table 16.
Discussion
This review considers alternative topical drug treatments, but does not compare treatment regimens or delivery methods. We also excluded comparisons with steroids (with or without antibiotics; see steroids review) or systemic treatments, and presented results for quinolone and non‐quinolone antibiotics separately, to address focused and meaningful questions. These tighter comparisons and the addition of new trials, are why our conclusions differ to those of an earlier review (Acuin 1998). The earlier review included systemic non‐quinolones and antibiotic‐steroid combinations in this comparison, leading to a stronger effect in favour of topical quinolones than found here; meanwhile only three trials were included in the earlier antibiotic versus antiseptic comparison, one of which used steroids, and no statistically significant difference had been found. The following discusses the findings of this new review.
Topical antibiotics were superior to no drug treatment for curing CSOM at one week, when both groups received aural toilet. However, this was based on two small trials, both using quinolone antibiotics, with no longer‐term follow‐up.
Only two trials compared different topical non‐quinolone antibiotics; no statistically significant difference was detected between trimethoprim‐sulfacetamide‐polymyxin B (TSP) and gentamicin (for cure rates or level of hearing), although TSP was better than trimethoprim‐polymyxin B. However, these results included participants with a range of diagnoses, and rates differed between diagnoses.
Of the trials that compared topical quinolone and non‐quinolone antibiotics, only one found an effect in favour of topical quinolones (Tutkun 1995, reporting at day 11); the other trials found no statistically significant difference at one week or up to three weeks, although Kaygusuz 2002 did show a trend in favour of quinolones at two weeks but numbers were small with wide confidence intervals. Excluding Tutkun 1995 (which received 28% and 26% of the weight for results at two weeks and two to three weeks respectively) from the results for discharge gave pooled estimates detecting no statistically significant difference at any time‐point. This trial also assessed hearing but found no significant difference between treatments in the level of hearing improvement at day 11.
When compared to topical antiseptics, topical quinolone antibiotics were more effective at curing CSOM. However, the findings for non‐quinolones were more mixed, and any possible benefit decreased over time. Three of these trials included participants with a range of diagnoses, and treatment regimens varied widely. Other possible causes of the heterogeneity may be partly due to the different dropout rates between treatment groups for van Hasselt 1997 (see Table 6 for dropout rates), which was also the only study for the antiseptic comparisons that did not specify any blinding. Meanwhile Browning 1983a compared weekly insufflation of antiseptic after aural toilet by the otologist with daily non‐quinolone antibiotics, and followed participants later than the other trials (four weeks). This was the only trial to find a result in favour of antiseptic, although the effect was not statistically significant, with confidence intervals crossing the line of no‐effect.
Macfadyen 2005 dominated the quinolone versus antiseptic comparisons at two to four weeks, with 76% of the weight, due to its large size and narrow confidence intervals. Excluding Macfadyen 2005 from the quinolone versus antiseptics comparison increases the heterogeneity and confidence intervals, with a non‐significant effect after accounting for heterogeneity. This trial reduced the heterogeneity and consolidated the results (with confidence intervals overlapping all the other trials), to pull the effect significantly in favour of quinolone antibiotics over antiseptics. The lack of heterogeneity in the results for this trial, and its impact on the pooled results, suggests the findings for this trial may be applicable to other settings. Using older dedicated children as 'ear monitors' to treat the participants at school, under teacher supervision, may have helped minimise treatment errors or sharing of the treatment with other members of the household or community. The effectiveness of the treatments may therefore be slightly higher than might be found in practice in resource‐poor settings if treatment is to be applied at home. However, the resolution rates were lower than obtained by many trials in higher income settings, and/or in private clinics where treatment is done in the clinic. This may be partly due to better health and other environmental factors in richer settings, but also a reflection of the more pragmatic nature of this study than others, even with the child‐to‐child treatment approach. These rates are therefore more likely to reflect the actual resolution rates in developing countries than many other trials, and may also be more generalisable to higher‐income settings than might be anticipated.
Macfadyen 2005 found low healing rates with both quinolones antibiotic and antiseptic, although there was a trend towards a benefit of antibiotic over antiseptic by week two. This trial also reported improved hearing in both groups, with a greater improvement on antibiotic than antiseptic.
Antiseptics and non‐quinolone antibiotics are often cheaper and more widely available than quinolone eardrops, and the findings presented here may have important implications in resource‐poor settings.
Most trials did not report any adverse events. However, those that did discuss safety, supported a good profile for the topical treatments assessed; one trial also reported fewer local effects for quinolone antibiotic than for antiseptic in alcohol (Macfadyen 2005). None of the trials that assessed hearing in this review reported any evidence of ototoxicity; two reported a mild auditory disorder (Lorente 1995, one case) or increase in bone conduction thresholds (Tutkun 1995) after treatment with gentamicin (an aminoglycoside, non‐quinolone, antibiotic), but stated that the hearing losses were not clinically (Lorente 1995) or statistically (Tutkun 1995) significant. However, ototoxicity has been observed for various non‐quinolone antibiotics, and aminoglycoside antibiotics are not recommended in patients with a tympanic membrane perforation by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK (CSM 1997). One review of ototoxicity cases in humans reported that ototoxicity may be primarily due to vestibular damage (causing imbalance and dizziness) rather than cochlear (Marais 1998), so assessments of hearing thresholds for bone conduction alone would be unlikely to detect many cases (Marais 1998; Lancaster 1999).
The follow‐up period in most trials included here was short, with few assessing the longer‐term effects of treatment, and none reported time to resolution or to reappearance of discharge and perforation. As some conclusions changed over time, it may be important to investigate the longer‐term effects in future trials.
Most of the trials in this review were poor quality, with short follow‐up. Many included a range of participants (e.g. including otitis externa, or draining mastoid cavities; while some did not specify cholesteatomatous disease as an exclusion criterion). This may explain some of the heterogeneity found, since resolution rates may vary widely between diagnoses, and so make the results less applicable to any one type of disease. Trials were also inconsistent in approaches for handling and reporting bilateral disease. Where available, we have reported results for number of participants rather than ears; otherwise results for number of ears have been taken. The concern is that a trial analysed by ears rather than number of participants will have an underestimated standard error and therefore receive an inappropriate increased weight in a meta‐analysis. We will use the information obtained to attempt to address the issues of inflated weighting for trials reporting numbers of ears, in a later update of this review.
Authors' conclusions
Short courses of topical quinolone antibiotics are more effective than no drug treatment or topical antiseptics for the short‐term resolution of otorrhoea from uncomplicated CSOM. The effects of non‐quinolone antibiotics without steroids are less clear, when compared to antiseptics; studies were also inconclusive regarding any differences between quinolones and non‐quinolones, although indirect comparisons suggest a benefit of topical quinolones cannot be ruled out. Less is known about longer‐term outcomes (for dry ear, or to prevent complications, heal the eardrum, and improve hearing), or about treating complicated CSOM. Evidence regarding safety is also weak, and more research is needed to clarify the risk of ototoxicity with alternative topical treatments (about which there is much concern, particularly for aminoglycosides), and whether there may be fewer adverse events with topical quinolones. Treatment should therefore be accompanied by regular medical follow‐up and clinical vigilance, also monitoring for adverse effects of treatment (particularly for local effects or signs of ototoxicity), or for complications of the disease. Clinical staff should also advise patients and their caregivers on appropriate ear care, with aural toilet and effective instillation of the drops, ensuring the drops reach the site of infection to work effectively.
Further trials should clarify the effects of non‐quinolone antibiotics and what is the most appropriate topical treatment for CSOM. Adequately designed and powered clinical trials should focus on the effects on the longer‐term natural history of CSOM, such as healing of the tympanic membrane, hearing improvement, prevention of complications, and also on further safety assessments. Other more comprehensive systematic reviews should also assess safety further, particularly to verify the risks of ototoxicity for alternative treatments. Trialists should also consider how they handle bilateral disease and also the type of participants included (or stratify randomisation by diagnosis if various diagnoses are included), to ensure the results are clinically relevant to a particular patient group. The cost effectiveness of alternative treatments, preferably through economic evaluations alongside clinical trials, would be valuable in guiding both clinical practice and health policy.
Acknowledgements
The authors wish to thank Professor Paul Garner of the Cochrane Infectious Diseases Group, Liverpool School of Tropical Medicine, for his advice and support, and Gemma Healy and Carolyn Doree of the Cochrane Ear, Nose and Throat Disorders group for their help with the searches.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 OTITIS MEDIA SUPPURATIVE single term (MeSH) #2 OTITIS MEDIA explode all trees (MeSH) #3 otitis media #4 #2 OR #3 #5 SUPPURATION explode all trees (MeSH) #6 suppurat* OR purulen* OR PUS #7 #5 OR #6 #8 #4 AND #7 #9 CHRONIC DISEASE explode all trees (MeSH) #10 CHRONIC* OR PERSIST* #11 #9 OR #10 #12 #1 AND #11 #13 #8 AND #11 #14 #12 OR #13 #15 CHRONIC* NEAR DISCHARG* #16 PERSIST* NEAR DISCHARG* #17 #15 OR #16 #18 #4 AND #17 #19 CSOM OR OTORRH* OR OTORH* #20 #14 OR #18 OR #19 #21 MASTOIDITIS single term (MeSH) #22 MASTOIDITIS #23 TYMPANIC MEMBRANE PERFORATION single term (MeSH) #24 EAR* NEAR DRUM* OR EARDRUM* OR TYMPANIC #25 PERFORAT* OR RUPTUR* #26 #24 AND #25 #27 #20 OR #21 OR #22 OR #23 OR #26 #28 ANTI INFECTIVE AGENTS explode all trees (MeSH) #29 ACETIC ACID explode all trees (MeSH) #30 BORIC ACIDS explode all trees (MeSH). #31 antibiot* OR antibact* OR antisept* OR antiinfect* OR microbides OR bacteriocid* OR antimicrobial* OR antimycobact* #32 anti ADJ (biot* OR bact* OR sept* OR infect* OR mycobact* OR microbial*) #33 borax OR boric OR hydrogen peroxide OR iodine OR acetic acid OR burow* OR acetate* OR acetyl #34 #28 OR #29 OR #30 OR #31 OR #32 OR #33 #35 #27 AND #34 #36 OTITIS‐MEDIA‐SUPPURATIVE‐QT.DE. #37 #35 OR #36
Data and analyses
Comparison 1.
Topical antibiotics versus no drug treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent discharge at 1 week | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.34, 0.59] |
| 1.1 Quinolone versus no drug treatment (ear toilet both groups) | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.34, 0.59] |
Comparison 2.
Topical non‐quinolones: trimethoprim‐sulfacetamide‐polymyxin B (TSP) versus other non‐quinolone antibiotic
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent discharge | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Topical TSP versus topical trimethoprim‐polymyxin B (TP) after 3 months (all diagnoses) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.80] |
| 1.2 Topical TSP versus topical gentamicin after 6 months (all diagnoses) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.64, 6.22] |
Comparison 3.
Topical quinolone versus topical non‐quinolone antibiotic
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent discharge | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Discharge at 1 week | 3 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.32] |
| 1.2 Discharge at 2 weeks | 5 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.92] |
| 1.3 Discharge at 3 weeks | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.54, 1.72] |
| 1.4 Discharge at 2‐3 weeks | 6 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.04] |
Comparison 4.
Topical non‐quinolone antibiotic versus topical antiseptic
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent discharge | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Discharge at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Discharge at 2‐4 weeks | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5.
Topical quinolone antibiotic versus topical antiseptic
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent discharge | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Discharge at 1 week | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.41, 0.67] |
| 1.2 Discharge at 2‐4 weeks | 4 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.47, 0.72] |
| 2 Healing of the tympanic membrane | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Healing at 2 weeks | 1 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.52, 2.13] |
| 2.2 Healing at 4 weeks | 1 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.91, 2.61] |
What's new
| Date | Event | Description |
|---|---|---|
| 30 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 24 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Differences between protocol and review
The following changes were made to the eligibility criteria and outcome measures from those stipulated in the protocol:
1. ELIGIBILITY CRITERIA Types of studies: quasi‐randomised controlled trials were specified in the protocol but excluded from the review.
Types of participants: Protocol: People of any age with a diagnosis of CSOM meeting the WHO definition Review: any diagnosis of CSOM as defined by the trial authors.
Types of interventions ‐ the following were stipulated in the protocol: Intervention: topical (aural) antibiotics (all and individual) Comparator: no intervention; placebo; other topical antibiotics with and without steroids; systemic antibiotics (all and individual); combination of topical and systemic antibiotics; antiseptics.
