Abstract
Background
Inflammatory processes involving cytokines, prostaglandins, free radicals and glial cells have been implicated in the pathogenesis of Alzheimer's disease. Non‐steroidal anti‐inflammatory drugs such as indomethacin attenuate inflammatory reactions. Hence, there may be a role for some of these drugs in the treatment of Alzheimer's disease.
Objectives
To examine the efficacy of indomethacin in the treatment of patients suffering from Alzheimer's disease.
Search methods
The trials were identified from a search of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (which contains records from many different medical and trials databases) on 14 April 2004 using the terms "indomethacin", "indome*" and "NSAIDS". In addition two independent reviewers systematically searched relevant computerized databases and Internet sites. This was supplemented by hand searching and additional references sought from selected papers.
Selection criteria
Single or multi‐centre placebo‐controlled randomized trials examining the efficacy of indomethacin in patients diagnosed with Alzheimer's disease were eligible for selection for this review. Using a standard extraction form, inclusion/exclusion criteria were set to ensure design quality and lack of bias of all trials included.
Data collection and analysis
Data were collected independently by two reviewers and any discrepancies were subject to discussion. Corresponding authors were contacted for any missing data needed for statistical analysis.
Main results
Only one study was selected for this review (Rogers 1993). We detected no statistically significant difference between indomethacin treatment and placebo for the individual cognitive tests: Mini Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale ( ADAS), Boston Naming Test (BNT) and Token Test (TK). Dropouts and death rate were the only reported results that were amenable to evaluation. The dropout rate was higher in the indomethacin group (10/24) than in the control group (6/20). Gastrointestinal adverse events were more prevalent in the treatment group (5/24 compared with 1/20 in control group). There was no statistically significant difference in death rate between the two groups (p=0.9).
Authors' conclusions
On the basis of this one trial and subsequent analysis of data as reported by the authors, indomethacin cannot be recommended for the treatment of mild to moderate severity Alzheimer's disease. At doses of 100‐150 mg daily, serious side effects will limit its use.
Keywords: Humans; Alzheimer Disease; Alzheimer Disease/drug therapy; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Indomethacin; Indomethacin/therapeutic use
No evidence for efficacy and safety of indomethacin for treatment of mild to moderate Alzheimer's disease
Extensive evidence implicates inflammatory processes in the pathogenesis of Alzheimer's disease. Non‐steroidal anti‐inflammatory drugs such as indomethacin have been proposed for the treatment of patients with Alzheimer's disease. Only one study met criteria for inclusion. In this one selected trial, authors did not carry out statistical analyses on the absolute change from baseline, but on the percentage change from the baseline score. Taking into account the difficulties in evaluating a single trial, at present there is no indication for treatment of mild to moderate Alzheimer's disease with indomethacin.
Background
The deposition of beta‐amyloid in the brain is a defining event in the pathogenesis of Alzheimer's disease (AD). Senile plaques seen in AD contain activated microglial cells (O'Banion 1996) which generate cytokines such as interleukin‐1 (IL‐1) and interleukin‐6 (IL‐6). These in turn induce glial cells such as astrocytes to produce more substances that perpetuate further the inflammatory process (Floyd 1999). The mediators of toxicity in AD are also enhanced by a process of "reciprocal induction" between beta‐amyloid and cytokines leading to a corresponding increase in the levels of these substances (Tabet 2000). In addition, beta‐amyloid fibrils bind acute phase proteins and activate the complement cascade (Kalaria 1996). Complement activation products in the brain then contribute to inflammation and stimulate microglia to produce neurotoxic free radicals (Eikelenboom 1996).