However, we have divided these trials into the following three reviews: * Topical treatment with antibiotics (THIS REVIEW) comparisons: no treatment or aural toilet, topical antiseptics, various topical antibiotics, excluding steroids * Systemic versus topical treatments for CSOM comparisons: any systemic treatment against any topical treatment excluding steroids * Systemic or topical steroids, as monotherapy or combination therapy comparisons: no treatment or aural toilet, topical antiseptics, topical antibiotics, systemic antibiotics
2. OUTCOMES Types of outcome measures ‐ the following primary outcomes have been changed: Protocol: * Resolution of CSOM at 2 to 4 weeks and after 4 weeks, according to the following findings: a) No report of otorrhoea b) Disappearance of discharge on otoscopy c) Healing of the eardrum perforation on otoscopy d) Time to resolution of CSOM according to any other definition of resolution made by the authors, including improvement in mucosal appearance
Review: * Resolution of CSOM at 2 to 4 weeks, and after 4 weeks, according to the investigators' criteria. We have also analysed results for treatment failure rather than success.
The others were analysed as separate, secondary outcomes (where reported by trialists): * Healing of perforation at 2 to 4 weeks, and after 4 weeks; * Time to resolution of CSOM as defined by the investigators.
The other protocol outcomes were unchanged, except that results before 2 weeks were also included and reported separately.
3. SEARCH STRATEGY The protocol stated that we would obtain all relevant studies regardless of publication status. However, trials reported in conference proceedings or on posters have not yet been sought, but will be sought for inclusion in an update of this review. Additionally, the further potential sources that we will search for future updates of this review, were also specified in the protocol for this review.
4. METHODS ‐ REVIEWERS' CONTRIBUTIONS The protocol stated that we would follow the statistical guidelines in the Cochrane ENT Group Guidelines for Reviewers (CochraneENTGuideline, updated November 2000), and that two reviewers (CM and JA) would independently: * select trials (at least for electronic searches), review the titles and abstracts of articles identified by the search strategy, and assess full texts for eligibility; * assess the methodological quality of all trials identified as eligible for inclusion (and report the level of agreement between the two reviewers); * extract data of study characteristics, including methods, participants, interventions and outcomes, and record these on standard forms; and * enter data onto Review Manager 4.2.
However, while CM and JA independently reviewed titles and abstracts of articles identified by the search strategy, only CM assessed the full texts for eligibility and methodological quality, extracted data, and entered data onto Review Manager 4.2. JA and CG provided a second opinion on trials CM had selected for inclusion; CG reviewed those where there was any ambiguity about the methods used, for the methodological quality and data extraction; and JA provided further information where this had been obtained from authors of trials included in the previous review 'Interventions for chronic suppurative otitis media' (Acuin 1998). The three authors resolved any disagreements through discussion.
5. MINIMISING CONFLICTS OF INTERESTS The protocol also stated that where one of the authors has worked on a trial in the review, we will seek help from another person at the ENT editorial base, to minimise bias due to potential conflict of interests. However, CM and CG both worked on one trial, Macfadyen 2005; no third party input was enlisted.
6. ASSESSMENT OF METHODOLOGICAL QUALITY The protocol stated that allocation concealment, and inclusion of randomised participants, would be assessed as inadequate if an allocation concealment approach was not reported, or it was not clear how many people were originally randomised into the trial, respectively. However, we have classed these as unclear.
We assessed the methodological quality of the trials using the following dimensions and criteria based on four methodological aspects.
a) Generation of allocation sequence
Adequate: if sequences are suitable to prevent selection bias and the method used is described. Adequate methods include random numbers generated by computer, table of random numbers, drawing of lots or envelopes, tossing a coin, shuffling cards, throwing dice, or other methods of allocation that appear to be unbiased.
Unclear: stated but method not described. The trial describes itself as being randomised but no further information is given.
Inadequate: if sequence could be related to prognosis. Inadequate methods include case record number, date of birth, time, day, month or year of admission.
b) Allocation concealment
Adequate: if participants and investigators enrolling participants cannot foresee assignment. Adequate measures include a priori numbered or coded containers of identical appearance, central randomisation; sequentially numbered, opaque, sealed envelopes; or other descriptions that contained convincing elements of concealment.
Inadequate: trials in which the authors reported an approach that could not be considered adequate (e.g. methods of allocation such as alternation methods or use of case record numbers are not concealed).
Unclear: trials in which the authors either did not report an allocation concealment approach at all or allocation concealment was stated but method not described.
Baseline comparison of experimental groups will confirm whether treatment arm allocation appears to be unbiased.
c) Blinding
Double blind: the trial uses a placebo, or a double dummy technique such that neither the participant or care provider/assessor know which treatment is given.
Single blind: the participant or care provider/assessor is aware of the treatment given.
Open: all parties are aware of treatment.
d) Inclusion of all randomised participants
Adequate: More than 90% of people randomised in the trial were included in the analysis.
Inadequate: Less than 90% of those randomised in to the trial were included in the analysis.
Unclear: It is not clear how many people were originally randomised into the trial or analysed.
7. DATA ANALYSIS The protocol stated that we would only include the results for CSOM participants where available, for studies that also enrolled people with otitis externa, draining surgical cavities or acute otitis media. However, we only did so if the authors reported stratifying randomisation by diagnosis. Where information was not reported regarding whether alternative diagnostic groups were stratified at randomisation, we included all participants (groups may be unbalanced, and decisions by trialists to report subgroups may have been linked to trend).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | See Table 03 75 adults over 16 years old, with active chronic otitis media randomised; 51 analysed. 19/51 (37%) analysed participants had previously undergone modified radical mastoidectomy ‐ results were not presented separately, so included in this review. 38/51 analysed participants included in this review (receiving topical antibiotics or antiseptics); 13/51 receiving systemic antibiotics are not discussed here. |
|
| Interventions | See Table 04 1) Topical non‐quinolone antibiotic eardrops: chloramphenicol or gentamicin, 3 or 4 times daily, respectively. 2) Weekly insufflation of topical antiseptics (boric acid and iodine powder) after aural toilet. * Other comparison not included in this review (n = 13/51 analysed participants) 3) Systemic non‐quinolone antibiotics: oral cephalexin, flucloxacillin, cloxacillin or amoxicillin, 1 to 2g/day. * Duration (all treatments): 4 weeks. Aural toilet (all groups; confirmed by trial authors): weekly aural toilet by otologist, using microscopic vision and suction aspiration when necessary. Participants with Pseudomonas species were randomised to topical antibiotic or antiseptics only (groups 1 or 2). Choice of antibiotics depended on sensitivity of bacteria isolated at baseline. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) |
|
| Notes | Setting: Secondary referrals at Department of Otolaryngology, Glasgow Royal Infirmary. Location: Glasgow, UK. Trial in other CSOM reviews: systemic versus topical treatments. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | See Table 03 139 randomised ears (20 CSOM); 102 ears (15 CSOM) analysed. Participants with otorrhoea caused by otitis externa and mastoid cavities were also included in the trial. Randomisation was not described as stratified by diagnosis, so all cases are included in this review (although results were presented separately in the trial publication). |
|
| Interventions | See Table 04 1) Topical non‐quinolone antibiotic eardrops: gentamicin sulphate 0.3% 2) Topical antiseptic eardrops: Aluminium acetate 8% Both treatments: 3 times daily after self mopping, for 3 weeks. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) NB: Trial reported improved or not improved only; numbers with complete cure were not reported. Excluded participants who failed to attend clinics or failed to comply with treatment. * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: ENT Department, Bradford Royal Infirmary. Location: UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT | |
| Participants | See Table 03 51 participants (aged 18 to 73), contributing 60 ears with purulent disease were randomised; 54 ears analysed. 3 ears had surgical perforations and no perforation could be seen in 8 ears due to granulation tissue ‐ not analysed separately. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: ciprofloxacin hydrochloride 2) Topical non‐quinolone antibiotic eardrops: tobramycin 3) Topical antiseptic eardrops: 1% Burow aluminium acetate solution (weak antiseptic, as designated "placebo" by the trialists) All treatments: 3 times daily, for 3 weeks. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) ** 2) Improvement in hearing threshold ** * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: outpatient clinic of the otolaryngology department of a university teaching hospital. Location: Haifa, Israel. Study time‐period: 01 January 1994 ‐ 01 December 1995. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT Individually randomised by Taves' minimisation method. Crossover design ‐ failures at 6 months were crossed over to the alternative treatment and reassessed 6 months later. |
|
| Participants | See Table 03 91 analysed participants (all ages above 2 months) with otorrhoea, contributing 100 ears (CSOM 50 ears). 11 participants (12 ears; 7 CSOM ears) then crossed over to the alternative treatment. Unless otherwise indicated, only numbers before crossover have been used for this review. Participants with otorrhoea from post‐operative or mastoid cavity infections, subacute otitis media, and otitis externa were also included in the trial. Type of infection was the principal criteria for randomisation (minimisation), but it is unclear whether this relates to diagnosis (balanced across treatments) or bacteriology, so all cases are included in this review (although results were presented separately in the trial publication). |
|
| Interventions | See Table 04 1) Topical non‐quinolone antibiotic eardrops: 0.1% trimethoprim‐sulfacetamide‐polymyxin B (TSP) 2) Topical non‐quinolone antibiotic eardrops: 0.3% gentamicin (Garamycin) Both treatments: twice daily with suction and mopping. Pneumatic otoscope sometimes used to instil the drops. Duration: up to 3 weeks plus 3 more weeks if required. Failed ears or not dry at 6 months crossed over to the other treatment for 3 weeks; follow‐up for 6 months as before. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge after 4 weeks) Results were at 6 months. Negative bacterial culture included in the definition of cure. 2) Improvement in hearing threshold 3) Adverse events (including assessment of ototoxicity) Information relevant to the following were also reported: 4) Time to resolution of CSOM 5) Time to reappearance of discharge and perforation * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: outpatient visit ‐ appears to be a private clinic. Location: Winnipeg, Manitoba, Canada. Source of support: study conducted in conjunction with Burroughs Wellcome Ltd (manufacturer of TSP and collected details of Adverse Events). Article in French. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | See Table 03 60 eligible participants (all ages) with otorrhoea, contributing 68 ears were analysed (CSOM 27/68 ears). Participants with otorrhoea from post‐operative or mastoid cavity infections, subacute otitis media, and otitis externa were also included in the trial. Randomisation was not described as stratified by diagnosis, so all cases are included in this review (although results were presented separately in the trial publication). |
|
| Interventions | See Table 04 1) Topical non‐quinolone antibiotic eardrops: trimethoprim‐polymyxin B‐sulfacetamide (TSP) 2) Topical non‐quinolone antibiotic eardrops: trimethoprim‐polymyxin B (TP) Both treatments: twice daily with suction and mopping. Pneumatic otoscope sometimes used to instil the drops. Duration: up to 14 days depending on response. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge after 4 weeks) Results were at 3 months. Negative bacterial culture included in the definition of cure. ** 2) Improvement in hearing threshold ** (Reported negative statement for no ototoxicity only) 3) Adverse events (including assessment of ototoxicity) * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting and location: not reported (appears to be a private clinic in Winnipeg, Canada). Conducted: October 1978 ‐ March 1979. Sources of support: Burroughs Wellcome Ltd assisted in the compilation and statistical analysis of the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT | |
| Participants | See Table 03 40 randomised participants (over 10 years old), with actively discharging CSOM‐tubotympanic disease; 36 assessed at week 4. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: 0.3% ciprofloxacin hydrochloride 2) Topical antiseptic eardrops: 5% Povidone‐iodine (PVP‐I, Betadine) Both treatments: 3 times daily, after dry‐mopping, for 10 days. Suction cleaning (both groups): before initial treatment for all participants; at weekly visits if discharge present. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) ** 2) Improvement in hearing threshold ** (Reported negative statement for no ototoxicity only) 3) Adverse events (including assessment of ototoxicity) * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: Academic tertiary medical centre ‐ otolaryngology outpatient department. Location: Vellore, India. Study time period: March ‐ November 2000. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT | |
| Participants | See Table 03 50 adult participants randomised (aged 21 to 66), with perforated tympanic membranes (> 3 months) and mucopurulent otorrhoea; 35 were analysed. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: ciprofloxacin (250 micrograms/mL) 2) Topical placebo eardrops: saline Both treatments: 3 times daily for at least 7 days, with ear cleaning at each visit (days 1, 4 and 7). |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge) Follow‐up only reported to Day 7. ** 2) Improvement in hearing threshold ** (Reported negative statement for no ototoxicity only) 3) Adverse events (including assessment of ototoxicity) * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: Otolaryngology outpatient clinic at Ramathibodi Hospital. Location: Bangkok, Thailand. Time period: October 1993 ‐ December 1993. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT | |
| Participants | See Table 03 80 adults included (aged 18 to 60) contributing 103 ears with CSOM (perforated eardrum and discharge for over 3 months). 40/80 analysed participants included in this review (receiving topical antibiotics without steroids); 40/80 receiving topical antibiotics + steroids are not discussed here. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: ciprofloxacin 0.3% 2) Topical non‐quinolone antibiotic eardrops: tobramycin 0.3% * Other comparisons not included in this review (n = 40/80 participants)* *3) Topical quinolone antibiotic plus steroid eardrops: ciprofloxacin plus dexamethasone* *4) Topical non‐quinolone antibiotic plus steroid eardrops: tobramycin plus dexamethasone* All treatments: 2 drops 3 times daily, with aspiration once daily, for 3 weeks. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) Also reported results at Day 7. * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: University hospital, outpatient clinic. Location: Firat University, Elalzig, Turkey. Time period: 2000‐2001. Article in Turkish. Trial in other CSOM reviews: steroids. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | See Table 03 308 analysed adult participants (aged 18 to 65 years) with simple CSOM. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: 0.3% ciprofloxacin 2) Topical non‐quinolone antibiotic eardrops: 0.3% gentamicin Both treatments: 3 times daily for 8 days. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge) Follow‐up only reported to Day 8 ** 2) Improvement in hearing threshold ** (Reported negative statement for no ototoxicity only) 3) Adverse events (including assessment of ototoxicity) * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: Multicenter outpatient trial. Location: Barcelona, Spain. Sources of support: conducted with Laboratorios SALVAT, SA, Barcelona (manufacturer of ciprofloxacin 0.3% drops). Article in Spanish. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | See Table 03 427 randomised schoolchildren (at least 5 years old, but range was 4 to 19) with CSOM; 394 participants analysed for resolution at 4 weeks. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: ciprofloxacin hydrochloride (0.3%) 2) Topical antiseptic eardrops: 2% boric acid in 45% alcohol Both treatments: Child‐to‐child treatment twice daily with dry mopping for 10 schooldays (not weekends). Dry mop only for weeks 2 to 4 if discharge persists. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) 2) Healing of the tympanic membrane 3) Improvement in hearing threshold 4) Adverse events Results were given for weeks 2 and 4. All analyses followed the intention‐to‐treat principle. |
|
| Notes | Setting and location: 165 rural primary schools, Kisumu District, West Kenya. Study time period: May to August 2002 school term. Sources of support: Wellcome Trust funded (but no role in any stage from design to submission for publication). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT | |
| Participants | See Table 03 44 participants (at least 9 years old) with purulent chronic otorrhoea analysed for primary outcome. 4 participants with normal flora at baseline (all ciprofloxacin) were excluded from the hearing analyses. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: ciprofloxacin hydrochloride, 200 micrograms/mL 2) Topical non‐quinolone antibiotic eardrops: gentamicin sulfate, 5mg/mL Both treatments: 3 times daily for 10 days. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks). Result was for Day 11 Negative bacterial culture included in the definition of cure. 3) Improvement in hearing threshold 4) Adverse events * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: Marmara University Hospital ENT and audiology departments. Location, Istanbul, Turkey. Dates: Nov 1993 to June 1994 (Nov 1993 to March 1994 for Ozagar 1997). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | See Table 03 96 children with CSOM randomised; 93 ears in 69 children analysed. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: 0.3% ofloxacin 2) Topical non‐quinolone antibiotic eardrops: 0.5% neomycin ‐ 0.1% polymyxin‐B 3) Topical antiseptic eardrops: 2% acetic acid in 25% spirit and 30% glycerin. All groups: 3 times daily for 2 weeks; suction cleaning at weeks 0, 1 and 2 visits. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) Also reported for 1 week. Discrepancies for results at week 2 existed between the 1997 CBM report (see graphs and results text) and 2002 paper (see results text). |
|
| Notes | Location: Rural Nkota Kota District, Malawi. Sources of support: conducted for Christian Blind Mission. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | See Table 03 Randomised 107 participants (mainly children) with 151 active ears (>2 months CSOM); analysed 138 ears at week 2. |
|
| Interventions | See Table 04 2 antibiotics, each with 2 alternative administration regimens, were compared: 1) Topical quinolone antibiotic eardrops, 0.3% ofloxacin: 1a) twice daily 1b) once weekly 2) Topical non‐quinolone antibiotic eardrops, neomycin/polymyxin B: 2a) twice daily 2b) once weekly All treatments for 2 weeks with suction cleaning once weekly. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge at 2 to 4 weeks) Results at weeks 1, 2 and 8. * Outcomes not included in this review were also assessed ‐ see table 05 * |
|
| Notes | Setting: Community‐based. Location: Rural area in Nkota Kota District, Malawi Sources of support: conducted for Christian Blind Mission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | See Table 03 253 analysed ears with CSOM. |
|
| Interventions | See Table 04 1) Topical quinolone antibiotic eardrops: 0.075% ofloxacin in 1.5% HPMC 2) Topical antiseptic eardrops: 1% povidone iodine in 1.5% HPMC 3) 1.5% HPMC All treatments: Single application after suction cleaning. HPMC (hydroxypropyl methyl‐cellulose, hypromellose): delivery vehicle providing prolonged contact with the middle ear mucosa and sustained release of antibiotic or antiseptic. |
|
| Outcomes | See Table 05 Review outcomes assessed: 1) CSOM resolution/failure (persistent discharge) Follow‐up was for 1 week only. |
|
| Notes | Setting: Pilot study in rural Malawi. Location: Malawi. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Dummy variable created for van Hasselt 1998 (comparisons tables/graphs) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Dummy variable created for van Hasselt 1998 (comparisons tables/graphs) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
* Not included under any treatments/outcomes specified for this review * ** Measured but not reported results **
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 2000 | PARTICIPANTS Participants had acute otitis media with effusion, with no perforation of the tympanic membrane. INTERVENTION No participants received topical treatment (systemic antibiotics only) |
| Akisada 1997 | INTERVENTION No participants received topical treatment (oral antibiotics only). |
| Anon 1970a | ALLOCATION (Trial 1 of 2 reported in the paper): Included participants with chronic otorrhoea, but was not a randomised controlled trial (no comparator group). |
| Anon 1970b | PARTICIPANTS (Trial 2 of 2 reported in the paper): Randomised controlled trial for participants with acute otitis media only. |
| Arguedas 1994 | ALLOCATION Participants were not randomised. INTERVENTION No topical antimicrobial treatments were used (systemic antibiotics only). |
| Aslan 1998 | ALLOCATION Participants were divided into two groups, but allocation not described as randomised. |
| Baba 1982a | ALLOCATION Not a randomised controlled trial (no comparator group). INTERVENTION No participants received topical treatment (intravenous antibiotics only). |
| Baba 1982b | INTERVENTION No participants received topical treatment (oral antibiotics only). |
| Baba 1984 | INTERVENTION No participants received topical treatment (systemic treatments only). |
| Baba 1995 | INTERVENTION No participants received topical treatment (oral treatments only). |
| Blekher 1967 | ALLOCATION No mention of how the decision was taken to assign individual participants to different treatment groups. |
| Block 2000 | PARTICIPANTS Participants had acute otitis media for one week or less, without tympanic membrane perforation. INTERVENTION No participants were allocated topical antibiotics (systemic antibiotics only). |
| Brook 2001 | PARTICIPANTS Participants suffered from recurrent otitis media, not chronic suppurative otitis media. INTERVENTION Participants received oral antibiotics or no treatment ‐ no participants were allocated topical antibiotics. |
| Browning 1983b | ALLOCATION Participants were not described as randomised. INTERVENTION No topical treatments were allocated (systemic antibiotics only). |
| Browning 1984 | ALLOCATION Editorial; not a randomised controlled trial. |
| Browning 1988 | (INTERVENTION) For steroids review (topical gentamicin‐hydrocortisone + steroid versus placebo). |
| Chaput 1982 | PARTICIPANTS Participants had acute otitis media, not chronic suppurative otitis media. INTERVENTION No participants were allocated topical antibiotic treatment (systemic antibiotics only). |
| Coates 2002 | This is an editorial, not a randomised controlled trial. |
| Coates 2003 | ALLOCATION Not a randomised controlled trial ‐ paper discusses 2 studies; 1 randomised controlled trial (Couzos 2003, for Steroids review) and a controlled clinical trial. |
| Colletti 1983 | ALLOCATION Not a randomised controlled trial ‐ no comparator group. INTERVENTION All participants received intramuscular ribostamycin ‐ no topical treatment group. |
| Connolly 1997 | (INTERVENTION) For review on delivery methods (compares alternative delivery methods of topical neomycin sulphate + dexamethasone (Otomize): drops versus spray). |
| Cooke 1974 | INTERVENTION No participants were allocated topical treatment (given ear toilet with or without systemic (oral) antibiotics only). |
| Couzos 2003 | (INTERVENTION) For steroids review (topical ciprofloxacin versus topical framycetin, gramicidin and dexamethasone (Sofradex)). |
| Cronin 1974 | ALLOCATION Participants were not described as randomised. |
| Crowther 1991 | (INTERVENTION) For steroids review (topical antibiotic‐steroid, gentamicin‐hydrocortisone versus topical steroid, betamethasone). |
| Damoiseaux 2004 | This is a letter regarding treatment of acute otitis media, not a randomised controlled trial for CSOM treatment. |
| de Miguel 1999 | (INTERVENTION) For other CSOM reviews: systemic versus topical treatments, and steroids (compares systemic quinolone versus systemic + topical quinolone versus topical quinolone alone (2 doses) versus non‐quinolone + steroid drops. Does not compare 2 alternative topical antibiotics without steroids). |
| Deguchi 1985 | ALLOCATION 'Double blind study' described in part of this paper does not appear to be a randomised controlled trial. |
| Deguchi 1986 | ALLOCATION Not a randomised controlled trial ‐ five references described within the paper, one of which is apparently a double‐blind test, but further information not available. |
| Deguchi 1992 | Review of several MIC studies, one of which is a phase III double‐blind study, but reference not provided to confirm eligibility. |
| Di Brino 1967 | INTERVENTION No participants were allocated topical treatments (oral antibiotics only). |
| Eason 1986 | (INTERVENTION) For other reviews instead: aural toilet, antiseptics, and steroids (comparisons are: no treatment; aural toilet alone; aural toilet + topical boric acid; aural toilet + topical Sofradex (antibiotic+steroid); aural toilet + topical Sofradex + oral clindamycin antibiotic). |
| Esposito 1990 | (INTERVENTION) For systemic versus topical treatments review (comparisons: oral ciprofloxacin (quinolone) versus oral + topical ciprofloxacin, versus topical ciprofloxacin). |
| Esposito 1992 | (INTERVENTION) For systemic versus topical treatments review (comparisons: intramuscular gentamicin sulfate (non‐quinolone antibiotic) versus topical ciprofloxacin (quinolone)). |
| Federspil 1969 | ALLOCATION Not a randomised controlled trial ‐ topical or systemic gentamicin administered to a series of unselected participants, with choice of allocation usually based on in vitro sensitivity tests. |
| Fliss 1990 | INTERVENTION No participants received topical antimicrobial agents (allocated 1 of 2 different systemic antibiotics or no treatment). |
| Fontanel 1998 | ALLOCATION Not a randomised controlled trial. |
| Foshee 1992 | PARTICIPANTS Two trials reported in this one article. In both trials, participants had acute otitis media with effusion, not chronic suppurative otitis media. INTERVENTION Participants were not allocated topical antibiotics in either trial (oral antibiotics only). |
| Garcia‐Rodriguez 93a | ALLOCATION Participants divided into two treatment groups (0.2% or 0.5% ciprofloxacin drops) but allocation was not described as randomised. |
| Garcia‐Rodriguez 93b | ALLOCATION Participants divided into three treatment groups (oral and/or topical ciprofloxacin) but allocation was not described as randomised. INTERVENTION Only 1 group received topical antibiotic without systemic treatment. |
| Gasmanne 1972 | (INTERVENTION) For systemic antibiotics review? Comparisons were oral Bactrim or Ledermycin ‐ no topical treatment group. |
| Ghosh 2001 | This is a review of studies assessing oral antibiotics vs placebo for acute otitis media, not a trial for CSOM. |
| Gyde 1982 | (INTERVENTION) For steroids review (comparisons: topical non‐quinolone antibiotic (gentamicin) versus topical non‐quinolone antibiotic + steroid (colistin, neomycin + hydrocortisone)) |
| Halsted 1967 | PARTICIPANTS Participants had acute otitis media, without a ruptured tympanic membrane, not chronic suppurative otitis media. INTERVENTION No participants were allocated topical treatment (oral antibiotics or placebo only). |
| Howard 1976 | PARTICIPANTS Participants had acute otitis media, not chronic suppurative otitis media. INTERVENTION No participants were allocated topical treatment (systemic antibiotics only). |
| Howie 1971 | PARTICIPANTS Participants had recurrent acute otitis media with presence of middle ear fluid. INTERVENTION No participants were allocated topical antibiotics (oral only). |
| Howie 1974 | PARTICIPANTS Participants had acute otitis media with the presence of middle ear fluid. INTERVENTION No participants were allocated topical antibiotics (oral only). |
| Howie 1985 | PARTICIPANTS Participants had acute or recurrent acute otitis media. INTERVENTION No participants were allocated topical treatment (systemic antibiotics only). |
| Indudharan 1997 | (INTERVENTION) For Steroids review: comparisons were topical gentamicin (antibiotic) versus gentamicin‐betamethasone (antibiotic‐steroid) drops. |
| Jang 2004 | ALLOCATION Participants received topical vancomycin or gentamicin drops but allocation was not described as randomised. |
| John 1983 | PARTICIPANTS Participants were not described to have chronic suppurative otitis media. INTERVENTION Participants were randomised to oral antibiotic syrups; no participants received topical antibiotics. |
| Johnston 2003 | (INTERVENTION) (Unpublished trial) For steroids review ‐ comparisons are: non‐quinolone antibiotic + steroid (Otomize TM spray; dexamethasone 0.1%, neomycin sulphate 3250 units/ml), with antiseptic (glacial acetic acid 2%) versus antiseptic (Earcalm TM Spray; glacial acetic acid 2%). |
| Kaga 1997 | INTERVENTION No participants were allocated topical treatments (oral antibiotics only). |
| Kantawala 1976 | ALLOCATION Treatment allocation not described as random (cases were selected at random, but unclear how control group was selected). INTERVENTION Trial tests a mucolytic agent, Acetylcysteine; no comparisons with topical antibiotics or topical versus systemic treatment. |
| Karabaev 1997 | INTERVENTION All participants received oral antioxidants ‐ no topical treatment or comparator drug. |
| Kashiwamura 2004 | ALLOCATION Not a randomised controlled trial ‐ no comparator group. INTERVENTION No antibiotic group (topical antiseptic only). |
| Kawamura 1985 | INTERVENTION No participants were allocated topical treatments (oral antibiotics only). |
| Khanna 2000 | ALLOCATION Quasi‐randomised trial. INTERVENTION No topical eardrops were prescribed except in participants with fungal infections. |
| Kilcoyne 1973 | ALLOCATION Not a randomised controlled trial ‐ no comparator treatment group (topical gentamicin hydrocortisone only). |
| Kiris 1998 | INTERVENTION Method of treatment delivery and aspiration daily or once only are compared, and not the actual treatment: daily topical ciprofloxacin following aspiration administered by clinic personnel at the clinic, versus topical quinolone self‐administered at home after the first treatment with aspiration at the clinic only. |
| Kriukov 1996 | Study 1 of 2: ALLOCATION Not described as a randomised controlled trial ‐ oral rovamycin compared to normal standard treatment. Study 2 of 2: ALLOCATION Treatment allocation not described as randomised. INTERVENTION Bacterial study of oral amoxyclav compared to alternative oral treatments for a range of inflammatory ENT infections. |
| Lancaster 1999 | ALLOCATION Not a randomised controlled trial (all participants received topical gentamicin with comparisons made between their diseased and non‐diseased ears before and after treatment). |
| Lancaster 2003 | ALLOCATION Participants received antibiotic drops or spray, but treatment allocation was not described as randomised. |
| Legent 1994 | INTERVENTION No participants were allocated topical treatments (oral antibiotics only). |
| Leiberman 1989 | (INTERVENTION) For systemic antibiotics review ‐ participants were randomised to intravenous Mezlocillin or Ceftazidime; no topical antimicrobials were used during the study. |
| Lildholdt 1986 | INTERVENTION For surgery review. No participants were allocated topical treatments (systemic antibiotics versus no drug treatment). |
| Linder 1997 | Comment on two trials (one on otitis media with effusion, and Smith 1996 trial); not a randomised controlled trial itself. |
| Mendonca 1969 | ALLOCATION Not a randomised controlled trial ‐ no comparator treatment group (topical gentamicin only). |
| Merifield 1993 | ALLOCATION Participants were categorised into treatment groups, but not described as randomised. PARTICIPANTS Participants had tympanostomy tubes accompanied by chronic suppurative otitis media. |
| Mesure 1973 | (INTERVENTION) For systemic antibiotics review ‐ participants were randomised to systemic Bactrim or demethylchlortetracycline; no topical treatment groups. |
| Mira 1992 | (INTERVENTION) For systemic versus topical treatments review (comparisons: intramuscular ceftizoxime (non‐quinolone antibiotic) + topical saline versus intramuscular + topical ceftizoxime) |
| Miro 2000 | (INTERVENTION) For steroids review (comparisons: topical quinolone, ciprofloxacin, versus topical non‐quinolone antibiotic and steroid, polymyxin B, neomycin and hydrocortisone. |
| Miskovska 2004 | PARTICIPANTS Participants had acute otitis media, not CSOM. INTERVENTION No topical treatment group; participants received systemic penicillin, surgery, or both. |
| Moreno Martínez 1988 | PARTICIPANTS Participants treated for a range of ENT infections, including otitis media with fever, not CSOM. INTERVENTION No topical treatment groups (systemic only). |
| Occhiuzzi 1972 | INTERVENTION For systemic antibiotis review? Both groups of participants received intramuscular treatment (no topical treatment groups). |
| Ott 2001 | (ALLOCATION) Not a randomised controlled trial (tutorial). |
| Papastavros 1989 | (INTERVENTION) For systemic versus topical treatments reveiw ‐ participants received systemic antibiotic or topical antiseptic; no participants were allocated topical antibiotics. |
| Picozzi 1983 | (INTERVENTION) For steroids review (comparisons: topical non‐quinolone antibiotic + steroid, gentamicin + hydrocortisone versus placebo; aural toilet both groups). |
| Picozzi 1984 | (INTERVENTION) For steroids review (comparisons: topical non‐quinolone antibiotic + steroid, gentamicin + hydrocortisone, versus topical gentamicin + hydrocortisone + systemic non‐quinolone antibiotic, metronidazole; aural toilet both groups). |
| Povedano 1995 | (INTERVENTION) For systemic versus topical treatments review ‐ comparisons are: systemic versus topical quinolone, ciprofloxacin. |
| Pugliese 1972 | PARTICIPANTS Participants had acute otitis media. INTERVENTION No participants were allocated topical treatment (oral antibiotics only). |
| Quick 1975 | PARTICIPANTS Participants suffered from a range of ENT disorders including acute otitis media, but none described to have chronic suppurative otitis media. INTERVENTION No participants were allocated topical treatments (systemic antibiotics only). |
| Rostein 1978 | INTERVENTION No topical treatment group ‐ alll participants received oral sulfamethoxazol‐trimethoprime. |
| Roy 2003 | (INTERVENTION) (Unpublished trial) For steroids review ‐ comparisons are topical quinolone, ciprofloxacin ear drops, versus topical non‐quinolone + steroid, Gentisone HC (hydrocortisone) ear drops. |
| Salzberg 1972 | ALLOCATION Not described as randomised. PARTICIPANTS Includes a range of upper respiratory tract infections, not specific to CSOM. INTERVENTION No participants were allocated topical treatments (oral antibiotics only). |
| Sambe 1977 | INTERVENTION For systemic antibiotics review? Participants were allocated systemic Pipdemic acid or Aminobenzyl penicillin; no topical treatment groups. |
| Schaad 1986 | INTERVENTION No topical treatment group ‐ participants were allocated oral bacterial lysate or placebo. |
| Schechkin 1978 | ALLOCATION Not a randomised controlled trial (no comparator group). |
| Shah 2000 | Not a randomised controlled trial ‐ analyses of bacteriology and drug sensitivity only; no treatment arm or effectiveness analyses of participants. |
| Shenderey 1985 | PARTICIPANTS Participants suffered from acute respiratory tract infections, including otitis media; none described to have chronic suppurative otitis media. INTERVENTION No participants were allocated topical treatments (oral antibiotics only). |
| Smith 1996 | (INTERVENTION) For steroids review? (Comparisons: dry mopping alone; versus dry mopping plus topical non‐quinolone antibiotic + steroid, framycetin, gramicidin + dexamethasone (Sofradex) plus systemic non‐quinolone antibiotic, oral amoxicillin; versus no specific treatment). |
| Somekh 2000 | INTERVENTION No participants were allocated topical antibiotics (both groups received intravenous antibiotics). |
| Stechenberg 1976 | PARTICIPANTS Participants had acute otitis media, not chronic otitis media. INTERVENTION No participants were allocated topical treatments (systemic antibiotics only). |
| Sugiyama 1981 | ALLOCATION Participants were divided into two groups (receiving oral or topical antibiotic), but not described as randomised. INTERVENTION Only one group received topical antibiotic without systemic treatment. |
| Supiyaphun 1995 | ALLOCATION Not a randomised controlled trial. INTERVENTION All participants received topical 0.3% ofloxacin, with no comparator group. |
| Supiyaphun 2000 | (INTERVENTION) For systemic versus topical treatments review (comparisons systemic + topical non‐quinolone antibiotics, amoxicillin + chloramphenicol, versus topical quinolone, ofloxacin). |
| Tachibana 1986 | INTERVENTION No participants were allocated topical treatment (administered intravenously in both groups). |
| Tong 1996 | (INTERVENTION) For steroid review (comparisons: topical quinolone, ofloxacin, versus topical non‐quinolone antibiotic + steroid, neomycin‐polymyxin B‐hydrocortisone drops). |
| Tong 2002 | (Intervention) For surgery review ‐ results before surgery were not provided. |
| Van de Heyning 1988 | ALLOCATION Not a randomised controlled trial. INTERVENTION All participants received oral ciprofloxacin, with no comparison group. |
| Verhoeff 2003 | INTERVENTION No topical treatment group (comparisons were systemic antibiotic versus placebo). |
| Wilde 1995 | INTERVENTION Mode of delivery of treatment under investigation, not actual treatment (regular antibiotic‐steroid ointment (Tri‐Adcortyl) dressing versus Tri‐Adcortyl ointment instilled into the ear once only). |
| Willis 1979 | PARTICIPANTS The trial described within this discussion paper is for colorectal and bowel surgery, not otitis media. |
| Yuen 1994 | (INTERVENTION) For systemic versus topical treatments review (comparisons: systemic non‐quinolone antibiotic, amoxicillin‐clavulanic acid, versus topical quinolone, ofloxacin). |
| Zbären 1983 | INTERVENTION Only systemic antibiotics were given; no topical treatment group. |
Contributions of authors
Jose Acuin (JA) was the primary author for the Cochrane Review 'Interventions for chronic suppurative otitis media', that this review replaces.
Carolyn Macfadyen (CM) designed the current review, in consultation with JA.
CM and JA worked with Gemma Healy (GH) and Carolyn Doree (CD), the Cochrane ENT Group Trials Search Co‐ordinators, for the search strategy development. GH ran the initial electronic searches, and performed a preliminary screen of the search results; CD ran the search update in March 2005. Both searches were carried out independently.
CM and JA independently reviewed the titles and abstracts identified during the search for preliminary assessment, and CM retrieved and reviewed the full papers for all potentially relevant studies. CM will organise retrieval of future unpublished studies, and will contact authors for additional information or clarifications where needed.
CM assessed the methodological quality of all trials identified for inclusion, extracted data, and entered data into Review Manager 4.2 for analysis. JA and Carrol Gamble (CG) provided a second opinion on trials CM had selected for inclusion; CG reviewed those where there was any ambiguity about the methods used, for the methodological quality and data extraction; and JA provided further information where this had been obtained from authors of trials included in the previous review 'Interventions for chronic suppurative otitis media' (Acuin 1998). The three authors resolved any disagreements through discussion.
CM and CG have worked on one trial in the review (Macfadyen 2005); no third party input was enlisted for this trial.
CM wrote the final review, with statistical and clinical input from CG and JA. All three authors provide the methodological perspective; CG also provides the statistical perspective and JA the clinical perspective.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
Cochrane Infectious Diseases Group, UK.