Prostaglandins, which are derived from arachidonic acid through the action of cyclooxygenase, are additional significant players in chronic inflammation. Prostaglandins are known to enhance toxicity and induce cytokine synthesis (Hull 2000). Levels of prostaglandins can be increased by inflammatory mediators such as nitric oxide and IL‐1 (Dayton 1996; Fiebich 1997; Clancy 2000). Inhibition of the enzymatic activity of cyclooxygenase by non steroidal anti‐inflammatory drugs (NSAIDs) results in a subsequent attenuation of the inflammatory process through a decrease in the release of prostaglandins, cytokines and other inflammatory mediators (Fiebich 1997). Indomethacin, which crosses the blood brain barrier, is a potent non‐selective cyclooxygenase inhibitor and was one of the earliest NSAIDs. It has been shown to reduce inflammatory reactions by decreasing IL‐6 through the inhibition of prostaglandin E2 (Bour 2000). Indomethacin might also inhibit production of IL‐1 and nitric oxide and significantly attenuates the activation of microglia in response to beta‐amyloid infusion in rat brain (Netland 1998). Indomethacin has anti‐inflammatory, analgesic and antipyretic actions. It is rapidly absorbed from the gut with peak plasma levels occurring 30‐120 minutes after intake. It is 90‐94% protein‐bound and is metabolised by bio‐transformation in the liver (Hellberg 1981).
In addition to animal and human autopsy brain studies, evidence supporting a role for inflammatory process in AD comes from retrospective epidemiological surveys of people who have received NSAIDs in the past or had been diagnosed with rheumatoid arthritis (RA), for which these drugs are routinely prescribed. McGeer 1996 reviewed 17 such studies and reported that both people taking anti‐inflammatory drug treatment and people with RA are less likely to develop AD. In a longitudinal study of 1686 people, Stewart 1997 found that AD risk is lower for people who had taken NSAIDs for longer. However, Beard 1998, who undertook a retrospective case‐control study, found no statistically significant inverse association between the use of NSAIDs and AD; nonetheless, the data were suggestive of a protective effect of such drugs against AD. In addition, in a recent prospective, population‐based cohort study of subjects 55 years of age or older who were free of dementia at baseline, long‐term use of NSAIDs could protect against AD but not against vascular dementia (int' Veld 2001). It is clear that only controlled randomized trials using NSAIDs such as indomethacin can determine the real effectiveness of such drugs for AD sufferers. In this paper we review published trials to ascertain whether the use of indomethacin is justified for AD patients.
Objectives
The objective of this review was to assess the safety and efficacy of indomethacin treatment in people diagnosed with AD.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, double‐blind unconfounded trials comparing indomethacin with placebo were selected.
Types of participants
Individuals with probable Alzheimer's disease fulfilling standard diagnostic criteria including NINCDS‐ADRDA (McKhann 1984), DSM (APA 1994) and ICD (WHO 1992) were eligible for inclusion. Patients recruited for the trial were to have had no known extensive prior use of indomethacin.
Types of interventions
Comparisons of indomethacin treatment at any dose with placebo.
Types of outcome measures
Clinical global impression of change
Cognition (objective psychometric rating instruments, for example the Alzheimer's Disease Assessment Scale, cognitive subscale (ADAS‐COG))
Global severity of dementia
Mood/depression
Behavioural symptoms
Activities of daily living
Physical disability affecting mobility
Institutionalization
Death
Search methods for identification of studies
The trials were identified from a last updated search of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group on 14 April 2004 using the terms "indomethacin", "indome*", "NSAID".
The Specialized Register at that time contained records from the following databases:
CENTRAL: The Cochrane Library Issue 1, 2004;
MEDLINE (January 1966 to February 2004);
EMBASE (January 1980 to February 2004);
PsycINFO (January 1887 to January 2004);
CINAHL (January 1982 to January 2004);
SIGLE: Grey Literature in Europe (January 1980 to December 2002);
ISTP: Index to Scientific and Technical Proceedings (to May 2000);
INSIDE: British Library database of Conference Proceedings and Journals (to June 2000);
Aslib: Index to Theses (UK and Ireland theses) (January 1970 to March 2003);
Dissertation Abstract (USA): (January 1861 to March 2003);
ADEAR: Alzheimer's Disease Clinical Trials Database (to March 2004);
National Research Register: Issue 1, 2004;
Current Controlled trials (last searched March 2004) which includes:
Alzheimer Society
GlaxoSmithKline
HongKong Health Services Research Fund
Medical Research Council (MRC)
NHS R&D Health Technology Assessment Programme
Schering Health Care Ltd
South Australian Network for Research on Ageing
US Dept of Veterans Affairs Cooperative Studies
National Institutes of Health (NIH)
ClinicalTrials.gov (last searched March 2004);
LILACS: Latin American and Caribbean Health Science Literature (last searched April 2003).