External sources
Cochrane Ear, Nose and Throat Disorders Group, UK.
Department for International Development, UK.
Declarations of interest
None known. However, Carolyn Macfadyen and Carrol Gamble conducted and analysed a trial comparing a topical aural antiseptic, boric acid, with a topical aural antibiotic, ciprofloxacin (Macfadyen 2005).
Notes
This review, 'Topical antibiotics for chronically discharging ears with underlying eardrum perforations' is one in a series of reviews, which replaces the review 'Interventions for chronic suppurative otitis media'. Reviews of other interventions will follow.
REVIEW HISTORY Issue review first published: 1998/4 (Interventions for chronic suppurative otitis media). Date of most recent amendment: Information not available. Date of most recent SUBSTANTIVE amendment: 12 February 1998.
Most recent changes: February 1998. October 2001: Cochrane review 'Interventions for chronic suppurative otitis media' split into a series of Cochrane review titles, each focusing on particular interventions.
Issue 1, 2004: Publish protocol for component review 'Topical antibiotics for chronically discharging ears with underlying eardrum perforations'.
DEVIATIONS FROM THE PROTOCOL: see Differences between protocol and review.
Edited (no change to conclusions)
References
References to studies included in this review
- Browning GG. Correspondence1997. ; Browning GG. Correspondence2005. ; Browning GG, Picozzi GL, Calder IT, Sweeney G. Controlled trial of medical treatment of active chronic otitis media. British Medical Journal (Clinical Research Edition) 1983 Oct 8;287(6398):1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton MI, Osborne JE, Rutherford D, Rivron RP. A double‐blind, randomized, prospective trial of a topical antiseptic versus a topical antibiotic in the treatment of otorrhea. Clinical Otolaryngology and Allied Sciences 1990 Feb;15(1):7‐10. [DOI] [PubMed] [Google Scholar]
- Fradis M, Brodsky A, Ben‐David J, Srugo I, Larboni J, Podoshin L. Chronic otitis media treated topically with ciprofloxacin or tobramycin. Archives of Otolaryngology ‐ Head and Neck Surgery 1997 Oct;123(10):1057‐60. [DOI] [PubMed] [Google Scholar]; Podoshin L, Brodzki A, Fradis M, Ben‐David J, Larboni J, Srugo I. [Local treatment of purulent chronic otitis media with ciprofloxacin]. Harefuah 1998 Jan 1;134(1):32‐6, 78. Hebrew. [PubMed] [Google Scholar]
- Gyde MC, Randall RF. Double‐blind comparative study of trimethroprim‐sulphacetamide‐polymyxin b and gentamicin in the treatment of otorrhoea (author's transl) [Etude comparative a double insu de la trimethoprime‐sulfacetamide‐polymyxine B et de la gentamicine dans le traitement de l'otorrhée]. Annales d'Oto‐laryngologie et de Chirurgie Cervico‐faciale 1978 Jan‐Feb;95(1‐2):43‐55. [PubMed] [Google Scholar]
- Gyde MC. A double‐blind comparative study of trimethoprim‐polymyxin B versus trimethoprim‐sulfacetamide‐polymyxin B otic solutions in the treatment of otorrhea. Journal of Laryngology and Otology 1981 Mar;95(3):251‐9. [DOI] [PubMed] [Google Scholar]; Gyde MC. [Double‐blind comparative trial of trimethoprim‐polymyxin B and trimethoprim‐sulphacetamide‐polymyxin B ear drops in the treatment of otorrhoea (author's transl)]. Annales d' Oto‐Laryngologie et de Chirurgie Cervico‐Faciale 1981;98(1‐2):37‐40. French. [PubMed] [Google Scholar]
- Jaya C, Job A, Mathai E, Antonisamy B. Evaluation of Topical Povidone‐Iodine in Chronic Suppurative Otitis Media. Archives of Otolaryngology ‐ Head and Neck Surgery 2003 Oct;129(10):1098‐100. [DOI] [PubMed] [Google Scholar]
- Kasemsuwan L, Clongsuesuek P. A double blind, prospective trial of topical ciprofloxacin versus normal saline solution in the treatment of otorrhoea. Clinical Otolaryngology and Allied Sciences 1997 Feb;22(1):44‐6. [DOI] [PubMed] [Google Scholar]
- Kaygusuz I, Karlidag T, Gok U, Yalcin S, Keles E, Demirbag E, Kaygusuz TO. [Efficacy of topical ciprofloxacin and tobramycin in combination with dexamethasone in the treatment of chronic suppurative otitis media]. Kulak Burun Bogaz Ihtisas Dergisi : KBB [Journal of Ear, Nose, and Throat] 2002 Mar‐Apr;9(2):106‐11. Turkish. [PubMed] [Google Scholar]
- Lorente J, Sabater F, Maristany M, Jimenez R, Menem J, Vinas J, Quesada P, Traserra J, Dicenta M, Abello P, Villar E. Multicenter study comparing the efficacy and tolerance of topical ciprofloxacin (0.3%) versus topical gentamicin (0.3%) in the treatment of simple, non‐cholesteatomatous chronic otitis media in the suppurative phase [Estudio multicentrico comparativo de la eficacia y tolerancia de ciprofloxacino topico (0,3%) versus gentamicina topica (0,3%) en el tratamiento de la otitis media cronica simple no colesteatomatosa en fase supurativa]. Anales Otorrinolaringologicos Ibero‐Americanos 1995;22(5):521‐33. [PubMed] [Google Scholar]; Sabater F, Maristany M, Mensa J, Villar E, Traserra J. Prospective double‐blind randomized study of the efficacy and tolerance of topical ciprofloxacin vs topical gentamicin in the treatment of simple chronic otitis media and diffuse external otitis [Estudio prospectivo doble‐ciego randomizado de la eficacia y tolerancia de ciprofloxacino topico versus gentamicina topica en el tratamiento de la otitis media cronica supurada simple y de la otitis externa difusa]. Acta Otorrinolaringologica Española 1996 May‐Jun;47(3):217‐20. [PubMed] [Google Scholar]
- Macfadyen C, Gamble C, Garner P, Macharia I, Mackenzie I, Mugwe P, Oburra H, Otwombe K, Taylor S, Williamson P. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Tropical Medicine and International Health 2005 Feb;10(2):190‐7. [DOI] [PubMed] [Google Scholar]
- Ozagar A, Koc A, Ciprut A, Tutkun A, Akdas F, Sehitoglu MA. Effects of topical otic preparations on hearing in chronic otitis media. Otolaryngology ‐ Head and Neck Surgery 1997 Oct;117(4):405‐8. [DOI] [PubMed] [Google Scholar]; Tutkun A, Ozagar A, Koc A, Batman C, Uneri C, Sehitoglu MA. Treatment of chronic ear disease. Topical ciprofloxacin vs topical gentamicin. Archives of Otolaryngology ‐ Head and Neck Surgery 1995 Dec;121(12):1414‐6. [DOI] [PubMed] [Google Scholar]
- Hasselt P. CBM 1997: Pilot trial of treatment of Chronic Suppurative Otitis Media (CSOM) with several types of ear drops in Nkota Kota District, Malawi. Internal Report of Christian Blind Mission Int., Bensheim, Germany1997. ; Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002 Mar 15;63(1):49‐56. [DOI] [PubMed] [Google Scholar]
- Hasselt P. A controlled trial of the treatment of CSOM in rural Malawi. Presentation at the Conference of the Pan‐African Federation of Otorhinolaryngological Societies (PAFOS) in Nairobi1998. ; Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002 Mar 15;63(1):49‐56. [DOI] [PubMed] [Google Scholar]
- Hasselt P. Management of chronic suppurative otitis media in developing countries. Proceedings of the 2nd European Congress on Tropical Medicine. Liverpool. 1998:57. [Google Scholar]; Hasselt P, Kregten E. Treatment of chronic suppurative otitis media with ofloxacin in hydroxypropyl methylcellulose ear drops: a clinical/bacteriological study in a rural area of Malawi. International Journal of Pediatric Otorhinolaryngology 2002 Mar 15;63(1):49‐56. [DOI] [PubMed] [Google Scholar]
- Dummy variable created for van Hasselt 1998a/02.
- Dummy variable created for van Hasselt 1998a/02.
References to studies excluded from this review
- Adler M, McDonald PJ, Trostmann U, Keyserling C, Tack K. Cefdinir vs. amoxicillin/clavulanic acid in the treatment of suppurative acute otitis media in children. Pediatric Infectious Disease Journal 2000 Dec;19(12 Suppl):S166‐70. [DOI] [PubMed] [Google Scholar]
- Akisada T, Orita Y, Sato Y, Handa T, Yoshihiro T, Kawai A, Urabe Y, Aihara T, Mori Y. [Clinical evaluation of Sairei‐to to chronic otitis media and cholesteatomas after tympanoplasty]. Practica Otologica 1997;92(Supplement):55‐60. Japanese. [Google Scholar]; Akisada T, Orita Y, Yoshihiro T, Kawai A, Urabe Y, Take K, Okumoto K, Hidaka T, Aihara T, Hirai S. [Clinical evaluation of Sairei‐to for chronic otitis media and cholesteatoma after a tympanoplasty: Part II]. Practica Otologica 1998;98(Supplement):44‐8. Japanese. [Google Scholar]
- Anonymous. Local treatment with a corticosteroid‐antibiotic preparation in infections of the ear. The Practitioner 1970;205(229):691‐5. [PubMed] [Google Scholar]
- Anonymous. Local treatment with a corticosteroid‐antibiotic preparation in infections of the ear. The Practitioner 1970;205(229):691‐5. [PubMed] [Google Scholar]
- Arguedas A, Loaiza C, Herrera JF, Mohs E. Antimicrobial therapy for children with chronic suppurative otitis media without cholesteatoma. Pediatric Infectious Disease Journal 1994 Oct;13(10):878‐82. [DOI] [PubMed] [Google Scholar]; Arguedas AG, Herrera JF, Faingezicht I, Mohs E. Ceftazidime for therapy of children with chronic suppurative otitis media without cholesteatoma. Pediatric Infectious Disease Journal 1993 Mar;12(3):246‐8. [DOI] [PubMed] [Google Scholar]
- Aslan A, Altuntas A, Titiz A, Arda HN, Nalca Y. A new dosage regimen for topical application of ciprofloxacin in the management of chronic suppurative otitis media. Otolaryngology ‐ Head and Neck Surgery 1998 Jun;118(6):883‐5. [DOI] [PubMed] [Google Scholar]
- Baba S, Maruo T, Sasaki T, Miyano K, Maruyama H, Miyake H, Sakai M, Fujii K, Saito N, Takimoto I, Inafuku S, Nomura T, Yamashita K, Sato K, Ogawa A, Ueda T, Yoshizumi T, Nakai Y, Sugiyama M, Ohashi Y, Goto K, Ohta F, Murata K, Boku S, Miyamae M, Yonei K, Harada Y, Yajin K, Chikuie D, Hirata K. [The clinical efficacy of cefsulodin to the infection of otorhinolaryngological field]. Japanese Journal of Antibiotics 1982 Dec;35(12):2851‐60. Japanese. [PubMed] [Google Scholar]
- Baba S, et al. [A comparative double blind evaluation of AM‐715 with ABPC in acute suppurative otitis media and acute exacerbation of chronic otits media]. Practica Otologica ‐ Kyoto 1982;75(9):1835‐58. Japanese. [Google Scholar]
- Baba S, Kinoshita H, Mori Y, Mori Y, Iwasawa T, et al. [A comparative double blind study of AT‐2266 with pipemidic acid in the treatment of suppurative otitis media]. Chemotherapy ‐ Tokyo 1984;32(Suppl. 3):1061‐83. Japanese. [Google Scholar]
- Baba S, Miyamoto N, Hogo S, Itabishi T, Nishiya M, Ichikawa G, Wada M, et al. [Evaluation and clinical efficiacy of ritipenem acoxil for the treatment of otitis media and external otitis]. Japanese Journal of Chemotherapy 1995;43(Suppl. 3):374‐86. Japanese. [Google Scholar]
- Blekher LB. [Methods of treatment of chronic suppurative otitis]. Zhurnal Ushnykh, Nosovykh i Gorlovykh Boleznei [The Journal of Otology, Rhinology, and Laryngologie [sic]] 1967 Jan‐Feb;27(1):87‐90. Russian. [PubMed] [Google Scholar]