The search strategies used to identify relevant records in MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS can be found in the Group's module on The Cochrane Library.
In addition two independent reviewers systematically searched relevant computerized databases and Internet sites. This was supplemented by hand searching and additional references sought from selected papers.
Data collection and analysis
SELECTION OF STUDIES Two reviewers (NT, HF) assessed all references identified in the search and independently selected studies for inclusion. Any disparity between the reviewers' lists was resolved by discussion.
QUALITY ASSESSMENT The quality of the selected trials was assessed using methods described in the Cochrane Reviewers' Handbook (2001).
DATA EXTRACTION Data for the systematic review are based on summary statistics for each study. For the intention‐to‐treat analyses we sought data for each outcome measure on every patient randomized, irrespective of compliance. For the analyses of completers we sought data on every patient who completed a study on treatment or placebo.
For continuous variables, or ordinal variables that could be approximated to continuous variables, the main outcome of interest is the final assessment and the change from baseline at final assessment. For some ordinal and binary outcomes, the endpoint category relative to baseline category is the outcome of interest. For others, such as the global impression of change, the endpoint itself is relevant as all patients would be by definition at the same baseline score. The baseline assessment is defined as the latest available assessment prior to randomization, but no longer than two months before.
DATA ANALYSIS A vast number of rating scales and tests has been devised to assess outcomes in clinical trials testing treatments for dementia. There is much duplication, in so far as each scale purports to assess one of the five or six main characteristics of dementia, but with varying procedures. Systematic reviews are fairly straightforward in the situation where the included studies use the same outcome measures and the method of weighted mean difference can be used.
When different scales have been used in the studies, we have used the method of standardized mean difference. For continuous or ordinal variables, such as psychometric test scores, clinical global impression scales, and quality of life scales there are two possible approaches. If ordinal scale data appear to be approximately normally distributed, or if the analyses reported by the investigators suggest that parametric methods and a normal approximation are appropriate, then the outcome measures can be treated as continuous variables. The second approach, which may not exclude the first, is to concatenate the data into two categories which best represent the contrasting states of interest, and to treat the outcome measure as binary. For binary outcomes, the endpoint itself is of interest and the Peto method of the typical odds ratio can be used. A test for heterogeneity of treatment effect between the trials can be made using a chi‐square statistic. If no heterogeneity is indicated then a fixed effect parametric approach can be taken.
Results
Description of studies
The reviewers only found one trial that met the selection criteria. This was a six‐month, double‐blind, placebo‐controlled study testing the efficacy of indomethacin for people with mild to moderate AD (Rogers 1993). 66 patients had a preliminary diagnosis of AD and 44 of these met the selection criteria that included clinical diagnosis of AD according to the NINCDS‐ADRDA (McKhann 1984) and a Mini‐Mental State Examination (MMSE) (Folstein 1975) score of 16 or greater. Patients were randomly assigned to one of two groups, placebo or indomethacin. Indomethacin was taken three times a day with a total daily dose of 100‐150 mg, depending on body weight. All patients were assessed at baseline using MMSE score, Alzheimer's Disease Assessment Scale (ADAS) (Rosen 1984), Boston Naming Test (BNT) and Token Test (TK). Rogers 1993 refers to ADAS under the general heading of cognitive tests and so we assume that here ADAS means ADAS‐COG. The MMSE is the most widely used screening test of cognitive abilities. It is brief, easy to use and has a high inter‐rater reliability. A score of 24 has been used as a cut‐off point. The ADAS‐COG is a valuable but more extensive screening test which has been used for the diagnosis and staging of dementia disorders, especially AD. BNT is widely used in aphasia patients and consists of 60 line drawings. In this test standard stimulus and phonetic cues are given if items are unnamed. The TK is a sensitive and reliable test of auditory comprehension (Hodges 1994).
We note that Rogers (Rogers 1993) reversed the sign on the change in ADAS so that a positive change indicates improvement. This was to be consistent with the other three cognitive tests and although not mentioned in the paper was confirmed by Rogers (personal communication).
The indomethacin and placebo groups were found to be similar at baseline for age, sex and scores on the four outcome measures selected. A caregiver was entrusted with the tablets and bottles were checked at three months and six months after the start of the study. Indomethacin and placebo were given for six consecutive months and all patients completing the protocol were assessed immediately after the end of the study for the same four outcome measures (MMSE, ADAS‐COG, BNT and TK). For each patient, for each of the four outcomes scores, the percentage change from baseline was calculated ((final score‐baseline score)/baseline score) and the sum of the four percentage changes. The efficacy of indomethacin in the treatment of AD compared with placebo was evaluated using each percentage change separately, and by using the sum of the percentage changes.
Risk of bias in included studies
Patients were randomly assigned to either group (indomethacin or placebo) in a double‐blind protocol. No further details of the randomization process were given. Patients withdrew before completion of treatment because of side effects, loss of carers, or death.
Effects of interventions
There was only one included trial (Rogers 1993) and all results are from that trial. Rogers reported results of four cognitive tests (ADAS‐COG, MMSE, BNT and TK), side effects and withdrawals at six months. Rogers 1993 analysed the percentage changes in cognitive tests from baseline. We repeated these analyses and the results are shown in the analyses (Comparison 01, outcomes 05, 06, 07, 08), but the difference between treatment and placebo has been tested using a two‐sided t‐test, not the one‐sided test used by Rogers 1993. There were no statistically significant differences between treatment and placebo except for ADAS‐COG where benefit was seen for treatment (MD 14.70, 95% Confidence Interval (CI) 0.11, 29.29, P=0.05). We did not repeat the analysis of the sum total of the four tests.
Of the 16 patients who withdrew from the study before the 6 months end point, 10 were in the indomethacin group and 6 in the placebo group, but there was no statistical difference between the groups, (odds ratio 1.64, 95% CI 0.48 to 5.54, P=0.4).
There were no statistically significant differences between indomethacin and placebo groups for: ‐ withdrawals due to adverse physical events (8/24 indomethacin compared with 2/20 placebo ) (odds ratio 3.66, 95% CI 0.9 to 14.85; P=0.07), ‐ withdrawals due to gastrointestinal adverse events (indomethacin 5/24 compared with 1/20 placebo) (odds ratio 3.72, 95% CI 0.67 to 20.56, P=0.13), ‐ withdrawals due to headache (indomethacin 1/24 compared with 0/20 placebo) (odds ratio 6.25, 95% CI 0.12 to 320.4, P=0.36), ‐ withdrawals due to stroke (indomethacin 1/24 compared with 0/20 placebo) (odds ratio 6.25, 95% CI 0.12 to 320.4, P=0.36), ‐ death (indomethacin 1/24 compared with 1/20 placebo) (odds ratio 0.83, 95% CI 0.05 to 13.85, P=0.9), There was a difference in favour of indomethacin for withdrawals due to behavioural problems (indomethacin 0/24 compared with 4/20 placebo) (odds ratio 0.09, 95% CI 0.01 to 0.72, P=0.02).