- Block SL, McCarty JM, Hedrick JA, Nemeth MA, Keyserling CH, Tack KJ, Cefdinir Otitis Media Study Group. Comparative safety and efficacy of cefdinir vs. amoxicillin/clavulanate for treatment of suppurative acute otitis media in children. Pediatric Infectious Disease Journal 2000 Dec;19(12 Suppl):S159‐65. [DOI] [PubMed] [Google Scholar]
- Brook I, Shah K. Effect of amoxycillin with or without clavulanate on adenoid bacterial flora. Journal of Antimicrobial Chemotherapy 2001 Aug;48(2):269‐73. [DOI] [PubMed] [Google Scholar]
- Browning GG, Picozzi G, Sweeney G, Calder IT. Role of anaerobes in chronic otitis media. Clinical Otolaryngology and Allied Sciences 1983 Feb;8(1):47‐51. [DOI] [PubMed] [Google Scholar]
- Browning GC. Medical management of chronic mucosal otitis media. Clinical Otolaryngology and Allied Sciences 1984 Jun;9(3):141‐4. [DOI] [PubMed] [Google Scholar]
- Browning GG, Gatehouse S, Calder IT. Medical management of active chronic otitis media: a controlled study. Journal of Laryngology and Otology 1988 Jun;102(6):491‐5. [DOI] [PubMed] [Google Scholar]
- Chaput de Saintonge DM, Levine DF, Savage IT, Burgess GWS, Sharp J, Mayhew SR, Sadler MG, Moody R, Griffiths R, Griffiths S, Meadows G. Trial of three‐day and ten‐day courses of amoxycillin in otitis media. British Medical Journal (Clinical Research Edition) 1982 Apr 10;284(6322):1078‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates HL, Morris PS, Leach AJ, Couzos S. Otitis media in Aboriginal children: tackling a major health problem. Medical Journal of Australia 2002 Aug;177(4):177‐8. [DOI] [PubMed] [Google Scholar]
- Coates H. Topical treatment of chronic suppurative otitis media in aboriginal children. Ear, Nose & Throat Journal 2003 Aug;82(8 Suppl 2):13. [PubMed] [Google Scholar]
- Colletti V, Sittoni V, Crosara C. Ribostamycin use in otomastoiditis [Impiego della Ribostamicina nell'otomastoidite]. Giornale Di Clinica Medica 1983 Jul‐Aug;64(7‐8):284‐94. Italian. [PubMed] [Google Scholar]
- Connolly AA, Picozzi GL, Browning GG. Randomized trial of neomycin/dexamethasone spray vs drop preparation for the treatment of active chronic mucosal otitis media. Clinical Otolaryngology and Allied Sciences 1997 Dec;22(6):529‐31. [DOI] [PubMed] [Google Scholar]
- Cooke ET, Raghuvaran G. Clindamycin in conjunction with surgery of the chronic suppurative ear. British Journal of Clinical Practice 1974 Feb;28(2):57‐9. [PubMed] [Google Scholar]
- Couzos S, Lea T, Mueller R, Murray R, Culbong M. Effectiveness of ototopical antibiotics for chronic suppurative otitis media in Aboriginal children: a community‐based, multicentre, double‐blind randomised controlled trial. Medical Journal of Australia 2003 Aug 18;179(4):185‐90. [DOI] [PubMed] [Google Scholar]
- Cronin J, Dogra TS, Khan IA. Conservative management of chronic ear disease. Journal of Laryngology and Otology 1974 Feb;88(2):113‐8. [DOI] [PubMed] [Google Scholar]
- Crowther JA, Simpson D. Medical treatment of chronic otitis media: steroid or antibiotic with steroid ear‐drops?. Clinical Otolaryngology and Allied Sciences 1991 Apr;16(2):142‐4. [DOI] [PubMed] [Google Scholar]
- Damoiseaux RAMJ, Oudesluys‐Murphy AM, Semmekrot BA, Rovers MM, Schilder AGM, Zielhuis GA, Rosenfeld RM. Otitis media (2) (multiple letters). Lancet 2004 Apr 17;363(9417):1324‐5. [DOI] [PubMed] [Google Scholar]
- Miguel Martinez I, Vasallo Morillas JR, Ramos Macias A. Antimicrobial therapy in chronic suppurative otitis media [Terapeutica antimicrobiana en otitis media cronica supurada]. Acta Otorrinolaringologica Espanola 1999 Jan‐Feb;50(1):15‐9. Spanish. [PubMed] [Google Scholar]
- Deguchi K, Fukayama S, Nishimura Y, Nishike A, Oda S, Sato S, Matsumoto Y, Ikegami R, Yokota N, Tanaka S, et al. [Study of clinical bacteriological efficacy in a cefmenoxime ototopical solution]. Japanese Journal of Antibiotics 1985 Jul;38(7):1739‐49. Japanese. [PubMed] [Google Scholar]
- Deguchi K, Yokota N, Tanaka S, Fukayama S, Nishimura Y, Yoshihara H, Oda S, Matsumoto Y, Ikegami R, Sato K, et al. [A clinical bacteriological efficacy study on a fosfomycin otic solution]. Japanese Journal of Antibiotics 1986 Sep;39(9):2344‐54. Japanese. [PubMed] [Google Scholar]
- Deguchi K, Yokota N, Koguchi M, Suzuki Y, Suzuki K, Fukayama S, Ishihara R, Oda S. [Bacteriological evaluation of ofloxacin otic solution]. Japanese Journal of Antibiotics 1992 Oct;45(10):1342‐55. Japanese. [PubMed] [Google Scholar]
- Brino M, Baldiserri L. [Chlormethylencycline in otorhinolaryngologic diseases. (Clinical trials)]. Atti Della Accademia dei Fisiocritici in Siena 1967;16(1):329‐41. Italian. [PubMed] [Google Scholar]
- Eason RJ, Harding E, Nicholson R, Nicholson D, Pada J, Gathercole J. Chronic suppurative otitis media in the Solomon Islands: a prospective, microbiological, audiometric and therapeutic survey. New Zealand Medical Journal 1986 Oct;99(812):812‐5. [PubMed] [Google Scholar]
- Esposito S, D'Errico G, Montanaro C. Topical and oral treatment of chronic otitis media with ciprofloxacin. A preliminary study. Archives of Otolaryngology ‐ Head and Neck Surgery 1990 May;116(5):557‐9. [DOI] [PubMed] [Google Scholar]
- Esposito S, Noviello S, D'Errico G, Montanaro C. Topical ciprofloxacin vs intramuscular gentamicin for chronic otitis media. Archives of Otolaryngology ‐ Head and Neck Surgery 1992 Aug;118(8):842‐4. [DOI] [PubMed] [Google Scholar]
- Federspil P. Clinical use of gentamicin in ear, nose and throat infections. Journal of Infectious Diseases 1969 Apr‐May;119(4):465‐70. [DOI] [PubMed] [Google Scholar]
- Fliss DM, Dagan R, Houri Z, Leiberman A. Medical management of chronic suppurative otitis media without cholesteatoma in children. Journal of Pediatrics 1990 Jun;116(6):991‐6. [DOI] [PubMed] [Google Scholar]
- Fontanel JP. Role of ciprofloxacin in the treatment of chronic otitis media [Place de la ciprofloxacine dans le traitement des otites moyennes chroniques]. La Presse Medicale 1998 Oct 3;27(29):1506‐8. French. [PubMed] [Google Scholar]
- Foshee WS, Qvarnberg Y. Comparative United States and European trials of loracarbef in the treatment of acute otitis media. Pediatric Infectious Disease Journal 1992 Aug;11(8 Suppl):S12‐9. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez JA, Carnizo A, Garcia Sanchez JE, Garcia Garcia MI, Miguel Martinez I, Munoz Bellido JL, Garcia Sanchez E, Ramos A. Efficacy of 2 regimens of local ciprofloxacin in the treatment of ear infections. Drugs 1993;45(Suppl. 3):327‐8. [Google Scholar]
- Garcia Rodriguez JA, Garcia Sanchez JE, Garcia Garcia MI, Garcia Sanchez E, Munoz Bellido JL, Ramos Macias A. Efficacy of topical ciprofloxacin in the treatment of ear infections in adults. Journal of Antimicrobial Chemotherapy 1993 Mar;31(3):452‐3. [DOI] [PubMed] [Google Scholar]
- Gasmanne P. Double‐blind clinical study of bactrim in otorhinolaryngology [Etude clinique a double insu du bactrim en oto‐rhino‐laryngologie]. Bruxelles Medical 1972 Sep;52(9):639‐42. French. [PubMed] [Google Scholar]
- Ghosh A, Jackson R. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Antibiotics for otitis media. Emergency Medicine Journal 2001 Mar;18(2):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyde MC, Norris D, Kavalec EC. The weeping ear: clinical re‐evaluation of treatment. Journal of International Medical Research 1982;10(5):333‐40. [DOI] [PubMed] [Google Scholar]
- Halsted C, Lepow ML, Balassanian N, Emmerich J, Wolinsky E. Otitis media. Clinical observations, microbiology, and evaluation of therapy. American Journal of Diseases of Children 1968;115(5):542‐51. [DOI] [PubMed] [Google Scholar]; Halsted C, Lepow ML, Balassanian N, Emmerich J, Wolinsky E. Otitis media: microbiology and evaluation of therapy. Annals of the New York Academy of Sciences 1967 Sep 27;145(2):372‐8. [DOI] [PubMed] [Google Scholar]
- Howard JE, Nelson JD, Clahsen J, Jackson LH. Otitis media of infancy and early childhood. A double‐blind study of four treatment regimens. American Journal of Diseases of Children 1976 Sep;130(9):965‐70. [DOI] [PubMed] [Google Scholar]
- Howie VM, Ploussard JH. Bacterial etiology and antimicrobial treatment of exudative otitis media: relation of antibiotic therapy to relapses. Southern Medical Journal 1971 Feb;64(2):233‐9. [DOI] [PubMed] [Google Scholar]
- Howie VM, Ploussard JH, Sloyer J. Comparison of ampicillin and amoxicillin in the treatment of otitis media in children. Journal of Infectious Diseases 1974 Jun;129(0):(suppl):S181‐4. [DOI] [PubMed] [Google Scholar]
- Howie VM, Dillard R, Lawrence B. In vivo sensitivity test in otitis media: efficacy of antibiotics. Pediatrics 1985 Jan;75(1):8‐13. [PubMed] [Google Scholar]
- Indudharan R, Ashraful Haq J, Mohamad H, Mohamad Z. Topical antibiotic versus antibiotic steroid combination in the management of chronic suppurative otitis media. Sydney '97: Otorhinolaryngology head and neck surgery: World congress; 16th. XVI World Congress of Otorhinolaryngology Head and Neck Surgery. 1997 (2‐7 March); Vol. 2:1153‐8. [Google Scholar]
- Jang CH, Song CH, Wang PC. Topical vancomycin for chronic suppurative otitis media with methicillin‐resistant Staphylococcus aureus otorrhoea. Journal of Laryngology and Otology 2004 Aug;118(8):645‐7. [DOI] [PubMed] [Google Scholar]
- John WR, Valle‐Jones JC. Treatment of otitis media in children. A comparison between cefaclor and amoxycillin. The Practitioner 1983 Dec;227(1386):1805‐9. [PubMed] [Google Scholar]
- Mr M Johnston. Randomised Single‐Blind Controlled Trial of Otomize TM vs Earcalm TM Spray in Active CSOM, Infected Mastoid Cavities and Otitis Externa. National Research Register 6 February 2003. [Serial number at source: N0077075335]
- Kaga K, Okuno T, Fukaya S, Yamane M, Funai H, Yano J, Sugimoto M, Hara M. [Efficacy of CPFX vs CPDX‐PR in the treatment of exacerbation of chronic otitis media]. Practica Otologica ‐ Kyoto 1997;90(10):1169‐79. Japanese. [Google Scholar]
- Kantawala S A, Rege S R, Shah K L. Use of acetylcysteine in middle ear suppuration. Indian Journal of Otolaryngology 1976 Dec;28(4):176‐7. [Google Scholar]
- Karabaev K E, Antoniv V F, Bekmuradov R U. [Pathogenetic validation of optimal antioxidant therapy in suppurative inflammatory otic diseases in children]. Vestnik Otorinolaringologii 1997;1:5‐7. Russian. [PubMed] [Google Scholar]
- Kashiwamura M, Chida E, Matsumura M, Nakamaru Y, Suda N, Terayama Y, Fukuda S. The efficacy of Burow's solution as an ear preparation for the treatment of chronic ear infections. Otology and Neurotology 2004 Jan;25(1):9‐13. [DOI] [PubMed] [Google Scholar]
- Kawamura S, et al. [Clinical evaluation of TMS‐19‐Q.GC tablet in suppurative otitis media. A comparative double blind study with midecamycin]. Practica Otologica ‐ Kyoto 1985;78(3):439‐63. Japanese. [Google Scholar]