Discussion
Only one study (Rogers 1993) is included in this systematic review of indomethacin treatment in AD patients. 44 selected patients had mild to moderate disease and were randomized to treatment with indomethacin 100 to 150 mg daily for six months or placebo. The patients were assessed at baseline and at six months. Cognitive function was assessed using four rating scales, ADAS‐COG, MMSE, TK and BNT. There were several ways to adjust the analyses for random variation between people at baseline and Rogers 1993 chose the change from baseline as a percentage of the baseline score. The reason given was to improve the distributional properties of the variables analysed, presumably to ensure that they met the assumptions of the methods used. Analysing the percentage changes is likely to introduce problems as the baseline scores are variable and those with lower baseline values show greater change compared with the same absolute change at a higher baseline value. It would be preferable to perform the analysis with a baseline adjustment carried out either by using the baseline as a covariate in the analysis, or by analysing the change from baseline. Using the data as reported by Rogers 1993 there was no statistical difference between indomethacin and placebo for MMSE, BNT and TK, and a significant difference for ADAS‐COG (P=0.05). Due to our reservations about the method of analysis used by Rogers 1993, we conclude that there is no evidence of difference between indomethacin and placebo.
More patients were withdrawn from the study in the indomethacin group than in the placebo group, but the difference was not statistically significant. Interestingly, the reason that four patients from the placebo group were withdrawn was because of the development of behavioural problems, and none from the indomethacin group were withdrawn for this reason. Although indomethacin has been associated with the development of confusional states which may result in significant behavioural problems, this is much less likely to be a side effect of a placebo.
Indomethacin is a classical NSAID with a significant risk of side effects, especially for old people. This point should be taken into account before recommending indomethacin for treatment of AD patients. In this study a third of patients receiving indomethacin withdrew due to adverse events (stroke, gastrointestinal, headache and death). However, when compared with patients who were on placebo (excluding those with behavioural problems) this did not reach significant levels.
Although this is a ground‐breaking double‐blind randomized trial, interpretation of the results must take into account three important facts. Firstly, only 14 patients completed the study in each group over a 6‐month period. Secondly, no statistically significant benefits for the indomethacin group were observed in any of the four outcome measures separately. Thirdly, and perhaps most importantly, the side effects of indomethacin will limit its use among older people. In view of these observations, indomethacin cannot be recommended for the routine treatment of AD patients. Further research is needed on the efficacy of NSAIDs, especially those with fewer side effects such as the new generation cox‐2 inhibitors, on the manifestations and progression of AD.
Authors' conclusions
There is currently no evidence supporting the use of indomethacin in mild to moderate Alzheimer's disease.
Pro‐inflammatory agents such as free radicals and cytokines may participate in "reciprocal induction" of toxicity in AD, a process which may be initiated and maintained by beta‐amyloid deposition. NSAIDs may contribute to the treatment of AD by limiting toxicity.
This first published clinical trial on indomethacin for people with AD has demonstrated the feasibility of trials of this agent. Because the results do not rule out the possibility of important beneficial effects, there is a need to proceed to larger scale studies of NSAIDs measuring effects on both the manifestations of the disease and on its rate of progression. Any beneficial effects detected will need to be considered together with the known side effects of indomethacin and other NSAIDS.
Acknowledgements
We wish to thank Jacqueline Birks for her valued contribution and advice on the preparation of this review and Delores Williams for her input as Consumer editor.
Data and analyses
Comparison 1.
Indomethacin vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of withdrawals before end of treatment due to physical adverse events (excluding behavioural problems) | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.66 [0.90, 14.85] |
| 2 Number of withdrawals before end of treatment | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [0.48, 5.54] |
| 3 Number of deaths before end of treatment | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.05, 13.85] |
| 4 Number of withdrawals before end of treatment due to stroke | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.25 [0.12, 320.40] |
| 5 Number of withdrawals before end of treatment due to gastrointestinal side effect | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.72 [0.67, 20.56] |
| 6 Number of withdrawals before end of treatment due to headache | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.25 [0.12, 320.40] |
| 7 Number of withdrawals before end of treatment due to behavioural problems | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.09 [0.01, 0.72] |
| 8 ADAS, percentage change at 6 months relative to baseline | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 14.70 [0.11, 29.29] |
| 9 MMSE, percentage change at 6 months relative to base line | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 12.5 [‐0.29, 25.29] |
| 10 Boston Naming Test, percentage change at 6 months relative to base line | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 11.00 [‐1.99, 23.99] |
| 11 Token Test, percentage change at 6 months relative to baseline | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [‐5.13, 6.93] |
Analysis 1.1.
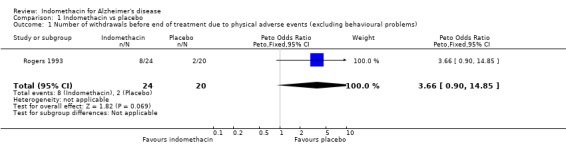
Comparison 1 Indomethacin vs placebo, Outcome 1 Number of withdrawals before end of treatment due to physical adverse events (excluding behavioural problems).
Analysis 1.2.
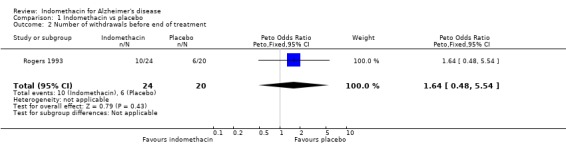
Comparison 1 Indomethacin vs placebo, Outcome 2 Number of withdrawals before end of treatment.
Analysis 1.3.
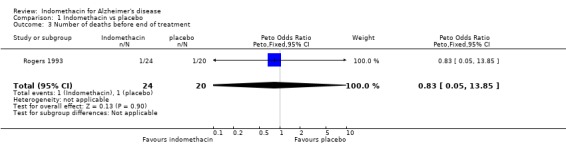
Comparison 1 Indomethacin vs placebo, Outcome 3 Number of deaths before end of treatment.
Analysis 1.4.
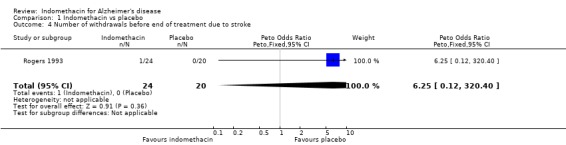
Comparison 1 Indomethacin vs placebo, Outcome 4 Number of withdrawals before end of treatment due to stroke.
Analysis 1.5.
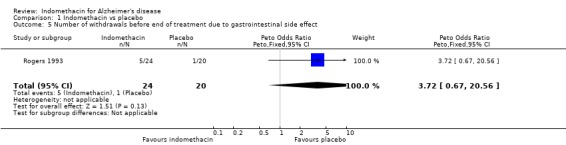
Comparison 1 Indomethacin vs placebo, Outcome 5 Number of withdrawals before end of treatment due to gastrointestinal side effect.
Analysis 1.6.
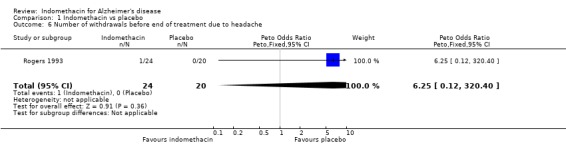
Comparison 1 Indomethacin vs placebo, Outcome 6 Number of withdrawals before end of treatment due to headache.
Analysis 1.7.
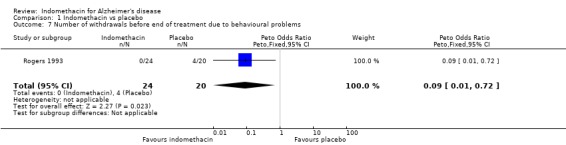
Comparison 1 Indomethacin vs placebo, Outcome 7 Number of withdrawals before end of treatment due to behavioural problems.
Analysis 1.8.
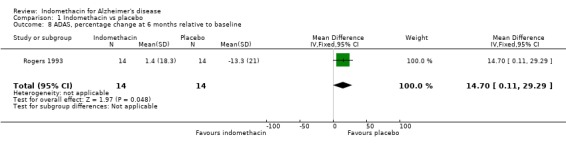
Comparison 1 Indomethacin vs placebo, Outcome 8 ADAS, percentage change at 6 months relative to baseline.
Analysis 1.9.