- Khanna V, Chander J, Nagarkar NM, Dass A. Clinicomicrobiologic evaluation of active tubotympanic type chronic suppurative otitis media. Journal of Otolaryngology 2000 Jun;29(3):148‐53. [PubMed] [Google Scholar]
- Kilcoyne AG. Gentamicin‐hydrocortisone ear‐drops in chronic infections. The Practitioner 1973 Jul;211(261):91‐2. [PubMed] [Google Scholar]
- Kiris M, Berktas M, Egeli E, Kutluhan A. The efficacy of topical ciprofloxacin in the treatment of chronic suppurative otitis media. Ear, Nose & Throat Journal 1998 Nov;77(11):904‐5, 909. [PubMed] [Google Scholar]
- Kriukov AI, Poliakova TS, Ogorodnikov DS, Gaivoronskaia GA. [Use of rovamycin and amox‐clav in patients with infectious‐inflammatory pathology of the ORL organs]. Vestnik Otorinolaringologii 1996 Mar‐Apr;2:42‐5. Russian. [PubMed] [Google Scholar]
- Lancaster JL, Mortimore S, McCormick M, Hart CA. Systemic absorption of gentamicin in the management of active mucosal chronic otitis media. Clinical Otolaryngology and Allied Sciences 1999 Sep;24(5):435‐9. [DOI] [PubMed] [Google Scholar]
- Lancaster J, Matthews J, Williams RS, Thussey C, Kent SE. Comparison of compliance between topical aural medications. Clinical Otolaryngology 2003 Aug;28(4):331‐4. [DOI] [PubMed] [Google Scholar]
- Legent F, Bordure PH, Beauvillain C, Berche P. Controlled prospective study of oral ciprofloxacin versus amoxycillin/clavulanic acid in chronic suppurative otitis media in adults. Chemotherapy 1994;40 Suppl 1:16‐23. [DOI] [PubMed] [Google Scholar]
- Leiberman A, Fliss D, Dagan R. Selected papers from a conference on the eustachian tube and middle ear diseases. Geneva, Switzerland, October 26‐29, 1989 [Therapeutic aspects of chronic suppurative otitis media without cholesteatoma in children]. The Eustachian Tube, Clinical Aspects. 1989:75‐8. [Google Scholar]
- Lildholdt T, Felding J, Juul A, Kristensen S, Schouenborg P. Efficacy of perioperative ceftazidime in the surgical treatment of chronic otitis media due to Pseudomonas aeruginosa. Preliminary report of a prospective, controlled study. Archives of Oto‐Rhino‐Laryngology 1986;243(3):167‐9. [DOI] [PubMed] [Google Scholar]
- Linder T. The effectiveness of antibiotics in chronic middle ear effusion and chronic suppurative otitis media. Comment on 2 contributions from Lancet [Die Wirksamkeit von Antibiotika beim chronischen Tubenmittelohrkatarrh und bei der eitrigen Otitis media chronica. Uber zwei Arbeiten aus dem Lancet]. HNO 1997 Mar;45(3):107‐9. German. [PubMed] [Google Scholar]
- Mendonca D. The topical use of gentamicin in otorrhoea. The Practitioner 1969 Dec;203(218):786‐8. [PubMed] [Google Scholar]
- Merifield DO, Parker NJ, Nicholson NC. Therapeutic management of chronic suppurative otitis media with otic drops. Otolaryngology ‐ Head and Neck Surgery 1993 Jul;109(1):77‐82. [DOI] [PubMed] [Google Scholar]
- Mesure R. Double‐blind study of the association of sulfamethoxazole and trimethoprim in otorhinolaryngological infections [Essai en double aveugle de l'association de sulfaméthoxazole et de triméthoprime dans les infections oto‐rhino‐laryngologiques]. Acta Oto‐Rhino‐Laryngologica Belgica 1973;27(1):27‐33. French. [PubMed] [Google Scholar]
- Mira E, Benazzo M. Ceftizoxime as local therapy in the treatment of recurrences of chronic suppurative otitis media. Journal of Drug Development Supplement 1993;6(Suppl 2):39‐44. [Google Scholar]; Mira E, Benazzo M. Clinical evaluation of ceftizoxime (EposerinR) as local therapy in the treatment of recurrences of chronic suppurative otitis media [Uso Topico Delle Cefalosporine Nel Trattamento Delle Otiti Medie Purulente: Valutazione Della Ceftizoxima (Eposerin R)]. Rivista‐Italiana‐di‐Otorinolaringologia‐Audiologia‐e‐Foniatria 1992;12(4):219‐25. [Google Scholar]
- Miro N, The Spanish ENT Study Group. Controlled multicenter study on chronic suppurative otitis media treated with topical applications of ciprofloxacin 0.2% solution in single‐dose containers or combination of polymyxin B, neomycin, and hydrocortisone suspension. Otolaryngology ‐ Head and Neck Surgery 2000 Nov;123(5):617‐23. [DOI] [PubMed] [Google Scholar]
- Miskovska O, Tnokovski T, Gjorsevski S, Dzolonga V, Dimovski V. Treatment of acute suppurative otitis media. International Journal of Pediatric Otorhinolaryngology. Abstract No. P.1.21. 6th International Conference on Pediatric Otorhinolaryngology, Athens, Greece, 16‐19 May 2004. 2004; Vol. 68, issue 5:687. [Google Scholar]
- Moreno Martínez J. Amoxillin, comparative double‐blind study in ENT infection. Clinical evaluation of diclofenac‐potassium vs. placebo [Manejo de las enfermedades ORL bajo estudio doble ciego con amoxicilina. Evaluación clínica con diclofenaco potásico y placebo]. Compendium De Investigaciones Clinicas Latinoamericanas 1988;8(1):50‐6. Spanish. [Google Scholar]
- Occhiuzzi L. Double‐blind controlled research on symptomatic effects of an antibiotic‐antiphlogistic combination in otorhinolaryngologic infections [Ricerca controllata doppio‐cieca sugli effetti sintomatici di una combinazione antibiotico‐antiiflogistica nelle infezioni di pertinenza ORL]. Oto‐Rino‐Laringologia Italiana 1972;39(1):51‐62. Italian. [PubMed] [Google Scholar]
- Ott MC, Lundy LB. Tympanic membrane perforation in adults. How to manage, when to refer. Postgraduate Medicine 2001 Nov;110(5):81‐4. [DOI] [PubMed] [Google Scholar]
- Papastavros T, Giamarellou H, Varlejides S. Preoperative therapeutic considerations in chronic suppurative otitis media. The Laryngoscope 1989 Jun;99(6 Pt 1):655‐9. [DOI] [PubMed] [Google Scholar]
- Picozzi GL, Browning GG, Calder IT. Controlled trial of gentamicin and hydrocortisone ear drops in the treatment of active chronic otitis media. Clinical Otolaryngology and Allied Sciences 1983;8:367‐8. [Google Scholar]
- Picozzi GL, Browning GG, Calder IT. Controlled trial of gentamicin and hydrocortisone ear drops with and without systemic metronidazole in the treatment of active chronic otitis media. Clinical Otolayngology and Allied Sciences 1984;9:305. [Google Scholar]
- Povedano Rodriguez V, Seco Pinero MJ, Jurado Ramos A, Lopez Villarejo P. Efficacy of topical ciprofloxacin in the treatment of chronic otorrhea [Eficacia del ciprofloxacino topico en el tratamiento de la otorrea cronica]. Acta Otorrinolaryngologica Española 1995 Jan‐Feb;46(1):15‐8. Spanish. [PubMed] [Google Scholar]
- Pugliese WM, Mesches DN, Wiersum J, Henriquez CL 3rd, Krivda JF. Double‐blind study: Cleocin Palmitate and Erythrocin Pediatric in otitis media in children. Current Therapeutic Research, Clinical and Experimental 1972 Jan;14(1):31‐4. [PubMed] [Google Scholar]
- Quick CA. Comparison of penicillin and trimethoprim‐sulfamethoxazole in the treatment of ear, nose and throat infections. CMAJ: Canadian Medical Association Journal 1975 (June 14);112(13 Spec No):83S‐86S. [PMC free article] [PubMed] [Google Scholar]; Quick CA, Wagner D. Trimethoprim‐sulfamethoxazole in the treatment of infections of the ears, nose and throat. Journal of Infectious Diseases 1973 Nov;128:Suppl:S696‐700. [DOI] [PubMed] [Google Scholar]
- Rostein D. Clinical study of sulfamethoxazole trimethoprim in ENT for suppurative otitis and rhinosinusitis [Etude clinique du sulfaméthoxazol triméthoprime en O.R.L. dans les otites et les rhinosinusites suppurées.]. Revue de Laryngologie ‐ Otologie ‐ Rhinologie 1978 Jan‐Feb;99(1‐2):157‐8. French. [PubMed] [Google Scholar]
- Mr Dr Roy. A double blind randomised controlled trial of topical ciprofloxacin ear drops in the treatment of chronic suppurative otitis media. National Research Register 6 February 2003. [Serial number at source: N0077075335]
- Salzberg R. Comparative clinical and bacteriological studies with Bactrim and ampicillin in the pediatrics [Vergleichende klinische und bakteriologische Untersuchungen mit Bactrim und Ampicillin in der padiatrischen Praxis]. Schweizerische Rundschau fur Medizin Praxis 1972 Aug 15;61(33):1051‐2. German. [PubMed] [Google Scholar]
- Sambe B, Baba S, Iwasawa T. [Double blind controlled trial of pipemidic acid in the treatment of chronic suppurative otitis media]. Otologia Fukuoka 1977;23(6):807‐27. Japanese. [Google Scholar]
- Schaad UB, Farine JC, Fux T. Prospective placebo‐controlled double‐blind study using a bacterial lysate in infections of the respiratory tract and ENT region in children [Prospektive placebo‐kontrollierte Doppelblindstudie mit einem Bakterienlysat bei Infektionen der Atemwege und des ORL‐Bereiches im Kindersalter]. Helvetica Paediatrica Acta 1986 May;41(1‐2):7‐17. German. [PubMed] [Google Scholar]
- Shchechkin VN, Khokhlova PV. [Treatment of chronic suppurative otitis media]. Vestnik Otorinolaringologii 1978 Sep‐Oct;5:64‐7. Russian. [PubMed] [Google Scholar]
- Shah A, Amatya RCM, Tuldhar NR, Joshi MP. Tubotympanic chronic suppurative otitis media: Bacteriology and drug sensitivity. 2nd International SAARC ENT Congress. Extending ENT Service to the Community. 2000:Abstract 37. [Google Scholar]
- Shenderey K, Marsh BT, Talbot DJ. A multi‐centre general practice comparison of a fixed‐dose combination of pivmecillinam plus pivampicillin with amoxycillin in respiratory tract infections. Pharmatherapeutica 1985;4(5):300‐5. [PubMed] [Google Scholar]
- Smith AW, Hatcher J, Mackenzie IJ, Thompson S, Bal I, Macharia I, Mugwe P, Okoth‐Olende C, Oburra H, Wanjohi Z. Randomised controlled trial of treatment of chronic suppurative otitis media in Kenyan schoolchildren. Lancet 1996 Oct 26;348(9035):1128‐33. [DOI] [PubMed] [Google Scholar]
- Somekh E, Cordova Z. Ceftazidime versus aztreonam in the treatment of pseudomonal chronic suppurative otitis media in children. Scandinavian Journal of Infectious Diseases 2000;32(2):197‐9. [DOI] [PubMed] [Google Scholar]
- Stechenberg BW, Anderson D, Chang MJ, Dunkle L, Wong M, Reken D, Pickering LK, Feigin RD. Cephalexin compared to ampicillin treatment of otitis media. Pediatrics 1976 Oct;58(4):532‐6. [PubMed] [Google Scholar]
- Sugiyama M, Tanabe K, Chang KC, Nakai Y. Variation in bacterial count in otorrhea from cases of chronic otitis media depending upon the method of antibiotic administration. Acta Otolaryngologica 1981 Sep‐Oct;92(3‐4):285‐91. [DOI] [PubMed] [Google Scholar]
- Supiyaphun P, Tonsakulrungruang K, Chochaipanichnon L, Chongtateong A, Samart Y. The treatment of chronic suppurative otitis media and otitis externa with 0.3 per cent ofloxacin otic solution: a clinico‐microbiological study. Journal of the Medical Association of Thailand 1995 Jan;78(1):18‐21. [PubMed] [Google Scholar]
- Supiyaphun P, Kerekhanjanarong V, Koranasophonepun J, Sastarasadhit V. Comparison of ofloxacin otic solution with oral amoxycillin plus chloramphenicol ear drop in treatment of chronic suppurative otitis media with acute exacerbation. Journal of the Medical Association of Thailand 2000 Jan;83(1):61‐8. [PubMed] [Google Scholar]
- Tachibana M, Ohshima W, Mizukoshi O, Yanohara K, Nishimura T. [Clinical trial of BRL 28500 (clavulanic acid‐ticarcillin) in the treatment of chronic suppurative otitis media]. Chemotherapy 1986 Oct;34(Suppl. 4):1120‐4. Japanese. [Google Scholar]
- Tong MC, Woo JK, Hasselt CA. A double‐blind comparative study of ofloxacin otic drops versus neomycin‐polymyxin B‐hydrocortisone otic drops in the medical treatment of chronic suppurative otitis media. Journal of Laryngology and Otology 1996 Apr;110(4):309‐14. [DOI] [PubMed] [Google Scholar]
- Tong MC, Yue V, Ku PK, Hasselt CA. Preoperative topical ofloxacin solution for tympanoplasty: a randomized, controlled study. Otology and Neurotology 2002 Jan;23(1):18‐20. [DOI] [PubMed] [Google Scholar]
- Heyning PH, Pattyn SR, Valcke HD, Caeckenberghe DL, Jans HW, Boerema BJ, Chysky V. Use of Ciprofloxacin in Chronic Suppurative Otitis. Reviews of Infectious Diseases 1988;10(Suppl 1):S250‐1. [Google Scholar]
- Verhoeff M, Rovers MM, Sanders EAM, Schilder AGM. Abstracts of the Netherlands Society for Otorhinolaryngology and Cervico‐Facial Surgery, November 2003 meeting. The COCO study. A prospective, double‐blind, randomized study on the efficacy of co‐trimoxazole in children with chronic otitis media [Een prospectieve, gerandomiseerde, dubbelblinde studie naar de effectiviteit van co‐trimoxazol bij kinderen met chronische otitis media. COCO‐studie]. Nederlands Tijdschrift voor KNO‐Heelkunde 2003;9(3):129‐30. [Google Scholar]; Verhoeff M, Rovers MM, Sanders EAM, Schilder AGM. Abstracts of the Netherlands Society for Otorhinolaryngology and Cervico‐Facial Surgery, November 2003 meeting. The COCO‐study: a randomized clinical trial of the efficacy of Trimethoprim‐sulfamethoxazole (Co‐trimoxazole) in children with chronic suppurative otitis media. Clinical Otolaryngology 2004 Aug;29(4):460. [Google Scholar]
- Wilde AD, England J, Jones AS. An alternative to regular dressings for otitis externa and chronic suppurative otitis media?. Journal of Laryngology and Otolology 1995 Feb;109(2):101‐3. [DOI] [PubMed] [Google Scholar]
- Willis AT, Jones PH. Anaerobic infections and their treatment ‐ Recent trends. Journal of Pharmacotherapy 1979;2(3):122‐32. [Google Scholar]
- Yuen PW, Lau SK, Chau P, Hui Y, Wong SF, Wong S, Wei WI. Ofloxacin eardrop treatment for active chronic suppurative otitis media: prospective randomized study. American Journal of Otology 1994 Sep;15(5):670‐3. [PubMed] [Google Scholar]
- Zbären P, Pradervand M. Comparative double‐blind clinical study of co‐tetroxazine and amoxicillin in common ENT infections [Etude clinique comparative en double aveugle entre co‐tétroxacine et amoxicilline dans les infections ORL courantes]. Schweizerische Rundschau fur Medizin Praxis 1983 Nov 22;72(47):1491‐3. French. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Andersen JB. Otitis media and antibiotics [Otitis media of antibiotika]. Ugeskrift for Laeger 2002 Aug 12;164(33):3876‐7. [PubMed] [Google Scholar]
- Iarlykov SA, Poliakova SD, Zemskov AM. [Correction of immunologic disorders in patients with chronic suppurative otitis media]. Vestnik Otorinolaringologii 1995 Jan‐Feb;95(1):9‐11. Russian. [PubMed] [Google Scholar]
- Khakimov AM, Arifov SS, Faizulaeva FN. Effectiveness of antioxidant therapy of acute and chronic otitis media purulenta. Vestnik Otorinolaringologii 97;5:16‐9. [PubMed] [Google Scholar]
- McKelvie P, Johnstone I, Jamieson I, Brooks C. The effect of gentamycin ear drops on the cochlea. British Journal of Audiology 1975;9(2):45‐7. [Google Scholar]
- Nawasreh O, Fraihat A. Topical ciprofloxacin versus topical gentamicin for chronic otitis media. Eastern Mediterranean Health Journal 2001 Jan‐Mar;7(1‐2):26‐30. [PubMed] [Google Scholar]
- Ramos A, Ayudarte F, Miguel I, Cuyas J M, Cenjor C. Use of topical ciprofloxacin in chronic suppurating otitis media [Utilización del ciprofloxacino tópico en la otitis media crónica supurada]. Acta Otorrinolaringologica Espanola 2003 Aug‐Sep;54(7):485‐90. Spanish. [DOI] [PubMed] [Google Scholar]
- Sattelkau G. The Treatment of Purulent Otitis with Chloramphenicol Ear‐drops [Die Behandlung eitgriger Otitiden mit Chloramphenicol‐Ohrentropfen]. Medizinische Klinik 1962;57(50):2105‐7. [Google Scholar]
- Xu Y, Yu J, Song Q. [Clinical and experimental study on treatment of chronic pyogenic tympanitis with shenlian ear‐drops]. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional and Western Medicine / Zhongguo Zhong Xi Yi Jie He Xue Hui, Zhongguo Zhong Yi Yan Jiu Yuan Zhu Ban] 1999 Aug;19(8):477‐80. Chinese. [PubMed] [Google Scholar]
Additional references
- Acuin J, Smith A, Mackenzie I. Interventions for chronic suppurative otitis media. The Cochrane Library 1998, Issue 2. [Google Scholar]
- Acuin J. Chronic suppurative otitis media. Clinical Evidence 2004 Dec;12:710‐29. [PubMed] [Google Scholar]
- Barnett ED, Klein JO, Pelton SI, Luginbuhl LM. Otitis media in children born to human immunodeficiency virus‐infected mothers. Pediatric Infectious Disease Journal 1992 May;11(5):360‐4. [DOI] [PubMed] [Google Scholar]
- Berman S. Otitis media in developing countries. Pediatrics 1995 Jul;96(1 Pt 1):126‐31. [PubMed] [Google Scholar]
- Bluestone CD, Klein JO. Chapter 24. Intratemporal Complications and Sequelae of Otitis Media. In: Bluestone CD, Stool SE, Kenna MA editor(s). Pediatric Otolaryngology. Third Edition. Vol. 1, London: WB Saunders Company, 1996:583‐635. [Google Scholar]
- Bluestone CD. Epidemiology and pathogenesis of chronic suppurative otitis media: implications for prevention and treatment. International Journal of Pediatric Otorhinolaryngology 1998 Jan;42(3):207‐23. [DOI] [PubMed] [Google Scholar]
- Browning GG. Aetiopathology of inflammatory conditions of the external and middle ear. In: Booth JB (General Editor Kerr AG) editor(s). Scott‐Brown's Otolaryngology. Sixth. Vol. 3, Oxford: Butterworth Heinemann, 1997:3/3/1‐3/3/37. [Google Scholar]
- Browning GG. Personal Correspondence2003.
- Clarke M, Oxman AD, editors. Optimal search strategy. In: Alderson P, Green S, Higgins J, editors. Cochrane Reviewers' Handbook 4.1.6 [updated January 2003]; Appendix 5c. In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
- Cochrane Ear, Nose and Throat Disorders Group. Cochrane ENT Group Guidelines for Reviewers. November 2000.
- Couzos S, Metcalf S, Murray RB. Systematic Review of Existing Evidence and Primary Care Guidelines on the Management of Otitis Media in Aboriginal and Torres Strait Islander Populations. National Aboriginal Community Controlled Health Organisation (NACCHO). Report for the Office for Aboriginal and Torres Strait Islander Health, Commonwealth Department of Health and Aged Care, Canberra, ACT (www.health.gov.au/oatsih/pubs/index.htm). Indigenous and Public Health Media Unit, Commonwealth Department of Health and Aged Care, March 2001.
- Committee on Safety of Medicines. Reminder: ototoxicity with aminoglycoside eardrops. Current Problems in Pharmacovigilance 1997 Dec;23:14. [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG, editors. Analysing and presenting results. In: Alderson P, Green S, Higgins J, editors. Cochrane Reviewers’ Handbook 4.2.2 [updated March 2004]; Section 8. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
- Dhillon RS, East CA. The Ear. Ear, Nose and Throat and Head and Neck Surgery ‐ an illustrated colour text. Second Edition. Edinburgh: Churchill Livingstone (Harcourt Publishers Limited), 1999:12‐17 (1‐28). [Google Scholar]
- Hatcher J, Smith A, Mackenzie I, Thompson S, Bal I, Macharia I, Mugwe P, Okoth‐Olende C, Oburra H, Wanjohi Z, Achola N, Mirza N, Hart A. A prevalence study of ear problems in school children in Kiambu district, Kenya, May 1992. International Journal of Pediatric Otorhinolaryngology 1995 Nov;33:197‐205. [DOI] [PubMed] [Google Scholar]
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. British Medical Journal 2001 Jul;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbwang J, Pattou C. TDR/TDP/SOP/99.1. http://www.who.int/tdr/publications/publications/pdf/sop.pdf viewed 17 June 2003 [Standard Operating Procedures for Clinical Investigators. Final SOP]. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR)1999.
- Klein JO. The burden of otitis media. Vaccine 2000 Dec 8;19 Suppl 1:S2‐8. [DOI] [PubMed] [Google Scholar]
- Ludman H. Complications of suppurative otitis media. In: Booth JB (General Editor Kerr AG) editor(s). Scott‐Brown's Otolaryngology. Sixth. Vol. 3, Oxford: Butterworth Heinemann, 1997:3/12/1‐3/12/29. [Google Scholar]
- Marais J, Rutka JA. Ototoxicity and topical eardrops. Clinical Otolaryngology and Allied Sciences 1998 Aug;23(4):360‐7. [DOI] [PubMed] [Google Scholar]
- McPherson B, Swart SM. Childhood hearing loss in sub‐Saharan Africa: a review and recommendations. International Journal of Pediatric Otorhinolaryngology 1997 May 4;40(1):1‐18. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. Review Manager (RevMan). Version Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
- Singh A, Georgalas C, Patel N, Papesch M. ENT presentations in children with HIV infection. Clinical Otolaryngology 2003 Jun;28(3):240‐3. [DOI] [PubMed] [Google Scholar]
- The Uppsala Monitoring Centre. http://www.who‐umc.org/ viewed 17 June 2003. The UMC ‐ the Global Intelligence Network for Benefits and Risks in Medicinal Products. WHO Collaborating Centre for International Drug Monitoring2003.
- World Health Organisation. WHO/PDH/98.4. http://www.who.int/pbd/deafness/en/chronic_otitis_media.pdf [Prevention of hearing impairment from chronic otitis media. Report of a WHO/CIBA Foundation Workshop, held at The CIBA Foundation, London, UK, 19‐21 November 1996]. 1998. [Google Scholar]
- World Health Organization. WHO/PBD/PDH/00.10 http://www.who.int/pbd/deafness/activities/en/capetown_final_report.pdf [Report of the International Workshop on Primary Ear and Hearing Care, Cape Town, South Africa, 12‐14 March 1998. Co‐spondored by The WHO Regional Office for Africa, Harare, Zimbabwe; Prevention of Blindness and Deafness, WHO, Geneva, Switzerland; and The University of Cape Town, South Africa]. 2000. [Google Scholar]
- World Health Organization. http://www.who.int/whr/2002/whr2002_annex2.pdf and http://www.who.int/whr/2002/whr2002_annex3.pdf [Annex Tables 2 and 3, 'Deaths', and 'Burden of disease in DALYs', respectively, 'by cause, sex and mortality stratum in WHO Regions, estimates for 2001']. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. 2002:186‐97. [Google Scholar]
- The International Bank for Reconstruction and Development / The World Bank. Appendix B. The global burden of disease, 1990. World development Report 1993: Investing in Health. Oxford: Oxford University Press, 1993:213‐25. [Google Scholar]