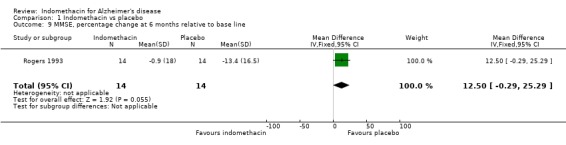
Comparison 1 Indomethacin vs placebo, Outcome 9 MMSE, percentage change at 6 months relative to base line.
Analysis 1.10.
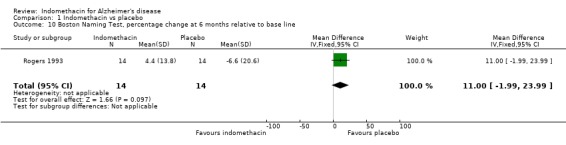
Comparison 1 Indomethacin vs placebo, Outcome 10 Boston Naming Test, percentage change at 6 months relative to base line.
Analysis 1.11.
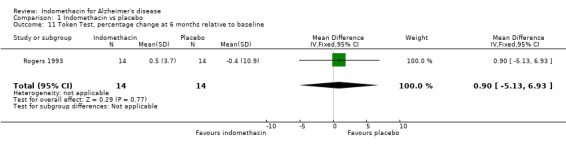
Comparison 1 Indomethacin vs placebo, Outcome 11 Token Test, percentage change at 6 months relative to baseline.
What's new
| Date | Event | Description |
|---|---|---|
| 6 May 2008 | Review declared as stable | This review will be withdrawn as it will be subsumed by the review on Aspirin and anti‐inflammatory drugs for Alzheimer's disease which is in preparation |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 24 May 2004 | New search has been performed | An update search was run in April 2004, which retrieved no new studies for inclusion/exclusion |
| 27 February 2002 | New citation required and conclusions have changed | Substantive amendment |
Contributions of authors
‐NT: was the main reviewer who searched the literature, selected studies, performed data analysis, wrote the first draft and amended subsequent drafts accordingly. He is also responsible for updates. ‐HF: was the co‐reviewer who searched the literature, selected studies and reviewed drafts.
‐Consumer editor: Delores Williams
‐CDCIG Contact editor: Lon Schneider
Sources of support
Internal sources
Department of Old Age Psychiatry, Maudsley Hospital, UK.
External sources
NHS, UK.
Declarations of interest
Dr Feldman has been a paid consultant and received honoraria serving on advisory boards for Pfizer, Janssen, Eisai, Lilly, Novartis, Servier, Sanofi Synthelabo and Tarqacept.
Notes
When the full review of "Aspirin and anti‐inflammatory drugs for Alzheimer's disease" is published, it will replace the previously published reviews "Ibuprofen for Alzheimer's disease", "Indomethacin for Alzheimer's disease", and the previously published protocol "Naproxen for Alzheimer's disease".
At that time, these reviews and this protocol will be withdrawn from The Cochrane Library.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
- Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, Zalinski J, Cofield M, Mansukhani L, Willson P, Kogan F. Clinical trial of indomethacin in Alzheimer's disease. Neurology 1993;43(8):1609‐1611. [DOI] [PubMed] [Google Scholar]
Additional references
- APA. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington DC: APA, 1994. [Google Scholar]
- Beard CM, Waring SC, O'Brien PC, Kurland LT, Kokmen E. Nonsteroidal anti‐inflammatory drug use and Alzheimer's disease: a case‐control study in Rochester, Minnesota, 1980 through 1984. Mayo Clinic Proceedings 1998;73:951‐955. [DOI] [PubMed] [Google Scholar]
- Bour AM, Westendorp RG, Laterveer JC, Bollen EL, Remarque EJ. Interaction of indomethacin with cytokine production in whole blood. Potential mechanism for a brain‐protective effect. Experimental Gerontology 2000;35(8):1017‐1024. [DOI] [PubMed] [Google Scholar]
- Clancy R, Varenika B, Huang W, Ballou L, Attur M, Amin AR, Abramson SB. Nitric oxide synthase/cox cross‐talk: nitric oxide activates COX‐1 but inhibits COX‐2 derived prostaglandin production. Journal of Immunology 2000;165(3):1582‐1587. [DOI] [PubMed] [Google Scholar]
- Dayton ET, Major EO. Recombinant human interleukin 1b induces production of prostaglandins in primary human fetal astrocytes and immortalized human fetal astrocyte cultures. Journal of Neuroimmunology 1996;71:11‐18. [DOI] [PubMed] [Google Scholar]
- Dollery C. Indomethacin. In: Dollery C editor(s). Therapeutic Drugs. Vol. 1, Unknown: Unknown, 1991:135‐138. [Google Scholar]
- Du ZY, Li XY. Inhibitory effect of indomethacin in interleukin‐1 and nitric oxide production in rat microglia in vitro. International Journal of Immunopharmacology 1999;21(3):219‐225. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Veerhuis R. The role of complement and activated microglia in the pathogenesis of Alzheimer's disease. Neurobiology of Aging 1996;17:673‐680. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Hull M, Lieb K, Gyufko K, Berger M, Bauer J. Prostaglandin E2 induces interleukin‐6 synthesis in human astrocytoma cells. Journal of Neurochemistry 1997;68:704‐709. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Neuroimmunology processes are important in neurodegenerative diseases: an hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radical Biology and Medicine 1999;26:1346‐1355. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 'Mini‐mental State': A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
- Hellberg K. Clinical pharmacokinetics of indomethacin. Clinical Pharmacokinetics 1981;6:245‐258. [DOI] [PubMed] [Google Scholar]
- Hodges JR. Cognitive assessment for clinicians. Oxford: Oxford Medical Publications, 1994. [Google Scholar]
- Hull M, Lieb K, Fiebich BL. Anti‐inflammatory drugs: a hope for Alzheimer's disease?. Expert Opinion on Investigational Drugs 2000;9(4):671‐683. [DOI] [PubMed] [Google Scholar]
- ICD10. ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Description and Diagnostics Guidelines.. Vol. none, Geneva: World Health Organisation, 1992. [Google Scholar]
- int' Veld BA, Ruitenberg A, Hofman A, Launer LJ, Duijin CM, Stijnen T, Breteler MMB, Stricker BHC. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. New England Journal of Medicine 2001;345:1515‐1521. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Harshbarger‐kelly M, Cohen DL, Premkumar DRD. Molecular aspects of inflammatory and immune responses in Alzheimer's disease. Neurobiology of Aging 1996;17:687‐693. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti‐inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiological studies. Neurology 1996;47:425‐432. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;4:933‐944. [DOI] [PubMed] [Google Scholar]
- Netland EE, Newton JL, Majocha RE, Tate BA. Indomethacin reverses the microglial response to amyloid beta‐protein. Neurobiology of Aging 1998;19(3):201‐204. [DOI] [PubMed] [Google Scholar]
- O'Banion MK, Finch CE. Inflammatory mechanisms and anti‐inflammatory therapy in Alzheimer's disease. Neurobiology of Aging 1996;17:669‐671. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356‐64. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology 1997;48:626‐632. [DOI] [PubMed] [Google Scholar]
- Tabet N, Mantle D, Orrell M. Free radicals as mediators of toxicity in Alzheimer's disease: a review and hypothesis. Adverse Drug Reactions and Toxicological Reviews 2000;19(2):127‐152. [PubMed] [Google Scholar]
- World Health Organisation. International Classification of Disease (ICD‐10). Geneva: WHO, 1992. [Google Scholar]
References to other published versions of this review
- Tabet N, Feldman H. Indomethacin for Alzheimer's disease. Cochrane Database of Systematic Reviews 2, Issue 2 10.1002/14651858.CD003673 10.1002/14651858.CD003673. [DOI: 10.1002/14651858.CD003673] [DOI] [PMC free article] [PubMed] [Google Scholar]


